Abstract
Breast cancer is a highly heterogeneous group of diseases posing a significant challenge in biomarker-driven research and the development of effective targeted therapies. Especially the treatment of metastatic breast cancer poses even more challenges, as we still lose more than 42,000 women and men each year in the United States alone. New biological insight helps to improve breast cancer treatment through early detection, adaptation to chemotherapy resistance, and tailoring to find the right size of care. This review focuses on existing and new areas of predictive biomarkers under development to tailor the management of breast cancer and the application of integrative approaches that have resulted in the promising candidate biomarker discovery. Furthermore, we review new methods to detect metastatic progression using imaging, and blood-based assays. We hope to increase the attention and awareness of a new generation of therapeutic development strategies in metastatic breast cancer.
Keywords: biomarkers, emerging therapy resistance, metastatic breast cancer, translational research
Introduction
Unlike hematologic malignancies, where subsets of patients with disseminated disease achieve long-term remission with combinations or high-dose chemotherapy followed by autologous transplant or an infusion of chimeric antigen receptor T cells (CAR-T cells), metastatic breast cancer (MBC) remains an incurable disease to date. Several classes of chemotherapy and targeted therapies as a single agent or in combination have improved the outcome of the patient over the last several decades. Yet, numerous mechanisms can lead to systemic therapy resistance. The source of resistance includes intrinsic tumor factors such as genomic and epigenomic alterations, alterations of functioning proteins, and interference from the tumor immune microenvironment (TME) or other host factors like the immune system. Currently used biomarkers in breast cancer are heavily focused on diagnosing and treating early-stage breast cancers. At the same time, complex mechanisms of emerging resistance to systemic therapy in MBC remain largely untapped. Targeting the genomic alterations such as PIK3CA mutation has led to the approval of targeted therapies in MBC. Still, the proportion of patients with MBC who benefit from this approach is limited to date. Here we review under-represented yet critical mechanisms of systemic therapy resistance, related biomarkers, and new ways to detect the metastatic progression that can further guide new therapeutics development focusing on the MBC.
Resistance mechanism of chemotherapy
Despite the successful development of targeted therapies and immunotherapy in breast cancers, the mainstay of treatment remains to be chemotherapy both in early-stage and metastatic settings in MBC. However, not many resistances mechanism to chemotherapeutics has been elucidated to date. Here, we review several less understood resistances that may lead to discovering novel therapeutic targeting.
Drug efflux transporters
Drug efflux transporters have long been recognized as crucial chemotherapy resistance mechanisms. Early studies have identified drug efflux proteins, ATP-binding cassette (ABC) transporters to be involved with chemotherapy resistance in breast cancer and include multidrug-resistant protein-1 (MRP1 or ABCC), breast cancer resistance protein (BCRP or ABCG2) for doxorubicin and p-glycoprotein (MDR1, ABCB1, or CD243) which results in various chemotherapeutic agents including paclitaxel (Table 1). Expression levels of drug efflux pumps are significantly increased in chemo-resistant cancers, particularly after exposure to a chronic sub-lethal drug level. These drug transporters are promiscuous, and upregulation can lead to upfront resistance to unrelated drug compounds.1,2
Table 1.
Cytotoxic chemotherapy agents and the main mechanism of resistance in breast cancer.
| Anthracyclines | Doxorubicin, liposomal doxorubicin, epirubicin | P-glycoprotein substrate Mitochondrial re-structuring |
| Taxanes | Paclitaxel, nab-paclitaxel, docetaxel | P-glycoprotein substrate |
| Platinum salts | Carboplatin, cisplatin | Less associated with drug efflux transporters |
| Alkylating agents | Cyclophosphamide | Not well determined |
| Anti-metabolites | Capecitabine and 5-fluorouracil (pyrimidine analogs), methotrexate (folic acid analog) | Methotrexate is a moderate substrate for several ABC transporters |
| Microtubule inhibitors | Eribulin, vinorelbine, ixabepilone | P-glycoprotein substrate |
| Nucleoside analog | Gemcitabine | Not well determined |
Antibody-drug conjugates (ADCs), a new category of drugs that expanded the therapeutic options for patients with MBC in recent years, 3 deliver potent chemotherapeutic payloads into the cytoplasm and drug efflux transporters may also contribute to resistance. Drug efflux transporters such as MRP1 may mediate resistance to ADCs such as ado-trastuzumab emtansine (T-DM1), which consists of the monoclonal antibody trastuzumab covalently linked to cytotoxic chemotherapy emtansine and approved for use in HER2+ breast cancer.4,5
