Abstract
Some evidence indicates endometriosis and migraine have a common genetic predisposition in sex-hormone genes, which could have important implications for the treatment of these two heterogenous conditions. To date, the genes responsibility remains unknown. Based on the biological hypothesis that polymorphisms of genes involved in sex-hormone pathways may influence estrogen levels and phenotypes of both disorders, we did a literature search for candidate sex-hormone genes and genes involved in the metabolism of estradiol. The aim was to review the evidence for shared sex-hormone-related polymorphisms between endometriosis and migraine and provide an exhaustive overview of the current literature. We included case-control studies investigating associations between candidate sex-hormone-related genes and the disorders endometriosis and migraine, respectively. Results showed three overlapping sex-hormone-associated polymorphisms in estrogen receptor genes that are associated with both conditions. To confirm possible associations with other sex-hormone genes, larger studies are needed.
Keywords: endometriosis, migraine, polymorphism, sex hormone genes, sex hormone receptor
Introduction
Endometriosis and migraine are two distinctive disorders associated with chronic pain, inflammation and a high grade of disability. Endometriosis is a common gynecological disease where endometrium-like tissue is manifested outside the uterine cavity. 1 Migraine, on the other hand, is a neurological disorder distinguished by recurrent headache attacks. 2 Based on the high prevalence in women during their reproductive life phase, it is assumed that estrogens or other female sex hormones might play a crucial role in the pathophysiology of both conditions. There is also some evidence that both conditions might share a common genetic background.3,4 The lifetime prevalence of endometriosis is at least 10% and the global prevalence of migraine in women is 18.9%, which corresponds to over a billion women suffering worldwide.5,6 Both conditions generate a huge social and economic burden and exert a significant negative impact on women’s quality of life.7–9 Migraine is the number one cause of disability in women during their reproductive years (aged 15 - 49 years).10,11 Large twin studies indicate that both disorders have a heritable trait, with heritability estimated to be between 30–60% for migraine and circa 50% for endometriosis, indicating that genes are of importance in the etiology of both conditions.12–16 Therefore, it has been suggested that endometriosis and migraine might be comorbidities, at least in a subset of women. 3 Menstruation is a major trigger for endometriosis-associated pain and menstrual-related migraine.17,18 In women, migraine typically starts during puberty and resolves during menopause. More than 50% of women report an association between migraine and their menstrual bleeding. 19 Both disorders are estrogen-dependent and may lead to exceptionally strong symptoms during menstruation.18,20
The role of female sex hormones in the pathogenesis of migraine is well established. 21 The drop of estrogen levels at the end of the menstrual cycle and at the beginning of the hormone-free interval in users of exogenous estrogens plays a crucial role in the pathophysiology of migraines in women.22–24 In addition, it has been shown that the use of combined oral contraceptives in the standard regimen may initiate and worsen migraine in predisposed women.25–28 Interestingly, a positive impact on the frequency of migraine episodes and the intensity of pain was observed in a continuous regimen with Desogestrel, a progestin-only contraceptive.29,30 Likewise, endometriosis symptoms can be successfully treated with hormones, in particular progestins.31,32
In line with the biological hypothesis that polymorphisms of genes involved in sex-hormone pathways may influence estrogen levels, we aimed to study the main sex-hormone genes (ESR1, ESR2, PGR, FSHR, AR, SHBG) and selected the most regularly studied genes affecting the metabolism of estradiol (COMT, NRIP1, CYP1A1, CYP17A1, CYP19A1). 33 There are two types of estrogen receptor (ESR) genes; estrogen receptor 1 (ESR1 or ER-alpha) and estrogen receptor 2 (ESR2 or ER-beta). Estrogen levels rise in the early phase of the cycle as a result of increasing levels of follicle stimulating hormone (FSH). During the menstrual cycle, not only estrogen levels but also levels of FSH, progesterone, and androgens fluctuate. Therefore, FSH receptor (FSHR), progesterone receptor (PGR), and androgen receptor (AR) genes must also be investigated as a potential source for a genetic predisposition. The PGR gene has two protein isoforms that modulate the biological action of progesterone: isoform A (PRA), which is capable of inhibiting the activation of the estrogen receptors, and isoform B (PRB), which has the capacity to activate the estrogen receptors. 34 Similar to the ESR, the PGR can undergo ligand-independent activation and is involved in various intracellular signaling pathways. A specific polymorphism in the PGR gene called PROGINS seems to impact the ligand-binding and the entire signaling pathway. 35 Moreover, the sex hormone-binding globulin (SHBG) gene, which codes for a glycoprotein that binds to androgens and estrogens, is of interest. For the final step of estrogen biosynthesis, aromatase enzymes such as Cytochrome P450, family 19, subfamily A, polypeptide 1 (CYP19A1), which is involved in the conversion of androgen to estrogen, are essential. Another enzyme of this family, Cytochrome P450, Family 17, subfamily A, polypeptide 1 (CYP17A1), mediates both 17-alpha-hydroxylase and 17,20-lyase, which play a key role in androgen biosynthesis. 36 Finally, Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) participates in this process by catalyzing estrogen hydroxylation in extrahepatic tissues. 37 Other genes involved in estrogen metabolism, like catechol-estrogen or its products, including catechol-O-methyl-transferase (COMT) and nuclear receptor interacting protein 1 (NRIP1), could also have an impact. NRIP1 negatively regulates the transcription of estrogen receptors, particularly ESR1. 38 Catechol-O-methyltransferase (COMT) is an omnipresent enzyme of the estrogen metabolizing pathway that catalyzes O-methylation and subsequently inactivates estradiol metabolites. 39
The association between most of these sex hormone gene variants (ESR1, ESR2, PGR, FSHR, AR, SBHG) and estradiol-metabolizing enzymes (NRIP1, COMT, and CYP family members) has been studied in women with migraine or endometriosis, however mostly for each disorder separately.40–42 One large Australian family study with 815 monozygotic and 457 dizygotic twin-pair sisters with surgically confirmed endometriosis investigated whether the combination of the two conditions is the result of chance, selection bias, or common environmental and genetic factors. The findings suggest that endometriosis and migraine have common genetic predispositions with a bivariate heritability of 17%. 3
To our knowledge, no previous study has specifically assessed the shared polymorphism between endometriosis and migraine. A possible genetic link could lead to better therapeutic care of these patients who suffer from chronic pain.
The aim of this narrative review is to obtain evidence for shared sex-hormone related polymorphisms in migraine and endometriosis. We aspire to present a comprehensive review of the existing literature and rationale for new research. We included studies focusing on sex-hormone receptor polymorphisms as well as those investigating enzymes affecting the metabolism of estradiol.
Materials and methods
Search strategy
We searched the PubMed database for publications from between January 2000 and July 2021 on sex hormone polymorphisms in endometriosis and migraine, respectively. To search and include as many related studies as possible, we used the MeSH terms “endometriosis” and “migraine disorders” with different combinations of the keywords for each sex hormone receptor. For Estrogen Receptor 1, for example, we used: ESR1, ESR1 gene, ESR alpha, ESR alpha gene, ESR1 polymorphism, ESR alpha polymorphism, estrogen receptor alpha polymorphism, estrogen receptor 1 polymorphism. We did the same for ESR2, FSHR, PGR, AR, SHBG and the estrogen-metabolizing association genes NRIP1, CYP1A1, CYP17A1, CYP19A1 and COMT. For each sex hormone-related gene included in the present study, a literature search was performed to find case-control studies and potential previous reviews and/or meta-analyses in order to identify potential additional records through other sources.
Study selection
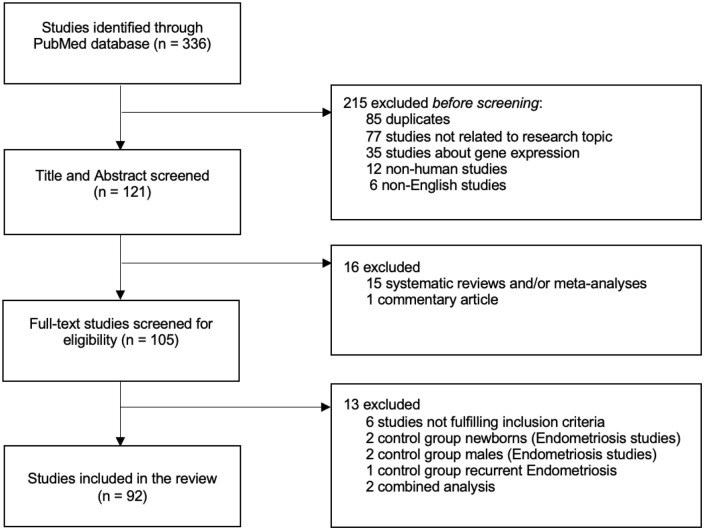
The eligibility of each publication was assessed independently by two reviewers (JFV and GM). After the titles and abstracts were screened, the full text of articles eligible for our review was examined. Studies had to meet the following inclusion criteria: 1) published between January 2000—July 2021, 2) original case-control study investigating associations between polymorphisms in the candidate sex hormone genes (ESR1, ESR2, FSHR, PGR, AR, SHBG) and the estradiol metabolizing association genes (NRIP1, CYP1A1, CYP17A1, CYP19A1, COMT) and 3) diagnosis of endometriosis confirmed by surgery and/or histology and diagnosis of migraine according to the criteria of the International Headache Society (IHS). 2 While reviewing publications, a novel gene, the FSH beta-subunit (FSHB) gene, consisting of a FSH beta-subunit (FSHβ), was added to our gene selection. 43 A new literature search for this FSHB gene was carried out. We excluded systematic reviews and/or meta-analyses, commentary articles, and duplicated studies. In the endometriosis studies, control groups with unrelated males or neonates were excluded, considering endometriosis is a disease occurring in women of reproductive age. However, we included one study with a very large sample size that included both parents of the women with endometriosis, due to their comparable hereditary genetic makeup. Studies with self-reported or self-diagnosed endometriosis or migraine were excluded. We excluded two case-control studies that investigated the combination of two sex hormone polymorphisms44,45 (Supplemental Table S1). Figure 1 summarizes the study selection process for identifying eligible studies.
Figure 1.
Flow diagram of the literature search and study selection process.
