Abstract
Background
Rice (Oryza sativa L.) is the major source of daily caloric intake for more than 30% of the human population. However, the sustained productivity of this staple food crop is continuously threatened by various pathogens and herbivores. Breeding has been successful in utilizing various mechanisms of defense by gene pyramiding in elite cultivars, but the continuous resurgence of highly resistant races of pathogens and herbivores often overcomes the inherent capacity of host plant immunity. MicroRNAs (miRNAs) are endogenous, short, single-stranded, non-coding RNA molecules that regulate gene expression by sequence-specific cleavage of target mRNA or suppressing target mRNA translation. While miRNAs function as upstream regulators of plant growth, development, and host immunity, their direct effects on growth and development in the context of balancing defenses with agronomic potential have not been extensively discussed and explored as a more viable strategy in breeding for disease and pest resistant cultivars of rice with optimal agronomic potentials.
Results
Using the available knowledge in rice and other model plants, this review examines the important roles of miRNAs in regulating host responses to various fungal, bacterial, and viral pathogens, and insect pests, in the context of gains and trade-offs to crop yield. Gains from R-gene-mediated resistance deployed in modern rice cultivars are often undermined by the rapid breakdown of resistance, negative pleiotropic effects, and linkage drags with undesirable traits. In stark contrast, several classes of miRNAs are known to efficiently balance the positive gains from host immunity without significant costs in terms of losses in agronomic potentials (i.e., yield penalty) in rice. Defense-related miRNAs such as Osa-miR156, Osa-miR159, Osa-miR162, Osa-miR396, Osa-530, Osa-miR1432, Osa-miR1871, and Osa-miR1873 are critical in fine-tuning and integrating immune responses with physiological processes that are necessary to the maintenance of grain yield. Recent research has shown that many defense-related miRNAs regulate complex and agronomically important traits.
Conclusions
Identification of novel immune-responsive miRNAs that orchestrate physiological processes critical to the full expression of agronomic potential will facilitate the stacking of optimal combinations of miRNA-encoding genes to develop high-yielding cultivars with durable resistance to disease and insect pests with minimal penalties to yield.
Keywords: Rice, MicroRNA, Plant immunity, Defense-yield trade-off, Genome editing
Background
The world population is increasing at an alarming rate, with projections of 10 billion by 2050 [1]. At least 60% more food grains will have to be produced to ensure that the global needs for staple foods are secured in the twenty-first century and beyond [1]. Rice (Oryza sativa L.) is one of the major staple food crops that provide calories to more than one-third of the human population. Rice productivity is constantly challenged by the negative impacts of pathogens, insect herbivores, and other parasites. These biotic stresses, particularly pathogens, account for 20–30% of losses in global rice yields [2].
Pathogens and their host plants continuously compete for dominance in a co-evolutionary battle. Plants have evolved multi-layered defense strategies against pathogen invasion [3, 4]. Host plants induce complex defense mechanisms by activating or suppressing a large array of genes in response to pathogen attacks [5]. Such mechanisms are facilitated by the ability of a host plant to recognize a myriad of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) during the initial stages of pathogen or herbivore invasion [6]. The pathogen-triggered PAMP or DAMP is usually recognized by pattern-recognition receptors (PRRs) on the surface of host cells. The PAMPs or DAMPs, in turn, activate the PAMP-triggered immunity (PTI) response by inducing many types of defense-related genes [7, 8].
Host-plant immunity is dependent on the successful activation of defense-related genes. Successful virulent pathogens often overcome the PTI by mediating effector-triggered susceptibility (ETS), which leads to disease development [9]. In response, host plants develop a secondary immune response known as effector-triggered immunity (ETI), mediated by intracellular receptor proteins encoded by R-genes [10]. The product of R-genes (R-proteins) binds to specific pathogen effectors producing a more complex and robust hypersensitive response (HR), which further mediates cell death to restrict the growth of the pathogen at the sites of infection [3]. In rice, many R-genes against pathogens and insect pests have been identified and characterized. Most of these R-genes are effectively utilized to enhance resistance through introgression breeding. However, their efficacies are often overcome within a certain period of time due to the evolution of new resistant races that can no longer be recognized by the R-gene products. Moreover, unregulated expression of R-genes imposes a substantial demand on cellular resources, which negatively affects plant growth with trade-offs to productivity in terms of penalty to grain yield [11–13]. Therefore, in addition to R-genes, exploring other types of genetic defenses, including those that are mediated by other regulatory molecules such as microRNAs (miRNAs) is a potentially important strategy for balancing efficient disease and pest management with sustainable rice production.
MicroRNAs (miRNAs) are short (~ 22 nucleotides), endogenous, single-stranded, non-coding RNAs that form a characteristic stem-loop structure. Their function is to negatively regulate the expression of their target genes (i.e., usually a regulatory gene such as a transcription factor) at the post-transcriptional level. Importantly, microRNAs act as crucial modulators of various cellular and biological processes, including plant growth, development, reproduction, and responses to biotic and abiotic stresses [14–16]. Plant miRNAs are transcribed as primary miRNA (pri-miRNA) from miRNA-encoding genomic loci (MIR loci) by RNA polymerase II [17]. The long stem of the looped pri-miRNAs is subsequently processed by RNAse III enzymes called DICER-like 1 (DCL1) proteins in association with hyponastic leaves 1 (HYL1) and serrate (SE) into double-stranded miRNA-miRNA* (*passenger strand of miRNA) duplex [18, 19]. The miRNA/miRNA* duplex is transported from the nucleus into the cytoplasm and subsequently methylated by HUA ENHANCER 1 (HEN1) to prevent degradation [20]. The guide miRNA is loaded into ARGONAUTE1 (AGO1) to form a functional RNA-induced silencing complex (RISC) [21]. Complementarity between the silencer miRNA and its target transcript allows the RISC complex to trigger complete inhibition of protein synthesis either through the degradation of the mRNA or inhibition of its translation.
In the past few years, the rapid development of next-generation sequencing (NGS) technologies and powerful algorithms for the prediction and modelling of interactions at the genetic, genomic, and molecular levels opened new paths in miRNA discovery. Molecular genetic approaches like 5’RACE, degradome sequencing, stem-loop RT-PCR, reporter gene analysis, loss- or gain-of-function mutation experiments have led to the discovery and unraveling of the function of the large number of miRNAs [22]. In rice, numerous miRNAs that fine-tune host immune response and also those that regulate plant growth have been identified, cloned, and functionally validated through loss-of-function or gain-of-function mutation experiments. In this mini-review, we specifically focus on the regulatory impacts of functionally characterized defense-related miRNAs in rice and their roles in fine-tuning complex traits of agronomic importance. Cautionary aspects of innovative strategies for miRNA manipulation towards understanding the balance and trade-offs between defense and productivity in terms of yield are discussed. In the subsequent sections, we highlight the role of miRNAs in mediating resistance to various pathogens and herbivores (Fig. 1, Table 1).
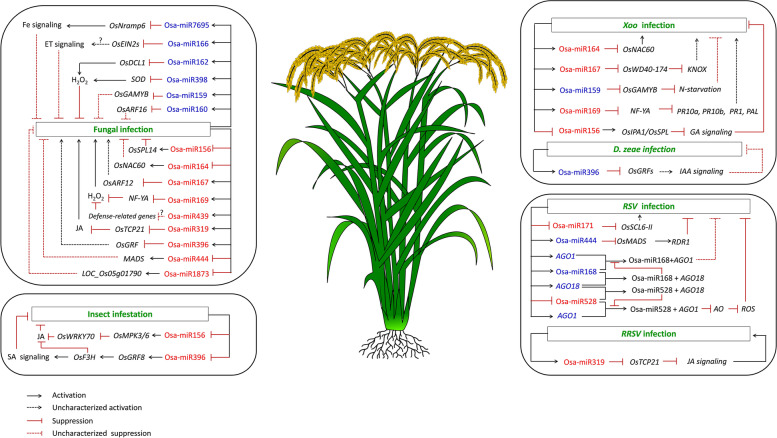
Fig. 1.
Summary of known miRNA-mediated defense mechanisms against pathogens and herbivores in rice. The left panel indicates an overview of the miRNAs responsive to fungal pathogens and insects. The right panel shows the miRNAs involved in resistance to bacterial and viral diseases. The miRNAs written in blue and red represent the positively and negatively regulated miRNAs, respectively. Xoo- Xanthomonas oryzae pv. oryzae; D. zeae- Dickey Zeae, RSV- Rice stripe virus; RRSV- Rice ragged stunt virus
Table 1.
