Summary
Screening for aorto‐iliac stenosis is important in kidney transplant candidates as its presence affects pre‐transplantation decisions regarding side of implantation and the need for an additional vascular procedure. Reliable imaging techniques to identify this condition require contrast fluid, which can be harmful in these patients. To guide patient selection for these imaging techniques, we aimed to develop a prediction model for the presence of aorto‐iliac stenosis. Patients with contrast‐enhanced imaging available in the pre‐transplant screening between January 1st, 2000 and December 31st, 2018 were included. A prediction model was developed using multivariable logistic regression analysis and internally validated using bootstrap resampling. Model performance was assessed with the concordance index and calibration slope. Three hundred and seventy‐three patients were included, 90 patients (24.1%) had imaging‐proven aorto‐iliac stenosis. Our final model included age, smoking, peripheral arterial disease, coronary artery disease, a previous transplant, intermittent claudication and the presence of a femoral artery murmur. The model yielded excellent discrimination (optimism‐corrected concordance index: 0.83) and calibration (optimism‐corrected calibration slope: 0.91). In conclusion, this prediction model can guide the development of standardized protocols to decide which patients should receive vascular screening to identify aorto‐iliac stenosis. External validation is needed before this model can be implemented in patient care.
Keywords: cardiovascular disease, kidney transplantation, recipient screening
The presence of aorto‐iliac stenosis affects pre‐transplant decisions regarding the side of implantation and the need for an additional vascular procedure. Our prediction model can guide the development of standardized protocols regarding which patients should be screened with excellent discrimination and calibration.

Introduction
Kidney transplantation is the best treatment for end‐stage renal disease (ESRD) because it improves life expectancy and quality of life [1]. Many renal disease patients suffer from cardiovascular disease or it is accidentally discovered when screening for kidney transplantation. This is not surprising as many of the important risk factors for cardiovascular disease are present in the ESRD population [2]. Therefore, a personalized cardiovascular work‐up is crucial to identify and treat underlying cardiovascular disease to optimize post‐transplant outcomes. Aorto‐iliac stenosis is one of these conditions that should be identified before transplantation as it affects pre‐transplantation decisions. Transplantation of the donor kidney below a stenosis can cause arterial inflow problems to the transplant, which may lead to inadequate perfusion of the donor kidney resulting in allograft dysfunction and steal syndrome [3]. Therefore, in the case of an ipsilateral stenosis, the kidney is ideally transplanted on the contralateral side. In the case this is not possible or in the case of a bilateral stenosis, an additional vascular procedure is needed. Depending on stenosis severity, a pre‐transplantation percutaneous transluminal angioplasty (PTA), endarterectomy or vascular bypass is performed [4, 5, 6]. These procedures should be planned before or together with the transplantation, which accentuates the need for pre‐transplantation vascular screening [5, 6, 7].
Selection of patients that should be screened for aorto‐iliac stenosis remains difficult because important guidelines, such as the Kidney Disease Improving Global Outcomes (KDIGO), lack clear recommendations [8]. Duplex ultrasound is a non‐invasive method to detect aorto‐iliac stenosis with acceptable sensitivity. However, due to the growing prevalence of obesity, this method becomes less reliable [9, 10, 11]. Contrast‐enhanced imaging is required to identify aorto‐iliac stenosis with high sensitivity, but this should be administered carefully in patients with chronic kidney disease stages 4 and 5 [12]. Contrast‐induced nephropathy is prevalent in these patients with a reported incidence of 27% [13]. This is detrimental for the remaining kidney function, especially in pre‐emptive kidney transplant candidates. Therefore, careful patient selection for these imaging techniques is not only important from a cost‐effectiveness perspective, but also because of potential adverse effects.
A recent survey among 161 transplant surgeons found that kidney transplant candidates are mostly empirically selected for vascular screening based on various risk factors for vascular disease [14]. Twenty‐five percent answered that no vascular screening protocol is in place while 17.4% answered that all patients above a certain age are screened [14]. These differences in current practice accentuate the need to develop standardized screening protocols that minimize unnecessary exposure of patients to the risk of radiation and contrast. To guide development of these protocols, the aim of the current study was to develop a simple prediction model for aorto‐iliac stenosis in kidney transplant candidates based on patient characteristics, history and physical examination.
Methods
Study design and population
This retrospective, single‐centre, cohort study included all adult kidney transplant recipients, transplanted between 2000 and 2018, who received contrast‐enhanced imaging in the pre‐transplantation work‐up. Patients were divided into two groups, based on the presence or absence of a haemodynamically significant aorto‐iliac stenosis. The study was executed at Erasmus MC University Medical Center in Rotterdam, the Netherlands. The study was approved by the local Ethics Committee and was conducted in accordance with the provisions of the Declaration of Helsinki (MEC 2017‐1039).
Assessment of the outcome
Contrast‐enhanced abdominal magnetic resonance angiography (MRA), abdominal computed tomography angiography (CTA) and conventional angiography were used for the assessment of aorto‐iliac stenosis. All imaging was evaluated by a radiologist on the presence of aorto‐iliac stenosis. A stenosis had to cause at least 50% lumen narrowing to be haemodynamically significant. At the time of the study, there was no standardized protocol in place which patients should undergo contrast‐enhanced imaging and the decision to perform additional imaging was up to the transplant surgeon or referring nephrologist.