Data extraction
For each eligible study, data were extracted by one investigator (JFV) using a standardized Microsoft Excel spreadsheet and checked by a second investigator (GM). Discrepancies were resolved through discussion. The following data were listed for each study: Author, year of publication, country in which the research was conducted, ethnicity of the women, sample size, sex hormone gene, dbSNP (RefSNP or rs), 46 associated alleles if available, and the type of genetic analysis. For the endometriosis studies, we also extracted the type of control population and the endometriosis stage according to the American Society for Reproductive Medicine (ASRM) classification or the American Fertility Society score (AFS) respectively, if available. 47
Results
In total, 92 studies fulfilled the inclusion criteria for this review (Figure 1). The characteristics of the included studies are listed in Table 1. For some of the candidate genes, a broad variety of SNPs were investigated. SNPs that were mentioned more than once in our literature search are grouped, see Table 1. Other SNPs that have been mentioned only once in our search are listed at the end. In the CYP17A1 gene, SHBG gene, and FSHB gene, the SNPs have only been assessed in one of both conditions. Since we are looking for overlapping SNPs between both conditions, these SNPs will not be described in our results but can be found in Table 1.
Table 1.
Summary table of the included studies according to sex-hormone genes.
| Gene | Polymorphism | Allele | Association | Disease | Ref. citation | Cases/Controls | Ethnicity | Country | Analysis | Cases Surgery Yes/No | Control Surgery Yes/No | ASRM | IHS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR1 | rs2234693, Pvull | T > C | Yes | Endometriosis | Hsieh et al. 48 | 112/110 | Asian | Taiwan | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2234693, Pvull | T > C | Yes | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2234693, Pvull | C > T | Yes | Endometriosis | Paskulin et al. 50 | 98/115 | Caucasian | Brazil | Taqman assay | Yes | Yes | Yes | |
| ESR1 | rs2234693, Pvull | * | Yes | Endometriosis | Kitawaki et al. 51 | 109/179 | Asian | Japan | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2234693, Pvull | T > C | No | Endometriosis | Govindan et al. 52 | 110/115 | Asian | India | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2234693, Pvull | T > C | No | Endometriosis | Renner et al. 53 | 98/98 | Caucasian | Germany | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2234693, Pvull | * | No | Endometriosis | Xie et al. 54 | 214/160 | Asian | China | PCR-RFLP | Yes | No | No | |
| ESR1 | rs2234693, Pvull | * | Yes | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR1 | rs2234693, Pvull | C > T | Yes | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| ESR1 | rs2234693, Pvull | T > C | Yes | Migraine | Joshi et al. 57 | 217/217 | Asian | India | PCR-RFLP | Yes | |||
| ESR1 | rs2234693, Pvull | C > T | No | Migraine | Colson et al. 58 | 240/240 | Caucasian | Australia | PCR-RFLP | Yes | |||
| ESR1 | rs9340799, Xbal | A > G | Yes | Endometriosis | Hsieh et al. 48 | 112/110 | Asian | Taiwan | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs9340799, Xbal | A > G | Yes | Endometriosis | Xie et al. 54 | 214/160 | Asian | China | PCR-RFLP | Yes | No | No | |
| ESR1 | rs9340799, Xbal | A > G | No | Endometriosis | Renner et al. 53 | 98/98 | Caucasian | Germany | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs9340799, Xbal | A > G | No | Endometriosis | Paskulin et al. 50 | 98/115 | Caucasian | Brazil | Taqman assay | Yes | Yes | Yes | |
| ESR1 | rs9340799, Xbal | * | Yes | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR1 | rs9340799, Xbal | * | No | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| ESR1 | TA repeat | (TA)n | Yes | Endometriosis | Kim et al. 59 | 180/165 | Asian | Korea | PCR-RFLP | Yes | Yes | Yes | |
| ESR1 | TA repeat | (TA)n | Yes | Endometriosis | Hsieh et al. 60 | 119/108 | Asian | Taiwan | PCR-RFLP | Yes | Yes | Yes | |
| ESR1 | TA repeat | (TA)n | Yes | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs2228480 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| ESR1 | rs2228480 | G > A | Yes | Migraine | Colson et al. 62 | 224/224 | Caucasian | Australia | PCR | Yes | |||
| ESR1 | rs2228480 | G > A | Yes | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Oterino et al. 65 | 599/232 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Corominas et al. 66 | 210/210 | Caucasian | Spain | PCR-RFLP | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| ESR1 | rs2228480 | G > A | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | PCR-RFLP | Yes | |||
| ESR1 | rs1801132 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| ESR1 | rs1801132 | C > G | Yes | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs1801132 | G > C | Yes | Migraine | Oterino et al. 65 | 599/232 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| ESR1 | rs1801132 | G > C | Yes | Migraine | Oterino et al. 68 | 356/374 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| ESR1 | rs1801132 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR1 | rs1801132 | C > G | No | Migraine | Colson et al. 58 | 240/240 | Caucasian | Australia | PCR-RFLP | Yes | |||
| ESR1 | rs1801132 | * | No | Migraine | Corominas et al. 66 | 210/210 | Caucasian | Spain | PCR-RFLP | Yes | |||
| ESR1 | rs1801132 | * | No | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| ESR1 | rs1801132 | C > G | No | Migraine | Joshi et al. 57 | 217/217 | Asian | India | PCR-RFLP | Yes | |||
| ESR1 | rs1801132 | * | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | PCR-RFLP | Yes | |||
| ESR1 | rs1801132 | * | No | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR1 | rs3798573 | A > G | Yes | Endometriosis | Wang W et al. 69 | 312/357 | Asian | China | PCR-RFLP | Yes | Yes | Yes | |
| ESR1 | rs2077647 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| ESR1 | rs3853250 | * | No | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs3853251 | * | No | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| ESR1 | rs1159327 | A > G | No | Endometriosis | Wang W et al. 69 | 312/357 | Asian | China | PCR-RFLP | Yes | Yes | Yes | |
| ESR1 | rs3020348 | A > C | No | Endometriosis | Wang W et al. 69 | 312/357 | Asian | China | PCR-RFLP | Yes | Yes | Yes | |
| ESR1 | rs1884049 | * | No | Endometriosis | Matsuzaka et al. 70 | 100/143 | Asian | Japan | PCR | Yes | Yes | Yes | |
| ESR1 | rs1884053 | * | No | Endometriosis | Matsuzaka et al. 70 | 100/143 | Asian | Japan | PCR | Yes | Yes | Yes | |
| ESR1 | rs1884054 | * | No | Endometriosis | Matsuzaka et al. 70 | 100/143 | Asian | Japan | PCR | Yes | Yes | Yes | |
| ESR1 | Intron 1 HaeIII | GGCC > GGCT | No | Endometriosis | Sato et al. 71 | 105/125 | Caucasian | Brazil | PCR-RFLP | Yes | No | Yes | |
| ESR1 | Exon 1 MspI | CCGG > CTGG | No | Endometriosis | Sato et al. 71 | 105/125 | Caucasian | Brazil | PCR-RFLP | Yes | No | Yes | |
| ESR1 | IVS1 –401 > C | * | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| ESR1 | rs6557170 | G > A | Yes | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs6557171 | C > T | Yes | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs2347867 | A > G | Yes | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs4870062 | T > G | Yes | Migraine | Kaunisto et al. 64 | 898/900 | Caucasian | Finland | Multiplex-PCR | Yes | |||
| ESR1 | rs726281 | * | Yes | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR1 | rs2295193 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR1 | rs3798577 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR1 | rs2077647 | * | No | Migraine | Corominas et al. 66 | 210/210 | Caucasian | Spain | PCR-RFLP | Yes | |||
| ESR2 | rs4986938, AluI | G > A | Yes | Endometriosis | Bianco et al. 73 | 108/210 | Caucasian | Brazil | PCR-RFLP | Yes | Yes | Yes | |
| ESR2 | rs4986938, AluI | G > A | Yes | Endometriosis | Szaflik et al. 74 | 100/100 | Caucasian | Poland | Sanger sequencing | Yes | No | Yes | |
| ESR2 | rs4986938, AluI | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| ESR2 | rs4986938, AluI | G > A | No | Endometriosis | Lee et al. 75 | 239/287 | Asian | Korea | PCR-RFLP | Yes | Yes | Yes | |
| ESR2 | rs4986938, AluI | * | Yes | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | Taqman assay | Yes | |||
| ESR2 | rs4986938, AluI | * | Yes | Migraine | Oterino et al. 68 | 356/374 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| ESR2 | rs4986938, AluI | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR2 | rs4986938, AluI | * | No | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR2 | rs1256049 | * | Yes | Endometriosis | Silva et al. 76 | 54/46 | Caucasian | Brazil | PCR | Yes | No | Yes | |
| ESR2 | rs1256049 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| ESR2 | rs1256049 | * | No | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| ESR2 | rs1256049 | * | No | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| ESR2 | rs17179740 | * | Yes | Endometriosis | Smolarz et al. 77 | 200/200 | Caucasian | Poland | HRM | Yes | No | Yes | |
| ESR2 | rs17179740 | A > G | No | Endometriosis | Wang W et al. 69 | 312/357 | Asian | China | PCR-RFLP | Yes | Yes | Yes | |
| ESR2 | rs928554, A > G | A > G | Yes | Endometriosis | Szaflik et al. 74 | 100/100 | Caucasian | Poland | Sanger sequencing | Yes | No | Yes | |
| ESR2 | rs944052 | * | Yes | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| ESR2 | CA repeat | (CA)n | Yes | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| ESR2 | rs1255998 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| ESR2 | rs1271572 | * | No | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| PGR | PROGINS Alu ins | * | Yes | Endometriosis | Costa et al. 78 | 54/45 | Caucasian | Brazil | PCR-RFLP | Yes | No | No | |
