Abstract
Xenorhabdus spp. and Photorhabdus spp. are major insect bacterial pathogens symbiotically associated with nematodes. These bacteria are transported by their nematode hosts into the hemocoel of the insect prey, where they proliferate within hemolymph. In this work we report that wild strains belonging to different species of both genera are able to produce hemolysin activity on blood agar plates. Using a hemocyte monolayer bioassay, cytolytic activity against immunocompetent cells from the hemolymph of Spodoptera littoralis (Lepidoptera: Noctuidae) was found only in supernatants of Xenorhabdus; none was detected in supernatants of various strains of Photorhabdus. During in vitro bacterial growth of Xenorhabdus nematophila F1, two successive bursts of cytolytic activity were detected. The first extracellular cytolytic activity occurred when bacterial cells reached the stationary phase. It also displayed a hemolytic activity on sheep red blood cells, and it was heat labile. Among insect hemocyte types, granulocytes were the preferred target. Lysis of hemocytes by necrosis was preceded by a dramatic vacuolization of the cells. In contrast the second burst of cytolytic activity occurred late during stationary phase and caused hemolysis of rabbit red blood cells, and insect plasmatocytes were the preferred target. This second activity is heat resistant and produced shrinkage and necrosis of hemocytes. Insertional inactivation of flhD gene in X. nematophila leads to the loss of hemolysis activity on sheep red blood cells and an attenuated virulence phenotype in S. littoralis (A. Givaudan and A. Lanois, J. Bacteriol. 182:107–115, 2000). This mutant was unable to produce the early cytolytic activity, but it always displayed the late cytolytic effect, preferably active on plasmatocytes. Thus, X. nematophila produced two independent cytolytic activities against different insect cell targets known for their major role in cellular immunity.
The genus Xenorhabdus consists of the specific bacterial symbionts of the entomopathogenic nematodes of the family Steinernematidae (40) and was separated from the genus Photorhabdus (11), which contains the symbionts of the entomopathogenic nematodes of the family Heterorhabditidae. Both genera are entomopathogenic gram-negative bacteria belonging to the Enterobacteriaceae. The nematodes carry their bacterial symbionts monoxenically in a special vesicle of the infective stage (L3 juveniles) in Steinernematidae (8) and throughout the whole intestine of Heterorhabditidae (20). These bacteria are transported by their nematode hosts into the hemocoel of the insect prey, which is killed, probably via a combination of toxin action and septicemia. The bacterial symbionts also contribute to the symbiotic relationship by establishing and maintaining suitable conditions for nematode reproduction (31). Recently, isolation of some Photorhabdus strains from infected humans in Australia and the United States was reported (21, 30), and the strains from the United States were classified as Photorhabdus asymbiotica (23).
The form of the bacterium that is normally isolated from symbiotic infective-stage nematodes is referred to as phase I. Like many pathogenic bacteria, Xenorhabdus and Photorhabdus strains spontaneously produce colonial variants which have been called phase II variants (10). The two variants of the bacteria have generally been shown to be equally pathogenic for the larvae of the greater wax moth, Galleria mellonella (3). However, Volgyi et al. (42) described for the first time a phase II variant that showed reduced virulence in the tobacco hornworm, Manduca sexta.
Xenorhabdus nematophila and Photorhabdus luminescens are highly pathogenic to insects, and 50% insect mortality has been reported with direct infection with fewer than 20 bacteria per larva (5). The bacterial factors involved in killing of the insect or in overcoming the insect immune reactions are still under investigation. Following invasion of the insect host by the nematodes, both bacteria produce potential virulence factors, including lipase, protease, lecithinase, and lipopolysaccharides (LPSs), in the hemocoel (for a review, see reference 24). It was shown that purified LPS, Photorhabdus protease fractions, or Xenorhabdus lecithinase isomers showed no toxic effect following injection into insect hemocoel (12, 16, 39). Recently, a novel toxin complex with both oral and injectable activities against a wide range of insects was identified in a supernatant of P. luminescens (13). Purified toxin complex a (Tca) has specific effects on the midgut epithelium of the insect (9). In order to study Xenorhabdus and Photorhabdus virulence in insects, a genetic approach was also used. Avirulent mutants of X. nematophila have been isolated by transposon mutagenesis (Tn5). These mutants were pleiotropic, but all five mutants that tested as avirulent in G. mellonella were nonmotile and partially impaired in blood hemolysis (43). It was also shown that a homoserine lactone autoinducer restored virulence to one avirulent X. nematophila strain and stimulated the level of bacterial lipase activity (17). Recently we reported that flhDC, the flagellar master operon of X. nematophila, controls flagellin expression. Furthermore we revealed that lipolytic and extracellular hemolysin activity is flhD dependent. We also showed that the flhD null mutant displayed an attenuated virulence phenotype in the common cutworm, Spodoptera littoralis, compared to the wild-type strain (25). The recently published partial genome sequence of P. luminescens (22) revealed a diverse array of genes that putatively encodes potential virulence factors. These factors include exoenzymes (proteases, lipases, and chitinases), a type III secretion system (Yop homolog), and several classes of toxins (insecticidal toxin complex, Rtx-like toxins, and hemolysin and cytotoxin homologs) (22). Until now, studies examining hemolytic activity of both genera have not been reported.
Cytolysins are proteins which cause lysis of red blood cells (RBC) as well as nucleated cell types by hydrolysis (lipases, phospholipases, or proteases) or by forming pores in the plasma membrane. Surfactants may also cause cytolysis by solubilization of the target cell membrane. Bacterial cytolysins are usually recognized as hemolysin on blood agar where a transparent zone appears around colonies. The production by a few strains of Xenorhabdus and Photorhabdus of hemolysin has been detected on agar supplemented with sheep blood (6, 21, 25).
Apart from their phoretic location inside infective juvenile nematodes, these bacteria are only observed in insect hemolymph, where they enter their growth cycle. Here the bacteria are in contact with hemocytes which achieve defense reactions in insects. Some of these cells are immunocompetent cells able to engulf (phagocytosis) or to isolate and kill (nodule formation) bacteria. We hypothesize that hemolytic activities could target the immunocompetent cells in insect hemolymph. In this study, we report that different cytolytic activities were found in supernatants of Xenorhabdus whereas none was detected in supernatants of various strains of Photorhabdus. We have studied the kinetics of the production of cytolytic activities over the course of in vitro bacterial growth. We also provide evidence on the characteristics and on the specificity of each of these cytolytic activities against mammalian RBC and insect hemocyte types.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains used in this study and their sources are listed in Table 1. For each subculture, phase status was determined by differential absorption of dye when the strains were grown on NBTA (nutrient agar supplemented with 25 mg of bromothymol blue and 40 mg of triphenyltetrazolium chloride per liter), by measuring antibacterial activity against Micrococcus luteus (from the culture collection of the Institut Pasteur, Paris, France), and by bioluminescence production for Photorhabdus. Phase I colonies are blue on NBTA, produce agar-diffusible antibiotics, and are bioluminescent for Photorhabdus strains while phase II colonies are red and produce reduced or no antibacterial activity. Bacterial cells were grown at 28°C in 100 ml of Luria-Bertani (LB) broth for liquid cultures and on nutrient agar (Difco) for solid cultures.
