Abstract
The bopXYZ genes from the gram-positive bacterium Rhodococcus sp. strain 19070 encode a broad-substrate-specific benzoate dioxygenase. Expression of the BopXY terminal oxygenase enabled Escherichia coli to convert benzoate or anthranilate (2-aminobenzoate) to a nonaromatic cis-diol or catechol, respectively. This expression system also rapidly transformed m-toluate (3-methylbenzoate) to an unidentified product. In contrast, 2-chlorobenzoate was not a good substrate. The BopXYZ dioxygenase was homologous to the chromosomally encoded benzoate dioxygenase (BenABC) and the plasmid-encoded toluate dioxygenase (XylXYZ) of gram-negative acinetobacters and pseudomonads. Pulsed-field gel electrophoresis failed to identify any plasmid in Rhodococcus sp. strain 19070. Catechol 1,2- and 2,3-dioxygenase activity indicated that strain 19070 possesses both meta- and ortho-cleavage degradative pathways, which are associated in pseudomonads with the xyl and ben genes, respectively. Open reading frames downstream of bopXYZ, designated bopL and bopK, resembled genes encoding cis-diol dehydrogenases and benzoate transporters, respectively. The bop genes were in the same order as the chromosomal ben genes of P. putida PRS2000. The deduced sequences of BopXY were 50 to 60% identical to the corresponding proteins of benzoate and toluate dioxygenases. The reductase components of these latter dioxygenases, BenC and XylZ, are 201 residues shorter than the deduced BopZ sequence. As predicted from the sequence, expression of BopZ in E. coli yielded an approximately 60-kDa protein whose presence corresponded to increased cytochrome c reductase activity. While the N-terminal region of BopZ was approximately 50% identical in sequence to the entire BenC or XylZ reductases, the C terminus was unlike other known protein sequences.
For many years, investigations of the prokaryotic degradation of aromatic compounds focused almost exclusively on the metabolism of gram-negative bacteria. Nevertheless, some gram-positive bacteria, such as the actinomycetes, can mineralize a wide array of hydrocarbons, including n-alkanes and aromatic compounds (1, 7, 8, 18, 29, 45–47). For example, in a study of 34 strains of actinomycetes representing nine genera, Rhodococcus sp. strain 19070 was found to use at least six different long-chain alkanes as the sole source of carbon and energy (17, 27). In addition, each of the following aromatic compounds supported the growth of this strain: toluene, m-xylene, p-xylene, o-xylene, trimethylbenzene, benzyl alcohol, and benzoate. Relatively little is known about the biochemistry or genetics of the catabolic routes for these compounds in Rhodococcus.
To exploit the microbial potential for bioremediation and environmental detoxification and to understand more fully how catabolic pathways have evolved in diverse microorganisms, more information is needed about aromatic compound degradation by actinomycetes. By analogy with catabolic pathways of gram-negative bacteria, some of the aromatic compounds listed above that serve as carbon sources for Rhodococcus sp. strain 19070 might be converted to catechol or a substituted catechol (22, 23). Aromatic ring cleavage could then be mediated by either an intradiol or extradiol ring-cleavage dioxygenase. Both intradiol and extradiol catechol dioxygenases have been studied from several Rhodococcus strains (12, 26, 41).
The well-characterized degradation of toluene, xylenes, benzyl alcohol, and related compounds occurs via a plasmid-encoded pathway in gram-negative Pseudomonas species. The TOL plasmid pWW0 of Pseudomonas putida mt-2 encodes broad-substrate-specific enzymes that convert the initial growth substrate to either benzoate or a substituted benzoate (51). Dihydroxylation of the benzoate ring by the xylXYZ-encoded dioxygenase then yields a nonaromatic cis-diol which is converted to catechol by a xylL-encoded dehydrogenase. The TOL plasmid also encodes enzymes for the extradiol cleavage of catechol and a “meta-cleavage” pathway that feeds catabolites into the tricarboxylic acid cycle (19, 22).
Pseudomonas strains carrying TOL plasmids also have a chromosomally encoded pathway for the degradation of benzoate but not substituted benzoates. The chromosomal benABC genes, which are evolutionarily related to xylXYZ, encode a relatively narrow substrate-specific aromatic ring hydroxylating benzoate dioxygenase (20, 34). The product of the BenABC-catalyzed reaction is converted to catechol by the benD-encoded dehydrogenase (32). Catechol is then cleaved by an intradiol ring-cleavage dioxygenase, and catabolism proceeds via the β-ketoadipate pathway (23).
In this study, the xylXYZ genes of the TOL plasmid pWW0 were used as hybridization probes to isolate homologs from Rhodococcus sp. strain 19070. The xylXY counterparts from Rhodococcus 19070 were expressed in Escherichia coli and enabled rapid transformations of benzoate, anthranilate, and m-toluate. In addition, adjacent genes were isolated and found to be homologous to genes involved in benzoate degradation. There were sequence similarities to both the xyl and ben genes of pseudomonads. The apparent broad substrate range of the Rhodococcus enzyme was reminiscent of the xylXYZ-encoded toluate dioxygenase, yet the gene order matched that of the chromosomal ben genes of P. putida. Since it was not clear which designation was most appropriate for the Rhodococcus genes, they were given the distinct name bop (benzoate oxidation participation). The characterization of these genes enabled the construction of phylogenetic trees to evaluate the evolution of enzymes involved in benzoate degradation by bacteria.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Rhodococcus sp. strain ATCC 19070 was grown at 37°C in Luria-Bertani broth or M9 minimal medium (39). Carbon sources were provided at 4mM. Toluene, as a carbon source, was placed in the arm of a sidearm flask. Growth conditions for Acinetobacter sp. strain ADP1, also known as strain BD413 (25), have been described (42). E. coli strains XL-1 Blue (Stratagene), DH5α (Life Technologies), and MC1061 (4) were used as hosts. DNA from TOL plasmid pWW0 was isolated from P. putida mt-2 (48). Cloning vectors pEMBL8b (9), pUC19, M13mp18, and M13mp19 (50) were used. The bopZ gene from Rhodococcus sp. strain 19070 genomic DNA was PCR amplified and ligated into pUC19 to form plasmid pSAM3. Plasmid pSAM4 is a derivative of pSAM3, created by deleting a 1-kb PstI restriction fragment within the bopZ gene. Plasmid pIB1354 carries the benABC genes of Acinetobacter sp. strain ADP1 (34).
