Abstract
Introduction. Physical activity (PA) can reduce blood pressure (BP) in hypertensives through possibly interacting with the renin-angiotensin-aldosterone system (RAAS). We conducted a nested-cohort analysis to determine if self-reported PA was associated with BP responsiveness to acute angiotensin converting enzyme inhibition (ACEi). Methods. Data were extracted from the HyperPATH dataset, a cohort designed to identify mechanisms of cardiometabolic risk. Hypertensives that completed a self-assessed PA questionnaire, hormonal assessments (aldosterone [ALDO]), and BP to a single dose of an ACEi (captopril, 25 mg) were included. All participants (n = 144) were studied on a controlled diet for 7 days. PA was recorded as no PA, or little, moderate, or high amounts of exercise. Analyses were adjusted for age, sex, race, and body mass index. Results. Individuals who reported high amounts of PA displayed a greater BP lowering effect from ACEi compared to those who reported moderate (−14.8 ± 8.1 vs −8.4 ± 9.9 mm Hg, P < .01) or no additional PA (−14.8 ± 8.1 vs −2.6 ± 9.9 mm Hg, P < .001). Exploratory analyses indicated high amounts of PA were associated with a reduced heart rate (54 ± 8 vs 66 ± 10 bpm, P < .001) and blunted ALDO (β = 0.44, 95% confidence interval = 0.19-0.70). Conclusions. Higher self-reported PA was associated with an augmented BP lowering effect to acute ACEi in hypertensive patients.
Keywords: blood pressure, exercise, renin-angiotensin-aldosterone system, ACE inhibitor
‘Despite the alarmingly high prevalence of hypertension, only half of individuals successfully attain blood pressure (BP) lowering targets.’
Hypertension is a complex health condition that afflicts 77.9 million people in the United States, contributing to a significant portion of excess cardiovascular death.1,2 Despite the alarmingly high prevalence of hypertension, only half of individuals successfully attain blood pressure (BP) lowering targets.1-3 Reasons for this shortfall include lack of adherence, poor tolerance, and heterogeneous response to prescribed lifestyle and medication programs.2,3 Furthermore, BP elevation itself is a physiologic indicator of systemic dysregulation with variable cardiometabolic consequences dependent on underlying pathological basis.4-6 Thus, efforts to clarify mechanisms leading to hypertension could help improve identification of individuals at particular risk and programs specific to this population, which should improve both adherence and efficacy.
Two fruitful areas of research include lifestyle modification through increased physical activity (PA) and hormonal manipulation targeting the renin-angiotensin-aldosterone system (RAAS). 6 Several lines of investigation suggest that at the intersection of these 2 programs lies an opportunity of synergistic benefit.7-13
Trials involving angiotensin converting enzyme inhibition (ACEi) report a systolic BP lowering effect between 4 and 12 mm Hg in hypertensive populations.7,14,15 ACEi therapy efficacy on BP lowering has been shown to be associated with measures of RAAS activity.7-9 Of note, PA has been shown to influence RAAS activity.10-13 Specifically, PA has been shown to increase plasma renin activity (PRA) and genetic polymorphisms associated with the RAAS improved BP reduction following aerobic exercise.10-13 In addition, PA has been shown to reduce systolic blood pressure (SBP) by 4 to 9 mm Hg in hypertensives.16,17 Thus, it seems inherently possible that manipulation of the RAAS may result in a more favorable BP lowering response in individuals who are more physically active. In other words, PA could prime the effects of ACEi on BP reduction.
Thus, the purpose of this study was to investigate the association between self-reported levels of PA and the hemodynamic and hormonal responses to ACEi in human hypertension.
Materials and Methods
Ethical Approval
In total, 5 international centers have contributed to this data set: Brigham and Women’s Hospital (Boston, MA), University of Utah Medical Center (Salt Lake City, UT), Hospital Broussais (Paris, France), Vanderbilt University (Nashville, TN), and University of La Sapiena (Rome, Italy). Each institutional review board approved the uniform HyperPATH protocol, and written informed consent was obtained from every participant prior to enrollment. Only data from Brigham and Women’s Hospital and Hospital Broussais were used in this analysis due to availability of required data points (reported PA and completion of an ACEi study).
