Abstract
Early childhood is a sensitive period for learning and social skill development. The maturation of cerebral regions underlying social processing lays the foundation for later social‐emotional competence. This study explored myelin changes in social brain regions and their association with changes in parent‐rated social‐emotional development in a cohort of 129 children (64 females, 0–36 months, 77 White). Results reveal a steep increase in myelination throughout the social brain in the first 3 years of life that is significantly associated with social‐emotional development scores. These findings add knowledge to the emerging picture of social brain development by describing neural underpinnings of human social behavior. They can contribute to identifying age‐/stage‐appropriate early life factors in this developmental domain.
Abbreviations
- ACC
Anterior cingulate cortex
- AIC
Akaike information criterion
- ASQ:SE‐2
Ages and Stages Questionnaire: Social‐Emotional, 2nd edition
- dlPFC
dorsolateral prefrontal cortex
- EEG
electroencephalography
- ERP
event‐related potential
- FDR
False Discovery Rate
- HPA
Hypothalamic–pituitary–adrenal
- IFG
inferior frontal gyrus
- MCR
multicomponent relaxometry
- mPFC
medial prefrontal cortex
- MRI
Magnetic Resonance Imaging
- MWF
myelin water fraction
- NICU
neonatal intensive care unit
- SE
social‐emotional
- SPGR
spoiled gradient
- SSE
sum of square error
- SSFP
steady‐state‐free precession
- STS
Superior temporal sulcus
- ToM
theory of Mind
- TPJ
temporoparietal junction
Along with sensorimotor and cognitive‐language functions, social‐emotional (SE) development is one of the primary domains of healthy child development (Walker et al., 2007). SE development generally refers to the ability to express, recognize, and control one’s own feelings as well as to accurately read and respond to the feelings of others. Key contributors to SE processing, therefore, include sociability, autonomy, attachment, self‐regulation, empathy, and morality (Chen et al., 2018; Scharf et al., 2016; Squires et al., 2002). SE skills are foundational to the development of cognition, language, and adaptive life skills, and are fundamental in shaping our social lives. They begin to emerge during infancy with the infant’s bonding to parents or caregivers, and gradually mature through childhood and adolescence (Case‐Smith, 2013). SE skills in early childhood, thus, lay the foundation for later competencies and benefits, and have been linked to school readiness and the ability to adapt in school, academic skills, emotional well‐being, mental health, and ethical decision making (Alducin et al., 2014; Denham & Brown, 2010; Huber et al., 2019; Landy, 2009; National Scientific Council on the Developing Child, 2004). When mature, these social abilities facilitate the interaction and cooperation with others across different life domains and contribute to perspective‐taking, justice, and morality (Chen et al., 2018).
Developmental stages of social‐emotional development
Children develop social‐emotional skills with increasing complexity from birth onwards allowing for increasing autonomy, self‐regulation, and relationships. Denham et al., (2009) propose social competence, attachment, emotional competence, self‐perceived competence, and temperament/personality as key domains in which developmental tasks or milestones are accomplished. In the first 6 months, infants learn to detect as well as attend to faces, biological motion and eye‐gaze (Missana et al., 2015), they form attachment bonds with adults (Denham et al., 2009) and start smiling spontaneously in response to pleasurable sensory input (Scharf et al., 2016). From 6 to 12 months, infants begin to discriminate between emotions from faces, voices, and bodies and display tendencies of joint attention (Grossman, 2013; Grossmann et al., 2007; Missana et al., 2014) and start using sounds to get attention (Scharf et al., 2016). From 12 to 36 months, toddlers learn to understand others’ beliefs, intentions, show prosocial tendencies, and develop complex emotions such as empathy (Hoffman, 1977; Vaish et al., 2010; Warneken & Tomasello, 2007). Toddlers can initiate play with age mates and play alongside them. Their capacity to self‐sooth evolves with adult assistance and the expression of more social emotions like shame and empathy emerge (Denham et al., 2009; Scharf et al., 2016). At around 4 years of age, children learn to predict the actions of others, prosocial behaviors, and interactions as well as friendships emerge. Temperament begins to differentiate into personality (Denham et al., 2009; Scharf et al., 2016). Attainment of these milestones and developmental tasks is critical as deviations could result in developmental difficulties.
Assessment of social‐emotional development in infants and toddlers
SE development, like other domains of child development, can be assessed and monitored behaviorally using clinical interviews, direct behavior observations, and rating systems as well as parent‐ and teacher‐report questionnaires. Relevant SE dimensions for developmental assessments typically include social competence, attachment, emotional competence, self‐perceived competence, and temperament (Denham et al., 2009). Age‐appropriate measures for very young children include the Minnesota Preschool Affect Checklist as an observational measure as well as the Infant‐Toddler Social‐Emotional Assessment, the Brief Infant‐Toddler Social‐Emotional Assessment, and the Ages and Stages Questionnaire: Social‐Emotional as parent‐ and teacher‐rated questionnaires (for an overview of measures see Denham et al., 2009; McCrae & Brown, 2018). The latter assesses SE health across seven key social‐emotional areas: self‐regulation, compliance, social‐communication, adaptive functioning, autonomy, affect, and interaction with people. It has been widely used in research studies, particularly for SE development screenings (e.g., Briggs et al., 2012; McCrae & Brown, 2018).
At the level of the brain, human SE development has been studied using neuroimaging techniques to identify neural substrates of SE skills.
Neural substrates of the social brain
Social skills and behaviors evolve progressively from basic to complex behaviors and mature alongside underlying physiological systems that provide important drivers for SE development. Those systems include the maturation of the autonomous nervous system, which regulates stress and emotional responses in a social context (Porges & Furman, 2011), the hypothalamic–pituitary–adrenal (HPA) axis, which integrates environmental information and the downstream release of glucocorticoids to cause adaptive behavioral responses to stimuli (Rubenstein & Hofmann, 2015), or the development of brain areas and connections involved in social information processing and coordinating social behaviors (Crafa, 2015; Soto‐Icaza et al., 2015).
Neuroanatomically, social behaviors are associated with neural circuits that comprise regions involved in understanding and interacting with others—the “social brain.” These regions include the superior temporal sulcus (STS) involved in recognition of others; anterior cingulate cortex (ACC) involved in emotion and response selectivity; medial prefrontal cortex (mPFC); temporoparietal junction (TPJ) involved in knowledge of self and others and thinking about mental states; inferior frontal gyrus (IFG) involved in self‐other mapping; and the anterior insula and amygdala, both specialized emotion areas (Blakemore, 2008; Crafa, 2015; Sherwin et al., 2019). These social brain areas have been reported consistently for children, adolescents and adults in functional neuroimaging studies, as well as identified through lesion studies in patient populations (Blakemore, 2010, 2012). For example, neuroimaging studies investigating fairness, trust, and reciprocity considerations in social decision making in children and adolescents have highlighted the involvement of the anterior mPFC in decision‐making processes in which one evaluates other and self‐related goals; the dorsolateral prefrontal cortex (dlPFC) in rejection of unfair offers; and the insula in social norm violations (Güroğlu et al., 2009). In young adults, the mPFC has been shown to underly both the perception of social communication indicated by facial expressions and the feeling of personal involvement indicated by eye gaze, with a differential contribution ventral and dorsal parts of the mPFC to social cognition. Activation of the ventral mPFC has been shown during the analysis of social content, while the dorsal mPFC is active in the detection of self‐relevance (Schilbach et al., 2006).