Comprehensive list of miRNAs involved in rice immunity against pathogens and herbivores
| Organisms | MicroRNAs | Target genes | Regulation | Pathogens/ herbivores | References |
|---|---|---|---|---|---|
| Fungus | Osa-miR156fhl-3p | OsSPL14 | Negative | M. oryzae | [23] |
| Osa-miR159a | OsGAMYB | Positive | M. oryzae | [24] | |
| Osa-miR160a | ARF16 | Positive | M. oryzae | [25] | |
| Osa-miR162a | OsDCL1 | Positive | M. oryzae | [26] | |
| Osa-miR164a | OsNAC60 | Negative |
M. oryzae, R. solani |
[27] | |
| Osa-miR166k-166 h | EIN 2 | Positive |
M. oryzae, F. fujikuroi |
[28] | |
| Osa-miR167d | ARF12 | Negative | M. oryzae | [29] | |
| Osa-miR169 | NF-YA | Negative | M. oryzae | [30] | |
| Osa-miR319 | OsTCP21 | Negative | M. oryzae | [31] | |
| Osa-miR396 | OsGRFs | Negative | M. oryzae | [32] | |
| Osa-miR398b | SOD | Positive | M. oryzae | [33] | |
| Osa-miR439a | Defense-related genes | Negative | M. oryzae | [34] | |
| Osa-miR444 | MADS | Negative | M. oryzae | [35] | |
| Osa-miR530 | HDFR-TS | Negative | M. oryzae | [36] | |
| Osa-miR1432 | OsEFH1 | Negative | M. oryzae | [37] | |
| Osa-miR1871 | OsMFAP1 | Negative | M. oryzae | [38] | |
| Osa-miR1873 | LOC_Os05g01790 | Negative | M. oryzae | [39] | |
| Osa-miR7695 | OsNramp6 | Positive | M. oryzae | [40] | |
| Bacteria | Osa-miR156 | OsSPLs | Negative | X. oryzae | [41] |
| Osa-miR159b | OsGAMYB | Positive | X. oryzae | [42] | |
| Osa-miR164a | OsNAC60 | Negative | X. oryzae | [42] | |
| Osa-miR167d-5p | OsWD40–174 | Negative | X. oryzae | [42] | |
| Osa-miR169o | NF-YA | Negative | X. oryzae | [43] | |
| Osa-miR396f | OsGRFs | Positive | Dickeya zeae | [44] | |
| Viruses | Osa-miR168 | AGO1 | Positive | RSV | [45] |
| Osa-miR171b | OsSCL6-II | Negative | RSV | [46] | |
| Osa-miR319 | OsTCP21 | Negative | RRSV | [47] | |
| Osa-miR444 | MADS Box | Positive | RSV | [48] | |
| Osa-miR528 | L-ascorbate oxidase | Negative | RSV | [49, 50] | |
| Herbivores | Osa-miR156 | OsMPKs | Negative | BPH | [51] |
| Osa-miR396 | OsGRF8 | Negative | BPH | [52] |
OsSPL- SQUAMOSA promoter-binding protein-like transcription factor; ARF- Auxin responsive factor; DCL- Dicer-like, EIN- Ethylene insensitive; NF-YA- Nuclear Factor Y-A; OsTCP- Teosinte branched/Cycloidea/Proliferating cell factor; OsGRF- Growth regulating factors; SOD- Superoxide dismutase, DHFR-TS - Dihydrofolate reductase/thymidylate synthase; OsEFH1- EF-hand family protein 1; OsMFAP1- Microfibrillar-associated protein 1; OsNramp- Natural resistance-associated macrophage pathogen, AGO- ARGONAUTE 1; OsSCL- Scarecrow-like; RSV- Rice stripe virus; RRSV- Rice ragged stunt virus; BPH-Brown planthopper
MicroRNAs against fungal pathogens
In rice, the two-layered immune system (PTI and ETI) has been shown to play important roles in defense against fungal pathogens such as Magnaporthe oryzae [53]. Pattern Recognition Receptors (PRRs) such as CEBiP, LYP4, and LYP6 are known to recognize the pathogen-associated molecular patterns (PAMP) and induce the PAMP-triggered immunity [54, 55]. Whereas the products of R-genes recognize divergent pathogen effectors that activate effector-triggered immunity (ETI), their functionality for recognizing the target effectors depends on several structural features. However, resistance conferred by a single R-gene is quickly overcome by the emergence of new pathotypes that can evade the effects of the R-gene products. Therefore, pyramiding multiple R-genes in the same genetic background represents a more robust approach to develop rice cultivars with broad-spectrum resistance. The caveat to this approach is that R-gene pyramiding by conventional breeding (even with a marker-assisted approach) requires multiple rounds of hybridization and selection that are often confounded by the negative effects of linkage drags. A random combination of multiple R-genes in the same genetic background may not always produce positive or optimal effects, thus the trade-off effects between resistance and yield have been a major challenge in maximizing and optimizing the gains from such an approach for yield improvement. The use of miRNAs provides an alternative strategy to develop broad-spectrum resistance against fungal pathogens in rice cultivars. The increasing number of evidence support that miRNAs also regulate the ETI and PTI [56, 57]. In particular, it has been shown that certain miRNAs fine-tune the expression of innate immunity in certain cultivars through the integration of R-gene regulation, hormone signaling, callose deposition, and production of reactive oxygen species (ROS) such as superoxide radicals (O• 2 −), hydroxyl radicals (OH·) and hydrogen peroxide (H2O2) (Fig. 2).
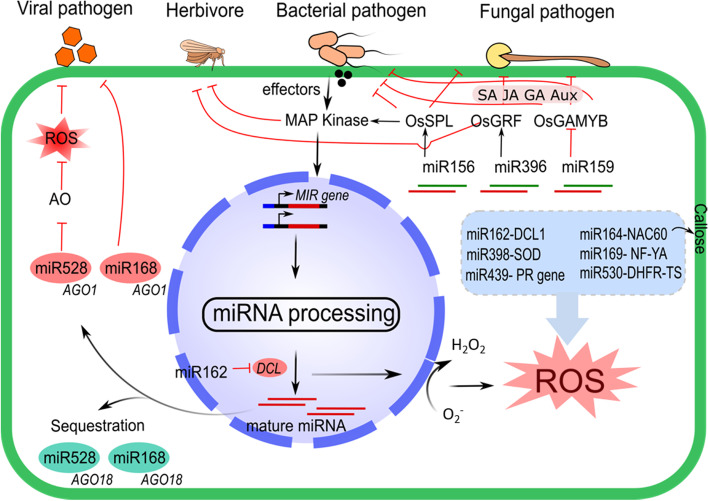
Fig. 2.
Functionally characterized miRNAs associated with the immune response against bacterial, fungal, and viral pathogens, as well as insect herbivores. Pathogen-derived effector molecules elicit the expression of MIR genes by RNA polymerase II via mitogen-activated protein (MAP) kinase signaling. The long hairpin transcript is processed by Dicer-like protein (DCL). The miR162 targets the DCL and produces ROS. Various miRNAs such as miR162, miR164, miR169, miR398, and miR439 also participate in the immune response mechanism via ROS. On the other hand, a tug of war between AGO1 and AGO18 for binding to miR528 and miR168 facilitates the expression of strong resistance against the invading viral pathogen through the production of reactive oxygen species (ROS). The miR156, miR396, and miR159 confer resistance to pathogens and insect pests by targeting transcription factors through a mechanism modulated by phytohormones
MicroRNAs as positive regulators of immunity against blast disease in rice
Several miRNAs, including Osa-miR159, Osa-miR160, Osa-miR162a, Osa-miR166k, Osa-miR166h, Osa-miR398b, and Osa-miR7695 have been shown to function as positive regulators of defenses against the rice blast disease caused by fungus Magnaporthe oryzae [26]. For instance, the Osa-miR159a fine-tunes host plant growth and immunity by inhibiting its three target genes, OsGAMYB, OsGAMYBL, and OsZF. The OsGAMYB and OsGAMYBL are transcriptional regulators of gibberellic acid signaling, while OsZF is a C3HC4- domain-containing zinc finger protein involved in ubiquitin-protein transferase activity. Transgenic rice plants overexpressing a short tandem target mimic (STTM) to inhibit the Osa-miR159a showed enhanced host susceptibility to the disease. In contrast, the knock-down mutation of the target genes conferred resistance to M. oryzae.
In addition, the Osa-miR159-GAMYB module orchestrates the reproductive developmental process by its direct role in the regulation of flower, pollen, and seed development [24]. It is apparent that Osa-159a must be precisely spatiotemporally regulated to coordinate plant development and immunity. Similarly, leaf and seed development in rice has been shown to be regulated by Osa-miR160 via auxin signaling, which also acts as a positive regulator of rice immunity against M. oryzae. Transgenic plants overexpressing Osa-miR160 displayed significantly stronger resistance to blast disease. The Osa-miR160a enhances resistance to blast by suppressing the AUXIN RESPONSE FACTOR 16 (ARF16), which in turn silences the indole-3-acetic acid (IAA) signaling [25]. Diverse cellular and physiological processes in plants are controlled by miR166, which belongs to a highly conserved family of miRNA molecules. The miR166 targets the class III homeodomain-leucine zipper family (HD ZIP III) of transcription factors [58, 59]. In rice specifically, overexpression of the polycistronic miRNA containing two of the miR166-family members, i.e., Osa-miR166k and Osa-miR166h, has been shown to cause stronger immunity against M. oryzae and Fusarium fujikuroi, through targeting of the ethylene insensitive 2 (EIN 2) gene via cross-regulation. This mechanism has been shown to occur without negative effects on plant growth [28].
