Abstract
Background
Tumor infiltrating lymphocytes (TILs) have been shown to be associated with the prognosis of breast ductal carcinoma in situ (DCIS). In this systematic review and meta-analysis, we investigated the role of TILs and TIL subsets in predicting the recurrence risk of DCIS.
Method
PubMed, Medline, Web of Science, Embase and Cochrane were searched to identify publications investigating the prognostic role of TILs in DCIS. After study screening, data extraction and risk of bias assessment, a meta-analysis was performed to assess the association between TILs (total TILs, CD4+, CD8+, FOXP3+, PD-L1+ TILs) and the risk of DCIS recurrence.
Results
A pooled analysis indicated that dense stromal TILs in DCIS were associated with a higher recurrence risk (HR 2.11 (95% CI 1.35–3.28)). Subgroup analysis showed that touching TILs (HR 4.73 (95% CI 2.28–9.80)) was more precise than the TIL ratio (HR 1.49 (95% CI 1.11–1.99)) in estimating DCIS recurrence risk. Moreover, the prognostic value of TILs seemed more suitable for patients who are diagnosed with DCIS and then undergo surgery (HR 2.77, (95% CI 1.26–6.07)) or surgery accompanied by radiotherapy (HR 2.26, (95% CI 1.29–3.95)), than for patients who receive comprehensive adjuvant therapies (HR 1.16, (95% CI 1.35–3.28)). Among subsets of TILs, dense stromal PD-L1+ TILs were valuable in predicting higher recurrence risk of DCIS.
Conclusion
This systematic review and meta-analysis suggested a non-favorable prognosis of TILs and stromal PD-L1+ TILs in DCIS and indicated an appropriate assessment method for TILs and an eligible population.
Keywords: Tumor infiltrating lymphocytes (TILs), PD-L1, ductal carcinoma in situ (DCIS), recurrence risk, prognosis
Introduction
Widespread use of mammographic screening has largely increased the detection rate of breast ductal carcinoma in situ (DCIS), which accounts for 20–25% of newly diagnosed breast cancer [1]. Theoretically, surgical dissection is adequate for DCIS treatment. For patients receiving surgery alone, the local recurrence risk ranges from 10.5 to 18% [2], and invasive cancer events occur in 19.2% of high-grade DCIS patients [3]. In addition, the mortality risk for patients who experience recurrence is 18 times higher than that for those who do not [4]. Thus, it is crucial to identify risk factors in predicting the recurrence risk of DCIS in order to carry out appropriate management strategies.
DCIS is a heterogeneous disease, and its recurrence is a complex process caused by the coevolution of cancer cells and the immune microenvironment. Cells of the tumor microenvironment mainly include tumor infiltrating lymphocytes (TILs), NK cells, macrophages, dendritic cells and myeloid lineage cells [5]. In recent years, accumulating evidence has suggested that TILs in the immune microenvironment are associated with better prognosis in basal-like and HER2-positive invasive breast cancers [6]. In contrast to invasive breast cancer, the role of total TILs and specific subtypes of TILs in DCIS remain ambiguous.
Tumor-infiltrating lymphocytes are an important component of tumor environment and play an essential role in cancer progression. In DCIS, dense TILs were shown to be associated with many clinical factors, including younger age, higher tumor grade, comedo necrosis and molecular subtype [7]. However, it remains ambiguous whether dense TILs in DCIS are associated with aggressive tumor features and tumor recurrence risk. A few previous studies have reported that there was no significant difference between dense and sparse TILs groups in tumor recurrence [8, 9]. Some other studies recently put forward that dense TILs are associated with higher recurrence risk [10–13]. Moreover, some research proposed that the value of TILs in predicting recurrence risk is associated with TIL assessment methods [14] and included patient therapy strategies [15].
Along with total TILs, different subsets of TILs also exhibit diverse functions in cancer progression. The TILs in DCIS are generally composed of CD3+ T cells, followed by CD4+ T cells, CD8+ T cells, CD20+ B cells and FOXP3+ regulatory T cells (Tregs) [16]. Among these cells, it is now believed that CD8+ and CD4+ T cells are involved in the effective immune response, and FOXP3+ regulatory T cells (Tregs) are associated with the suppression of antitumor immunity [17]. However, the exact prognostic role of each subset of lymphocytes in DCIS has not yet been clarified. In addition to the above subsets, the expression of PD-L1 in immune cells is also associated with DCIS subtypes and their recurrence [18]. Considering that the PD-1/PD-L1 axis is effective in triple-negative invasive breast cancer immunotherapy [19], the prognostic and therapeutic value of PD-L1 in DCIS remains to be further investigated.
In order to illustrate whether TILs have prognostic value in DCIS, we performed a systematic review and meta-analysis to investigate the prognostic roles of TILs and CD4+, CD8+, FOXP3+ and PD-L1 TIL subtypes in DCIS. We aimed to identify potential pathological biomarkers about TILs and TIL subsets in DCIS that can be used to predict patient recurrence risk.
Method
The present systematic review and meta-analysis were performed in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Search strategy and study selection
We performed an extensive literature search of electronic databases including Pubmed, Medline, Web of Science, Excerpta Medica Database (Embase) and Cochrane up to 5 April 2021 by two investigators. The search strategy was in line with published articles, and the following determinant domains were used: (“Ductal Carcinoma in Situ” OR “DCIS” OR “Intraductal Carcinoma”) AND (“Tumor infiltrating lymphocyte” OR “Infiltrating lymphocyte” OR “Immune” OR “Immune cell” OR “Immunology” OR “TILs” OR “TIL assessment” OR “lymphocyte” OR “CD4” OR “CD8” OR “FOXP3” OR “PD-L1”) AND (“Prognosis” OR “Survival” OR “recurrence”). In addition, All the proceedings in scientific meetings and references of the selected articles were searched to identify associated data. The title and abstract of each study in the search were scanned by two independent reviewers, clearly irrelevant studies were excluded.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1). Patients diagnosed with DCIS or DCIS with micro-invasive lesions confirmed with pathological examination; (2). Total TILs and specific subtypes of TILs were measured according to HE and IHC staining; (3). Original research articles; (4). Correlation of TILs with tumor recurrence was illustrate with Hazard Ratio (HR) and a 95% confidence interval (95% CIs).
Exclusion criteria were as follows: (1). Overlapping articles or repeat analysis; (2). Studies lacking sufficient data for assessing Hazard Ratio (HR) and a 95% confidence interval (95% CIs); (3). Study with missing data and unavailable HR; (4). Types of Case reports, reviews, letters, comments and nonclinical studies.
Data extraction
All the data from candidate studies were evaluated and extracted by two independent investigators. Disagreements in data extraction were discussed and resolved by consensus. The following data were obtained from each study: year of publication, first author, country of the population studied, pathology of studied samples, total number of included cases, method of TIL’s detection, cutoff of dense TIL’s classification, cell type of studied TILs, treatment strategy, time of follow-up, deadline (type of recurrence).