Precursors of the social brain emerge in infancy
Very few studies have investigated the precursors of social brain development during infancy, a developmental stage that typically spans the first year of life. However, research suggests that already the first months of life may play a critical role in laying the foundation for later social skills. During infancy, and thus earlier than often assumed, important neurobiological as well as behavioral precursors of the social brain emerge that are critical for successfully navigating complex social environments and relationships in later childhood as well as adult life. These neurobiological and behavioral precursors of the social brain start appearing in the first year of life with the infant’s ability to process faces, emotions, biological motion and to engage in joint attention (Grossmann & Johnson, 2007). Infants develop the capacity to perceive and discriminate between negative and positive emotions from faces, voices, and body expressions at around 7 months (Grossman, 2013; Grossmann et al., 2007; Missana et al., 2014). To show the relevance of those early life milestones for later behaviors Grossmann et al., (2018) conducted a longitudinal study which demonstrated a link between infants’ attention to fearful faces at 7 months and their altruistic abilities during toddlerhood at 14 months. More specifically, infants that looked longer at fearful faces at 7 months showed an increase in helping behaviors at 14 months. For the origins of empathy, morality and justice, Chen et al., (2018) summarize a developmental trajectory from early life distress perception in infants, as shown by the infant’s brain capacity to discriminate distressful and threat‐related voices from emotionally neutral sounds already in the first months of life, to affective arousal and emotional sharing, a basic form of empathy, and to harm sensitivity, inequity aversion, and fairness in young children. Those basic forms of empathy, sympathy, and fairness are early precursors of later empathy and pro‐social behaviors and appear to arise much earlier during development than previously concluded (Chen et al., 2018).
Emerging evidence on infants’ neural correlates of processing social information, mainly based on electroencephalography (EEG)/event‐related potential (ERP) studies, supports the idea of early life precursors of the human social brain as well as the assumption of a functional connectedness of those regions emerging during infancy, for example, related to face, gaze, emotion, biological motion, action, and joint attention (Grossman & Johnson, 2007).
The still limited findings from research in infants indicate that the human brain is fundamentally adapted to develop within a social context and most parts of the social brain can be activated in infants, still displaying differences to adults related to specialization, localization, and functional differentiation. However, the early functioning of cortical structures involved in perceiving other humans, preferential attention to conspecifics and their interactions in newborns allows for necessary input to developing related cortical circuitry over the first few months of life. With experience, social brain structures become more differentiated and specialized, resulting in the specialized activation patterns observed in the adult social brain (Grossmann & Johnson, 2007).
Social brain networks become functionally distinct in toddlers and young children
Evidence from functional neuroimaging studies provides further understanding of the developmental dynamics of the social brain across childhood and adolescence. A functional Magnetic Resonance Imaging (fMRI) study in N = 122 3‐ to 12‐year‐old children, including 65 children between 3 and 6 years of age, reports cortical networks involved in reasoning about others’ minds (Theory of Mind [ToM] network) and bodies (pain network) to be functionally distinct by age 3 and to be related to behavioral changes in ToM reasoning ability. The authors considered bilateral TPJ, precuneus, and dorso‐, middle‐, and ventromedial prefrontal cortex as ToM brain regions as well as bilateral medial frontal gyrus, insula, and secondary sensory cortex, and dorsal anterior middle cingulate cortex as pain network. An increase in functional specialization was demonstrated to occur throughout childhood as well as an anti‐correlation in older children and adults between the two networks (Richardson et al., 2018).
In adolescents, network activation closely resembles that of adults while differences in activation dominance are still present, shifting from anterior areas, including the anterior mPFC in children and adolescents to more posterior areas, such as STS and TPJ in adults (Blakemore, 2008; Güroğlu et al., 2009). Heightened activation in the mPFC in children has been shown, for example, in response to social evaluation, particularly to negative social feedback, in 7‐ to 10‐year‐old children suggesting social motivation to already be highly salient in middle childhood (Achterberg et al., 2017). Ten to 12‐year‐old children still showed increased activity in the subgenual ACC in the context of social exclusion, an area associated with negative affective processing, compared to adolescents who in turn show no differences to young adults (Moor et al., 2012). The decrease in activity between adolescence and adulthood is often suggested as being due to developmental reductions in gray matter volume, presumably related to synaptic pruning (Blakemore, 2008, 2010). Functional connectivity findings further support late childhood as an age stage by which the social brain network organization and its functional architecture are in place. No age‐related difference in social brain network organization was found, for example, in a recent study of N = 50 8‐ to 16‐year‐old participants. Study results moreover demonstrated that social brain regions not only show strong functional relationships between each other but also interface with non‐social regions (McCormick et al., 2018).
Those functional changes in childhood and adolescence parallel structural changes in the social brain. A longitudinal MRI study of N = 288 participants between 7 and 30 years of age highlights developmental changes in the structure of the social brain. Participants underwent at least two MRI scans approximately 2 years apart. Regions of interest in that study were medial Brodmann Area 10 (mBA10), TPJ, posterior STS, and anterior temporal cortex. Results show a decrease in gray matter volume and cortical thickness in mPFC, TPJ, and posterior STS from late childhood into the early twenties, an increase in gray matter volume until adolescence and in cortical thickness until early adulthood in the anterior temporal cortex, and a peak in surface area trajectories for each region in early or pre‐adolescence before decreasing into the early twenties (Mills et al., 2014). The study did not investigate the link of the investigated structures to social skills, nor the link between the developmental structural changes and the evolution of social skills in that age group. It yet provides an important contribution to the understanding of the structural development of the social brain.
The role of myelination in the developing brain
Myelination is a developmental process ensuring coordinated communication between brain cells and networks and is both a cornerstone of human structural neurodevelopment as well as an expression of the functional maturity of the brain (Barkovich, 2005; Dean et al., 2014). Beginning in utero in the cerebellum and brain stem and continuing into the Second and third decades of life (Bartzokis et al., 2010), myelination follows a characteristic pattern from deep to superficial brain regions and a posterior‐to‐anterior arc. The protracted nature of myelination imparts a high degree of plasticity to developing neural systems. However, the protracted development also poses a prolonged risk of injury or insult (Rodier, 1995). Myelinated white matter matures alongside cognitive and learning abilities and has been shown to be associated with cognitive performance both in infants and children (Deoni et al., 2014) as well as in adults (Scholz et al., 2009). In turn, alterations in myelination timing and/or extent can significantly affect behavioral and cognitive outcomes (Fields, 2008), and are consistent findings in many neurological, psychiatric, and intellectual disorders (Flynn et al., 2003; Hendry et al., 2006; Wolff et al., 2012; Xiao et al., 2014). Regarding social aspects, adult rodent models indicate social isolation stress and chronic social defeat stress to be associated with social avoidance and myelin abnormalities in the prefrontal cortex, for example, reduced myelin thickness in the mPFC (Antontseva et al., 2020; Bonnefil et al., 2019; Liu et al., 2012). Furthermore, a human study in Major Depressive Disorder has demonstrated reduced myelin levels in prefrontal regions compared to healthy control participants (Sacchet & Gotlib, 2017). However, myelination of the social brain in healthy development has not yet been investigated.