Potential candidate predictors
The following patient characteristics were collected from the electronic patient files as potential predictors: age, sex, body mass index (BMI), smoking history, diabetes mellitus, chronic obstructive pulmonary disease (COPD), peripheral arterial disease (PAD), history with cerebrovascular disease [cerebrovascular accident (CVA), transient ischemic attack (TIA) or amaurosis fugax] or coronary artery disease, dyslipidaemia, hypertension, previous transplant, duration of renal replacement therapy and intermittent claudication complaints upon screening. In addition, weak inguinal pulsations and the presence of a femoral artery murmur were included as candidate predictors. Coronary artery disease included acute coronary syndrome (unstable angina pectoris, non‐ST and ST elevation myocardial infarction) or a diagnosis of single, double or triple coronary artery disease confirmed with coronary angiography. Peripheral arterial disease was defined as stenotic arterial disease below Poupart’s ligament including Fontaine stage II–IV [15]. This included patients requiring endovascular or surgical revascularization and patients treated conservatively with exercise therapy. Intermittent claudication was defined as pain in the calf, thigh or buttock, which is provoked by exertion and relieved after a few minutes of rest.
Statistical analysis
For continuous variables, a normal distribution of the sample means was assumed because of the large sample size according to the central limit theorem. Therefore, continuous baseline characteristics were presented as mean with standard deviation (SD) and compared with unpaired t‐tests. Categorical baseline characteristics were presented as number with percentage and compared with chi‐square tests. Because of missing values in the covariates, the building of the prediction model required proper handling of missing data. In this study, an MI‐Boot strategy was used to build the model [16]. Missing values in the dataset were assumed to be missing at random and were imputed using fully conditional specification multiple imputation. The number of imputed datasets was based on the average percentage of missing values per variable with missing values [17]. Logistic regression analysis was used for the imputation of binary variables, and multiple linear regression was used for the imputation of normally distributed variables. Predictive mean matching was used for imputation of non‐normally distributed variables. Pooling of the data was performed using Rubin’s rules [18]. The number of iterations needed was until the sampling distribution converged, which was observed with a trace plot. The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement was followed for reporting the development of the prediction model [19]. The approach as described by Hosmer and Lemeshow was adopted for model development [20]. A univariable logistic regression analysis was performed to assess associations between the candidate predictors and the outcome variable. Candidate predictors with a P‐value below 0.2 on univariable analysis were selected for multivariable analysis. Variables with a P‐value > 0.1 were one‐by‐one deleted from the full model in a backward elimination approach unless the likelihood ratio test was significant or a shift of ≥20% in coefficients of the remaining variables was observed. Consequently, all candidate predictors that were previously left out were one‐by‐one added if they added significance to the model. After this step, the preliminary main effects model was reached. The assumption of linearity for continuous predictors was assessed by plotting the values against the log odds. If the linearity assumption was violated, natural cubic splines with three degrees of freedom were added. Multicollinearity between the predictor variables was analysed using the variance inflation factor (VIF). VIF values <4 were considered acceptable [21]. Clinically plausible interactions between selected variables in the preliminary main effects model were tested using forward selection with a P‐value of 0.01 for all two‐way interactions. After reaching the final model, internal validation was performed with bootstrapping using 250 resamples [22]. Several measures of model performance were assessed for the final model. Nagelkerke’s R 2 was assessed as a measure of explained variation [23]. Harrell’s concordance index, that is the area under the Receiver‐Operating‐Characteristic (ROC) curve, was calculated as a measure of how well the predictions discriminated between the presence and absence of the outcome [24]. The calibration, that is the agreement between observed outcomes and predictions, was visualized with a calibration plot and assessed with the calibration slope. Based on the ROC curve, the optimal cut‐off value for the probability of aorto‐iliac stenosis was determined that maximizes the Youden index (sensitivity + specificity − 1) [25]. Finally, performance measures were also determined for a model containing only age because a recent survey found that 17.4% of respondents used this as a sole criterion for vascular screening [14]. r statistical software (version 4.0.4) was used for all analyses (packages: ‘mice’, ‘psfmi’, ‘VIM’, ’finalfit’).
Results
Baseline characteristics of the study cohort
A total number of 2263 patients were transplanted in the study period, of which 373 patients underwent contrast‐enhanced imaging prior to transplantation. Ninety of these patients (24.1%) had imaging‐proven aorto‐iliac stenosis of which 20 (22.2%) required a pre‐operative or peri‐operative vascular intervention. Median time between imaging and kidney transplantation was 4.6 months [interquartile range (IQR) 1.4–10.4]. Baseline characteristics are presented in Table 1. Patients with aorto‐iliac stenosis were older [mean age (SD) with stenosis: 63.9 (8.5), without stenosis: 58.3 (13.4); P‐value < 0.001] and had more often a smoking history (with stenosis: 13.3% never smoked, without stenosis: 35.0%; P < 0.001). Peripheral arterial disease was more common in patients with aorto‐iliac stenosis (46.7% compared to 15.2%; P‐value < 0.001), as well as a history with coronary artery disease (38.9% compared to 22.3%; P‐value = 0.003). Patients with aorto‐iliac stenosis presented more often with intermittent claudication complaints (32.2% compared to 6.4%; P‐value < 0.001), a femoral artery murmur (41.1% compared to 11.3%; P‐value < 0.001) and weak inguinal pulsations (27.8% compared to 10.6%; P‐value < 0.001). No statistically significant differences were observed with regard to the prevalence of diabetes mellitus, pre‐transplant dialysis or dialysis duration and whether a living donor or deceased donor kidney transplant was performed. The percentage missing data per variable are also demonstrated in Table 1. Only five variables were found to have missing data: BMI, smoking, intermittent claudication, femoral artery murmur and weak inguinal pulsations. Inspection of the missing data pattern for the variables intermittent claudication, femoral artery murmur and weak inguinal pulsations can be found in Tables S1–S3. Missing values for intermittent claudication were dependent on the presence of diabetes mellitus, peripheral arterial disease and femoral artery murmur (Table S1). Missing values for femoral artery murmur were dependent on weak inguinal pulsations (Table S2) and missing values for weak inguinal pulsations were dependent on dialysis duration (Table S3). Our missing data analysis suggests that the assumption of missing at random holds and that multiple imputation can be performed. Intermittent claudication was the variable with the highest missing rate (41.8%) while the average rate of missing values per variable with missing values was 17.6%. Therefore, 20 imputed datasets were used for multiple imputation. Diagnostics of the imputation procedure showed no concerns with regard to the imputation procedure (Fig. S1a–c).
