Abstract
Background
Both inflammation and thrombotic/hemostatic mechanisms may play a role in acute ischemic stroke (AIS) pathogenesis, and a biomarker, such as the platelet-to-lymphocyte ratio (PLR), considering both mechanisms may be of clinical utility.
Objectives
This meta-analysis sought to examine the effect of PLR on functional outcomes, early neurological changes, bleeding complications, mortality, and adverse outcomes in AIS patients treated with reperfusion therapy (RT).
Design
Systematic Review and Meta-Analysis
Data Sources and Methods
Individual studies were retrieved from the PubMed/Medline, EMBASE and Cochrane databases. References thereof were also consulted. Data were extracted using a standardised data sheet, and systematic reviews and meta-analyses on the association of admission (pre-RT) or delayed (post-RT) PLR with defined clinical and safety outcomes were conducted. In the case of multiple delayed PLR timepoints, the timepoint closest to 24 hours was selected.
Results
Eighteen studies (n=4878) were identified for the systematic review, of which 14 (n=4413) were included in the meta-analyses. PLR collected at admission was significantly negatively associated with 90-day good functional outcomes (SMD=−.32; 95% CI = −.58 to −.05; P=.020; z=−2.328), as was PLR collected at delayed timepoints (SMD=−.43; 95% CI = −.54 to −.32; P<.0001; z=−7.454). PLR at delayed timepoints was also significantly negatively associated with ENI (SMD=−.18; 95% CI = −.29 to −.08; P=.001. Conversely, the study suggested that a higher PLR at delayed timepoints may be associated with radiological bleeding and mortality. The results varied based on the type of RT administered.
Conclusions
A higher PLR is associated with worse outcomes after stroke in terms of morbidity, mortality, and safety outcomes after stroke.
Keywords: stroke, endovascular therapy, meta-analysis, platelet-lymphocyte ratio, reperfusion therapy
Background
Acute ischemic stroke (AIS) forms the vast majority of stroke and has a large burden of disease and death, with sizeable case-fatality and disability rates.1,2 As such, the identification of a prognostic biomarker that could inform treatment decision making in AIS has attracted great research interest. 3 Blood-based biomarkers are of particular clinical interest because they are easier and cheaper to obtain than imaging-based biomarkers. 4 The platelet-lymphocyte ratio (PLR) is such a biomarker that has shown utility in emergency medicine and trauma settings, 5 acute illnesses such as acute coronary syndrome, 6 and cardiovascular reperfusion.7,8 It is particularly promising as it could potentially provide insight into both inflammation and thrombotic/hemostatic mechanisms thought to play a role in AIS pathogenesis, whereas other biomarkers shown to have prognostic value such as C-Reactive Protein, platelet count and the neutrophil-lymphocyte ratio encompass only one of these mechanisms.4,9-11 While there has been some evidence showing benefit of PLR in predicting clinical outcomes,12-19 mortality12,16,17,19-21 and bleeding risk12,17,18,22,23 in AIS patients treated with reperfusion therapy (RT), this is yet to be clearly elucidated; to the best of our knowledge there is no systematic review or meta-analysis currently in the literature evaluating this. As such, this study aims to investigate the association of PLR at admission and PLR collected at delayed timepoints through a systematic review and meta-analysis of published literature, and to gauge the clinical utility thereof in prognostication and clinical decision-making.
Our underlying research questions are as follows; in AIS patients receiving RT:
•Is lower baseline or delayed PLR associated with (1) good functional outcomes; (2) modified Rankin scale (mRS) 0-1; (3) favourable recanalisation outcomes; (4) early neurological improvement (ENI), and (5) dramatic ENI?
•Is higher baseline or delayed PLR associated with: (1) mortality; (2) intracerebral haemorrhage (ICH) (3) symptomatic ICH (sICH); (4) early neurological deterioration (END) and (5) stroke associated infection (SAI)?
Methods
The study was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 24 flowchart (Figure 1) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Checklist (Supplemental Table 3). 25 Ethics approval was not required as this study was a systematic review and meta-analysis of previously published studies.
Figure 1.
PRISMA Diagram. Note: The PRISMA flowchart shows the main characteristics of the included studies. Outcomes for which a meta-analysis could successfully be carried out also have the number of patients shown. Abbreviations: mRS=Modified Rankin Scale; SAI=Stroke Associated Infection; SAP=Stroke Associated Pneumonia; sICH=symptomatic intracerebral hemorrhage; PH=Parenchymal Hematoma; PLR=Platelet-Lymphocyte Ratio; N=Number of Included Studies; n=number of patients.
Literature Search: Identification and Selection of Studies
The following databases were searched: Embase, PubMed/Medline, and Cochrane Library until 30 October 2021. Keywords used included a combination of terms including “acute stroke”, “cerebrovascular accident”, “brain ischemia”, “reperfusion”, “endovascular therapy,” “endovascular thrombectomy”, “thrombolysis”, “PLR” and “platelet-lymphocyte ratio.” Full search strategies and a complete list of keywords are provided in the Supplementary Information (search strategy). In addition, references of related articles were also examined to retrieve studies relevant to our analysis.
Inclusion and Exclusion Criteria
Following Inclusion criteria were applied: (1) patients aged 18 years or above; (2) patients diagnosed with AIS; (3) patients who received RT; and (4) studies with good methodological design (including sufficient sample size, defined as >20 patients). The exclusion criteria were: (1) animal/preclinical studies; (2) duplicated publications; (3) studies with smaller sample sizes or shorter study periods, where multiple studies from overlapping centres with varying study periods reporting similar outcomes were present; (4) full-text articles not available; (5) systematic reviews or meta-analyses, conference abstracts, letters and case reports or series; and (6) studies presented as abstracts, with relevant PLR or outcome data not reported.
Data Extraction
Titles and abstracts were first reviewed on Endnote to exclude articles mismatched to eligibility criteria. The remaining articles underwent thorough full text examination to determine if they were to be included in the systematic review or meta-analysis as per the eligibility criteria. Reviews, former systematic reviews and meta-analyses and opinion articles were kept separately for discussion in the manuscript. Two authors conducted the screening independently, and any disagreements were discussed until a consensus was made. Data from each study/trial were extracted independently using a standardised data extraction sheet to obtain the following information: (1) baseline demographics: author, country, and year of publication; (2) study population: age of patients, sample size, characteristics of acute stroke patients, and RT type (EVT/IVT); (3) PLR; (4) time of blood collection (preintervention vs postintervention; for delayed timepoints, the timepoint closest to 24 hours was included); (5) outcome measures: primary and secondary outcomes, including clinical outcomes, angiographic outcomes, and mortality; and (6) adverse effects/safety outcomes. The primary outcome was in terms of morbidity: 90-day good functional outcomes, defined as mRS 0-2 across all studies. One study looked at mRS 0-1 and was considered separately. Mortality was defined at 90 days in all studies. Regarding angiographic outcomes, successful recanalisation was defined as mTICI≥2b across all included studies, and where applicable, the first pass effect (FPE) as complete recanalisation (mTICI 3) achieved with a single pass. In all studies, ENI was defined in terms of improvements in NIHSS score, with this generally being 4 points in 24 hours, unless otherwise indicated (Table 2). Dramatic ENI was defined as improvement in NIHSS score by 8 points across all studies reporting this outcome. END was conversely defined as NIHSS score worsening across all studies, with any specifications on this indicated (Table 2). sICH was determined by neurological decline along with imaging confirmation across all studies, with criteria such as European Cooperative Acute Stroke Study-I (ECASS-I) and Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) used. The radiological bleed outcome was defined as any radiological evidence of bleeding, with CT being the most common imaging modality used to ascertain this, and some studies using additional MRI.