No restrictions regarding study design, observational studies, including cohort study and case-control study, were included. The patients diagnosed with DCIS, DCIS mixed with micro-invasive breast cancer or DCIS mixed with invasive breast cancer were all included without restriction of patients’ clinical characteristics and patients’ adjuvant treatments. The total TILs were assessed with HE staining, and TIL subsets as well as PD-L1+ tumor cells were assessed with immunohistochemical staining. The level of the total TILs, CD4+ TILs, CD8+ TILs, FOXP3+ TILs, stromal PD-L1+ TILs and PD-L1+ tumor cells were evaluated with TILs percentage or the number of touching-TILs. Recurrence was defined as any in situ or invasive carcinoma relapse in ipsilateral breast, contralateral breast, axilla, or chest and distant metastasis.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of each included study and the risk of bias in each study. The quality assessment was performed by two investigators independently. The NOS consists of three items including selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). NOS scored more than 7 were assigned as high-quality studies.
Statical analysis
The meta-analysis calculated the pooled HR and corresponding 95% CIs to evaluate the prognostic value of TILs in DCIS. All statistical analyses were performed with STATA version 15. Higgins I-squared statistic were used to estimate the heterogeneity of the included studies. Random-effect model was adopted in our analysis and heterogeneity analysis was assessed by I2 and P heterogeneity (P < 0.10 or I2 > 50% was indicative of statistically significant heterogeneity). Sensitivity analysis and meta-regression were used to explore the origin of heterogeneity. Publication bias was assessed by Egger test and Begg funnel plot. All statistical tests were two-sided, P value < 0.05 was considered statistically significant.
Results
Literature research
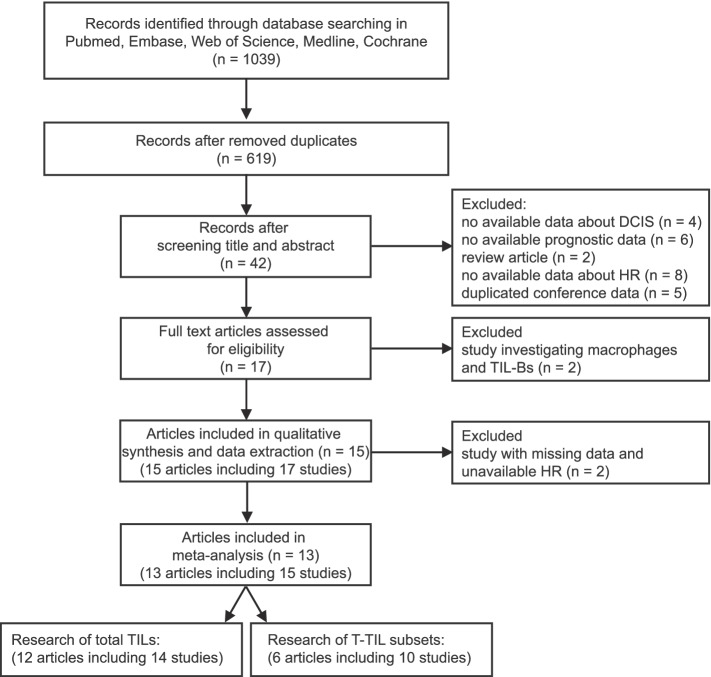
A total of 1039 records were searched in Medline, PubMed, Embase, Cochrane and Web of Science. After excluding duplicates, 619 records remained. Next, we screened the titles and abstracts of the 619 papers, and only 42 papers were included for further full-text review. Among these papers, 20 papers were excluded because they did not provide relevant data in estimating TILs in DCIS, and another 5 were conference abstracts that displayed the same data as other included papers. Next, 2 papers were excluded because they focused on infiltrating macrophage cells and TIL-Bs in DCIS; and 2 articles were excluded because their missing data and unavailable HR. Finally,13 articles including 15 sets of studies were used for following meta-analysis. Among these 13 studies, 12 studies including 14 studies investigated the prognostic role of total TILs in DCIS, and 6 articles containing 10 studies explored the value of TIL subsets in DCIS (Fig. 1).
Fig. 1.
Flow diagram of study selection and identification
Included studies’ characteristics
Detailed characteristics of the included articles are listed in Table 1. These articles were conducted in the United States (2), China (1), Europe (1), Australia (2), the United Kingdom (3), Singapore (1), the Netherlands (1), Italy (1) and Belgium (1), including approximately 4843 participations. All 13 articles were retrospective cohort studies, 1 of the 13 was a conference abstract, and the others were full-reported articles. Among these, 12 articles, which included 14 sets of studies, evaluated the relationship between TILs and DCIS recurrence, and 6 articles investigated the prognostic value of TIL subtypes (CD4+, CD8+, FOXP3+, PD-L1) in DCIS.
Table 1.
Characteristics of included studies in meta-analysis
| Year | First author | Country | Type of DCIS | Number of participants | TIL’s detection method | Cutoff of TIL’s point | Subtype of TILs | Treatment | Median follow up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | Gaynor J. Bates et al. | UK | p-DCIS | 62 | IHC | 15 | FOXP3+ TILs | S & AT (RT/ HT) | 5.4 y | AR | [20] |