The present study
The highlighted research suggests that structural and functional changes throughout the mPFC, TPJ, the insula, and ACC throughout childhood and adolescence underpin SE skill development and predict successful social interaction. Young children develop a sophisticated understanding of others’ desires, thoughts, and emotions, as distinct from their bodily reflexes and pains (Richardson et al., 2018). This early period of child development is a particularly sensitive period for brain, cognitive, and behavioral maturation. In SE development and social cognition, much of this development occurs before first grade (age ~6). Recent studies have highlighted the importance of linking changes in neural substrates to behavioral development to better understand the mechanisms underlying the developing social brain and sociocognitive skills for better understanding of the development of social interactions and relationships. In addition, myelination is a particularly dynamic neurodevelopmental process that has been linked to cognitive development in infants and children, however, no study investigated the link between developmental myelination, the social brain and related social behaviors in healthy young children. This exploratory study, therefore, adds to previous research by examining the structural changes in toddlers’ and young children’s social brains related to myelination and the relation to changes in SE development as rated by parents.
METHOD
Participants
Data used in this study were drawn from a large observational study of healthy child brain and cognitive development. General demographic details and visual timelines of study visits of included children are presented in Table 1 and Figure 1. Children were recruited as a community sample through flyers, radio and print advertisements, school and daycare information sessions, and at local community events. As a longitudinal study, enrolled children participated in study visits at approximately 6‐month intervals between birth and 2 years of age, and 12‐month intervals from two through adolescence. Children born pre‐term (<37 weeks); small for gestational age (<1500 g); non‐singleton pregnancy; with complicated delivery requiring a stay in the neonatal intensive care unit (NICU) or 5 min APGAR scores <8, or with in utero exposure to cigarettes, alcohol, or illicit substances; a family history of learning or psychiatric disorders (including major depressive disorder in the mother requiring medication during pregnancy); or neurological trauma or disorder (e.g., epilepsy) were excluded to help ensure a neurotypical population.
TABLE 1.
General demographic profile of the included study sample
| Sample size | Males | 65 |
| Females | 64 | |
| Race | White | 73 |
| African American | 22 | |
| Asian | 2 | |
| Hispanic | 32 | |
| Maternal education | High school complete | 15 |
| Partial college | 39 | |
| College or University | 34 | |
| Professional degree | 26 | |
| Not reported | 15 | |
| Birth weight (lbs.) | 7.1 (1.2) | |
| Gestation (days) | 273 (14) | |
| Mean age (days) | 235 (229) |
FIGURE 1.
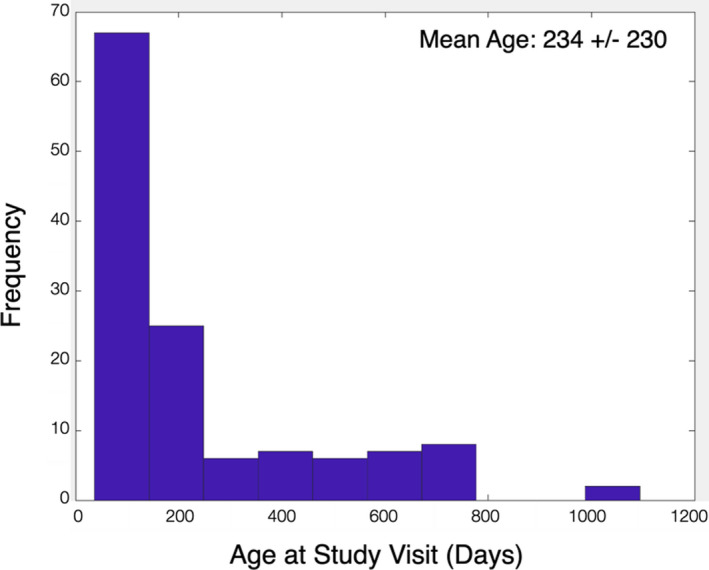
Histogram of child ages at assessment (corrected to a 40‐week gestation)
In the current analyses, N = 129 children (64 females) were drawn from this larger cohort who were between 2 and 36 months of age and who had myelin and social‐emotional assessments performed at the same study visit. While the larger study is longitudinal in nature, for these analyses we used only a single visit for each child. The distribution of child age on each included study visit is shown in Figure 1.
MR image data acquisition
Neuroimaging was performed on a Siemens 3T Trio and myelination was assessed using mcDESPOT multicomponent relaxometry (MCR) (Deoni et al., 2013). MCR aims to decompose the measured MRI signal into contributions from discrete anatomically separated water pools based on their signal relaxation properties (MacKay & Laule, 2016). In human brain, MCR analysis consistently reveals the presence of at least two water compartments that through histological comparisons have been attributed to the intra and extra‐cellular water, and water trapped within the lipid bilayers of the myelin sheath. Through appropriate analysis of MRI data, the relative volume fractions of these water pools can be estimated, with the myelin compartment fraction termed the myelin water fraction (MWF) and having strong associations with histological assessments of myelin content (Laule et al., 2006). mcDESPOT imaging involves the acquisition of eight T1‐weighted spoiled gradient (SPGR) images acquired over a range of flip angles; and 16 T1/T2‐weighted balanced steady‐state‐free precession (SSFP) images, also acquired of a range of flip angles and with different radio‐frequency phase‐cycling patterns (Deoni et al., 2013). Two additional magnetization‐prepared (IR‐) SPGR images are also acquired. From this collection of data, B0 and B1 field inhomogeneities are first calibrated and corrected; and MWF estimates are calculated using a stochastic region‐contraction approach. General imaging parameters for infants are provided in Table 2.
TABLE 2.
Acquisition parameters for the mcDESPOT MRI acquisitions
| Sequence | Parameter | 0–9 months | 9–24 months | >24 months |
|---|---|---|---|---|
| SPGR | FOV | 16 × 16 cm2 | 19 × 19 cm2 | 22 × 22 cm2 |
| X resolution | 96 | 112 | 128 | |
| Slice thickness | 1.7 | 1.7 | 1.7 | |
| # of slices | 80 | 84 | 92 | |
| TE/TR | 5.8/14 ms | 5.4/14 ms | 5.4/12 ms | |
| Flip angle | 3, 4, 5, 6, 7, 9, 11, 14 | 3, 4, 5, 6, 7, 9, 11, 14 | 3, 4, 5, 6, 7, 9, 11, 14 | |
| Bandwidth | 350 hz/pixel | 350 hz/pixel | 350 hz/pixel | |
| IR SPGR #1 | FOV | 16 × 16 cm2 | 19 × 19 cm2 | 22 × 22 cm2 |
| X resolution | 64 | 64 | 64 | |
| Slice thickness | 3.4 | 3.4 | 3.4 | |
| # of slices | 40 | 42 | 40 | |
| TE/TR/TI | 5.8/14/950 ms | 5.4/14/900 ms | 5.4/12/850 ms | |
| Flip angle | 5 | 5 | 5 | |
| Bandwidth | 350 hz/pixel | 350 hz/pixel | 350 hz/pixel | |
| IR SPGR #2 | FOV | 16 × 16 cm2 | 19 × 19 cm2 | 22 × 22 cm2 |
| X resolution | 64 | 64 | 64 | |
| Slice thickness | 3.4 | 3.4 | 3.4 | |
| # of slices | 40 | 42 | 40 | |
| TE/TR/TI | 5.8/14/650 ms | 5.4/14/600 ms | 5.4/12/500 ms | |
| Flip angle | 5 | 5 | 5 | |
| Bandwidth | 350 hz/pixel | 350 hz/pixel | 350 hz/pixel | |
| SSFP | FOV | 16 × 16 cm2 | 19 × 19 cm2 | 22 × 22 cm2 |
| X resolution | 96 | 112 | 128 | |
| Slice thickness | 1.7 | 1.7 | 1.7 | |
| # of slices | 80 | 84 | 92 | |
| T/TR | 6/12 ms | 6/12 ms | 6/12 ms | |
| Flip angle | 12, 16, 19, 23, 27, 35, 50, 70 | 12, 16, 19, 23, 27, 35, 50, 70 | 12, 16, 19, 23, 27, 35, 50, 70 | |
| Bandwidth | 350 hz/pixel | 350 hz/pixel | 350 hz/pixel |
Infants and toddlers in this study were imaged at night during natural and non‐sedated sleep (Dean et al., 2014) to help minimize intra‐scan motion. Children were swaddled with an infant or pediatric MedVac vacuum immobilization bag (CFI Medical Solutions) and foam cushions were packed around their head. Scanner noise was reduced by limiting the peak gradient amplitudes and slew rates. A noise‐insulating insert (Quiet Barrier HD Composite, UltraBarrier) was also fitted to the inside of the scanner bore. MiniMuff pediatric ear protectors and electrodynamic headphones (MR Confon) were used for all children. A pediatric pulse‐oximetry system and infrared camera were used to continuously monitor children during scanning, and parents were allowed to sit with their child in the scanner suite provided they had no MRI counter indications.
