Abstract
Background
The human monoclonal antibody dupilumab blocks interleukin (IL)‐4 andIL‐13, key and central drivers of type 2 inflammation. Dupilumab, on background mometasone furoate nasal spray (MFNS), improved outcomes in the phase III SINUS‐52 study (NCT02898454) in patients with severe chronic rhinosinusitis with nasal polyps (CRSwNP). This posthoc analysis of SINUS‐52 examined whether eosinophilic status of CRSwNP was a predictor of dupilumab efficacy.
Methods
Patients were randomized 1:1:1 to dupilumab 300 mg every 2 weeks (q2w) until week 52; dupilumab 300 mg q2w until Week 24, then 300 mg every 4 weeks until week 52; or placebo (MFNS) until week 52. Coprimary endpoints were change from baseline in nasal polyps score (NPS), nasal congestion (NC), and Lund‐Mackay score assessed by CT (LMK‐CT) at week 24. Patients (n = 438) were stratified by eosinophilic chronic rhinosinusitis (ECRS) status according to the Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis algorithm.
Results
Dupilumab significantly improved NPS, NC, and LMK‐CT scores versus placebo at week 24 in all ECRS subgroups (p < 0.001), with improvements maintained or increased at week 52 (p < 0.001). There was no significant interaction between ECRS subgroup (non‐/mild or moderate/severe) and dupilumab treatment effect for all endpoints at weeks 24 and 52 (p > 0.05), except LMK‐CT at week 24 (p = 0.0275). Similar results were seen for the secondary endpoints. Dupilumab was well tolerated across all ECRS subgroups.
Conclusion
Dupilumab produced consistent improvement in symptoms of severe CRSwNP irrespective of ECRS status. Therefore, blood eosinophil level may not be a suitable biomarker for dupilumab efficacy in CRSwNP.
Keywords: biologics, ENT (rhinitis, sinusitis, nasal polyps), eosinophils, inflammation, sinusitis
Patients with CRSwNP from SINUS‐52 were classified by eosinophilic status (ECRS subgroup; JESREC criteria). Although moderate/severe ECRS shows greater baseline disease burden than mild/no ECRS, improvements in disease control, symptom burden, sense of smell, and HRQoL with dupilumab versus placebo are unaffected by ECRS status. Eosinophilic status is not a biomarker for dupilumab efficacy in CRSwNP patients meeting SINUS‐52 inclusion criteria. Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; EOS, eosinophil; HRQoL, health‐related quality of life; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LMK‐CT, Lund‐Mackay score assessed by CT; SINUS‐52, controlled clinical study of dupilumab in patients with nasal polyps; SNOT‐22, 22‐item sinonasal outcome test; VAS, visual analog scale.

1. INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic inflammatory disease of the nasal passages and paranasal sinuses associated with a high symptom burden and poor health‐related quality of life (HRQoL). 1 , 2 , 3 , 4 , 5 CRSwNP pathophysiology is predominantly characterized by type 2 inflammation with interleukin (IL)‐4, IL‐13, and IL‐5 as prominent cytokines, and tissue infiltration by eosinophils, lymphocytes, basophils, and mast cells. 2 , 3 , 6 , 7 , 8 , 9
In Japan and East Asia, neutrophil infiltration has been traditionally dominant in CRSwNP. 10 , 11 , 12 , 13 However, in recent years, cases of eosinophilic CRSwNP have increased in these regions. The reasons for this are not entirely clear, but may include environmental factors, changes in diet and hygiene, and the switch from oral to inhaled corticosteroids in the treatment of asthma. 13 , 14 , 15 , 16 It is thought that eosinophilic chronic rhinosinusitis (ECRS) and CRSwNP develop in a Thelper 2‐dominant environment. 3 A study demonstrating that tissue eosinophilia in nasal polyps dramatically increased over a 10–20 year period in the same study cohorts in Asian countries provides support for the involvement of environmental factors, such as air pollution, microbiome, and the use of antibiotics in mucosal inflammatory patterns in patients with chronic rhinosinusitis (CRS). 12
The Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis (JESREC) 14 developed and validated an algorithm that enables diagnosis of populations of patients with eosinophilic CRSwNP currently widely used in Japan, and classification of the severity of eosinophil status is expected to lead to better treatment outcomes. According to this JESREC algorithm, CRSwNP may be classified as “non‐ECRS,” “mild ECRS,” “moderate ECRS,” and “severe ECRS” (see Figure 1 for more detail on the classifications). 14 In addition, according to the JESREC study, 45.2% of Japanese patients with CRS postendoscopic sinus surgery have noneosinophilic (type 2‐low) disease. 14
FIGURE 1.
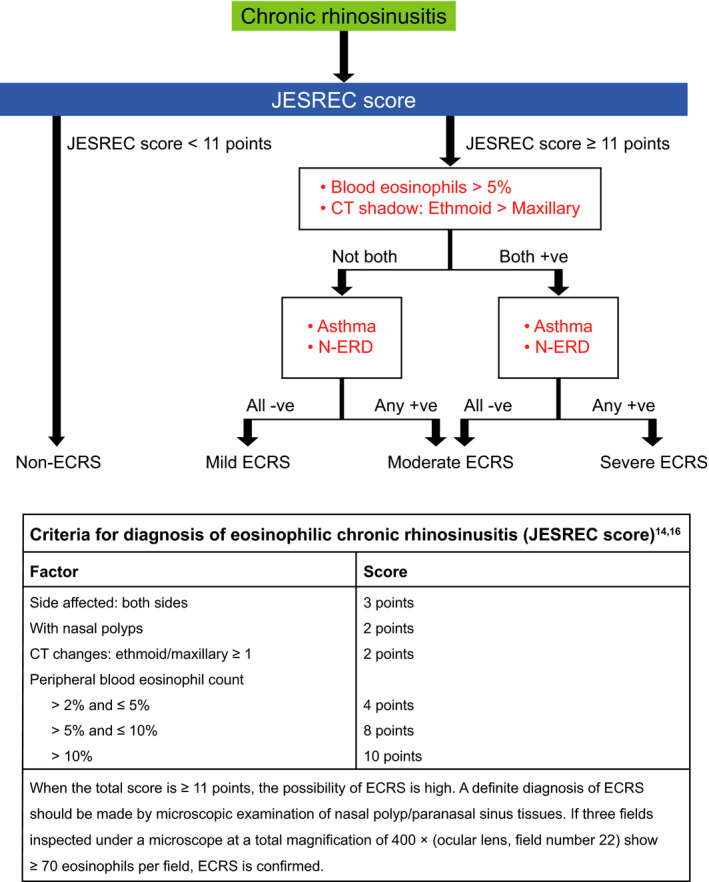
Any treatment arm. ECRS, eosinophilic chronic rhinosinusitis; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; MedDRA PT, Medical Dictionary for Regulatory Activities Preferred Term; q2w, every 2 weeks; q4w, every 4 weeks; TEAE, treatment‐emergent adverse event. Figure 1 JESREC algorithm. CT, computed tomography; ECRS, eosinophilic chronic rhinosinusitis; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; NSAID‐ERD, nonsteroidal antiinflammatory drug‐exacerbated respiratory disease. Figure from: Allergy 75 (2020) 3087–3099 © 2020Asano K, et al. Allergy. Published by John Wiley and Sons Ltd
Severe eosinophilic asthma is also driven by type 2 inflammation and characterized by sputum eosinophilia and mild‐to‐moderate increases in blood eosinophil counts. 17 Targeting IL‐5 with biologic agents for the treatment of severe asthma in patients with elevated peripheral eosinophil counts has been shown to reduce the number of exacerbations. Thus, in the setting of eosinophilic asthma, eosinophilia represents a potential biomarker for biologic therapy. Whether eosinophilic status also represents a biomarker for biologic therapy in CRSwNP has not been established.