Plants produce H2O2 in response to biotic and abiotic stresses. It has been shown that Osa-miR162a fine-tunes the host’s innate immunity against M. oryzae by targeting the Dicer-like 1 (OsDCL1) gene through the accumulation of intracellular H2O2 thereby facilitating cell death at the infection site. This mechanism also regulates other physiological processes that are critical to yield maintenance. Overexpression of Osa-miR162a showed enhanced resistance to M. oryzae by positively regulating many other defense-related genes [26]. Growing evidence suggests that loss-of-function of OsDCL1 showed developmental defects in rice at the seedling stage, including dwarfism, root and shoot abnormality, and wilting of leaves. All of these effects indicate that Osa-miR162a optimizes growth and immunity without any yield penalty. ROS homeostasis is regulated by several key enzymes, including superoxide dismutase (SOD). Consistent with earlier reports, elevated levels of cellular H2O2 has been observed with the suppression of the Cu/Zn superoxide dismutase (SOD) genes by the Osa-miR398b. This process positively regulates host immunity against blast by negatively regulating the components of ROS production and homeostasis [25].
Upon M. oryzae infection, higher activity of SOD is associated with accumulation of H2O2, which leads to enhanced resistance to blast [33]. ROS is also produced by an excess of iron (Fe), an essential micro-element required for photosynthesis and chloroplast maintenance. This process is critically regulated upon pathogen infection as the host and pathogen compete for available Fe. Recently, Osa-miR7695 has been shown to function as a positive regulator of rice immune response by mediating the trade-off between defense and iron homeostasis. Rice plants overexpressing Osa-miR7695 showed increased resistance to M. oryzae and stronger Fe accumulation at the site of infection. It was proposed that upon infection with M. oryzae, expression of Osa-miR7695 leads to the suppression of the target gene OsNramp 6 (Natural resistance-associated Macrophage Protein 6), which encodes an iron transporter [40, 60].
Negative regulation of defenses against rice blast by miRNAs
The Osa-miR156fhl-3p, Osa-miR164a, Osa-miR167d, Osa-miR169a, Osa-miR319, Osa-miR396, Osa-439a, Osa-miR444, Osa-miR530, Osa-miR1432, and Osa-miR1873 have been identified as negative regulators of rice innate immunity against M. oryzae. These miRNAs have been reported to control rice innate immunity by targeting critical transcription factors. The miR156 belongs to a conserved family that regulates plant growth, development, and yield by targeting the SQUAMOSA promoter-binding protein-like transcription factor 14 (SPL14) and WRKY45 transcription factor. In rice, overexpression of Osa-miR156fhl-3p in a target mimic mutant showed stronger resistance to blast by virtue of enhanced expression of the target genes SPL14 and WRKY45 transcription factors [23]. Expression of the OsNAC60 transcription factor is negatively regulated by the suppression of Osa-miR164a upon M. oryzae infection, which leads to the enhancement of defense responses [27]. Abolition of the entire Osa-miR164a/OsNAC60 regulatory module has been shown to result in a susceptible phenotype. The Osa-miR164a has also been involved in controlling the sheath blight-causing fungus Rhizoctonia solani by activating the salicylic acid (SA) signaling pathway and expression of associated defense-related genes. Expression of Osa-miR169a has been shown to condition a strong resistance against M. oryzae by suppressing its target gene nuclear factor Y-A (NF-YA). Significant accumulation of Osa-miR169a has been documented in a susceptible genotype of rice with a somewhat decreased resistance level. Transgenic rice plants overexpressing Osa-miR169a have been shown to exhibit a higher level of susceptibility, which was associated with the downregulation of target genes and reduced accumulation of intracellular H2O2 [30]. The Osa-miR530 controls H2O2 production by regulating the expression of dihydrofolate reductase/thymidylate synthase (DHFR-TS). It is noted that DHFR-TS participates in the maintenance of redox balance by producing the nicotinamide adenine dinucleotide phosphate (NADPH) and ROS. Subsequently, ROS is converted into H2O2 by SOD. Perturbation of Osa-miR530 enhances resistance to blast disease through its effects on H2O2 accumulation. It is important to mention that blocking the Osa-miR530 also positively affects flowering and seed maturation [36]. The Osa-miR439a negatively affects immunity by inhibiting the expression of defense-related genes and H2O2 production Suppression of the Osa-miR439a using target mimic mutants has been shown to compromise susceptibility to M. oryzae by induction of H2O2 [34].
The Osa-miR319 modulates host immune response in the rice-M. oryzae interaction in a negative manner. Accumulation of Osa-miR319 has been observed in susceptible genotypes as indicated by the suppression of the target gene OsTCP21 upon M. oryzae infection. Blocking the conversion of a-linoleic acid (LnA) to hydroperoxy-octadecadienoic acid (HPODE) has been shown to inhibit the jasmonic acid (JA) signaling pathway [31]. The Osa-miR444b.2 has also been identified among the many negative regulators of rice immunity against M. oryzae. Overexpression of Osa-miR444b.2 enhances susceptibility to M. oryzae but with little impact on its target transcription factors MADS27b and MADS57. Altered expression of the target mimicry of Osa-miR444b.2 resulted in enhanced resistance to blast [35]. The Osa-miR444a has been shown to positively regulate immunity against the rice stripe virus (RSV) by upregulating OsRDR1 expression, which is facilitated by suppressing target MADS-box genes [48].
The Osa-miR1432 has been shown to fine-tune resistance to disease as well as yield potential by targeting the OsEFH1 (EF-hand family protein 1). Overexpression of Osa-miR1432 has been shown to compromise resistance to M. oryzae with concomitant adverse effects on yield. On the other hand, inhibition of Osa-miR1432 expression has enhanced resistance with positive effects on yield, attributed to the enhancement of PTI responses [37]. During M. oryzae infection, immunity and yield in rice are also balanced by the Osa-miR1871. Inhibition of Osa-miR1871 expression greatly enhances resistance and yield, by virtue of the effects on the targetOsMFAP1 (Microfibrillar-associated protein 1) gene. In contrast, overexpression of Osa-miR1871 had been shown to cause susceptibility to blast, with concomitant negative effects on grain yield [38].
The Osa-miR1873 appeared to regulate resistance to rice blast in a negative manner. It has been shown that expression of Osa-miR1873 leads to disease susceptibility through the suppression of a target gene with an unknown function (i.e., LOC_Os05g01790, which encodes a DUF868 domain-containing protein). Suppression of Osa-miR1873 through the expression of the target mimicry mutant has been shown to enhance defense response against blast by fine-tuning host plant immunity and growth [39]. The Osa-miR167d modulates plant developmental and stress responses by targeting auxin response factor (ARF) genes. The Osa-miR167d also seemed to play a negative role in rice immunity against M. oryzae by blocking ARF12 genes. Suppression of Osa-miR167d by overexpressing a target disease mimic mutant led to enhanced resistance to blast [29]. Characterization of Osa-miR396 revealed its negative effect on defense response against M. oryzae by silencing multiple OsGRF genes. Overexpression of Osa-miR396 promotes susceptibility to blast through the downregulation of OsGRFs genes. Suppression of Osa-miR396 by expressing the target disease mimics mutant enhanced resistance to blast with concomitant improvement in yield [32].
MicroRNAs regulating responses to bacterial pathogens
Five classes of miRNAs have been shown to modulate resistance against bacterial blight (BB) caused by Xanthomonas oryzae pv. oryzae (Xoo). Another class of miRNAs has been shown to positively regulate resistance against the bacterial foot rot disease caused by Dickeya zeae. Of the miRNAs involved in responses to BB, the Osa-miR159b has been shown to function as a positive regulator, while the others function as negative regulators. The Osa-miR159b enhances resistance to BB by repressing its target transcription factor OsGAMYB involved in gibberellic acid (GA) signaling. This process leads to negative effects on nitrogen assimilation as an outcome of the repression of the GA signaling pathway [42].
The Osa-miR167 has also been shown to be involved in rice immunity to bacterial pathogens [57, 61]. For instance, Osa-miR167d abates immunity upon Xanthomonas oryzae pv. oryzae (Xoo) infection by suppressing the target gene OsWD40–174, which downregulates the lignin biosynthetic gene OsKNOX during leaf development [42]. Downregulation of KNOX hinders lignin biosynthesis [62]. Transgenic plants overexpressing Osa-miR164a showed enhanced susceptibility to Xoo by virtue of the downregulation of the OsNAC60 transcription factor [42]. The role of the miR164a/OsNAC60 regulatory module in blast resistance has been discussed earlier in this review. The Osa-miR169o represents the coordinated crosstalk regulation between BB and Nitrogen-use efficiency (NUE) in rice. Overexpression of the miR169o enhances NUE and promotes susceptibility to BB by suppressing the nuclear factor Y-A (NF-YA), which in turn causes significant downregulation of several defense-related genes, including PR10b, PR1b, PR10a, and PAL [43]. The Osa-miR156 negatively regulates immunity against bacterial blight [41]. Transgenic plants overexpressing the target gene IPA1 (Ideal Plant Architecture1) under the control of a pathogen-inducible promoter of OsHEN1 have been shown to enhance both disease resistance and yield.