| 2016 | G. Pruneri et al. | Europe | DCIS | 1488 (set1: n = 1391; set2: n = 620) | HE | set1: < 1% vs. 35–50% | total p-TILs | S & AT (RT/ HT) | 8.2 y | IR | [8] |
| set2: < 1% vs. > 50% | |||||||||||
| 2015 | Elizabeth Thompson et al. | USA | p-DCIS & i-DCIS | total p-TILs (n = 27) PD-L1+ TILs (n = 27) | HE & IHC | 50% | total p-TILs | S & AT | 80 m | IR | [9] |
| PD-L+ TILs | |||||||||||
| 2017 | Shona Hendry et al. | Australia | p-DCIS | total p-TILs (n = 138) PD-L1+ TILs (n = 79) PD-L1+ TC(n = 79) | HE & IHC | TILs: 2%. PD-L1: 1% | total p-TILs | S & AT (RT/ HT) | TILs: 89 m PD-L1:39 m | IR | [7] |
| PD-L1+ TILs | |||||||||||
| PD-L1+ TC | |||||||||||
| 2018 | Michael S. Toss et al. | UK | training set: | training set | HE | training set: 5% | training set: | training set: | training set: 161 m | IR | [14] |
| p-DCIS | (n = 150) | total p-TILs | S/ S & RT | ||||||||
| validation set: | validation set | validation set: 20 | validation set: | validation set: | validation set: 109 m | ||||||
| p-DCIS | (n = 534) | total t-TILs | S/ S & RT | ||||||||
| 2019 | Rajiv Dave et al. | Australia | p-DCIS | 423 | HE | 5% | total p-TILs | BCS | 119 m | IR | [21] |
| 2020 | Marie Colombe Agehozo et al. | Netherlands | p-DCIS | total p-TILs (n = 466) PD-L1+ TILs (n = 115) PD-L1+ TC(n = 115) | HE & IHC | 30% | total p-TILs | BCS/ MAS | 98 m | IR | [22] |
| PD-L1+ TILs | |||||||||||
| PD-L1+ TC | |||||||||||
| 2019 | Farbod Darvishian et al. | USA | p-DCIS | 69 | HE | 45% | total p-TILs | BCS | 6.7 y | IR | [11] |
| 2019 | Mieke Van Bockstal et al. | Belgium | p-DCIS | 211 | HE | 50% | total p-TILs | S & AT (RT/ HT) | 124 m | IR | [10] |
| 2020 | Aye Aye THIKE et al. | Singapore | DCIS | total p-TILs (n = 198) CD4+ TILs (n = 198) | HE & IHC | 20% | total p-TILs | S (BCS/ MAS) | TILs: 7.9 y CD4+: 7.2 y | AR | [23] |
| CD4+ TILs | |||||||||||
| 2020 | Michael S. Toss et al. | UK | p-DCIS | total p-TILs (n = 508) FOXP3+ TILs(n = 406) PD-L1+ TILs (n = 383) CD4+ TILs (n = 403) CD8+ TILs (n = 402) | HE & IHC | 20 | total t-TILs | BCS | unknown | IR | [12] |
| FOXP3+ TILs | |||||||||||
| PD-L1+ TILs | |||||||||||
| CD4+ TILs | |||||||||||
| CD8+ TILs | |||||||||||
| 2020 | Alberto Farolfi et al. | Italy | p-DCIS | 496 | HE | 5% | total p-TILs | S/ S & RT | 56.4 y | IBE | [15] |
| 2021 | Fei-Fei Xu et al. | China | p-DCIS & | 135 | HE | 5 | total t-TILs | BCS/ BCS & RT | 53 m | IR | [13] |
| m-DCIS |
UK United Kingdom, USA United States of America, p-DCIS pure DCIS, i-DCIS pure DCIS mixed with IDC, m-DCIS pure DCIS mixed with microinvasive cancer, HE Hematoxylin and eosin staining, IHC Immunohistochemistry staining, TILs Tumor-infiltrating lymphocytes, vt-TILs Stromal touching TILs, p-TILs percentage of stromal TILs, TC Tumor cell, S Surgery, BCS Breast conserving surgery, MAS Mastectomy, AT Adjuvant therapy, RT Radiation therapy, HT Hormonal therapy, IR Lipsilateral recurrence; AR All kinds of recurrence, IBE Ipsilateral breast cancer events
Study quality and risk of bias
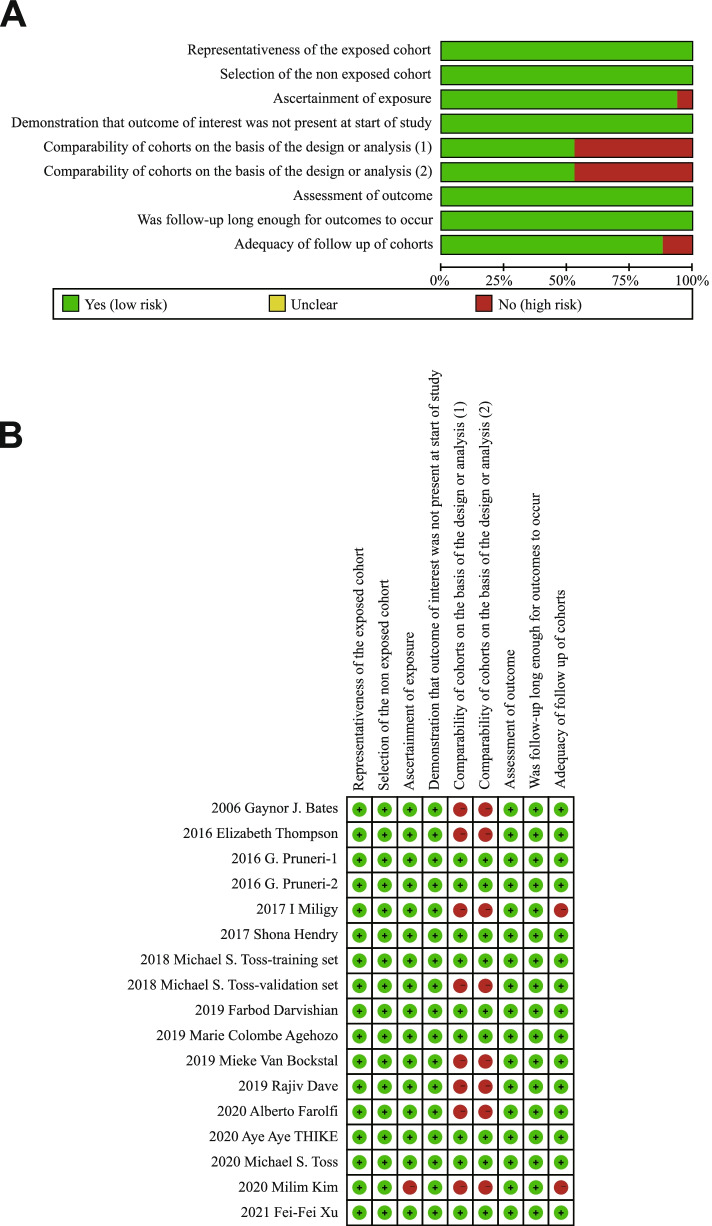
After full-text review, we performed critical assessment for each study by NOS, and the quality of each study is summarized in Fig. 2. Most of the studies exhibit excellent quality with more than six stars. Two studies scored fewer than 7 stars due to missing data, unclear TIL assessment method and univariate analysis. Studies with missing data and unavailable HR were excluded from our meta-analysis; therefore, 15 studies were eligible for inclusion in the meta-analysis.
Fig. 2.
Risk of bias assessment of included studies. A Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies; B Risk of bias summary: review authors’ judgments about each risk of bias item for each included study
The value of total TILs in the recurrence of DCIS
A total of 14 studies in 12 sets of articles containing 4843 patients were included in our meta-analysis to evaluate the prognostic value of total TILs in DCIS. The results showed that dense TILs in DCIS indicates a higher recurrence risk. The pooled HR was 2.11 (95% CI, 1.35–3.28) for the total TIL level (dense vs. sparse), with statistically significant heterogeneity (I2 = 78.3%, P = 0.000) (Fig. 3A).
Fig. 3.
