Abstract
Background:
This study aimed to investigate the role of circulating tumor cells (CTCs) and circulating cancer stem-like cells (cCSCs) before and after one cycle of chemotherapy and assessed the effects of early changes in CTCs and cCSCs on the outcomes of patients with metastatic breast cancer.
Methods:
Patients with stage IV invasive ductal carcinoma of the breast who received first-line chemotherapy between April 2014 and January 2016 were enrolled. CTCs and cCSCs were measured before the first cycle of chemotherapy (baseline) and on day 21, before the second cycle of chemotherapy commenced; a negative selection strategy and flow cytometry protocol were employed.
Results:
CTC and cCSC counts declined in 68.8 and 45.5% of patients, respectively. Declines in CTCs and cCSCs following the first chemotherapy cycle were associated with superior chemotherapy responses, longer progression-free survival (PFS), and longer overall survival (OS). An early decline in cCSCs remained an independent prognostic indicator for OS and PFS in multivariate analysis.
Conclusions:
A cCSC decline after one cycle of chemotherapy for metastatic breast cancer is predictive of a superior chemotherapy response and longer PFS and OS, implying that cCSC dynamic monitoring may be helpful in early prediction of treatment response and prognosis.
Keywords: breast cancer, cancer stem cells, CD133, circulating tumor cells, chemotherapy, prognosis
Introduction
Treatment for metastatic breast cancer has advanced considerably, as exemplified by innovations in targeted therapy, checkpoint inhibitors, hormone therapy, and chemotherapy, as well as an overall drop in mortality of approximately 40% from 1989 to 2015. 1 The response rate to first-line treatment varies from 50 to 80% of patients, 2 and some patients still do not respond very well to the treatment. The treatment goal is palliative because metastatic breast cancer is primarily incurable. Individual therapy responses in terms of toxicity should be closely monitored in this setting, and ineffective therapy should be avoided. In clinical practice, parameters such as tumor markers and radiological responses are widely used to evaluate individual response; however, 2–3 months are usually required to conduct such evaluations. Moreover, the availability of reliable real-time disease monitoring tools is limited. To minimize the use of inefficient therapy and unnecessary treatment-related toxicity, identifying biomarkers correlated with anti-cancer therapy response and survival is crucial.
Circulating tumor cells (CTCs) constitute a valuable biomarker in cancer research for monitoring disease status and treatment response. CTC measurement has been reported as a viable technique for tracking therapeutic response in patients with metastatic breast cancer. Several researchers have reported that the detection of CTCs in peripheral blood before treatment is linked to poor prognosis.3,4 During therapy, elevated CTC levels may be predictive of poor treatment response. 5 CTC analysis may be a more reliable and earlier indicator of disease state than standard clinical imaging procedures are. Knowledge regarding cancer stem cells, however, has evolved, leading to the creation of novel diagnostic tools. 6 CTCs obtained from patients with metastatic breast cancer frequently overexpress stem cell markers, suggesting that a subgroup of CTCs that express the cancer stem cell marker is responsible for metastasis. 7 Tumor cells that undergo more aggressive change following the epithelial-to-mesenchymal transition (EMT) may promote cancer spread and metastasis. 8 EMT in these cancer cells is potentially linked to stem cell-like features, 9 thus enhancing their differentiation and proliferation. This contributes to the spread of metastatic disease. 10 Cancer stem cells may be crucial to disease recurrence, disease progression, and drug resistance in patients with cancer. 11 A particular subpopulation of CTCs has been revealed to exhibit stem cell markers (e.g. CD44 12 or CD133 12 ) and contain cells that have the characteristics of cancer stem cell13,14; they can therefore be regarded as circulating cancer stem-like cells (cCSCs). Research on metastatic breast cancer has revealed that CTCs undergoing the EMT and expressing cancer stem cell markers are more likely to be discovered in patients refractory to chemotherapy than in patients responding to treatment. 7 Hsu et al. 15 also reported CTCs and cCSCs (defined as CD133 + CTC) before treatment are associated with poor treatment outcome. Because CD133 is the most commonly used marker for identifying cCSCs, 16 we used it to enhance our comparisons and the validity of our results.
CTCs are linked to cancer progression and tumor spread, whereas cCSCs are associated with drug resistance. Several studies have reported baseline CTCs are strongly related to the prognosis of breast cancer and dynamic CTCs status and change can serve as an indicator to monitor the effectiveness of treatments.3,4,5,17 Regarding cCSC part, we have previously reported that the higher baseline cCSCs (above median of the cohort) is one of the prognostic factors in metastatic breast cancer. 17 However, few studies have investigated the role of cCSC counts and their changes throughout chemotherapy as a tool monitoring therapeutic resistance in real time. Therefore, the data of dynamic monitoring of cCSCs during chemotherapy to demonstrate the association of drug resistance is an unmet need. Hence, we hypothesis that cCSCc decline after chemotherapy immediately may be predictive of therapeutic response and better outcome. Herein, we conducted another prospective trial investigating the role of both CTCs and cCSCs before and after one cycle of chemotherapy and examined the association with early CTC and cCSC changes on the progression-free survival (PFS), overall survival (OS), and chemotherapy response rate of patients with metastatic breast cancer.