More recently, Osa-miR396f was shown to play a positive role in conferring resistance against bacterial foot rot disease caused by Dickeya zeae. Overexpression of Osa-miR396f in the susceptible rice variety Nipponbare showed enhanced immunity to D. zeae by suppressing the target gene OsGRFs (Growth-Regulating Factors). However, the precise molecular mechanism underpinning the immunity against bacterial foot roots mounted by OsGRFs is still unknown [44].
MicroRNAs control resistance to viral pathogens
The miRNA-mediated gene regulation has emerged as a novel strategy to enhance antiviral defenses in crop plants, including rice [63]. Plants have evolved different RNA silencing mechanisms to respond to diverse classes of viral infections. Antiviral RNA silencing is triggered by virus-derived double-stranded RNA (dsRNA) that are directly recognized and processed by the host’s dicer-like proteins (DCL) to form virus-derived small-interfering RNAs (vsiRNAs) [64]. The vsiRNAs are incorporated into the ARGONAUTE (AGO) proteins that form the core component of the RNA-induced silencing complex (RISC). The functional RISC either cleaves the viral RNA or arrests the viral protein translation. Single-stranded viral RNAs require endogenous RNA-dependent RNA polymerases (RDRs) to synthesize dsRNA that serves as the substrate for DCLs to produce secondary vsiRNAs. To counteract the defense response of host plants, most viruses have developed specialized proteins known as viral suppressors of RNA silencing (VSRs) that impede the antiviral RNA silencing pathway and suppress the defense response [65].
MicroRNAs provide an additional layer of intrinsic defense against viral attacks. In rice, the Osa-miR528 is well characterized and known to be involved in regulating defenses against the rice stripe virus (RSV). In cleavage-defective AGO18 mutants of rice, the Osa-miR528 is sequestered away from AGO1, upon RSV infection. These, in turn, prevent the formation of a functional RNA-induced silencing complex. These events further lead to the enhanced expression of downstream gene AO, encoding an L-ascorbate oxidase and functions in the initiation of defense response against RSV through the accumulation of ROS [49]. Further research suggested that the transcription factor OsSPL9 specifically regulates the expression of the Osa-miR528-AO module by binding to the promoter of the Osa-miR528. Mutation of OsSPL9 has been shown to cause the dramatic downregulation of Osa-miR528, thereby inducing AO expression that leads to enhanced resistance to RSV [50].
The AGO18 also sequesters the Osa-miR168 upon RSV infection. It has been shown that AGO18 competes with AGO1 for binding to Osa-miR168, resulting in an elevated level of AGO1-mediated resistance [45]. RSV infection triggers the expression of Osa-miR444, which positively regulates immune response. Transgenic plants overexpressing the Osa-miR444 displayed a broad-spectrum resistance to RSV by inducing OsRDR1 and by silencing several MADS-box transcription factors [48].
RSV infection of host rice plants perturbs the expression of Osa-miR171. Such impaired expression has been shown to severely affect plant height and chlorophyll content, thereby causing RSV-like symptoms. It has been demonstrated that plants expressing miR171b were less susceptible to RSV characterized by attenuated RSV symptoms [46]. Similarly, the Osa-miR319 acts as a negative regulator of immunity against the ragged stunt virus (RRSV) in rice. Transgenic plants overexpressing the Osa-miR319 have exhibited severe disease-like symptoms by negatively affecting the target gene OsTCP21 (TEOSINTE BRANCHED/CYCLOIDEA/PCF) and subsequently suppressing JA-mediated plant defense pathways [47].
MicroRNAs for insect resistance
Rice is host to more than 200 insect pests at different stages of its life cycle. Advances in genomics have greatly expanded the understanding of the role of miRNAs in the regulation of immune response to insect pests. During brown planthopper (BPH) infestation of rice, differentially expressed miRNAs have been identified by deep sequencing in an introgression line carrying the resistance gene Bph15 in comparison to a susceptible genotype. Several known miRNAs, along with 183 other novel BPH-responsive miRNAs, have been identified to be involved in the regulation of either basal defenses or targeted resistance to BPH [66]. Similarly, integrated analysis of miRNA and mRNA expression profiles identified numerous BPH-responsive miRNAs that are differentially expressed between transgenic lines carrying the resistance gene Bph6 and wild-type [67]. Osa-miR156 was found to function as a negative regulator of the BPH resistance. Sequestration of the Osa-miR156 using a target mimic mutant of Osa-miR156 displayed enhanced resistance to BPH by positively regulating the OsMPK3 and OsMPK6 genes. The transcript level of OsWRKY70 transcription factor was found to be significantly reduced in the Osa-miR156-sequestered target mimic mutant that represses jasmonic acid (JA) biosynthesis and signaling [51]. Another miRNA, Osa-miR396, also acts as a negative regulator of BPH resistance. Downregulation of Osa-miR396 enhances the expression of the growth-regulating factor 8 (OsGRF8) gene, which in turn positively regulates the flavanone 3-hydroxylase (OsF3H) gene involved in the flavonoid biosynthesis. The OsF3H positively regulates the BPH resistance through its positive effects on the salicylic acid (SA) pathway and negative effects on the jasmonic acid (JA) pathway [52].
MicroRNA-mediated regulation of R-genes: lessons from rice and other models
Modern cultivars are being developed by pyramiding multiple R-genes as well as other types of genes involved in quantitative mechanisms as a major strategy to avoid substantial losses to crop yield due to diseases and pests. However, it has been shown that constitutive expression of R-genes often imposes high fitness costs, mainly with deleterious consequences to plant growth and development. Therefore, the expression of R-genes must be optimized in the right spatial and temporal manner to minimize trade-offs.
Emerging evidence suggests that miRNAs and secondary siRNA play important roles in the regulation of R-gene function by silencing the immune-response receptors when the host plant is not under attack by the pathogen. This mechanism is important in stabilizing basal transcript levels to limit the fitness costs of an overactive immune response. Plants have evolved specific miRNAs that can target the conserved domains of the R-gene in two major pathways, either by direct targeting of R-gene or indirect targeting of R-gene via phasiRNA (Fig. 3). In the pathway for direct targeting of R-genes, a mature miRNA produced from MIR loci interacts with AGO1 protein. The AGO1-miRNA complex binds and cleaves the R-gene transcripts in a sequence-specific manner and prevents the R-gene mediated autoimmunity in the absence of the pathogen.
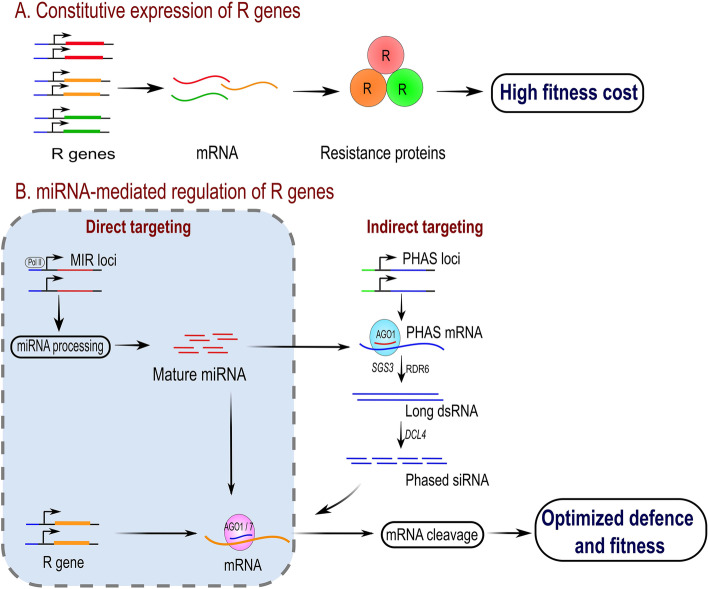
Fig. 3.