TABLE 1.
Bacterial strains used in this study and their cytolytic activities
| Taxon | Strain | Source (host nematode or hospital strain) or reference | Sheep blood hemolysisa | Cytolytic activityb of cell supernatants from stationary phase cultures with:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| SRBC
|
RRBC
|
IHs
|
|||||||
| Earlyc | Lated | Early | Late | Early | Late | ||||
| Xenorhabdus nematophila | F1/1 | Steinernema carpocapsae | T+ | + | − | − | + | + | + |
| F1/2 | 25 | − | − | − | − | − | − | − | |
| ΩIA (F1/1 flhD::Ω) | 25 | AH | − | − | − | + | − | + | |
| Xenorhabdus japonica | JP02 | Steinernema kushidai | T+ | ++ | + | − | + | ++ | ++ |
| Xenorhabdus bovienii | F3 | Steinernema affine | T+W | +W | +W | − | − | ++ | ++ |
| Xenorhabdus poinarii | G6 | Steinernema glaseri | T+W | + | − | ++ | + | − | − |
| Xenorhabdus beddingii | Q58 | Steinernema sp. | T+W | − | − | − | +W | − | + |
| Xenorhabdus sp. | SaV | Steinernema arenarium | T+W | − | +W | +W | +W | − | + |
| Photorhabdus luminescens subsp. luminescens | Hb/1 (ATCC 29999T) | Heterorhabditis bacteriophora group Brecon | P | − | − | +W | − | − | − |
| Hb/2 | Laboratory collection | − | − | − | − | − | − | − | |
| Photorhabdus luminescens subsp. akhurstii | FRG04 (CIP 105564T) | H. indica | V | − | − | − | − | − | − |
| Photorhabdus luminescens subsp. laumondii | TT01 (CIP 105565T) | H. bacteriophora group HP88 | T+W | − | − | +W | − | − | − |
| Photorhabdus temperata | Meg | H. megidis Nearctic group | V | − | − | − | − | − | − |
| Photorhabdus temperata subsp. temperata | XlNach (CIP 105563T) | H. megidis Palaearctic group | P | − | − | − | − | − | − |
| Photorhabdus asymbiotica | 3265–86 (ATCC 43950T) | CDCe Atlanta | AH | − | − | − | − | − | − |
| 1216–79 (ATCC 43948) | CDC Atlanta | AH | − | − | − | − | − | − | |
All blood agar plates were cultured for 2 days at 28°C before assays were interpreted. Interpretations: T, total hemolysis; P, partial hemolysis; AH, annular hemolysis; +, clearing halo up to 5 mm; +W, perceptible halo with size less than 5 mm; −, hemolysis not detected; V, variable (annular hemolysis or total hemolysis, depending on the plates).
Erythrocyte cytolysis and IH cytolysis were expressed as cytolytic units and percent dead hemocytes, respectively (see Materials and Methods for calculation). ++, value up to 0.75; +, value between 0.25 and 0.75; +W, value less than 0.25; −, cytolytic activity not detected.
Supernatants were collected when bacterial cultures reached an OD540 of about 2.5 (20-h-old cultures).
Supernatants were collected when bacterial cultures reached an OD540 up to 4 (48-h-old cultures).
CDC, Centers for Disease Control and Prevention.
RBC hemolytic activity.
Hemolytic activity was determined using blood agar plates and a liquid hemolytic assay (35). (i) Bacteria were grown on Trypticase soy (bioMérieux, Marcy L'Etoile, France) with 5% (vol/vol) defibrinated sheep blood (bioMérieux); hemolysis was determined by the observation of a clearing surrounding bacteria grown on standard sheep blood agar plates. (ii) Determination of the hemolytic activities in bacterial supernatant was achieved using a liquid assay. Briefly, bacterial cells were harvested during growth until 3 days. After centrifugation and ultrafiltration (0.22-μm-pore-size filter; Millipore), extracts (50 μl) were mixed with a suspension (25 μl) of phosphate-buffered saline (PBS)-washed sheep RBC (SRBC) (bioMérieux) or rabbit RBC (RRBC) (bioMérieux) at a final concentration of 5%. The mixture was incubated at 37°C for 1 h. After centrifugation to remove unlysed cells and cell membranes, the released hemoglobin present in the samples was measured at an optical density at 540 nm (OD540). The percent hemolysis was calculated by the following formula: [(A540 for the sample with hemolysin − A540 for the control without hemolysin)/(A540 for the complete lysis caused by mixing ultrapure-grade water)] × 100. One cytolytic unit is defined as the release of 100% of hemoglobin.
In vitro insect hemocyte (IH) monolayers and evaluation of IH cytotoxic (IHC) activity.
The common cutworm, S. littoralis was reared with a photoperiod of 12 h on an artificial diet at 24°C. Two-day-old sixth-instar larvae were selected and surface sterilized with 70% (vol/vol) ethanol prior to collection of hemolymph in test tubes filled with sterile anticoagulant buffer (62 mM NaCl, 100 mM glucose, 10 mM EDTA, 30 mM trisodium citrate, 26 mM citric acid) (28). Approximately 1 volume of hemolymph was collected in 5 volumes of the buffer. After centrifugation (800 × g for 15 s) the hemocyte pellet was rinsed in PBS (bioMérieux) and resuspended in the same buffer. Hemocyte suspension (20 μl) was layered on heat-sterilized (220°C for 2 h) glass coverslips. Hemocytes were allowed to adhere on glass for 15 min in a moist chamber at room temperature and then were gently rinsed with PBS before use as monolayers.
In tests for cytotoxic activity, excess PBS was pipetted off the coverslip and replaced by 20 μl of the solution under study, and monolayers were incubated in a moist chamber at 23°C for 1 h. Hemocyte mortality was checked under phase-contrast microscopy by adding 2 μl of trypan blue dye (0.4% in PBS) and allowing 5 min more of incubation. Results were expressed as a percentage of dead cells for each hemocyte type (differential count) or as a percentage of dead hemocytes relative to total hemocyte count, depending on the experiment. Means were compared using the Student t test after arcsin transformation.
Preparation of cytolytic extracts and resistance to physical and enzymatic factors.
X. nematophila F1/1 cells were cultivated at 28°C in a 100-ml LB broth liquid culture for 3 days. Cells were removed by centrifugation (6,000 × g, 10 min, 4°C) over the course of bacterial growth. The filter (0.22-μm pore size)-sterilized supernatants were used as cytolytic extracts. Temperature and trypsin resistance were respectively assayed by 1-h incubations at 60 to 100°C or with a solution of trypsin (30 U in final volume) (Sigma) before cytolytic assays on RBC and IHs.
Characterization of cytolytic activities.
The effects of different experimental parameters on RBC cytolytic activity were assessed.
(i)Effect of incubation at low temperature.
RBC suspensions (5%) in PBS were incubated with cytolytic extracts at 4°C for 2 h. After centrifugation, the supernatant was tested for hemolysis as described above. The pellet was rapidly washed in PBS, suspended in the same buffer, and incubated for 1 h more at 37°C, and cytolytic activity was determined.