Methods
Patient enrollment and cancer status evaluation
We performed this prospective study at three medical facilities: Chang Gung Memorial Hospital (Linkou, TuCheng, and Keelung branches) in Taiwan. CTC and cCSC analysis was conducted in Circulating Tumour Cell Lab at Linkou Chang Gung Memorial Hospital. The Institutional Review Boards of Chang Gung Memorial Hospital (approval ID: 103-5322B) approved of the study protocols. Written informed consent was obtained from patients for protocols that required ethical approval. Patients with surgically unresectable or metastatic invasive ductal carcinoma of the breast, as confirmed through histology or cytopathology [stage IV, per the American Joint Committee on Cancer (AJCC) Staging Manual, Seventh Edition] were eligible for this study. The other criteria for enrollment were as follows: (a) age ⩾ 20 years, (b) capacity to comprehend the content of the consent form and independently sign it, (c) kidney and liver function and blood cell counts sufficient for chemotherapy, and (d) previous failed endocrine therapy in those with positive hormone receptor (HR) status. First-line chemotherapy prescription without previous endocrine therapy was allowed if the physician had provided a potential visceral crisis diagnosis. Patients with multiple cancers and those with cancer in the 5-year period preceding enrollment were excluded. All of the patients underwent baseline examinations for gathering demographic information, clinical histories, computed tomography (CT) results, pathological features, and biochemical data. For disease staging and management, existing treatment protocols compliant with institutional guidelines were employed. The systemic anticancer therapies prescribed by physicians were as follows: pertuzumab plus trastuzumab plus docetaxel, trastuzumab plus docetaxel, doxorubicin plus cyclophosphamide, and paclitaxel plus gemcitabine. All patients with human epidermal growth factor receptor 2 (HER-2) positive tumor received at least one HER-2 targeted agent. We assessed tumor response through CT, positron emission tomography, or both, in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 18 ; a multidisciplinary breast tumor board subsequently determined the tumor response. We calculated OS and PFS from the date blood was drawn for CTC testing until death or disease progression, respectively, after chemotherapy. We followed up all of the patients until their deaths or until 31 December 2020.
Blood sample drawing during chemotherapy
In this prospective observational study, the clinical significance of changes in CTCs and cCSCs following first-line palliative chemotherapy was assessed in patients with metastatic breast cancer. Blood sample was drawn no more than 7 days prior to the start of the first chemotherapy cycle (baseline) and on day 21, before the second cycle started. The findings are reported in accordance with the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines. 19 All procedures complied in full with the relevant guidelines.
Measurement of peripheral CTCs and cCSCs
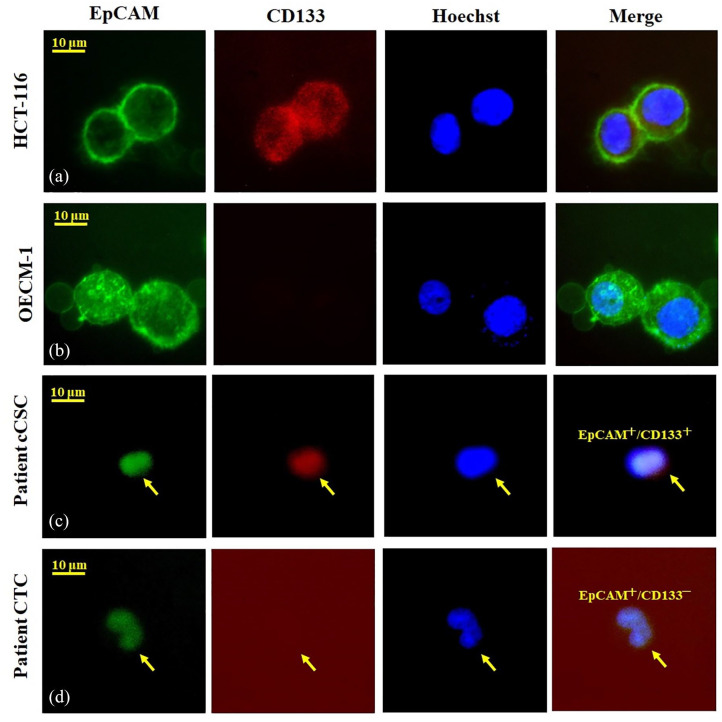
We employed positive detection and negative selection strategies to identify CTCs; these approaches had been validated in our previous studies.17,20–23 Specifically, a negative selection protocol with a CD45 depletion kit was employed to deplete leukocytes after standard red blood cell lysis; subsequently, flow cytometry was employed to quantitatively identify and count CTCs (EpCAM+Hoechst+CD45−) and cCSCs (CD133+EpCAM+Hoechst+CD45−). The CTC tests were carried out using 4 mL samples of peripheral blood after discarding of the original 4 mL of blood to avoid epithelial contamination. Red blood cells were lysed within 72 h, and the EasySep Human CD45 Depletion Cocktail (25 L/mL; STEMCELL Technologies, Vancouver, BC, Canada) and EasySep Magnetic Nanoparticles (50 L/mL; STEMCELL) were used for further negative selection. Thereafter, we spiked the immunomagnetically enriched samples with OECM1/HCT116 cells labeled with an Alexa Fluor 488-conjugated monoclonal antibody to EpCAM (1:400; Cell Signaling Technology, Danvers, MA, USA) and an Alexa Fluor 647-conjugated monoclonal antibody to CD133 (1:200; Novus Biologicals, Littleton, CO, USA), and we stained the samples with Hoechst 33342 (20 mM; Thermo Fisher Scientific, Waltham, MA, USA), a blue fluorescent stain specific to DNA. As the internal control, we employed an isotype control antibody as well as 4-mL peripheral blood samples from healthy individuals that were spiked and not spiked with 1000 OECM1/HCT-166 cells acquired from Taiwan’s Food Industry Research and Development Institute. Performance recovery was defined as the proportion of OECM1/HCT-116 cells detected by flow cytometry (BD FACSCalibur; BD Biosciences, San Jose, CA, USA) to the number of spiked OECM1/HCT-116 cells; a related study reported a stable coefficient of variation (CV) value calculation. 