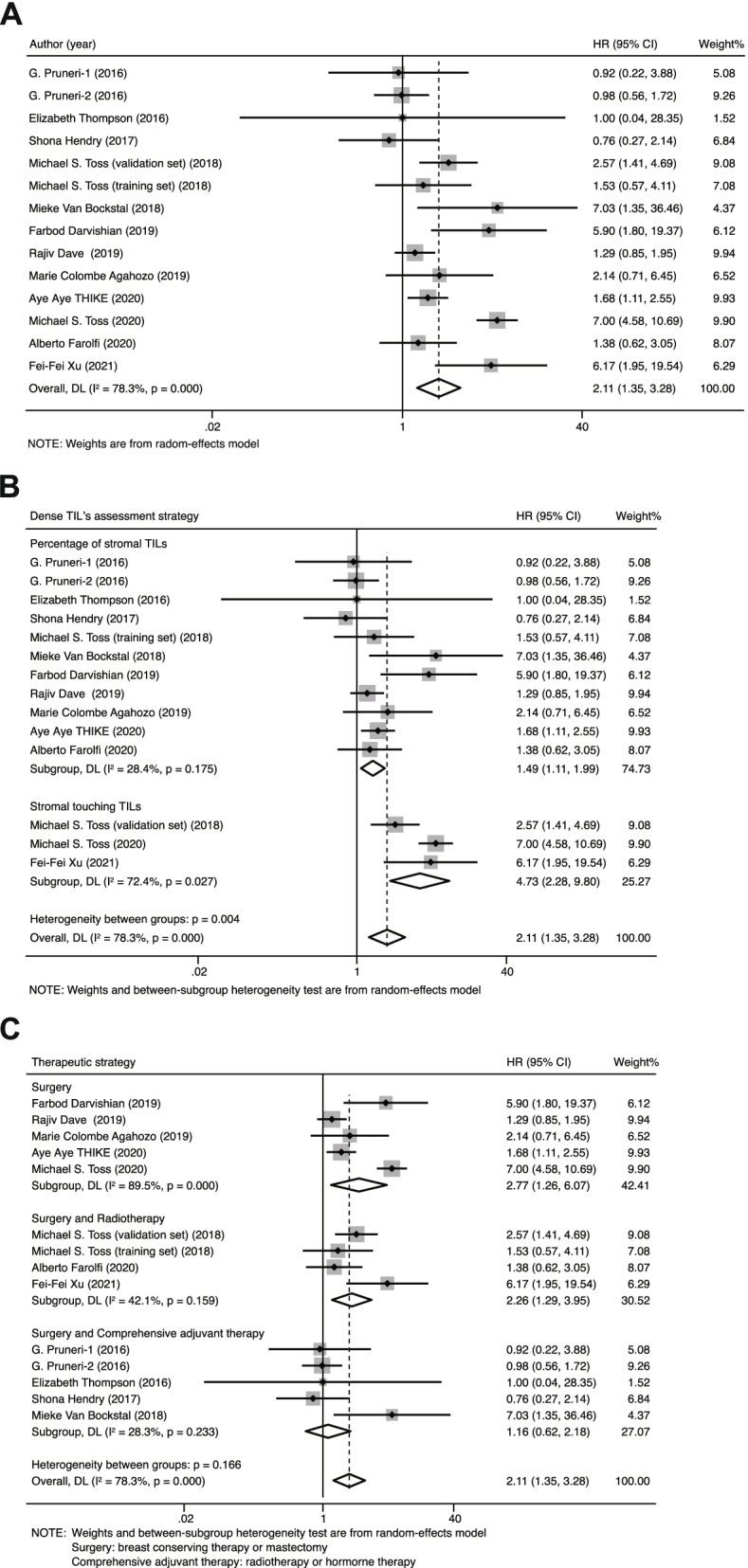
Forest plots of the prognostic value of TILs in patients diagnosed with DCIS. A Forest plots of prognostic value of total TILs in DCIS; B Forest plots of prognostic value of TILs assessed with different strategies on recurrence risk of DCIS; C Forest plots of prognostic value of TILs on patients who receiving different therapies
To further investigate the heterogeneity in our meta-analysis, we performed meta-regression and subgroup analyses (Table 2). Through meta-regression analysis, we identified “TIL assessment method” as the main cause of heterogeneity (P > |t| = 0.005). Furthermore, in subgroup analysis, we also observed that the assessment method of TILs in different studies may affect the prognostic value of TILs in DCIS. The pooled HR for 11 studies (N = 3666) using TIL ratio classification was 1.49 (1.11–1.99), with no obvious heterogeneity between the results of the studies (I2 = 28.4%, P = 0.175). In the other 3 studies (N = 1177) that employed touching-TIL classification, the pooled HR was 4.73 (2.28–9.8), with substantial heterogeneity (I2 = 72.4%, P = 0.027). The pooled HR indicated that stromal touching TILs in DCIS were associated with recurrence more closely than the stromal TIL ratio (Fig. 3B). In addition, we observed that the therapeutic strategy is also significant for the evaluation of the prognostic value of TILs. The pooled HRs for those patients who underwent surgery only or surgery accompanied by radiotherapy were 2.77 (95% CI: 1.26–6.07, I2 = 89.5%, P = 0.00) and 2.26 (95% CI: 1.29–3.95, I2 = 42.1%, P = 0.159), which analyzed with 5 (N = 1690) and 4 (N = 1315) studies, respectively. Five studies (N = 1864) were used to investigate the prognostic role of TILs in those patients who experienced comprehensive adjuvant therapy, no prognostic effect on recurrence risk was observed with an HR of 1.16 (95% CI: 0.62–2.18, I2 = 28.3%, P = 0.233) (Fig. 3C). The value of TILs in predicting DCIS recurrence is more suitable for patients who receive surgery only or surgery accompanied by radiotherapy.
Table 2.
Summary of the prognostic value of TILs obtained from the subgroup analysis including dense TIL’s assessment, Deadline, Variable, Location of TILs, Cutoff of TILs, Therapeutic approach, Pathology and subsets of TILs
| Subgroup | Number of studies | Number of participants | Random-effects model | Fixed-effects model | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | I2 | Ph | |||
| Dense TIL’s assessment | 14 | 4843 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0.001 | 78.30% | 0 |
| percentage of stromal TILs | 11 | 3666 | 1.49 (1.11–1.99) | 0.008 | 1.45 (1.16–1.80) | 0 | 28.40% | 0.175 |
| number of touching TILs | 3 | 1177 | 4.73 (2.28–9.80) | 0 | 5.1 (3.66–7.11) | 0 | 72.40% | 0.027 |
| Outcome | 14 | 4843 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| ipsilateral recurrence | 12 | 4149 | 2.26 (1.31–3.91) | 0.004 | 2.33 (1.89–2.88) | 0 | 80.60% | 0 |
| all kinds of recurrence | 2 | 694 | 1.61 (1.11–2.33) | 0.011 | 1.61 (1.11–2.33) | 0.011 | 0 | 0.662 |
| Variable | 14 | 4335 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| univariate analysis | 5 | 1307 | 1.42 (1.02–1.99) | 0.039 | 1.42 (1.02–1.99) | 0.039 | 0.00% | 0.42 |
| multivariate analysis | 9 | 3028 | 2.30 (1.27–4.17) | 0.006 | 2.52 (2.03–3.14) | 0 | 83.40% | 0 |
| Location of TILs | 14 | 4843 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| touching TILs | 2 | 204 | 6.04 (2.64–13.81) | 0 | 6.04 (2.64–13.81) | 0 | 0 | 0.958 |
| stromal TILs | 12 | 4639 | 1.81 (1.13–2.90) | 0.013 | 2.02 (1.67–2.43) | 0 | 79.40% | 0 |
| Cutoff of TILs | 14 | 5366 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| 1–5% | 6 | 2625 | 1.24 (0.90–1.70) | 0.186 | 1.24 (0.90–1.70) | 0.186 | 0 | 0.933 |
| 6–30% | 2 | 664 | 1.73 (1.17–2.56) | 0.006 | 1.73 (1.17–2.56) | 0.006 | 0 | 0.69 |