Model depicting the possible roles of miRNAs and phased secondary siRNAs (phasiRNA) in the regulation of R-genes. A Under normal conditions, when the host plant is not challenged, constitutive and unregulated expression of R-genes results in high fitness costs; B In the direct targeting pathway of R-genes by miRNA, the MIR loci produce miRNA transcripts that are processed to mature miRNAs. Subsequently, mature miRNA is complexed with AGO1/7 and directly binds to R-gene transcript followed by cleavage, resulting in basal resistance response and concomitant effects on fitness cost (box with dashed line). In the indirect targeting pathway of R-genes by miRNAs, the mature miRNA is produced from MIR loci and interacts with AGO1. The AGO1-miRNA complex binds to PHAS transcripts produced from the coding region of PHAS loci and cleaves the PHAS transcripts in a sequence-specific manner. SGS3 and RDR6 convert the single-stranded RNA to long double-stranded RNA which is processed by DCL4 to phased siRNA (phasiRNA). AGO1/7-phasiRNA complex cleaves R- gene transcripts and maintain the basal level of R-gene expression to achieve optimized and well-balanced resource usage for defense and maintenance of plant fitness by robust growth and development. R genes-resistance genes; AGO1-ARGONAUTE 1; SGS3-SUPPRESSOR OF GENE SILENCING 3; RDR6- RNA-DEPENDENT RNA POLYMERASE 6; DCL4- DICER-LIKE 4
The miR472 was the first to be involved in the direct targeting of the CC-NBS-LRRs domain-containing immune receptor genes in Arabidopsis [68]. Since its discovery, many other miRNAs involved in R-gene regulation that optimize the defense-fitness trade-off have been reported. For example, the sequence-specific cleavage of the TIR-NB-LRR immune receptor of N gene transcripts that conditions resistance to tobacco mosaic virus (TMV) has been shown to be mediated by nta-miR6019 and nta-miR6020 modules in the Solanaceae family [69]. The miR482/2118 super-family targeting NB-LRR genes have also been well characterized in tomato, and its prime importance in the regulation of immune response is well documented [70]. A recent report suggested that the miR1885 is involved in a dynamic balance between plant growth and immunity to viral pathogen in Brassica by direct silencing the R-gene [71]. More recently, in rice, Osa-miR1876 has been shown to epigenetically regulate the expression of the NBS8R gene encoding an NB-ARC domain protein that confers resistance to X. oryzae [72].
Besides the direct targeting of NLRs, miRNAs have also been shown to indirectly regulate R-genes by targeting other genes that are part of the R-gene networks [73]. For example, the NBS-LRR genes, which are not primarily recognized by miRNA, trigger the production of a phased array (in a sequential, head-to-tail manner, according to the miRNA cleavage site) of 21-nt secondary small interfering RNAs (phasiRNAs) to amplify the silencing effects [74]. These phasiRNAs act in trans to silence the addition of R-gene transcripts. In rice, no phasiRNA that regulates R-genes has so far been characterized. However, phasiRNAs regulating reproductive development has been reported.
The phasiRNA targeting R-genes remains unknown in rice. The phasiRNA that targets the conserved motifs of CC-NBS-LRR has been explored in diverse plant species, including spruce, grapevine, poplar, cotton, Arabidopsis, and citrus [75, 76]. General observations indicate that the highly conserved siRNAs may be important in optimizing the expression of NBS-LRR genes, which may compromise plant fitness. However, the precise role of phasiRNA in regulating NBS-LRR immune receptors in rice is yet to be fully understood [77]. Furthermore, Fine-tuning of NBS-LRR protein expression by phasiRNA inhibits the constitutive expression of many other R-genes, which could potentially be detrimental to plant growth, leading to trade-offs to productivity when not regulated optimally. In the model legume Medicago truncatula, 22-nt miRNAs including miR2275, miR2109, and miR2118 have been shown to trigger the production of phasiRNA that are specifically associated with the regulation of other genes encoding NBS-LRR immune receptors [77, 78]. These miRNAs are highly conserved in both legumes and non-legume plant species.
MicroRNAs fine-tune immunity and trade-offs to yield
High-yielding rice cultivars with resistance to multiple pathogens and pests are paramount to sustainable production. With major accomplishments by breeding, the caveat appeared to be that strong defense responses often come with unintended trade-offs in terms of significant losses to yield as the photosynthetic source-sink dynamics that should favor plant growth tend to be diverted towards defense-related processes [13]. Modern approaches in plant breeding such as marker-assisted selection, transgenics, and genome-editing are being applied to develop resistant cultivars with the minimal penalty to yield. In the past few decades, many R-genes in rice have been tagged with molecular markers for efficient selection and use in pyramiding. However, a limited set of genes are deployed in crop improvement programs since many of them exhibit pleiotropic effects, unwanted linkage drags that undermine agronomic traits, and low heritability [16]. Furthermore, because of the rapid breakdown of R-gene-mediated mechanisms along with their associated fitness costs, the potential use of miRNAs with major roles in regulating the immunity of host rice plants has gained more interest as a more effective means to develop resistant cultivars with optimal balance between resistance and trade-offs to plant vegetative and reproductive growth.
Recent reports have shown that many miRNAs facilitate the maintenance of fitness and resistance with minimal or no penalty to yield. General observations indicate that miRNAs enhance the immunity to pathogens and herbivores with significant positive effects on yield maintenance in most cases. For example, the Osa-miR159, Osa-miR162, Osa-miR396, Osa-miR530, Osa-miR1432, and Osa-1871 have been shown to have positive effects on yield component traits even when the plant is challenged by pathogens [24, 26, 32, 36–38]. For instance, the miRNA, Osa-miR156, fine-tunes resistance without significant penalty to yield, while Osa-miR1873 confers resistance with only a minor penalty to yield [23, 39].
The Osa-miR162 synergizes the mechanisms involved in resistance to blast with the genetic potential for yield through the OsDCL1 that causes the accumulation of intracellular H2O2. Nevertheless, overexpression of Osa-miR162 showed significantly narrower grains, lower seed weight, and poor seed set, leading to a significant penalty to grain yield. Silencing of Osa-miR162 showed positive effects on yield by increasing the number of grains per panicle during M. oryzae infection [26]. Similarly, blocking the expression of Osa-miR530 induces early flowering and seed maturation, with positive effects on grain number per panicle and grain weight. The underlying regulatory networks that balance yield-related processes and immunity is an area that is currently under investigation [36].
Likewise, downregulation of Osa-miR1432 enhances both immunity and yield by targeting the OsEFH1 protein [37]. The resistance and yield trade-off are also fine-tuned by the miR1871-MFAP1 module. Transgenic rice overexpressing Osa-miR1871 as well as the mfap1 mutants exhibit significant reductions in grain yield. Conversely, downregulation of Osa-miR1871 displayed the opposite effects. These results suggested that Osa-miR1871 regulates yield-related traits and immunity through the function of MFAP1 [38]. Suppression of Osa-miR396 induces multiple growth-regulating factor (GRF) genes that enhance resistance to blast and BPH [32, 52]. The Osa-miR396 also plays important roles in the regulation of cellular processes that are critical in the maintenance of inherent potentials for grain size, grain yield, inflorescence development, panicle branching, as well as tolerance to saline and alkaline soil and water [79–81].
The Osa-miR156 negatively regulates host immunity against blast, bacterial blight, and BPH by targeting the IPA1 (Ideal Plant Architecture 1), OsSPLs (SQUAMOSA Promoter-binding protein-like transcription factors), and several OsWRKY genes. Sequestering Osa-miR156 by target mimicry led to enhanced resistance to all three biotic stressors by affecting the accumulation of target transcripts, including the products of defense-related genes [23, 41, 51]. However, overexpression of modified OsSPL14 promotes the panicle branching, which leads to improvements in the number of spikelets per panicle, stronger culm, and tiller reduction. These effects contributed to a significant enhancement of grain yield [82, 83].
Transgenic studies showed that inducible expression of the IPA1 gene under the control of bacterial effector-induced promoter leads to enhanced resistance to BB, with concomitant improvement in grain size, plant architecture, and grain yield [41]. The Osa-miR1873 has been shown to balance the cellular processes involved in the expression of resistance to blast and maintenance of plant growth. Overexpression of Osa-miR1873 has been shown to compromise resistance with additional negative effects to plant growth and developments as manifested by a significant reduction in yield potential due to the reduction of seed-set as indicated by the proportion of filled grains per panicle. In contrast, studies that sequestered Osa-miR1873 showed that proper regulation causes no significant effects on yield [39]. Other miRNAs such as Osa-miR159, Osa-miR160, Osa-miR164, Osa-miR167, and Osa-miR398 have been shown to affect different traits that are a critical component of yield potential, independently.
MicroRNAs coordinate immune response with other cellular processes that determine agronomic potential
MicroRNAs rarely work independently. Single miRNA-encoding loci often function in the regulation of multiple cellular processes, hence more than one trait. Recent studies in rice showed that the role of miRNAs is not limited to the regulation of defense response mechanisms, but they are also involved in the regulation of complex traits (Table 2). For example, the Osa-miR156 is known to regulate a total of eleven (11) SPL genes involved in diverse biological and developmental processes in rice. The Ideal Plant Architecture 1, which encodes a SQUAMOSA Promoter-binding protein-like transcription factors is known to be targeted by Osa-miR156. Overexpression of OsSPL14 enhanced resistance to bacterial blight accompanied by a substantial improvement in yield caused by the reduction in the number of unproductive tillers and enhancement of panicle branching [41]. Mutation in OsSPL14 perturbs Osa-miR156-mediated regulation of OsSPL14, leading to positive gains in yield maintenance as indicated by the reduction of unproductive tillers and stronger culm [82]. Furthermore, overexpression of OsSPL14 also enhanced the number of grains per panicle by increasing the panicle branching [83].
Table 2.