(ii)Effect of RBC concentration.
The same amounts of cytolytic extracts were incubated with different RBC concentrations (5, 10, and 20%) for 2 h, a time course longer than that necessary for saturation of the extent of the effects. Hemolysis was calculated as a percentage of total hemolysis at each RBC concentration.
(iii) Effect of calcium deprivation.
The effect of calcium deprivation was determined using the protocol previously described (41). Briefly, cytolytic extracts were collected from supernatant of bacterial cells grown in 100 ml of LB broth supplemented with 10 μM EGTA (Sigma). EGTA extracts were mixed with a suspension of 5% SRBC or RRBC or IHs washed three times in PBS (without Ca and Mg) supplemented with 10 mM EGTA. Thereafter, the number of cytolytic units was calculated as described above.
Effect of LPS on cytolysis in vitro.
RBC or hemocyte monolayers were incubated in graded solutions of LPS from Escherichia coli (serotype 0111:B4 [Sigma]; maximum concentration, 5 · 105 U/ml [that is, 1 mg/ml]) in PBS.
Electron microscopy.
Hemocytes were collected in anticoagulant buffer and then incubated for 1 h with cytolytic extracts diluted 1/3 (vol/vol) with PBS. After incubation, hemocytes were fixed in 5% glutaraldehyde in phosphate buffer (pH 7.2), pelleted by gentle centrifugation, postfixed in 1% osmic acid in the same buffer, dehydrated in a graded series of ethanol, and embedded in Epon 812. Ultrathin sections were stained according to the method of Reynolds (32) and examined in a JEOL 200-CX transmission electron microscope at 70 kV.
RESULTS
Hemolysin- and cytolysin-producing strains among Xenorhabdus and Photorhabdus strains.
Two methods (blood agar plate and liquid hemolytic assays) were used to check for the production of cytotoxic factors by the bacteria. Blood agar assays showed that all Xenorhabdus and Photorhabdus wild strains were able to produce hemolysin activity on plates. Xenorhabdus strains produced a total discoloration around the colony of bacteria, whereas Photorhabdus strains displayed different hemolysis patterns (Table 1). An unusual type of hemolysis showing a partial hemolysis immediately around the colony and a thin line of complete hemolysis at some distance from the colony has been designated “annular hemolysis” (6) as previously described by Farmer et al. (21). This type of hemolysis was clearly found in P. asymbiotica isolated from clinical specimens, but it was irregularly observed on plates with other Photorhabdus strains (FRG04 and Meg) isolated from nematodes (Table 1). As previously described (25), the flhD mutant from X. nematophila F1 also produces an annular hemolysis reaction on blood agar (Table 1).
The screening of extracellular cytolytic activity among Xenorhabdus and Photorhabdus wild strains allowed us to distinguish three groups of insect pathogen bacteria: (i) the cytolysin-producing strains which were positive on the three kinds of eukaryotic cells (SRBC, RRBC, and IHs), (ii) the cytolysin-producing strains which were positive on two cell types, and (iii) the cytolysin nonproducers or the weaker producers active only on RRBC (Table 1). All Photorhabdus strains belonged to the third category, while Xenorhabdus strains belonged to both other categories of cytolysin-producing bacteria. No cytolytic activity against IH was detected in Xenorhabdus poinarii G6.
Both phase II variants from X. nematophila and P. luminescens failed to generate any discoloration of blood and to produce any cytolysis activity with RBC or IHs.
Cytolysin production during broth growth of X. nematophila F1.
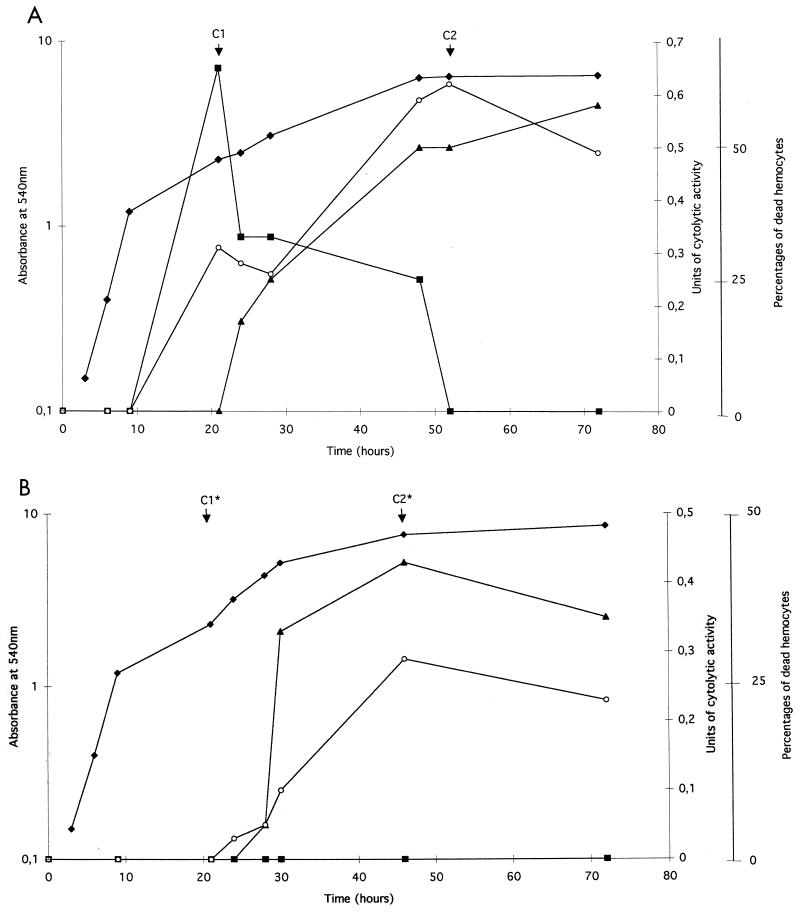
Figure 1A shows that production of extracellular cytolysin from X. nematophila F1/1 was growth phase dependent. No cytolysin activity was observed during the exponential growth phase. Cytolysin production by the X. nematophila wild type occurred after 10 h of incubation when cells reached the stationary phase (Fig. 1A). However, different kinetics of production were observed according to the target cell. When growth began to slow down and entered the stationary phase, there was a sudden burst of cytolysis on IHs and hemolysis on SRBC. When this first burst rapidly rose to a maximum level, SRBC cytolysis progressively stopped in prolonged bacterial cultures, whereas a second burst of IHC activity appeared concomitantly with the RRBC cytolytic activity after 20 h of growth (Fig. 1A).
FIG. 1.
Relationship between extracellular cytolytic activity and growth in LB broth liquid culture of X. nematophila F1/1 (A) and flhD null mutant (ΩIA) (B). Cytolysis of RBC was expressed as cytolytic units by measuring the release of hemoglobin at OD540 (see Materials and Methods for calculation). Cytolysis of IHs was expressed as percentages of dead hemocytes relative to total hemocyte counts using trypan blue assay (see Materials and Methods for calculation). The extracellular cytolytic extracts selected for further characterization are indicated at the top of the graph and are designated C1 and C2 for X. nematophila F1/1 and C1* and C2* for the flhD null mutant. Symbols: ⧫, growth measured as OD600; ■, cytolytic activity on SRBC; ○, cytolytic activity on IHs from S. littoralis; ▴, cytolytic activity on RRBC.