Following image acquisition, the mcDESPOT data were visually assessed for motion artifacts (e.g., blurring and ghosting) and MWF myelin content measures were estimated throughout the brain. The calculated MWF images were nonlinearly aligned to MNI space using a multi‐step, multi‐scale approach (Avants et al., 2011).
Assessment of social‐emotional development
Social‐emotional development was assessed within 1 week of successful MRI, using the Ages and Stages Questionnaires: Social‐Emotional, second edition (ASQ:SE‐2). The ASQ:SE‐2 is a parent‐rated questionnaire that measures social and emotional development in children between 0 and 6 years of age in seven key social‐emotional areas: self‐regulation, compliance, social‐communication, adaptive functioning, autonomy, affect, interaction with people (Squires et al., 2002).
Statistical analysis
Population‐derived masks corresponding to SE‐relevant brain regions were superimposed on the MNI‐aligned MWF images and mean values were calculated. To identify these regions, masks were used from the Destrieux atlas (Destrieux et al., 2010) for right and left hemispheres of the following SE‐relevant brain regions: anterior cingulate (identified from the parcellation file as regions 1002 and 2002), posterior cingulate (regions 1023 and 2023), insula (regions 1035 and 2035), medial orbital frontal (regions 1013 and 2013), middle frontal (regions1003 and 2003), rostral middle frontal (regions 1027 and 2027), precuneus (regions 1025 and 2025), superior temporal cortices (regions 1030 and 2030). Amygdala segmentation was performed using FSL‐FIRST. In addition, mean MWF values were also calculated in right and left hemisphere posterior central cortex (regions 1022 and 2022) and cerebellar cortices (regions 8 and 47)—regions associated with motor functions but not with socioemotional functions. These data were included to test the regional specificity of our analysis.
FreeSurfer processing (Fischl, 2012) was performed on all children >1 year of age and labeled cortical regions from each were converted to volumes using the mri_label2vol command in FreeSurfer. These regions were aligned to MNI‐space using the registration transformation calculated above, averaged and threshold at 0.85 to create mean region templates. These template volumes were superimposed on each individual’s MWF data. To visualize all results, p‐value maps were projected to a surface in FreeSurfer using the mri_vol2surf command, with the mid‐point projected (Figure 2).
FIGURE 2.
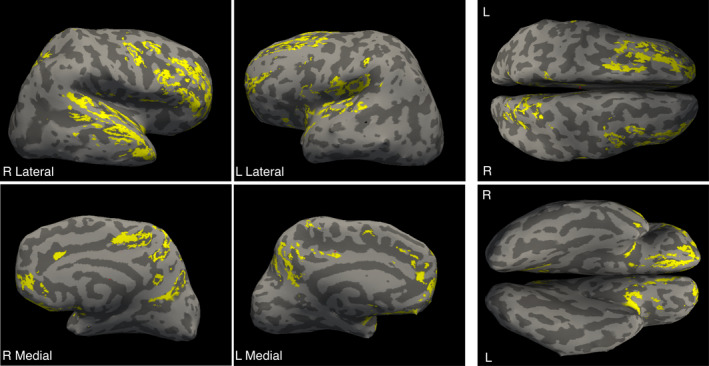
Overlay of social brain cortical regions on a representative child’s inflated cortical surface
The analyses presented represent an exploratory approach in a subset of children from a larger observational cohort and in a rather novel domain aimed to test the hypothesis that social‐emotional behaviors are associated with cortical myelin development in specific brain regions. We tested a series of linear models that related ASQ:SE‐2 scores to child age, gender, and with and without SE brain region MWF, that is,
| (1) |
and
| (2) |
The sum of square error (SSE) and the Akaike information criterion (AIC) were calculated and compared between the models for each anatomical region as well as the average MWF from the complete network. For regions where the AIC measure was smaller for the myelin‐included model, we calculated the False Discovery Rate (FDR) adjusted p values for the MWF term to determine is MWF was a significant model predictor.
This analysis was repeated for the control motor and cerebellar cortex data to test our hypothesis that there would be no significant association with ASQ:SE‐2 scores and these regions.
A potential confound of the above analysis is the nonlinear development of MWF throughout infancy. To help account for this, we built on past work and first modeled the mean MWF trajectory using a Gompertz growth model with a bootstrap approach to estimate the age‐wise standard deviation. Using these data, we then transformed the mean MWF estimates to MWF residual Z‐scores and repeated the above analyses substituting individual Z‐score for MWF in Equation (2).
RESULTS
Patterns of myelin development within the delineated SE brain regions are shown in Figure 3, corresponding to just the children included in this analysis who had a corresponding ASQ:SE‐2 assessment, and across all children in our larger child study.
FIGURE 3.
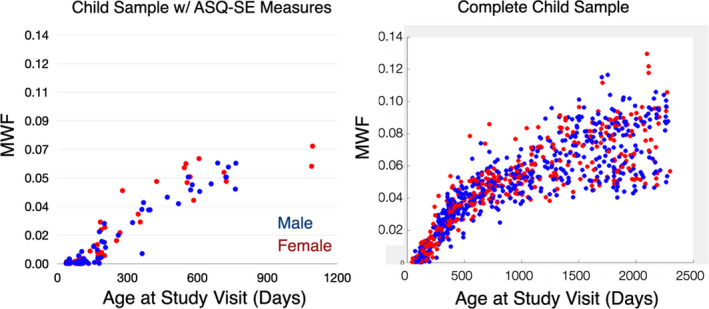
Patterns of social brain myelination in (left) children included in this study and (right) children drawn from a larger study of child health and development. Both present a characteristic pattern of rapid myelination over the first 2–3 years of life, followed by slower development over later childhood
For each of the nine social brain regions per hemisphere, and the complete network overall, we found that the myelin‐included model provided improved goodness‐of‐fit measures with lower AIC values in all brain regions (Table 3).