Dupilumab is a fully human VelocImmune®‐derived monoclonal antibody that blocks the shared receptor component for IL 4 and IL 13, which are key and central drivers of type 2 inflammation. 18 , 19 , 20 It is approved in Japan as an add‐on maintenance treatment in adult patients with inadequately controlled CRSwNP. In the SINUS‐52 (NCT02898454) phase III study, the coprimary endpoints of changes from baseline to week 24 in nasal polyp score (NPS), nasal congestion/obstruction (NC), and Lund‐Mackay score assessed by CT (LMK‐CT) were met. Dupilumab, on a background of mometasone furoate nasal spray (MFNS) versus MFNS alone (placebo), significantly improved endoscopic, radiographic, clinical, and patient‐reported outcomes in patients with severe CRSwNP refractory to standard‐of‐care therapy. 21 The aim of this study was to determine whether eosinophilic status in CRSwNP, classified using the JESREC algorithm, was a predictor of dupilumab efficacy compared with placebo in patients with severe CRSwNP enrolled in the SINUS‐52 study.
2. METHODS
2.1. Study design
SINUS‐52 was a multinational, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study of dupilumab in patients with severe uncontrolled CRSwNP. The methods for SINUS‐52 have been reported elsewhere. 21 Briefly, adults (≥18 years) with bilateral endoscopic NPS ≥5 with ≥2 for each nostril and ≥2 chronic rhinosinusitis symptoms were randomized 1:1:1 to dupilumab 300 mg subcutaneous (SC) every 2 weeks (q2w) for 52 weeks; dupilumab 300 mg SC q2w for 24 weeks, then 300 mg SC every 4 weeks (q4w) for 28 weeks; or placebo q2w for 52 weeks.
The current analysis is a posthoc analysis of SINUS‐52 that uses the JESREC algorithm to classify patients into non‐ECRS, mild ECRS, moderate ECRS, and severe ECRS subgroups. 14 The cut‐off value for ECRS is a score of 11 points, with a sensitivity of 83% and specificity of 66%. 14 The JESREC algorithm is shown in Figure 1. 22
2.2. Outcome measures
Coprimary efficacy endpoints in SINUS‐52 were change from baseline at week 24 in NPS, NC score of ≥2 of 3 and a weekly average score of ≥1 at randomization (0 = no symptoms, 1 = mild, 2 = moderate, and 3 = severe);LMK‐CT score was a third coprimary endpoint in Japan only. Changes in these parameters from baseline at 52 weeks were secondary endpoints. 21 Additional secondary efficacy endpoints were changes from baseline (weeks 24 and 52) in the 22 item Sinonasal Outcome Test (SNOT‐22) score, patient‐reported Total Symptom Score (TSS; a composite severity score consisting of the sum of daily symptoms of NC, loss of smell, and anterior or posterior rhinorrhea), CRSwNP severity (visual analog scale [VAS]) score, and University of Pennsylvania Smell Identification Test (UPSIT) score. 21 The SNOT‐22 is a validated disease‐specific tool consisting of 22 items evaluating the patient‐perceived health burden of rhinosinusitis. 23 Each item is scored from 0 (no problem) to 5 (worst symptom) with a maximum score of 110 and a change of 8,9 points regarded as the minimally important difference. The VAS is a 10‐point scale capturing patients’ perception of how troublesome their symptoms are, ranging from 0 (not troublesome at all) to 10 (worst thinkable troublesome). A score of 0–3 is classified as mild, >3–7 as moderate, and >7 as severe. 4 , 24
2.3. Statistical methods
For least squares (LS) mean changes from baseline, each of the imputed complete data was analyzed by fitting an analysis of covariance (ANCOVA) model with change from baseline at the corresponding visit as the response variable, and the corresponding baseline value, treatment group, asthma/nonsteroidal antiinflammatory drug‐exacerbated respiratory disease (NSAID‐ERD) status, prior surgery history, and regions as covariates. Data collected after treatment discontinuation were included. Data after systemic corticosteroids or sinonasal surgery were set to missing and imputed by worst observation carried forward, and other missing data were imputed by multiple imputation.
3. RESULTS
3.1. Baseline demographic and disease characteristics
A total of 448 patients were randomized to study treatment in SINUS‐52, but after randomization, one patient was excluded because no study drug was administered, and a further nine patients were excluded because no LMK score was obtained, and thus ECRS severities were not specified. This post hoc analysis was performed using the remaining 438 patients (intention‐to‐treat [ITT] population).