Table 2.
Study Characteristics for studies included in the meta-analysis.
| Id | Author | Year | Study Type (R/P) | Country | Reperfusion | Cohort Size | PLR Blood Collection Time-point | PLR (Mean, SD) | Outcome Proportions (n (%)) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENI | DENI | END | GFOs | mRS 0-1 | Mortality | sICH | RB | SR | SAI/SAP | |||||||||
| 1 | Yi et al 50 | 2021 | R | NS^ | EVT±IVT | 440 | Admission | 127.27 (117.37) | 245 (55.68) | 32 (7.27) | 106 (24.09) | 399 (90.68) | ||||||
| 2 | Chen, lin et al 13 | 2019 | P | China | IVT | 241 | Admission | 140.1 (66.5) | 29** (12.03) | 136 (56.43) | 25 (10.37) | 14 (5.81) | 56 (23.24) | 65 (26.97) | ||||
| 18 to 24 hr | 165.4 (85.4) | |||||||||||||||||
| 3 | Ferro et al 23 | 2021 | R | Portugal | All | 325 | After RT, within 24 hours of onset | 189.3 (126.59) | 85 (26.15) | 147 (45.23) | 149 (78.42) | 48 (14.80) | ||||||
| 4 | Ozgen et al 16 | 2020 | R | Turkey | EVT±IVT | 150 | Admission | 146.73 (78.75) | 58 (38.7) | 33 (22) | 122*** (81.3) | |||||||
| 5 | Topcuoglu et al 18 | 2020 | R | Turkey | IVT | 165 | Before IVT | 138.45 (86.15) | 86 (52.12) | 47 (28.48) | 81 (49.09) | 54 (32.73) | 11 (6.67) | 42 (25.45) | ||||
| 24 hours after IVT | 184.77 (130.00) | |||||||||||||||||
| 6 | Xu et al 19 | 2019 | R | China | IVT±EVT | 286 | Within 24 hours of onset | 155 (88.67) | 166 (58.04) | 38 (13.29) | ||||||||
| 7 | Feng et al 22 | 2020 | R | China | EVT±IVT | 90 | Admission | 204.50 (135.80) | 43 (47.78) | 34 (37.78) | 76& (84.44) | |||||||
| 8 | Sengeze et al 34 | 2020 | R | China | EVT±IVT### | 91 | Admission | 156.51 (97.57) | 28 (30.77) | 35 (38.46) | 19 (20.88) | 43 (47.78) | ||||||
| 9 | Sarioglu et al 35 | 2020 | R | Turkey | EVT±IVT | 83 | Admission | 155.36 (92.09) | 49 (59.04) | 13 (15.66) | 17 (20.48) | 65 (78.31) | ||||||
| 10 | Gong et al 15 | 2021 | P | China | IVT±EVT | 1060 | Admission | 140.62 (62.18) | 398# (37.55) | 193## (18.21) | 82 (7.74) | |||||||
| 11 | Deng et al 49 | 2020 | P | China | IVT | 337 | Admission | 191.67 (99.03) | 141 (41.84) | |||||||||
| EVT±IVT | 333 | Before EVT | 143 (62.54) | 296 (88.89) | 219 (65.77) | |||||||||||||
| 12 | Chen, ren et al 20 | 2021 | R | China | TPA | 280 | 24 hours | 130.47 (54.54) | 194 (69.28) | 27 (9.64) | 6 (2.14) | |||||||
| 13 | Eren et al 14 | 2021 | R | Turkey | IVT | 250 | Admission | 136.59 (78.38) | 114&& (45.6) | 27 (10.8) | ||||||||
| 14 | Lee et al 33 | 2021 | R | Korea | EVT±IVT | 282 | At hospital admission, before EVT | 129.28 (73.07) | 224 (79.43) | |||||||||
| 15* | Inanc & inanc 21 | 2018 | R | Turkey | EVT±IVT | 56 | Admission | 29.95 (57.15) | 23 (41.07) | 24 (42.86) | 33&&& (58.93) | |||||||
| 16* | Altintas et al 12 | 2016 | R | Turkey | EVT only | 57 | Admission | 32.55 (1306.81) | 23 (40.35) | 17 (29.82) | 19 (.33) | 42 (73.68) | ||||||
| 17* | Diestro et al 32 | 2021 | R | Canada | EVT±IVT | 252 | Pre-EVT | 181.75 (162.56) | SITS-MOST: 16 (6.35) NINDS: 25 (9.92)^^ | 74 (29.37) | 208 (82.54) | |||||||
| 18* | Chen, Li et al 51 | 2021 | R | Taiwan | IVT±EVT | 100 | Baseline | 123 (108.3) | 42 (42) | 9 (9) | 26^^^ (72.22) | |||||||
| Post rtTPA | 167.7 (123.3) | |||||||||||||||||
All values were provided to 2 decimal places where rounding was required. Where data was not available, this was left blank. Definitions of outcomes were as outlined in text unless specified otherwise.
*Only included in the systematic review.
**Alternate definition was NIHSS recovery to 0-1 at 24 hours after treatment.
***Definition not clearly specified.
&Definition via eTICI scale.
&&Improvement in NIHSS by 5 points at discharge.
&&&Definition via Thrombolysis in Brain Ischemia Scale.
#Alternate definition was complete recovery at 24 hours after treatment.
##NIHSS decrease defined as 4 points.
###Some patients were also given IA tPA.
^All authors were affiliated with institutions in Korea.
^^Both SITS and NINDS criteria used.
^^Only 36 patients received EVT; percentage reflects this.
Abbreviations: R=Retrospective; P=Prospective; ENI=Early Neurological Improvement; DENI=Dramatic ENI; END=Early Neurological Decline; GFOs=Good Functional Outcomes; PLR=Platelet-Lymphocyte Ratio; sICH=symptomatic intracerebral haemorrhage; RB=Radiological Bleed; SR=Successful Recanalisation; SAI=Stroke Associated Infection; SAP=Stroke Associated Pneumonia; NS=Not Specified; EVT=Endovascular Therapy; IVT=Intravenous Thrombolysis; RT=Reperfusion Therapy; NS=Not Specified; SITS=Safe Implementation of Thrombolysis in Stroke-Monitoring Study; NINDS=National Institute of Neurological Disorders and Stroke.