We previously showed that the disruption of the flhD gene abolished expression of SRBC cytolytic activity in X. nematophila (25). One question that arises from this work is the dependence of RRBC and IHC activities on flhD. Figure 1B confirmed that no SRBC cytolysis was detected in the bacterial supernatant from ΩIA, whatever the time of bacterial growth, whereas the burst of IHC activity appeared in the late stationary phase of growth concomitantly with the RRBC cytolysis. This showed that the second burst of IH cytolysis and the RRBC cytolysis were flhD independent.
No cytolytic activity was detected during 3 days of broth growth of the phase II variant from X. nematophila F1 (data not shown).
Biochemical properties of X. nematophila cytolytic extracts.
Two cytolytic extracts from F1/1 supernatant were chosen according to the kinetics of cytolysin production (Fig. 1A). Cytolytic extract C1 displayed the highest SRBC activity and no RRBC activity, whereas cytolytic extract C2 showed no SRBC activity but a high RRBC activity (Table 2). Both C1 and C2 cytolytic extracts had high IHC activities.
TABLE 2.
Cytolytic activities of extracts C1 and C2 for different target cells and effects of different treatments
| Target cells | % Cytolytic activity under indicated conditiona
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytolytic extract C1
|
Cytolytic extract C2
|
LPS | |||||||||||||
| Control | 60°C | 100°C | With trypsin | With EGTA | After incubationb at 4°C
|
Control | 60°C | 100°C | With trypsin | With EGTA | After incubationb at 4°C
|
||||
| PEL | SUP | PEL | SUP | ||||||||||||
| SRBC | 100 | 0 | 0 | 20 | 99 | 4 | 94 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RRBC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 143 | 221 | 100 | 110 | 48 | 46 | 0 |
| IH | 100 | 0 | 0 | NDc | 100 | ND | ND | 100 | 80 | 50 | ND | 100 | ND | ND | 0 |
Cytolytic activities obtained in control experiments (see Materials and Methods) were arbitrarily set at 100%. Each experiment was performed in triplicate.
After incubation at 4°C for 2 h with C1 or C2 extracts, RBC were centrifuged, and the hemolytic activity remaining in the resulting pellet (PEL) and supernatant (SUP) was tested. Remaining hemolysis is expressed as a percentage of hemolysis before incubation at 4°C.
ND, not done.
Each extract was stable as demonstrated by recovery of the whole cytolytic activity after freezing at −20°C. Both SRBC and IHC activities were completely lost in C1 extract after long-term storage (several weeks) at room temperature or after protease (trypsin) or temperature (60°C, 1 h) treatment, whereas C2 extract was always stable after such treatments. An increase of RRBC activity was even observed following heat incubations, suggesting a heat sensitivity of cytolysin inhibitor. Surprisingly, a negative effect of heat treatment was observed on IHC activity of C2 extract (Table 2). The same resistance to the treatments was obtained using supernatant obtained after long-term growth (C2∗ extract) from the flhD null mutant, ΩIA (data not shown).
Characterization of hemolytic and cytolytic activities from X. nematophila. (i) Calcium independence.
X. nematophila F1/1 cells were grown in LB broth depleted of calcium by the addition of 10 μM EGTA. The kinetics of production of the different cytolytic activities in LB broth culture supernatants with (Fig. 1A) or without (data not shown) calcium were similar. Cytolytic activities of the C1 and C2 extracts from calcium-depleted cultures against SRBC, RRBC, and IH in the presence of EGTA gave approximately the same values as those obtained from control assays without calcium depletion (Table 2). These results suggest that the molecules involved in cytolytic activities were produced independently of calcium and that calcium was not required for activity.
(ii) Binding to cell membranes at low temperatures.
In a series of experiments, RBC were incubated with C1 or C2 extracts at 4°C for 2 h. No hemolytic activity was recorded at this temperature. After centrifugation, the pellet of RBC and the supernatant were separately assayed for cytolytic activity at 37°C (Table 2). With C1 extract, all the activity on SRBC was recovered in the supernatant (Table 2), suggesting that there was no fixation of the hemolytic factors on the cell membrane at low temperatures. In contrast, the C2 extract's hemolytic activity was recovered both in the pellet and in the supernatant (Table 2).
(iii) Effect of RBC concentration.
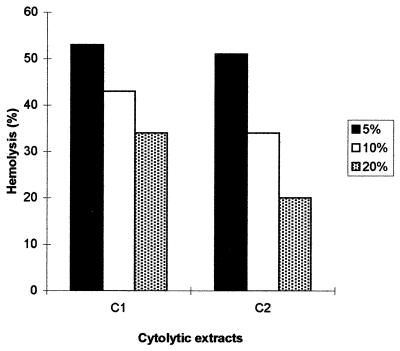
In order to assay the recycling of hemolytic factors, constant amounts of C1 and C2 extracts were incubated with increasing concentrations (5, 10, and 20%) of SRBC and RRBC, respectively. Figure 2 shows that the percentage of lysis, expressed as a percentage of the total lysis, decreased with increasing target cell concentration. These results demonstrate that hemolytic factors in C1 and C2 extracts were not recycled.
FIG. 2.
Effect of increasing concentrations of RBC on percent hemolysis. Equal amounts of C1 and C2 were incubated with three different concentrations (5, 10, and 20%) of SRBC and RRBC, respectively. Hemolysis is expressed as a percentage of complete hemolysis achieved by mixing ultrapure-grade water in each RBC concentration. Data represent mean values for duplicate determinations from one of three similar experiments.
(iv) LPS assay.
In a series of experiments, SRBC, RRBC, or hemocytes were incubated in graded concentrations of LPS solution in PBS. No hemocyte lysis was recorded in the LPS assay. With RBC no hemolytic activity was recorded, regardless of the origin of the RBC (Table 2).
Cell specificity of cytolytic extracts.