List of known microRNAs involved in the integration of defense-related responses with growth and development-related responses
| miRNAs | Biotic stresses | Traits | References |
|---|---|---|---|
| Osa-miR156 | Blast, BB, BPH | Grain size, grain yield, grain quality, panicle branching, Seed germination, tillering, plant architecture | [82–88] |
| Osa-miR159 | Blast, BB | Floral development, stem elongation, leaf development, grain size | [89–91] |
| Osa-miR160 | Blast | Rice growth and development, tillering, seed setting rate | [92, 93] |
| Osa-miR162 | Blast | Drought tolerance, plant development | [94, 95] |
| Osa-miR164 | Blast, BB | Drought tolerance, plant architecture, grain yield | [96–98] |
| Osa-miR166 | Blast | Nutrient ion uptake, drought tolerance, cadmium tolerance | [99, 100] |
| Osa-miR167 | Blast, BB | Auxin response, tiller number, grain weight | [101–103] |
| Osa-miR169 | Blast, BB | Nitrogen-use efficiency, salt stress | [43, 104] |
| Osa-miR319 | Blast, RRSV | Leaf morphogenesis, cold tolerance, plant height | [105–107] |
| Osa-miR396 | Blast, BPH, Foot rot | Grain size, grain yield, floral development, stem elongation, salt, and alkali tolerance | [79, 80, 87, 108–112] |
| Osa-miR398 | Blast | Panicle length, grain number, grain size, abiotic stress | [88, 113] |
| Osa-miR444 | Blast, RSV | Tillering, nitrate signalling, root development | [114–116] |
| Osa-miR528 | RSV | Flowering time, pollen development, arsenite tolerance, cold tolerance | [117–120] |
BB Bacterial blight, BPH Brown planthopper, RRSV Rice ragged stunt virus, RSV Rice stripe virus
It was recently shown that downregulation of Osa-miR156fhl-3p enhances host plant immunity to M. oryzae by positively affecting OsSPL14 and WRKY45 [23]. Furthermore, recent reports also showed that Panicle blast 1 (Pb1), a panicle blast resistance gene encoding a coiled-coil, nucleotide-binding site, leucine-rich repeat (CC-NBS-LRR) protein, interacts with a WRKY45 transcription factor, which plays a critical role in the expression of induced resistance through the salicylic acid signaling pathway regulated by the ubiquitin-proteasome system [121]. Evidence also supports that suppression of Osa-miR156 could promote seed dormancy by inducing the expression of OsSPL14 and repressing the GA pathway [84]. Expression of OsSPL13 promotes yield in rice by improving grain size and panicle length [122]. The OsSPL16 improves grain size by binding to GW7 [85, 123]. The OsSPL18 binds to the promoter of DEP1 and negatively regulates the cellular process for grain number potential [124, 125]. Overexpression of OsSPL7 negatively affects tiller number and positively affects plant height [86]. In rice, the OsSPL9 is involved in the regulation of Osa-miR528, which promotes flowering under long-day conditions by repressing the Red and Far-red Insensitive 2 (OsRFI2) gene [119]. The OsSPL9 regulates the Osa-miR528/L-Ascorbate Oxidase (AO) transcriptional module, which enhances anti-viral defenses [50]. Moreover, Osa-miR528 regulates pollen intine formation by targeting the uclacyanin gene OsUCL23 [120]. Likewise, in creeping bentgrass, it has been shown that constitutive expression of Osa-miR528 enhances tolerance to salinity and nitrogen-starvation [126].
Hormones are the foundations of cellular signaling that integrate growth-related and defense-related responses [11]. In rice, genes involved in auxin signaling particularly the auxin response factors (ARFs) are known to be targeted by Osa-miR160 and Osa-miR167. The Osa-miR160 is known to positively regulate blast resistance, whereas Osa-miR167 acts in an antagonistic fashion. Furthermore, alteration in auxin signaling in rice through the upregulation of Osa-miR160-resistant OsARF18 has been shown to cause severe defects in overall plant growth as well as reproductive development, with negative effects on seed size and seed set [92, 93]. The Osa-miR167 promotes an efficient response to auxin, which translates to the concomitant enhancement of tiller number and grain weight [101–103]. The growth regulating factors (GRFs) targeted by Osa-miR396 regulates diverse biological processes. It has been shown that manipulating the Osa-miR396-OsGRFs module substantially increases grain size and yield. Specifically, mutation of the OsGRF4 (GS2) perturbs the function of Osa-miR396 and its regulatory role over OsGRF4 [79, 87, 108]. Additionally, Osa-miR396 promotes panicle branching by regulating OsGRF6 [80].
Several immune-responsive miRNAs play a critical role in regulating cellular processes that determine nutrient uptake as well as responses to other types of abiotic stresses. For example, the Osa-miR164, which is important in processes that control grain yield and other plant architecture traits in rice, has been shown to affect drought tolerance [96, 97]. The Osa-miR166 is involved in the regulation of cellular processes determining nutrient and ion uptake, drought tolerance, and cadmium tolerance [99, 100, 127]. The pathogen–responsive Osa-miR169 has been shown to regulate nitrogen uptake and salinity tolerance in rice [43, 104]. The Osa-miR319 regulates leaf morphogenesis, plant height, and improves cold tolerance [105–107]. The Osa-miR398 not only modulates panicle length, grain number, and grain size but also regulates various abiotic stresses [88, 113]. The Osa-miR444 modulates MADS-box transcription factors to regulate tillering and nitrate signaling [114–116]. The RSV-responsive Osa-miR528 regulates tolerance to arsenite and low temperature in rice [117–120].
Manipulation of miRNAs as a novel approach for the development of high-yielding disease and insect-resistant rice cultivars
Knock-out mutation of miRNAs is an important approach to validating cellular functions through the identification of their downstream target genes. Furthermore, miRNAs belong to conserved families comprised of multiple members with potentially redundant functions. Therefore, loss-of-function analysis of miRNA-encoding loci has become a major challenge. Traditional methods of mutagenesis (chemical/radiation/T-DNA insertion) are largely ineffective due to the small size of miRNA molecules. Currently, target mimics (TMs), short tandem target mimics (STTMs), molecular sponges (SPs), and artificial miRNAs (amiRNAs) are the most commonly used techniques for loss-of-function analysis of miRNAs. Among them, the miRNA decoy techniques such as TMs and STTMs have been widely adopted for functional analysis [128]. TMs and STTMs efficiently suppress the endogenous activity of highly abundant miRNA molecules and nullify the suppression effects to the target gene. This in turn changes the phenotype through the accumulation of target transcripts. However, neither of these approaches provides the most efficient silencing effects to the endogenous activity of the cognate miRNA genes.
Genome editing technologies such as zinc finger nuclease (ZFN), transcription activator-like effector nucleases (TALEN), and clustered regularly interspaced short palindromic repeats (CRISPR)-based system are the more recent cutting-edge approaches for the control of endogenous miRNA abundance [129]. These strategies work based on the principle of binding an exonuclease to a target region in the genome, creating a double-stranded break (DSB). To stabilize the genome, these breaks are repaired by one of two methods: Non-Homologous End-Joining (NHEJ) and Homology Directed Repair (HDR), which causes insertions and deletions (indels) or incorporation of a larger sequence at the repair site. When these changes occur within the miRNA coding region, they can reduce the rate of miRNA biogenesis leading to incomplete loss of function or production of new miRNAs. In contrast, large deletions can cause a complete loss of function of specific miRNAs by producing null mutants. Editing the target gene also hinders miRNA activity due to errors in binding.
Zinc-finger nucleases (ZFNs) are artificial restriction enzymes generated by fusing zinc-finger-based DNA-recognition modules with the DNA-cleavage domain. Each zinc finger typically recognizes and binds to a nucleotide triplet, and fingers are often assembled into groups to bind to specific DNA sequences. TALENS is equipped with the same tenet as ZFN with a target specificity from the protein-DNA association. In addition, it can recognize a single nucleotide using specific amino acid repeats to recognize a single nucleotide, thus providing more choices for selecting the target locus. CRISPR-mediated genome editing has revolutionized nuclease targeting [130–133]. In the CRISPR-Cas system, a single guide RNA (sgRNA) or double guide-RNA (dgRNAs) recognizes the target sequence, and the double-strand break caused by Cas9 (CRISPR associated protein 9) or Cpf1(CRISPR from Prevotella and Francisella 1) nuclease, induces insertion or deletion through the process of DNA repair. The CRISPR-based system has higher efficacy and efficiency than the other methods.
Recently, CRISPR/Cas9 was efficiently used in rice to introduce heritable mutations in mature miRNAs [134–136]. Knockout mutations in Osa-miR396e and Osa-miR396f using the CRISPR/Cas9 system have been shown to create genetic gains through the enhancing effects to yield component traits such as grain size and panicle branching, particularly under nitrogen deficit conditions [81]. CRISPR/Cas9-mediated mutagenesis of Osa-miR396ef has been shown to promote the efficiency of GA signaling, with concomitant enhancement in grain yield contributed by improvements in grain size, leaf blade and sheath anatomy and morphology [112]. Targeted mutagenesis of a single miRNA locus (e.g., Osa-miR408, Osa-miR528) or entire miRNA gene families (e.g., Osa-miR815, Osa-miR820) has been achieved by a CRISPR/Cas9 mediated strategy in rice. It has been shown that under salinity stress, larger deletion in Osa-miR528 led to elevated transcript levels from the target genes [134]. Similarly, targeted mutagenesis of OsSPL9 using CRISPR)/Cas9 led to a significant reduction in Osa-miR528 expression, suggesting a critical role of OsSPL9 in transcriptional regulation of Osa-miR528 [119].