Quality Assessment of Included Studies
The methodological quality of each study was assessed using the modified Jadad scale by two researchers independently. 26 The scale evaluates study quality based on the following evaluation criteria: randomisation, blinding, withdrawals, dropouts, inclusion/exclusion criteria, adverse effects, and statistical analysis. A double-blind study received 1 score; a single blind study received .5 scores. The total score for each study ranged from 0 to 8 points, and studies were divided into low-quality (0-3 points) and high-quality (4-8 points) levels.
The risk of funding bias in the included studies was evaluated independently from the quality assessment by using the scoring test developed by Saunders et al 27 (2017), which analyses the declaration of funding sources and conflicts of interest. A score of 1-2 was considered to indicate moderate potential for bias. The absence of industry funding was not considered to signify an absence of bias, but the presence of industry funding or conflicts of interest was assumed to indicate bias.
Statistical Analysis
All statistical analyses were performed using STATA (Version 13.0, StataCorp LLC, College Station, Texas, USA). Forest plots were generated to present the standard mean difference (SMD), P values, 95% confidence intervals (CI), percentage weight and heterogeneity between studies included in the meta-analysis. Meta-analyses were split by admission PLR and delayed PLR (pre- and postintervention, respectively). In cases where there were multiple delayed PLR timepoints, the timepoint closest to 24 hours was taken (Table 2). I2 statistics and P values were used to assess heterogeneity between studies, with <40%, 30-60%, 50-90% and 75-100% representing low, moderate, substantial, and considerable heterogeneity, respectively. 28 Random-effects modelling was used across all subgroup analyses. Subgroup analyses stratified by treatment groupxxxs were performed for all outcomes, with any adjunct treatment indicated by ‘±‘. Baseline characteristics of patient populations were synthesized from all included studies (Table 1). Where applicable, median, and interquartile ranges were converted to mean and standard deviation using Wan et al.‘s (2012) method, and median and ranges were converted to mean and standard deviation using the methods described by Luo et al (2018) 29 and Wan et al (2014), 30 respectively. For studies where SD was not available, we used the method proposed by Walter and Yao 31 (2007) to determine the SD, assuming data were normally distributed. Combined means were calculated where applicable. A (Begg’s) funnel plot was used to visually detect the presence of publication bias in the meta-analysis; asymmetry was indicative of publication bias. This was confirmed using Egger’s test of effect sizes for publication bias. The command “metaling” was used in STATA to determine the impact of individual studies on the overall meta-analysis (Supplemental Figure 1). P values <.05 were considered statistically significant.
Table 1.
Summary of combined clinical characteristics, risk factors and stroke etiologies across all included studies.
| Factor | Number of Patients for Whom Data was Available | Number of Patients with Factor | % or Mean (±SD) |
|---|---|---|---|
| Age (yrs.) | 4788 | N/A | 69.67 ± 13.25 |
| Male gender | 4713 | 2784 | 59.07 |
| Baseline NIHSS | 4348 | N/A | 11.51 ± 7.67 |
| Baseline PLR | 4553 Excluding Inanc & Inanc:* 4497 |
91.23 ± 168.51 Excluding Inanc & Inanc:* 91.99 ± 169.29 |
|
| Delayed PLR | 1111 | 166.66 ± 106.70 | |
| BSBP | 2936 | 149.60 ± 24.62 | |
| Etiology | |||
| LAA | 3533 | 1134 | 32.10 |
| CE | 3533 | 1260 | 35.66 |
| SVO | 2721 | 440 | 16.17 |
| Other and/or undetermined | 3533 (as reported) 2384 (excluding studies not reporting SVO to avoid overlap) | 571 (as reported) 384 (excluding studies not reporting SVO to avoid overlap) | 16.16 (as reported) 16.11 (excluding studies not reporting SVO to avoid overlap) |
| Risk factors | |||
| CAD | 3476 | 758 | 21.81 |
| AF | 4306 | 1270 | 29.49 |
| HTN | 4361 | 2869 | 65.79 |
| DM | 4713 | 1173 | 24.89 |
| HL/DL | 3757 | 1255 | 33.40 |
| Smoking | 3111 | 916 | 29.44 |
| PS/TIA | 3877 | 757 | 19.53 |
*Inanc & Inanc was excluded, and the analysis was repeated to ensure that this did not skew the data because it was an outlier compared to other studies.
Abbreviations: LAA=Large Artery Atherosclerosis; CE=Cardioembolic; SVO=Small Vessel Occlusion; CAD=coronary artery disease; AF=Atrial Fibrillation; HTN=Hypertension; DM=Diabetes Mellitus; HL=Hyperlipidemia; DL=Dislipidemia; PS=Previous Stroke; TIA=Transient Ischemic Event; BSBP=Baseline Systolic Blood Pressure; PLR=Platelet-Lymphocyte Ratio.
Results
Description of Included Studies
The total number of patients considered in the meta-analysis was 4413. An additional four studies, with 465 patients, were included in the systematic review. The mean age was 69.67 (SD 13.25) years. The clinical characteristics of all studies included in the meta-analysis are shown in Table 1, details about outcomes in all studies in Table 2, and details about PLR stratified by outcome in Table 3. The results of the methodological quality and funding bias assessment are provided in Supplemental Table 1.
Table 3.
Platelet-Lymphocyte Values Stratified by Outcome.