Table 1.
Baseline characteristics of transplant candidates presenting with or without aorto‐iliac stenosis.
| Variable | All (n = 373) | With stenosis (n = 90) | Without stenosis (n = 283) | P‐value |
|---|---|---|---|---|
| Age, mean (SD) | 59.6 (12.6) | 63.9 (8.5) | 58.3 (13.4) | <0.001* |
| Male sex, n (%) | 252 (67.6) | 64 (71.1) | 188 (66.4) | 0.486 |
| BMI in kg/m2, mean (SD) | 26.4 (4.7) | 25.9 (4.7) | 26.5 (4.6) | 0.281 |
| Missing, n (%) | 4 (1.1) | 1 (1.1) | 3 (1.1) | |
| Smoking | ||||
| Never, n (%) | 111 (29.8) | 12 (13.3) | 99 (35.0) | <0.001* |
| Currently, n (%) | 85 (22.8) | 30 (33.3) | 55 (19.4) | |
| Quit, n (%) | 175 (46.9) | 48 (53.3) | 127 (44.9) | |
| Missing, n (%) | 2 (0.5) | 0 (0) | 2 (0.7) | |
| Diabetes mellitus, n (%) | 133 (35.7) | 36 (40.0) | 97 (34.3) | 0.389 |
| COPD, n (%) | 32 (8.6) | 11 (12.2) | 21 (8.8) | 0.457 |
| Peripheral arterial disease, n (%) | 85 (22.8) | 42 (46.7) | 43 (15.2) | <0.001* |
| Cerebrovascular disease | ||||
| None, n (%) | 316 (84.7) | 71 (78.9) | 245 (86.6) | 0.067 |
| TIA, n (%) | 20 (5.4) | 9 (10.0) | 11 (3.9) | |
| CVA, n (%) | 37 (9.9) | 10 (11.1) | 27 (9.5) | |
| Coronary artery disease, n (%) | 98 (26.3) | 35 (38.9) | 63 (22.3) | 0.003* |
| Dyslipidaemia, n (%) | 167 (44.8) | 46 (51.1) | 121 (42.8) | 0.205 |
| Hypertension, n (%) | 344 (92.2) | 83 (92.2) | 261 (92.2) | 1.00 |
| Previous transplant, n (%) | 35 (9.4) | 13 (14.4) | 22 (7.8) | 0.092 |
| Pre‐emptive transplant, n (%) | 64 (17.2) | 11 (12.2) | 53 (18.7) | 0.206 |
| Total dialysis duration, mean (SD) | 30.1 (29.8) | 29.1 (29.3) | 33.1 (31.3) | 0.278 |
| Intermittent claudication, n (%) | 47 (12.6) | 29 (32.2) | 18 (6.4) | <0.001* |
| Missing, n (%) | 156 (41.8) | 27 (30.0) | 129 (45.6) | |
| Femoral artery murmur, n (%) | 69 (18.5) | 37 (41.1) | 32 (11.3) | <0.001* |
| Missing, n (%) | 114 (30.6) | 28 (31.1) | 86 (30.4) | |
| Weak inguinal pulsations, n (%) | 55 (14.7) | 25 (27.8) | 30 (10.6) | <0.001* |
| Missing, n (%) | 44 (11.8) | 10 (11.1) | 34 (12.0) | |
| Reason for end‐stage renal disease | ||||
| ADPKD, n (%) | 43 (11.5) | 11 (12.2) | 32 (11.3) | 0.099 |
| Hypertension, n (%) | 92 (24.7) | 30 (33.3) | 62 (21.9) | |
| DM, n (%) | 87 (23.3) | 22 (24.4) | 65 (23.0) | |
| Glomerulonephritis, n (%) | 19 (5.1) | 5 (5.6) | 14 (4.9) | |
| IgA nephropathy, n (%) | 11 (2.9) | 4 (4.4) | 7 (2.5) | |
| VUR, n (%) | 11 (2.9) | 2 (2.2) | 9 (3.2) | |
| Auto‐immune, n (%) | 13 (3.5) | 0 (0.0) | 13 (4.6) | |
| Congenital, n (%) | 8 (2.1) | 0 (0.0) | 8 (2.8) | |
| Other, n (%) | 89 (23.9) | 16 (17.8) | 73 (25.8) | |
| Type of imaging | ||||
| Angiography, n (%) | 27 (7.2) | 14 (15.6) | 13 (4.6) | <0.001* |
| Contrast‐enhanced CT‐scan, n (%) | 313 (83.9) | 64 (71.1) | 249 (88.0) | |
| MRA, n (%) | 33 (8.8) | 12 (13.3) | 21 (7.4) | |
| Donor type | ||||
| Living, n (%) | 229 (61.4) | 55 (61.1) | 174 (61.5) | 0.950 |
| Deceased, n (%) | 144 (38.6) | 35 (38.9) | 109 (38.5) | |
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CTA, computed tomography angiography; CVA, cerebrovascular accident; DM, diabetes mellitus; MRA, magnetic resonance angiography; SD, standard deviation; TIA, transient ischaemic attack; VUR, vesicoureteral reflux.