| PGR | PROGINS Alu ins | * | Yes | Endometriosis | De Carvalho et al. 79 | 121/281 | Caucasian | Brazil | PCR-RFLP | Yes | No | Yes | |
| PGR | PROGINS Alu ins | * | Yes | Endometriosis | Lattuada et al. 80 | 131/127 | Caucasian | Italy | PCR-RFLP | Yes | Yes | Yes | |
| PGR | PROGINS Alu ins | * | Yes | Endometriosis | Wieser et al.39,81 | 95/107 | Caucasian | Austria | PCR-RFLP | Yes | Yes | Yes | |
| PGR | PROGINS Alu ins | * | No | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| PGR | PROGINS Alu ins | * | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | PROGINS Alu ins | * | No | Endometriosis | Gimenes et al. 84 | 148/179 | Caucasian | Brazil | PCR-RFLP | Yes | Yes | Yes | |
| PGR | PROGINS Alu ins | * | No | Endometriosis | Govindan et al. 85 | 100/108 | Asian | India | PCR | Yes | No | No | |
| PGR | PROGINS Alu ins | * | Yes | Migraine | Colson et al. 86 | 300/300 | Caucasian | Australia | PCR | Yes | |||
| PGR | PROGINS Alu ins | * | No | Migraine | Corominas et al. 66 | 210/210 | Caucasian | Spain | PCR | Yes | |||
| PGR | PROGINS Alu ins | * | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | PCR | Yes | |||
| PGR | rs1042838 | * | No | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| PGR | rs1042838 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| PGR | rs1042838 | G > T | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs1042838 | * | No | Endometriosis | Van Kaam et al. 87 | 72/102 | Caucasian | Netherlands | PCR-RFLP | Yes | Yes | Yes | |
| PGR | rs1042838 | * | No | Migraine | Joshi et al. 57 | 217/217 | Asian | India | PCR | Yes | |||
| PGR | rs1042838 | G > T | No | Migraine | Palmirotta et al. 88 | 380/185 | Caucasian | Italy | Sanger sequencing | Yes | |||
| PGR | rs10895068 | G > A | No | Endometriosis | Van Kaam et al. 87 | 72/102 | Caucasian | Netherlands | PCR-RFLP | Yes | Yes | Yes | |
| PGR | rs10895068 | G > A | No | Endometriosis | Gentilini et al. 89 | 199/300 | Caucasian | Italy | PCR-RFLP | Yes | Yes | Yes | |
| PGR | rs10895068 | G > A | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs10895068 | G > A | No | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs500760 | A > G | Yes | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs1042839 | C > T | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs2008112 | G > A | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs2020880 | C > T | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs3740754 | G > C | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| PGR | rs518162 | G > A | No | Endometriosis | Treloar et al.82,83 | 980/2940 | Caucasian | Australia | PCR-RFLP | Yes | No | Yes | |
| AR | CAG repeat | (CAG)n | Yes | Endometriosis | Hsieh et al. 90 | 110/99 | Asian | Taiwan | PCR | Yes | No | No | |
| AR | CAG repeat | (CAG)n | Yes | Endometriosis | Shaik et al. 91 | 90/101 | Asian | India | PCR | Yes | No | No | |
| AR | CAG repeat | (CAG)n | Yes | Endometriosis | Shin et al. 92 | 421/349 | Asian | Korea | PCR | Yes | Yes | Yes | |
| AR | CAG repeat | (CAG)n | No | Endometriosis | Lattuada et al. 93 | 105/92 | Caucasian | Italy | PCR | Yes | Yes | Yes | |
| AR | CAG repeat | (CAG)n | No | Endometriosis | Tong et al. 94 | 24/114 | Asian | China | PCR | Yes | No | Yes | |
| AR | CAG repeat | (CAG)n | No | Migraine | Colson et al. 86 | 275/275 | Caucasian | Australia | PCR | Yes | |||
| FSHR | rs6165 | * | Yes | Endometriosis | Kerimoglu et al. 95 | 100/100 | Asian | Turkey | PCR-RFLP | Yes | Yes | Yes | |
| FSHR | rs6165 | * | Yes | Endometriosis | Liaqat et al. 96 | 156/208 | Asian | Pakistan | PCR | Yes | No | No | |
| FSHR | rs6165 | G > A | No | Endometriosis | Andre et al. 97 | 352/510 | Caucasian | Brazil | Taqman assay | Yes | Yes | Yes | |
| FSHR | rs6165 | A > G | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| FSHR | rs6166 | * | Yes | Endometriosis | Kerimoglu et al. 95 | 100/100 | Asian | Turkey | PCR-RFLP | Yes | Yes | Yes | |
| FSHR | rs6166 | * | No | Endometriosis | Liaqat et al. 96 | 156/208 | Asian | Pakistan | PCR | Yes | No | No | |
| FSHR | rs6166 | A > G | No | Endometriosis | Andre et al. 97 | 352/510 | Caucasian | Brazil | Taqman assay | Yes | Yes | Yes | |
| FSHR | rs6166 | A > G | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| FSHR | rs6166 | A > G | No | Endometriosis | Schmitz et al. 99 | 67/65 | Caucasian | Brazil | PCR-RFLP | Yes | Yes | Yes | |
| FSHR | rs6166 | * | Yes | Migraine | Oterino et al. 68 | 356/374 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| FSHR | rs6166 | * | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | MALDI-TOF MS | Yes | |||
| FSHR | rs6166 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| FSHB | rs11031006 | * | Yes | Endometriosis | Angioni et al. 100 | 72/41 | Caucasian | Italy | Sanger sequencing | Yes | Yes | Yes | |
| FSHB | rs11031006 | * | Yes | Endometriosis | Matalliotakis et al. 101 | 166/150 | Caucasian | Greece | Taqman assay | Yes | No | Yes | |
| SHBG | rs6259 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| NRIP1 | rs2229741 | G > A | No | Endometriosis | Caballero et al. 102 | 59/141 | Caucasian | Spain | Real-Time PCR assay | Yes | No | Yes | |
| NRIP1 | rs2229741 | * | Yes | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | MALDI-TOF MS | Yes | |||
| NRIP1 | rs2229741 | * | No | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| NRIP1 | rs2229741 | * | No | Migraine | Oterino et al. 68 | 356/374 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| NRIP1 | rs2229742 | C > G | Yes | Endometriosis | Caballero et al. 102 | 59/141 | Caucasian | Spain | Real-Time PCR assay | Yes | No | Yes | |
| NRIP1 | rs2506142 | * | Yes | Migraine | Pollock et al. 103 | 235/140 | Caucasian | Australia | PCR-RFLP | Yes | |||
| COMT | rs4680 | * | No | Endometriosis | Christofolini et al.44,104 | 198/168 | Caucasian | Brazil | Taqman assay | Yes | Yes | Yes | |
| COMT | rs4680 | * | No | Endometriosis | Trabert et al. 40 | 255/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| COMT | rs4680 | G > A | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| COMT | rs4680 | G > A | No | Endometriosis | Wieser et al.39,81 | 91/92 | Caucasian | Australia | PCR-RFLP | Yes | Yes | Yes | |
| COMT | rs4680 | G > A | No | Endometriosis | Juo et al. 105 | 105/312 | Asian | China | PCR-RFLP | Yes | No | No | |
| COMT | rs4680 | G > A | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| COMT | rs4680 | G > A | Yes | Migraine | Emin Erdal et al. 106 | 62/64 | Asian | Turkey | PCR-RFLP | Yes | |||
| COMT | rs4680 | * | Yes | Migraine | Sullivan et al. 107 | 1740/1132 | Caucasian | USA | Illumina sequencing | Yes | |||
| COMT | rs4680 | * | No | Migraine | De Marchis et al. 108 | 380/132 | Caucasian | Italy | Sanger sequencing | Yes | |||
| COMT | rs4680 | * | No | Migraine | Todt et al. 109 | 270/272 | Caucasian | Germany | Taqman assay | Yes | |||
| COMT | rs4680 | G > A | No | Migraine | Sutherland et al. 110 | 268/140 | Caucasian | Australia | PCR-RFLP | Yes | |||
| COMT | rs4680 | G > A | No | Migraine | Takigawa et al. 111 | 223/191 | Asian | Japan | PCR-RFLP | Yes | |||
| COMT | rs4680 | G > A | No | Migraine | Hagen et al. 112 | 982/1468 | Caucasian | Norway | PCR | Yes | |||
| COMT | rs4680 | G > A | No | Migraine | Park et al. 113 | 97/94 | Asian | Korea | PCR | Yes | |||
| COMT | rs1544325 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs165774 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs4633 | C > T | No | Migraine | Takigawa et al. 111 | 223/191 | Asian | Japan | PCR-RFLP | Yes | |||
| COMT | rs4646316 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs4818 | * | No | Migraine | De Marchis et al. 108 | 380/132 | Caucasian | Italy | Sanger sequencing | Yes | |||
| COMT | rs6267 | G > T | No | Migraine | Takigawa et al. 111 | 223/191 | Asian | Japan | PCR-RFLP | Yes | |||
| COMT | rs740601 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs740603 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs9332377 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs933271 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| COMT | rs2020917 | * | No | Migraine | Corominas et al. 66 | 259/287 | Caucasian | Spain | SNPlex assay | Yes | |||
| CYP1A1 | rs4646903 | * | Yes | Endometriosis | Arvanitis et al. 114 | 275/346 | Caucasian | Greece | PCR | Yes | No | Yes | |
| CYP1A1 | rs4646903 | T > C | Yes | Endometriosis | Barbosa et al. 115 | 52/42 | Caucasian | Brazil | PCR-RFLP | Yes | Yes | Yes | |
| CYP1A1 | rs4646903 | T > C | No | Endometriosis | Babu et al. 116 | 310/215 | Asian | India | PCR-RFLP | Yes | Yes | Yes | |
| CYP1A1 | rs4646903 | T > C | No | Endometriosis | Rozati et al. 117 | 97/102 | Asian | India | PCR-RFLP | Yes | Yes | Yes | |
| CYP1A1 | rs4646903 | T > C | No | Endometriosis | Juo et al. 105 | 105/312 | Asian | China | PCR-RFLP | Yes | No | No | |
| CYP1A1 | rs4646903 | T > C | No | Endometriosis | Babaki et al. 118 | 93/139 | Asian | Iran | PCR-RFLP | Yes | Yes | Yes | |
| CYP1A1 | rs4646903 | T > C | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| CYP1A1 | rs4646903 | * | No | Endometriosis | Wu et al. 119 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| CYP1A1 | rs4646903 | T > C | No | Migraine | Sutherland et al. 110 | 268/140 | Caucasian | Australia | PCR-RFLP | Yes | |||
| CYP1A1 | rs1048943 | A > G | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| CYP1A1 | rs1048943 | A > G | No | Migraine | Sutherland et al. 110 | 268/140 | Caucasian | Australia | PCR-RFLP | Yes | |||