TABLE 3.
Summary measures from the model fitting relating social brain regions MWF with ASQ:SE‐2 measures
| Brain region | Non‐myelin model AIC | Myelin model AIC | FDR p value | |
|---|---|---|---|---|
| Left hemisphere | Amygdala | 877.573141 | 863.152746 | .009861 |
| Anterior cingulate | 877.573141 | 869.409600 | .030481 | |
| Insula | 877.573141 | 864.577008 | .013420 | |
| Medial orbitofrontal | 877.573141 | 868.255657 | .025644 | |
| Middle frontal | 877.573141 | 869.171738 | .026255 | |
| Posterior cingulate | 877.573141 | 869.096751 | .027711 | |
| Precuneus | 877.573141 | 867.005320 | .018748 | |
| Rostral middle frontal | 877.573141 | 869.077897 | .027390 | |
| Superior temporal | 877.573141 | 867.730716 | .012962 | |
| Right hemisphere | Amygdala | 877.573141 | 866.855480 | .016322 |
| Anterior cingulate | 877.573141 | 868.490108 | .027421 | |
| Insula | 877.573141 | 867.190345 | .017930 | |
| Medial orbitofrontal | 877.573141 | 866.900949 | .014296 | |
| Middle frontal | 877.573141 | 867.499344 | .013019 | |
| Posterior cingulate | 877.573141 | 869.182772 | .032014 | |
| Precuneus | 877.573141 | 865.983459 | .019293 | |
| Rostral middle frontal | 877.573141 | 867.280473 | .017476 | |
| Superior temporal | 877.573141 | 867.553060 | .015732 | |
| Social brain regions | 877.573141 | 867.256645 | .017131 | |
In contrast to the above results, Table 4 contains the results from the hypothesized non‐SE involved brain regions (motor and cerebellar cortices). Here we note that while the MWF‐included models provided improved goodness of fits, MWF was not a significant predictor of SE scores.
TABLE 4.
Summary measures from the model fitting relating hypothesized non‐social brain regions MWF with ASQ:SE‐2 measures
| Brain region | Non‐myelin model AIC | Myelin model AIC | FDR p value | |
|---|---|---|---|---|
| Left hemisphere | Posterior central cortex | 877.573141 | 1231.872481 | .246536 |
| Cerebellar cortex | 877.573141 | 1233.185712 | .2293 | |
| Right hemisphere | Posterior central cortex | 877.573141 | 1233.311049 | .214329 |
| Cerebellar cortex | 877.573141 | 1231.104478 | .23105 | |
Finally, Table 5 shows results from analysis using residual Z‐score in place of raw MWF measures to help ensure results presented in Table 3 were not biased by nonlinear patterns of MWF development. We find a replication of our prior results.
TABLE 5.
Summary measures from the model fitting relating myelin residual Z‐score with ASQ:SE‐2 measures
| Brain region | Non‐myelin model AIC | Myelin model AIC | FDR p value | |
|---|---|---|---|---|
| Left hemisphere | Amygdala | 877.573141 | 865.405714 | .004911 |
| Anterior cingulate | 877.573141 | 869.700308 | .039027 | |
| Insula | 877.573141 | 867.989063 | .012823 | |
| Medial orbitofrontal | 877.573141 | 869.111039 | .046861 | |
| Middle frontal | 877.573141 | 869.755577 | .040719 | |
| Posterior cingulate | 877.573141 | 869.740139 | .047423 | |
| Precuneus | 877.573141 | 869.092164 | .025083 | |
| Rostral middle frontal | 877.573141 | 869.716549 | .045920 | |
| Superior temporal | 877.573141 | 869.408177 | .032997 | |
| Right hemisphere | Amygdala | 877.573141 | 868.123089 | .010058 |
| Anterior cingulate | 877.573141 | 869.088320 | .044340 | |
| Insula | 877.573141 | 868.853327 | .021369 | |
| Medial orbitofrontal | 877.573141 | 868.583164 | .029035 | |
| Middle frontal | 877.573141 | 869.410968 | .035654 | |
| Posterior cingulate | 877.573141 | 869.360401 | .032735 | |
| Precuneus | 877.573141 | 868.678251 | .020469 | |
| Rostral middle frontal | 877.573141 | 869.305313 | .031317 | |
| Superior temporal | 877.573141 | 869.320955 | .031238 | |
| Social brain regions | 877.573141 | 869.189432 | .033844 | |
DISCUSSION
This is the first study to show the myelin trajectory of the social brain in young children, indicating the same steep increase in myelin characteristic of infancy and throughout the first 3 years of life as reported in previous developmental myelin trajectory studies for global brain myelination. Patterns of myelin development within the delineated SE brain regions (Figure 3) reveal a rapid increase in myelination throughout the first 2–3 years of life and showing in older childhood. In addition, we also note an increase in population variability with child age, potentially underlying individual differences in social‐emotional processing.
While prior studies have mapped out the social brain areas in children which include the amygdala, anterior cingulate, insula, medial orbitofrontal, middle frontal, posterior cingulate, precuneus, rostral middle frontal, superior temporal, anterior cingulate, medial orbitofrontal, middle frontal, posterior cingulate, precuneus, rostral middle frontal, superior temporal (Blakemore, 2008; Crafa, 2015; Sherwin et al., 2019), the structural changes in toddlers’ and young children’s social brain areas related to myelination and their links to changes in SE development remained largely unexplored. This study examined this link by investigating changes in myelination of social brain areas and their subsequent links to SE behavior in children. The results show a significant correlation between this pattern of myelination and their social‐emotional development as observed and rated by parents, validating the social brain areas previously described in adolescents and adults (e.g., Blakemore, 2008; Crafa, 2015; Sherwin et al., 2019). While myelination has been described as a brain maturation marker in young children (Dean et al., 2015a, 2015b; Deoni et al., 2012) and has closely linked to emerging general functions (Deoni et al., 2014; McKenzie et al., 2014; O’Muircheartaigh et al., 2013, 2014), this marks the first investigation of social‐emotional development in this context. It is, however, a particularly relevant developmental domain for infants and toddlers as their social environments begin to expand beyond immediate family members. Their learning experiences also concurrently occur more routinely in social environments such as day cares and activity camps.
Our myelination findings complement results from functional imaging studies (e.g., Achterberg et al., 2017; Güroğlu et al., 2009; Richardson et al., 2018) that have highlighted the developmental dynamics of social brain activation across childhood, adolescence, and young adulthood.
To date, neuroimaging data linked to social brain and social skill development in healthy young children seems scarce. To our knowledge, this study is one of the first to establish a link between social brain development and concurrent socioemotional skill development as rated by the parents via the ASQ:SE‐2. While the ASQ:SE‐2 has been previously used to track children’s development of SE behaviors (Alwaely et al., 2020) and to observe links between maternal stress & children’s SE development (Briggs et al., 2014; Salomonsson & Sleed, 2010; Sikander et al., 2019), so far no study has investigated the link between the social brain areas and SE development as captured by ASQ:SE‐2. Thus, the data may provide a new and additional angle to linking brain growth and behavioral development. The trajectory analyses allow for identifying sensitive windows of social brain and social‐emotional development.