Statistically significant.
Model development
The results from univariable logistic regression analysis are shown in Table 2. Variables associated with aorto‐iliac stenosis on univariable analysis were age [odds ratio (OR) per 10 years: 1.53 (95% confidence interval (95%‐CI) 1.21–1.93)], smoking [ever smoked: OR 3.57 (95%‐CI 1.85–6.89)], peripheral arterial disease [OR 4.88 (95%‐CI 2.88–8.28)], coronary artery disease [OR 2.22 (95%‐CI 1.33–3.70)], intermittent claudication [OR 4.90 (95%‐CI 2.41–9.97)], weak inguinal pulsations [OR 3.31 (95%‐CI 1.84–5.96)] and the presence of a femoral artery murmur [OR 7.32 (95%‐CI 3.87–13.86)]. Other variables that were not statistically significant but had a P‐value below 0.2 were a history with cerebrovascular disease [OR 1.73 (95%‐CI 0.93–3.18)], a previous transplant [OR 2.00 (95%‐CI 0.96–4.17) and dyslipidaemia [OR 1.40 (95%‐CI 0.87–2.26)]. Subsequently, a full multivariable logistic regression model was constructed including all variables with a P‐value below 0.2 on univariable analysis. As we aimed to construct a user‐friendly prediction model based on the most parsimonious model that provided the best fit to the data, we used stepwise backward elimination starting with removal of the variable with the highest P‐value. Our final model contained the following predictor variables: age per 10 years [OR 1.33 (95%‐CI 0.97–1.82)], smoking [ever smoked: OR 2.58 (95%‐CI 1.14–5.82)], the presence of peripheral arterial disease [OR 2.06 (95% CI 1.07–3.97)], the presence of coronary artery disease [OR 1.80 (95%‐CI 0.97–3.35)], a previous transplant [OR 4.62 (95% CI 1.78–11.97)], intermittent claudication complaints [OR 3.43 (95% CI 1.50–7.81)] and the presence of a femoral artery murmur [OR 5.18 (95% CI 2.67–10.08)] (Table 3). All VIF values for the final model were below 2, indicating no multicollinearity (Table S4). The addition of a natural cubic spline for age with knots at the 25th, 50th and 75th percentile did not improve model fit significantly (likelihood ratio test: P‐value 0.339), indicating that the nonlinearity assumption for age was not violated. The addition of interaction terms between age and smoking and intermittent claudication and a femoral artery murmur did not improve the model significantly (likelihood ratio test: P‐value 0.368).
Table 2.
Univariable logistic regression analysis for the risk of aorto‐iliac stenosis.
| Variable | Odds ratio (95% confidence Interval) | P‐value |
|---|---|---|
| Age (per 10 years) | 1.53 (1.21–1.93) | <0.001* |
| Sex | ||
| Male | Reference | 0.410 |
| Female | 0.80 (0.48–1.35) | |
| BMI in kg/m2 | 0.97 (0.92–1.02) | 0.284 |
| Smoking | ||
| Never | Reference | <0.001* |
| Ever | 3.57 (1.85–6.89) | |
| Diabetes mellitus | ||
| No | Reference | 0.325 |
| Yes | 1.28 (0.78–2.09) | |
| COPD | ||
| No | Reference | 0.346 |
| Yes | 1.44 (0.68–3.06) | |
| Peripheral arterial disease | ||
| No | Reference | <0.001* |
| Yes | 4.88 (2.88–8.28) | |
| Cerebrovascular disease | ||
| No | Reference | 0.081 |
| Yes, TIA or CVA | 1.73 (0.93–3.18) | |
| Coronary artery disease | ||
| No | Reference | 0.002* |
| Yes | 2.22 (1.33–3.70) | |
| Dyslipidaemia | 1.40 (0.87–2.26) | 0.167 |
| Hypertension | ||
| No | Reference | 0.999 |
| Yes | 1.00 (0.41–2.43) | |
| Previous transplant | 2.00 (0.96–4.17) | 0.063 |
| Total dialysis duration (per month) | 1.00 (1.00–1.01) | 0.261 |
| Intermittent claudication | ||
| No | Reference | <0.001* |
| Yes | 4.90 (2.41–9.97) | |
| Weak inguinal pulsations | ||
| No | Reference | <0.001* |
| Yes | 3.31 (1.84–5.96) | |
| Femoral artery murmur | ||
| No | Reference | <0.001* |
| Yes | 7.32 (3.87–13.86) | |
| Diabetic nephropathy | ||
| No | Reference | 0.773 |
| Yes | 1.09 (0.62–1.89) | |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; TIA, transient ischaemic attack.
Statistically significant.
Table 3.
Final model for the prediction of aortoiliac stenosis derived from the full model by using stepwise backward elimination of not significant variables.
| Variable | Final model | P‐value |
|---|---|---|
| Odds ratio (95% confidence interval) | ||
| Age (per 10 years) | 1.33 (0.97–1.82) | 0.073 |
| Smoking | ||
| Never | Reference | 0.023* |
| Ever | 2.58 (1.14–5.82) | |
| Peripheral arterial disease | ||
| No | Reference | 0.031* |
| Yes | 2.06 (1.07–3.97) | |
| Coronary artery disease | ||
| No | Reference | 0.062 |
| Yes | 1.80 (0.97–3.35) | |
| Previous transplant | 4.62 (1.78–11.97) | 0.002* |
| Intermittent claudication | ||
| No | Reference | 0.004* |
| Yes | 3.43 (1.50–7.81) | |
| Femoral artery murmur | ||
| No | Reference | <0.001* |
| Yes | 5.18 (2.67–10.08) | |
Statistically significant.