| CYP1A1 | rs4646422 | G > A | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| CYP17A1 | rs743572 | T > C | Yes | Endometriosis | Bozdag et al. 120 | 46/39 | Asian | Turkey | PCR | Yes | Yes | Yes | |
| CYP17A1 | rs743572 | * | Yes | Endometriosis | Hsieh et al. 60 | 119/108 | Asian | Taiwan | PCR | Yes | Yes | No | |
| CYP17A1 | rs743572 | A > G | Yes | Endometriosis | Szczepańska et al. 121 | 115/197 | Caucasian | Poland | HRM | Yes | Yes | Yes | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | Vietri et al. 122 | 104/86 | Caucasian | Italy | PCR | Yes | Yes | Yes | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | De Carvalho et al. 79 | 121/281 | Caucasian | Brazil | PCR-RFLP | Yes | No | No | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | Vietri et al. 122 | 104/86 | Caucasian | Italy | PCR | Yes | Yes | Yes | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | Juo et al. 105 | 105/312 | Asian | China | PCR-RFLP | Yes | No | No | |
| CYP17A1 | rs743572 | * | No | Endometriosis | Kado et al. 36 | 140/177 | Asian | Japan | PCR-RFLP | Yes | No | Yes | |
| CYP17A1 | rs743572 | * | No | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| CYP17A1 | rs743572 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | Yes | Yes | |
| CYP17A1 | rs619824 | G > T | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs3740397 | C > G | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs4919687 | G > A | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs6163 | C > A | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs6162 | G > A | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs743572 | T > C | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs2486758 | T > C | No | Endometriosis | Zhao et al. 123 | 768/768 | Caucasian | Australia | MALDI-TOF MS | Yes | Yes | Yes | |
| CYP17A1 | rs10786712 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| CYP19A1 | rs10046 | * | No | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| CYP19A1 | rs10046 | C > T | No | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | rs10046 | C > T | No | Endometriosis | Szaflik et al. 74 | 100/100 | Caucasian | Poland | Sanger sequencing | Yes | No | Yes | |
| CYP19A1 | rs10046 | * | No | Endometriosis | Szczepańska et al. 122 | 115/197 | Caucasian | Poland | HRM | Yes | Yes | Yes | |
| CYP19A1 | rs10046 | T > C | No | Endometriosis | Wang L et al. 124 | 146/225 | Asian | China | PCR | Yes | No | No | |
| CYP19A1 | rs10046 | * | Yes | Migraine | Coşkun et al. 63 | 142/141 | Asian | Turkey | PCR | Yes | |||
| CYP19A1 | rs10046 | * | Yes | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| CYP19A1 | rs10046 | * | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | MALDI-TOF MS | Yes | |||
| CYP19A1 | rs10046 | * | No | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| CYP19A1 | rs10046 | * | No | Migraine | Oterino et al. 68 | 356/374 | Caucasian | Spain | Real-Time PCR assay | Yes | |||
| CYP19A1 | rs4646 | C > A | No | Endometriosis | Szaflik et al. 74 | 100/100 | Caucasian | Poland | Sanger sequencing | Yes | No | Yes | |
| CYP19A1 | rs4646 | * | Yes | Migraine | Ghosh et al. 56 | 334/200 | Asian | India | PCR-SSCP | Yes | |||
| CYP19A1 | rs4646 | * | No | Migraine | An et al. 55 | 494/533 | Asian | China | MALDI-TOF MS | Yes | |||
| CYP19A1 | rs4646 | * | No | Migraine | Rodriguez-Acevedo et al. 67 | 282/155 | Caucasian | Australia | MALDI-TOF MS | Yes | |||
| CYP19A1 | rs700519 | C > T | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| CYP19A1 | rs700519 | C > T | No | Endometriosis | Tsuchiya et al. 37 | 75/57 | Asian | Japan | PCR | Yes | Yes | Yes | |
| CYP19A1 | rs700519 | C > T | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| CYP19A1 | rs700519 | C > T | No | Migraine | Sutherland et al. 110 | 268/140 | Caucasian | Australia | PCR-RFLP | Yes | |||
| CYP19A1 | rs2236722 | T > C | No | Endometriosis | Wang HS et al. 98 | 300/337 | Asian | Taiwan | MALDI-TOF MS | Yes | No | Yes | |
| CYP19A1 | rs2236722 | T > C | No | Endometriosis | Wang L et al. 124 | 146/225 | Asian | China | PCR | Yes | No | No | |
| CYP19A1 | TTTA repeat | (TTTA)n | Yes | Endometriosis | Arvanitis et al. 114 | 275/346 | Caucasian | Greece | PCR | Yes | No | Yes | |
| CYP19A1 | TTTA repeat | (TTTA)n | Yes | Endometriosis | Kado et al. 36 | 140/177 | Asian | Japan | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | TTTA repeat | (TTTA)n | No | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | TTTA repeat | (TTTA)n | No | Endometriosis | Hur et al. 125 | 224/188 | Asian | Korea | PCR | Yes | Yes | Yes | |
| CYP19A1 | TTTA repeat | (TTTA)n | No | Endometriosis | Wang L et al. 124 | 146/225 | Asian | China | PCR | Yes | No | No | |
| CYP19A1 | Val80 | G > A | Yes | Endometriosis | Vietri et al. 122 | 104/86 | Caucasian | Italy | PCR | Yes | Yes | Yes | |
| CYP19A1 | Val80 | G > A | No | Endometriosis | Hur et al. 125 | 224/188 | Asian | Korea | PCR | Yes | Yes | Yes | |
| CYP19A1 | C1558T | C > T | Yes | Endometriosis | Vietri et al. 122 | 104/86 | Caucasian | Italy | PCR | Yes | Yes | Yes | |
| CYP19A1 | C1558T | C > T | No | Endometriosis | Huber et al. 72 | 32/790 | Caucasian | Austria | Multiplex-PCR | Yes | No | Yes | |
| CYP19A1 | rs1004982 | * | Yes | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | rs1870049 | * | Yes | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | rs936307 | * | Yes | Endometriosis | Trabert et al. 40 | 256/567 | Caucasian | USA | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | rs8042086 | * | Yes | Endometriosis | Wu et al. 61 | 121/171 | Asian | Taiwan | Taqman assay | Yes | No | Yes | |
| CYP19A1 | TCT ins/del | * | No | Endometriosis | Lamp et al. 49 | 150/199 | Caucasian | Estonia | PCR-RFLP | Yes | No | Yes | |
| CYP19A1 | 115T > C | * | No | Endometriosis | Hur et al. 125 | 224/188 | Asian | Korea | PCR | Yes | Yes | Yes | |
| CYP19A1 | 1531C > T | * | No | Endometriosis | Hur et al. 125 | 224/188 | Asian | Korea | PCR | Yes | Yes | Yes | |
| CYP19A1 | rs700518 | A > G | No | Endometriosis | Wang L et al. 124 | 146/225 | Asian | China | PCR | Yes | No | No | |
| CYP19A1 | rs2899470 | * | Yes | Endometriosis | Smolarz et al. 77 | 200/200 | Caucasian | Poland | HRM | Yes | No | Yes |
ASRM: American Society for Reproductive Medicine; IHS: International Headache Society; ESR: estrogen receptor; PCR: polymerase chain reaction; PCR-SSCP: polymerase chain reaction-single-strand conformation polymorphism; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; HRM: high resolution melt; MALDI TOF MS: matrix-assisted laser desorption ionization-time-of-flight mass spectrometry; SNP: single nucleotide polymorphism; PGR: progesterone receptor; AR: androgen receptor; FSH: follicle stimulating hormone; FSHR: follicle stimulating hormone receptor; FSHB: FSH beta-subunit; SHBG: sex hormone-binding globulin; NRIP: nuclear receptor interacting protein; COMT: Catechol-O-methyl-transferase.
Not mentioned.
ESR1 gene
We found two overlapping polymorphisms in the ESR1 gene that could possibly play a role in the common genetic cause (Table 2).
Table 2.
Overview of overlapping polymorphisms associated with endometriosis and migraine.
| Association Endometriosis | Association Migraine | ||||
|---|---|---|---|---|---|
| Gene | SNP | Caucasian | Asian | Caucasian | Asian |
| ESR1 | rs2234693, Pvull | probable | probable | no | yes |
| ESR1 | rs9340799, Xbal | no | yes | more studies needed | possible |
| ESR1 | TA repeat | possible | yes | no data | no data |
| ESR1 | rs2228480 | no data | more studies needed | no | no |
| ESR1 | rs1801132 | no data | more studies needed | probable | no |
| ESR2 | rs4986938, AluI | yes | no | yes | no |
| ESR2 | rs1256049 | no data | more studies needed | no data | no |
| ESR2 | rs17179740 | more studies needed | more studies needed | no data | no data |
| PGR | PROGINS Alu ins | possible | more studies needed | no | more studies needed |
| PGR | rs1042838 | no | more studies needed | more studies needed | no data |
| PGR | rs10895068 | no | no data | no data | no data |
| AR | CAG repeat | more studies needed | yes | more studies needed | more studies needed |
| FSHR | rs6165 | more studies needed | probable | no data | no data |
| FSHR | rs6166 | more studies needed | no | possible | no data |
| FSHB | rs11031006 | yes | no data | no data | no data |
| SBHG | rs6259 | no data | no data | no data | more studies needed |
| NRIP1 | rs2229741 | no data | no data | possible | more studies needed |
| COMT | rs4680 | no | no | possible | no |
| CYP1A1 | rs4646903, MspI | more studies needed | no | more studies needed | no data |
| CYP17A1 | rs743572, MspA1 | no | no | no data | no data |
| CYP19A1 | rs10046 | no | more studies needed | no | possible |
| CYP19A1 | rs4646 | more studies needed | no data | more studies needed | possible |
| CYP19A1 | rs700519 | no data | more studies needed | more studies needed | no data |
| CYP19A1 | TTTA repeat | probable | possible | no data | no data |
SNP: single nucleotide polymorphism; ESR: estrogen receptor; PGR: progesterone receptor; AR: androgen receptor; FSHR: follicle stimulating hormone receptor; FSHB: FSH beta-subunit; NRIP: nuclear receptor interacting protein; COMT: Catechol-O-methyl-transferase.
Yes: all studies showed an association.
Probable: ⩾ 50% with association AND largest sum of sample sizes.
Possible: ⩽ 50% with association BUT largest sum of sample sizes.
No: ⩾ 75% without association AND/OR large sample size without association.
More studies needed: ⩾ 2 studies needed for each disorder to make a statement.