Multiple oncogenic pathways also mediate the upregulation of drug efflux transporters. The PI3K/Akt pathway aberrations are common across all subtypes of breast cancer, and pathway activation leads to upregulation of p-glycoprotein. PIK3/Akt activation leads to upregulation of survivin, 6 an anti-apoptosis protein that can also upregulate p-glycoprotein. 7 It has been proposed that the transcriptional regulation of p-glycoprotein may occur through Wnt/ß–catenin pathway activation, specifically via overexpression of Wnt5A and Pygo2.8–11 In addition to regulating drug efflux transporters, these pathways crosstalk and mediate important cellular processes in oncogenesis and metastasis, thereby can be utilized as an indirect targeting possibility. To date, two PIK3/Akt/mTOR pathway inhibitors are approved for use in metastatic hormone receptor-positive breast cancer, alpelisib for PIK3CA-mt 12 and everolimus in mutant or wildtype disease in combination with endocrine therapy, 13 however, their role in chemo-sensitization remains elusive. 14
Since the discovery of drug efflux transporters, several generations of inhibitors have been developed but have not made their way into clinical practice due to toxicity and limited therapeutic benefit, possibly due to compensatory resistance mechanisms. For instance, a phase III trial with BMS-217380-01 (tesmilifene), a third-generation inhibitor, resulted in minor hematologic toxicity and no benefit when combined with anthracycline-containing chemotherapy. 15 Several herbal and natural agents have been found to modulate drug efflux transporters, such as tea polyphenols, artemisinin, and curcumin, with mixed results.16–18
Other approaches include novel delivery systems such as nanoparticles that encapsulate and deliver one or more cytotoxic chemotherapeutic agents. In MBC treatment, encapsulated liposomal doxorubicin (Doxil) and albumin-bound paclitaxel (Abraxane) are actively used in therapeutics. 19
Extrachromosomal DNA
The assessment of the resistance mechanism to chemotherapy is confounded by the changes in mutational frequency overtime during treatment and subclone specific factors that promote metastatic outgrowth in different organs. However, non-conventional DNAs have been able to detect the effect of chemotherapy-associated with chemotherapy resistance. For example, the extrachromosomal DNA (ecDNA) results from multiple double-strand breaks and chromothripsis; however, the exact mechanisms remain unknown. ecDNA leads to oncogene amplification such as EGFR, MYC, CCND1, CDK4, and MDM2, which are common amplifications found in breast cancer, and accelerates intratumoral heterogeneity. ecDNA is present in up to 20–25% of breast cancers and across solid tumors frequently detected in aggressive tumor subtypes and is associated with poorer survival.20,21 The contribution of ecDNA drug resistance has been best described as copy number amplification of dihydrofolate reductase gene amplification and resistance to methotrexate.
Mitochondrial restructuring and metabolism
Mitochondria is a critical intracellular organelle that produces vital energy sources for the cells and regulates the cells’ core metabolism through the TCA cycle and oxidative phosphorylation. Accumulating data supports the dynamic contribution of the mitochondria to systemic therapy resistance by several mechanisms. The first is through metabolic alteration. When cancer cells are challenged with systemic therapy, this can pressure the cells to shift the metabolic pathways to alternative ones, such as lipid metabolism, Oxphos, and glutamate-dependent metabolism. Proteomic assays such as reverse phosphoprotein assay or mass-spectrometry allow the measurement of metabolites in the tissue and blood. In addition, a structural formation of the mitochondria itself may contribute to the resistance/sensitivity to the systemic therapy. 22 When cancer cells undergo genotoxic stress from chemotherapeutics, mitochondrial units fuse to form a large unit of mitochondria (mitochondrial fusion). Therefore, targeting the anatomical structure of mitochondria regulation itself can provide a therapeutic opportunity. Additionally, direct/indirect regulation of apoptosis proteins in the mitochondrial membrane can cause the resistance to systemic therapy, therefore potentially therapeutically targetable. 23
Predictive biomarkers for immunotherapy resistance
PD-L1 as a biomarker
Two immune checkpoint inhibitors (ICIs), atezolizumab and pembrolizumab, in combination with chemotherapy, are approved for metastatic PD-L1 + TNBC in the first-line setting,24,25 neoadjuvant therapy for early Triple Negative Breast Cancer (TNBC). 26 Translational findings in prospective trials also have identified Lactate dehyrogenase (LDH), Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), target lesion size, and liver metastasis as predictors of response to the atezolizumab. 27 The greatest focus has been on Programmed Death Ligand-1 (PD-L1) expression on tumor and immune cells (ICs) present in the TME. In both studies, PD-L1 positivity is a predictive factor for response accompanying Ventana SP-142 assay ⩾1 and Combined Positive Score (CPS) score ⩾10. In Impassion130 patients with PD-L1 + tumors ⩾ 1% median progression-free survival (PFS) was 7.5 months versus 5.0 months with chemotherapy alone [hazard ratio (HR) 0.62, CI 0.49–0.78, p < 0.001] and an overall survival (OS) benefit of 25 months versus 15.5 months (HR 0.62, CI 0.45–0.86). With pembrolizumab, the median PFS was 9.7 months for patients with CPS ⩾ 10 and 5.6 months with chemotherapy alone (HR 0.74, CI 0.61–0.90, p = 0.0012). In both studies, the treatment effect of ICI blockade increased with PD-L1 enrichment. Now with the approval of these two agents, clinicians must decide between the CPS score measurement and the Ventana SP142 measurement of ICs. The analytical concordance of these two assays has been examined in Impassion130 with an overall percent agreement of 69%, positive percent agreement of 98%, and negative percent agreement of 45%. Patients with Ventana SP142 PD-L1+ and Dako 22C3 IHC PD-L1+ derived the greatest clinical benefit with a combination of atezolizumab and nab-paclitaxel. 28