Patients from the ITT population were stratified by ECRS status at baseline according to the JESREC algorithm (Figure 1) 14 365 (83.3%) patients had an ECRS phenotype (mild n = 61 [16.7%], moderate n = 144 [39.5%], and severe n = 160 [43.8%]) and 73 (16.7%) did not (non‐ECRS). Proportions in each subgroup were similar for Japanese patients (n = 49): 40 (81.6%) with ECRS (mild n = 2 [5.0%], moderate n = 18 [45.0%], and severe n = 20 [50.0%]) and nine (18.4%) without ECRS. At baseline, patients tended to have more severe disease characteristics with increasing ECRS severity (Table 1). Statistically significant differences between non‐/mild and moderate/severe ECRS were shown for NPS (p = 0.0266), LMK‐CT score (p < 0.0001), LoS score (p = 0.0009), UPSIT score (p < 0.0001), SNOT‐22 score (p = 0.012) and CRSwNP VAS (p = 0.0175). In the patient subgroups with moderate/severe ECRS, there was a significantly greater proportion of females (p = 0.0013) and significantly higher mean peripheral blood eosinophil count (p < 0.0001) and mean blood immunoglobulin E (IgE) levels (p = 0.0489) compared with those in the non‐/mild ECRS subgroups.
TABLE 1.
Baseline demographic and disease characteristics by JESREC subgroup
| Non‐ECRS (n = 73) | Mild ECRS (n = 61) | Moderate ECRS (n = 144) | Severe ECRS (n = 160) | p value (non‐/mild versus moderate/severe ECRS) a | |
|---|---|---|---|---|---|
| Age, years | 53.10 (12.87) | 54.07 (11.28) | 51.39 (12.21) | 51.46 (12.93) | 0.1022 |
| Male sex, n (%) | 50 (68.5) | 48 (78.7) | 91 (63.2) | 82 (51.3) | 0.0013 |
| Weight, kg | 79.64 (14.74) | 84.98 (17.68) | 81.06 (18.74) | 76.82 (18.39) | 0.0824 |
| Bilateral endoscopic NPS, b range 0–8 | 5.88 (1.25) | 5.98 (1.21) | 6.27 (1.16) c | 6.14 (1.25) d | 0.0266 |
| Daily NC score, b range 0–3 | 2.46 (0.61) | 2.35 (0.62) | 2.41 (0.60) | 2.47 (0.56) | 0.5932 |
| LMK‐CT score, b range 0–24 | 16.44 (4.33) | 15.87 (3.49) | 18.33 (3.33) | 19.11 (3.40) | <0.0001 |
| TSS, b range 0–9 | 7.21 (1.52) | 6.88 (1.50) | 7.13 (1.53) | 7.45 (1.31) | 0.1220 |
| LoSS, b range 0–3 | 2.71 (0.57) | 2.52 (0.63) | 2.75 (0.59) | 2.85 (0.36) | 0.0009 |
| Smell test (UPSIT) score, b range 0–40 | 15.14 (8.96) e | 17.37 (8.90) f | 13.33 (7.78) g | 11.60 (6.53) h | <0.0001 |
| SNOT‐22 total score, b range 0–110 | 47.14 (19.23) | 49.20 (22.10) | 54.06 (21.60) c | 53.05 (20.39) i | 0.0120 |
| CRSwNP severity (VAS) score, b range 0–10 cm | 7.65 (2.23) j | 7.64 (2.18) | 8.22 (2.07) k | 8.12 (1.98) h | 0.0175 |
| Patients with comorbid asthma, n (%) | 28 (38.4) | 0 | 81 (56.3) | 153 (95.6) | <0.0001 |
| Patients with comorbid NSAID‐ERD, n (%) | 14 (19.2) | 0 | 43 (29.9) | 60 (37.5) | <0.0001 |
| Blood eosinophils, Giga/L | 0.13 (0.09) | 0.24 (0.15) | 0.41 (0.31) | 0.67 (0.37) | <0.0001 |
|
Eosinophils ≥150 cells/μl, n (%) |
19 (26.0) | 55 (90.2) | 137 (95.1) | 160 (100) | <0.0001 |
|
Eosinophils ≥300 cells/μl, n (%) |
7 (9.6) | 9 (14.8) | 87 (60.4) | 152 (95.0) | <0.0001 |
|
Eosinophils ≤2%, n (%) |
55 (75.3) | 0 | 0 | 0 | |
|
Eosinophils >2%–≤ 5%, n (%) |
18 (24.7) | 59 (96.7) | 76 (52.8) | 4 (2.5) | |
|
Eosinophils >5%–≤10%, n (%) |
0 | 1 (1.6) | 54 (37.5) | 100 (62.5) | |
|
Eosinophils >10%, n (%) |
0 | 1 (1.6) | 14 (9.7) | 56 (35.0) | <0.0001 |
| Periostin, ng/ml | 91.27 (37.76) | 90.26 (30.71) l | 113.52 (46.89) c | 123.26 (53.47) d | <0.0001 |
| Total IgE, IU/ml | 198.89 (251.85) | 185.38 (268.77) l | 266.83 (428.23) c | 260.09 (319.26) | 0.0489 |
Data are presented as mean (SD) unless otherwise stated.
Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; IgE, immunoglobulin E; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LMK‐CT, Lund‐Mackay score assessed by CT scan; LoSS, loss of sense of smell; NC, nasal congestion; NPS, nasal polyp score; NSAID‐ERD, nonsteroidal antiinflammatory drug‐exacerbated respiratory disease; SD, standard deviation; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
p values were obtained using a t‐test for equality of variance. In cases where the equality of variance assumption was not met, Satterthwaite's p value was calculated. p values were obtained using the chi‐square test. In cases with an expected cell frequency <5, Fisher's exact test was used.
Higher scores indicate greater disease severity, except for UPSIT, where higher scores indicate lower disease severity.
n = 143.
n = 159.
n = 71.
n = 59.
n = 141.
n = 156.
n = 157.
n = 72.
n = 140.
n = 60.