24 Briefly, the platform could achieve a recovery rate of 44.6 ± 9.1% and a CV of 20.4%. The earlier platform reported in 2015 detected 13.1 ± 0.9 cells/mL in healthy individuals (n = 20), 24 a confusing result that may be attributable to a background signal (i.e. a false positive). With the use of an isotype control for each sample in the revised platform, 0.0–3.0 cells/mL was observed in healthy individuals in a study cohort of 20. 17 In the present study, we defined CTCs as cells those positive for Hoechst 33342 and EpCAM. Cells expressing CD133, EpCAM, and Hoechst were identified as cCSCs (Figure 1). All human cell lines have been authenticated using short tandem repeat (STR) profiling within the last 3 year.
Figure 1.
Demonstration of CD133 expression in circulating tumor cells (CTCs). The experimental control using HCT-116 [a human colon cancer cell line, CD133+EpCAM+Hoechst+, (a)] and OECM-1 [a head and neck cancer cell line, CD133−EpCAM+Hoechst+, (b)] by immunofluorescence staining. We further demonstrate a real breast cancer patient in the study cohort with CD133+ CTCs (c) and CD133− CTC (d).
Statistical analysis
We present patient demographic data as medians (ranges) and numbers (%) and for continuous and categorical variables, respectively. We conducted both univariate and multivariate analyses, and in the multivariate analysis, we examined all factors used in the univariate analysis, but only statistically significant factors were reported. Moreover, we used a multivariate Cox proportional hazards regression using a forward stepwise approach to determine how factors were correlated with PFS and OS. The Kaplan–Meier method was used for survival analysis, and the log-rank test was used to test for differences. The Pearson chi-squared or Fisher exact test was used to calculate the correlations of CTCs or cCSCs with treatment response. Statistical analyses were performed using SPSS for Windows (version 21; IBM, Armonk, NY, USA). We performed all statistical analyses in SPSS for Windows (version 21; IBM, Armonk, NY, USA), and all analyses were two sided, with a p value of <0.05 indicating significance.
Results
Fifty patients with metastatic breast cancer were prospectively enrolled between April 2014 and January 2016 before they received first-line palliative chemotherapy. Two patients withdrew from the study early; thus, we were unable to obtain second blood test results for CTCs or cCSCs or imaging studies for response assessment. The basic attributes of the recruited patients are listed in Table 1. The enrolled patients were all women and had a median age of 52 (range: 28–81) years. Most participants (86.0%) exhibited an Eastern Cooperative Oncology Group performance status between 0 and 1, and all patients had stage IV disease (based on the seventh edition of the AJCC criteria). In total, 36 patients (72.0%) tested positive for estrogen receptor and progesterone receptor, and 24 (48.0%) tested positive for HER-2. Among the participants, 8 (16.0%) had triple-negative breast cancer and 33 (66.0%) had ⩾2 metastatic sites. The most common metastatic site was the bones (72.0%), followed by the liver (36.0%), distant lymph nodes (34.0%), lungs (32.0%), brain (14.0%), and pleura (12.0%). Visceral metastases were found in 36 patients (72%). CTCs were identified in all enrolled patients and cCSCs were isolated in 98% of them. The baseline CTC and cCSC number (mean ± SD) of all the enrolled patients was 75.1 ± 108.0 [95% confidence interval (CI): 42.8–103.2] cells/mL and 18.9 ± 37.5 (95% CI: 8.0–29.9) %, respectively. After one cycle of chemotherapy, we observed a significant reduction in CTCs and cCSCs to 60.1 ± 93.4 (95% CI: 33.0–87.2) cells/mL and 16.6 ± 35.5 (95% CI: 5.8–27.5) %, respectively (p < 0.001). After a median follow-up of 36.2 months, 43 (86%) had exhibited disease progression, and 25 (50%) had died. The median PFS and OS were 11.1 and 42.3 (95% CI: 5.4–16.8 and 14.0–70.7) months, respectively.