No data.
rs2234693, Pvull
Four studies in endometriosis patients found an association of this polymorphism in Caucasian and Asian women,51,48–50 while three studies did not.52–54 This, in spite the sample sizes of the seven studies were comparable. Only two of four studies investigating the base-pair change T > C in different ethnicities found an association.48,49,52,53 Three out of four studies found an association with migraine, all conducted in Asian populations, while no association with migraine was found in the study with a Caucasian population.55–58 Only one study specified the affected base-pair change T > C. 57 Two large studies with Caucasian migraineurs did not find a significant association.58,67
rs9340799, Xbal
Two of four studies found an association with endometriosis, and one of two studies found a significant association with migraine (Table 1). The associations with endometriosis were found in trials with Asian women.48,54 The association was not confirmed in Caucasian women in studies with similar sample sizes.50,53 All four endometriosis studies investigated the same base-pair change, A > G. For migraine, the studies for this SNP showed contradictory results55,56 (Table 1).
rs2228480
In an Asian study, no association with endometriosis was found. 61 For migraine, an association was significant in two of eight studies with the same alleles G > A55,62 (Table 1).
rs1801132
No association with endometriosis was found and a significant association with migraine was reported in three studies including large sample sizes of Caucasian women, two of them investigating the base-pair change G > C. The largest study investigated a C > G change. 64 Seven studies did not find an association for this SNP with migraine, two also studying the C > G change.57,58 The majority of these seven studies included Asian populations.55–57,121
ESR2 gene
One overlapping polymorphism that could possibly play a role in the common genetic cause of the two diseases was found in the ESR2 gene (Table 2).
rs4986938
Endometriosis and migraine were both associated with this polymorphism in four of eight studies. Two studies with Caucasian endometriosis patients found a significant association with the base-pair change G > A,73,74 while such an association was not confirmed in women of Asian descent.61,75 Moreover, two studies in Caucasian migraineurs, but not those including Asian populations, reported an association with this SNP.55,67,68,121
rs1256049
One of two studies found an association with endometriosis. 76 A total of three Asian studies investigated this polymorphism, but none found an association with endometriosis or migraine.55,56,61
PGR gene
PROGINS
Four studies showed an association with endometriosis. All four studies included Caucasian participants.78,79,81,93 The sample sizes of these four studies were relatively small compared with the studies without an association.49,82,84,85One association was found in a migraine trial with Caucasian women. 86 Two other migraine trials with Caucasian women and slightly smaller sample sizes did not find an association.66,67
rs1042838
No associations were found, for neither endometriosis nor migraine.40,57,61,83,87,89
AR gene
CAG repeat
Three studies showed an association with endometriosis. All three studies consisted of participants of Asian ethnicity.90–92 Three other studies did not find an association, one of which was a study investigating an association with migraine.80,86,94
FSHR gene
rs6166
One of five studies found an association with endometriosis in Turkish women, and one of three studies found an association with Caucasian migraineurs.68,95
NRIP1 gene
rs2229741
No association was found with endometriosis in a small study with Caucasian women. 102 One of three studies showed an association in Caucasian migraineurs. 67
COMT gene
rs4680
In six studies investigating an association with endometriosis, none were found.39,40,72,98,104,105 Two of eight studies found an association with migraine. One of the trials included more than 1000 participants. 107 On the contrary, another very large trial did not find an association. 112 In both groups, the ethnicity was considered Caucasian. The other association was found in a small Turkish trial focused on the G > A pair change. 106 Six studies with female migraineurs did not find an association.107–109,111–113 Four of these six investigated the same base pair change, G > A.
CYP1A1 gene
rs4646903 (MspI)
Two of eight studies showed an association with endometriosis. The case-control study with the smaller sample size investigated the T > C change. 115 Four Asian studies also investigated this base pair change, but found no association.72,105,116–119 No association with migraine was found. 110
rs1048943
No associations were found in endometriosis patients or migraine patients.72,110
CYP19A1 gene
rs10046
Within five studies, no significant associations with endometriosis were found in either Asian or Caucasian study populations.49,61,74,124,126 Two of five studies, both of them in Asian populations, found an association with migraine. In contrast, a large Chinese case-control study did not find an association. 55
rs4646
No association with endometriosis was found in a Caucasian study population. 74 In one Indian study, migraine was associated with this polymorphism. 56 However, two other large studies found no association.55,67
rs700519
None of the three studies examined found an association with endometriosis, and neither did one study with migraineurs.37,72,98,110
Discussion
The objectives of the present study were to provide an extensive review of sex hormone-related polymorphisms studied in endometriosis and migraine. Improved understanding of this comorbidity might facilitate early diagnosis and specific therapy. We found many probable overlapping SNPs in the candidate genes (Table 2), with mostly contradictory results, presumably due to some limitations of the included studies.
Both endometriosis and migraine are complex conditions with a variety of phenotypes. The heterogeneity of the control groups in the endometriosis studies constitutes a source of critique, as some women who have had a laparoscopy might not be representative of the normal population, and women in control groups without a laparoscopy might suffer from asymptomatic endometriosis with a probability of 2–11%. 5 Preferably, controls would be pain-free, fertile women in whom the absence of endometriosis is confirmed by surgery. 126 Such a control group will not only be difficult to establish in large study populations, but is also not acceptable for ethical reasons. A recent systematic review of polymorphisms and endometriosis confirmed the importance of being cautious regarding the criteria for selecting the control population. 33 To reduce heterogeneity, we excluded studies with self-reported endometriosis, recurrent endometriosis, female newborns, and men as controls. Arguably, including postmenopausal women in control groups is associated with the problem that these now asymptomatic women might have suffered from endometriosis in their reproductive years. Postmenopausal women could still have the genetic makeup predisposing them to endometriosis.
Another possible explanation is failed replication in subsequent studies due to the candidate-gene approach. Candidate-gene association studies have been widely used in the genetics of complex traits and diseases. This approach is based on the a priori selection of candidate genes with a hypothetical role in the pathogenesis of the disease and uses indirect genotyping methods. 127 Nowadays, these methods are mostly obsolete due to the rise of direct-sequencing technologies. 128 Indirect genotyping methods have a higher chance of yielding false positives compared to direct-sequencing technologies. In a simulation study, 968 of 1000 simulations (96.8%) produced at least one false positive. 129 Other drawbacks of candidate-gene studies are the small sample sizes and the lack of standardized genotyping methodologies. However, there is no universal genotyping method because choosing a suitable genotyping method for a certain variant depends on multiple factors, for example, the number of variants in the specified gene. Finally, problems with population stratification can occur in candidate-gene studies when cases and controls are poorly matched and, consequently, are responsible for significant associations. 130 We are aware that this approach has a limited ability to include all possible causative genes and polymorphisms. However, from a clinical point of view, we carefully decided to only include studies examining the candidate sex-hormone genes in question to better understand the role of these sex-hormones and to enable more targeted treatments for the comorbidity. Although subject to criticism, this approach still proves to be a robust tool for studying the genetic makeup, especially for diseases with complex traits. 131
We found three overlapping sex hormone-associated polymorphisms in the estrogen receptor genes (ESR1 and ESR2), in particular the SNPs rs2234693 (Pvull), rs9340799 (Xbal) and rs4986938 (AluI)48–51,54–57,67,68,73,74 (Table 2). Both Caucasian and Asian women with endometriosis showed an association between the disease and rs2234693 (Pvull), but for migraine the association was found only in Asian women.55–58 While rs9340799 (Xbal) was associated with endometriosis and migraine in Asian women, no association was found in Caucasian women.50,53 Both conditions were significantly associated with rs4986938 (AluI) in specifically Caucasian women. The differences between the findings in Asian and Caucasian populations demonstrate the importance of ethnicity when performing and interpreting genetic studies. The prevalence of endometriosis seems to differ among Asian and Caucasian populations. Asian women are significantly more likely to be diagnosed with endometriosis (OR 1.63, 95% CI 1.03–2.58). 132
Some studies did not specify the allelic change in the polymorphism. However, one SNP can have several different base-pair changes and allele frequencies can vary in different ethnicities. This information was reviewed in the current dbSNP database. 46
In the ESR1 gene, rs1801132 has been found to have an association with migraine in women, but no studies have investigated this polymorphism in women with endometriosis. Three repeat polymorphisms, TA repeat (ESR1 gene), CAG repeat (AR gene), and TTTA repeat (CYP19A1 gene) all have probable associations with endometriosis, but have not yet been investigated in migraineurs. We found overlapping polymorphisms for PROGINS (PGR gene) and rs6166 (FSHR gene), but more powered studies are required to understand if there is a significant association. Regarding the FSHB gene, rs11031006 is associated with endometriosis, but migraine studies are needed. General overlaps in the metabolizing enzymes have been assessed in our literature search for COMT, NRIP1, CYP1A1, CYP19A1, but no overlapping SNPs have been found and more data is needed (Table 2).
The strengths of this study were the strict inclusion and exclusion criteria to reduce selection bias. We included only case-control studies. To ensure the diagnosis, we only included studies with endometriosis diagnosed by laparoscopy and/or histology, and the majority was ASRM-classified. Migraineurs were diagnosed based on the criteria specified by the IHS. 2 A limitation of our study was the stratification into two ethnic groups, resulting in recruitment bias. Arguably, this could have been more specific–for example, stratifying for European, Hispanic, and Turkish populations. It is important to note that ethnicity and race have different definitions, although within medical literature these are often used interchangeably. 132 In female lifetime, the clinical pattern of migraine is linked to reproductive events with an increase around puberty, a peak during fertile age, and a decrease after menopause. 133 Age was considered, but studies for women in menopause or before menarche were not available and therefore could not be included. Other mechanisms might be involved in childhood migraine.
Migraines appear to occur more commonly in patients with endometriosis than in the general population. 134 The prevalence of migraine is significantly higher in women with endometriosis as compared to women without endometriosis.135–137 We hypothesize that endometriosis and migraine are comorbidities in a subset of women. A recent study confirmed this comorbidity and suggested a non-causal relationship between the two traits. 4 Nevertheless, mechanistic insights for both conditions are still lacking. In addition to differences on the hormone receptor level or hormone metabolism level, this also could be a shared problem in the immune response in women with the comorbidities.
Table 2 shows an overview of overlapping genes in both conditions, which were only found in estrogen receptor genes. It seems estrogen plays a central role in the genetic link underlying the comorbidity of endometriosis and migraine. Biologically, estrogens exert their effects via the estrogen receptors localized in epithelial, stromal, and vascular cells. Progestins antagonize estrogen actions in the reproductive tissues, brain, and nerve cells by reducing estrogen receptor expression. 138 Women with overlapping estrogen receptor polymorphisms could experience a higher improvement in symptoms with estrogen-suppression by continuous treatment with progestins. The majority of women in the reproductive years use hormonal contraception. However, combined hormonal contraception frequently has a negative impact on migraine. 139 Whereas treatment with progestin-only has a positive impact on both conditions.30,140–143 Therefore, patients with the comorbidity of migraine and endometriosis would profit if their attending physician could identify the co-occurrence to optimize hormonal treatment.