| Id | Author | PLR Time-point | Outcome Group | Patients (n) | PLR Value (Mean (SD)) |
|---|---|---|---|---|---|
| 1 | Yi et al 50 | Admission | GFOs | 245 | 119.2 (108.5) |
| No GFOs | 195 | 137.4 (127.2) | |||
| 2 | Chen, lin et al 13 | Admission | GFOS | 136 | 134.9 (60.6) |
| No GFOs | 105 | 146.7 (73.3) | |||
| Delayed | GFOS | 136 | 148.4 (78.9) | ||
| No GFOs | 105 | 187.5 (88.6) | |||
| 3 | Ferro et al 23 | Delayed | GFOS | 147 | 163.67 (104.82) |
| No GFOs | 178 | 215 (133.79) | |||
| END | 85 | 230.67 (157.62) | |||
| No END | 240 | 229.33 (152.89) | |||
| RB* | 168 | 214.32 (143.79) | |||
| No RB* | 160 | 168.00 (109.97) | |||
| 4 | Ozgen et al 16 | Admission | GFOS | 92 | 123.54 (44.13) |
| No GFOs | 58 | 183.5 (104.2) | |||
| Mortality | 33 | 178.71 (79.60) | |||
| No mortality | 117 | 144.76 (103.77) | |||
| 5 | Topcuoglu et al 18 | Admission | mRS 0-1** | 54 | 131 (79) |
| No mRS 0-1** | 110 | 142 (89) | |||
| GFOs | 81 | 130 (79) | |||
| No GFOs | 84 | 147 (92) | |||
| RB | 43 | 155 (105) | |||
| No RB | 122 | 134 (78) | |||
| sICH | 11 | 215 (112) | |||
| No sICH | 154 | 134 (81) | |||
| ENI | 86 | 126 (82) | |||
| No ENI | 79 | 152 (89) | |||
| DENI | 47 | 133 (85) | |||
| No DENI | 118 | 141 (87) | |||
| Delayed | mRS 0-1 | 54 | 141 (62) | ||
| No mRS 0-1 | 110 | 207 (146) | |||
| GFOs | 81 | 146 (66) | |||
| No GFOs | 84 | 223 (162) | |||
| RB | 43 | 246 (185) | |||
| No RB | 122 | 163 (95) | |||
| sICH | 11 | 427 (271) | |||
| No sICH | 154 | 168 (93) | |||
| ENI | 86 | 157 (96) | |||
| No ENI | 79 | 215 (154) | |||
| DENI | 47 | 147 (75) | |||
| No DENI | 118 | 199 (145) | |||
| 6 | Xu et al 19 | Delayed | GFOS | 166 | 143.83 (73.66) |
| No GFOs | 120 | 172.27 (100.33) | |||
| Mortality | 38 | 202 (124.42) | |||
| No mortality | 151.83 | 87.62 | |||
| 7 | Feng et al 22 | Admission | RB | 34 | 260.92 (181.35) |
| No RB | 56 | 170.25 (83.35) | |||
| 8 | Sengeze et al 34 | Admission | SR | 43 | 144.77 (80.30) |
| No SR | 48 | 167.03 (110.59) | |||
| 9 | Sarioglu et al 35 | Admission | FPE | 32 | 103.17 (37.06) |
| No FPE | 51 | 195.35 (101.49) | |||
| 10 | Gong et al 15 | Admission | END | 193 | 184.1 (96.35) |
| No END | 867 | 130.94 (46.41) | |||
| ENI | 398 | 134.6 (48.88) | |||
| NO ENI | 662 | 144.24 (68.73) | |||
| 11 | Deng et al 49 | Admission (IVT only) | SAI | 141 | 168 (89.88) |
| No SAI | 196 | 127.91 (52.52) | |||
| Admission (EVT±IVT) | SAI | 219 | 208 (117.91) | ||
| No SAI | 114 | 174.33 (93.11) | |||
| 12 | Chen, ren et al 20 | Delayed | GFOS | 194 | 125.27 (53.28) |
| No GFOs | 86 | 146.97 (5 | |||
| Mortality | 27 | 165.5 (111.81) | |||
| No mortality | 253 | 127.91 (52.52) | |||
| 13 | Eren et al 14 | Admission | ENI | 136 | 129.03 (70.58) |
| No ENI | 114 | 145.61 (86.23) | |||
| RB | 27 | 129.6 (61.98) | |||
| No RB | 223 | 137.45 (80.20) | |||
| 14 | Lee et al 33 | Admission | SR | 224 | 120.97 (65.59) |
| No SR | 58 | 161.37 (90.39) | |||
| 15* | Inanc & inanc 21 | Admission | Mortality | 23 | 72.68 (107.67) |
| No mortality | 33 | 22.78 (19.17) | |||
| RB | 24 | 24.10 (20.51) | |||
| No RB | 32 | 61.56 (100.45) | |||
| 16* | Altintas et al 12 | Admission | |||
| 17* | Diestro et al 32 | Admission | |||
| 18* | Chen, Li et al 51 | Admission | |||
| Delayed |
All values were provided to 2 decimal places where rounding was required. Outcome groups where data were available included only. Definitions of outcomes were as outlined in the text and the caption of Figure 2.
*Total numbers for this outcome were not congruent with other outcomes. However, numbers provided in data tables were used for relevant analyses.
**Data missing for one patient.
**Only included in the systematic review.
Abbreviations: n=Number of patients in each outcome group; ENI=Early Neurological Improvement; DENI=Dramatic ENI; END=Early Neurological Decline; GFOs=Good Functional Outcomes; PLR=Platelet-Lymphocyte Ratio; sICH=symptomatic intracerebral haemorrhage; RB=Radiological Bleed; SR=Successful Recanalisation; SAI=Stroke Associated Infection; SAP=Stroke Associated Pneumonia; FPE=First Pass Effect.
Association of PLR With 90-day Good Functional Outcomes
Four studies (n=996) reported admission PLR levels for this outcome, and 5 studies (n=1297) reported PLR collected at delayed timepoints. All studies defined this outcome as mRS 0-2.
Admission PLR
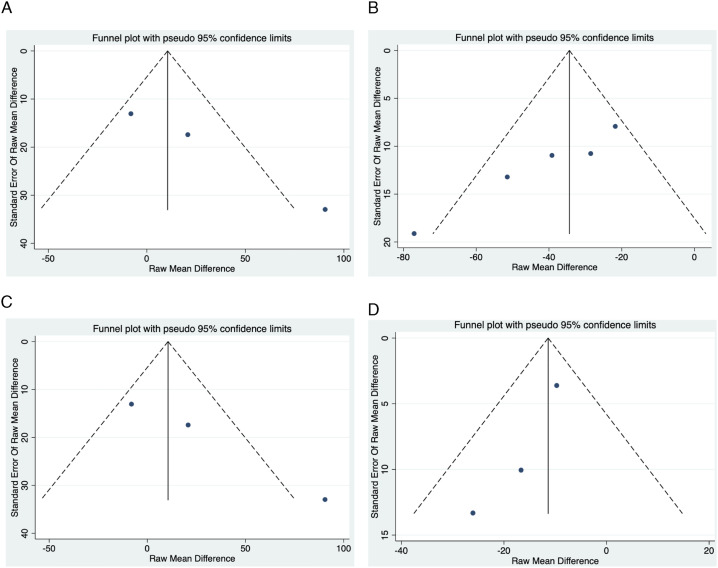
The meta-analysis showed a significant negative association of admission PLR with 90-day good functional outcomes (SMD=−.32; 95% CI = −.58 to −.05; P=.020; z=−2.328; Figure 2). However, considering treatment provided, a nonsignificant effect was observed in both subgroups, including IVT-treated patients (SMD=−.19; 95% CI = −.38 to .01; P=.063; z=−1.860) and patients who received EVT±IVT (SMD=−.47; 95% CI = −1.12 to .18; P=.155; z=−1.423). There was nonsignificant heterogeneity between groups (P=.410) and substantial to considerable overall heterogeneity (I2=75.0%, P=.007). No evidence of publication bias was observed by visual inspection of the funnel plot (Figure 4), but this was not consistent with Egger’s test (Supplemental Table 2 and Figure 2). Notably, omitting the study by Ozgen et al 16 markedly reduced the magnitude of the trend, especially in comparison to removing other studies (SMD=−.17; 95% CI = −.31 to −.03), although significance was maintained (Supplemental Figure 1).
Figure 2.
Forest plots showing association of platelet lymphocyte ratio (PLR) with good functional outcomes. Abbreviations: GFOs=Good Functional Outcomes; PLR=Platelet-Lymphocyte Ratio; IVT=Intravenous Thrombolysis; EVT=Endovascular Thrombectomy.
Figure 3.