Study Design
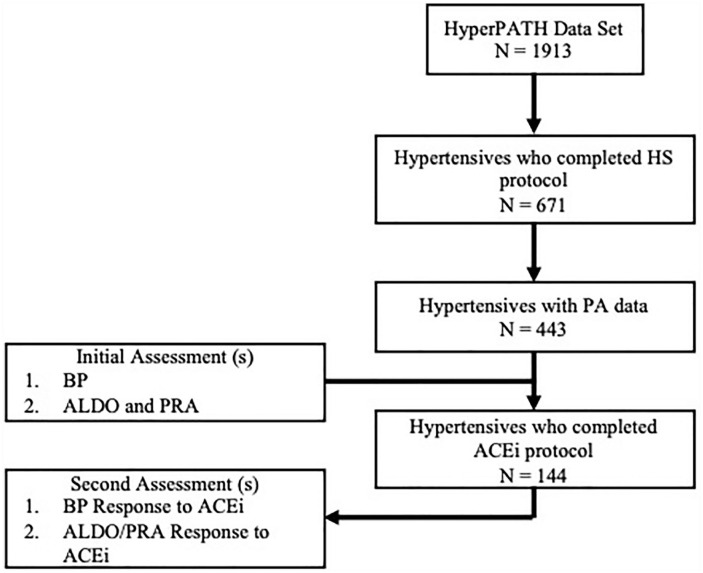
Data were extracted from the HyperPATH data set, which is an ongoing research program to identify genetic mechanisms underlying cardiometabolic risk. 18 The steps in data extraction are described in Figure 1. The initial analysis in RAAS outcomes was conducted in the subset of 443 hypertensives with PA data reported. Following the identification of a possible relationship between PA and RAAS, we analyzed the effects of acute ACEi (1 dose of 25 mg captopril) within the same HyperPATH cohort (n = 144). This program includes participants with hypertension who completed a series of physiological manipulations and assessments under rigorous conditions to reduce possible confounding factors. These manipulations included antihypertensive medication washout, a controlled diet run-in period, and utilization of an inpatient clinical research center to control body posture, fasting, and diurnal variation.
Figure 1.
Analytical selection of participants from the HyperPATH Cohort. BP, blood pressure; ALDO, aldosterone; HS, high salt diet; PA, reported physical activity; ACEi, angiotensin converting enzyme inhibitor (captopril 25 mg, once).
Participants
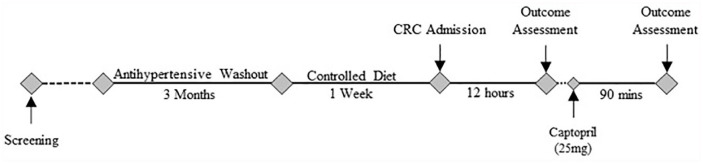
The standardized HyperPATH protocol, along with inclusion and exclusion criteria, were previously described.19-21 In brief, all subjects underwent a detailed screening process that included a history and physical and laboratory evaluations. All subjects were between 18 and 65 years old, and race was self-reported. Subjects with coronary heart disease, heart failure, chronic kidney disease, known causes of secondary hypertension, and active malignancy were excluded. Study participants were considered hypertensive if they had one or several of the following conditions: (1) untreated seated diastolic blood pressure (DBP) >100 mm Hg on no antihypertensive medications, (2) DBP >90 mm Hg with one or more antihypertensive medications, or (3) use of 2 or more antihypertensive medications. The HyperPATH cohort has been an ongoing research project for the last 20 years, and thus these criteria were selected at the initiation of the project.21,22 Subjects on 4 or more antihypertensive medications were excluded from participating in the study protocol due to safety concerns during the required medication washout period. Subjects taking antihypertensive medications were managed according to the following washout protocol: (1) all angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists were discontinued 3 months before study procedures and/or (2) β-blockers were withdrawn 1 month before study procedures (Figure 2). All medications were stopped 3 weeks prior to study procedures. BP was monitored on a daily basis by a study physician throughout the washout period. Intolerance of washout according to predetermined approved BP cutoffs led to discontinuation of the study and resumption of prior antihypertensive program.
Figure 2.
Study schema. CRC, inpatient clinical research center. Outcome Assessment includes study of hormonal and hemodynamics measurements.