| 31–50% | 3 | 900 | 3.03 (0.71–12.97) | 0.134 | 1.57 (0.97–2.56) | 0.068 | 81.20% | 0.005 |
| counts (5 or 15 or 20) | 3 | 1177 | 4.73 (2.28–9.80) | 0 | 5.10 (3.66–7.11) | 0 | 72.40% | 0.027 |
| Therapeutic approach | 14 | 4869 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| breast conserving therapy or mastectomy | 5 | 1690 | 2.77 (1.26–6.07) | 0.011 | 2.51 (1.99–3.17) | 0 | 89.50% | 0 |
| breast conserving therapy + radiotherapy | 4 | 1315 | 2.26 (1.29–3.95) | 0.004 | 2.24 (1.49–3.35) | 0 | 42.10% | 0.159 |
|
surgery + adjuvant therapies (hormonal therapy, radiotherapy) |
5 | 1864 | 1.16 (0.62–2.18) | 0.645 | 1.07 (0.69–1.68) | 0.753 | 28.30% | 0.233 |
| Pathology | 14 | 4843 | 2.11 (1.35–3.28) | 0.001 | 2.13 (1.77–2.55) | 0 | 78.30% | 0 |
| pure DCIS | 9 | 2995 | 2.37 (1.31–4.28) | 0.004 | 2.54 (2.02–3.18) | 0 | 81.90% | 0 |
| pure DCIS & pure DCIS mixed with microinvasive or invasive breast cancer | 2 | 162 | 5.03 (1.63–15.52) | 0.005 | 5.09 (1.71–15.13) | 0.003 | 1.60% | 0.313 |
| DCIS | 3 | 1686 | 1.32 (0.88–1.97) | 0.184 | 1.36 (0.98–1.89) | 0.064 | 22.60% | 0.275 |
| subset of TILs | 10 | 2190 | 2.29 (1.31–3.99) | 0.003 | 2.55 (2.06–3.16) | 0 | 77.80% | 0 |
| CD4+ TILs | 2 | 601 | 1.98 (1.14–3.44) | 0.015 | 1.97 (1.31–2.96) | 0.001 | 45.80% | 0.174 |
| CD8+ TILs | 1 | 402 | 0.90 (0.47–1.71) | 0.747 | 0.90 (0.47–1.71) | 0.747 | 0.00% | – |
| FOXP3+ TILs | 2 | 468 | 1.83 (1.23–2.70) | 0.003 | 1.83 (1.23–2.70) | 0.003 | 0.00% | 0.382 |
| PD-L1+ TILs | 5 | 719 | 6.21 (4.26–9.06) | 0 | 6.21 (4.26–9.06) | 0 | 0.00% | 0.708 |
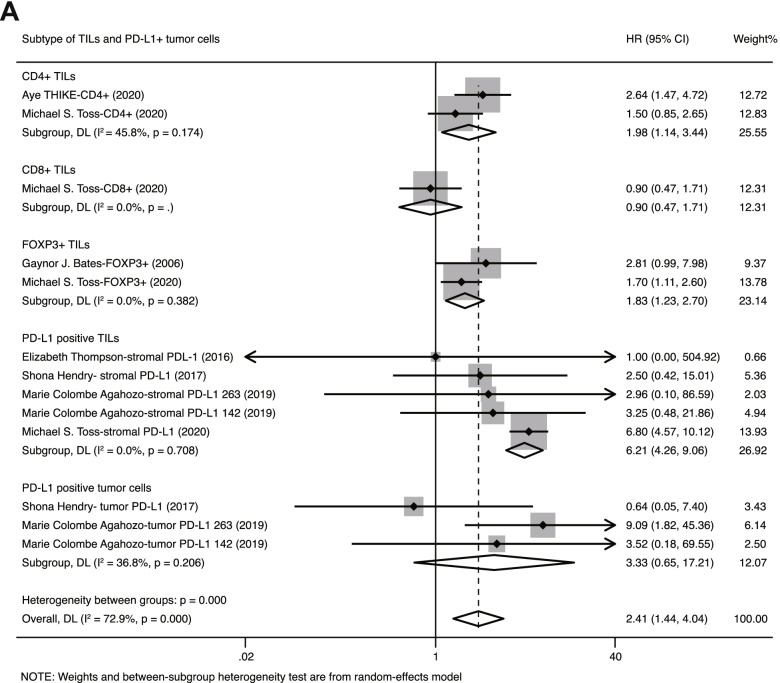
Different subtypes of TILs play different roles in the recurrence of DCIS
Aside from total TILs, we also investigated the prognostic role of CD4+, CD8+, FOXP3+ and PD-L1+ TILs in DCIS. There were 2 studies (N = 601) investigating CD4+ TILs, and 2 studies (N = 468) investigating FOXP3+ TILs. The pooled HRs of CD4+ and FOXP3+ TILs were estimated to be 1.98 (95% CI: 1.44–3.44) and 1.83 (95% CI: 1.23–2.70), respectively, with no considerable heterogeneity between studies (CD4+: I2 = 45.8%, P = 0.174; FOXP3+: I2 = 0%, P = 0.382). This indicates that dense CD4+, FOXP3+ TILs in DCIS are associated with a higher recurrence risk. In addition to CD4+ and FOXP3+ TILs, we also evaluated the prognostic value of PD-L1+ TILs in DCIS with 5 studies (N = 719). The pooled HR for stromal PD-L1 TILs was 6.21 (95% CI: 4.26–9.06, I2 = 0.0%, P = 0.708). Considering that some studies observed positive expression of PD-L1 in intraductal cancer cells in DICS, we further investigated the association between PD-L1+ tumor cells and the recurrence risk of DCIS with 3 studies (N = 309). The pooled HR for PD-L1+ tumor cells was 3.33 (95% CI: 0.65–17.21), without apparent heterogeneity (I2 = 36.8%, P = 0.206). Through the above integrated analysis, we observed that both PD-L1+ TILs and PD-L1+ tumor cells are associated with the recurrence risk of DCIS. Regarding CD8+ TILs, an insufficient number of studies provided data to perform a meta-analysis, and only 1 study (N = 402) with such data provided showed no significant association between CD8+ TILs and patient recurrence (HR 0.90, 95% CI: 0.47–1.71) (Fig. 4). Taken together, CD4+, FOXP3+, PD-L1 TILs and PD-L1 tumor cells possess the potential to predict the recurrence risk of DCIS, and the stromal PD-L1 is more valuable than the others in evaluating DCIS recurrence risk.
Fig. 4.
Forest plots of the prognostic value of different subsets of T-TILs and PD-L1+ tumor cells in DICS
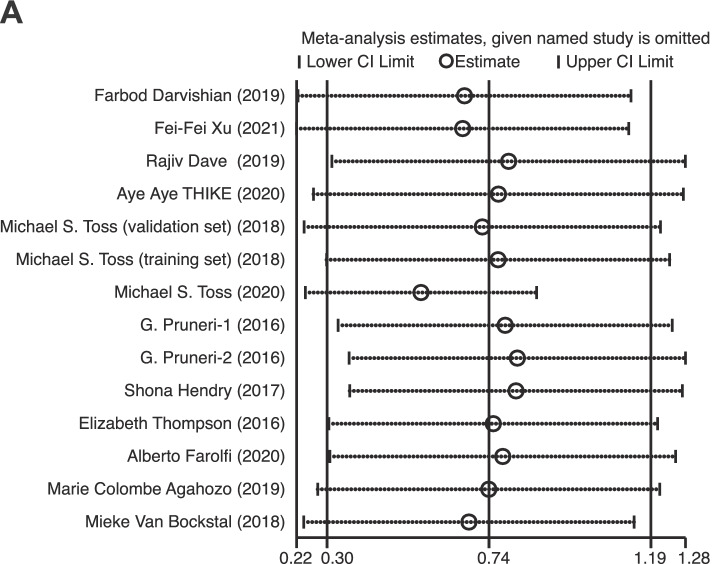
Sensitivity analysis
Sensitivity analysis was performed in our study to estimate the effect of each study on pooled HR by consecutive deletion of each study. The results show that no eligible study exhibited a significant influence on the pooled HR of total TILs (Fig. 5).
Fig. 5.