Conclusion
This literature review gives an overview of the shared sex hormone polymorphisms in women with migraine and endometriosis. Furthermore, we have identified SNPs potentially related to these conditions, which are relevant for future research. To confirm possible associations with other sex-hormone genes, larger studies are needed, in which ethnicity needs to be taken into account. We hypothesize that ESR1 and ESR2 may play a role in the genetic cause of endometriosis and migraine. For optimal treatment and patient care, we recommend actively exploring the comorbidity of migraine and endometriosis.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221111315 for Sex hormone-related polymorphisms in endometriosis and migraine: A narrative review by Joy-Fleur van der Vaart and Gabriele Susanne Merki-Feld in Women’s Health
Footnotes
Author contribution(s): Joy-Fleur van der Vaart: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review and editing. Gabriele Susanne Merki-Feld: Conceptualization; Methodology; Supervision.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: According to the cantonal Swiss Ethic Committee of Zürich http://www.kek.zh.ch/ and Swiss Association of Research Ethics Committees https://swissethics.ch/en/ ethics approval is not applicable for literature reviews.
ORCID iD: Joy-Fleur van der Vaart  https://orcid.org/0000-0002-2544-210X
https://orcid.org/0000-0002-2544-210X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Zondervan KT, Becker CM, Koga K, et al. Endometriosis. Nat Rev Dis Primers 2018; 4(1): 9. [DOI] [PubMed] [Google Scholar]
- 2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38(1): 1–211. [DOI] [PubMed] [Google Scholar]
- 3. Nyholt DR, Gillespie NG, Merikangas KR, et al. Common genetic influences underlie comorbidity of migraine and endometriosis. Genet Epidemiol 2009; 33(2): 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adewuyi EO, Sapkota Y, International Endogene Consortium (IEC), et al. Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes 2020; 11(3): 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020; 382(13): 1244–1256. [DOI] [PubMed] [Google Scholar]
- 6. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17(11): 954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buse DC, Fanning KM, Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache 2019; 59(8): 1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache 2018; 58(suppl. 1): 17–32. [DOI] [PubMed] [Google Scholar]
- 9. Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012; 27(5): 1292–1299. [DOI] [PubMed] [Google Scholar]
- 10. Vos T, Barber RM, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386(9995): 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin 2019; 37(4): 631–649. [DOI] [PubMed] [Google Scholar]
- 12. Treloar SA, O’Connor DT, O’Connor VM, et al. Genetic influences on endometriosis in an Australian twin sample. sueT@qimr.edu.au. Fertil Steril 1999; 71(4): 701–710. [DOI] [PubMed] [Google Scholar]
- 13. Saha R, Pettersson HJ, Svedberg P, et al. Heritability of endometriosis. Fertil Steril 2015; 104(4): 947–952. [DOI] [PubMed] [Google Scholar]
- 14. Honkasalo ML, Kaprio J, Winter T, et al. Migraine and concomitant symptoms among 8167 adult twin pairs. Headache 1995; 35(2): 70–78. [DOI] [PubMed] [Google Scholar]
- 15. Mulder EJ, Van Baal C, Gaist D, et al. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 2003; 6(5): 422–431. [DOI] [PubMed] [Google Scholar]
- 16. Polderman TJ, Benyamin B, De Leeuw CA, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 2015; 47(7): 702–709. [DOI] [PubMed] [Google Scholar]
- 17. Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10(5): 261–275. [DOI] [PubMed] [Google Scholar]
- 18. Vetvik KG, MacGregor EA. Menstrual migraine: a distinct disorder needing greater recognition. Lancet Neurol 2021; 20(4): 304–315. [DOI] [PubMed] [Google Scholar]
- 19. MacGregor EA. Menstrual migraine: a clinical review. J Fam Plann Reprod Health Care 2007; 33(1): 36–47. [DOI] [PubMed] [Google Scholar]
- 20. Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev 2019; 40(4): 1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017; 16(1): 76–87. [DOI] [PubMed] [Google Scholar]
- 22. MacGregor EA, Frith A, Ellis J, et al. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006; 67(12): 2154–2158. [DOI] [PubMed] [Google Scholar]
- 23. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 1972; 22(4): 355–365. [DOI] [PubMed] [Google Scholar]
- 24. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47(1): 27–37. [DOI] [PubMed] [Google Scholar]
- 25. Aegidius K, Zwart JA, Hagen K, et al. Oral contraceptives and increased headache prevalence: the Head-HUNT Study. Neurology 2006; 66(3): 349–353. [DOI] [PubMed] [Google Scholar]
- 26. Teepker M, Peters M, Kundermann B, et al. The effects of oral contraceptives on detection and pain thresholds as well as headache intensity during menstrual cycle in migraine. Headache 2011; 51(1): 92–104. [DOI] [PubMed] [Google Scholar]
- 27. Massiou H, MacGregor EA. Evolution and treatment of migraine with oral contraceptives. Cephalalgia 2000; 20(3): 170–174. [DOI] [PubMed] [Google Scholar]
- 28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health 2014; 23(4): 310–317. [DOI] [PubMed] [Google Scholar]
- 29. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia 2013; 33(5): 340–346. [DOI] [PubMed] [Google Scholar]
- 30. Merki-Feld GS, Imthurn B, Gantenbein AR, et al. Effect of desogestrel 75 µg on headache frequency and intensity in women with migraine: a prospective controlled trial. Eur J Contracept Reprod Health Care 2019; 24(3): 175–181. [DOI] [PubMed] [Google Scholar]
- 31. Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod 2010; 25(3): 633–641. [DOI] [PubMed] [Google Scholar]
- 32. Caruso S, Iraci M, Cianci S, et al. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 µg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest 2016; 39(8): 923–931. [DOI] [PubMed] [Google Scholar]
- 33. Méar L, Herr M, Fauconnier A, et al. Polymorphisms and endometriosis: a systematic review and meta-analyses. Hum Reprod Update 2020; 26(1): 73–102. [DOI] [PubMed] [Google Scholar]
- 34. Fabjani G, Tong D, Czerwenka K, et al. Human progesterone receptor gene polymorphism PROGINS and risk for breast cancer in Austrian women. Breast Cancer Res Treat 2002; 72(2): 131–137. [DOI] [PubMed] [Google Scholar]
- 35. Patel BG, Rudnicki M, Yu J, et al. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96(6): 623–632. [DOI] [PubMed] [Google Scholar]
- 36. Kado N, Kitawaki J, Obayashi H, et al. Association of the CYP17 gene and CYP19 gene polymorphisms with risk of endometriosis in Japanese women. Hum Reprod 2002; 17(4): 897–902. [DOI] [PubMed] [Google Scholar]
- 37. Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 2005; 227(2): 115–124. [DOI] [PubMed] [Google Scholar]
- 38. Docquier A, Garcia A, Savatier J, et al. Negative regulation of estrogen signaling by ERβ and RIP140 in ovarian cancer cells. Mol Endocrinol 2013; 27(9): 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wieser F, Wenzl R, Tempfer C, et al. Catechol-O-methyltransferase polymorphism and endometriosis. J Assist Reprod Genet 2002; 19(7): 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trabert B, Schwartz SM, Peters U, et al. Genetic variation in the sex hormone metabolic pathway and endometriosis risk: an evaluation of candidate genes. Fertil Steril 2011; 96(6): 1401.e3–1406.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schürks M, Rist PM, Kurth T. Sex hormone receptor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia 2010; 30(11): 1306–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao Y, Zhu R, Xiao T, et al. Genetic variants in migraine: a field synopsis and systematic re-analysis of meta-analyses. J Headache Pain 2020; 21(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sapkota Y, Steinthorsdottir V, Morris AP, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun 2017; 8: 15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christofolini DM, Vilarino FL, Mafra FA, et al. Combination of polymorphisms in luteinizing hormone β, estrogen receptor β and progesterone receptor and susceptibility to infertility and endometriosis. Eur J Obstet Gynecol Reprod Biol 2011; 158(2): 260–264. [DOI] [PubMed] [Google Scholar]
- 45. Cardoso JV, Machado DE, Ferrari R, et al. Combined effect of the PGR +331C > T, CYP17A1 -34A > G and CYP19A1 1531G > a polymorphisms on the risk of developing endometriosis. Rev Bras Ginecol Obstet 2017; 39(6): 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001; 29(1): 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997; 67(5): 817–821. [DOI] [PubMed] [Google Scholar]
- 48. Hsieh YY, Wang YK, Chang CC, et al. Estrogen receptor alpha-351 XbaI*G and -397 PvuII*C-related genotypes and alleles are associated with higher susceptibilities of endometriosis and leiomyoma. Mol Hum Reprod 2007; 13(2): 117–122. [DOI] [PubMed] [Google Scholar]
- 49. Lamp M, Peters M, Reinmaa E, et al. Polymorphisms in ESR1, ESR2 and HSD17B1 genes are associated with fertility status in endometriosis. Gynecol Endocrinol 2011; 27(6): 425–433. [DOI] [PubMed] [Google Scholar]
- 50. Paskulin DD, Cunha-Filho JS, Paskulin LD, et al. ESR1 rs9340799 is associated with endometriosis-related infertility and in vitro fertilization failure. Dis Markers 2013; 35(6): 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitawaki J, Obayashi H, Ishihara H, et al. Oestrogen receptor-alpha gene polymorphism is associated with endometriosis, adenomyosis and leiomyomata. Hum Reprod 2001; 16(1): 51–55. [DOI] [PubMed] [Google Scholar]
- 52. Govindan S, Shaik NA, Vedicherla B, et al. Estrogen receptor-alpha gene (T/C) Pvu II polymorphism in endometriosis and uterine fibroids. Dis Markers 2009; 26(4): 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Renner SP, Strick R, Oppelt P, et al. Evaluation of clinical parameters and estrogen receptor alpha gene polymorphisms for patients with endometriosis. Reproduction 2006; 131(1): 153–161. [DOI] [PubMed] [Google Scholar]