Limitations and future directions
Our study has notable strengths, including a relatively big sample with high‐quality myelin imaging and social‐emotional data in early childhood. However, this study also suffers limitations that merit consideration. The first is our use of parent‐reported measures of infant social skills. Parent reports may be prone to certain biases that do not reflect objective developmental changes. However, the ASQ:SE‐2 has good validity (Squires et al., 2002). Future studies may consider a wider range of social skill measures and assessments to minimize this bias while also providing a more holistic measurement of social skill development. Being competent in social situations depends not only on social‐emotional abilities but also cognitive abilities such as social cognition, which allow us to interact with others and understand their intentions, feelings, thoughts, and behaviors (e.g., Grossmann & Johnson, 2007).
Second, the narrow assessment focus and the exploratory nature of our study does not consider the interplay with other potential physiological pathways that may underlie social behavior. These may include neural circuits related to the brain reward system, gut‐brain axis‐related pathways, neuroendocrine regulation, gene expression, epigenetic regulation, and genome structure (Blumstein et al., 2010; Chen et al., 2018; Rubenstein & Hofmann, 2015; Sherwin et al., 2019). To these latter points, potential associations between social brain myelination to the microbiota‐gut‐social brain axis (Sherwin et al., 2019) may provide new insights into healthy neurodevelopment as well as potential targets for early life factors.
While statistically significant and meaningful, our correlational findings do not imply causality nor allow for any predictions for the impact of early myelination on later social‐emotional outcomes. Future studies may include prediction models and or mediation analyses to further validate the relevance of myelin early in life, during its formative peak years, as an important brain structural contributor that lays the foundation for future mental health and development potential. It should also be noted that the study sample has a relatively high socioeconomic status background, which may limit generalization of the data to other populations from different backgrounds. Additional replication of these results in a more diverse and generally representative community sample is warranted.
CONCLUSIONS
This study is the first to investigate myelin trajectories of the social brain in toddlers and young children and linking it to SE development. The data contribute to the important crosstalk between neuroimaging and behavioral disciplines (Grossmann & Johnson, 2007) and may add knowledge to the emerging picture of the developing social brain by describing neural underpinnings of human social behavior. While several neural processes contribute to social skill development, myelin is a non‐invasive, feasible, and relevant early life measure as demonstrated by the significant association between social brain myelin and behaviorally rated SE development in this study. Understanding those developmental dynamics, their peaks and plateaus at the level of the brain and behavior are relevant for supporting healthy development as well as at risk development by providing targets for early stimulation or intervention strategies that fit with behavioral as well as neural windows of sensitivity for SE development. Furthermore, the study findings may be relevant for understanding school readiness and learning beyond intellectual abilities as well as social norm compliance and/or violations development (e.g., Bas Hoogendam et al., 2017).
CONFLICT OF INTEREST
EG is employee of Nestlé Enterprises SA; NS is employee of the Société des Produits Nestlé SA; and SCLD receives funding from the Société des Produits Nestlé SA.
Schneider, N. , Greenstreet, E. , & Deoni, S. C. L. (2022). Connecting inside out: Development of the social brain in infants and toddlers with a focus on myelination as a marker of brain maturation. Child Development, 93, 359–371. 10.1111/cdev.13649
Funding information
Environmental Influences on Child Health Outcomes (ECHO) National Institutes of Health (SCD UG3OD023313) and National Institutes of Health (SCD R34‐DA050284).
REFERENCES
- Achterberg, M. , van Duijvenvoordea, A. C. K. , van der Meulen, M. , Euser, S. , Bakermans‐Kranenburg, M. J. , & Crone, E. A. (2017). The neural and behavioral correlates of social evaluation in childhood. Developmental Cognitive Neuroscience, 24, 107–117. 10.1016/j.dcn.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alducin, N. , Huffman, L. C. , Feldman, H. M. , & Loe, I. M. (2014). Executive function is associated with social competence in preschool‐aged children born preterm or full term. Early Human Development, 90(6), 299–306. 10.1016/j.earlhumdev.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwaely, S. A. , Yousif, N. B. A. , & Mikhaylov, A. (2020). Emotional development in preschoolers and socialization. Early Child Development and Care, 1–10. 10.1080/03004430.2020.1717480 [DOI] [Google Scholar]
- Antontseva, E. , Bondar, N. , Reshetnikov, V. , & Merkulova, T. (2020). The effects of chronic stress on brain myelination in humans and in various rodent models. Neuroscience, 441, 226–238. 10.1016/j.neuroscience.2020.06.013 [DOI] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. J. , Song, G. , Cook, P. A. , Klein, A. , & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich, A. J. (2005). Magnetic resonance techniques in the assessment of myelin and myelination. Journal of Inherited Metabolic Disease, 28(3), 311–343. 10.1007/s10545-005-5952-z [DOI] [PubMed] [Google Scholar]
- Bartzokis, G. , Lu, P. H. , Tingus, K. , Mendez, M. F. , Richard, A. , Peters, D. G. , Oluwadara, B. , Barrall, K. A. , Finn, J. P. , Villablanca, P. , Thompson, P. M. , & Mintz, J. (2010). Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiology of Aging, 31(9), 1554–1562. 10.1016/j.neurobiolaging.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas Hoogendam, J. M. , van Steenbergen, H. , Kreuk, T. , van der Wee, N. J. A. , & Westenberg, P. M. (2017). How embarrassing! The behavioral and neural correlates of processing social norm violations. PLoS One, 12(4), e0176326. 10.1371/journal.pone.0176326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, S.‐J. (2008). The social brain in adolescence. Neuroscience, 9(4), 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Blakemore, S.‐J. (2010). The developing social brain: Implications for education. Neuron, 65(6), 744–747. 10.1016/j.neuron.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, S.‐J. (2012). Development of the social brain in adolescence. Journal of the Royal Society of Medicine, 105(3), 111–116. 10.1258/jrsm.2011.110221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein, D. T. , Ebensperger, L. A. , Hayes, L. D. , Vásquez, R. A. , Ahern, T. A. , Burger, J. R. , Dolezal, A. G. , Dosmann, A. , González‐Mariscal, G. , Harris, B. N. , Herrera, E. A. , Lacey, E. A. , Mateo, J. , McGraw, L. A. , Olazábal, D. , Ramenofsky, M. , Rubenstein, D. R. , Sa, S. A. , Saltzman, W. , … Young, L. J. (2010). Toward an integrative understanding of social behavior: New models and new opportunities. Frontiers in Behavioral Neuroscience, 4, 1–9. 10.3389/fnbeh.2010.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefil, V. , Dietz, K. , Amatruda, M. , Wentling, M. , Aubry, A. V. , Dupree, J. L. , Temple, G. , Park, H.‐J. , Burghardt, N. S. , Casaccia, P. , & Liu, J. (2019). Region‐specific myelin differences define behavioral consequences of chronic social defeat stress in mice. eLife, 8, e40855. 10.7554/eLife.40855.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, R. D. , Silver, E. J. , Krug, L. M. , Mason, Z. S. , Schrag, R. D. , Chinitz, S. , & Racine, A. D. (2014). Healthy steps as a moderator: The impact of maternal trauma on child social‐emotional development. Clinical Practice in Pediatric Psychology, 2(2), 166. 10.1037/cpp0000060 [DOI] [Google Scholar]