Forest plots showing association of platelet lymphocyte ratio (PLR) with radiological bleed and early neurological improvement (ENI). Abbreviations: PLR=Platelet-Lymphocyte Ratio; IVT=Intravenous Thrombolysis; EVT=Endovascular Thrombectomy; ENI=Early Neurological Improvement.
Delayed PLR
A significant negative association was observed between PLR collected at delayed timepoints and 90-day good functional outcomes (SMD=−.43; 95% CI = −.54 to −.32; P<.0001; z=−7.454; Figure 2). This was consistent in the group comprising patients receiving IVT only (SMD=−.47; 95% CI = −.63 to −.32; P<.0001; z=−5.911). There was only one study in both the IVT±EVT (SMD=−.33; 95% CI = −.57 to −.09; P=.006; z=−2.746) and all treatment combination groups (SMD=−.42; 95% CI = −.64 to −.20; P<.0001; z=−3.748), both of which provided a significant effect. There was no significant heterogeneity between groups (P=.615) and nonsignificantly low overall heterogeneity (I2=.0%, P=.680). Some evidence of publication bias was observed by visual inspection of the funnel plot (Figure 4), and this was confirmed by Egger’s test (Supplemental Table 2 and Figure 2).
Association of PLR With 90-day Mortality
One study (n = 150) reported admission PLR values for this outcome, and 2 studies (n = 566) reported PLR values collected at delayed timepoints. A meta-analysis could not be carried out for either due to the limited number of studies.
Admission PLR
A meta-analysis could not be performed. However, the systematic review indicated that a higher admission PLR could be associated with 90-day mortality. However, this was a significant difference in only the results of Ozgen et al and not Inanc & Inanc.16,21 Altintas et al 12 did not provide PLR values by outcome but carried out an analysis stratified by an optimal PLR value determined from receiver operating curve analysis, where mortality was significantly higher in the group with a higher PLR than in the group with a lower PLR.
Delayed PLR
A meta-analysis could not be performed. However, a systematic review indicated that a higher admission PLR could be associated with 90-day mortality, with both Chen, Ren et al 20 and Xu et al 19 reporting this association. The difference in delayed PLR between groups was significant in both studies.
Association of PLR With Radiological Bleed
There were 3 studies (n=505) reporting admission PLR values for this outcome and 2 studies (n=490) reporting PLR collected at delayed timepoints. A meta-analysis was not carried out for the latter due to a lack of studies.
Admission PLR
The meta-analysis showed that although there was a positive association of admission PLR with radiological bleeding, this was not significant (SMD=.27; 95% CI = −.15 to .70; P=.209; z=1.256; Figure 3). Considering the treatment provided, a nonsignificant effect was also observed in IVT-treated patients (SMD=.09; 95% CI = −.25 to .42; P=.614; z=.505). There was only one study reporting on patients who received EVT±IVT (SMD=.70; 95% CI = .26 to 1.14; P=.002; z=3.138), which showed a significant effect. There was significant heterogeneity between groups (P=.029) and significant substantial to considerable overall heterogeneity (I2=71.6%, P=.030). No major evidence of publication bias was observed by visual inspection of the funnel plot (Figure 4), and this was confirmed by Egger’s test (Supplemental Table 2 and Figure 2). Notably, omitting the study by Eren et al 14 provided a result not crossing the line of no effect (SMD=.45; 95% CI = .01 to .90).
Figure 4.
Funnel Plots for each meta-analysis. Note: Funnel plots for each meta-analysis. A: Admission PLR association with Good Functional Outcomes; B: Delayed PLR association with Good Functional Outcomes; C: Admission PLR association with Radiological Bleed; D: Admission PLR association with ENI.
Delayed PLR
A meta-analysis could not be performed. However, a systematic review indicated that a higher admission PLR could be associated with radiological bleeding, with both Ferro et al 23 and Topcuoglu et al 18 reporting this. Ferro et al.‘s results, split into grades of radiological bleeding, while significant in univariate analyses, did not provide a significant result in multivariate modelling. Topcuoglu et al showed a significant difference in delayed PLR between groups.
Association of PLR With Early Neurological Improvement (ENI)
There were 3 studies (n=1475) reporting admission PLR values and 1 study (n=165) reporting relevant data for PLR collected at delayed timepoints. A meta-analysis was not carried out for the latter group.
Admission PLR
A significant decrease in admission PLR was associated with ENI (SMD=−.18; 95% CI = −.29 to −.08; P=.001; Figure 2). This significant effect was observed in both patients receiving IVT only (SMD=−.25; 95% CI = −.44 to −.06; P=.012) and bridging therapy (SMD=−.16; 95% CI = −.28 to −.03; P=.014), although the latter group had only one study. There was no significant heterogeneity between groups (P=.426) and nonsignificantly low overall heterogeneity (I2=.0%, P=.656). No major evidence of publication bias was observed by visual inspection of the funnel plot (Figure 4), and this was confirmed by Egger’s test (Supplemental Table 2 and Figure 2).
Delayed PLR
A meta-analysis could not be performed. However, the systematic review indicated mixed results. A lower delayed PLR was observed in patients with ENI in both studies included in the systematic review, but this reached statistical significance only in the results of Topcuoglu et al and not Inanc & Inanc.18,21
Association of PLR With Early Neurological Deterioration (END)
Only one study each for both admission (n=1060) and delayed (n=325) PLR was available with relevant data for consideration in the meta-analysis.
Admission PLR
A meta-analysis was not possible. However, the systematic review provided mixed results, with Inanc & Inanc reporting nonsignificantly higher admission PLR values in patients with END than in those without END, but Gong et al reporting a significantly higher admission PLR in the END group than in the ENI and neither ENI nor END groups.15,21
Delayed PLR
Only one study was available for this outcome for PLR collected at delayed timepoints, and thus, neither a meta-analysis nor systematic review was possible.
Association of PLR With Dramatic Early Neurological Improvement (DENI)
For this outcome, only one study (n=165) reported relevant data for consideration in meta-analyses, providing both admission and delayed PLR data.
Admission PLR
A meta-analysis was not possible. The systematic review indicated a possible association, with Inanc & Inanc reporting significantly lower admission PLR values in patients with DENI compared to those without, and Topcuoglu et al reporting the same trend but no statistical significance.18,21
Delayed PLR
Only one study was available for this outcome for PLR collected at delayed timepoints, and thus, neither a meta-analysis nor systematic review was possible.
Association of PLR With 90-day mRS 0-1
Only one study (n=165) was available with relevant data for consideration in the meta-analyses for this outcome, providing both admission and delayed PLR data.
Admission PLR
A meta-analysis was not possible. However, the systematic review indicated that admission PLR was not associated with 90-day mRS 0-1, with both Topcuoglu et al and Inanc & Inanac reporting nonsignificant differences between groups, although PLR was lower in both the mRS 0-1 group in both studies.18,21
Delayed PLR
Only one study was available for this outcome for PLR collected at delayed timepoints, and thus, neither a meta-analysis nor systematic review was possible.