Isolation of the bop genes, DNA sequence, and analysis.
Total DNA from Rhodococcus sp. strain 19070 (24) was digested with EcoRI and used to generate a partial genomic library (39). Fragments ranging from 0.5 to 6.0 kb were extracted with phenol from low-melting-temperature agarose and were ligated to pUC19. Following the transformation of E. coli, plasmids from 156 transformants were purified and digested with EcoRI. Individual Rhodococcus DNA inserts were isolated, and samples were fixed on nitrocellulose membranes for hybridization. The 8.2-kb SacI-D restriction fragment of the P. putida mt-2 TOL pWW0 plasmid, containing xylXYZLTEGFJ (14), was radioactively labeled. It hybridized to the 2.3-kb Rhodococcus insert from plasmid pSAM1.
This 2.3-kb EcoRI fragment, labeled as a probe, was used in standard colony hybridizations (39) to identify adjacent genomic DNA. Rhodococcus DNA digested with BglII and in the 5- to 12-kb size range was ligated to pUC19 and transformed into E. coli MC1061. Approximately 2,000 transformants were screened, and four colonies hybridized to the probe. Three contained an identical 7.4-kb BglII fragment of Rhodococcus DNA, and one contained a 9.0-kb insert. Southern hybridization indicated that these two BglII fragments contained genomic DNA flanking each end of the 2.3-kb EcoRI fragment on pSAM1. A 0.9-kb BamHI-EcoRI fragment immediately upstream of the bopY gene was inserted into pSAM1. This generated plasmid pSAM2 with the complete bopXY and partial bopZ genes, as was confirmed by nucleotide sequencing.
Some single-stranded DNA, prepared from M13mp19 and M13mp18 clones (39), was sequenced by the Sanger method (40) with the T7 Sequenase 7-deaza-dGTP DNA sequencing kit (Promega). Some DNA was sequenced at the Molecular Genetics and Instrumentation Facility at the University of Georgia. Computer-assisted analysis was done with AssemblyLign and MacVector software (6.5 ed; Oxford Molecular, Ltd.) Homology searches (BLAST) were carried out at the network server of the National Center for Biotechnology Information. Amino acid sequences were aligned using the Pileup program of the Genetics Computer Group package (10). Phylogenetic trees were generated with the Fitch-Margoliash or neighbor-joining method from a distance matrix created by PROTDIST of the PHYLIP program package (13).
Southern hybridization and DNA labeling.
DNA fragments were labeled by nick translation with [α-32P]dATP (39) or by random-primed labeling with digoxigenin (DIG DNA labeling kit; Roche). Hybridizations were done at 42°C for 12 to 16 h in 3× SSC (1× SSC buffer is 0.15 M NaCl plus 0.015 M sodium citrate; pH 7.4) with 50% formamide (39). Wash conditions after hybridization of the xyl probes to Rhodococcus DNA consisted of two 5-min washes with 2× SSC plus 0.1% sodium dodecyl sulfate (SDS) at room temperature, three washes for 45 min in 0.1× SSC plus 0.1% SDS at 55°C, and four rinses with 0.1× SSC. Higher-stringency conditions after hybridization of Rhodococcus probes to Rhodococcus target DNA used 70°C rather than 55°C washes.
Analysis of metabolites.
E. coli (pSAM2) were grown overnight in 5 ml of M9 medium with glucose (10 mM) and ampicillin (50 mg/ml). Wild-type Acinetobacter ADP1 was grown overnight in ADP1 minimal medium with 10 mM succinate. On the addition of 1 mM benzoate, anthranilate, m-toluate, or o-chlorobenzoate to the cultures, a 1-ml culture sample was collected. Isopropyl-β-d-thiogalactopyranoside (IPTG) was then added to each E. coli culture to a final concentration of 1 mM to induce expression from the lac promoter of recombinant plasmids. Samples of the culture medium (1 ml) were collected at various time points and filtered through 0.22-μm (pore-size) nylon filters (MSI) to remove whole cells. Metabolites were detected by high-performance liquid chromatography (HPLC). The samples were analyzed with a Bio-Rad 2800 HPLC system with an AS-100 HPLC sampler. The samples were resolved on a reversed-phase Bio-Sil QDS-5S HPLC column (BioRad; 250 by 4 mm). Elution at a rate of 0.8 ml/min was carried out with 30% acetonitrile containing 1% acetic acid. The eluant was monitored by UV detection at 230 nm. Under these conditions, the retention times were as follows: benzoate (13.3 min), 2-hydro-1,2-dihydroxybenzoate (cis-diol) (3.9 min), anthranilate (8.8 min), catechol (6.9 min), m-toluate (23.3 min), and o-chlorobenzoate (17.1 min). Authentic chemical samples were analyzed to assure proper peak identification. A sample of the benzoate cis-diol was kindly provided by A. Reiner.
Expression of bop genes in E. coli.
Single colonies of E. coli with plasmids were used to inoculate 2 ml of medium (Luria-Bertani broth with 150 μg of ampicillin per ml). Cultures at an optical density at 600 nm (OD600) of 0.1 were then used to inoculate 50 ml of medium. To these cultures (at an OD600 of 0.3 to 0.4), IPTG was added to a final concentration of 1 mM. Five hours later, cells were harvested by centrifugation. Cell pellets were stored frozen at −20°C.
Electrophoresis.
Proteins were analyzed by SDS–8% polyacrylamide gel electrophoresis (PAGE) with Coomassie blue staining (39). Approximately 20 μg of total protein from each sample was loaded per well. DNA for pulsed-field gel electrophoresis (PFGE) was prepared as described elsewhere (15, 43). A CHEF DRIII system (Bio-Rad) was used to run 1% agarose gels at 4°C with a 150-V/cm electric field for 38 h. Pulse times increased from 50 to 120 s, and the field angle was 120°. For hybridization, DNA was transferred to a nylon membrane (TurboBlotter; Schleicher & Schuell).