Model performance and optimal cut‐off value
Harrel’s concordance index of the final model was 0.85 (0.83 after optimism correction), showing that the model could discriminate well between patients with and without the outcome. The corresponding ROC curve is shown in Fig. 1. The calibration plot with a slope of 1.00 (0.91 after optimism correction) revealed excellent agreement between the observed and predicted risk of the outcome (Fig. 2). The explained variation (R 2) of the model was 0.39 (0.34 after optimism correction), indicating that 39% of the variation in the outcome variable could be explained based on the selected predictors in the model. To use this score in clinical practice, we aimed to identify the optimal cut‐off value for the risk of stenosis that maximized sensitivity and specificity using the Youden index. The highest Youden index was obtained by using 0.214 as cut‐off value, which corresponds with a 21.4% probability of stenosis. Sensitivity of this cut‐off value was 0.83 with a specificity of 0.73. The model containing age alone had a low discriminative capacity with a concordance index of 0.62 (0.62 after optimism correction) and a low explained variation with an R 2 of 0.06 (0.06 after optimism correction). The Youden index identified an optimal cut‐off value of 56 years of age, with a sensitivity of 0.82 and a specificity of 0.38.
Figure 1.
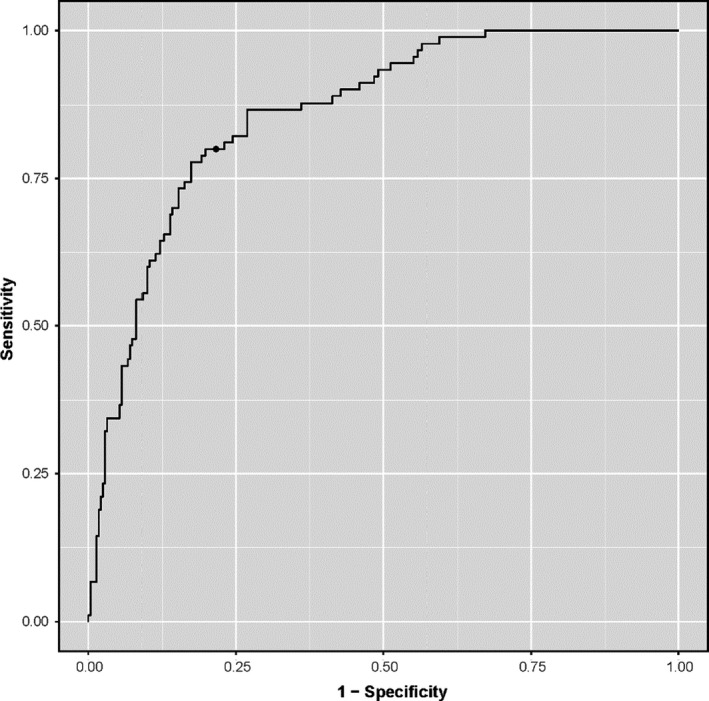
Receiver‐Operating‐Characteristic (ROC) curve of the final model. With the chosen cut‐off value (the dot on the curve), sensitivity and specificity were 0.83 and 0.73, respectively. The cut‐off value can be used to determine whether contrast enhanced imaging is advised.
Figure 2.
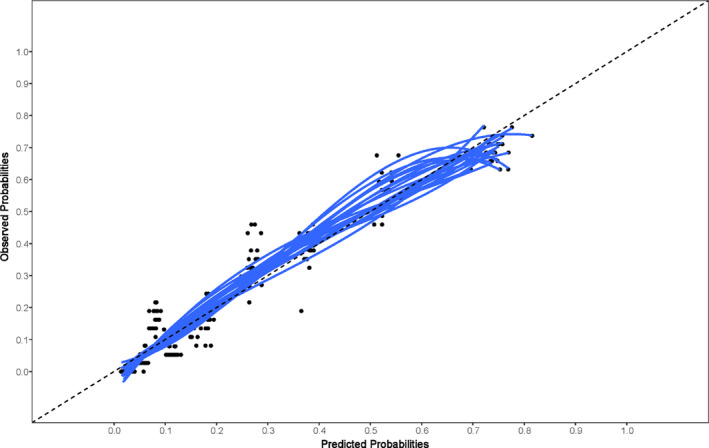
Calibration plot for the final model, which visualizes the observed probability of the outcome in comparison to the predicted probability based on the model. The blue lines represent each one of the 20 imputed datasets. The dashed line in the middle represents perfect calibration.
Online risk calculator and patient examples
The underlying model is published online as a risk calculator for usage in clinical practice (Prediction of aorto‐iliac stenosis in the screening for kidney transplantat ‐ Evidencio). We present two examples to demonstrate the use of the risk calculator and proposed cut‐off value which can be found in Fig. 3. Patient 1 is a 55‐year‐old woman who presents at the outpatient clinic for her first transplant. She is currently smoking and had a myocardial infarction one year before. She has no history of peripheral arterial disease and no claudication complaints or femoral artery murmurs. Based on the risk calculator, her risk of aorto‐iliac stenosis is 11%. According to our cut‐off value, there is no need for further contrast‐enhanced imaging. Patient 2 is a 62‐year‐old man who is screened for a second transplant. He has an extensive smoking history, but no intermittent claudication complaints. He has no history with peripheral arterial disease or ischaemic heart disease. Upon physical examination, he has a femoral artery murmur. The calculated risk of aorto‐iliac stenosis is 66%, indicating that contrast‐enhanced imaging is advised.