- 54. Xie J, Wang S, He B, et al. Association of estrogen receptor alpha and interleukin-10 gene polymorphisms with endometriosis in a Chinese population. Fertil Steril 2009; 92(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 55. An X, Fang J, Lin Q, et al. New evidence for involvement of ESR1 gene in susceptibility to Chinese migraine. J Neurol 2017; 264(1): 81–87. [DOI] [PubMed] [Google Scholar]
- 56. Ghosh J, Joshi G, Pradhan S, et al. Potential role of aromatase over estrogen receptor gene polymorphisms in migraine susceptibility: a case control study from North India. PLoS One 2012; 7(4): e34828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joshi G, Pradhan S, Mittal B. Role of the oestrogen receptor (ESR1 PvuII and ESR1 325 C->G) and progesterone receptor (PROGINS) polymorphisms in genetic susceptibility to migraine in a North Indian population. Cephalalgia 2010; 30(3): 311–320. [DOI] [PubMed] [Google Scholar]
- 58. Colson NJ, Lea RA, Quinlan S, et al. No role for estrogen receptor 1 gene intron 1 Pvu II and exon 4 C325G polymorphisms in migraine susceptibility. BMC Med Genet 2006; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SH, Choi YM, Jun JK, et al. Estrogen receptor dinucleotide repeat polymorphism is associated with minimal or mild endometriosis. Fertil Steril 2005;84(3): 774–777. [DOI] [PubMed] [Google Scholar]
- 60. Hsieh YY, Chang CC, Tsai FJ, et al. Estrogen receptor alpha dinucleotide repeat and cytochrome P450c17alpha gene polymorphisms are associated with susceptibility to endometriosis. Fertil Steril 2005;83(3): 567–572. [DOI] [PubMed] [Google Scholar]
- 61. Wu CH, Yang JG, Chang YJ, et al. Screening of a panel of steroid-related genes showed polymorphisms of aromatase genes confer susceptibility to advanced stage endometriosis in the Taiwanese Han population. Taiwan J Obstet Gynecol 2013; 52(4): 485–492. [DOI] [PubMed] [Google Scholar]
- 62. Colson NJ, Lea RA, Quinlan S, et al. The estrogen receptor 1 G594A polymorphism is associated with migraine susceptibility in two independent case/control groups. Neurogenetics 2004; 5(2): 129–133. [DOI] [PubMed] [Google Scholar]
- 63. Coşkun S, Yůcel Y, Çim A, et al. Contribution of polymorphisms in ESR1, ESR2, FSHR, CYP19A1, SHBG, and NRIP1 genes to migraine susceptibility in Turkish population. J Genet 2016; 95(1): 131–140. [DOI] [PubMed] [Google Scholar]
- 64. Kaunisto MA, Kallela M, Hämäläinen E, et al. Testing of variants of the MTHFR and ESR1 genes in 1798 Finnish individuals fails to confirm the association with migraine with aura. Cephalalgia 2006; 26(12): 1462–1472. [DOI] [PubMed] [Google Scholar]
- 65. Oterino A, Pascual J, Ruiz de Alegría C, et al. Association of migraine and ESR1 G325C polymorphism. Neuroreport 2006;17(1): 61–64. [DOI] [PubMed] [Google Scholar]
- 66. Corominas R, Ribasés M, Cuenca-León E, et al. Lack of association of hormone receptor polymorphisms with migraine. Eur J Neurol 2009; 16(3): 413–415. [DOI] [PubMed] [Google Scholar]
- 67. Rodriguez-Acevedo AJ, Smith RA, Roy B, et al. Genetic association and gene expression studies suggest that genetic variants in the SYNE1 and TNF genes are related to menstrual migraine. J Headache Pain 2014; 15(1): 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oterino A, Toriello M, Cayón A, et al. Multilocus analyses reveal involvement of the ESR1, ESR2, and FSHR genes in migraine. Headache 2008; 48(10): 1438–1450. [DOI] [PubMed] [Google Scholar]
- 69. Wang W, Li Y, Maitituoheti M, et al. Association of an oestrogen receptor gene polymorphism in Chinese Han women with endometriosis and endometriosis-related infertility. Reprod Biomed Online 2013;26(1):93–98. [DOI] [PubMed] [Google Scholar]
- 70. Matsuzaka Y, Kikuti YY, Izumi S, et al. Failure to detect significant association between estrogen receptor-alpha gene polymorphisms and endometriosis in Japanese women. Environ Health Prev Med 2012;17(5):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sato H, Nogueira-de-Souza NC, D’Amora P, et al. Intron 1 and exon 1 alpha estrogen receptor gene polymorphisms in women with endometriosis. Fertil Steril 2008;90(6): 2086–2090. [DOI] [PubMed] [Google Scholar]
- 72. Huber A, Keck CC, Hefler LA, et al. Ten estrogen-related polymorphisms and endometriosis: a study of multiple gene-gene interactions. Obstet Gynecol 2005; 106(5 pt 1): 1025–1031. [DOI] [PubMed] [Google Scholar]
- 73. Bianco B, Christofolini DM, Mafra FA, et al. +1730 G/A polymorphism of the estrogen receptor beta gene (ERbeta) may be an important genetic factor predisposing to endometriosis. Acta Obstet Gynecol Scand 2009; 88(12): 1397–1401. [DOI] [PubMed] [Google Scholar]
- 74. Szaflik T, Smolarz B, Romanowicz H, et al. Polymorphisms in the 3’UTR region of ESR2 and CYP19A1 genes in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 2020; 250: 241–245. [DOI] [PubMed] [Google Scholar]
- 75. Lee GH, Kim SH, Choi YM, et al. Estrogen receptor beta gene +1730 G/A polymorphism in women with endometriosis. Fertil Steril 2007; 88(4): 785–788. [DOI] [PubMed] [Google Scholar]
- 76. Silva RCPC, Costa IR, Bordin BM, et al. RsaI polymorphism of the ERβ gene in women with endometriosis. Genet Mol Res 2011; 10(1): 465–470. [DOI] [PubMed] [Google Scholar]
- 77. Smolarz B, Romanowicz H. Association between single nucleotide polymorphism of the CYP19A1 and ESR2 genes and endometriosis. Arch Gynecol Obstet 2021;304(2):439–445. [DOI] [PubMed] [Google Scholar]
- 78. Costa IR, Silva RCPC, Frare AB, et al. Polymorphism of the progesterone receptor gene associated with endometriosis in patients from Goias, Brazil. Genet Mol Res 2011; 10(3): 1364–1370. [DOI] [PubMed] [Google Scholar]
- 79. De Carvalho CV, Nogueira-De-Souza NC, Costa AM, et al. Genetic polymorphisms of cytochrome P450cl7alpha (CYP17) and progesterone receptor genes (PROGINS) in the assessment of endometriosis risk. Gynecol Endocrinol 2007; 23(1): 29–33. [DOI] [PubMed] [Google Scholar]
- 80. Lattuada D, Viganò P, Somigliana E, et al. Androgen receptor gene cytosine, adenine, and guanine trinucleotide repeats in patients with endometriosis. J Soc Gynecol Investig 2004; 11(4): 237–240. [DOI] [PubMed] [Google Scholar]
- 81. Wieser F, Schneeberger C, Tong D, et al. PROGINS receptor gene polymorphism is associated with endometriosis. Fertil Steril 2002; 77(2): 309–312. [DOI] [PubMed] [Google Scholar]
- 82. Treloar SA, Zhao ZZ, Armitage T, et al. Association between polymorphisms in the progesterone receptor gene and endometriosis. Mol Hum Reprod 2005; 11(9): 641–647. [DOI] [PubMed] [Google Scholar]
- 83. Treloar SA, Wicks J, Nyholt DR, et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet 2005; 77(3): 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gimenes C, Bianco B, Mafra FA, et al. The progins progesterone receptor gene polymorphism is not related to endometriosis-associated infertility or to idiopathic infertility. Clinics 2010; 65(11): 1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Govindan S, Ahmad SN, Vedicherla B, et al. Association of progesterone receptor gene polymorphism (PROGINS) with endometriosis, uterine fibroids and breast cancer. Cancer Biomark 2007; 3(2): 73–78. [DOI] [PubMed] [Google Scholar]
- 86. Colson NJ, Lea RA, Quinlan S, et al. Investigation of hormone receptor genes in migraine. Neurogenetics 2005; 6(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 87. Van Kaam KJ, Romano A, Schouten JP, et al. Progesterone receptor polymorphism +331G/A is associated with a decreased risk of deep infiltrating endometriosis. Hum Reprod 2007; 22(1): 129–135. [DOI] [PubMed] [Google Scholar]
- 88. Palmirotta R, Barbanti P, Ialongo C, et al. Progesterone receptor gene (PROGINS) polymorphism correlates with late onset of migraine. DNA Cell Biol 2015; 34(3): 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gentilini D, Vigano P, Carmignani L, et al. Progesterone receptor +331G/A polymorphism in endometriosis and deep-infiltrating endometriosis. Fertil Steril 2008;90(4): 1243–1245. [DOI] [PubMed] [Google Scholar]
- 90. Hsieh YY, Chang CC, Tsai FJ, et al. Androgen receptor trinucleotide polymorphism in endometriosis. Fertil Steril 2001; 76(2): 412–413. [DOI] [PubMed] [Google Scholar]
- 91. Shaik NA, Govindan S, Kodati V, et al. Polymorphic (CAG)n repeats in the androgen receptor gene: a risk marker for endometriosis and uterine leiomyomas. Hematol Oncol Stem Cell Ther 2009; 2(1): 289–293. [DOI] [PubMed] [Google Scholar]
- 92. Shin JJ, Choi YM, Choi HY, et al. Androgen receptor cytosine, adenine, and guanine trinucleotide repeat polymorphism in Korean patients with endometriosis: a case-control study. Eur J Obstet Gynecol Reprod Biol 2017; 218: 1–4. [DOI] [PubMed] [Google Scholar]
- 93. Lattuada D, Somigliana E, Viganò P, et al. Genetics of endometriosis: a role for the progesterone receptor gene polymorphism PROGINS? Clin Endocrinol 2004; 61(2): 190–194. [DOI] [PubMed] [Google Scholar]
- 94. Tong D, Deng J, Sun H, et al. The relationship between CAG repeat length polymorphism and infertility in Southern Chinese Han women. J Endocrinol Invest 2010; 33(8): 559–563. [DOI] [PubMed] [Google Scholar]
- 95. Kerimoglu OS, Yılmaz SA, Pekin A, et al. Follicle-stimulating hormone receptor gene polymorphisms in women with endometriosis. Arch Gynecol Obstet 2015; 291(6): 1411–1416. [DOI] [PubMed] [Google Scholar]
- 96. Liaqat I, Arifa NA, Asif S, et al. Association of FSHR gene polymorphisms with endometriosis in women visiting tertiary-care hospitals of Lahore, Pakistan. J Pak Med Assoc 2021;71(4): 1118–1122. [DOI] [PubMed] [Google Scholar]
- 97. André GM, Martins Trevisan C, Pedruzzi IN, et al. The impact of FSHR gne polymorphisms Ala307Thr and Asn680Ser in the endometriosis development. DNA Cell Biol 2018;37(6): 584–591. [DOI] [PubMed] [Google Scholar]