Table 1.
Basic characteristics of enrolled patients.
| N | % | |
|---|---|---|
| Age, median, years (range) | 52 (28–81) | |
| Sex | ||
| Female | 50 | 100.0 |
| Staging (AJCC Seventh Edition) | ||
| Stage IV | 50 | 100.0 |
| Performance status (ECOG) | ||
| 0–1 | 43 | 86.0 |
| ⩾2 | 7 | 14.0 |
| Receptor status | ||
| ER and/or PR positive | 36 | 72.0 |
| HER-2/neu positive | 24 | 48.0 |
| Triple-negative (ER/PR/HER-2) | 8 | 16.0 |
| Number of metastases | ||
| Single metastasis | 17 | 34.0 |
| ⩾2 metastases | 33 | 66.0 |
| Site of distant metastasis at study enrollment | ||
| Visceral metastasis † | 36 | 72.0 |
| Nonvisceral metastasis | 14 | 28.0 |
Visceral sites include the lungs, liver, brain, adrenal glands, and pleura (with or without effusion). Nonvisceral sites were defined as the breast, lymph nodes, chest wall, bones, and skin.
AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; CI: confidence interval; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; OS: overall survival; PFS: progression-free survival; PR, progesterone receptor; SD: standard deviation.
We investigated potential associations of chemotherapy response rate, OS, and PFS with changes in CTCs and cCSCs after one cycle of chemotherapy. CTC decline and cCSC decline were defined as a 30% decline from baseline. Overall, CTC and cCSC counts declined in 33 (66%) and 22 (44%) patients, respectively. In subgroup analysis, the delince in CTC count (61.5% versus 79.2%, p = 0.212) and cCSC count (38.1% versus 52.2%, p = 0.383) were not significantly between the HER-2 positive and HER-2 negative patients.
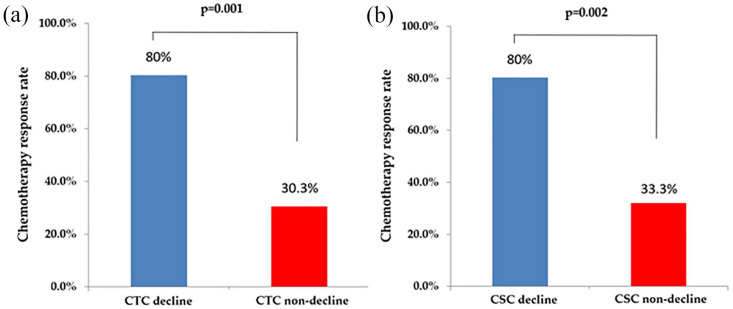
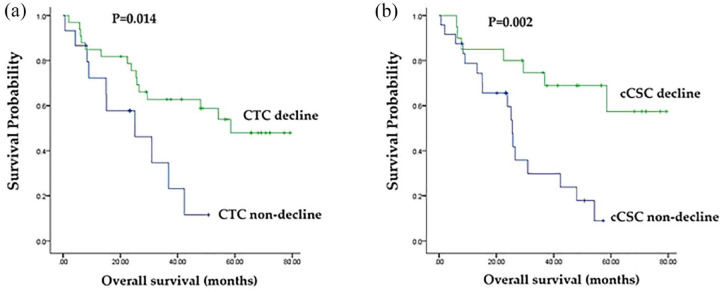
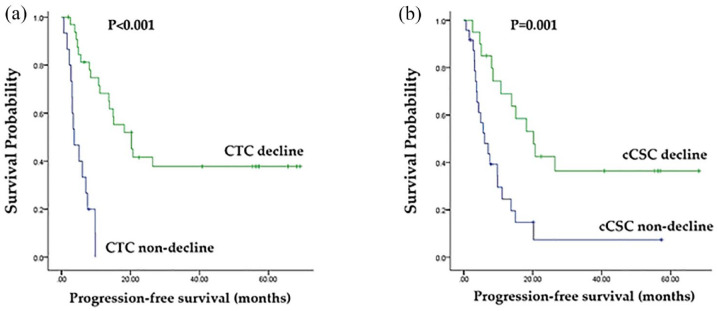
As displayed in Figures 2 to 4, the decline in CTC count was correlated with an increased tumor response rate [80.0% versus 30.3%, p = 0.001; Figure 2(a)] as well as longer PFS [median, 20.2% versus 3.7%, p < 0.001; Figure 3(a)] and OS [58.5% versus 25.0%, p = 0.014; Figure 4(a)] relative to those of patients without a CTC decline. A decline in cCSC count was associated with a higher tumor response rate [80.0% versus 33.3%, p = 0.002; Figure 2(b)] as well as longer PFS [median, 20.2 versus 6.0 months, p = 0.001; Figure 3(b)] and OS [median, not reached versus 25.5 months, p = 0.002; Figure 4(b)] relative to patients without a cCSC decline. Multivariate analysis revealed that a decline in CTC count and a decline in cCSC count were independent prognostic indicators for PFS when combined with other clinical parameters. Regarding OS, CTC level at baseline and a decline in cCSCs were identified as independent prognostic factors (Table 2).