Sensitivity analysis of the meta-analysis of total TILs in DCIS
Publication bias
Funnel plots display symmetrical distribution and did not indicate any obvious publication bias affecting the HR for cancer recurrence in the included studies (PBegg = 0.228, PEgger = 0.931) (Fig. 6A-B).
Fig. 6.
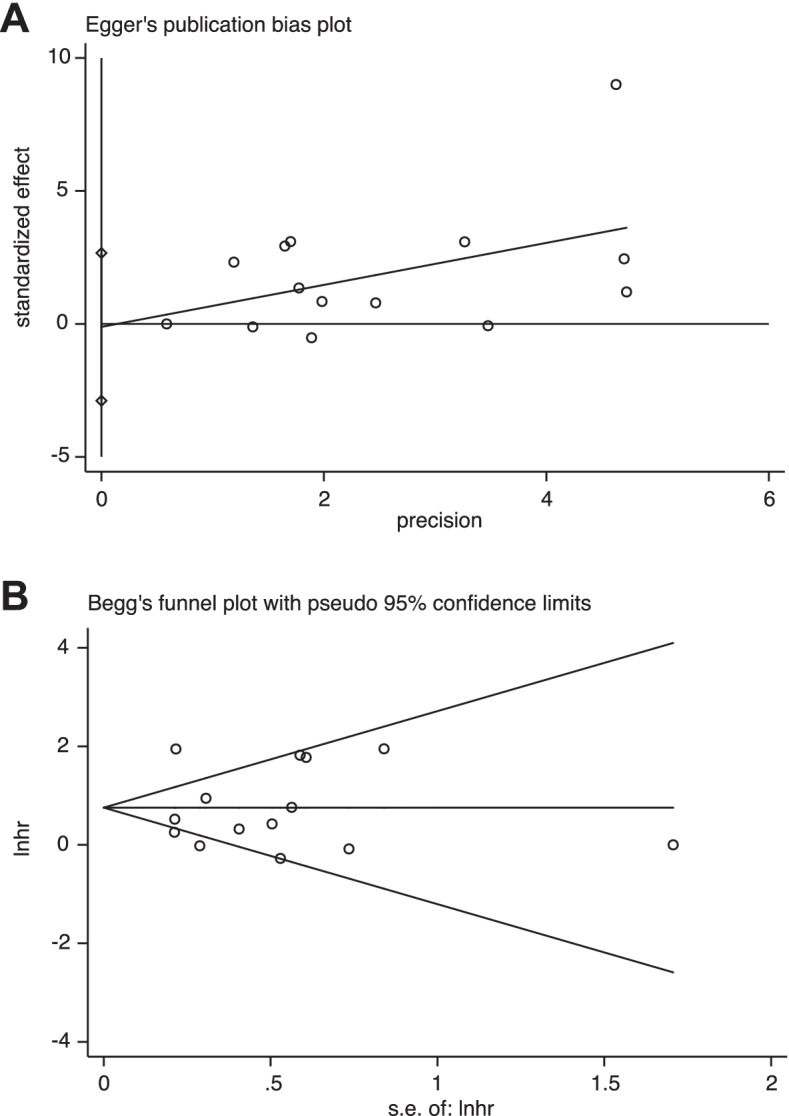
Funnel plots of potential publication bias with Egger’s test (A) and Begg’s test (B)
Discussion
Some studies have investigated the prognostic role of tumor infiltrating lymphocytes (TILs) in ductal carcinoma in situ (DCIS), however it is still controversial whether total TILs and subtypes of TILs can indicate recurrence risk of DCIS. Therefore, we performed a systematic review and meta-analysis to evaluate the prognostic value of total TILs and some subsets of TILs, including CD4+, CD8+, FOXP3+ and PD-L1, in DCIS.
Our meta-analysis performed the integrative analysis of TILs with 14 reports, with 4843 patients, which revealed that dense stromal TILs in DCIS indicates higher recurrence risk (HR: 2.11, 95% CI: 1.35–3.28, p < 0.001, I2 = 78.3%). Subgroup analyses showed that touching TILs are more associated with patient recurrence-free survival compared with the stromal TIL ratio. Moreover, the prognostic value of TILs is influenced by the treatment strategies adopted by patients. Only in the subgroup of patients who underwent surgery or surgery accompanied by radiotherapy, TILs are valuable in evaluating patients’ recurrence risk. Subsequent analysis of TIL subsets suggested that PD-L1-positive stomal lymphocytes in DCIS are correlated with recurrence risk more closely than the other subset of TILs.
Through comparative analysis, our observation about the enrichment of TILs in DCIS is generally consistent with invasive breast cancer. Compared with luminal breast cancer, high TILs are more frequent in TNBC and HER-2, which are biologically more aggressive, with high immunogenic characteristics. TILs are considered to reflect the immunogenicity of tumor, and previous study proposed that aggressive tumors may be more immunogenic [24]. Therefore, it was speculated that TILs may be more likely to react to aggressive clones. Additionally, it was observed that the unfavorable prognostic role of TILs in DCIS is in line with luminal breast cancer rather than TNBC [25]. Considering that TILs are the most important immune cells that affect tumor development, it was supposed that the opposite prognostic value of TILs in breast cancer may attribute to the complex interaction between cancer cells and immune cells. When the cancer cells are under the control of immune system, high level of TILs indicate better prognosis. On the contrary, immune cells in luminal breast cancer and DCIS are insufficient to affect tumor cells’ biological characteristics. Therefore, dense TILs are related to tumor cell’s aggressive features and patients’ poor prognosis. Some studies proposed that immune microenvironment may contribute to the ethnical disparity in breast cancer [26]. Compared with Western patients, the favorable prognosis in Asian patients may attribute to the abundance of TILs [27]. Regarding to DCIS, the prognostic value of TILs was observed in both Asian (Aye Aye THIKE et.al’s study [28]) and White (Farbod Darvishian et.al [11] and Elizabeth Thompson et.al’s study [9]). However, considering that the limited studies included in our meta-analysis display patients’ ethnicity, subgroup analysis was not conducted. Whether TILs and the compositions of TILs in DCIS exhibit significantly difference between patients with diverse ethnicity, and whether they have a diverse impact on patients’ mortality still remain to be investigated in the future.
Aside from total TILs, we also performed a meta-analysis for TIL subsets. Considering limited research using IHC staining to investigate subsets of TILs in DCIS, our meta-analysis is restricted. In our study, only CD4+ TILs and FOXP3+ TILs were marginally associated with the recurrence risk of DCIS. In addition to the T-TIL subset study contained in our meta-analysis, some studies also investigated B lymphocytes (TIL-Bs) and tumor-associated macrophages (TAMs), and tried to use the TIL ratio to assess DCIS recurrence risk. In I Miligy’s study, CD20+/CD19+ TIL-Bs were demonstrated to be associated with a shorter recurrence-free interval [29]; the prognostic value of CD68+ and CD163+ TAMs were also proposed in Xiao-Yang Chen [30] and Aye Aye Thike’s studies [31]. In addition, the ratio of FOXP3+/CD8+ and FOXP3+/CD4+ cells in Milim Kim’s study [18], and CD8HLADR+/− and CD115+ cells in Michael J. Campbell’s study [32], were associated with recurrence-free survival in DCIS. Although some of the studies observed positive results, related studies are still limited, and there is a lack of uniform and suitable assessment methods for the TIL ratio. Thus, no studies about TIL-B, macrophage and TIL ratios can be included in our meta-analysis. In recent years, accumulating evidence showed that PD-L1 expression is a novel prognostic marker for breast cancer [33]. In our subgroup analysis, it was also found that PD-L1 staining in DCIS is valuable, and stromal PD-L1 in immune cells is associated with recurrence risk closely than tumor cell PD-L1 staining. The limited cancer cells in DCIS exhibit PD-L1 expression, and PD-L1 staining in immune cells is partly positive, thus leading to the superior prognostic value of PD-L1 in TILs [7, 9].