3.2. Efficacy of dupilumab by ECRS subgroup
Dupilumab treatment was associated with significant improvements in each ECRS subgroup (including non‐ECRS) for the coprimary endpoints of change from baseline in NPS, NC, and LMK‐CT scores at weeks 24 and 52, consistent with the overall population of patients with CRSwNP recruited to the SINUS‐52 study. As shown in Figure 2, LS mean (95% confidence interval[CI]) differences with dupilumab 300 mg q2w at 24 weeks were all statistically significantly improved versus placebo for NPS, NC, and LMK‐CT scores across all ECRS subgroups (all p values <0.001). Improvements in NPS, NC, and LMK‐CT scores, with dupilumab 300 mg versus placebo were maintained or had increased through week 52, irrespective of ECRS status (Figure 2 and Figure S1). There was no apparent subgroup‐by‐treatment interaction for any of the primary or secondary outcomes when evaluating the interaction between ECRS subgroup (non‐/mild ECRS or moderate/severe ECRS) and the treatment effect of dupilumab versus placebo (Table 2; p values all >0.05). The only exception was for LMK‐CT at Week 24, where the calculated p value was 0.0275, suggesting a greater effect of dupilumab in the moderate/severe ECRS subgroup compared with the non‐/mild ECRS subgroup for this endpoint.
FIGURE 2.
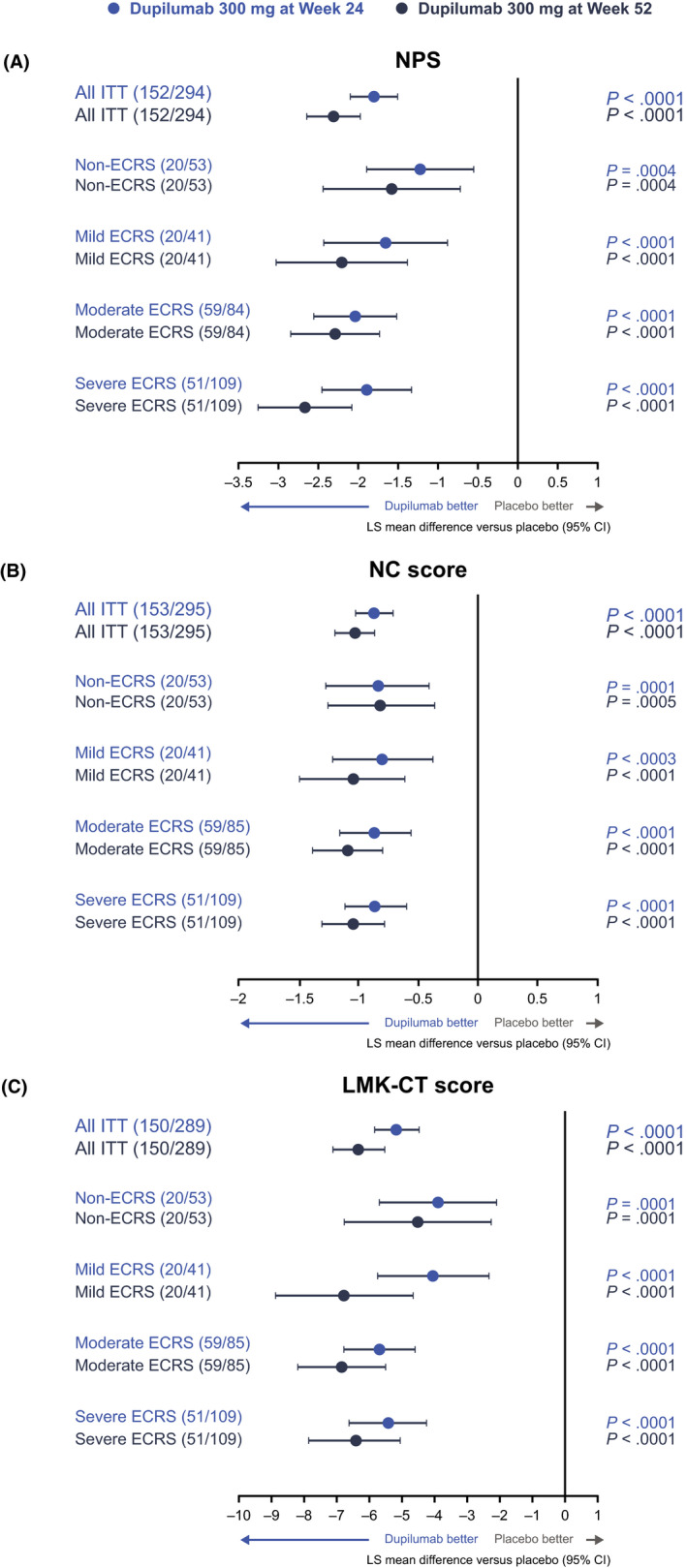
Effect of dupilumab 300 mg versus placebo on change from baseline in symptom scores at weeks 24 and 52 by ECRS subgroup: (A) NPS, (B) NC, and (C) LMK‐CT scores. ECRS subgroups were defined according to the JESREC algorithm (Figure 1). Data for dupilumab 300 mg q2w and q2w–q4w treatment arms are pooled. CI, confidence interval; ECRS, eosinophilic chronic rhinosinusitis; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LMK‐CT, Lund‐Mackay score assessed by CT; LS, least squares; NC, nasal congestion; NPS, nasal polyp score; q2w, every 2 weeks; q4w, every 4 weeks
TABLE 2.
Interaction between ECRS subgroup and dupilumab treatment effect versus placebo at weeks 24 and 52
| Endpoint | LS mean of treatment effect of dupilumab versus placebo (95% CI) | p value | |
|---|---|---|---|
| Non‐/mild ECRS | Moderate/severe ECRS | ||
| Week 24 | |||
| NPS | −1.37 (−1.88, −0.86) | −1.99 (−2.36, −1.61) | 0.0945 |
| NC | −0.79 (−1.09, −0.48) | −0.92 (−1.11, −0.72) | 0.5073 |
| LMK‐CT | −3.82 (−5.07, −2.57) | −5.59 (−6.38, −4.80) | 0.0275 |
| TSS | −1.94 (−2.77, −1.11) | −2.70 (−3.20, −2.20) | 0.1205 |
| UPSIT | 8.45 (5.68, 11.23) | 11.74 (9.83, 13.64) | 0.0692 |
| SNOT‐22 | −14.78 (−21.03, −8.54) | −19.16 (−23.49, −14.83) | 0.2802 |
| CRSwNP VAS | −2.41 (−3.45, −1.36) | −3.28 (−3.90, −2.67) | 0.1462 |
| Week 52 | |||
| NPS | −1.83 (−2.42, −1.23) | −2.50 (−2.90, −2.10) | 0.0911 |
| NC | −0.93 (−1.24, −0.62) | −1.11 (−1.31, −0.91) | 0.3374 |
| LMK‐CT | −5.39 (−6.97, −3.82) | −6.64 (−7.61, −5.67) | 0.1995 |
| TSS | −2.68 (−3.56, −1.80) | −3.32 (−3.83, −2.81) | 0.1768 |
| UPSIT | 8.35 (5.42, 11.28) | 11.63 (9.75, 13.51) | 0.0733 |
| SNOT‐22 | −18.65 (−24.88, −12.42) | −23.92 (−28.20, −19.65) | 0.1676 |
| CRSwNP VAS | −3.24 (−4.32, −2.16) | −3.94 (−4.61, −3.28) | 0.2723 |
ECRS subgroups were defined according to the JESREC algorithm (Figure 1).