Enzyme assays.
Cells were sonicated, and cell extracts were prepared (42). Protein concentrations were determined with bovine serum albumin as the standard (2). Catechol 1,2- or 2,3-dioxygenase activity was assayed spectrophotometrically by monitoring the increase in cis,cis-muconate concentration at A260 or the increase in 2-hydroxymuconic semialdehyde at A375, respectively (31, 35, 38). Treatment with 40 mM hydrogen peroxide inactivated catechol 2,3-dioxygenase (31). Heating at 55°C for 10 min inactivated catechol 1,2-dioxygenase (30). The NADH-dependent reduction of cytochrome c was detected by an increase in A550 (49). The specific activities were averages of at least two independent repetitions done in duplicate. Standard deviations were less than 20% of the reported value.
Nucleotide sequence accession number.
The DNA sequence of bopXYZLK has been submitted to GenBank under accession no. AF279141.
RESULTS AND DISCUSSION
Identification of the Rhodococcus sp. 19070 bopXYZ genes and gene products.
Rhodococcus sp. strain 19070 can grow on benzoate and substituted benzoates, as well as other aromatics such as toluene and xylenes that might be metabolized via benzoate or methylated benzoates. This ability suggested that strain 19070 would possess at least one aromatic ring-hydroxylating dioxygenase similar to the toluate dioxygenase encoded by the P. putida xylXYZ genes. Consistent with this possibility, the xyl genes were found to hybridize to a 2.3-kb EcoRI restriction fragment of Rhodococcus DNA on plasmid pSAM1. DNA sequence analysis revealed a complete open reading frame (ORF) on pSAM1 with significant similarity to xylY and two incomplete ORFs which resembled xylX and xylZ. Labeled DNA from pSAM1 was then used to isolate the adjacent regions of the Rhodococcus genome. Sequence determination of a 5.8-kb region, encompassing the EcoRI fragment of pSAM1, revealed three complete ORFs, designated bopXYZ, that seemed likely to encode a dioxygenase.
The deduced sequences of BopXY were approximately 60% identical to XylXY and BenAB, which are the alpha and beta subunits of the terminal oxygenase components of two-component benzoate dioxygenases. Sequence identity was also significant, i.e., approximately 40 to 55%, between BopXY and AntAB or CbdAB. The latter proteins comprise the alpha and beta subunits of the terminal oxygenase of a two-component anthranilate (2-aminobenzoate) dioxygenase or a chlorobenzoate dioxygenase (3, 16).
In each case, the second component of the two-component dioxygenase is a reductase that transfers electrons from NADH to the terminal oxygenase. The XylZ, BenC, and AntC reductases are all similar in size, i.e., ca. 337 amino acid residues (39 kDa), and all share a common evolutionary ancestor (11). The bopZ ORF was considerably longer than all of its counterparts, and it was predicted to encode a 60-kDa protein with an additional 201 amino acid residues beyond that corresponding to the C terminus of XylZ. The first 337 residues of the deduced BopZ sequence were approximately 50% identical to the entire XylZ and BenC sequences. In contrast, homology searches using the BopZ C-terminal region did not identify any significant similarity to sequences in current databases.
To investigate the Bop protein sizes, cell extracts of E. coli cultures carrying recombinant plasmids were analyzed by SDS-PAGE. DNA sequencing predicted that plasmid pSAM2, with complete Rhodococcus bopXY genes, carried only part of the bopZ gene. This portion of bopZ, however, would have been sufficient to encode a reductase that was the size of xylZ or benC. Consistent with the DNA sequence analysis, E. coli cells carrying pSAM2 produced high levels of proteins of the expected sizes for BopX (56 kDa) and BopY (20 kDa), but not BopZ (60 kDa) (data not shown). Moreover, in the size range expected for a XylZ-type of reductase (39 kDa), there were no notable differences in the protein profiles of cells that did or did not carry pSAM2 (data not shown).
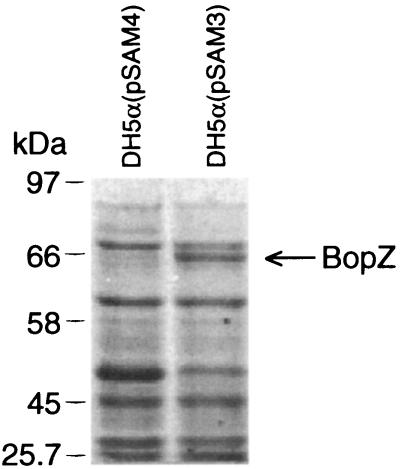
To enable BopZ synthesis, a plasmid (pSAM3) with the entire coding region was constructed. A second plasmid, pSAM4 was derived from pSAM3 by deleting the central portion of bopZ. E. coli cells carrying pSAM3, but not pSAM4, had high levels of an approximately 65-kDa protein (Fig. 1). Additional controls with different plasmids and plasmid-free cells confirmed that the 65-kDa protein was correlated with the presence of an intact bopZ gene (data not shown). Therefore, although the apparent size was slightly larger than predicted, the protein was inferred to be BopZ. The amount of BopZ relative to other cellular proteins was variable, however. It appeared that expression or stability of this protein in E. coli was sensitive to slight variations in experimental conditions.
FIG. 1.
SDS-PAGE of proteins from plasmid-containing E. coli strain DH5α. Plasmid pSAM3 encodes the entire bopZ gene and pSAM4 has a deletion in its bopZ allele. The BopZ protein and the sizes of protein standards (in kilodaltons) are indicated.
Function of bopXY genes expressed in E. coli.