Association of PLR With Symptomatic Intracerebral Hemorrhage (sICH)
Only one study (n=165) was available for consideration in the meta-analyses for this outcome, providing both admission and delayed PLR data.
Admission PLR
A meta-analysis was not possible. However, the systematic review provided unclear results; although a higher PLR was observed in patients with sICH by both Topcuoglu et al and Inanc & Inana, this was only significant in the former study.18,21 Additionally, Diestro et al 32 reported that admission PLR was not significantly associated with sICH in adjusted analyses.
Delayed PLR
Only one study was available for this outcome for PLR collected at delayed timepoints, and thus, neither a meta-analysis nor systematic review was possible.
Association of PLR With Successful Recanalisation
Only two studies provided relevant admission PLR data for consideration of the meta-analysis for this outcome (n=373), with the same definition of successful recanalisation (mTICI 2b-3).33,34 The systematic review indicated that admission PLR may be associated with recanalisation outcomes, as the aforementioned studies both reported lower admission PLR in patients with a favorable recanalisation outcome, as did Inanc & Inanc, 21 who defined this in terms of complete recanalisation or percentages thereof using the Thrombolysis in Brain Ischemia (TIBI) scale. Of these three studies, the differences between groups were significant only in Lee et al. 33 ‘s work. Another study, reporting on the first pass effect (FPE), where complete recanalisation (mTICI 3) was achieved with a single pass, showed a significantly higher PLR in the non-FPE group, 35 further supporting an association of admission PLR with recanalisation outcomes.
Only one study was available for this outcome for PLR collected at delayed timepoints, and thus, neither a meta-analysis nor systematic review was possible.
Association of PLR With Stroke Associated Infection (SAI)
Only one study was available for this outcome, providing only admission PLR data, and thus no systematic review or meta-analysis was possible.
Discussion
This study investigated the association of PLR with outcomes after AIS in patients receiving RT and is, to the best of our knowledge, the first of its kind. We demonstrate that a lower admission PLR is significantly associated with ENI, and a lower PLR collected at both admission and delayed timepoints is significantly associated with 90-day good functional outcomes. Additionally, our meta-analysis suggests that higher PLR collected at delayed timepoints may be associated with 90-day mortality and radiological bleeding. As such, there is a role for PLR in predicting outcomes and informing treatment decisions.
PLR is a beneficial biomarker owing to its low cost and availability from standard blood panels,4,10 and its ability to provide insight into both the hemostatic/thrombotic and inflammatory pathways underlying AIS pathogenesis. 22 It is of particular interest in patients receiving RT due to the association of platelets with increased recruitment and activation of immune cells, including neutrophils, which have a role in ischaemia–reperfusion injury (IRI), a phenomenon where stroke continues to progress despite resolution of the occlusion, along with the no-reflow phenomenon, whereby blood flow is not restored despite removal of the occlusion. The role of platelets in formation of neutrophil extracellular traps may contribute to this.4,36-38 Indeed, several studies pertaining to cardiovascular reperfusion show a strong association of elevated PLR with the no-reflow phenomenon.7,8 This may explain our observation that lower PLR levels collected at delayed timepoints were associated with good functional outcomes and higher delayed PLR levels with mortality in studies included in the systematic review, but admission PLR showed nonsignificant results in each of the treatment subgroups, as all included patients received RT and hence these phenomena may have occurred. These results should be interpreted with caution due to the publication bias detected in the delayed timepoints analysis. Similar trends were seen in the systematic review component of our study looking at association of admission PLR with mRS score 0-1. Although both included studies showed nonsignificant results, this should be interpreted in light of the small sample size and retrospective study design. There were no studies considering PLR collected at delayed timepoints. Drawing conclusions about utility of PLR in predicting recanalisation outcomes was restricted by similar issues for both admission and delayed PLR timepoints. As such, there is a pressing need for further prospective high-powered studies in this space, as this may help determine which patients would benefit from recanalisation, and could assist in monitoring patients at high risk of IRI and no-reflow injury more closely, and inform long-term follow up of high risk patients.4,10 Considering recent data showing a sizeable recurrence rate in AIS, the latter is of enormous significance for early intervention and initiating aggressive preventive measures. 1 There is also a pressing need for further studies with regards to bridging therapy or EVT only, as most studies included in these analyses had cohorts where patients received IVT only; it is well established that treatment with EVT has the potential to improve outcomes, 39 and hence, understanding the role of PLR in these patients is critical to determining its putative role in clinical practice and treatment decision-making.
We also showed that patients with ENI had lower admission PLR levels. This may be related to previous hypotheses that higher PLR levels may reflect a higher burden of high-risk plaques and atherosclerotic risk and thus may be associated with worse prognosis due to plaque instability. 12 Additionally, it may also reflect less immune-mediated inflammation, which has a notable role in early neurological changes.4,13 It has also recently been reported that PLR may reflect initial stroke severity, and this may have been a contributing factor to our results, 40 Though only 2 studies included in the meta-analysis for ENI provided stroke severity data, and future research into this area is necessary. Most studies did not consider delayed PLR values for early neurological change outcomes, likely owing to this outcome generally being measured at 24 hours, which is when most delayed PLR measurements are taken.15,18 However, a meta-analysis could not be carried out to determine the role of admission PLR in predicting other early neurological outcomes, END and DENI, and further primary research is necessary for this. Interestingly, the results reported by Gong et al (n=1060) indicated that END was statistically significantly associated with admission PLR, which is consistent with an understanding that platelets can drive early stroke inflammation via cross-talk mechanisms with deleterious immune cells.4,15,38,41 However, the other study in the systematic review for this outcome had only 56 patients, and thus, further research is critical to validate these results. PLR has also been shown to have utility in the coronavirus disease 2019 (COVID-19) setting, with higher PLR associated with increased clinical deterioration and thrombosis risk, and thus, considering reports of increased stroke risk in the milieu of COVID-19, along with involvement of the clotting cascade, PLR could have a putative role in predicting short-term neurological outcomes.42-45 As we have reported previously, such biomarkers may also help facilitate treatment owing to the increasing reliance on telemedicine to keep up with increased healthcare demands in the COVID-19 era.4,10,42,46
There has been a particular interest in using PLR for predicting bleeding outcomes considering previous reports that thrombocytopenic patients have a higher risk of sICH than patients with a normal platelet count. 47 However, our meta-analysis showed somewhat diverging results, with radiological bleeding seemingly indicated by a higher PLR collected at delayed timepoints. This could be attributed to previous observations that platelets can, in conjunction with neutrophils and fibrinogen, cause blood brain barrier (BBB) damage after initial occlusion and thus may predispose patients to hemorrhagic transformation. 22 We observed no statistically significant association of admission PLR with radiological bleeding and that removing Eren et al caused the effect to no longer cross the line of no effect. This was surprising, as this was the study with the longest follow-up CT scanning, extending up to 72 hours, more than twice the next highest included study. 14 This could be related to stroke etiology, as PLR, due to it being a marker of high-risk plaques, has been purported as being more relevant in LAA stroke, 23 which formed only 32.10% of our included patient population, with relevant etiology data only available for one of the 3 studies included in the admission PLR association with radiological bleed meta-analysis. Again, there is a pressing need for further studies considering bridging therapy and EVT-treated patients to further validate the utility of PLR in AIS patients in informing treatment decisions. A meta-analysis could not be carried out for sICH or SAI, and the systematic review for the former was limited by small underpowered studies. Neither a systematic review nor a meta-analysis could be conducted for SAI, but this area that warrants further attention, especially with evidence suggesting SAI may impact immune cell counts and the thromboinflammatory role of platelets.4,48
The major strength of our study is that, to the best of our knowledge, it is the first of its kind. Additionally, our use of SMD to account for the continuous nature of PLR allows us to overcome the issue of varying thresholds in the published literature. We also considered admission and delayed PLR separately, using pre- and postintervention definitions, thereby accounting for the role of platelets in IRI and no-reflow injury in clinical and recanalisation outcomes and in potential BBB damage leading to HT,22,38 as well as the dynamicity of immune cells in AIS pathogenesis.4,10 By considering treatment modalities separately, we also clearly show that there are differences in treatment modalities, which can be used not only to determine the utility of PLR in different patient subgroups but also to guide further research.