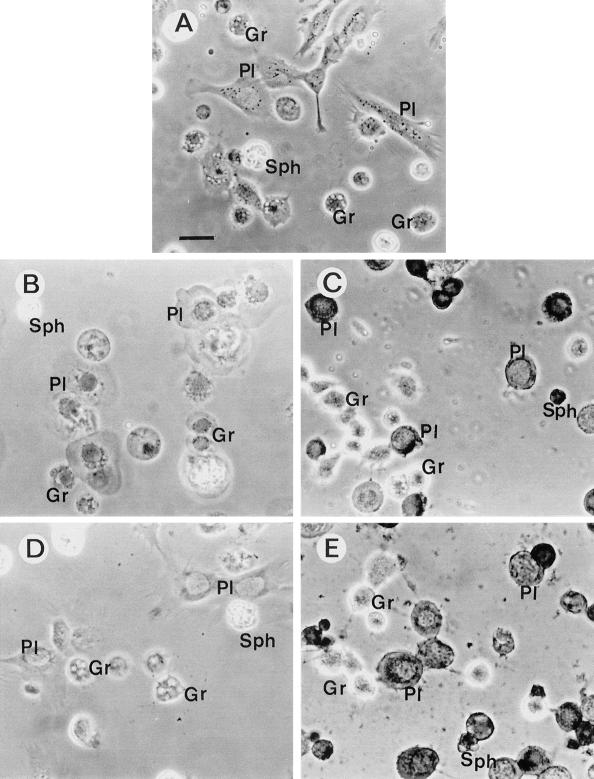
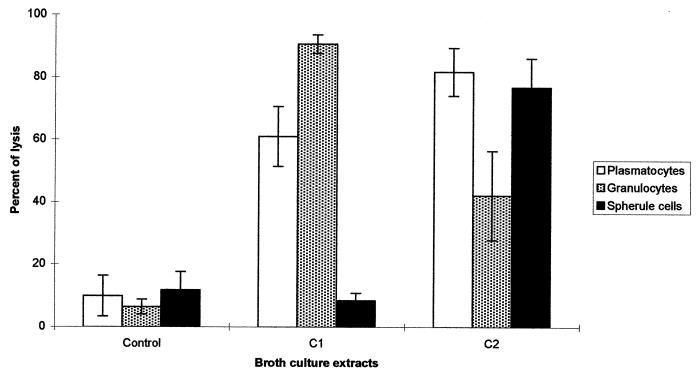
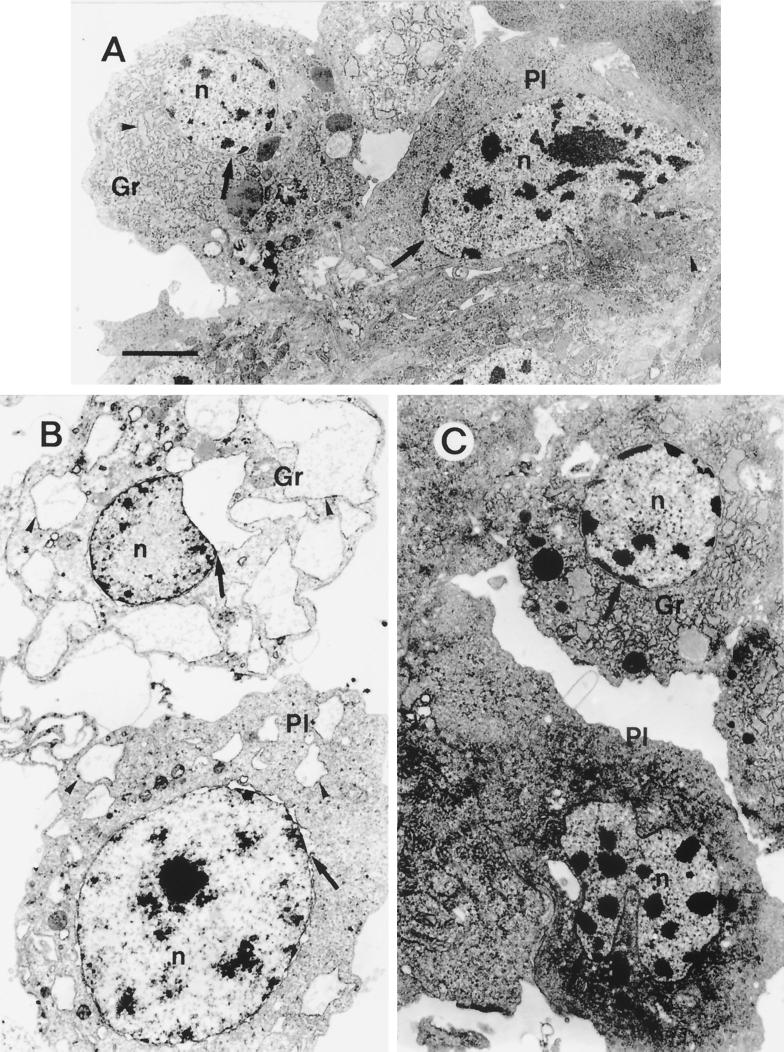
C1 and C2 extracts from X. nematophila F1/1 exhibited different specificities with regard to the IH types. According to Brehélin and Zachary (14), six different hemocyte types were characterized in S. littoralis larvae. Plasmatocytes (PL) and granulocytes (type 1 granular hemocytes [GR]) are the most numerous cell types, representing almost 90% of total hemocytes present in hemolymph. As in other lepidopteran species, they were endowed with immune reactions. GR are phagocytic cells and are the functional equivalent of mammalian macrophages, whereas PL are the main cell type which forms capsules around foreign bodies of a large size. Spherule cells (SPH), which have unknown function, represented 5% of total hemocytes, whereas prohemocytes, oenocytoids, and cells with large granules comprised the remaining 5% (C. Ribeiro and M. Brehélin, unpublished data). On cell monolayers, after incubation in PBS on slides, hemocytes rapidly spread and took characteristic shapes (Fig. 3A), with extended lamellipodia for PL and numerous short filopodia for GR (34). GR were the main hemocyte target in C1 extract (Fig. 3B and 4). After 1 h of incubation with twofold-diluted C1 extract, more than 90% of GR and almost 60% of PL were stained with trypan blue when no cytolytic activity was detected on SPH (Fig. 4). Transmission electron microscopic studies using fourfold-diluted C1 extract showed extensive distensions of endoplasmic reticulum (ER) vesicles and of the perinuclear cisterna in GR (Fig. 5B). PL showed vacuoles of a small size, but as in GR, these vacuoles were also dilated vesicles of the ER.
FIG. 3.
Phase-contrast light micrographs of trypan blue-stained S. littoralis hemocyte monolayers, after 1 h of incubation in different extracts at 22°C. Bar = 10 μm. (A) Incubation in uncultivated sterile broth diluted 1/1 (vol/vol) in PBS (control). (B) Incubation with C1 extract (1/1 [vol/vol] in PBS) from X. nematophila F1/1. All hemocytes are swollen dead cells (trypan blue-stained nuclei), except for the SPH (left top). (C) Incubation with C2 extract (1/1 [vol/vol] in PBS) from strain F1/1. Most PL are dead cells (trypan blue staining) and look like shrunken cells devoid of their lamellipodia. Most GR are very refringent live cells. (D) Incubation with C1∗ extract (1/1 [vol/vol] in PBS) from the flhD null mutant. PL, GR, and SPH look like cells in the control (panel A). (E) Incubation with C2∗ extract (1/1 [vol/vol] in PBS) from the same mutant. Hemocytes look like cells in C2 extract (see panel C) from the wild strain F1/1.
FIG. 4.
Percentages of lysis of S. littoralis hemocytes of each type after 1 h of incubation with supernatants of X. nematophila F1/1. C1 or C2 extract was diluted 1/3 (vol/vol) with PBS. Uncultivated sterile medium diluted 1/3 (vol/vol) in PBS was used as the control. Means and standard deviations (error bars) are shown for duplicate experiments with six larvae. For each hemocyte type after arcsin transformation, differences in percentages are highly significant (P < 0.001) except for SPH in the control and C1 extract.
FIG. 5.