To test whether the BopXY proteins expressed from pSAM2 conferred any aromatic ring hydroxylating abilities to E. coli, HPLC methods were used to monitor metabolite transformations. E. coli does not have known genes or enzyme activities that correspond to those of benzoate dioxygenases. E. coli cultures that contained pSAM2, no plasmid, or a plasmid vector with no heterologous DNA were provided with 1 mM amounts of either benzoate, m-toluate, anthranilate, or o-chlorobenzoate as a substrate for hydroxylation. After incubation for variable amounts of time ranging from 1 to 18 h, whole cells were removed, and the amount of aromatic acid remaining in the medium was assessed. Only in E. coli cultures containing pSAM2 did the concentration of substrates decrease over time.
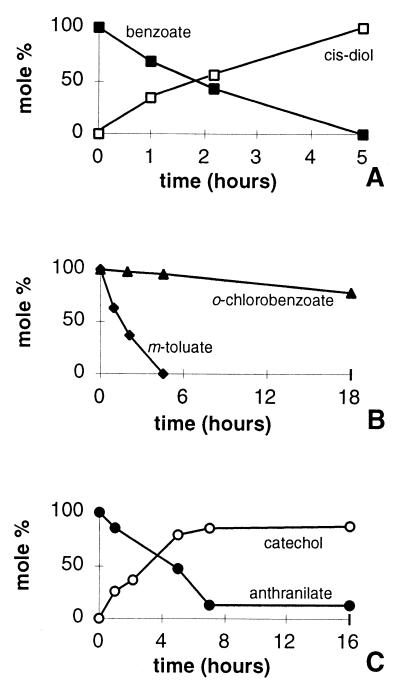
E. coli expressing bopXY quantitatively converted 1 mM benzoate to the cis-diol, 2-hydro-1,2-dihydroxybenzoate, in approximately 5 h (Fig. 2). This cis-diol is the product of benzoate dihydroxylation by either benzoate 1,2- or toluate 1,2-dioxygenases (21, 36, 37). E. coli (pIB1354) with the benABC genes of Acinetobacter sp. strain ADP1 were also able to carry out this transformation in a similar amount of time (34). Whole cells of Acinetobacter sp. strain ADP1 were able to remove benzoate from the medium, but the cis-diol did not accumulate, presumably because this bacterium is able to mineralize benzoate completely (data not shown). When m-toluate was used as a substrate, E. coli cultures expressing the bopXY genes were able to remove the substrate at a rate similar to that of benzoate (Fig. 2). The disappearance of the m-toluate peak on the HPLC chromatographs corresponded over time to the broadening of a different peak with a retention time of 4.4 min. The transformation product was not identified, because this compound was not clearly resolved under the experimental conditions that were used.
FIG. 2.
Metabolites in the culture medium of DH5α(pSAM2) provided with benzoate, m-toluate, o-chlorobenzoate, or anthranilate (1 mM each). (A) Conversion of benzoate (■) to cis-diol (□) (B) Consumption of m-toluate (⧫) or o-chlorobenzoate (▴). (C) Conversion of anthranilate (●) to catechol (○) Compounds were identified by HPLC analysis.
Expression of bopXY in E. coli enabled the removal of anthranilate, albeit at a rate slightly slower than for benzoate or m-toluate (Fig. 2). Under the same conditions, E. coli expressing the Acinetobacter benABC genes did not transform anthranilate to catechol (data not shown). Most likely, the dihydroxylation of anthranilate by BopXY produces a cis-diol that spontaneously deaminates and decarboxylates to yield catechol. The dihydroxylation of benzoate or toluate produces a more stable cis-diol that requires the action of a dehydrogenase to form catechol (3). With o-chlorobenzoate as substrate, the bopXY genes enabled a small amount of the substrate (0.2 mM) to be removed from the medium during a relatively long 18-h incubation period.
The expression of bopXY without bopZ was sufficient to transform the substrates tested. Previous studies demonstrate that the cognate reductase components of several two-component dioxygenases from gram-negative bacteria are not required for activity of the oxygenase in E. coli (28). For example, the AntAB oxygenase component of the Acinetobacter ADP1 anthranilate 1,2-dioxygenase is active in E. coli without AntC (11). Similarly, it appears that in the absence of BopZ, an endogenous reductase in E. coli transfers electrons to the BopXY oxygenase.
Function of bopZ expressed in E. coli.
To confirm that the bopZ gene of pSAM2 was incomplete, cytochrome c reductase activity was measured. Previously characterized reductases can transfer electrons from NADH to cytochrome c in the absence of their terminal oxygenases (49). This activity was measured in extracts of plasmid-free E. coli and E. coli carrying pSAM2, pUC19, or pSAM4 (with a deletion in the bopZ allele). The specific activities of all of these samples were comparable (≤20 ± 5 nmol/min per mg of protein). Thus, the truncated bopZ on pSAM2 was not sufficient to encode a functional NADH-dependent cytochrome c reductase in E. coli. In contrast, either the entire bopZ gene (pSAM3) or benC (pIB1354) increased the reductase activity in E. coli (100 ± 11 or 600 ± 76 nmol/min per mg of protein, respectively). The unusually large BopZ, therefore, appeared to have an activity similar to that of the smaller XylZ and BenC proteins.
Organization of the bop gene cluster and putative functions of adjacent genes.
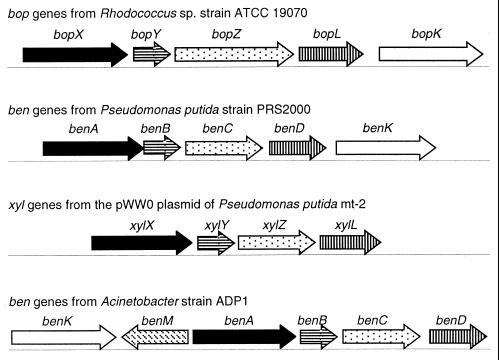
Sequence analyses of genes in the vicinity of bopXYZ were consistent with the likelihood that they are all involved in benzoate catabolism (Fig. 3). The deduced amino acid sequence of the ORF immediately downstream of bopXYZ, designated bopL, was approximately 60% identical to that of XylL or BenD. The latter proteins are cis-diol dehydrogenases that use as their substrates the products of XylXYZ- or BenABC-catalyzed reactions, respectively. By analogy, BopL may be a dehydrogenase that converts the products of BopXYZ-catalyzed reactions to catechol or substituted catechols. The ORF immediately downstream of bopL was designated bopK based on its sequence similarity to benK. In Acinetobacter sp. strain ADP1, BenK is a benzoate transporter that is encoded by a gene upstream of the operon containing the benABCD genes (Fig. 3) (5). A recently identified benK homolog in P. putida PRS2000 lies immediately downstream of the benABCD genes (6) in the same arrangement as the bop genes (Fig. 3). No benK homolog, however, has been identified near the xylXYZL genes.