Data were analyzed using an ANCOVA model with the corresponding baseline value, treatment group, asthma/N‐ERD status, prior surgery history, and regions (except for the subgroups of Region and Territory) as covariates, plus the subgroup variable and the subgroup‐by‐treatment interaction.
Abbreviations: ANCOVA, analysis of covariance; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LMK‐CT, Lund‐Mackay score assessed by CT; LS, least squares; NC, nasal congestion; NPS, nasal polyp score; NSAID‐ERD, nonsteroidal antiinflammatory drug‐exacerbated respiratory disease; SNOT‐22,22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
Dupilumab treatment was associated with similar improvements in each ECRS subgroup for the secondary endpoints of change from baseline in UPSIT, SNOT‐22, TSS, and CRSwNP severity (VAS) scores at weeks 24 and 52 (Figure 3). For all secondary efficacy endpoints (except SNOT‐22 at week 52 in the non‐ECRS subgroup with dupilumab q2w treatment [p =0.0786]), LS mean (95% CI) differences to weeks 24 and 52 were significantly greater with dupilumab 300 mg q2w and q2w–q4w than placebo (p values ≤0.0271) across ECRS subgroups.
FIGURE 3.
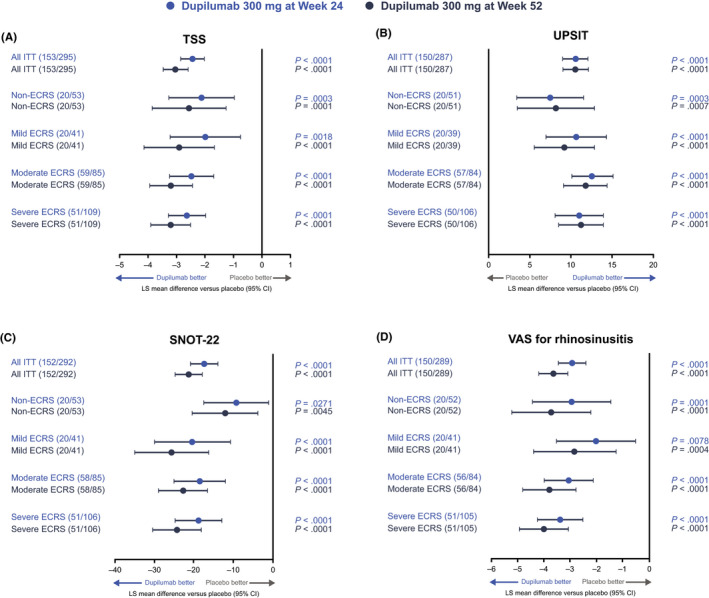
Effect of dupilumab 300 mg versus placebo on changes from baseline in secondary efficacy endpoints at weeks 24 and 52 by ECRS subgroup: (A) TSS, (B) UPSIT, (C) SNOT‐22, and (D) CRSwNP severity (VAS) scores. ECRS subgroups were defined according to the JESREC algorithm (Figure 1). Data for dupilumab 300 mg q2w and q2w–q4w treatment arms are pooled. Higher scores indicate greater disease severity, except for UPSIT, where higher scores indicate lower disease severity. CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LS, least squares; q2w, every 2 weeks; q4w, every 4 weeks; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale
3.3. Effect of dupilumab on eosinophils
A subanalysis was conducted to determine the effect of dupilumab on blood eosinophil counts in each ECRS subgroup. As eosinophil count was a safety analysis item, the data were handled using last observation carried forward, and the interaction between placebo and dupilumab in each ECRS severity subgroup was examined using ANCOVA. Overall, no significant interaction was observed (p = 0.06).
3.4. Safety of dupilumab by ECRS subgroup
Dupilumab was generally well tolerated over 52 weeks across JESREC subgroups, with a safety profile similar to that observed among the overall ITT population (Table 3). The number of serious treatment‐emergent adverse events (TEAEs) reported by patients receiving dupilumab 300 mg q2w and q2w–q4w was low with no trends observed across ECRS subgroups. There was only one death reported, which occurred in the dupilumab 300 mg q2w–q4w group, which was deemed unrelated to the study drug and has been reported previously. 20 Nasopharyngitis was the most frequently reported TEAE in the non‐, moderate, and severe ECRS subgroups (non‐ECRS: dupilumab q2w 15% and dupilumab q2w–q4w 33% versus placebo 10%; moderate ECRS: 22% and 23% versus 24%; severe ECRS: 19% and 20% versus 35%) and sinusitis was the most frequent (17% and 17% versus 5%)in the mild ECRS subgroup.
TABLE 3.