- Briggs, R. D. , Stettler, E. M. , Silver, E. J. , Schrag, R. D. A. , Nayak, M. , Chinitz, S. , & Racine, A. D. (2012). Social‐emotional screening for infants and toddlers in primary care. Pediatrics, 129(2), e377–e384. 10.1542/peds.2010-2211 [DOI] [PubMed] [Google Scholar]
- Case‐Smith, J. (2013). Systematic review of interventions to promote social–emotional development in young children with or at risk for disability. American Journal of Occupational Therapy, 67(4), 395–404. 10.5014/ajot.2013.004713 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Martínez, R. M. , & Cheng, Y. (2018). The developmental origins of the social brain empathy, morality, and justice. Frontiers in Psychology, 9, 2584. 10.3389/fpsyg.2018.02584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafa, D. (2015). Neural correlates of social development. In International encyclopedia of the social & behavioral sciences, Vol. 16 (pp. 600–605). Elsevier. 10.1016/B978-0-08-097086-8.23206-6 [DOI] [Google Scholar]
- Dean, D. C. , Dirks, H. , O’Muircheartaigh, J. , Walker, L. , Jerskey, B. A. , Lehman, K. , Han, M. , Waskiewicz, N. , & Deoni, S. C. L. (2014). Pediatric neuroimaging using magnetic resonance imaging during non‐sedated sleep. Pediatric Radiology, 44(1), 64–72. 10.1007/s00247-013-2752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean III, D. C. , O'Muircheartaigh, J. , Dirks, H. , Waskiewicz, N. , Lehman, K. , Walker, L. , Piryatinsky, I. , & Deoni, S. C. L. (2015a). Estimating the age of healthy infants from quantitative myelin water fraction maps. Human Brain Mapping, 36, 1233–1244. 10.1002/hbm.22671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean III, D. C. , O'Muircheartaigh, J. , Dirks, H. , Waskiewicz, N. , Walker, L. , Doernberg, E. , Piryatinsky, I. , & Deoni, S. C. L. (2015b). Characterizing longitudinal white matter development during early childhood. Brain Structure and Function, 220, 1921–1933. 10.1007/s00429-014-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham, S. A. , & Brown, C. (2010). “Plays nice with others”: Social‐emotional learning and academic success. Early Education and Development, 21(5), 652–680. 10.1080/10409289.2010.497450 [DOI] [Google Scholar]
- Denham, S. A. , Wyatt, T. M. , Bassett, H. H. , Echeverria, D. , & Knox, S. S. (2009). Assessing social‐emotional development in children from a longitudinal perspective. Journal of Epidemiology and Community Health, 63(Suppl 1), i37–i52. 10.1136/jech.2007.070797 [DOI] [PubMed] [Google Scholar]
- Deoni, S. C. L. , Dean III, D. C. , O'Muircheartaigh, J. , Dirks, H. , & Jerskey, B. A. (2012). Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage, 63(3), 1038–1053. 10.1016/j.neuroimage.2012.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni, S. C. L. , Matthews, L. , & Kolind, S. H. (2013). One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magnetic Resonance in Medicine, 70, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni, S. C. L. , O’Muircheartaigh, J. , Elison, J. T. , Walker, L. , Doernberg, E. , Waskiewicz, N. , Dirks, H. , Piryatinsky, I. , Dean III, D. C. , & Jumbe, N. L. (2014). White matter maturation profiles through early childhood predict general cognitive ability. Brain Structure and Function, 221(2), 1189–1203. 10.1007/s00429-014-0947-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux, C. , Fischl, B. , Dale, A. , & Halgren, E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R. D. (2008). White matter in learning, cognition and psychiatric disorders. Trends in Neuroscience, 31(7), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, S. W. , Lang, D. J. , Mackay, A. L. , Goghari, V. , Vavasour, I. M. , Whittall, K. P. , Smith, G. N. , Arango, V. , Mann, J. J. , Dwork, A. J. , Falkai, P. , & Honer, W. G. (2003). Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post‐mortem with analysis of oligodendrocyte proteins. Molecular Psychiatry, 8(9), 811–820. 10.1038/sj.mp.4001337 [DOI] [PubMed] [Google Scholar]
- Grossman, T. (2013). The early development of processing emotions in face and voice. In Belin P., Campanella S., & Ethofer T. (Eds.), Integrating face and voice in person perception (pp. 95–116). Springer. [Google Scholar]
- Grossmann, T. , & Johnson, M. H. (2007). The development of the social brain in human infancy. European Journal of Neuroscience, 25(4), 909–919. 10.1111/j.1460-9568.2007.05379.x [DOI] [PubMed] [Google Scholar]
- Grossmann, T. , Missana, M. , & Krol, K. M. (2018). The neurodevelopmental precursors of altruistic behavior in infancy. PLoS Biology, 16(9), e2005281. 10.1371/journal.pbio.2005281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann, T. , Striano, T. , & Friederici, A. D. (2007). Developmental changes in infants’ processing of happy and angry facial expressions: A neurobehavioral study. Brain and Cognition, 64(1), 30–41. 10.1016/j.bandc.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Güroğlu, B. , van den Bos, W. , & Crone, E. A. (2009). Neural correlates of social decision making and relationships: A developmental perspective. Annals of the New York Academy of Sciences, 1167(1), 197–206. 10.1111/j.1749-6632.2009.04502.x [DOI] [PubMed] [Google Scholar]
- Hendry, J. , DeVito, T. , Gelman, N. , Densmore, M. , Rajakumar, N. , Pavlosky, W. , Williamson, P. C. , Thompson, P. M. , Drost, D. J. , & Nicolson, R. (2006). White matter abnormalities in autism detected through transverse relaxation time imaging. NeuroImage, 29(4), 1049–1057. 10.1016/j.neuroimage.2005.08.039 [DOI] [PubMed] [Google Scholar]
- Hoffman, M. L. (1977). Empathy, its development and prosocial implications. Nebraska Symposium on Motivation, 25, 169–217. [PubMed] [Google Scholar]
- Huber, L. , Plötner, M. , & Schmitz, J. (2019). Behavioral observation of prosocial behavior and social initiative is related to preschoolers’ psychopathological symptoms. PLoS One, 14(11), e0225274. 10.1371/journal.pone.0225274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy, S. (2009). Pathways to competence: Encouraging healthy social and emotional development in young children (2nd ed.). Paul H Brookes Publishing. [Google Scholar]