FIG. 3.
Organization of the bop, ben, and xyl genes. Arrows indicate the direction of transcription. Gene products are known or are predicted to be: the α and β subunits of a terminal dioxygenase (solid black and horizontal lines, respectively), the reductase components of a two-component dioxygenase (dots), cis-diol dehydrogenases (vertical lines), membrane proteins involved in aromatic compound transport (white), and a transcriptional regulator (diagonal lines).
The similarity of the bop and ben clusters raised the possibility that Rhodococcus sp. strain 19070 might have a second genetic region, one corresponding more closely to the xyl genes, that encodes a benzoate dioxygenase distinct from BopXYZ. To test this possibility, a variety of restriction enzymes, including BglII, EcoRI, PstI, SacI, SalI, and SphI, were used to digest Rhodococcus DNA for Southern hybridizations. A labeled bopXY probe, with the same conditions that allow the Pseudomonas xyl genes to hybridize to the bop genes, did not detect DNA fragments other than those of the sizes expected for the bop genes (data not shown). Nevertheless, the presence of additional dioxygenase genes cannot be precluded.
Catabolic pathways for benzoate degradation in Rhodococcus sp. strain 19070.
One difference between the xyl- and ben-encoded catabolic pathways in pseudomonads is that catechols formed from xyl-encoded enzymes are cleaved by an extradiol-cleaving catechol dioxygenase (encoded by xylE). Catabolites are then channeled through the meta-cleavage pathway (22). In contrast, catechol formed by the ben-encoded enzymes is cleaved by an intradiol-cleaving catechol dioxygenase (encoded by catA). Catabolites are channeled through the ortho-cleavage pathway, also known as the β-ketoadipate pathway (23). The xylE gene of the TOL plasmid pWW0 is downstream of xylXYZL and is coexpressed with them (44). The catA genes of P. aeruginosa PAO1 and Acinetobacter sp. strain ADP1 are located near the benABCD genes (33, 52). In Rhodococcus sp. strain 19070 partial DNA sequencing in the regions near the bop genes did not reveal the presence of a gene encoding either an intradiol or extradiol catechol dioxygenase.
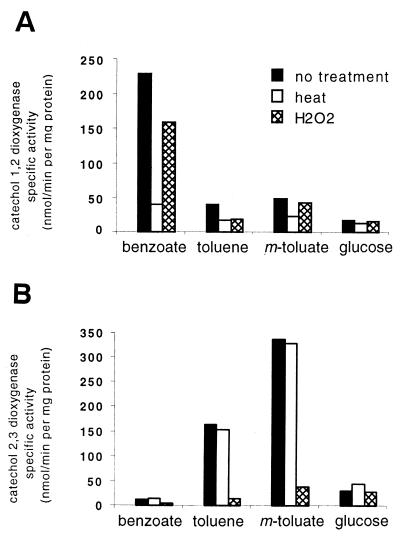
The activities of catechol 1,2-dioxygenase (intradiol) and catechol 2,3-dioxygenase (extradiol) can be distinguished by the distinct absorbance patterns of their ring cleavage products. Furthermore, in previous studies H2O2 has been shown to inactivate catechol 2,3-dioxygenase, whereas heat treatment inactivates catechol 1,2-dioxygenase (31). The activities of both enzymes were measured in cell extracts of Rhodococcus sp. strain 19070 grown on benzoate, toluene, m-toluate, or glucose as the sole carbon source. For each carbon source, the activity patterns in cell extracts were compared. As shown in Fig. 4, catechol 1,2-dioxygenase was induced by growth on benzoate, whereas catechol 2,3-dioxygenase was induced by growth on toluene or m-toluate. Similar results are observed for pseudomonads, in which the xylXYZ-encoded dioxygenase participates in toluene catabolism and the benABC-encoded dioxygenase participates in benzoate catabolism. Further work is needed to establish under which growth conditions the bop genes of Rhodococcus sp. strain 19070 are expressed. Nevertheless, this induction of catechol 1,2-and 2,3-dioxygenases indicates the presence of both ortho- and meta-cleavage pathways.
FIG. 4.
Catechol dioxygenase activity of Rhodococcus sp. strain 19070 grown with benzoate, toluene, m-toluate, or glucose as the sole carbon source. (A) Catechol 1,2-dioxygenase activity was assayed spectrophotometrically at 260 nm with the ring cleavage product inferred to be cis,cis-muconate. (B) Catechol 2,3-dioxygenase activity was assayed spectrophotometrically at 375 nm with the ring cleavage product inferred to be 2-hydroxymuconic semialdehyde. White bars indicate the activity in samples that were heat treated to inactivate catechol 1,2-dioxygenase. Cross-hatched bars indicate activity in samples that were treated with hydrogen peroxide to inactivate catechol 2,3-dioxygenase.
PFGE analysis of Rhodococcus sp. strain 19070.