Summary of adverse events over 52 weeks by ECRS subgroup
| ITT | Non‐ECRS | Mild ECRS | Moderate ECRS | Severe ECRS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 150) | Dupilumab | Placebo (n = 20) | Dupilumab | Placebo (n = 20) | Dupilumab | Placebo (n = 58) | Dupilumab | Placebo (n = 49) | Dupilumab | ||||||
| 300 mg q2w (n = 149) | 300 mg q2w–q4w (n = 148) | 300 mg q2w (n = 26) | 300 mg q2w–q4w (n = 27) | 300 mg q2w (n = 23) | 300 mg q2w–q4w (n = 18) | 300 mg q2w (n = 41) | 300 mg q2w–q4w (n = 44) | 300 mg q2w (n = 57) | 300 mg q2w–q4w (n = 54) | ||||||
| Any TEAE | 136 (90.7) | 124 (83.2) | 132 (89.2) | 15 (75.0) | 20 (76.9) | 24 (88.9) | 17 (85.0) | 19 (82.6) | 17 (94.4) | 53 (91.4) | 34 (82.9) | 40 (90.9) | 48 (98.0) | 49 (86.0) | 47 (87.0) |
| Any serious TEAE | 15 (10.0) | 8 (5.4) | 10 (6.8) | 1 (5.0) | 2 (7.7) | 3 (11.1) | 2 (10.0) | 3 (13.0) | 1 (5.6) | 3 (5.2) | 1 (2.4) | 2 (4.5) | 8 (16.3) | 2 (3.5) | 3 (5.6) |
| Any TEAE leading To death | 0 | 0 | 1 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.3) | 0 | 0 | 0 |
| Any TEAE leading to permanent treatment discontinuation | 17 (11.3) | 6 (4.0) | 2 (1.4) | 2 (10.0) | 0 | 1 (3.7) | 3 (15.0) | 2 (8.7) | 0 | 4 (6.9) | 2 (4.9) | 0 | 7 (14.3) | 2 (3.5) | 1 (1.9) |
| TEAEs occurring in ≥5% of patients in the ITT populationa (MedDRA PT) | |||||||||||||||
| Nasopharyngitis | 36 (24.0) | 30 (20.1) | 31 (20.9) | 2 (10.0) | 4 (15.4) | 9 (33.3) | 3 (15.0) | 6 (26.1) | 1 (5.6) | 14 (24.1) | 9 (22.0) | 10 (22.7) | 17 (34.7) | 11 (19.3) | 11 (20.4) |
| Upper respiratory tract infection | 19 (12.7) | 10 (6.7) | 8 (5.4) | 3 (15.0) | 2 (7.7) | 1 (3.7) | 1 (5.0) | 2 (8.7) | 2 (11.1) | 10 (17.2) | 2 (4.9) | 2 (4.5) | 4 (8.2) | 4 (7.0) | 3 (5.6) |
| Bronchitis | 8 (5.3) | 9 (6.0) | 9 (6.1) | 1 (5.0) | 2 (7.7) | 1 (3.7) | 0 | 1 (4.3) | 0 | 3 (5.2) | 5 (12.2) | 4 (9.1) | 4 (8.2) | 1 (1.8) | 4 (7.4) |
| Sinusitis | 17 (11.3) | 8 (5.4) | 13 (8.8) | 3 (15.0) | 0 | 1 (3.7) | 1 (5.0) | 4 (17.4) | 3 (16.7) | 10 (17.2) | 2 (4.9) | 5 (11.4) | 3 (6.1) | 2 (3.5) | 4 (7.4) |
| Headache | 18 (12.0) | 14 (9.4) | 16 (10.8) | 2 (10.0) | 3 (11.5) | 2 (7.4) | 3 (15.0) | 2 (8.7) | 3 (16.7) | 8 (13.8) | 2 (4.9) | 6 (13.6) | 4 (8.2) | 6 (10.5) | 4 (7.4) |
| Nasal polyps | 25 (16.7) | 8 (5.4) | 15 (10.1) | 1 (5.0) | 4 (15.4) | 1 (3.7) | 3 (15.0) | 0 | 0 | 12 (20.7) | 2 (4.9) | 5 (11.4) | 8 (16.3) | 2 (3.5) | 9 (16.7) |
| Epistaxis | 20 (13.3) | 13 (8.7) | 7 (4.7) | 3 (15.0) | 4 (15.4) | 1 (3.7) | 1 (5.0) | 1 (4.3) | 2 (11.1) | 12 (20.7) | 5 (12.2) | 1 (2.3) | 4 (8.2) | 3 (5.3) | 2 (3.7) |
| Cough | 8 (5.3) | 9 (6.0) | 9 (6.1) | 0 | 0 | 3 (11.1) | 1 (5.0) | 3 (13.0) | 1 (5.6) | 4 (6.9) | 2 (4.9) | 1 (2.3) | 3 (6.1) | 3 (5.3) | 3 (5.6) |
| Asthma | 19 (12.7) | 6 (4.0) | 13 (8.8) | 3 (15.0) | 0 | 1 (3.7) | 1 (5.0) | 0 | 0 | 7 (12.1) | 2 (4.9) | 3 (6.8) | 8 (16.3) | 4 (7.0) | 9 (16.7) |
| Injection‐site erythema | 11 (7.3) | 11 (7.4) | 10 (6.8) | 3 (15.0) | 3 (11.5) | 2 (7.4) | 1 (5.0) | 0 | 1 (5.6) | 2 (3.4) | 4 (9.8) | 3 (6.8) | 5 (10.2) | 3 (5.3) | 4 (7.4) |
| Injection‐site reaction | 3 (2.0) | 5 (3.4) | 8 (5.4) | 0 | 1 (3.8) | 1 (3.7) | 1 (5.0) | 0 | 3 (16.7) | 1 (1.7) | 2 (4.9) | 2 (4.5) | 1 (2.0) | 2 (3.5) | 1 (1.9) |
Values are n (%). ECRS subgroups were defined according to the JESREC algorithm.
Abbreviations: ECRS, eosinophilic chronic rhinosinusitis; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; MedDRA PT, Medical Dictionary for Regulatory Activities Preferred Term; q2w, every 2 weeks; q4w, every 4 weeks; TEAE, treatment‐emergent adverse event.
Any treatment arm.