Physical Activity Measurement and Diet
Self-reported PA was recorded at time of enrollment as part of a broad survey of environmental and demographic assessments. Participants reported PA levels according to the following scale: no participation in PA, some PA, a moderate amount of PA, or high amounts of PA. These descriptions were read to the participants through a script provided to the research assistant responsible for baseline health screening. One of the limitations of this study is this broad measurement of PA which may affect our results, and thus, these findings should be interpreted as hypothesis generating for future studies to investigate these associations. However, the HyperPATH cohort was designed to assess the genetic underpinnings of hypertension and therefore there was specific emphasis on control of other environmental factors such as medications and diet.21,22 The predetermined analytical plan was to assess the effect of self-reported PA on (1) BP, (2) BP response to acute ACEi, and (3) RAAS activity.
Participants consumed a high salt (HS) diet (≥200 mEq Na+/day), controlled for dietary potassium (100 mEq/day) and calcium (1000 mg/day), for 7 days prior to inpatient research center admission. Diet compliance and sodium balance was confirmed with a 24-hour urine collection prior to hemodynamic and hormonal assessments (sodium excretion >160 mmol/24 h). The HS diet was utilized in order to tease out physiological mechanisms in the RAAS and has been shown to represent the typical Western diet. 23
Outcome Assessment
Participants were then admitted to the Clinical Research Center and kept in a supine posture overnight for 12 hours prior to outcome testing (Figure 2). Baseline BP, as recorded with an automated device every 5 minutes (Dinamapp, Critikon, FL), was measured as the average of 3 repeated readings obtained over a 60-minute period from 8:00 am. A single dose of captopril (25 mg) was given and, similarly, the BP response was recorded 90 minutes after drug administration. Measures of plasma renin activity and aldosterone concentration were obtained under the same conditions in triplicate and reported as a mean value for data collection. Determination of hormone levels was performed at BWH Brigham Research Assay Core laboratory using validated assays. All assays performed in this study have been extensively reported previously.22,24
Statistical Analysis
Data are reported as means ± standard deviation unless otherwise stated. For determining the effect of captopril on SBP a dependent t test was used, and for analyzing self-reported PA on BP ANOVA was used. However, ANOVA with an interaction term was utilized for the primary outcome of BP, controlling for age, sex, race, and body mass index. To assess if PA was associated with reduced ALDO levels, a linear regression was performed controlling for age, sex, race, and body mass index. The other exploratory outcomes (PRA and heart rate) were analyzed by ANOVA with a Bonferroni adjustment in order to account for multiple comparisons. Data were analyzed using STATA (Version 15), and graphs were created using GraphPad Prism 5 software. Significance was set at .05, except as noted above for multiple comparisons. Power calculations performed with STATA indicate our study power at approximately 0.81 with an estimated mean difference of 30, α set at .05, and a sample size of 40.
Results
Participants within the HyperPATH cohort are subdivided into hypertensive, diabetic, and normotensive patients (n = 1913). This analysis included only participants diagnosed with hypertension. In total, 671 hypertensives completed the high-salt diet protocol, among whom 443 had reported levels of PA. Demographic information is provided in Table 1. Of the 443, a total of 144 participants had completed the captopril protocol. Thus, the total analytic population was 144 individuals for the analysis investigating captopril responsiveness.
Table 1.
| None | Mild | Moderate | High | |
|---|---|---|---|---|
| N | 115 | 149 | 120 | 59 |
| Age, years | 48.5 (8.3) | 50.2 (8.3) | 49.6 (8.2) | 48.4 (8.9) |
| Sex, female (%) | 71 (62) | 71 (48) | 60 (50) | 25 (42) |
| Weight, kg | 85.7 (13.9) | 83.6 (15.2) | 86.6 (17.6) | 81.7 (16.8) |
| Height, cm | 167.9 (8.8) | 170.9 (9.4) | 170.9 (7.7) | 172.4 (10.3) |
| BMI, kg/m2 | 28.9 (4.6) | 29.3 (4.6) | 28.5 (4.3) | 28.6 (4.1) |
| Serum creatinine, mg/dL | 0.88 (0.18) | 0.90 (0.16) | 0.84 (0.16) | 0.93 (0.27) |
| Total cholesterol, mg/dL | 203.8 (37.5) | 198.1 (44.4) | 193 (34.6) | 193.8 (37.6) |
| LDL cholesterol, mg/dL | 129.0 (37.7) | 126.3 (43.6) | 119.5 (29.7) | 114.8 (32.3) |
| HDL cholesterol, mg/dL | 41.1 (15.5) | 42.7 (13.0) | 43.6 (12.4) | 43.5 (12.8) |
| Blood glucose, mg/dL | 96.6* (16.9) | 95.7 (17.3) | 91.4* (15.7) | 90.2* (15.8) |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Baseline demographics stratified by reported levels of physical activity into none, mild, moderate, and high amounts of physical activity.