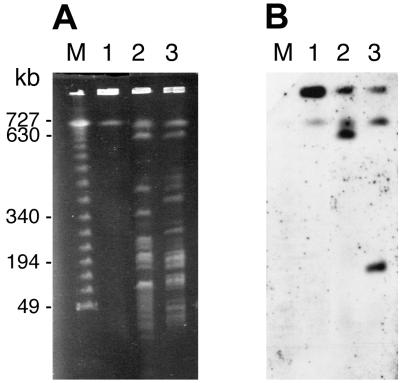
In P. putida mt-2, xylXYZL are on the TOL plasmid pWW0, while benABCD are on the chromosome. PFGE analysis of undigested genomic DNA of Rhodococcus sp. strain 19070 demonstrated that no DNA was visible below 700 kb (Fig. 5A, lane 1) or hybridized to a labeled bopXY probe (Fig. 5B, lane 1). Thus, it appeared that the bop genes are either chromosomal or on a very large plasmid. The bopXY probe hybridized to distinct fragments of circa 640 or 190 kb in DNA digested with SspI or XbaI, respectively (Fig. 5B, lanes 2 and 3). These hybridization patterns and the clear separation of molecular weight standards in the range of 50 to 730 kb suggest that the presence of the bop genes on a small plasmid could have been detected.
FIG. 5.
PFGE of genomic DNA from Rhodococcus sp. strain 19070. (A) DNA was either uncut (lane 1) or digested with SspI (lane 2) or XbaI (lane 3). The sizes of DNA markers are indicated adjacent to the corresponding DNA (lambda ladder from New England Biolabs) in lane M. (B) Results of Southern hybridization of gel in panel A with a bopXY probe.
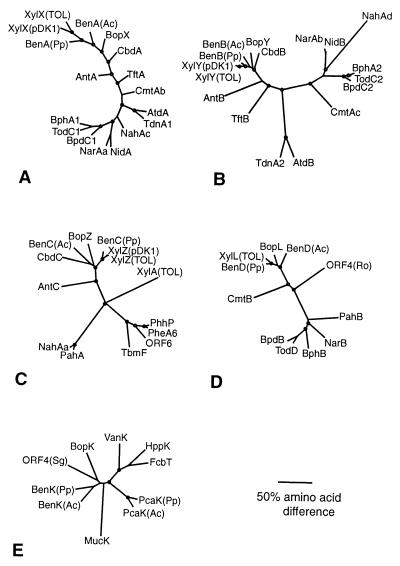
Phylogeny of Rhodococcus BopXYZLK.
The Bop protein sequences were aligned with those found by database searches to be most similar. Phylogenetic trees constructed from these alignments indicated that each of the BopXYZ proteins, from a gram-positive bacterium, was closely related to the corresponding component of the benzoate (Ben) and toluate (Xyl) dioxygenases from gram-negative bacteria (Fig. 6A, B, and C). Moreover, the close relationships among BopL, XylL, and BenD (Fig. 6D) and between BopK and BenK (Fig. 6E) support a role for the Bop proteins in benzoate degradation.
FIG. 6.
Phylogenetic trees based on comparisons with homologs of the Rhodococcus sp. strain 19070 Bop proteins. (A) BopX was aligned with alpha components of oxygenase subunits. (B) BopY was aligned with the corresponding beta components of oxygenases from Panel A (C) BopZ was aligned with available reductase components associated with oxygenases from panel A and other reductases or putative reductases. (D) The putative BopL dehydrogenase was aligned with dehydrogenases and putative dehydrogenases associated with the proteins displayed in trees A to C, as well as ORF4 (Ro). (E) BopK, a putative transport protein, was aligned with proteins that may be involved in transporting organic compounds. Gene clusters are associated with metabolism and/or transport of the indicated compounds: BopXYZLK, Rhodococcus sp. ATCC 19070, benzoate-toluate (AF279141), BenABCDK(Pp), P. putida strain PRS200, benzoate (AF218267), BenABCDK(Ac), Acinetobacter sp. strain ADP1, benzoate (AF009224), XylXYZ(pDK1), P. putida sp. plasmid pDK1, toluate (AF134348), XylXYZL(TOL), P. putida sp. TOL plasmid, benzoate (M64747), CbdABC, Burkholderia cepacia, halobenzoate (X79076), AntABC, Acinetobacter sp. strain ADP1, anthranilate (AF071556), TftAB, B. cepacia, 2,4,5-trichlorophenoxyacetic acid (U11420), AtdAB, Acinetobacter sp. plasmid pYA1, aniline (D86080), TdnA1B1, P. putida strain UCC22 (pTDN1) F1, aniline (D85415), CmtAbAcB, P. putida, p-cymene (U24215), NidAB, Rhodococcus sp. strain I24, indene (AF121905), NarAaAbB, Rhodococcus sp. strain NCIMB12038, naphthalene (AF082663), BphA1A2B, Rhodococcus sp. strain RHA1, biphenyl (D32142), NahAcAdAa, P. putida strain G7, napthalene (M83949), BpdC1C2B, Rhodococcus sp. strain M5, biphenyl-chlorobiphenyl (U27591), TodC1C2D, P. putida sp. strain F1, toluene (J04996), ORF6, A. calcoaceticus strain NCIB8250, phenol (Z36909), XylA, P. putida sp. TOL plasmid, xylene (M37480), PheA6, P. putida sp. strain BH, phenol (D28864), PhhP, P. putida sp. strain P35X (NCIB9869), phenol (X79063), TbmF, Pseudomonas sp. strain JS150, toluene-benzene (L40033), PahAB, P. aeruginosa strain PaK1, naphthalene (D84146), ORF4(Ro), Rhodococcus opacus sp. strain 1CP putative short-chain dehydrogenase (AF030176), PcaK(Ac), Acinetobacter sp. strain ADP1, protocatechuate transporter (L05770), ORF4(Sg), Streptomyces griseus, putative tyrosine transporter (AB022095), PcaK(Pp), P. putida sp. strain PRS2000, protocatechuate transporter (U10895), HppK, Rhodococcus globerulus sp. strain PWD1, putative 3-hydrox-yphenyl propionate transporter (U89712), FcbT, Arthrobacter sp. strain TM1, 4-chlorobenzoate transporter (AF042490), MucK, Acinetobacter sp. strain ADP1, cis,cis-muconate transporter (U87258), VanK, Acinetobacter sp. strain ADP1, vanillate transporter (AF009672). Accession numbers are indicated parenthetically. Circles represent branch points that occur with a frequency of 85 to 100%, respectively, as calculated by bootstrap analysis using 100 replicates.