4. DISCUSSION
Various factors have been proposed to explain the increasing prevalence of eosinophilic CRSwNP in Japan. 13 , 14 , 15 , 16 Changes in diet and nutrition may be involved, although there is no direct evidence for this. Fermented food may regulate coagulation and fibrinolysis in nasal mucosa. Increased consumption of fermented foods, along with environmental changes, may have led to alterations to the bacterial flora in the mouth, nasal cavity, and intestine, resulting in an increase of patients with ECRS among the Japanese population, although the precise causative mechanisms have not been established. 13 , 16 Oral corticosteroids were traditionally used to treat patients with moderate or severe asthma in Japan, but following publication of management guidelines, these have largely been replaced by inhaled corticosteroids. 25 Patients with ECRS often have asthma, including aspirin/nonsteroidal antiinflammatory drug‐induced asthma, and oral glucocorticosteroids (GCS) are more effective than topical GCS at suppressing blood eosinophil counts. Consequently, the reduction in the use of oral corticosteroids in asthma treatment may have contributed to the increase in ECRS, as the secondary benefit of oral corticosteroids among individuals with comorbid asthma has been lost. 16
In cases of CRSwNP with strong eosinophilic infiltration, nasal polyps often recur after sinus surgery. Eosinophilic CRSwNP was, therefore, described as ECRS, to differentiate it from CRSwNP that responds well to standard approaches to treatment. 14 According to the JESREC scoring system and algorithm for classifying chronic rhinosinusitis, cases of moderate‐to‐severe ECRS are considered intractable disease (Figure 1). 14 Consistent with previous studies showing that peripheral blood eosinophilia can be considered a biomarker for severe intractable disease, 26 , 27 , 28 , 29 in the current study, patients tended to have more severe disease characteristics with increasing ECRS severity at baseline. Previous reports have also observed that patients with moderate and severe ECRS were predominantly male, and had higher peripheral blood eosinophil count and blood IgE levels compared with those classified as having mild and non‐ECRS. 29 ECRS subtypes based on JESREC scores and mucosal eosinophil counts showed different inflammatory patterns, supporting use of the JESREC score and mucosal eosinophil counts in predicting ECRS endotypes. 30
Dupilumab has been shown to improve NPS, LMK‐CT score, nasal symptoms, quality‐of‐life scores, and olfactory function. 21 We report that dupilumab treatment led to significant improvements in coprimary and secondary endpoints associated with CRSwNP severity across all ECRS and non‐ECRS subgroups classified by the JESREC algorithm, which increased or were maintained for up to 52 weeks of treatment. This represents consistent improvement in objective (NPS and LMK‐CT) and subjective (NC, loss of smell, and SNOT‐22) assessment of CRSwNP disease control irrespective of ECRS status in the SINUS‐52 study. Improvement was evident at the time of first assessment in all disease scores and increased through subsequent assessments. In addition, the treatment effect of dupilumab was similar across all ECRS subgroups, even though baseline disease burden was higher in the moderate and severe subgroups classified as intractable disease. The statistical analysis indicated no significant interaction between dupilumab treatment effect and ECRS subgroup across all endpoints, with the single exception of LMK‐CT at week 24, where there was a suggestion of an increased dupilumab treatment effect in the moderate/severe ECRS subgroups versus the non‐/mild ECRS subgroups. Based on inclusion criteria, all patients had a baseline JESREC score of 5–7(bilateral disease, nasal polyps, and ethmoid opacification [Figure 1]), and therefore percentage blood eosinophils was the main distinguishing factor in the present study. All patients defined as non‐ECRS by the JESREC algorithm had ≤5% eosinophils in the peripheral blood (approximately equivalent to ≤300 cells/μl) at baseline (Table 1). A type 2 inflammatory phenotype in patients without asthma, but with blood eosinophils >300 cells/μl, is highly likely in mild ECRS. 31 , 32 Moreover, there are data to suggest that the proportion of patients with non‐ECRS driven predominantly by neutrophilic inflammation may be higher in Japanese populations than in Western populations in which ECRS predominates, although there is evidence that eosinophilic inflammation is on the rise in Japan and Southeast Asia. 16 , 33 As such, in the current study, the non‐ECRS subgroup likely included patients with and without a type 2 inflammatory phenotype. Therefore, improvements with dupilumab were observed in patients from SINUS‐52 with severe symptoms of CRSwNP as defined by an NPS ≥5, including those defined as non‐ECRS by the JESREC algorithm, irrespective of blood eosinophils. Although peripheral blood eosinophil count plays a role as one of the biomarkers for CRS classification, it does not have a dominant correlation with dupilumab efficacy in CRSwNP. By blocking the shared receptor component for IL 4 and IL 13 and inhibiting signaling pathways of both IL 4 and IL 13, key and central drivers of the type 2 inflammation underlying CRSwNP, dupilumab has a broad effect in several cells and cytokines involved the inflammatory process at the origin of CRSwNP.
Dupilumab was generally well tolerated in patients with severe CRSwNP, irrespective of ECRS status, in line with the overall population of patients recruited to the SINUS‐52 study. Consistent with the overall ITT population, the number of adverse events reported with dupilumab was similar to or lower than those reported with placebo.
These analyses were carried out in the overall ITT population because of the small sample size of the Japanese population (n = 49) in SINUS‐52. By using this approach, and classifying patients using an algorithm designed for the Japanese population, we were able to robustly study the effects of eosinophilic disease on efficacy and safety outcomes with dupilumab treatment, aiding understanding of the likely effects in Japanese patients. Improvements with dupilumab versus placebo in each ECRS subgroup were similar between ITT and Japanese populations (data not shown). Multiple imputation methods were applied for the missing values. The use of this approach results in less biased findings when dealing with missing covariate data. A separate subanalysis of Japanese patients enrolled in the SINUS‐52 study demonstrated that the efficacy of dupilumab on the primary measures of change from baseline in NPS, NP, and LMK‐CT scores at 24 weeks was consistent with the overall ITT population. 34
5. CONCLUSIONS
In patients with severe, uncontrolled CRSwNP, dupilumab as an add‐on to MFNS improved disease control, symptom burden, sense of smell, and HRQoL across all ECRS subgroups defined by the JESREC algorithm, consistent with the overall population of patients recruited to the SINUS‐52 study. Dupilumab was generally well tolerated across ECRS subgroups. These results enhance understanding of dupilumab treatment benefit, with improvements shown irrespective of eosinophilic disease, and suggest that blood eosinophil level may not be a suitable biomarker for dupilumab efficacy in CRSwNP with NPS ≥5.