- 98. Wang HS, Wu HM, Cheng BH, et al. Functional analyses of endometriosis-related polymorphisms in the estrogen synthesis and metabolism-related genes. PLoS One 2012; 7(11): e47374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schmitz CR, Souza CA, Genro VK, et al. LH (Trp8Arg/Ile15Thr), LHR (insLQ) and FSHR (Asn680Ser) polymorphisms genotypic prevalence in women with endometriosis and infertility. J Assist Reprod Genet 2015;32(6): 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Angioni S, D’Alterio MN, Coiana A, et al. Genetic characterization of endometriosis patients: Review of the literature and a prospective cohort study on a mediterranean population. Int J Mol Sci 2020;21(5). DOI: 10.3390/ijms21051765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Matalliotakis M, Zervou MI, Matalliotaki C, et al. The role of gene polymorphisms in endometriosis. Mol Med Rep 2017;16(5): 5881–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Caballero V, Ruiz R, Sainz JA, et al. Preliminary molecular genetic analysis of the Receptor Interacting Protein 140 (RIP140) in women affected by endometriosis. J Exp Clin Assist Reprod 2005; 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pollock CE, Sutherland HG, Maher BH, et al. The NRP1 migraine risk variant shows evidence of association with menstrual migraine. J Headache Pain 2018;19(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Christofolini DM, Teles JS, Vilarino FL, et al. COMT polymorphism and the risk of endometriosis-related infertility. Gynecol Endocrinol 2011; 27(12): 1099–1102. [DOI] [PubMed] [Google Scholar]
- 105. Juo SH, Wang TN, Lee JN, et al. CYP17, CYP1A1 and COMT polymorphisms and the risk of adenomyosis and endometriosis in Taiwanese women. Hum Reprod 2006; 21(6): 1498–1502. [DOI] [PubMed] [Google Scholar]
- 106. Emin Erdal M, Herken H, Yilmaz M, et al. Significance of the catechol-O-methyltransferase gene polymorphism in migraine. Brain Res Mol Brain Res 2001; 94(1–2): 193–196. [DOI] [PubMed] [Google Scholar]
- 107. Sullivan AK, Atkinson EJ, Cutrer FM. Hormonally modulated migraine is associated with single-nucleotide polymorphisms within genes involved in dopamine metabolism. Open J Genet 2013; 3(2): 38–45. [Google Scholar]
- 108. De Marchis ML, Barbanti P, Palmirotta R, et al. Look beyond Catechol-O-Methyltransferase genotype for cathecolamines derangement in migraine: the BioBIM rs4818 and rs4680 polymorphisms study. J Headache Pain 2015; 16: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Todt U, Netzer C, Toliat M, et al. New genetic evidence for involvement of the dopamine system in migraine with aura. Hum Genet 2009; 125(3): 265–279. [DOI] [PubMed] [Google Scholar]
- 110. Sutherland HG, Champion M, Plays A, et al. Investigation of polymorphisms in genes involved in estrogen metabolism in menstrual migraine. Gene 2017; 607: 36–40. [DOI] [PubMed] [Google Scholar]
- 111. Takigawa H, Kowa H, Nakashima K. No associations between five polymorphisms in COMT gene and migraine. Acta Neurol Scand 2017; 135(2): 225–230. [DOI] [PubMed] [Google Scholar]
- 112. Hagen K, Pettersen E, Stovner LJ, et al. The association between headache and Val158Met polymorphism in the catechol-O-methyltransferase gene: the HUNT Study. J Headache Pain 2006; 7(2): 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Park JW, Lee KS, Kim JS, et al. Genetic contribution of Catechol-O-methyltransferase polymorphism in patients with migraine without aura. J Clin Neurol 2007; 3(1): 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arvanitis DA, Koumantakis GE, Goumenou AG, et al. CYP1A1, CYP19, and GSTM1 polymorphisms increase the risk of endometriosis. Fertil Steril 2003;79 Suppl 1: 702–709. [DOI] [PubMed] [Google Scholar]
- 115. Barbosa AM, De Souza SR, Frare AB, et al. Association of CYP1A1 (cytochrome P450) MspI polymorphism in women with endometriosis. Genet Mol Res. Epub ahead of print 26 August 2016. DOI: 10.4238/gmr.15038389. [DOI] [PubMed] [Google Scholar]
- 116. Babu KA, Reddy NG, Deendayal M, et al. GSTM1, GSTT1 and CYP1A1 detoxification gene polymorphisms and their relationship with advanced stages of endometriosis in South Indian women. Pharmacogenet Genomics 2005; 15(3): 167–172. [DOI] [PubMed] [Google Scholar]
- 117. Rozati R, Reddy S, Giragalla S, et al. The CYP1A1 and GSTM1 genetic polymorphisms and susceptibility to endometriosis in women from South India. Fertil Steril 2008; 2: 105–112. [Google Scholar]
- 118. Babaki A, Mehrjardi EZ, Firouzabadi RD, et al. Relationship between MspI polymorphism of CYP1A1 gene and the risk of endometriosis in an Iranian population: a case-control study. Int J Reprod Biomed 2018; 16(10): 637–640. [PMC free article] [PubMed] [Google Scholar]
- 119. Wu CH, Guo CY, Yang JG, et al. Polymorphisms of dioxin receptor complex components and detoxification-related genes jointly confer susceptibility to advanced-stage endometriosis in the Taiwanese Han population. Am J Reprod Immunol 2012; 67(2): 160–168. [DOI] [PubMed] [Google Scholar]
- 120. Bozdag G, Alp A, Saribas Z, et al. CYP17 and CYP2C19 gene polymorphisms in patients with endometriosis. Reprod Biomed Online 2010;20(2): 286–290. [DOI] [PubMed] [Google Scholar]
- 121. Szczepańska M, Wirstlein P, Skrzypczak J, et al. Polymorphic variants of CYP17 and CYP19A and risk of infertility in endometriosis. Acta Obstet Gynecol Scand 2013; 92(10): 1188–1193. [DOI] [PubMed] [Google Scholar]
- 122. Vietri MT, Cioffi M, Sessa M, et al. CYP17 and CYP19 gene polymorphisms in women affected with endometriosis. Fertil Steril 2009;92(5): 1532–1535. [DOI] [PubMed] [Google Scholar]
- 123. Zhao ZZ, Nyholt DR, Le L, et al. Common variation in the CYP17A1 and IFIT1 genes on chromosome 10 does not contribute to the risk of endometriosis. Open Reprod Sci J 2008;1: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang L, Lu X, Wang D, et al. CYP19 gene variant confers susceptibility to endometriosis-associated infertility in Chinese women. Exp Mol Med 2014; 46: e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hur SE, Lee S, Lee JY, et al. Polymorphisms and haplotypes of the gene encoding the estrogen-metabolizing CYP19 gene in Korean women: no association with advanced-stage endometriosis. J Hum Genet 2007;52(9): 703–711. [DOI] [PubMed] [Google Scholar]
- 126. Greaves E, Critchley HOD, Horne AW, et al. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet Gynecol Scand 2017; 96(6): 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kwon JM, Goate AM. The candidate gene approach. Alcohol Res Health 2000; 24(3): 164–168. [PMC free article] [PubMed] [Google Scholar]
- 128. Hashim HO, Al-Shuhaib MBS. Exploring the potential and limitations of PCR-RFLP and PCR-SSCP for SNP detection: a review. J Appl Biotechnol Rep 2019; 6(4): 137–144. [Google Scholar]
- 129. Sullivan PF. Spurious genetic associations. Biol Psychiatry 2007; 61(10): 1121–1126. [DOI] [PubMed] [Google Scholar]
- 130. Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet 2002; 3(5): 391–397. [DOI] [PubMed] [Google Scholar]
- 131. Newton-Cheh C, Hirschhorn JN. Genetic association studies of complex traits: design and analysis issues. Mutat Res 2005; 573(1–2): 54–69. [DOI] [PubMed] [Google Scholar]
- 132. Bougie O, Yap MI, Sikora L, et al. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG 2019; 126(9): 1104–1115. [DOI] [PubMed] [Google Scholar]
- 133. Faubion SS, Batur P, Calhoun AH. Migraine throughout the female reproductive life cycle. Mayo Clin Proc 2018; 93(5): 639–645. [DOI] [PubMed] [Google Scholar]
- 134. Yang MH, Wang PH, Wang SJ, et al. Women with endometriosis are more likely to suffer from migraines: a population-based study. PLoS One 2012; 7(3): e33941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Maitrot-Mantelet L, Hugon-Rodin J, Vatel M, et al. Migraine in relation with endometriosis phenotypes: results from a French case-control study. Cephalalgia 2020; 40(6): 606–613. [DOI] [PubMed] [Google Scholar]
- 136. Miller JA, Missmer SA, Vitonis AF, et al. Prevalence of migraines in adolescents with endometriosis. Fertil Steril 2018; 109(4): 685–690. [DOI] [PubMed] [Google Scholar]
- 137. Ferrero S, Pretta S, Bertoldi S, et al. Increased frequency of migraine among women with endometriosis. Hum Reprod 2004; 19(12): 2927–2932. [DOI] [PubMed] [Google Scholar]
- 138. Jayaraman A, Pike CJ. Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol 2009; 21(1): 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bentivegna E, Luciani M, Scarso F, et al. Hormonal therapies in migraine management: current perspectives on patient selection and risk management. Expert Rev Neurother 2021; 21: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 140. Merki-Feld GS, Imthurn B, Seifert B, et al. Desogestrel-only contraception may reduce headache frequency and improve quality of life in women suffering from migraine. Eur J Contracept Reprod Health Care 2013; 18(5): 394–400. [DOI] [PubMed] [Google Scholar]
- 141. Niu X, Luo Q, Wang C, et al. Effects of Etonogestrel implants on pelvic pain and menstrual flow in women suffering from adenomyosis or endometriosis: results from a prospective, observational study. Medicine 2021; 100(6): e24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Samy A, Taher A, Sileem SA, et al. Medical therapy options for endometriosis related pain, which is better? A systematic review and network meta-analysis of randomized controlled trials. J Gynecol Obstet Hum Reprod 2021; 50(1): 101798. [DOI] [PubMed] [Google Scholar]
- 143. Tanmahasamut P, Saejong R, Rattanachaiyanont M, et al. Postoperative desogestrel for pelvic endometriosis-related pain: a randomized controlled trial. Gynecol Endocrinol 2017; 33(7): 534–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221111315 for Sex hormone-related polymorphisms in endometriosis and migraine: A narrative review by Joy-Fleur van der Vaart and Gabriele Susanne Merki-Feld in Women’s Health