Figure 2.
Chemotherapy response rate stratified by (a) circulating tumor cells (CTCs) and (b) circulating cancer stem-like cells (cCSCs) decline and non-decline.
Figure 4.
(a) Comparison of overall survival (OS; months) between patients with circulating tumor cell (CTC) counts decline and non-decline. (b) Comparison of OS (months) between patients with circulating cancer stem-like cell (cCSC) counts decline and cCSCs non-decline.
Figure 3.
(a) Comparison of progression-free survival (PFS; months) between patients with circulating tumor cell (CTC) counts decline and non-decline. (b) Comparison of PFS (months) between patients with circulating cancer stem-like cell (cCSC) counts decline and cCSCs non-decline.
Table 2.
Univariate and multivariate analyses for PFSand OS.
| Parameters | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age > 65 y | 0.995 (0.996–1.024) | 0.718 | 1.016 (0.979–1.054) | 0.398 | ||||
| Triple-negative | 0.531 (0.217–1.299) | <0.166 | 0.470 (0.172–1.290) | 0.172 | ||||
| Visceral metastasis† | 1.939 (0.833–4.512) | 0.124 | 2.130 (0.791–5.736) | 0.135 | ||||
| Baseline CTC | 1.003 (1.000–1.006) | 0.063 | 1.006 (1.003–1.009) | <0.001 | 1.017 (1.004–1.030) | 0.008 | ||
| Baseline cCSCs | 1.004 (0.994–1.014) | 0.480 | 1.012 (1.002–1.021) | 0.015 | ||||
| CTC decline | 8.209 (3.313–20.339) | <0.001 | 3.631 (1.187–11.110) | 0.024 | 2.776 (1.187–6.445) | 0.018 | ||
| cCSC decline | 3.207 (1.516–6.784) | 0.002 | 3.096 (1.002–9.569) | 0.050 | 8.236 (2.269–29.894) | 0.001 | 6.921 (1.770–27.054) | 0.005 |
Visceral sites include the lungs, liver, brain, adrenal glands, and pleura (with or without effusion).
cCSC: circulating cancer stem-like cells, which were defined as CD133+ CTCs in this study; CTCs: circulating tumor cells; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR: hazard ratio; OS: overall survival; PFS: progression-free survival; PR, progesterone receptor.
Discussion
We have previously reported that the higher baseline cCSCs (defined as above the median of the cohort) is one of the prognostic factors in metastatic breast cancer. 17 However, the impact of dynamic change of cCSCs association with chemotherapy response and survival was not well understood at that time. Therefore, we conducted this prospective study later to collect samples before and after one cycle of chemotherapy from the different cohort to establish a predictive model of early change CTCs and cCSC in patients with metastatic breast cancer receiving first-line chemotherapy. To the best of our knowledge, it is the first research to report the predictive value of early cCSC decline. In this study, we discovered numerous elementary but important findings regarding the clinical value of dynamic CTC and cCSC monitoring during first-line chemotherapy in metastatic breast cancer. Decreases in CTC and cCSC counts after one cycle (3 weeks) of chemotherapy can predict treatment response, PFS, and OS. In multivariate analysis, decreases in CTC and cCSC counts were independent predictors of PFS. Furthermore, CTC level at baseline and a decrease in cCSC level were independent predictors of OS.
Chemotherapy has been thought to kill both cancer cells and cancer stem cells; however, early resistance to chemotherapy has been observed in those with higher percentages or counts of CSCs.25–28 We observed a similar phenomenon in our previous report, which concluded that the higher baseline cCSC counts predict chemotherapy response in metastatic breast cancer. 17 The current study also found that CTC and cCSC count changes from the first chemotherapy date to the second blood drawing at the third week after the first dose of chemotherapy would also predict the response to systemic chemotherapy 3–4 months later (Figure 1). These results indicated that serial follow-up liquid biopsies during anti-cancer therapy could provide additional value in predicting tumor response to a specific anti-cancer therapy in breast cancer; this finding is consistent with those of studies in various types of cancer.29–33 However, few of these studies provided survival impacts from the early response of CTCs or cCSCs, which was supported in the present study (Figures 2 and 3).