Data analyzed with ANOVA and Bonferroni adjustment, *P < .05 compared to none, or no physical activity reported.
Higher reported levels of PA were associated with reduced RAAS activity prior to ACEi (Table 2). Patients reporting high amounts of PA were associated with reductions in ALDO levels (Coef = 0.44, 95% confidence interval [CI] = 0.19-0.70, P = .001). In addition, the relationship between ALDO levels on a high salt diet and SBP may be influenced by reported levels of PA (Coef = 0.28, 95% CI = 0.023-0.545, P = .03; Figure 3). Hypertensives reporting no PA had higher PRA when compared to those who reported moderate levels of PA (0.59 ± 0.53 vs 0.42 ± 0.48 ng/mL/h, P = .03), while PRA in reporters of high amounts of PA did not approach significance (P = .19). In the presence of ACEi, there were no differences in ALDO or PRA measures between reported levels of PA (P > .05).
Table 2.
| None | Mild | Moderate | High | |
|---|---|---|---|---|
| N | 115 | 149 | 120 | 59 |
| ALDO, ng/dL | 5.78 (4.06) | 4.49* (2.96) | 4.53* (2.89) | 4.12* (2.78) |
| PRA, ng/mL/h | 0.59 (0.53) | 0.45 (0.55) | 0.42* (0.49) | 0.45 (0.46) |
| Urinary Na, mEq/day | 197.56 (68.60) | 237.21* (81.51) | 240.53* (78.27) | 243.35* (66.75) |
Abbreviations: ALDO, plasma aldosterone; PRA, plasma renin activity.
The effects of self-reported physical activity on RAAS activity.
Data analyzed with ANOVA and Bonferroni adjustment, *P < .05 in comparison to none, or no physical activity reported.
Figure 3.
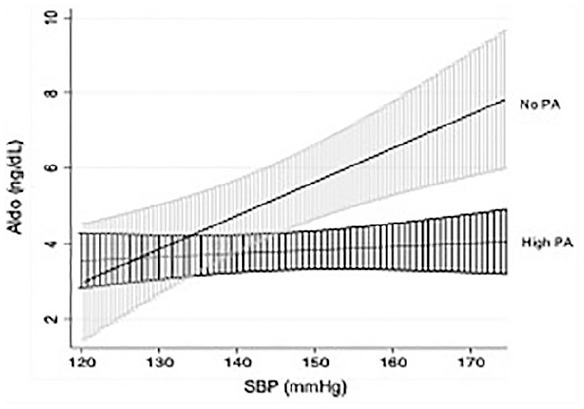
Relationship between plasma aldosterone (ALDO) concentration and systolic blood pressure according to reported level of physical activity (95% confidence interval).
At baseline (prior to captopril administration), higher self-reported levels of physical was associated with reduced SBP when compared to no reported PA (142.6 ± 20.2 vs 149.2 ± 18.8 mm Hg, P = .03). As expected, acute administration of captopril caused a signification reduction in BP in the cohort as a whole (captopril 139 ± 23 mm Hg vs baseline 143 ± 19 mm Hg, P = .004). However, individuals who reported high amounts of exercise displayed a greater BP lowering effect from captopril compared to those who reported moderate (−15 ± 8 vs −8 ± 10 mm Hg, P < .01) or no additional PA (−15 ± 8 vs −3 ± 10 mm Hg, P < .001; Figure 4). Interestingly, hypertensives with no reported levels of PA had minimal to no BP effect in response to acute ACEi. High amounts of PA were also associated with a reduced heart rate when compared to no PA (54 ± 8 vs 66 ± 10 bpm, P < .001) with similar, graded responses with lesser reported PA observed.