Limitations of our study include that most studies retrospective in design; we sought to minimise the ensuing heterogeneity through random-effects modelling. Additionally, some outcomes lack a breadth of published literature, with many low powered studies; hence, conclusions could not be drawn about the role of PLR in prognosticating these outcomes. Additionally, we were limited from making conclusions about bridging therapy and IVT because there were very few studies with cohorts of patients treated as such. The role of racial and ethnic differences in PLR should also not be discounted, but we were unable to consider this in our analysis due to minimal reporting of data stratified as such. 49 Notably, our findings relate to AIS patients receiving RT and thus, should be interpreted in this scope. Very few studies considered the association of dynamic PLR changes with outcomes, and thus, this could not be analysed. Finally, not all studies12,13,17,22,34,35 excluded patients with active infection, malignancy, or chronic disease, which can influence lymphocyte count via systemic inflammation.4,10
Conclusion
Our study clearly shows that there is a putative role for PLR in the management of AIS patients treated with RT, particularly regarding predicting long-term outcomes in these patients, which may be related to IRI and no-reflow injury and for predicting bleeding outcomes. As such, PLR could be beneficial in informing management decisions and predicting higher-risk patients who might need closer monitoring post RT. Implementing systems level changes for such monitoring could help address the AIS global disease burden. Given the rapid access and low costs, PLR values could be implemented on a systems level to stratify secondary prevention and improve ongoing surveillance as well as follow-up strategies for AIS patients at high-risk of poor morbidity and mortality. However, there is a pressing need for further prospective high-powered studies considering bridging therapy and EVT-only patients. Additionally, further experimental studies are required to better elucidate pathophysiological mechanisms underlying the role of PLR in AIS, as this may mediate clinical outcomes and bleeding complications.
Supplemental Material
Supplemental Material for Prognostic Role of the Platelet-Lymphocyte Ratio in Acute Ischemic Stroke Patients Undergoing Reperfusion Therapy: A Meta-Analysis by Divyansh Sharma and Sonu M. M. Bhaskar in Applied Spectroscopy.
Author Contributions: SMMB conceptualised the study, contributed to the planning, drafting and revision of the manuscript, and supervised the student. SMMB encouraged DS to investigate and supervise the findings of this work. DS and SMMB wrote the first draft of this paper and were involved in data extraction and analyses. All authors contributed to the revision of the manuscript. All authors approved the final draft of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the NSW Brain Clot Bank (Chief Investigator: S. M. M. B.) from the NSW Ministry of Health (2019-2022) is acknowledged.
Data Availability: The original contributions presented in the study are included in the article and online Supplementary Material/s, further inquiries can be directed to the corresponding author.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Sonu M. M. Bhaskar https://orcid.org/0000-0002-9783-3628
References
- 1.Tu WJ, Chao BH, Ma L, et al. Case-fatality, disability and recurrence rates after first-ever stroke: A study from bigdata observatory platform for stroke of China. Brain Res Bull 2021;175:130-135. [DOI] [PubMed] [Google Scholar]
- 2.Vos T. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi C, Killingsworth MC, Bhaskar SMM. Prognostic capacity of hyperdense middle cerebral artery sign in anterior circulation acute ischaemic stroke patients receiving reperfusion therapy: a systematic review and meta-analysis. Acta Neurol Belg 2021;122:1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma D, Spring KJ, Bhaskar SMM. Neutrophil–lymphocyte ratio in acute ischemic stroke: Immunopathology, management, and prognosis. Acta Neurol Scand 2021;144:486-499. [DOI] [PubMed] [Google Scholar]
- 5.Jo S, Jeong T, Lee JB, Jin Y, Yoon J, Park B. The prognostic value of platelet-to-lymphocyte ratio on in-hospital mortality in admitted adult traffic accident patients. PLoS One 2020;15:e0233838-e0233838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep 2017;7:40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badran HM, Fatah AA, Soltan G. Platelet/lymphocyte ratio for prediction of no-reflow phenomenon in ST-elevation myocardial infarction managed with primary percutaneous coronary intervention. J Clin Transl Res 2020;6:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yildiz A, Yuksel M, Oylumlu M, et al. The Utility of the Platelet–Lymphocyte Ratio for Predicting No Reflow in Patients With ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost 2014;21:223-228. [DOI] [PubMed] [Google Scholar]
- 9.Qu X, Shi J, Cao Y, Zhang M, Xu J. Prognostic Value of White Blood Cell Counts and C-reactive Protein in Acute Ischemic Stroke Patients After Intravenous Thrombolysis. Curr Neurovascular Res 2018;15:10-17. [DOI] [PubMed] [Google Scholar]
- 10.Sharma D, Spring KJ, Bhaskar SMM. Role of Neutrophil-Lymphocyte Ratio in the Prognosis of Acute Ischaemic Stroke After Reperfusion Therapy: A Systematic Review and Meta-analysis. J Cent Nerv Syst Dis 2022;14:11795735221092518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M, Pan Y, Li Z, et al. Platelet Count Predicts Adverse Clinical Outcomes After Ischemic Stroke or TIA: Subgroup Analysis of CNSR II. Front Neurol 2019;10:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altintas O, Altintas MO, Tasal A, Kucukdagli OT, Asil T. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res 2016;38:759-765. [DOI] [PubMed] [Google Scholar]
- 13.Chen S-Y, Lin YS, Cheng YF, Wang H, Niu XT, Zhang WL. Mean Platelet Volume-To-Lymphocyte Ratio Predicts Poor Functional Outcomes Among Ischemic Stroke Patients Treated With Intravenous Thrombolysis. Front Neurol 2019;10:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eren F, Demir A, Eren G. The Platelet/Lymphocyte Ratio in Patients with Ischemic Stroke Treated with Intravenous Thrombolysis and Its Relationship with Mortality, Disability, and Prognosis. Istanbul Medical Journal 2021;22:161-167. [Google Scholar]