Tumor-infiltrating lymphocytes
Although tumor-infiltrating lymphocytes (TILs) and CD8+ T cells have been established as prognostic markers for breast cancer treated with chemotherapy,29–33 there are mixed reports of TILs serving as a predictive marker for ICI. For example, one study found increased TILs or CD8+ T cells to be predictive of Overall Response Rate (ORR) and OS with atezolizumab monotherapy. Another found no significant response to atezolizumab with nab-paclitaxel.34,35 Additionally, on-treatment changes versus baseline PD-L1 expression, CD8+ T cells, or stromal TILs were associated with the clinical response. 36 Since the identification of TILs as a robust prognostic marker of chemotherapy sensitivity in early-stage breast cancer, there has been increasing evidence of how cytotoxic chemotherapy may also activate adaptive antitumor immune responses by inducing resistant mediated cell death.29,37 However, the detailed mechanism of the contribution of the TIL to the chemotherapy resistance is less understood. As one of the new potential biomarkers/therapeutic targets, CD73 has been studied more. CD73 converts adenosine triphosphate (ATP) to adenosine and suppresses CD8+ T cells and is associated with increased resistance to doxorubicin in early-stage breast cancer, and doxorubicin can increase CD73 expression. In murine models, CD73 silencing did not affect anti-apoptosis pathways or p-glycoprotein expression, suggesting an alternative method of chemotherapy resistance. 38
Recently, advanced technology and assays such as single-cell genomics and spatial transcriptomics, have been applied to improve the quality of detailed assessment of the TILs, by dissecting into functional TILs, regulatory T cells, and exhausted T cells that can explain the difference response to the ICI therapy in breast cancer treatments.39,40 Application of complex biomarker assessment is inevitable, given the wide adaptation of the ICI in the field of breast oncology; therefore, more data on the use of functional TILs is expected to emerge in the near future. 41
Imaging approaches to expand the tumor stroma investigation
Imaging approaches are also under development. One promising approach is positron-emission tomography (PET) with antibodies to PD-L1. One such study included patients with metastatic TNBC, non-small cell lung cancer, and bladder cancer with zirconium-89-labeled atezolizumab PET imaging at baseline before the start of atezolizumab therapy and uptake better correlated with clinical response compared to PD-L1+ by Immunohistochemistry (IHC). 42 An ongoing trial (NCT04222426) is examining the role of zirconium-89-labeled atezolizumab in metastatic lobular breast cancer receiving treatment with carboplatin combined with atezolizumab.
DNA damage repair pathway
There has been significant interest in BRCA1/2 and germline and somatic mutation of other DNA damage repair (DDR) genes as a predictive response to ICI. Interestingly, in a retrospective analysis, BRCA2 truncating mutations may be predictive of response to ICI in breast and other solid tumors. 43 This may be due to differences in the TME imposed by BRCA1/2 mutant detected on single-cell sequencing and differences in types of DNA aberrations. BRCA2 increases the number of single nucleotide variants and InDels, which may increase neoantigen load resulting in enhanced immunogenicity. MSK-IMPACT, a database of patients treated with ICI, included 44 breast cancer patients identified a 44% clinical benefit rate in this population when the ICI was used, suggesting the potential of integrating DDR into the ICI predictive biomarker. 43
Developing mechanisms of resistance to CDK4/6 inhibitors
Amplification of CDK4
Cyclin-dependent kinase (CDK)4/6 inhibitor revolutionized the life of patients who suffer from Estrogen Receptor (ER) + MBC. Initial efforts to select predictive biomarkers on pre-treatment specimens have been largely unsuccessful. Yet, potential mechanisms of resistance after the patients progressed on CDK4/6 inhibitor are continued to be unraveled thanks to numerous efforts from researchers. More conventional ways to detect the mechanism of resistance use genomic analysis. For example, in a recently reported French study, SAFIR-breast02, 44 the analysis of the post-CDK4/6 inhibitor progressed luminal breast cancer samples revealed alterations in ZMIZ1, FOXM1, AGR2, TACC1, CPNE3, ATG16L2, CDK4, LGR5, NFKBIA, CCL1, KCNG1, LINC00686, and NSL1. Although many of these genes’ copy number alterations call for further investigation, the amplification of CDK4 was particularly interesting. Amplification or mutation in the targets of effective therapeutics is a commonly observed phenomenon, as a mechanism of emerging resistance. This observation provides a rationale for studying enhanced targeting of CDK4, as well as the potential ground of targeting downstream molecules approach.