Figure 4.
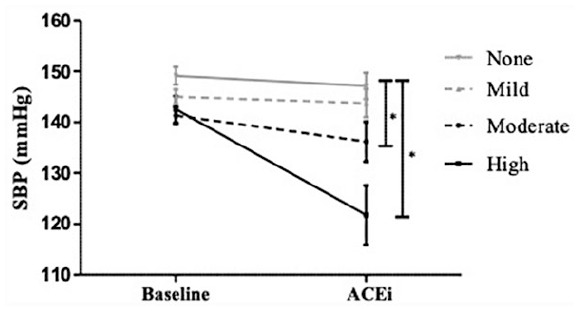
Blood pressure response to acute ACE inhibition (25 mg captopril) according to reported level of physical activity (None, Mild, Moderate, or High). Baseline = prior to ACE inhibition, *P < .05. Data are shown as means (standard error).
Discussion
The results of these analyses indicate that self-reported levels of PA are associated with an improved hemodynamic and RAAS response to acute ACE inhibition in hypertension. While acute ACE inhibition and reported levels of PA were individually associated with reduced BP as has been previously reported, this study observed a combined effect whereby individuals who reported higher levels of PA appeared to have greater BP reduction and lower aldosterone levels in comparison to individuals reporting reduced PA. The response appeared to be graded in effect based on level of PA, wherein those individuals who reported little additional PA seemed to have minimal BP lowering effect from captopril. The results are remarkable considering the nature of self-reported data assessments, which would inherently favor the null hypothesis and obscure identification of potential relationships. 25 The associations between PA and the BP response to ACE inhibition indicate that there may be a possible synergistic relationship related to altered RAAS activity.
Previous studies have investigated the combined effects of PA and ACEi on parameters of physical function in older adults and responsiveness to an acute bout of exercise.26,27 The work by Predel and colleagues indicated that ACEi blunted the BP increase that occurs following acute bouts of exercise. 26 This finding indicates that there may be some interplay between PA and pharmaceutical drugs that impact RAAS function. However, work conducted by Sumukadas and colleagues did not see a synergistic effect with PA and ACEi on improving physical function. 27 Thus, there seems to be conflicting evidence between the possible synergism between PA and ACEi.26,27
The mechanism by which exercise reduces BP is currently theorized to include alterations in nitric oxide physiology, changes in baroreceptors, or alterations in RAAS.28,29 Although this study did not include a direct analysis of nitric oxide or baroreceptors, we did measure heart rate, a known contributor to BP regulation. 30 We observed higher levels of PA were associated with a reduced heart rate similar to others who have reported strong evidence that PA reduces heart rate and sympathetic nervous system activity.14,31,32 Given the intertwined relationship between heart rate, BP, and cardiac output, it is difficult to determine which factors are causal versus reactive at this point.
One possible explanation for the observed relationship between PA and response to ACE inhibition is a direct interaction with the RAAS, a major contributor to BP regulation. 10 While exploratory in nature, the results of this study indicate that PA might be inversely associated with RAAS activity. In addition, our exploratory analyses suggest that hypertensives reporting higher levels of PA may reduce the impact that ALDO, a primary product of the RAAS, has on BP regulation. Furthermore, the possible association between ALDO and BP seemed stronger in hypertensives who report no PA. This might at first seem counterintuitive since one might anticipate that individuals with higher circulating RAAS components would be those who would be most susceptible to the actions of RAAS inhibition. However, the relationship between circulating levels of RAAS components and tissue activity is poor at best. 21 Stronger determinants of BP susceptibility seem to correlate better when tissue or organ function is assessed. 33 This can be particularly important under conditions of chronic activation which may reset receptor sensitivity as is seen in primary aldosteronism. 34
The results of this study indicate that there may be a possible synergistic relationship between PA and response to ACEi in reducing BP. Together, higher levels of PA when combined with ACEi resulted in a 17% reduction in systolic BP. This possible added benefit suggests that some hypertensive patients who are resistant to ACEi may see benefits from increased PA. The opposite may also be true; patients who see minimal benefits of exercise may combine lifestyle changes with pharmaceutical interventions for better effect. Also, the associations of reduced ALDO levels and PA could indicate potential benefit beyond BP lowering effects as reported in large clinical outcome trials with mineralocorticoid receptor antagonists.35,36
Strengths of this study include a rigorously controlled testing environment for evaluation of primary outcomes and relatively large sample size for exploratory comparisons. However, an important limitation of this study is the self-reporting of PA and the very informal and limited classification, although as noted above, this would be anticipated to obscure findings and bias the null hypotheses. 25 The nonspecific assessment of PA did demonstrate the known effects of exercise on BP reduction as seen in other studies. In addition, we only could evaluate the effects of acute ACE inhibition. It is not known how responses would evolve over time (durability). Thus, these results are observational in nature and should be taken as hypothesis driving with need for replication in a controlled clinical trial setting.