Transmission electron micrographs of PL and GR from S. littoralis after 1 h of incubation in cytolytic extracts of X. nematophila F1/1. n, nuclei; arrowhead, vesicle of ER; arrow, perinuclear cisterna. (A) Control, sterile LB. (B) C1 extract diluted 1/3 (vol/vol) in PBS. Note the dilated vesicles of ER (arrowheads) and perinuclear cisternae (arrows). (C) C2 extract diluted 1/3 (vol/vol) in PBS. Hemocytes are shrunken cells with a dense hyaloplasm. Chromatin is condensed in rounded masses, especially in PL (compare with panel A). Bar = 1.5 μm.
The effects of C2 extract on hemocyte monolayers were quite different from those of C1 extract. PL were much more sensitive than GR to extract C2 (Fig. 4). PL had lost their lamellipodia and often appeared as shrunken, rounded cells, sometimes difficult to distinguish from GR (Fig. 3C). SPH also appeared very sensitive to cytotoxic factors of the C2 extract (Fig. 4). In transmission electron microscopy, very few vesicles were seen in hemocytes after incubation with C2 extract (Fig. 5C). Hemocytes of the different types appeared as shrunken cells with a dense cytoplasm. Their chromatin was condensed in numerous small rounded masses. Vesicles of the ER were not distended, but mitochondria were slightly swollen (Fig. 5A and C).
Twofold-diluted extract obtained from short-term broth growth (20 h old) of the ΩIA mutant (C1∗ extract) showed no effect on hemocyte monolayers (Fig. 3D), whereas extracts obtained from long-term growth (C2∗ extract) (Fig. 1B) gave the same cytotoxicity, mainly on PL and on SPH (Fig. 3E), as did C2 extract from the wild strain F1 (Fig. 3C).
DISCUSSION
Hemolysis in Photorhabdus, formerly considered Xenorhabdus luminescens, was first described by Farmer et al. (21). An unusual reaction on a sheep blood plate, designated annular hemolysis (6), was at first considered to be a marker in recognizing P. asymbiotica strains isolated from clinical specimens (21). The present study confirms that P. asymbiotica strains clearly express this phenotype but other Photorhabdus strains isolated from nematodes are also annular hemolysis producers (Table 1). As previously described, the Xenorhabdus flhD mutant also produces an annular hemolysis reaction on blood agar. This unusual pattern is not unique. Bacillus cereus isolates display the same phenotype, which has been termed a “discontinuous hemolytic pattern.” The hemolysin BL, which causes this reaction, is a major virulence determinant of B. cereus in the nongastrointestinal infections (7).
Since the partial genome sequencing of P. luminescens revealed numerous sequences similar to genes encoding hemolysins (22), we could predict that the Photorhabdus strains would be likely strong hemolysin producers. Photorhabdus strains were hemolytic on blood agar plates (Table 1). Surprisingly, no extracellular cytolytic activity against SRBC and IHs was detected in Photorhabdus supernatants. These data may suggest (i) that insect cellular types other than hemocytes are the targets of Photorhabdus extracellular hemolysins, (ii) that these compounds should be processed like insecticidal toxin complexes (13), or (iii) that the contact between bacteria and target cells is necessary for cytolysis.
Unlike Photorhabdus, Xenorhabdus wild strains displayed strong cytolytic activities on Spodoptera hemocytes. Only X. poinarii, which is considered to be a weakly pathogenic species for Lepidoptera, with a 50% lethal dose in the range of 1,000 to 10,000 bacteria (4), was a cytolysin nonproducer (Table 1). Thus, there is a positive correlation between the presence of cytolysin active on insect immunocytes and Xenorhabdus virulence. Moreover, we have previously demonstrated that the flhD null mutant which has lost activity on SRBC displayed an attenuated virulence phenotype in S. littoralis (25). This study revealed that even though this mutant was unable to produce the early cytolytic activity when tested with sheep or insect blood cells, it always displayed an IHC activity during late stationary growth phase (Table 1; Fig 1B). These data may explain why the flhD null mutant remained virulent and why insects injected with the mutant take longer to die.
The toxicity of entomopathogenic bacteria for insect hemocytes has already been described for Pseudomonas aeruginosa (26) and for X. nematophila (18, 37, 38). These in vivo experiments were inappropriate for distinguishing a direct toxicity of factors to hemocytes (cytotoxins) or a lytic action involving the general host physiology as with LPSs in mammals (36). In vitro experiments using cultured insect cells (Sf-9 and mbn-2) and Bacillus thuringiensis as the pathogen showed that the bacterium (strain Bt 13) or its culture supernatant can kill the insect cells (44). In the present study we also conducted in vitro experiments, but rather than using cell lines, we used IH monolayers. Using this in vitro assay, two different cytolytic activities on insect immunocompetent cells were found in the supernatant of X. nematophila F1/1 broth growth. Both of these activities found in C1 and C2 extracts have been studied (Fig. 1A). The extracellular cytolytic activity of the C1 extract was (i) the most precocious, as it occurred when the bacterial culture reached the stationary phase; (ii) flhD dependent; (iii) calcium independent; and (iv) heat labile. These characteristics were also those of the hemolytic activity evidenced on SRBC, suggesting that the same factor(s) was responsible for hemolysis of SRBC in suspension and for cytolysis of IHs. In contrast, the second cytolytic activity (i) appeared late in the stationary phase, (ii) was flhD independent, (iii) was calcium independent, and (iv) was heat resistant, four characteristics of the hemolytic activity observed on RRBC suspensions.
In addition to their differences in lysis of RBC from two mammalian species, C1 and C2 extracts also showed strong differences in specificity for IHs. Among the three most-numerous hemocyte types, the macrophage-like cells of S. littoralis hemolymph (GR) were more sensitive than PL to C1 extract, SPH being the most resistant cells. In contrast, C2 extract was mainly cytotoxic for PL and SPH whereas GR were the most resistant hemocytes (Fig. 4). The specificity of C1 extract for IH types and the biochemical characteristics (Table 2) were exactly those evidenced for a cytotoxic factor (CyA) present in medium after incubation of the nematobacterial complex Steinernema-Xenorhabdus (33). The present study shows that the factor CyA characterized in the nematobacterial complex originates from the bacterium rather than from the nematode.
Finally, C1 and C2 also exhibited differences in the kinds of cytotoxic effects against insect immunocytes. Soon after incubation with C1, hemocytes developed large and numerous vacuoles and then appeared as swollen cells, whereas incubation with C2 induced a shrinkage of hemocytes. We show here that C1-induced vacuoles observed in PL and GR are the dilatation of the ER (Fig. 5). Similar vacuoles are also observed in mammalian cells treated by the pore-forming aerolysin of Aeromonas hydrophila (1). However, no cytolysis after several hours of incubation has been observed with the pore-forming aerolysin (2), whereas C1-induced vacuolization led to hemocyte lysis in a few minutes.