The presence of a benK-like gene within the bop gene cluster might indicate that BopXYZ is more similar to BenABC than XylXYZ. However, BopXYZ in E. coli, unlike BenABC of ADP1, was able to hydroxylate anthranilate. The rate of anthranilate hydroxylation was reduced relative to benzoate or m-toluate as a substrate, and the phylogenetic relationship to AntABC was more distant than to the Ben or Xyl proteins (Fig. 6A, B, and C). Therefore, while the primary role for BopXYZ is most likely not anthranilate catabolism, the substrate specificity of BopXYZ appears to be broader than that of BenABC. Further work may clarify whether BopXYZL converts benzoate to catechol for degradation via an ortho-cleavage pathway or whether BopXYZL converts toluates to methyl-catechols for degradation via a meta-cleavage pathway. An intriguing possibility, which remains to be investigated, is that it could carry out both functions.
The most unusual feature of the BopXYZ dioxygenase was the large size of BopZ. Whereas approximately two-thirds of the BopZ protein was closely related to the entire BenC and XylZ reductases (Fig. 6C), the C-terminal region of the protein did not resemble any known sequences. The ability of the BopXY terminal oxygenase to function without BopZ in E. coli is consistent with oxygenase components of aromatic-ring-hydroxylating dioxygenases having relaxed requirements for specific reductases. Therefore, the unusual C-terminal region of BopZ does not appear to be necessary for the hydroxylation activity of BopXY, and its specific function in strain 19070 is unclear. It will be interesting to discover whether this protein region is conserved among related reductases of gram-positive bacteria.
ACKNOWLEDGMENTS
We thank A. Reams for assistance with PFGE analysis. S. Haddad also gratefully acknowledges the support of J. W. Bennett and W. A. Toscano, Jr.
This work was supported by the National Science Foundation grant MCB-9808784 (to E.L.N.) and postdoctoral research fellowship in microbial biology DBI-0074398 (to S.H.).
REFERENCES
- 1.Andreoni V, Bernasconi S, Colombo M, van Beilen J B, Cavalca L. Detection of genes for alkane and naphthalene catabolism in Rhodococcus sp. strain 1BN. Environ Microbiol. 2000;2:572–577. doi: 10.1046/j.1462-2920.2000.00134.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Bundy B M, Campbell A L, Neidle E L. Similarities of the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadaban M J, Chou J, Cohen S N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier L S, Nichols N N, Neidle E L. benK Encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowles C E, Nichols N N, Harwood C S. BenR, a XylS homologue, regulates three different pathways of aromatic acid degradation in Pseudomonas putida. J Bacteriol. 2000;182:6339–6346. doi: 10.1128/jb.182.22.6339-6346.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Schrijver A, De Mot R. Degradation of pesticides by actinomycetes. Crit Rev Microbiol. 1999;25:85–119. doi: 10.1080/10408419991299194. [DOI] [PubMed] [Google Scholar]
- 8.Deeb R A, Alvarez-Cohen L. Temperature effects and substrate interactions during the aerobic biotransformation of BTEX mixtures by toluene-enriched consortia and Rhodococcus rhodochrous. Biotechnol Bioeng. 1999;62:526–536. [PubMed] [Google Scholar]
- 9.Dente L, Cesarini G, Cortese R. pEMBL: a new family of single-stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12(Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eby D M, Beharry Z M, Coulter E D, Kurtz D M, Neidle E L. Characterization and evolution of anthranilate 1,2-dioxygenase from Acinetobacter sp. strain ADP1. J Bacteriol. 2001;183:109–118. doi: 10.1128/JB.183-1.109-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eulberg D, Golovleva L A, Schlömann M. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenstein J. PHYLIP-Phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 14.Franklin F C H, Bagdasarian M, Bagdasarian M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gralton E M, Campbell A L, Neidle E L. Directed introduction of DNA cleavage sites to produce a high-resolution genetic and physical map of the Acinetobacter sp. strain ADP1 (BD413UE) chromosome. Microbiology. 1997;143:1345–1357. doi: 10.1099/00221287-143-4-1345. [DOI] [PubMed] [Google Scholar]
- 16.Haak B, Fetzner S, Lingens F. Cloning, nucleotide sequence, and expression of the plasmid-encoded genes for the two-component 2-halobenzoate 1,2-dioxygenase from Pseudomonas cepacia 2CBS. J Bacteriol. 1995;177:667–675. doi: 10.1128/jb.177.3.667-675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad S. Ph.D. thesis. New Orleans, La: Tulane University; 1998. [Google Scholar]
- 18.Hanne L F, Kirk L L, Appel S M, Narayan A D, Bains K K. Degradation and induction specificity in actinomycetes that degrade p-nitrophenol. Appl Environ Microbiol. 1993;59:3505–3508. doi: 10.1128/aem.59.10.3505-3508.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harayama S, Lerhbach P R, Timmis K N. Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J Bacteriol. 1984;160:251–255. doi: 10.1128/jb.160.1.251-255.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harayama S, Rekik M, Bairoch A, Neidle E L, Ornston L N. Potential DNA slippage structures acquired during evolutionary divergence of Acinetobacter calcoaceticus chromosomal benABC and Pseudomonas putida TOL pWW0 plasmid xylXYZ, genes encoding benzoate dioxygenases. J Bacteriol. 1991;173:7540–7548. doi: 10.1128/jb.173.23.7540-7548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harayama S, Rekik M, Timmis K N. Genetic analysis of a relaxed substrate specificity aromatic ring dioxygenase, toluate 1,2-dioxygenase, encoded by TOL plasmid pWW0 of Pseudomonas putida. Mol Gen Genet. 1986;202:226–234. doi: 10.1007/BF00331641. [DOI] [PubMed] [Google Scholar]
- 22.Harayama S, Timmis K N. Catabolism of aromatic hydrocarbons by Pseudomonas. In: Hopwood D A, Chater K, editors. Genetics of bacterial diversity. New York, N.Y: Academic Press, Inc.; 1989. pp. 151–174. [Google Scholar]