CDK2, Rb, and others
Cycline Dependent Kinase 2 (CDK2), another key CDK within the family member, has also been recognized as a resistance mechanism of the CDK4/6 inhibition. Cyclin E1 (CCNE1) and 2, constituent activation of E2F have shown to be related to resistance to CDK4/6 inhibitors. 45 CCNE1 gene amplification was also shown to induce resistance in the CDK4/6. CCNE2 gene amplification was also noted in clinically palbociclib progressed tumor samples as well. 13 In addition, the interaction between CDK2 and phosphorylated Rb (pRb) may also play important role in the primary CDK4/6 inhibitor. Based on phosphosite activity, the level of pRb clearly correlated with CDK4 and CDK6 kinase activity scores inferred from phosphosite data, confirming the importance of the intact Rb function for CDK4/6 inhibitor efficacy. Furthermore, in an unsupervised clustering of ER+ and TNBC tumors from the prospective CPTAC Breast Cancer Study based on pRB levels, CDK2 phosphosite, and E2F transcriptional targets showed that the HER2 negative tumors can be segregated into several clusters. The first cluster had low levels of target phosphorylation of CDK2 (suppression of the G1-S transition in the cell cycle) regardless of pRb status and was mainly distributed among luminal A patients. Luminal B and TNBC were clustered into two groups: the group with low levels of both CDK2 and pRB, indicating the abrogation of the G1-S cell cycle transition; and the second group with high levels of both CDK2 and pRb, releasing the activity of CDK2 inhibition. We do not know if these are directly related to CDK4/6 inhibitor efficacy, however, provide an insight as to how complex these pathways are interconnected. Further studies to unravel this complexity is warranted. Other co-mutation or copy number alterations 12 are also commonly observed in ER + MBC, and the detailed correlation with CDK4/6 inhibitor efficacy needs to be tested. Indeed, several clinical trials testing either CDK2 inhibitor alone or an inhibitor to block CDK2/4/6 are actively tested.
Imaging techniques to predict/monitor CDK4/6 inhibitors
Aside from the biological understudies, more practical approaches to detect the CDK4/6 inhibitor resistance using the imaging modality are also actively developed. Mechanistically exploiting progesterone receptor expression as a measure of intact estrogen receptor signaling upon estradiol challenge, a radiolabeled progestin analog, 21-[18F] fluorofuranylnorprogesterone (FFNP), predicts response to endocrine therapy in patients with metastatic HR+ disease determined by IHC with 100% sensitivity and specificity. In this small trial, 65% (28/43 patients) received a combination CDK4/6 inhibitor and endocrine therapy, and FFNP-PET identified 10 nonresponders. 46 A second imaging technique, 13C magnetic resonance spectroscopy may predict PI3Ka inhibitor resistance in PIK3CA-mutated ER+ disease by measuring the intratumoral lactate pool of catalyzed LDHA has been developed as well, and can be potentially utilized upon progression on CDK4/6 inhibitors. 47 The clinical utility of these imaging-based biomarkers is to be further validated.
Biomarker to detect the metastatic spread and novel approaches
Current barriers
Guidelines currently do not support routine surveillance imaging for detecting recurrent metastatic disease in asymptomatic patients since it has previously not been found to change outcomes. 48 However, detecting recurrent disease with a low metastatic burden or oligometastatic disease may change outcomes, particularly when definitive therapy with surgery or radiation is an option. Additionally, with newer agents under development and those recently approved for use, and the development of novel imaging techniques and non-invasive biomarkers, it is possible that initiating treatment sooner may change outcomes. A subgroup of patients may benefit from more tailored surveillance approaches beyond waiting for the development of symptoms. Additionally, in other solid tumors, there is evidence that the burden of disease may influence immunotherapy outcomes where early detection may result in direct patient benefit. 49
However, challenges remain ahead to determine if initiation of treatment and which treatment provides outcome benefit directly to a patient at earlier time points. Other solid tumors, such as prostate cancer, has faced similar challenges, where elevations in the biomarker prostate-specific antigen are detected before radiographic or symptomatic recurrence or progression. Tumor markers in breast cancer are yet to be validated and used as a routine practice. Prospective studies are needed to demonstrate benefits to justify morbidity, the expense of treatment and surveillance imaging studies, and patient education regarding a ‘watch and wait approach’ versus initiation of treatment.