In summary, higher self-reported levels of PA were associated with an improved hemodynamic response to acute ACEi administration and reduced aldosterone levels. This may provide additional insight into the mechanisms underlying BP regulation in hypertension and its relation to lifestyle modifications. Future investigations should explore whether acute effects observed in this analysis are durable in a controlled clinical trial setting.
Acknowledgments
We would like to thank all members of the Clinical Research Center at Brigham and Women’s Hospital, all past researchers that contributed to the HyperPATH Cohort, and all of the research participants who participated in the studies included in this analysis.
Footnotes
Author Contributions: SAM and JSW contributed to the conception or design of the work.
SAM, JSW, KMM, and GM contributed to the acquisition, analysis, or interpretation of data for the work; contributed to drafting of the work or revising it critically for important intellectual content; approved the final version of the manuscript; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. We would also like to thank the following NIH Grants: P50HL055000 (HyperPATh Cohort); UL1RR025758 Harvard Clinical and Translational Science Center; R01HL086907, R01HL11476, R01HL96208, R01HL4765, R01HL59424, R01HL59424, R01HL69208, and R01HL09518. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health.
Ethical Approval: This study was approved by the Institutional Review Board at Partners Healthcare, Boston, MA.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Stephen A. Maris  https://orcid.org/0000-0003-4083-3333
https://orcid.org/0000-0003-4083-3333
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043-2050. doi: 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292. doi: 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palomo-Piñón S, Rosas-Peralta M, Paniagua-Sierra JR. Treatment of hypertension in chronic kidney disease [in Spanish]. Rev Med Inst Mex Seguro Soc. 2016;54(suppl 1):s78-s88. [PubMed] [Google Scholar]
- 5.Neutel JM. Beyond the sphygmomanometric numbers: hypertension as a syndrome. Am J Hypertens. 2001;14(8 pt 2):250S-257S. doi: 10.1016/s0895-7061(01)02155-0 [DOI] [PubMed] [Google Scholar]
- 6.Weber MA. Hypertension as a risk factor syndrome: therapeutic implications. Am J Med. 1993;94(4A):24S-31S. [PubMed] [Google Scholar]
- 7.Jekell A, Kalani M, Kahan T. The effects of alpha 1-adrenoceptor blockade and angiotensin converting enzyme inhibition on central and brachial blood pressure and vascular reactivity: the doxazosin-ramipril study. Heart Vessels. 2017;32:674-684. doi: 10.1007/s00380-016-0924-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlin PR, Erlinger TP, Bohannon A, et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens. 2003;16(5 pt 1):337-342. doi: 10.1016/s0895-7061(03)00056-6 [DOI] [PubMed] [Google Scholar]
- 9.Conlin PR, Moore TJ, Swartz SL, et al. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461-465. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard BE, Tsongalis GJ, Guidry MA, et al. RAAS polymorphisms alter the acute blood pressure response to aerobic exercise among men with hypertension. Eur J Appl Physiol. 2006;97:26-33. doi: 10.1007/s00421-006-0142-8 [DOI] [PubMed] [Google Scholar]
- 11.Goessler K, Polito M, Cornelissen VA. Effect of exercise training on the renin-angiotensin-aldosterone system in healthy individuals: a systematic review and meta-analysis. Hypertens Res. 2016;39:119-126. doi: 10.1038/hr.2015.100 [DOI] [PubMed] [Google Scholar]
- 12.Hespel P, Lijnen P, Van Hoof R, et al. Effects of physical endurance training on the plasma renin-angiotensin-aldosterone system in normal man. J Endocrinol. 1988;116:443-449. [DOI] [PubMed] [Google Scholar]
- 13.M’Buyamba-Kabangu JR, Fagard R, Lijnen P, Amery A. Relationship between plasma renin activity and physical fitness in normal subjects. Eur J Appl Physiol Occup Physiol. 1985;53:304-307. [DOI] [PubMed] [Google Scholar]