Multiple clinicopathologic factors, including the age of onset, hormone status, tumor grade, general performance status, anti-cancer therapies, and supportive care, all contribute to the OS of patients with breast cancer.34–36 Furthermore, CTCs have been suggested to represent a predictive factor in individuals with metastatic breast cancer undergoing systemic therapy. 3 Some specific markers in CTCs, such as insulin growth factor-1 receptor, 37 EMT, 38 and CD133,12,39–41 were found to be prognostic for survival. These characteristics support CTCs and demonstrate heterogeneity in breast cancer to help identify potential metastatic seeds. 42 Identifying metastasis-initiating CTCs is essential for developing therapeutic strategies against metastatic breast cancer. 42 Our study further presented an interesting phenomenon: the changes in cCSC (CD133+ CTC) counts after anti-cancer therapy might be more effective predictor of PFS than are CTC or cCSC counts alone (Table 2). After a long-term follow-up (36.2 months in this study), baseline CTC remained an independent factor for OS. Notably, an early decline in cCSC counts can also serve as a prognostic factor for long-term OS (Table 2). We believe that these results highlight the importance of a strategy for reducing CTCs and rapidly and effectively suppressing cCSC counts at the beginning of systemic therapy. Therefore, anti-CD133 drugs might be a potential target for combination with current anti-cancer therapy in the future.
This study has several limitations. First, although this proof-of-concept prospective trial examined the prognostic significance of CTCs and cCSCs over a long-median follow-up period, the number of cases included was small. More large-scale research is needed. Second, four different chemotherapy regimens were prescribed in this study. Although the CTC and CSCs response rate were not affected by therapies in our cohort, it is still worthwhile to analysis the response between HER-2 positive and negative subgroup. In addition, HER-2 positive CTC detection is another important issue correlated with prognosis and deserve us for further exam in the future. Finally, cCSCs were defined as CD133+ CTCs in this study, which may not be a comprehensive definition. CD133 represents only one marker with stem-like characteristics.43,44 However, among all the surface markers in the literature, CD133 in breast tumors, particularly in the triple-negative phenotype,45,46 can directly regulate the expression of proteins implicated in cancer metastasis and drug resistance.45,46 More experimental strategies have been identified to restrain levels of CD133, which could be used to reduce malignancy and halt the progression of breast cancers. 41 As a result, although CD133 is not a perfect marker, it is nevertheless useful in predicting cancer behavior and may be a future target of metastatic cancer therapy.
Conclusion
We discovered that declines in CTCs and cCSCs during the first 3 weeks after chemotherapy initiation could independently predict PFS, whereas CTCs at baseline and an early decline in cCSCs could independently predict long-term OS. We propose close monitoring of tumor signals in peripheral blood through liquid biopsy to ensure prompt and personalized treatment.
Acknowledgments
The authors thank all members of the Cancer Centre, Chang Gung Memorial Hospital, for their invaluable help.
Footnotes
ORCID iDs: Pei-Hung Chang  https://orcid.org/0000-0002-5006-0277
https://orcid.org/0000-0002-5006-0277
Wen-Chi Chou  https://orcid.org/0000-0002-0361-4548
https://orcid.org/0000-0002-0361-4548
Jason Chia-Hsun Hsieh  https://orcid.org/0000-0002-5547-409X
https://orcid.org/0000-0002-5547-409X
Contributor Information
Pei-Hung Chang, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Keelung, Keelung City; College of Medicine, Chang Gung University, Taoyuan City.
Chun-Hui Lee, College of Medicine, Chang Gung University, Taoyuan City; Division of General Surgery, Department of Surgery, Chang Gung Memorial Hospital, Keelung, Keelung City.
Tyler Min-Hsien Wu, Circulating Tumour Cell Lab, Division of Medical Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Taoyuan City; Graduate Institute of Biomedical Engineering, Chang Gung University, Taoyuan City; Department of Chemical Engineering, Ming Chi University of Technology, New Taipei City.
Kun-Yun Yeh, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Keelung, Keelung City; College of Medicine, Chang Gung University, Taoyuan City.
Hung-Ming Wang, College of Medicine, Chang Gung University, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, Taoyuan City.
Wen-Kuan Huang, College of Medicine, Chang Gung University, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, Taoyuan City.
Sheng-Chieh Chan, Department of Nuclear Medicine, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien City.
Wen-Chi Chou, College of Medicine, Chang Gung University, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, Taoyuan City.
Feng-Che Kuan, Division of Hematology and Oncology, Department of Medicine, Chang Gung Memorial Hospital, Chiayi, Puzi City.
Hsuan-Chih Kuo, College of Medicine, Chang Gung University, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, New Taipei City Municipal TuCheng Hospital, New Taipei City.
Yung-Chia Kuo, College of Medicine, Chang Gung University, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, Taoyuan City; Division of Hematology-Oncology, Department of Internal Medicine, New Taipei City Municipal TuCheng Hospital, New Taipei City.