Recent years, to optimize DCIS management, molecular assays about DCIS were created. Similar to the molecular assays of Oncotype DCIS score [3] and DCISionRT [20], TILs in DCIS could also provide valuable prognostic information about the risk of recurrence with low cost and convenience. Additionally, in our observation, TILs were not only suitable to patients who received solely surgery, but also to patients who were treated with surgery and radiotherapy. Therefore, TILs in DCIS may also be conductive to the guidance of the additional adjuvant therapies and follow-up. Though TILs have been described as reliable, reproducible and inexpensive markers in breast cancer, and could up−/ down-stage traditional pathological-staging in early-stage TNBC [21], limited research was focused on the utility of TILs in DCIS. On account of the convenience of TILs’ assessment, patients in clinical trials like ECOG-E5194 [22, 23] can be used to re-analyze the association between TILs and ipsilateral breast events. Meanwhile, investigating whether TILs in DCIS could be used to stratify patients with different risk along with traditional risk factors such as tumor grade, tumor size, and age is also meaningful. Aside from pathological detection, according to Tiantian Bian et.al’s study, the preoperative MRI-based radiomics signatures are valuable in evaluating the TILs level in breast cancer [34]. Currently, NCT03495011 trial is trying to investigate the MRI-based radiomics signatures that correlate with pathologic markers in DCIS. Therefore, it is meaningful to take TILs into concern in NCT03495011 trial, aiming to assess whether MRI signatures are related to the TIL level in DCIS. In the future, if MRI could also be used to assess TIL level in DCIS preoperatively and identify low-risk DCIS, a proportion of patients with low-risk DCIS may avoid surgery.
We made an effort to conduct a comprehensive review for the prognostic value of TILs and subsets of TILs in DCIS, but there are several limitations in our study. The main limitation of our study is that the eligible studies were relatively small, and all of the included records were retrospective studies. Our study is a literature-based analysis. The cutoff value in category patients with “dense TILs” and “sparse TILs” are not consistent between studies. We checked the publication bias with both Egger’s and Begg’s tests and did not observe an indication of publication bias. While studies with positive results were more prone to be published, relative data that did not observe a positive correlation between TILs and DCIS recurrence risk were partly unavailable [35, 36].
Taken together, our meta-analysis observed a potential prognostic role of tumor infiltrating lymphocytes (TILs) in DCIS and proposed that the assessment method of TILs and patient adjuvant therapies are critical in evaluating TIL prognostic value. In addition, we identified PD-L1 in TIL subsets as a potential marker in predicting the higher recurrence risk of DCIS. To achieve clinical translation, further prospective clinical trials investigating TILs in DCIS are needed in the future.
Conclusions
For patients who received surgery or surgery accompanied with radiotherapy, dense stromal TILs in DCIS indicated higher recurrence risk. Among TIL subsets, dense stromal PD-L1+ TILs were valuable in predicting higher recurrence risk of DCIS.
Acknowledgements
Not applicable.
Abbreviations
- TILs
Tumor infiltrating lymphocytes
- t-TILs
Stromal touching TILs
- p-TILs
Percentage of stromal TILs
- TAMs
Tumor-associated macrophages
- DCIS
Ductal carcinoma in situ
- p-DCIS
Pure DCIS
- i-DCIS
Pure DCIS mixed with IDC
- m-DCIS
Pure DCIS mixed with microinvasive cancer
- HE
Hematoxylin and eosin staining
- IHC
Immunohistochemistry staining
- TC
Tumor cell
- S
Surgery
- BCS
Breast conserving surgery
- MAS
Mastectomy
- AT
Adjuvant therapy
- RT
Radiation therapy
- HT
Hormonal therapy
- IR
Ipsilateral recurrence
- AR
All kinds of recurrence
- SBE
Second breast cancer events
- HR
Hazard ratio
- CI
Confidence intervals
- PRISMA
Preferred reporting item for systematic reviews and meta-analyses
- Embase
Excerpta medica database
- NOS
The newcastle-ottawa scale
- UK
United Kingdom
- USA
United States of America
Authors’ contributions
SLW and XYM performed data acquisition and quality assessment, SLW contributed to data interpretation, statistical analysis and drafting the manuscript. SLW, XMY, XYM and FJ contributed to the study design and data interpretation. All the authors contributed to the article and approved the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China under Grant 82073282 and 81972791.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuang-Ling Wu, Email: slwu@cmu.edu.cn.
Xiaoyun Mao, Email: Xymao@cmu.edu.cn.
Feng Jin, Email: jinfeng@cmu.edu.cn.