- 14.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533-553. [DOI] [PubMed] [Google Scholar]
- 15.Snauwaert E, Walle JV, De Bruyne P. Therapeutic efficacy and safety of ACE inhibitors in the hypertensive paediatric population: a review. Arch Dis Child. 2017;102:63-71. doi: 10.1136/archdischild-2016-310582 [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31:639-648. doi: 10.1097/HJH.0b013e32835ca964 [DOI] [PubMed] [Google Scholar]
- 17.Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;33:853-856. doi: 10.1111/j.1440-1681.2006.04453.x [DOI] [PubMed] [Google Scholar]
- 18.Baudrand R, Pojoga LH, Vaidya A, et al. Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation. 2015;132:1825-1833. doi: 10.1161/CIRCULATIONAHA.115.016759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Ting PY, Yao TM, et al. Histone demethylase LSD1 and biological sex: impact on blood pressure and aldosterone production. J Endocrinol. 2019;240:111-122. doi: 10.1530/JOE-18-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pojoga L, Kolatkar NS, Williams JS, et al. Beta-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension. 2006;48:892-900. doi: 10.1161/01.HYP.0000244688.45472.95 [DOI] [PubMed] [Google Scholar]
- 21.Vaidya A, Pojoga L, Underwood PC, et al. The association of plasma resistin with dietary sodium manipulation, the renin-angiotensin-aldosterone system, and 25-hydroxyvitamin D3 in human hypertension. Clin Endocrinol (Oxf). 2011;74:294-299. doi: 10.1111/j.1365-2265.2010.03922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan JW, Gupta T, Manosroi W, et al. Dysregulated aldosterone secretion in persons of African descent with endothelin-1 gene variants. JCI insight. 2017;2:e95992. doi: 10.1172/jci.insight.95992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 24.Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472-4475. doi: 10.1210/jc.2007-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leppink J, O’Sullivan P, Winston K. Evidence against vs. in favour of a null hypothesis. Perspect Med Educ. 2017;6:115-118. doi: 10.1007/s40037-017-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Predel HG, Rohden C, Heine O, Prinz U, Rost RE. ACE inhibition and physical exercise: studies on physical work capacity, energy metabolism, and maximum oxygen uptake in well-trained, healthy subjects. J Cardiovasc Pharmacol. 1994;23(suppl 1):S25-S28. [PubMed] [Google Scholar]
- 27.Sumukadas D, Band M, Miller S, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69:736-743. doi: 10.1093/gerona/glt142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Bonham AC. Postexercise hypotension: central mechanisms. Exerc Sport Sci Rev. 2010;38:122-127. doi: 10.1097/JES.0b013e3181e372b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16:225-236. doi: 10.1038/sj.jhh.1001377 [DOI] [PubMed] [Google Scholar]
- 30.Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol (1985). 2000;89:1830-1836. doi: 10.1152/jappl.2000.89.5.1830 [DOI] [PubMed] [Google Scholar]
- 31.Kingsley JD, Figueroa A. Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging. 2016;36:179-187. doi: 10.1111/cpf.12223 [DOI] [PubMed] [Google Scholar]
- 32.Pearson MJ, Smart NA. Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2018;23:91-108. doi: 10.1007/s10741-017-9662-z [DOI] [PubMed] [Google Scholar]
- 33.Ryuzaki M, Morimoto S, Niiyama M, et al. The relationships between the differences in the central blood pressure and brachial blood pressure and other factors in patients with essential hypertension. Intern Med. 2017;56:587-596. doi: 10.2169/internalmedicine.56.7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funder JW. Primary aldosteronism: the next five years. Horm Metab Res. 2017;49:977-983. doi: 10.1055/s-0043-119802 [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717. doi: 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 36.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321. doi: 10.1056/NEJMoa030207 [DOI] [PubMed] [Google Scholar]