Patient stratification (positive versus negative cut point), the magnitude of expected change, the baseline distribution of the biomarker of interest, and variability in the biomarker measurement process and interpretation can significantly underpowered studies and require a large sample size.
New preclinical model developments
Preclinical modeling incorporating artificial learning and neural networks is inevitable to overcome this complexity. For the development of such artificial learning, the models are only as good as the data derived. Detailed characterization at a molecular level of each tumor is needed at a molecular level required in parallel to develop the best and utilize such technologies. 50 There are far too many combinations of therapies patient-related factors, including histological, stochastic flux of tissue biology imposed by selective pressure, and the variance of disease biology at the biopsy site. Identifying predictive biomarkers in clinical trials is difficult due to the collection of biopsies, on-treatment biopsies, and at progression and may not be powered for such identification.
To overcome this complexity, computational frameworks such as DrugCell are a powerful tool in identifying drug combinations derived from large-scale in vivo cell line drug screening resources and in vivo patient-derived xenograft models. 51 As a proof of concept, DrugCell examined 221 patients with ER + MBC previously treated with fulvestrant and CDK4/6 inhibitor fulvestrant or mTOR inhibitor everolimus and predicted sensitivity (48.2 versus 33.6 months, p = 0.018) and through unbiased PI3K signaling as an important response to mTOR inhibition and CDK activity for CDK4/6 inhibition. 51 Incorporating other ‘omics’ into machine learning algorithms will be necessary, such as large-scale reverse-phase protein array drug screens. 52 This will need to occur in parallel with the application of multilevel proteogenomic analysis with reverse-phase protein arrays, whole-exome sequencing, and RNA sequencing, and epigenetic modifications will also contribute to accurately profiling heterogeneous nature of breast cancer for therapeutic vulnerabilities. 53
Development of in vivo metastasis models with ‘barcoded’ cells is beginning to unravel the complexities of heterogeneity at the single-cell level and characteristics that promote metastasis to individual tissue sites, for instance, not only beyond ERBB2 gene amplification which has been well-established risk marker for CNS metastasis, but also PIK3CA-mt and PTEN loss and perhaps uniquely depending on CNS is metastasis is SREBF1-mt involved in lipid synthesis downstream of PIK3CA which needs additional validation. 54 Interestingly, olaparib decreases SREBF1 and the energy dependence of the cell from glycolysis to the lipid metabolism. 55 Therefore, an additional investigation should be carried out to identify agents that regulate lipid metabolism and undergo preclinical development in patients with aberrations.
Novel material for biomarker detection and processing
The discovery of biomarkers of therapeutic resistance has improved early diagnosis, prognosis, therapeutics, and systematized the escalation or de-escalation of therapy. There is increased demand for fresh tumor tissue in clinical trials for biomarker development, understanding resistance mechanisms, and evaluating response. The tissue biopsy is associated with anxiety and causes discomfort to the patients. Despite this, in oncology clinical trials from 2000 to 2015, only 50% of trials that included a research biopsy-related endpoint reported on these biopsy results. 56 Compared with diagnostic biopsies, patients are less likely to accept associated risks with research biopsies as the potential cost of time and biopsy risks are usually at the expense of the patient. 57
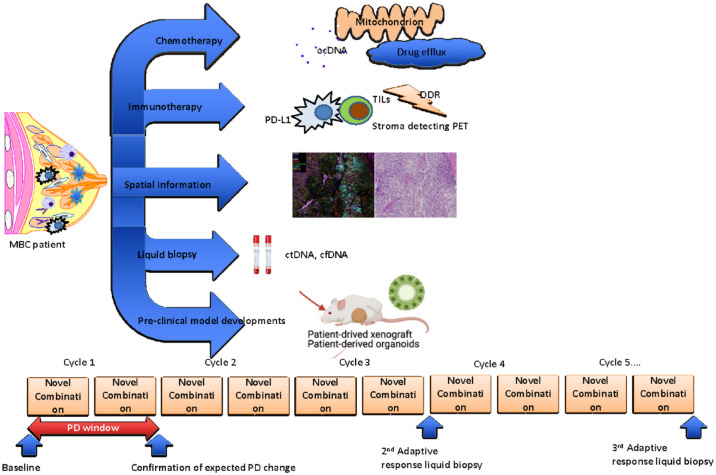
Additional considerations include the collection, handing, and processing of the specimen, research biopsy arrangement with interventional radiologists, and other barriers such as a lack of sufficient viable tumor, intratumoral heterogeneity. 58 An analysis of four historical clinical trials conducted at the National Cancer Institute’s Developmental Therapeutics Clinic showed that only 74% of samples collected met the required quality control criteria for the intended assay and for trials in which two adequate biopsied (pretreatment and post-treatment) resulted in a success rate of 50%.59,60 The suggested clinical study platform integrating novel approaches to detect and develop biomarkers based on biospecimen collection is illustrated in the Figure 1.