Furthermore, a series of CRISPR/Cas9-mediated mutations in Osa-miR156 have been investigated for their effects on seed dormancy [84]. CRISPR/Cas9 system has also been employed to introduce mutations in multiple superoxide dismutase (SOD) genes to examine their effects on Osa-miR398-mediated resistance to M. oryzae [33]. Targeted mutations were introduced in the target gene (OsARF12) of Osa-miR167d using the CRISPR/Cas9 method to investigate Osa-miR167-ARF12 interaction in immunity against rice blast disease [29].
Conclusion and future directions
Continuously emerging evidence from various experimental systems and approaches established the essentiality of miRNA-mediated regulatory mechanisms in integrating defense-related responses to fungal, bacterial, and viral pathogens, and insect herbivores, with growth and development-related responses. MicroRNA regulatory mechanisms have direct roles in host plant immunity by directly suppressing target genes with either negative or positive effects, or by indirectly inducing signaling pathways with different classes of phytohormones and other small molecules such as ROS. Genomics-enabled biology offers an excellent opportunity to identify more miRNAs involved in fine-tuning resistance to biotic stresses to minimize trade-offs to productivity. Harnessing these miRNAs could be useful in breeding disease and pest-resistant rice cultivars without the drags to the agronomic potential that are typically observed when manipulating R-genes alone.
Identifying new miRNAs associated with defense mechanisms against pests and diseases could be achieved by employing high-throughput sequencing technologies. Specifically, this approach involves the identification of differentially expressed miRNAs under stress via miRNA-Seq followed by in silico analysis and degradome sequencing to predict miRNA targets. Once the specific roles of the miRNAs and target genes are identified, it would be possible to engineer them through genome-editing technologies. Site-specific mutagenesis of target genes impairs the cleavage by miRNA, which allows the development of transgenic plants with disease and insect resistance. In addition to functional analysis, miRNAs are usually conserved in nature, hence limited sequence diversity. The miRNA binds to their target genes in a sequence-specific manner. Variation in miRNAs leads to a variable level of transcript accumulation that causes compounded phenotypes.
Acknowledgments
SNM acknowledges the Indian Council of Agricultural Research (ICAR), New Delhi, India for the Netaji Subhash Chandra Bose International Doctoral Fellowship. We also thank Jacobo Sanchez for his assistance in editing the manuscript.
Abbreviations
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Damage-associated molecular patterns
- PRRs
Pattern-recognition receptors
- PTI
PAMP-triggered immunity
- ETS
Effector-triggered susceptibility
- ETI
Effector-triggered immunity
- HR
Hypersensitive response
- DCL1
DICER-like 1
- ROS
Reactive oxygen species
- STTM
Short tandem target mimic
- ARF
Auxin response factor
- IAA
Indole-3-acetic acid
- EIN2
Ethylene insensitive 2
- SOD
Superoxide dismutase
- Nramp 6
Natural resistance-associated macrophage protein 6
- SPL
SQUAMOSA promoter-binding protein-like transcription factor
- NF-YA
Nuclear factor Y-A
- DHFR-TS
Dihydrofolate reductase/thymidylate synthase
- HPODE
Hydroperoxy-octadecadienoic acid
- GRFs
Growth-regulating factors
- TCP
TEOSINTE BRANCHED, cycloidea and PCF
- ZFN
Zinc finger nuclease
- TALEN
Transcription activator-like effector nucleases
- CRISPR
Clustered regularly interspaced short palindromic repeats
- NHEJ
Non-homologous end-joining
- HDR
Homology-directed repair
- R-genes
Resistance genes
- AGO1
Argonaute 1
- SGS3
Suppressor of gene silencing 3
- RDR6
RNA-dependent RNA polymerase 6
- RSV
Rice stripe virus
- RRSV
Rice ragged stunt virus
Authors’ contributions
BGDR and KN conceived the idea and content of this manuscript. KK, SNM drafted the manuscript. KN reviewed and proofread the manuscript. BGDR wrote, reviewed the manuscript and provided the intellectual direction for improving the manuscript. All the authors read and approved this manuscript.
Funding
Not applicable.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kishor Kumar and Swarupa Nanda Mandal contributed equally to this work.
Contributor Information
Kishor Kumar, Email: kishor.kumar@gm.rkmvu.ac.in.
Swarupa Nanda Mandal, Email: swarupa.mandal@ttu.edu.
Kumari Neelam, Email: kneelam@pau.edu.
Benildo G. de los Reyes, Email: benildo.reyes@ttu.edu
References
- 1.Gao C. Genome engineering for crop improvement and future agriculture. Cell. 2021;184(6):1621–1635. doi: 10.1016/j.cell.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3(3):430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 3.Dangl JL, Horvath DM, Staskawicz BJ. Pivoting the plant immune system from dissection to deployment. Science. 2013;341(6147):746–751. doi: 10.1126/science.1236011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JD, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354(6316):aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- 5.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 6.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11(8):539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Zhou JM. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3(5):783–793. doi: 10.1093/mp/ssq035. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35(7):345–351. doi: 10.1016/j.it.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Hatsugai N, Igarashi D, Mase K, Lu Y, Tsuda Y, Chakravarthy S, Wei HL, Foley JW, Collmer A, Glazebrook J, et al. A plant effector-triggered immunity signaling sector is inhibited by pattern-triggered immunity. EMBO J. 2017;36(18):2758–2769. doi: 10.15252/embj.201796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol. 2010;13(4):459–465. doi: 10.1016/j.pbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Huot B, Yao J, Montgomery BL, He SY. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant. 2014;7(8):1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson R, Wiesner-Hanks T, Wisser R, Balint-Kurti P. Navigating complexity to breed disease-resistant crops. Nat Rev Genet. 2018;19(1):21–33. doi: 10.1038/nrg.2017.82. [DOI] [PubMed] [Google Scholar]
- 13.Ning Y, Liu W, Wang GL. Balancing immunity and yield in crop plants. Trends Plant Sci. 2017;22(12):1069–1079. doi: 10.1016/j.tplants.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar-Agarwal S, Jin H. Role of small RNAs in host-microbe interactions. Annu Rev Phytopathol. 2010;48:225–246. doi: 10.1146/annurev-phyto-073009-114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khraiwesh B, Zhu JK, Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta. 2012;1819(2):137–148. doi: 10.1016/j.bbagrm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J, Chu C. MicroRNAs in crop improvement: fine-tuners for complex traits. Nat Plants. 2017;3:17077. doi: 10.1038/nplants.2017.77. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through dicer-like 1 protein functions. Proc Natl Acad Sci U S A. 2004;101(34):12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A. 2005;102(10):3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136(4):669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Basso MF, Ferreira PCG, Kobayashi AK, Harmon FG, Nepomuceno AL, Molinari HBC, Grossi-de-Sa MF. MicroRNAs and new biotechnological tools for its modulation and improving stress tolerance in plants. Plant Biotechnol J. 2019;17(8):1482–1500. doi: 10.1111/pbi.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Li Y, Zheng YP, Wang H, Yang X, Chen JF, Zhou SX, Wang LF, Li XP, Ma XC, et al. Expressing a target mimic of miR156fhl-3p enhances Rice blast disease resistance without yield penalty by improving SPL14 expression. Front Genet. 2020;11:327. doi: 10.3389/fgene.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JF, Zhao ZX, Li Y, Li TT, Zhu Y, Yang XM, Zhou SX, Wang H, Zhao JQ, Pu M, et al. Fine-tuning roles of Osa-miR159a in Rice immunity against Magnaporthe oryzae and development. Rice (N Y) 2021;14(1):26. doi: 10.1186/s12284-021-00469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Lu YG, Shi Y, Wu L, Xu YJ, Huang F, Guo XY, Zhang Y, Fan J, Zhao JQ, et al. Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol. 2014;164(2):1077–1092. doi: 10.1104/pp.113.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XP, Ma XC, Wang H, Zhu Y, Liu XX, Li TT, Zheng YP, Zhao JQ, Zhang JW, Huang YY, et al. Osa-miR162a fine-tunes rice resistance to Magnaporthe oryzae and yield. Rice (N Y) 2020;13(1):38. doi: 10.1186/s12284-020-00396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Xia Y, Lin S, Wang Y, Guo B, Song X, et al. Osa-miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Plant J. 2018;95:584-597. [DOI] [PubMed]