Ching-Chih Hu, Division of Hepatogastroenterology, Department of Internal Medicine, Chang Gung Memorial Hospital, Keelung, Keelung City.
Jason Chia-Hsun Hsieh, College of Medicine, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan, Taoyuan City 333; Circulating Tumour Cell Lab, Division of Medical Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, No. 5, Fuxing Street, Guishan District, Taoyuan City 333; Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, No. 5, Fuxing Street, Guishan District, Taoyuan City, 333; Division of Hematology-Oncology, Department of Internal Medicine, New Taipei City Municipal TuCheng Hospital, No. 6, Section 2, Jincheng Road, Tucheng District, New Taipei City 236.
Declarations
Ethics approval and consent to participate: The study as conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Boards of Chang Gung Memorial Hospital (approval ID: 103-0425B and 103-5322B). All participating patients provided written informed consent.
Consent for publication: Not applicable.
Author contribution(s): Pei-Hung Chang: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Chun-Hui Lee: Formal analysis; Funding acquisition; Writing – original draft.
Tyler Min-Hsien Wu: Formal analysis; Funding acquisition; Writing – review & editing.
Kun-Yun Yeh: Formal analysis; Supervision; Writing – review & editing.
Hung-Ming Wang: Conceptualization; Supervision; Writing – review & editing.
Wen-Kuan Huang: Investigation; Project administration; Software; Writing – review & editing.
Sheng-Chieh Chan: Formal analysis; Writing – review & editing.
Wen-Chi Chou: Methodology; Software; Validation; Writing – review & editing.
Feng-Che Kuan: Investigation; Writing – review & editing.
Hsuan-Chih Kuo: Investigation; Writing – review & editing.
Yung-Chia Kuo: Investigation; Writing – review & editing.
Ching-Chih Hu: Funding acquisition; Methodology; Writing – review & editing.
Jason Chia-Hsun Hsieh: Conceptualization; Formal analysis; Funding acquisition; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Chang Gung Memorial Hospital (CMRPG2D0171, CMRPG2D0172, CMRPG2G0681, CMRPG2G0682, CMRPG2G0683, CMRPG2J0061-3, and CMRPG2K0271, CMRPG2K0272 to PHC; CMRPG2D0173 to CHL; CMRPVVK0091, CMRPVVK0092, CMRPVVL0021 to JCHH; CMRPG3G1133, CMRPG3H0873 to HMW, CMRPVVL0261 to HCK). The funding source had no role in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Competing Interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data presented in this study are available on request from the corresponding author.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hattori M, Iwata H. Advances in treatment and care in metastatic breast cancer (MBC): are there MBC patients who are curable? Chin Clin Oncol 2018; 7: 23. [DOI] [PubMed] [Google Scholar]
- 3. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791. [DOI] [PubMed] [Google Scholar]
- 4. Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 2006; 33: S9–S14. [DOI] [PubMed] [Google Scholar]
- 5. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006; 12: 4218–4224. [DOI] [PubMed] [Google Scholar]
- 6. Todaro M, Francipane MG, Medema JP, et al. Colon cancer stem cells: promise of targeted therapy. Gastroenterology 2010; 138: 2151–2162. [DOI] [PubMed] [Google Scholar]
- 7. Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009; 11: R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onder TT, Gupta PB, Mani SA, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68: 3645–3654. [DOI] [PubMed] [Google Scholar]
- 9. Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawson DA, Bhakta NR, Kessenbrock K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015; 526: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313–319. [DOI] [PubMed] [Google Scholar]
- 12. Nadal R, Ortega FG, Salido M, et al. CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer 2013; 133: 2398–2407. [DOI] [PubMed] [Google Scholar]
- 13. Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett 2010; 288: 99–106. [DOI] [PubMed] [Google Scholar]
- 14. Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012; 14: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu CL, Tsai TH, Huang CK, et al. Monitoring levels of vimentin-positive circulating cancer stem cells and tumor cells in patients with advanced EGFR-mutated non-small cell lung cancer. Lung Cancer 2021; 156: 50–58. [DOI] [PubMed] [Google Scholar]
- 16. Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol 2011; 29: 1547–1555. [DOI] [PubMed] [Google Scholar]
- 17. Lee CH, Hsieh JC, Wu TM, et al. Baseline circulating stem-like cells predict survival in patients with metastatic breast cancer. BMC Cancer 2019; 19: 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 19. McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005; 93: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu CY, Fu JY, Wu CF, et al. Malignancy prediction capacity and possible prediction model of circulating tumor cells for suspicious pulmonary lesions. J Pers Med 2021; 11: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C-Y, Lee C-L, Wu C-F, et al. Circulating tumor cells as a tool of minimal residual disease can predict lung cancer recurrence: a longitudinal, prospective trial. Diagnostics 2020; 10: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang PH, Wu MH, Liu SY, et al. The prognostic roles of pretreatment circulating tumor cells, circulating cancer stem-like cells, and programmed cell death-1 expression on peripheral lymphocytes in patients with initially unresectable, recurrent or metastatic head and neck cancer: an exploratory study of three biomarkers in one-time blood drawing. Cancers (Basel) 2019; 11: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang HM, Wu MH, Chang PH, et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck 2019; 41: 2676–2687. [DOI] [PubMed] [Google Scholar]