- Laule, C. , Leung, E. , Lis, D. K. B. , Traboulsee, A. L. , Paty, D. W. , MacKay, A. L. , & Moore, G. R. W. (2006). Myelin water imaging in multiple sclerosis: Quantitative correlations with histopathology. Multiple Sclerosis Journal, 12(6), 747–753. 10.1177/1352458506070928 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Dietz, K. , DeLoyht, J. M. , Pedre, X. , Kelkar, D. , Kaur, J. , Vialou, V. , Lobo, M. K. , Dietz, D. M. , Nestler, E. J. , Dupree, J. , & Casaccia, P. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience, 15(12), 1621–1624. 10.1038/nn.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay, A. L. , & Laule, C. (2016). Magnetic resonance of myelin water: An in vivo marker for myelin. Brain Plasticity, 2(1), 71–91. 10.3233/BPL-160033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, E. M. , van Hoorn, J. , Cohen, J. R. , & Telzer, E. H. (2018). Functional connectivity in the social brain across childhood and adolescence. Social Cognitive and Affective Neuroscience, 13(8), 819–830 10.1093/scan/nsy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae, J. S. , & Brown, S. M. (2018). Systematic review of social‐emotional screening instruments for young children in child welfare. Research on Social Work Practice, 28(7), 767–788. 10.1177/1049731516686691 [DOI] [Google Scholar]
- McKenzie, I. A. , Ohayon, D. , Li, H. , Paes de Faria, J. , Emery, B. , Tohyama, K. , & Richardson, W. D. (2014). Motor skill learning requires active central myelination. Science Translational Medicine, 346(6207), 318–322. 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. L. , Lalonde, F. , Clasen, L. S. , Giedd, J. N. , & Blakemore, S.‐J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–131. 10.1093/scan/nss113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missana, M. , Atkinson, A. P. , & Grossmann, T. (2015). Tuning the developing brain to emotional body expressions. Developmental Science, 18(2), 243–253. 10.1111/desc.12209 [DOI] [PubMed] [Google Scholar]
- Missana, M. , Rajhans, P. , Atkinson, A. P. , & Grossmann, T. (2014). Discrimination of fearful and happy body postures in 8‐month‐old infants: An event‐related potential study. Frontiers in Human Neuroscience, 8, 531. 10.3389/fnhum.2014.00531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor, B. G. , Güroğlu, B. , Op de Macks, Z. A. , Rombouts, S. A. R. B. , Van der Molen, M. W. , & Crone, E. A. (2012). Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. NeuroImage, 59(1), 708–717. 10.1016/j.neuroimage.2011.07.028 [DOI] [PubMed] [Google Scholar]
- National Scientific Council on the Developing Child . (2004). Children’s emotional development is built into the architecture of their brains. Working Paper No. 2. http://www.developingchild.net
- O’Muircheartaigh, J. , Dean III, D. C. , Dirks, H. , Waskiewicz, N. , Lehman, K. , Jerskey, B. A. , & Deoni, S. C. L. (2013). Interactions between white matter asymmetry and language during neurodevelopment. The Journal of Neuroscience, 33(41), 16170–16177. 10.1523/JNEUROSCI.1463-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh, J. , Dean III, D. C. , Ginestet, C. E. , Walker, L. , Waskiewicz, N. , Lehman, K. , Dirks, H. , Piryatinsky, I. , & Deoni, S. C. L. (2014). White matter development and early cognition in babies and toddlers. Human Brain Mapping, 35(9), 4475–4487. 10.1002/hbm.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges, S. W. , & Furman, S. A. (2011). The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant and Child Development, 20, 106–118. 10.1002/icd.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, H. , Lisandrelli, G. , Riobueno‐Naylor, A. , & Saxe, R. (2018). Development of the social brain from age three to twelve years. Nature Communications, 9(1), 1027. 10.1038/s41467-018-03399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier, P. M. (1995). Developing brain as a target of toxicity. Environmental Health Perspectives, 103(Suppl 6), 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein, D. R. , & Hofmann, H. A. (2015). Proximate pathways underlying social behavior. Current Opinion in Behavioral Sciences, 6, 154–159. 10.1016/j.cobeha.2015.11.007 [DOI] [Google Scholar]
- Sacchet, M. D. , & Gotlib, I. H. (2017). Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Scientific Reports, 7(1), 2200. 10.1038/s41598-017-02062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson, B. , & Sleed, M. (2010). The ages & stages questionnaire: Social–emotional: A validation study of a mother‐report questionnaire on a clinical mother–infant sample. Infant Mental Health Journal, 31, 412–431. [DOI] [PubMed] [Google Scholar]
- Scharf, R. J. , Scharf, G. J. , & Stroustrup, A. (2016). Developmental milestones. Pediatrics Review, 37(1), 25–37. 10.1542/pir.2014-0103 [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Wohlschlaeger, A. M. , Kraemer, N. C. , Newenh, A. , Shah, N. J. , Fink, G. R. , & Vogeley, K. (2006). Being with virtual others: Neural correlates of social interaction. Neuropsychologia, 44(5), 718–730. 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Scholz, J. , Klein, M. C. , Behrens, T. E. , & Johansen‐Berg, H. (2009). Training induces changes in white matter architecture. Nature Neuroscience, 12(11), 1370–1371. 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin, E. , Bordenstein, S. R. , Quinn, J. L. , Dinan, T. G. , & Cryan, J. F. (2019). Microbiota and the social brain. Science, 366(6465), eaar2016. 10.1126/science.aar2016 [DOI] [PubMed] [Google Scholar]
- Sikander, S. , Ahmad, I. , Bates, L. M. , Gallis, J. , Hagaman, A. , O’Donnell, K. , Turner, E. L. , Zaidi, A. , Rahman, A. , & Maselko, J. (2019). Cohort profile: Perinatal depression and child socioemotional development; the Bachpan cohort study from rural Pakistan. British Medical Journal Open, 9(5), e025644. 10.1136/bmjopen-2018-025644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto‐Icaza, P. , Aboitiz, F. , & Billeke, P. (2015). Development of social skills in children: Neural and behavioral evidence for the elaboration of cognitive models. Frontiers in Neuroscience, 9, 333. 10.3389/fnins.2015.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires, J. , Bricker, D. D. , & Twombly, E. (2002). ASQ:SE‐2 user’s guide. Paul H. Brookes Publishing Co., Inc. [Google Scholar]
- Vaish, A. , Carpenter, M. , & Tomasello, M. (2010). Young children selectively avoid helping people with harmful intentions. Child Development, 81(6), 1661–1669. 10.1111/j.1467-8624.2010.01500.x [DOI] [PubMed] [Google Scholar]
- Walker, S. P. , Wachs, T. D. , Meeks Gardner, M. , Lozoff, B. , Wasserman, G. A. , Pollitt, E. , Carter, J. A. , & the International Child Development Steering Group . (2007). Child development: Risk factors for adverse outcomes in developing countries. Lancet, 369(9556), 145–157. 10.1016/S0140-6736(07)60076-2 [DOI] [PubMed] [Google Scholar]
- Warneken, F. , & Tomasello, M. (2007). Helping and cooperation at 14 months of age. Infancy, 11(3), 271–294. 10.1111/j.1532-7078.2007.tb00227.x [DOI] [PubMed] [Google Scholar]
- Wolff, J. J. , Gu, H. , Gerig, G. , Elison, J. T. , Styner, M. , Gouttard, S. , Botteron, K. N. , Dager, S. R. , Dawson, G. , Estes, A. M. , Evans, A. C. , Hazlett, H. C. , Kostopoulos, P. , McKinstry, R. C. , Paterson, S. J. , Schultz, R. T. , Zwaigenbaum, L. , & Piven, J. (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry, 169(6), 589–600. 10.1176/appi.ajp.2011.11091447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Z. , Qiu, T. , Ke, X. , Xiao, X. , Xiao, T. , Liang, F. , Zou, B. , Huang, H. , Fang, H. , Chu, K. , Zhang, J. , & Liu, Y. (2014). Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. Journal of Autism and Developmental Disorders, 44(7), 1633–1640. 10.1007/s10803-014-2033-x [DOI] [PMC free article] [PubMed] [Google Scholar]