It is clear that both cytolytic activities of X. nematophila were distinct and that their production was independently regulated. Nevertheless, the nature of molecules involved in the cytolysis and the mechanism of action against cell target remain unknown. The Xenorhabdus supernatants are rich in protease and lipolytic enzymes (10) which could account for hemolytic activity by proteolysis or phospholipid breakdown. For both cytolytic extracts, the percent hemolysis elaborated by a constant amount of extract decreased with increasing target cell concentration (Fig. 2). According to Rowe and Welch (35), this result shows that the hemolytic factor(s) was unable to be recycled, suggesting that its toxicity on RBC did not involve an enzymatic mechanism. Moreover, we showed (i) that the X. nematophila flhD null mutant which has lost the ability to produce heat-labile hemolytic activity (this study) remains a lecithinase and protease producer (25; A. Givaudan, unpublished data), (ii) that the phase II variant of X. nematophila which displayed a higher Tween lipase activity than did the wild type (25) was a hemolysin nonproducer (Table 1), and (iii) that addition of EGTA (an inhibitor of metalloprotease) to culture medium did not affect the cytolytic and hemolytic activity (Table 2). Taken together, all these results led us to conclude that the hemolytic and cytolytic activities in X. nematophila described in this study were not related to exoenzyme activities. In other respects, we have shown that no lysis occurred at 4°C with either cytolytic extract. These data suggest that this temperature-dependent lytic process could require energy to occur. A detergent-like activity has already been described as a hemolytic process (35). However, this mechanism is usually effective at low temperatures and unable to target particular types of cells, unlike both X. nematophila cytolytic activities. These data may suggest that pore-forming toxins were involved in the lytic process of both X. nematophila extracts as has been described for many bacteria (29).
Trypsin and heat sensitivities suggest that a proteinaceous component in C1 extract was required for cytolytic activity. Our preliminary results of patch-clamp experiments achieved with a prepurified C1 extract are also in accordance with the presence of a pore-forming-like molecule (Ribeiro and Brehélin, unpublished data). In contrast, heat and trypsin resistance of C2 extract is characteristic of LPS, which has been suggested to be toxic in vivo for G. mellonella (Lepidoptera) hemocytes (19). But Charalambidis et al. (15) have shown that solutions of LPS from E. coli (up to 500 μg/ml) had no toxic effect on IHs in vitro. Moreover, LPS extracted from X. nematophila was not toxic for lepidopteran hemocytes in vitro (33). These different studies are consistent with the data of inactivity of LPS on RBC and IHs as reported in the present work (Table 2). These data led us to conclude that LPS was not involved in the cytolysis against IHs and RBC observed with C2 heat-stable cytotoxic extracts. Finally, the active factors in C2 extracts can bind to RRBC membrane at 4°C and then achieve their hemolytic activity at 37°C, like most of the pore-forming bacterial toxins (29). Pore-forming cytotoxin isolated in the protozoan parasite Entamoeba histolytica is a polypeptide resistant to heat and proteolytic degradation that forms ion channels in target cell membranes (27). This may suggest that the active factor in C2 extract is also a pore-forming toxin.
Both insect cytolytic activities presented in this study destroyed the immunocompetent insect cells. If these bacterial factors are produced in insects during an infection with the nematobacterial complex, they could be involved in the infectious process by depression of some of the immune reactions. As the cellular targets were different from each other, the immune depression could be achieved by decreasing the number of cells available for two major immune reactions, capsule and nodule formation involving the PL and phagocytosis mediated by GR (34). Further work is required to construct a mutant deficient in both cytolytic activities in order to clarify the in vivo involvement of cytolysis against cellular defense responses of insects during the pathogenic interaction with Xenorhabdus.
ACKNOWLEDGMENTS
We thank Sylvie Pagès and Marc Ravalec for their precious assistance. We are also very grateful to John Scott (CSIRO, Montpellier, France) for revising the manuscript.
This work was supported by grants from Institut National de Recherche Agronomique (AIP no. 188). J.B. was funded by a MENRT grant (no. 98.5.11869), and C.R's. work in the EMIP laboratory was supported by grants from the CNRS (France), ICCTI, and PRAXIS XXI (BD/13935/97) (Portugal).
REFERENCES
- 1.Abrami L, Fivaz M, Decroly E, Seidah N G, Jean F, Thomas G, Leppla S H, Buckley J T, van der Goot F G. The pore-forming toxin proaerolysin is activated by furin. J Biol Chem. 1998;273:32656–32661. doi: 10.1074/jbc.273.49.32656. [DOI] [PubMed] [Google Scholar]
- 2.Abrami L, Fivaz M, van der Goot F G. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 2000;8:168–172. doi: 10.1016/s0966-842x(00)01722-4. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst R J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309. [Google Scholar]
- 4.Akhurst R J. Xenorhabdus nematophilus subsp. poinarii: its interaction with insect pathogenic nematodes. Syst Appl Microbiol. 1986;8:142–147. [Google Scholar]
- 5.Akhurst R J, Dunphy G B. Tripartite interactions between symbiotically associated entomopathogenic bacteria, nematodes, and their insect hosts. In: Beckage N E, Thompson S N, Federici B, editors. Parasites and pathogens of insects. Vol. 2. New York, N.Y: Academic Press; 1993. pp. 1–23. [Google Scholar]
- 6.Akhurst R J, Mourant R G, Baud L, Boemare N E. Phenotypic and DNA relatedness between nematode symbionts and clinical strains of the genus Photorhabdus (Enterobacteriaceae) Int J Syst Bacteriol. 1996;46:1034–1041. doi: 10.1099/00207713-46-4-1034. [DOI] [PubMed] [Google Scholar]
- 7.Beecher D J, Wong A C. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect Immun. 1994;62:980–986. doi: 10.1128/iai.62.3.980-986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird A F, Akhurst R J. The nature of the intestinal vesicle in nematodes of the family Steinernematidae. Int J Parasitol. 1983;13:599–606. [Google Scholar]
- 9.Blackburn M, Golubeva E, Bowen D, ffrench-Constant R H. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl Environ Microbiol. 1998;64:3036–3041. doi: 10.1128/aem.64.8.3036-3041.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boemare N E, Akhurst R J. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae) J Gen Microbiol. 1988;134:1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 11.Boemare N E, Akhurst R J, Mourant R G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol. 1993;43:249–255. [Google Scholar]
- 12.Bowen D, Blackburn M, Rocheleau T, Grutzmacher C, ffrench-Constant R H. Secreted proteases from Photorhabdus luminescens: separation of the extracellular proteases from the insecticidal Tc toxin complexes. Insect Biochem Mol Biol. 2000;30:69–74. doi: 10.1016/s0965-1748(99)00098-3. [DOI] [PubMed] [Google Scholar]