Figure 3.
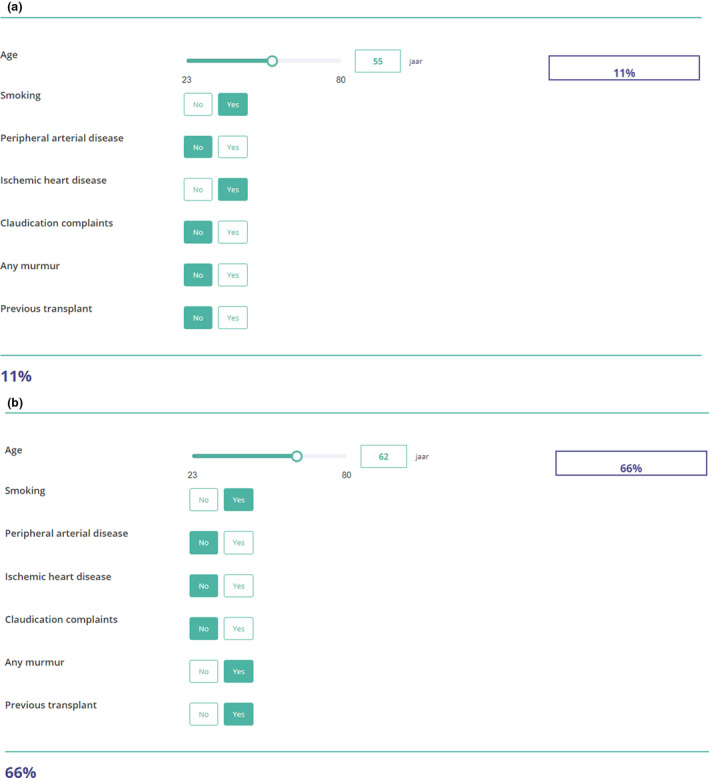
Example of the online risk calculator for (a) Patient 1. (b) Patient 2.
Discussion
In this study, we successfully developed a model to predict aorto‐iliac stenosis in kidney transplant candidates. Our final model has good discrimination and calibration and is easy to implement in the outpatient clinic using the risk calculator with the proposed cut‐off value. We chose to use patient information that is easily available for all physicians who are involved with patients who are screened for transplantation. Our proposed model has a much better performance than the model with only age, which is currently used in clinical practice by 17.4% of transplant surgeon respondents from a recent survey [14]. Vascular screening based on age alone will lead to over diagnostics due to the low specificity, indicating that many patients without aorto‐iliac stenosis are still selected for vascular screening leading to unnecessary radiation, contrast exposure and additional costs.
One very important predictor was the presence of a femoral artery murmur. The strength of the association in the model emphasizes the importance of femoral artery auscultation in routine physical examination. Weak inguinal pulsations were not associated with the presence of aorto‐iliac stenosis. This is somewhat surprising, because other studies suggested that weak pulsations in the legs were strongly associated with peripheral arterial disease [26]. One of the reasons for this may be the low inter‐observer agreement for this variable (kappa 0.53) resulting in misclassification, which underestimates the true strength of an existing association [26]. Another reason may be the high incidence of obesity, which may complicate palpation of the femoral artery [27]. We found evidence for this hypothesis in our data as we found a significantly higher BMI in patients with weak pulsations (mean 28.5 (5.0)) compared to strong pulsations (mean 26.1 (4.5), P‐value = 0.002). As expected, peripheral arterial disease was also a strong predictor for aorto‐iliac stenosis as it shares the same pathophysiology.
Interestingly, we did not find an association between diabetes mellitus and aorto‐iliac stenosis. A possible explanation for this may be that Mönckeberg calcification is more common in diabetes patients [28]. In contrast to calcification of the intima layer, this type of calcification is circumferential medial calcific sclerosis and not associated with luminal narrowing [29]. Another interesting finding is the strong association between a previous transplant and the risk of aorto‐iliac stenosis. This association may be observed because of two reasons. Firstly, patients who receive a retransplant have been on immunosuppressive drugs for a longer time. These drugs, and more specifically steroids and calcineurin inhibitors, promote endothelial dysfunction, which is one of the first steps in the development of atherosclerosis [30]. Secondly, it can be assumed that patients who are screened for a retransplant have had renal disease for a longer time that exposed them to cardiovascular risk factors associated with renal disease, such as a disturbed bone and mineral metabolism. Unfortunately, we could not objectify this in our dataset, as often the onset of renal disease was unknown because patients with renal disease stages I–III are often asymptomatic.
Our study has some limitations. The most important is selection bias due to the retrospective nature of the study combined with the absence of a standard screening protocol using contrast‐enhanced imaging. Patients without risk factors for aorto‐iliac stenosis were less likely to be selected for contrast‐enhanced imaging. Consequently, these patients were less likely to end up in our study cohort. This may affect the generalizability of the study as our cohort may not be representative of the kidney transplant population. As a result, effect estimates may be underestimated because risk factors for stenosis were overrepresented in patients without stenosis. Therefore, external validation is important before we can implement this prediction model in patient care. It may be suggested that our study is limited by a large proportion of missing data for one of the predictor variables (41.8% for intermittent claudication). However, data from simulation studies have shown that, even with large proportions of missing data up to 90%, unbiased estimates are obtained when using multiple imputation [31].