Figure 1.
Overview of drug-resistance mechanisms and novel methods to detect metastatic progression. There are several under-studied predictive biomarkers under development to tailor the management of breast cancer and the application of integrative approaches that have resulted in the promising candidate biomarker discovery. Spatial information, liquid biopsy, and new preclinical model developments are expected to expand the new ways to determine better therapeutic strategies in metastatic breast cancer.
Liquid biopsy
There is a significant need for noninvasive biomarkers such as protein, enzymes, circulating tumor cells (CTCs) or cell-free DNA (cfDNA), or nucleic acids released by the tumor cells.61,62
Liquid biopsy detects CTCs or cellular components released from cancer cells such as cfDNA, or specifically circulating tumor DNA (ctDNA), circulating extracellular-vesicles. Specific known mutations in the primary tumor can be detected in ctDNA using qPCR or digital Polymerase Chain Reaction (PCR) (BEAMING and droplet digital PCR). A second approach is through genome-wide analysis of CNAs or point mutation through next-generation sequencing. A combination of DNA methylation and fragmentation with targeted or wide-genome sequencing can improve the detection of ctDNA. Those currently under development primarily for the detection of early-stage disease utilize cell-free methylation profiling techniques that are both sensitive and specific.
Currently under development in early-cancer detection through the Circulating Cell-free Genome Atlas Study and awaiting validation in the ongoing STRIVE breast cancer study, it may be applied to detect recurrent metastatic disease (NCT03085888). 63 A similar cell-free methylated DNA technique called cfMeDIP-seq is also under development which characterizes the methylome without needing to sequence the whole genome or extensive discovery studies to find breast-unique methylation profiles of the genome informed by machine learning algorithms to develop panels to target specific regions. 64
Temporal/spatial characterization: propensity of organ-specific metastasis
Tumor heterogeneity is well known in different metastatic disease sites with loss or gain of essential molecular markers that may lead to changes in therapeutic options. Understanding tumor intrinsic mechanisms that drive site-specific metastasis may identify new therapeutic targets. Rapid autopsy protocols offer a unique opportunity to procure tissue for in-depth analysis across metastatic sites of disease to understand disease evolution and heterogeneity.35,65–67 Variability in the IC composition in the TME also differs and may contribute to organ-specific metastasis. A rapid autopsy study (post-morten) showed that there were differences in IC infiltration across different parts of lungs, such as differences in CD8+ tissue-resident T cells. 68
Conclusion and future direction
In understanding the natural history, molecular underpinnings, and introduction of genomic assays, use of biomarkers for treatment decision-making. Other emerging tumor-based markers could augment current clinical or molecular predictive classifiers to identify individuals at risk for resistant or sensitive disease in real-time beyond present tumor-based genomic signatures and standard clinical and pathologic features. Clinical validation of such approaches will provide a more extensive portfolio of therapeutic options for patients in need. Adaptation of Bayesian statistical models based on the predictive/prognostic markers may allow testing of such therapeutics to be tested in a rapid timeline for much-needed translations into the clinic.
Acknowledgments
None.
Footnotes
ORCID iD: Bora Lim  https://orcid.org/0000-0002-4182-6058
https://orcid.org/0000-0002-4182-6058
Contributor Information
Evthokia A. Hobbs, Hematology and Oncology, Oregon Health & Science University, Portland, OR, USA
Natalie Chen, Hematology and Oncology, Baylor College of Medicine, Houston, TX, USA.
Alphi Kuriakose, Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, TX, USA.
Elizabeth Bonefas, Surgical Oncology, Baylor College of Medicine, Houston, TX, USA.
Bora Lim, Hematology and Oncology, Baylor College of Medicine, One Baylor Plaza, BCM600, Houston, TX 70030, USA; Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, TX, USA.
Declarations
Ethics approval and consent to participate: NA (review article).
Consent for publication: We confirm that this manuscript has never been submitted to other journals. All authors fully consent to publish this work.
Author contribution(s): Evthokia A. Hobbs: Conceptualization; Data curation; Investigation; Writing – original draft.
Natalie Chen: Investigation; Writing – original draft.
Alphi Kuriakose: Data curation; Writing – original draft.
Elizabeth Bonefas: Data curation; Investigation; Writing – original draft.
Bora Lim: Conceptualization; Data curation; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing Interests: The corresponding author, BL reports research funding from Novartis, Genentech, Puma Biotechnology, Takeda Oncology, Celcuity, Merck. All other authors declare that there is no reportable COI.
Availability of data and materials: Not applicable.