- 23.Harwood C S, Parales R E. The B-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood D A, Bibb M J, Chater K F, Kiesser T, Bruton C J, Kiesser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces—a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 25.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulakov L A, Delcroix V A, Larkin M J, Ksenzenko V N, Kulakova A N. Cloning of new Rhodococcus extradiol dioxygenase genes and study of their distribution in different Rhodococcus strains. Microbiology. 1998;144:955–963. doi: 10.1099/00221287-144-4-955. [DOI] [PubMed] [Google Scholar]
- 27.Lofgren J, Haddad S, Kendall K. Metabolism of alkanes by Rhodococcus erythropolis. In: Tedder D W, Pohland F G, editors. Emerging technologies in hazardous waste management. V. Washington, D.C.: American Chemical Society; 1995. pp. 252–263. [Google Scholar]
- 28.Martin V J J, Mohn W W. A novel aromatic-ring-hydroxylating dioxygenase from the diterpenoid-degrading bacterium Pseudomonas abietaniphilia BKME-9. J Bacteriol. 1999;181:2675–2682. doi: 10.1128/jb.181.9.2675-2682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy A J, Williams S T. Actinomycetes as agents of biodegradation in the environment—a review. Gene. 1992;115:189–192. doi: 10.1016/0378-1119(92)90558-7. [DOI] [PubMed] [Google Scholar]
- 30.Murray K, Williams P A. Role of catechol and the methylcatechols as inducers of aromatic metabolism in Pseudomonas putida. J Bacteriol. 1974;117:1153–1157. doi: 10.1128/jb.117.3.1153-1157.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazawa T, Yokota T. Benzoate metabolism in Pseudomonas putida (arvilla) mt-2: demonstration of two benzoate pathways. J Bacteriol. 1973;115:262–267. doi: 10.1128/jb.115.1.262-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neidle E, Harnett C, Ornston L N, Bairoch A, Rekik M, Harayama S. cis-Diol dehydrogenases encoded by the TOL pWW0 plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur J Biochem. 1992;204:113–120. doi: 10.1111/j.1432-1033.1992.tb16612.x. [DOI] [PubMed] [Google Scholar]
- 33.Neidle E L, Hartnett C, Bonitz S, Ornston L N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol. 1988;170:4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neidle E L, Shapiro M K, Ornston L N. Cloning and expression in Escherichia coli of Acinetobacter calcoaceticus genes for benzoate degradation. J Bacteriol. 1987;169:5496–5503. doi: 10.1128/jb.169.12.5496-5503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngai K-L, Neidle E L, Ornston L N. Catechol and chlorocatechol 1,2-dioxygenase. Methods Enzymol. 1990;188:122–126. doi: 10.1016/0076-6879(90)88022-3. [DOI] [PubMed] [Google Scholar]
- 36.Reiner A M. Metabolism of benzoic acid by bacteria: 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is an intermediate in the formation of catechol. J Bacteriol. 1971;108:89–94. doi: 10.1128/jb.108.1.89-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiner A M, Hegeman G D. Metabolism of benzoic acid by bacteria. Accumulation of (−)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by a mutant strain of Alcaligenes eutrophus. Biochemistry. 1971;10:2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- 38.Sala-Trepat J M, Evans W C. The meta cleavage pathway of catechol by Azobacter species. Eur J Biochem. 1971;20:400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiner A, Fuchs K, Lottspeich F, Poth H, Lingens F. Degradation of 2-methylaniline in Rhodococcus rhodochrous: cloning and expression of two clustered catechol 2,3-dioxygenase genes from strain CTM. J Gen Microbiol. 1991;137:2041–2048. doi: 10.1099/00221287-137-8-2041. [DOI] [PubMed] [Google Scholar]
- 42.Shanley M S, Neidle E L, Parales R E, Ornston L N. Cloning and Expression of Acinetobacter calcoaceticus catBCDE Genes in Pseudomonas putida and Escherichia coli. J Bacteriol. 1986;165:557–563. doi: 10.1128/jb.165.2.557-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith C L, Cantor C R. Purification, specific fragmentation and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- 44.Spooner R A, Bagdasarian M, Franklin F C H. Activation of the xylDLEGF promoter of the TOL toluene-xylene degradation pathway by overproduction of the xylS regulatory gene product. J Bacteriol. 1987;169:3581–3586. doi: 10.1128/jb.169.8.3581-3586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uz I, Duan Y P, Ogram A. Characterization of the naphthalene-degrading bacterium, Rhodococcus opacus M213. FEMS Microbiol Lett. 2000;185:231–238. doi: 10.1111/j.1574-6968.2000.tb09067.x. [DOI] [PubMed] [Google Scholar]
- 46.Vanderberg L A, Krieger-Grumbine R, Taylor M N. Evidence for diverse oxidations in the catabolism of toluene by Rhodococcus rhodochrous strain OFS. Appl Microbiol Biotechnol. 2000;53:447–452. doi: 10.1007/s002530051640. [DOI] [PubMed] [Google Scholar]
- 47.Warhurst A M, Fewson C A. Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotech. 1994;14:29–73. doi: 10.3109/07388559409079833. [DOI] [PubMed] [Google Scholar]
- 48.Williams P A, Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974;120:416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi M, Fujisawa H. Characterization of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem. 1978;253:8848–8853. [PubMed] [Google Scholar]
- 50.Yanisch-Perron C J, Vieira J, Messing J. Improved M13 cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 51.Zeyer J, Lehrbach P R, Timmis K N. Use of cloned genes of Pseudomonas TOL plasmid to effect biotransformation of benzoates to cis-dihydrodiols and catechols by Escherichia coli cells. Appl Environ Microbiol. 1985;50:1409–1413. doi: 10.1128/aem.50.6.1409-1413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, Huang M, Holloway B W. Mapping of the ben, ant and cat genes of Pseudomonas aeruginosa and evolutionary relationship of the ben region of P. aeruginosa and P. putida. FEMS Microbiol Lett. 1993;108:303–310. doi: 10.1016/0378-1097(93)90560-o. [DOI] [PubMed] [Google Scholar]