Another limitation is that we could not take results from duplex ultrasound into account, as many of these patients did not receive this type of imaging. Imaging modalities used in our cohort to identify aorto‐iliac stenosis were contrast‐enhanced CT scan, MRA and angiography. Because not all patients underwent angiography, which is the golden standard, this may have led to misclassification. However, as both MRA (sensitivity: 97.5%, specificity 96.2%) and contrast‐enhanced CT scan (sensitivity 95%, specificity 96%) have excellent performance, the risk of misclassification is small and will dilute effect estimates towards the null [12, 32, 33]. Benefits of our study are the simplicity of the model and the accessibility of all variables for the clinician. Some of the variables we used were oversimplified, such as the classification of smoking as never/ever instead of using pack years. Due to the retrospective design, more detailed information was often not available or not reliable. Even though we used a very simplified model with only seven variables to reduce the risk of overfitting, performance measures showed a well‐calibrated model with an excellent concordance index.
This model is specifically designed for the prediction of aorto‐iliac stenosis and does not take aorto‐iliac calcification without stenosis into account. Even though aorto‐iliac calcification can complicate the surgical procedure by not finding a soft arterial spot to clamp, it does not directly affect perfusion of the graft. Therefore, the presence of severe aorto‐iliac calcification has less effect on pre‐operative clinical decisions as whether to perform an additional pre‐transplant vascular procedure or on which side the kidney should be implanted. Stenotic vascular disease, on the other hand, does affect perfusion of the graft if the graft is transplanted below the stenosis on the ipsilateral side. This concept has been shown in the case of renal transplant artery stenosis, which is a cause of graft dysfunction [34]. Because of this, the focus of this study was on stenotic vascular disease, as transplantation below a stenosis should be avoided by performing a pre‐ or peri‐transplant vascular procedure or contralateral implantation.
How can we use this model in clinical practice? The risk calculator with the proposed cut‐off value can be used in the outpatient clinic to decide whether a patient needs additional imaging for the presence of aorto‐iliac stenosis. Use of the risk calculator could reduce costs as it may prevent unnecessary contrast‐enhanced imaging procedures. It may also lead to less exposure of patients to potentially harmful contrast fluid and radiation. Non‐invasive imaging techniques, such as duplex ultrasound, could be an alternative to contrast‐enhanced imaging techniques as it has shown a moderate to high sensitivity for stenotic peripheral vascular disease [35]. However, it should be noted that this sensitivity could be lower for the aorto‐iliac trajectory because of obesity or over‐projection of bowel gas. A study found that 10.4% of all duplex images of the aorto‐iliac trajectory could not be evaluated because of these reasons [36]. Another study found a low inter‐observer agreement for duplex ultrasound in the case of the aorto‐iliac trajectory (kappa of 0.53) [37]. Especially in the case of borderline stenosis, substantial disagreement was found [37]. Obesity is a growing problem especially in the ESRD population making duplex ultrasound a less attractive modality in the Western world.
In conclusion, we successfully developed a model to predict aorto‐iliac stenosis in kidney transplant candidates, which could be useful in clinical practice to guide the development of standardized screening protocols for aorto‐iliac vascular disease. However, external validation is needed to see whether these results are generalizable outside of our dataset, preferably in a dataset where all kidney transplant candidates received contrast‐enhanced imaging.
Authorship
ER: participated in research design, data collection, performance of the research, data‐analysis and writing of the manuscript. HQ: assisted in data‐analysis and reviewed the statistical analysis paragraph. SB: participated in data collection. DCB: participated in data collection and critical review of the manuscript. HJANK: participated in critical review of the manuscript. JIR: participated in critical review of the manuscript. JNMI: participated in critical review of the manuscript. RCM: participated in research design, critical review of the manuscript and supervision of the project.
Funding
The authors have declared no funding.
Conflict of interest
The authors have declared no conflicts of interest.
Supporting information
Table S1. Investigation of the missing data pattern for intermittent claudication.
Table S2. Investigation of the missing data pattern for femoral artery murmur.
Table S3. Investigation of the missing data pattern for weak inguinal pulsations.
Table S4. VIF values for the final model.
Figure S1. Diagnostics of the multiple imputation procedure.