References
- 1. Nakagawa Y, Abe S, Kurata M, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol 2006; 81: 824–831. [DOI] [PubMed] [Google Scholar]
- 2. Sakurai K, Yamasaki S, Nakao K, et al. Crystal structures of multidrug efflux pump MexB bound with high-molecular-mass compounds. Sci Rep 2019; 9: 4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. New Engl J Med 2019; 380: 741–751. [DOI] [PubMed] [Google Scholar]
- 4. Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol 2013; 31: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 5. Torres S, Maralani P, Verma S. Activity of T-DM1 in HER-2 positive central nervous system breast cancer metastases. BMJ Case Rep 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao P, Meng Q, Liu LZ, et al. Regulation of survivin by PI3K/Akt/p70S6K1 pathway. Biochem Biophys Res Commun 2010; 395: 219–224. [DOI] [PubMed] [Google Scholar]
- 7. Liu F, Liu S, He S, et al. Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells. Oncol Rep 2010; 23: 1469–1475. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Song J, Zhao Z, et al. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Lett 2020; 470: 84–94. [DOI] [PubMed] [Google Scholar]
- 9. Williams KE, Bundred NJ, Landberg G, et al. Focal adhesion kinase and Wnt signaling regulate human ductal carcinoma in situ stem cell activity and response to radiotherapy. Stem Cells 2015; 33: 327–341. [DOI] [PubMed] [Google Scholar]
- 10. Sobel K, Tham M, Stark HJ, et al. Wnt-3a-activated human fibroblasts promote human keratinocyte proliferation and matrix destruction. Int J Cancer 2015; 136: 2786–2798. [DOI] [PubMed] [Google Scholar]
- 11. Shi B, Liang J, Yang X, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Cell Mol Biol 2007; 27: 5105–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, hormone receptor-positive advanced breast cancer. New Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 13. Hortobagyi GN, Chen D, Piccart M, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 2016; 34: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dent RK, Kim SB, Oliveira M, et al. Double-blind placebo-controlled randomized phase III trial evaluating first-line ipatasertib combined with paclitaxel for -PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer. In: 2020 San Antonio breast cancer symposium, 2020, abstract GS3-04, San Antonio, TX. [Google Scholar]
- 15. Reyno L, Seymour L, Tu D, et al. Phase III study of N,N-diethyl-2-[4-(phenylmethyl) phenoxy]ethanamine (BMS-217380-01) combined with doxorubicin versus doxorubicin alone in metastatic/recurrent breast cancer: National Cancer Institute of Canada Clinical Trials Group Study MA.19. J Clin Oncol 2004; 22: 269–276. [DOI] [PubMed] [Google Scholar]
- 16. Cao F, Liu T, Xu Y, et al. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int J Clin Exp Pathol 2015; 8: 6037–6045. [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Wang C, Bu G. Curcumin inhibits the growth of liver cancer stem cells through the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Exp Ther Med 2018; 15: 3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martel F, Guedes M, Keating E. Effect of polyphenols on glucose and lactate transport by breast cancer cells. Breast Cancer Res Treat 2016; 157: 1–11. [DOI] [PubMed] [Google Scholar]
- 19. FDA. FDA label information: Doxil. Ortho Biotech Products, LP Raritan, NJ, 2007. [Google Scholar]
- 20. Zeng X, Wan M, Wu J. ecDNA within tumors: a new mechanism that drives tumor heterogeneity and drug resistance. Signal Transduct Target Ther 2020; 5: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H, Nguyen N-P, Turner K, et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat Genet 2020; 52: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baek ML, Lee J, Pendleton KE, et al. Mitochondrial structure and function adaptation in residual triple negative breast cancer cells surviving chemotherapy treatment. bioRxiv, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-Mediated proteolysis induces selective cancer cell lethality. Cancer Cell 2019; 35: 721–737.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 44–59. [DOI] [PubMed] [Google Scholar]
- 25. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020; 396: 1817–1828. [DOI] [PubMed] [Google Scholar]
- 26. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. New Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 27. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019; 5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rugo HS, Loi S, Adams S, et al. PD-L1 immunohistochemistry assay comparison in atezolizumab plus nab-paclitaxel-treated advanced triple-negative breast cancer. J Natl Cancer Inst 2021; 113: 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018; 19: 40–50. [DOI] [PubMed] [Google Scholar]
- 30. Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 2016; 22: 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; 25: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 32. Miyashita M, Sasano H, Tamaki K, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2014; 148: 525–534. [DOI] [PubMed] [Google Scholar]
- 33. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31: 860–867. [DOI] [PubMed] [Google Scholar]
- 34. Gianni L, Huang C-S, Egle D. Abstract GS3-04: pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res 2020; 80: GS3-04. [DOI] [PubMed] [Google Scholar]
- 35. Smith EA, Hodges HC. The spatial and genomic hierarchy of tumor ecosystems revealed by single-cell technologies. Trends Cancer 2019; 5: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams S, Schmid P, Rugo HS, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol 2017; 35: 1008–1008. [Google Scholar]
- 37. Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 2018; 24: 986–993. [DOI] [PubMed] [Google Scholar]