- 15.Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation 2021;18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgen E, Guzel M, Akpinar CK, Yucel M, Demir MT, Baydin A. The relationship between neutrophil/lymphocyte, monocyte//lymphocyte, platelet/lymphocyte ratios and clinical outcomes after ninety days in patients who were diagnosed as having acute ischemic stroke in the emergency room and underwent a mechanical thro. Bratisl Lek Listy 2020;121:634-639. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y. Platelet-to-lymphocyte Ratio at 24h after Thrombolysis Is a Prognostic Marker in Acute Ischemic Stroke Patients. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topcuoglu MA, Pektezel MY, Yilmaz E, Arsava EM. Systemic Inflammation Indices in Patients With Acute Ischemic Stroke Treated With Intravenous Tissue Plasminogen Activator: Clinical Yield and Utility. Angiology 72, 279-284 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Xu J-H, He XW, Li Q, et al. Higher Platelet-to-Lymphocyte Ratio Is Associated With Worse Outcomes After Intravenous Thrombolysis in Acute Ischaemic Stroke. Front Neurol 2019;10:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Ren J, Yang N, et al. Eosinophil-to-monocyte ratio is a potential predictor of prognosis in acute ischemic stroke patients after intravenous thrombolysis. Clin Interv Aging 2021;16:853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inanc Y, Inanc Y. The effects of neutrophil to lymphocyte and platelet to lymphocyte ratios on prognosis in patients undergoing mechanical thrombectomy for acute ischemic stroke. Ann Ital Chir 2018;89:367-373. [PubMed] [Google Scholar]
- 22.Feng X, Ye G, Cao R, et al. Identification of Predictors for Hemorrhagic Transformation in Patients with Acute Ischemic Stroke After Endovascular Therapy Using the Decision Tree Model. Clin Interv Aging 2020;15:1611-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferro D, Matias M, Neto J, et al. Neutrophil-to-Lymphocyte Ratio Predicts Cerebral Edema and Clinical Worsening Early After Reperfusion Therapy in Stroke. Stroke 2021;52:859-867. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA 2000;283:2008-2012. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y-W, Zhang XM, Lv M, et al. Utility of gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a systematic review and meta-analysis. OncoTargets Ther 2015;8:3349-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders R, Struys MMRF, Pollock RF, Mestek M, Lightdale JR. Patient safety during procedural sedation using capnography monitoring: a systematic review and meta-analysis. BMJ Open 2017;7:e013402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deeks JJHJ, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2021. [Google Scholar]
- 29.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-1805. [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol 2007;60:849-852. [DOI] [PubMed] [Google Scholar]
- 32.Diestro JDB, Parra-Farinas C, Balas M, et al. Inflammatory Biomarkers and Intracranial Hemorrhage after Endovascular Thrombectomy. Can J Neurol Sci/J Can Sci Neurol 2021;1:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee S-H, Jang MU, Kim Y, et al. The Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Predict Reperfusion and Prognosis after Endovascular Treatment of Acute Ischemic Stroke. J Personalized Med 2021;11:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sengeze N, Giray S. The relationship between mean platelet volume, platelet count, platelet lymphocyte ratio, and recanalization success in first-pass thrombectomy of middle cerebral artery occlusions. Turk Noroloji Dergisi 2020;26:133-137. [Google Scholar]
- 35.Sarioglu O, Capar AE, Bas Sokmez DF, Topkaya P, Belet U. Relationship between the first pass effect and the platelet-lymphocyte ratio in acute ischemic stroke. Intervent Neuroradiol : Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences 2020;27:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in Stroke Brain Damage. Stroke 2016:47:1165-1172. [DOI] [PubMed] [Google Scholar]
- 37.Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018;315:H550-H562. [DOI] [PubMed] [Google Scholar]
- 38.Stegner D, Klaus V, Nieswandt B. Platelets as Modulators of Cerebral Ischemia/Reperfusion Injury. Front Immunol 2019;10:2505-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol 2018;18:8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kömürcü HF, Gozke E, Dogan Ak P, Kalyoncu Aslan I, Salt I, Ozgenc Bier CI. Changes in neutrophil, lymphocyte, platelet ratios and their relationship with NIHSS after rtPA and/or thrombectomy in ischemic stroke. J Stroke Cerebrovasc Dis: The Official Journal of National Stroke Association 2020;29:105004. [DOI] [PubMed] [Google Scholar]
- 41.Rawish E, Nording H, Münte T, Langer HF. Platelets as Mediators of Neuroinflammation and Thrombosis. Front Immunol 2020;11:2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhaskar S, Sharma D, Walker AH, et al. Acute Neurological Care in the COVID-19 Era: The Pandemic Health System REsilience PROGRAM (REPROGRAM) Consortium Pathway. Front Neurol 2020;11:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol 2020;11:1648-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandor-Keri J, Benedek I, Polexa S, Benedek I. The Link between SARS-CoV-2 Infection, Inflammation and Hypercoagulability-Impact of Hemorheologic Alterations on Cardiovascular Mortality. J Clin Med 2021;10:3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma D, Bhaskar SMM. Prevalence of paediatric hyperinflammatory conditions in paediatric and adolescent hospitalized COVID-19 patients: a systematic review and meta-analysis. APMIS 2022;130:101-110. [DOI] [PubMed] [Google Scholar]
- 46.Sharma D, Bhaskar S. Addressing the Covid-19 Burden on Medical Education and Training: The Role of Telemedicine and Tele-Education During and Beyond the Pandemic. Front Public Health 2020;8:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gensicke H, Al Sultan AS, Strbian D, et al. Intravenous thrombolysis and platelet count. Neurology 2018;90:e690-e697. [DOI] [PubMed] [Google Scholar]
- 48.Nording H, Langer HF. Complement links platelets to innate immunity. Semin Immunol 2018;37:43-52. [DOI] [PubMed] [Google Scholar]
- 49.Deng QW, Gong PY, Chen XL, et al. Admission blood cell counts are predictive of stroke-associated infection in acute ischemic stroke patients treated with endovascular therapy. Neurol Sci : Official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2020;42:2397-2409. [DOI] [PubMed] [Google Scholar]
- 50.Yi HJ, Sung JH, Lee DH. Systemic Inflammation Response Index and Systemic Immune-Inflammation Index Are Associated with Clinical Outcomes in Patients Treated with Mechanical Thrombectomy for Large Artery Occlusion. World neurosurgery 2021;153:e282-e289. [DOI] [PubMed] [Google Scholar]
- 51.Chen CT, Li LH, Su PY, et al. Neutrophil-to-lymphocyte ratio in predicting neurologic outcome of patients with acute ischemic stroke treated with intravenous thrombolytics. J Chin Med Assoc: J Chin Med Assoc 2021;1:102-108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Prognostic Role of the Platelet-Lymphocyte Ratio in Acute Ischemic Stroke Patients Undergoing Reperfusion Therapy: A Meta-Analysis by Divyansh Sharma and Sonu M. M. Bhaskar in Applied Spectroscopy.