Acknowledgements
None.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093. [DOI] [PubMed] [Google Scholar]
- 2. Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018; 33(suppl_3): iii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhri Y, Horrow MM, Zaki R. Vascular steal of renal transplant: sonographic diagnosis. J Ultrasound Med 2006; 25: 939. [DOI] [PubMed] [Google Scholar]
- 4. Norgren L, Hiatt WR, Dormandy JA, et al. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45: S5. [DOI] [PubMed] [Google Scholar]
- 5. Brekke LB, Lien B, Sødal G, et al. Aortoiliac reconstruction in preparation for renal transplantation. Transpl Int 1993; 6: 161. [DOI] [PubMed] [Google Scholar]
- 6. Tsivian M, Neri F, Nardo B, et al. Aortoiliac surgery concomitant with kidney transplantation: a single center experience. Clin Transplant 2009; 23: 164. [DOI] [PubMed] [Google Scholar]
- 7. Piquet P, Berland Y, Coulange C, Olmer M, Mercier C, Rampal M. Aortoiliac reconstruction and renal transplantation: staged or simultaneous. Ann Vasc Surg 1989; 3: 251. [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Transplant Candidate Work Group . KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020; 104: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koelemay MJ, den Hartog D, Prins MH, Kromhout JG, Legemate DA, Jacobs MJ. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg 1996; 83: 404. [DOI] [PubMed] [Google Scholar]
- 10. Lundin P, Svensson Å, Henriksen E, et al. Imaging of aortoiliac arterial disease. Duplex ultrasound and MR angiography versus digital subtraction angiography. Acta Radiol 2000; 41: 125. [DOI] [PubMed] [Google Scholar]
- 11. Claudon M, Plouin PF, Baxter GM, Rohban T, Devos DM. Renal arteries in patients at risk of renal arterial stenosis: multicenter evaluation of the echo‐enhancer SH U 508A at color and spectral Doppler US. Levovist Renal Artery Stenosis Study Group. Radiology 2000; 214: 739. [DOI] [PubMed] [Google Scholar]
- 12. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta‐analysis. JAMA 2009; 301: 415. [DOI] [PubMed] [Google Scholar]
- 13. Modi K, Padala SA, Gupta M. Contrast‐induced nephropathy, 2020. [PubMed]
- 14. Rijkse E, Kimenai D, Dor F, Ijzermans J, Minnee R. The management of aorto‐iliac vascular disease in candidates for kidney transplantation: a worldwide survey among transplant surgeons. Transplantation 2020; 104: S350. [DOI] [PubMed] [Google Scholar]
- 15. Fontaine R, Kim M, Kieny R. [Surgical treatment of peripheral circulation disorders] Die chirurgische Behandlung der peripheren Durchblutungsstorungen. Helv Chir Acta 1954; 21: 499. [PubMed] [Google Scholar]
- 16. Heymans MW, van Buuren S, Knol DL, van Mechelen W, de Vet HC. Variable selection under multiple imputation using the bootstrap in a prognostic study. BMC Med Res Methodol 2007; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Buuren S. Flexible Imputation of Missing Data. Boca Raton: Chapman & Hall/Crc Interdisciplinary Statistics Taylor & Francis, 2012. [Google Scholar]
- 18. Rubin DB. Multiple Imputation for Nonresponse in Surveys. London: Wiley, 1987. [Google Scholar]
- 19. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD). Ann Intern Med 2015; 162: 735. [DOI] [PubMed] [Google Scholar]
- 20. Hosmer DW, Lemeshow S, May S. Applied Survival Analysis. Hoboken, NJ: Wiley & Sons, 2008. [Google Scholar]
- 21. Hair J, Black W, Babin B, Anderson R. Multivariate data analysis, 2010.
- 22. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001; 54: 774. [DOI] [PubMed] [Google Scholar]
- 23. Gerds TA, Cai T, Schumacher M. The performance of risk prediction models. Biom J 2008; 50: 457. [DOI] [PubMed] [Google Scholar]
- 24. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361. [DOI] [PubMed] [Google Scholar]
- 25. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32. [DOI] [PubMed] [Google Scholar]
- 26. McGee SR, Boyko EJ. Physical examination and chronic lower‐extremity ischemia: a critical review. Arch Intern Med 1998; 158: 1357. [DOI] [PubMed] [Google Scholar]
- 27. Myers KA, Scott DF, Devine TJ, Johnston AH, Denton MJ, Gilfillan IS. Palpation of the femoral and popliteal pulses: a study of the accuracy as assessed by agreement between multiple observers. Eur J Vasc Surg 1987; 1: 245. [DOI] [PubMed] [Google Scholar]
- 28. Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non‐insulin‐dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 1996; 16: 978. [DOI] [PubMed] [Google Scholar]
- 29. Jeffcoate WJ, Rasmussen LM, Hofbauer LC, Game FL. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia 2009; 52: 2478. [DOI] [PubMed] [Google Scholar]
- 30. Nickel T, Schlichting CL, Weis M. Drugs modulating endothelial function after transplantation. Transplantation 2006; 82(1 Suppl): S41. [DOI] [PubMed] [Google Scholar]
- 31. Madley‐Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 2019; 110: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visser K, Hunink MG. Peripheral arterial disease: gadolinium‐enhanced MR angiography versus color‐guided duplex US – a meta‐analysis. Radiology 2000; 216: 67. [DOI] [PubMed] [Google Scholar]
- 33. Chen Q, Galfalvy H, Duan N. Effects of disease misclassification on exposure‐disease association. Am J Public Health 2013; 103: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol 2004; 15: 134. [DOI] [PubMed] [Google Scholar]
- 35. Bueno A, Acin F, Canibano C, Fernandez‐Casado JL, Castillo E. Diagnostic accuracy of contrast‐enhanced magnetic resonance angiography and duplex ultrasound in patients with peripheral vascular disease. Vasc Endovascular Surg 2010; 44: 576. [DOI] [PubMed] [Google Scholar]
- 36. Muela Mendez M, Morata Barrado PC, Blanco Canibano E, Garcia Fresnillo B, Guerra RM. Preoperative mapping of the aortoiliac territory with duplex ultrasound in patients with peripheral arterial occlusive disease. J Vasc Surg 2018; 68: 503. [DOI] [PubMed] [Google Scholar]
- 37. Ubbink DT, Fidler M, Legemate DA. Interobserver variability in aortoiliac and femoropopliteal duplex scanning. J Vasc Surg 2001; 33: 540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Investigation of the missing data pattern for intermittent claudication.
Table S2. Investigation of the missing data pattern for femoral artery murmur.
Table S3. Investigation of the missing data pattern for weak inguinal pulsations.
Table S4. VIF values for the final model.
Figure S1. Diagnostics of the multiple imputation procedure.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