- 13.Bowen D, Rocheleau T A, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant R H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 14.Brehélin M, Zachary D. Insect haemocytes: a new classification to rule out the controversy. In: Brehélin M, editor. Immunity in invertebrates: cells, molecules, and defense reactions. Berlin, Germany: Springer-Verlag; 1986. pp. 36–48. [Google Scholar]
- 15.Charalambidis N D, Zervas C G, Lambropoulou M, Katsoris P G, Marmaras V J. Lipopolysaccharide-stimulated exocytosis of nonself recognition protein from insect hemocytes depend on protein tyrosine phosphorylation. Eur J Cell Biol. 1995;67:32–41. [PubMed] [Google Scholar]
- 16.Clarke D J, Dowds B C A. Virulence mechanisms of Photorhabdus sp. strain K122 toward wax moth larvae. J Invertebr Pathol. 1995;66:149–155. [Google Scholar]
- 17.Dunphy G, Miyamoto C, Meighen E. A homoserine lactone autoinducer regulates virulence of an insect pathogenic bacterium, Xenorhabdus nematophilus (Enterobacteriaceae) J Bacteriol. 1997;179:5288–5291. doi: 10.1128/jb.179.17.5288-5291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunphy G B, Webster J M. Interaction of Xenorhabdus nematophilus subsp. nematophilus with the haemolymph of Galleria mellonella. J Insect Physiol. 1984;30:883–889. [Google Scholar]
- 19.Dunphy G B, Webster J M. Lipopolysaccharides of Xenorhabdus nematophilus (Enterobacteriaceae) and their haemocyte toxicity in non-immune Galleria mellonella (Insecta: Lepidoptera) larvae. J Gen Microbiol. 1988;134:1017–1028. [Google Scholar]
- 20.Endo B Y, Nickle W R. Ultrastructure of the intestinal epithelium, lumen and associated bacteria in Heterorhabditis bacteriophora. J Helminthol Soc Wash. 1991;58:202–212. [Google Scholar]
- 21.Farmer J J, III, Pierce G V, Poinar G O, Jr, Grimont P A D, Carter G P, Ageron E, Akhurst R J, Hickman-Brenner F W, Jorgensen J H, Wilson K L, Smith J A. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J Clin Microbiol. 1989;27:1594–1600. doi: 10.1128/jcm.27.7.1594-1600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ffrench-Constant R H, Waterfield N, Burland V, Perna N T, Daborn P J, Bowen D, Blattner F R. A genomic sample sequence of the entomopathogenic bacterium Photorhabdus luminescens W14: potential implications for virulence. Appl Environ Microbiol. 2000;66:3310–3329. doi: 10.1128/aem.66.8.3310-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer-Le Saux M, Viallard V, Brunel B, Normand P, Boemare N E. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov., and P. asymbiotica sp. nov. Int J Syst Bacteriol. 1999;49:1645–1656. doi: 10.1099/00207713-49-4-1645. [DOI] [PubMed] [Google Scholar]
- 24.Forst S, Nealson K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol Rev. 1996;60:21–43. doi: 10.1128/mr.60.1.21-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Givaudan A, Lanois A. flhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J Bacteriol. 2000;182:107–115. doi: 10.1128/jb.182.1.107-115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horohov D W, Dunn P E. Role of hemocytotoxins in the pathogenicity of Pseudomonas aeruginosa in larvae of the tobacco hornworm, Manduca sexta. J Invertebr Pathol. 1983;43:297–298. [Google Scholar]
- 27.Leippe M. Ancient weapons: NK-lysin, is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell. 1995;83:17–18. doi: 10.1016/0092-8674(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 28.Mead G P, Rateliffe N A, Renwrantz L. The separation of insect haemocyte types on Percoll gradients: methodology and problems. J Insect Physiol. 1986;13:167–177. [Google Scholar]
- 29.Menestrina G, Schiavo G, Montecucco C. Molecular mechanisms of action of bacterial protein toxins. Mol Aspects Med. 1994;15:79–193. doi: 10.1016/0098-2997(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 30.Peel M M, Alfredson D A, Gerrard J G, Davis J M, Robson J M, McDougall R J, Scullie B L, Akhurst R J. Isolation, identification, and molecular characterization of strains of Photorhabdus luminescens from infected humans in Australia. J Clin Microbiol. 1999;37:3647–3653. doi: 10.1128/jcm.37.11.3647-3653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poinar G O., Jr The presence of Achromobacter nematophilus in the infective stage of a Neoaplectana sp. (Steinernematidae: Nematoda) Nematologica. 1966;12:105–108. [Google Scholar]
- 32.Reynolds E S. The use of lead citrate at high pH as an electron opaque staining in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro C, Duvic B, Oliveira P, Givaudan A, Palha F, Simões N, Brehélin M. Insect immunity: effects of factors produced by a nematobacterial complex on immunocompetent cells. J Insect Physiol. 1999;45:677–685. doi: 10.1016/s0022-1910(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro C, Simões N, Brehélin M. Insect immunity: the haemocytes of the armyworm Mythimna unipuncta (Lepidopera: Noctuidae) and their role in defense reactions. In vivo and in vitro studies. J Insect Physiol. 1996;42:815–822. [Google Scholar]
- 35.Rowe G E, Welch R A. Assays of hemolytic toxins. Methods Enzymol. 1994;235:657–667. doi: 10.1016/0076-6879(94)35179-1. [DOI] [PubMed] [Google Scholar]
- 36.Schletter J, Heine H, Ulmer A J, Rietschel E T. Molecular mechanism of endotoxin activity. Arch Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 37.Seryczynska H. Changes in the ultrastructure of the haemolymph cells of Galleria mellonella L. under the influence of nematodes Neoplectana carpocapsae Weiser. Bull Acad Pol Sci. 1971;20:45–47. [PubMed] [Google Scholar]
- 38.Seryczynska H, Kamoniek M, Sandner H. Defensive reactions of caterpillars of Galleria mellonella L. in relation to bacteria Achromobacter nematophilus Poinar et Thomas (Eubacteriales: Achromobacteriacae) and bacteria-free nematodes Neoaplectana carpocapsae Weiser (Nematoda: Steinernematidae) Bull Acad Pol Sci. 1974;22:193–196. [Google Scholar]
- 39.Thaler J O, Duvic B, Givaudan A, Boemare N. Isolation and entomotoxic properties of the Xenorhabdus nematophilus F1 lecithinase. Appl Environ Microbiol. 1998;64:2367–2373. doi: 10.1128/aem.64.7.2367-2373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas G M, Poinar G O., Jr Xenorhabdus gen. nov., a genus of entomopathogenic nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Bacteriol. 1979;29:352–360. [Google Scholar]
- 41.van Leengoed L A, Dickerson H W. Influence of calcium on secretion and activity of the cytolysins of Actinobacillus pleuropneumoniae. Infect Immun. 1992;60:353–359. doi: 10.1128/iai.60.2.353-359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volgyi A, Fodor A, Szentirmai A, Forst S. Phase variation in Xenorhabdus nematophilus. Appl Environ Microbiol. 1998;64:1188–1193. doi: 10.1128/aem.64.4.1188-1193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J M, Olsen M E, Kahn M L, Hurlbert R E. Characterization of Tn5-induced mutants of Xenorhabdus nematophilus ATCC 19061. Appl Environ Microbiol. 1991;57:1173–1180. doi: 10.1128/aem.57.4.1173-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M Y, Lovgren A, Landen R. Adhesion and cytotoxicity of Bacillus thuringiensis to cultured Spodoptera and Drosophila cells. J Invertebr Pathol. 1995;66:46–51. doi: 10.1006/jipa.1995.1059. [DOI] [PubMed] [Google Scholar]