- 24. Su P-J, Wu M-H, Wang H-M, et al. Circulating tumour cells as an independent prognostic factor in patients with advanced oesophageal squamous cell carcinoma undergoing chemoradiotherapy. Sci Rep 2016; 6: 31423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X, Strietz J, Bleilevens A, et al. Chemotherapeutic stress influences epithelial-mesenchymal transition and stemness in cancer stem cells of triple-negative breast cancer. Int J Mol Sci 2020; 21: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yousefnia S, Seyed Forootan F, Seyed Forootan S, et al. Mechanistic pathways of malignancy in breast cancer stem cells. Front Oncol 2020; 10: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elbaiomy MA, Akl T, Atwan N, et al. Clinical impact of breast cancer stem cells in metastatic breast cancer patients. J Oncol 2020; 2020: 2561726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Du F, Fujii T, Kida K, et al. EpCAM-independent isolation of circulating tumor cells with epithelial-to-mesenchymal transition and cancer stem cell phenotypes using ApoStream® in patients with breast cancer treated with primary systemic therapy. PLoS One 2020; 15: e0229903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pantano F, Rossi E, Iuliani M, et al. Dynamic changes of receptor activator of nuclear factor-kappaB expression in circulating tumor cells during denosumab predict treatment effectiveness in metastatic breast cancer. Sci Rep 2020; 10: 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boonstra PA, Wind TT, van Kruchten M, et al. Clinical utility of circulating tumor DNA as a response and follow-up marker in cancer therapy. Cancer Metastasis Rev 2020; 39(3): 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vogl TJ, Riegelbauer LJ, Oppermann E, et al. Early dynamic changes in circulating tumor cells and prognostic relevance following interventional radiological treatments in patients with hepatocellular carcinoma. PLoS One 2021; 16: e0246527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ntzifa A, Strati A, Kallergi G, et al. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci Rep 2021; 11: 2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zapatero A, Gomez-Caamano A, Cabeza Rodriguez MA, et al. Detection and dynamics of circulating tumor cells in patients with high-risk prostate cancer treated with radiotherapy and hormones: a prospective phase II study. Radiat Oncol 2020; 15: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geurts YM, Witteveen A, Bretveld R, et al. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat 2017; 165: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phung MT, Tin Tin S, Elwood JM. Prognostic models for breast cancer: a systematic review. BMC Cancer 2019; 19: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Astvatsaturyan K, Yue Y, Walts AE, et al. Androgen receptor positive triple negative breast cancer: clinicopathologic, prognostic, and predictive features. PLoS One 2018; 13: e0197827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gennari A, Foca F, Zamarchi R, et al. Insulin-like growth factor-1 receptor (IGF-1R) expression on circulating tumor cells (CTCs) and metastatic breast cancer outcome: results from the TransMYME trial. Breast Cancer Res Treat 2020; 181: 61–68. [DOI] [PubMed] [Google Scholar]
- 38. Zhou J, Zhu X, Wu S, et al. Epithelial-mesenchymal transition status of circulating tumor cells in breast cancer and its clinical relevance. Cancer Biol Med 2020; 17: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savelieva OE, Tashireva LA, Kaigorodova EV, et al. Heterogeneity of stemlike circulating tumor cells in invasive breast cancer. Int J Mol Sci 2020; 21: 2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bock C, Rack B, Huober J, et al. Distinct expression of cytokeratin, N-cadherin and CD133 in circulating tumor cells of metastatic breast cancer patients. Future Oncol 2014; 10: 1751–1765. [DOI] [PubMed] [Google Scholar]
- 41. Brugnoli F, Grassilli S, Al-Qassab Y, et al. CD133 in breast cancer cells: more than a stem cell marker. Journal of Oncology 2019; 2019: 7512632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menyailo ME, Tretyakova MS, Denisov EV. Heterogeneity of circulating tumor cells in breast cancer: identifying metastatic seeds. Int J Mol Sci 2020; 21: 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Schauwer C, Meyer E, Van de Walle GR, et al. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology 2011; 75: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 44. Zhao W, Li Y, Zhang X. Stemness-related markers in cancer. Cancer Transl Med 2017; 3: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao P, Lu Y, Jiang X, et al. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci 2011; 102: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 46. Sartelet H, Imbriglio T, Nyalendo C, et al. CD133 expression is associated with poor outcome in neuroblastoma via chemoresistance mediated by the AKT pathway. Histopathology 2012; 60: 1144–1155. [DOI] [PubMed] [Google Scholar]