CONFLICT OF INTEREST
Fujieda S: AstraZeneca, GlaxoSmithKline, Kyowa Hakko Kirin, and Sanofi – advisory board member; Kyorin, Mitsubishi Tanabe Pharma, and Taiho Pharma – speaker fees. Matsune S: Sanofi – advisory board member; Kyorin, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Sanofi, Taiho Pharma, and Tanabe Pharma – speaker fees. Takeno S: Sanofi – advisory board member; Sanofi – speaker fees. Ohta N: Mitsubishi Tanabe Pharma, Sanofi, and Taiho Pharma– speaker fees. Asako M: AstraZeneca, Sanofi, and Taiho Pharma – speaker fees. Bachert C: ALK, ASIT Biotech, AstraZeneca, Intrexon Actobiotics, Novartis, Sanofi, Stallergenes Greer – advisory board member. Inoue T, Takahashi Y, Fujita H, Rowe P, Li Y, Mannent LP: Sanofi – employees, may hold stock and/or stock options in the company. Deniz Y, Ortiz B: Regeneron Pharmaceuticals, Inc. – employees and shareholders.
Supporting information
Fig S1
ACKNOWLEDGMENTS
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT02898454 (SINUS‐52). Medical writing/editorial assistance provided by Zach Dixon, PhD, of Adelphi Group, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Fujieda S, Matsune S, Takeno S, et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS‐52 is unaffected by eosinophilic status. Allergy. 2022;77:186–196. 10.1111/all.14906
REFERENCES
- 1. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN) rhinosinusitis cohort: a large European cross‐sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32‐42. [DOI] [PubMed] [Google Scholar]
- 2. Workman AD, Kohanski MA, Cohen NA. Biomarkers in chronic rhinosinusitis with nasal polyps. Immunol Allergy Clin North Am. 2018;38:679‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin‐exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192:682‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1‐464. [DOI] [PubMed] [Google Scholar]
- 5. Klonaris D, Doulaptsi M, Karatzanis A, Velegrakis S, Milioni A, Prokopakis E. Assessing quality of life and burden of disease in chronic rhinosinusitis: a review. Rhinology Online. 2019;2:6‐13. [Google Scholar]
- 6. Boztepe F, Ural A, Paludetti G, De Corso E. Pathophysiology of chronic rhinosinusitis with nasal polyps. In: Cingi C, Bayar Muluk N, eds. All Around the Nose. Cham: Springer; 2020:333‐337. [Google Scholar]
- 7. Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro‐inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah SA, Ishinaga H, Takeuchi K. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm (Lond). 2016;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsunaga K, Katoh N, Fujieda S, Izuhara K, Oishi K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol Int. 2020;69:187‐196. [DOI] [PubMed] [Google Scholar]
- 10. Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(484):e1‐e2. [DOI] [PubMed] [Google Scholar]
- 11. Nakayama T, Yoshikawa M, Asaka D, et al. Mucosal eosinophilia and recurrence of nasal polyps–new classification of chronic rhinosinusitis. Rhinology. 2011;49:392‐396. [DOI] [PubMed] [Google Scholar]
- 12. Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17‐year period. Otolaryngol Head Neck Surg. 2013;149:431‐437. [DOI] [PubMed] [Google Scholar]
- 13. Takabayashi T, Schleimer RP. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J Allergy Clin Immunol. 2020;145:740‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344‐1353. [DOI] [PubMed] [Google Scholar]
- 16. Fujieda S, Imoto Y, Kato Y, et al. Eosinophilic chronic rhinosinusitis. Allergol Int. 2019;68:403‐412. [DOI] [PubMed] [Google Scholar]
- 17. Brussino L, Heffler E, Bucca C, et al. Eosinophils target therapy for severe asthma: critical points. Biomed Res Int. 2018;2018:758205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111:5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111:5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425‐437. [DOI] [PubMed] [Google Scholar]
- 21. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394:1638‐1650. [DOI] [PubMed] [Google Scholar]
- 22. Asano K, Ueki S, Tamari M, Fujieda S, Imoto Y, Taniguchi M. Adult‐onset eosinophilic airway diseases. Allergy. 2020;75:3087‐3099. [DOI] [PubMed] [Google Scholar]
- 23. Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22‐item sinonasal outcome test. Clin Otolarynol. 2009;34:447‐454. [DOI] [PubMed] [Google Scholar]
- 24. Lim M, Lew‐Gor S, Darby Y, et al. The relationship between subjective assessment instruments in chronic rhinosinusitis. Rhinology. 2007;45:144‐147. [PubMed] [Google Scholar]
- 25. Makino S, Miyamoto T, Nakajima S, et al. Survey of recognition and utilization of guidelines for the diagnosis and management of bronchial asthma in Japan. Allergy. 2000;55:135‐140. [DOI] [PubMed] [Google Scholar]
- 26. Matsuwaki Y, Ookushi T, Asaka D, et al. Chronic rhinosinusitis: Risk factors for the recurrence of chronic rhinosinusitis based on 5‐year follow‐up after endoscopic sinus surgery. Int Arch Allergy Immunol. 2008;146:77‐81. [DOI] [PubMed] [Google Scholar]
- 27. Sakuma Y, Ishitoya J, Komatsu M, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2011;38:583‐588. [DOI] [PubMed] [Google Scholar]
- 28. Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010;59:239‐245. [DOI] [PubMed] [Google Scholar]
- 29. Hu Y, Cao PP, Liang GT, Cui YH, Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122:498‐503. [DOI] [PubMed] [Google Scholar]
- 30. Nakayama T, Sugimoto N, Okada N, et al. JESREC score and mucosal eosinophilia can predict endotypes of chronic rhinosinusitis with nasal polyps. Auris Nasus Larynx. 2019;46:374‐383. [DOI] [PubMed] [Google Scholar]
- 31. Bachert C, Gevaert P, Corren J, et al. Omalizumab improves outcomes in patients with chronic rhinosinusitis with nasal polyps irrespective of asthma status. J Allergy Clin Immunol. 2020;145(2):13‐16. [Google Scholar]
- 32. Cardell LO, Stjärne P, Jonstam K, Bachert C. Endotypes of chronic rhinosinusitis: impact on management. J Allergy Clin Immunol. 2020;145:752‐756. [DOI] [PubMed] [Google Scholar]
- 33. Katotomichelakis M, Tantilipikorn P, Holtappels G, et al. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013;27:354‐360. [DOI] [PubMed] [Google Scholar]
- 34. Fujieda S, Matsune S, Takeno S, et al. The effect of dupilumab on intractable chronic rhinosinusitis with nasal polyps in Japan. Laryngoscope. 2021;131:E1770‐E1777. 10.1002/lary.29230 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