- 38. Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. OncoImmunology 2013; 2: e24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kagohara LT, Zamuner F, Considine M, et al. Integrated single cell and bulk multi-omics reveals heterogeneity and early changes in pathways associated with cetuximab resistance in HNSCC sensitive cell lines. bioRxiv, 2019. [Google Scholar]
- 40. Zhang S, Gong C, Ruiz-Martinez A, et al. Integrating single cell sequencing with a spatial quantitative systems pharmacology model spQSP for personalized prediction of triple-negative breast cancer immunotherapy response. Immunoinformatics (Amsterdam, Netherlands) 2021; 1–2: 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hudson WH, Sudmeier LJ. Localization of T cell clonotypes using spatial transcriptomics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 2021. [Google Scholar]
- 42. Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018; 24: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 43. Samstein RM, Krishna C, Ma X, et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nature Cancer 2021; 1: 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. André F, Gonçalves A, Filleron T, et al. Abstract GS1-10: clinical utility of molecular tumor profiling: results from the randomized trial SAFIR02-BREAST. Cancer Res 2022; 82: GS1–GS10. [Google Scholar]
- 45. Taylor-Harding B, Aspuria P-J, Agadjanian H, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget 2015; 6: 696–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dehdashti F, Wu N, Ma CX, et al. Association of PET-based estradiol-challenge test for breast cancer progesterone receptors with response to endocrine therapy. Nat Commun 2021; 12: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ros S, Wright AJ, D’Santos P, et al. Metabolic imaging detects resistance to PI3Kα inhibition mediated by persistent FOXM1 expression in ER+ breast cancer. Cancer Cell 2020; 38: 516–533.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ueno NT, Espinosa Fernandez JR, Cristofanilli M, et al. International consensus on the clinical management of Inflammatory Breast Cancer from the Morgan Welch Inflammatory Breast Cancer research program 10th anniversary conference. J Cancer 2018; 9: 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin X, Demere Z, Nair K, et al. A metastasis map of human cancer cell lines. Nature 2020; 588: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuenzi BM, Park J, Fong SH, et al. Predicting drug response and synergy using a deep learning model of human cancer cells. Cancer Cell 2020; 38: 672–684.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao N, Guo M, Wang K, et al. Identification of Pan-Cancer prognostic biomarkers through integration of multi-omics data. Front Bioeng Biotechnol 2020; 8: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krug K, Jaehnig EJ, Satpathy S. Proteogenomic landscape of breast cancer tumorigenesis and targeted therapy. Cell 2020; 183: 1436–1456.e1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park JH, de Lomana ALG, Marzese DM, et al. A systems approach to brain tumor treatment. Cancers 2021; 13: 3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehta AK, Cheney EM, Hartl CA, et al. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nature Cancer 2021; 2: 66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parseghian CM, Tam AL, Yao J, et al. Ellis LM, Raghav K, Overman MJ. Assessment of reported trial characteristics, rate of publication, and inclusion of mandatory biopsies of research biopsies in clinical trials in oncology. JAMA Oncol 2019; 5: 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lemech C, Dua D, Newmark J, et al. Patients’ perceptions of research biopsies in phase I oncology trials. Oncology 2015; 88: 95–102. [DOI] [PubMed] [Google Scholar]
- 58. Zhao T, Chiang ZD, Morriss JW, et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature 2022; 601: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parchment RE, Doroshow JH. Pharmacodynamic endpoints as clinical trial objectives to answer important questions in oncology drug development. Semin Oncol 2016; 43: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ferry-Galow KV, Ji J, Kinders RJ, et al. Pharmacodynamic analyses in a multi-laboratory network: lessons from the poly(ADP-ribose) assay. Semin Oncol 2016; 43: 492–500. [DOI] [PubMed] [Google Scholar]
- 61. O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 2018; 9: 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Allin DM, Shaikh R, Carter P, et al. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur J Cancer 2018; 103: 165–175. [DOI] [PubMed] [Google Scholar]
- 63. Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020; 31: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nassiri F, Chakravarthy A, Feng S, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med 2020; 26: 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Casasent AK, Schalck A, Gao R, et al. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 2018; 172: 205–217.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim C, Gao R, Sei E, et al. Chemoresistance evolution in Triple-negative breast cancer delineated by single-cell sequencing. Cell 2018; 173: 879–893.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lawson DA, Kessenbrock K, Davis RT, et al. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol 2018; 20: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Egelston C, Guo W, Yost S, et al. Pre-existing effector T-cell levels and augmented myeloid cell composition denote response to CDK4/6 inhibitor palbociclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. J Immunother Cancer 2021; 9: e002084. [DOI] [PMC free article] [PubMed] [Google Scholar]