References
- 1.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):139–141. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward EM, DeSantis CE, Lin CC, Kramer JL, Jemal A, Kohler B, Brawley OW, Gansler T. Cancer statistics: Breast cancer in situ. CA Cancer J Clin. 2015;65(6):481–495. doi: 10.3322/caac.21321. [DOI] [PubMed] [Google Scholar]
- 3.Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW, Jr, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1(7):888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 5.Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, Zi X, Kwak M, Bergholtz H, Su Y, et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017;7(10):1098–1115. doi: 10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 7.Hendry S, Pang JMB, Byrne DJ, Lakhani SR, Cummings MC, Campbell IG, Mann GB, Gorringe KL, Fox SB. Relationship of the Breast Ductal Carcinoma In Situ Immune Microenvironment with Clinicopathological and Genetic Features. Clin Cancer Res. 2017;23(17):5210–5217. doi: 10.1158/1078-0432.CCR-17-0743. [DOI] [PubMed] [Google Scholar]
- 8.Pruneri G, Lazzeroni M, Bagnardi V, Tiburzio GB, Rotmensz N, DeCensi A, Guerrieri-Gonzaga A, Vingiani A, Curigliano G, Zurrida S, et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann Oncol. 2017;28(2):321–328. doi: 10.1093/annonc/mdw623. [DOI] [PubMed] [Google Scholar]
- 9.Thompson E, Elwood H, Sharma R, Meeker A, Warzecha H, Argani P, Cimino-Mathews A. The immune microenvironment of breast ductal carcinoma in situ: A pilot study. Lab Investig. 2015;95:70A. doi: 10.1038/modpathol.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Bockstal M, Lambein K, Smeets A, Slembrouck L, Neven P, Nevelsteen I, Weltens C, Van Limbergen E, Christiaens MR, Van Ongeval C, et al. Stromal characteristics are adequate prognosticators for recurrence risk in ductal carcinoma in situ of the breast. Ejso. 2019;45(4):550–559. doi: 10.1016/j.ejso.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Darvishian F, Ozerdem U, Adams S, Chun J, Pirraglia E, Kaplowitz E, Guth A, Axelrod D, Shapiro R, Price A, et al. Tumor-Infiltrating Lymphocytes in a Contemporary Cohort of Women with Ductal Carcinoma In Situ (DCIS) Ann Surg Oncol. 2019;26(10):3337–3343. doi: 10.1245/s10434-019-07562-x. [DOI] [PubMed] [Google Scholar]
- 12.Toss MS, Abidi A, Lesche D, Joseph C, Mahale S, Saunders H, Kader T, Miligy IM, Green AR, Gorringe KL, et al. The prognostic significance of immune microenvironment in breast ductal carcinoma in situ. Br J Cancer. 2020;122(10):1496–1506. doi: 10.1038/s41416-020-0797-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu F-F, Zheng S-F, Xu C, Cai G, Wang S-B, Qi W-X, Wang C-F, Chen J-Y, Lu C. Prognostic and predictive significance of tumor infiltrating lymphocytes for ductal carcinoma in situ. Oncoimmunology. 2021;10(1):1875637. doi: 10.1080/2162402X.2021.1875637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toss MS, Miligy I, Al-Kawaz A, Alsleem M, Khout H, Rida PC, Aneja R, Green AR, Ellis IO, Rakha EA. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Modern Pathol. 2018;31(8):1226–1236. doi: 10.1038/s41379-018-0040-8. [DOI] [PubMed] [Google Scholar]
- 15.Farolfi A, Petracci E, Serra L, Ravaioli A, Bravaccini S, Ravaioli S, Tumedei MM, Ulivi P, Canale M, Puccetti M, et al. Tumor-Infiltrating Lymphocytes (TILs) and Risk of a Second Breast Event After a Ductal Carcinoma in situ. Front Oncol. 2020;10:1486. doi: 10.3389/fonc.2020.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X-Y, Yeong J, Thike AA, Bay BH, Tan PH. Prognostic role of immune infiltrates in breast ductal carcinoma in situ. Breast Cancer Res Treat. 2019;177(1):17–27. doi: 10.1007/s10549-019-05272-2. [DOI] [PubMed] [Google Scholar]
- 17.Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–250. doi: 10.1016/j.breast.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Chung YR, Kim HJ, Woo JW, Ahn S, Park SY. Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res. 2020;22(1):32. doi: 10.1186/s13058-020-01267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastaki S, Irandoust M, Ahmadi A, Hojjat-Farsangi M, Ambrose P, Hallaj S, Edalati M, Ghalamfarsa G, Azizi G, Yousefi M, et al. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. 2020;247:117437. doi: 10.1016/j.lfs.2020.117437. [DOI] [PubMed] [Google Scholar]
- 20.Bremer T, Whitworth PW, Patel R, Savala J, Barry T, Lyle S, Leesman G, Linke SP, Jirstrom K, Zhou W, et al. A Biological Signature for Breast Ductal Carcinoma In Situ to Predict Radiotherapy Benefit and Assess Recurrence Risk. Clin Cancer Res. 2018;24(23):5895–5901. doi: 10.1158/1078-0432.CCR-18-0842. [DOI] [PubMed] [Google Scholar]
- 21.Loi S, Salgado R, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria S, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer. 2022;8(1):3. doi: 10.1038/s41523-021-00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes LL, Wang M, Page DL, Gray R, Solin LJ, Davidson NE, Lowen MA, Ingle JN, Recht A, Wood WC. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solin LJ, Gray R, Hughes LL, Wood WC, Lowen MA, Badve SS, Baehner FL, Ingle JN, Perez EA, Recht A, et al. Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015;33(33):3938–3944. doi: 10.1200/JCO.2015.60.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loi S, Michiels S, Adams S, Loibl S, Budczies J, Denkert C, Salgado R. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol. 2021;32(10):1236–1244. doi: 10.1016/j.annonc.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrino B, Hlavata Z, Migali C, De Silva P, Aiello M, Willard-Gallo K, Musolino A, Solinas C. Luminal Breast Cancer: Risk of Recurrence and Tumor-Associated Immune Suppression. Mol Diagn Ther. 2021;25(4):409–424. doi: 10.1007/s40291-021-00525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim G, Pastoriza JM, Condeelis JS, Sparano JA, Filippou PS, Karagiannis GS, Oktay MH. The Contribution of Race to Breast Tumor Microenvironment Composition and Disease Progression. Front Oncol. 2020;10:1022. doi: 10.3389/fonc.2020.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CH, Lu YS, Cheng AL, Huang CS, Kuo WH, Wang MY, Chao M, Chen IC, Kuo CW, Lu TP, et al. Disparity in Tumor Immune Microenvironment of Breast Cancer and Prognostic Impact: Asian Versus Western Populations. Oncologist. 2020;25(1):e16–e23. doi: 10.1634/theoncologist.2019-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thike AA, Chen X, Koh VCY, Binte Md Nasir ND, JPS Y, Bay BH, Tan PH. Higher densities of tumour-infiltrating lymphocytes and CD4+ T cells predict recurrence and progression of ductal carcinoma in situ of the breast. Histopathology. 2020;76(6):852–864. doi: 10.1111/his.14055. [DOI] [PubMed] [Google Scholar]
- 29.Miligy I, Mohan P, Gaber A, Aleskandarany MA, Nolan CC, Diez-Rodriguez M, Mukherjee A, Chapman C, Ellis IO, Green AR, et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology. 2017;71(2):258–268. doi: 10.1111/his.13217. [DOI] [PubMed] [Google Scholar]
- 30.Chen XY, Thike AA, Nasir NDM, Koh VCY, Bay BH, Tan PH. Higher density of stromal M2 macrophages in breast ductal carcinoma in situ predicts recurrence. Virchows Arch. 2020;476(6):825–833. doi: 10.1007/s00428-019-02735-1. [DOI] [PubMed] [Google Scholar]
- 31.Thike AA, Koh V, Md Nasir ND, Tan PH. CD68 and CD163 predict recurrence and progression of breast ductal carcinoma in situ. Lab Investig. 2018;98:108. [Google Scholar]
- 32.Campbell MJ, Baehner F, O’Meara T, Ojukwu E, Han B, Mukhtar R, Tandon V, Endicott M, Zhu Z, Wong J, et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2017;161(1):17–28. doi: 10.1007/s10549-016-4036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;178(1):17–33. doi: 10.1007/s10549-019-05371-0. [DOI] [PubMed] [Google Scholar]
- 34.Bian T, Wu Z, Lin Q, Mao Y, Wang H, Chen J, Chen Q, Fu G, Cui C, Su X. Evaluating Tumor-Infiltrating Lymphocytes in Breast Cancer Using Preoperative MRI-Based Radiomics. J Magn Reson Imaging. 2022;55(3):772–784. doi: 10.1002/jmri.27910. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita N, Hisamatsu Y, Shigechi T, Tokunaga E, Saeki H, Oki E, et al. The immune microenvironment of ductal carcinoma in situ of the breast. Cancer Res. 2019;79(4). 10.1158/15387445.SABCS18-P5-07-07.
- 36.Beguinot-Cornillon M, Dauplat MM, Kwiatkowski F, Lebouedec G, Tixier L, Pomel C, Penault-Llorca F, Radosevic-Robin N. Analysis of tumor-infiltrating lymphocytes (TILs) reveals biologically different subgroups of breast ductal carcinoma in situ. Cancer Res. 2016;76(14):1465. doi: 10.1158/1538-7445.AM2016-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.