- 28.Salvador-Guirao R, Hsing YI, San Segundo B. The Polycistronic miR166k-166h positively regulates Rice immunity via post-transcriptional control of EIN2. Front Plant Sci. 2018;9:337. doi: 10.3389/fpls.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao ZX, Feng Q, Cao XL, Zhu Y, Wang H, Chandran V, Fan J, Zhao JQ, Pu M, Li Y, et al. Osa-miR167d facilitates infection of Magnaporthe oryzae in rice. J Integr Plant Biol. 2020;62(5):702–715. doi: 10.1111/jipb.12816. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Zhao SL, Li JL, Hu XH, Wang H, Cao XL, Xu YJ, Zhao ZX, Xiao ZY, Yang N, et al. Osa-miR169 negatively regulates Rice immunity against the blast fungus Magnaporthe oryzae. Front Plant Sci. 2017;8:2. doi: 10.3389/fpls.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Bao Y, Shan D, Wang Z, Song X, Wang Z, Wang J, He L, Wu L, Zhang Z, et al. Magnaporthe oryzae induces the expression of a MicroRNA to suppress the immune response in Rice. Plant Physiol. 2018;177(1):352–368. doi: 10.1104/pp.17.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandran V, Wang H, Gao F, Cao XL, Chen YP, Li GB, Zhu Y, Yang XM, Zhang LL, Zhao ZX, et al. miR396-OsGRFs module balances growth and rice blast disease resistance. Front Plant Sci. 1999;2018:9. doi: 10.3389/fpls.2018.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Cao XL, Zhu Y, Yang XM, Zhang KN, Xiao ZY, Wang H, Zhao JH, Zhang LL, Li GB, et al. Osa-miR398b boosts H2 O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019;222(3):1507–1522. doi: 10.1111/nph.15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junhua L, Xuemei Y, Jinfeng C, Tingting L, Zijin H, Ying X, Jinlu L, Jiqun Z, Mei P, Hui F. Osa-miR439 negatively regulates Rice immunity against Magnaporthe oryzae. Rice Sci. 2021;28(2):156–165. doi: 10.1016/j.rsci.2021.01.005. [DOI] [Google Scholar]
- 35.Xiao ZY, Wang QX, He W, Li JL, Zhao SL, Fan J, Li Y, Wang WM. MiR444b.2 regulates resistance to Magnaporthe oryzae and tillering in rice. Acta Phytopathol Sin. 2017;47(4):511–522. [Google Scholar]
- 36.Li Y, Wang LF, Bhutto SH, He XR, Yang XM, Zhou XH, Lin XY, Rajput AA, Li GB, Zhao JH, et al. Blocking miR530 improves Rice resistance, yield, and maturity. Front Plant Sci. 2021;12:729560. doi: 10.3389/fpls.2021.729560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zheng YP, Zhou XH, Yang XM, He XR, Feng Q, Zhu Y, Li GB, Wang H, Zhao JH, et al. Rice miR1432 fine-Tunes the balance of yield and blast disease resistance via different modules. Rice (N Y) 2021;14(1):87. doi: 10.1186/s12284-021-00529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Li TT, He XR, Zhu Y, Feng Q, Yang XM, Zhou XH, Li GB, Ji YP, Zhao JH, et al. Blocking Osa-miR1871 enhances rice resistance against Magnaporthe oryzae and yield. Plant Biotechnol J. 2022;20(4):646–659. doi: 10.1111/pbi.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou SX, Zhu Y, Wang LF, Zheng YP, Chen JF, Li TT, Yang XM, Wang H, Li XP, Ma XC, et al. Osa-miR1873 fine-tunes rice immunity against Magnaporthe oryzae and yield traits. J Integr Plant Biol. 2020;62(8):1213–1226. doi: 10.1111/jipb.12900. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Sanuy F, Peris-Peris C, Tomiyama S, Okada K, Hsing YI, San Segundo B, Campo S. Osa-miR7695 enhances transcriptional priming in defense responses against the rice blast fungus. BMC Plant Biol. 2019;19(1):563. doi: 10.1186/s12870-019-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Shi Z, Zhang X, Wang M, Zhang L, Zheng K, Liu J, Hu X, Di C, Qian Q, et al. Inducible overexpression of ideal plant Architecture1 improves both yield and disease resistance in rice. Nat Plants. 2019;5(4):389–400. doi: 10.1038/s41477-019-0383-2. [DOI] [PubMed] [Google Scholar]
- 42.Jia Y, Li C, Li Q, Liu P, Liu D, Liu Z, Wang Y, Jiang G, Zhai W. Characteristic dissection of Xanthomonas oryzae pv. Oryzae responsive MicroRNAs in Rice. Int J Mol Sci. 2020;21(3):785. doi: 10.3390/ijms21030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C, Chen Y, Cao Y, Chen H, Wang J, Bi YM, Tian F, Yang F, Rothstein SJ, Zhou X, et al. Overexpression of miR169o, an overlapping MicroRNA in response to both nitrogen limitation and bacterial infection, promotes nitrogen use efficiency and susceptibility to bacterial blight in Rice. Plant Cell Physiol. 2018;59(6):1234–1247. doi: 10.1093/pcp/pcy060. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Jia Y, Liu F, Wang F, Fan F, Wang J, Zhu J, Xu Y, Zhong W, Yang J. Integration analysis of small RNA and Degradome sequencing reveals MicroRNAs responsive to Dickeya zeae in resistant Rice. Int J Mol Sci. 2019;20(1):222. doi: 10.3390/ijms20010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Yang Z, Wang Y, Zheng L, Ye R, Ji Y, Zhao S, Ji S, Liu R, Xu L, et al. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. Elife. 2015;4:e05733. doi: 10.7554/eLife.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong A, Yuan Q, Wang S, Peng J, Lu Y, Zheng H, Lin L, Chen H, Gong Y, Chen J, et al. Altered accumulation of Osa-miR171b contributes to rice stripe virus infection by regulating disease symptoms. J Exp Bot. 2017;68(15):4357–4367. doi: 10.1093/jxb/erx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Ding Z, Wu K, Yang L, Li Y, Yang Z, Shi S, Liu X, Zhao S, Yang Z, et al. Suppression of Jasmonic acid-mediated defense by viral-inducible MicroRNA319 facilitates virus infection in Rice. Mol Plant. 2016;9(9):1302–1314. doi: 10.1016/j.molp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Jiao X, Kong X, Hamera S, Wu Y, Chen X, Fang R, Yan Y. A signaling Cascade from miR444 to RDR1 in Rice antiviral RNA silencing pathway. Plant Physiol. 2016;170(4):2365–2377. doi: 10.1104/pp.15.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Yang R, Yang Z, Yao S, Zhao S, Wang Y, Li P, Song X, Jin L, Zhou T, et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants. 2017;3:16203. doi: 10.1038/nplants.2016.203. [DOI] [PubMed] [Google Scholar]
- 50.Yao S, Yang Z, Yang R, Huang Y, Guo G, Kong X, Lan Y, Zhou T, Wang H, Wang W, et al. Transcriptional regulation of miR528 by OsSPL9 orchestrates antiviral response in rice. Mol Plant. 2019;12(8):1114–1122. doi: 10.1016/j.molp.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Ge Y, Han J, Zhou G, Xu Y, Ding Y, Shi M, Guo C, Wu G. Silencing of miR156 confers enhanced resistance to brown planthopper in rice. Planta. 2018;248(4):813–826. doi: 10.1007/s00425-018-2942-6. [DOI] [PubMed] [Google Scholar]
- 52.Dai Z, Tan J, Zhou C, Yang X, Yang F, Zhang S, Sun S, Miao X, Shi Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa) Plant Biotechnol J. 2019;17(8):1657–1669. doi: 10.1111/pbi.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Ronald PC. Innate immunity in rice. Trends Plant Sci. 2011;16(8):451–459. doi: 10.1016/j.tplants.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, Li JF, Ao Y, Qu J, Li Z, Su J, Zhang Y, Liu J, Feng D, Qi K, et al. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell. 2012;24(8):3406–3419. doi: 10.1105/tpc.112.102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010;64(2):204–214. doi: 10.1111/j.1365-313X.2010.04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312(5772):436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Zhang Q, Zhang J, Wu L, Qi Y, Zhou JM. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010;152(4):2222–2231. doi: 10.1104/pp.109.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Lian H, Zhao Q, He Y. MicroRNA166 monitors SPOROCYTELESS/NOZZLE for building of the anther internal boundary. Plant Physiol. 2019;181(1):208–220. doi: 10.1104/pp.19.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu H, Hu F, Wang R, Zhou X, Sze SH, Liou LW, Barefoot A, Dickman M, Zhang X. Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell. 2011;145(2):242–256. doi: 10.1016/j.cell.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campo S, Peris-Peris C, Sire C, Moreno AB, Donaire L, Zytnicki M, Notredame C, Llave C, San Segundo B. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol. 2013;199(1):212–227. doi: 10.1111/nph.12292. [DOI] [PubMed] [Google Scholar]
- 61.Yao J, Withers J, He SY. Pseudomonas syringae infection assays in Arabidopsis. Methods Mol Biol. 2013;1011:63–81. doi: 10.1007/978-1-62703-414-2_6. [DOI] [PubMed] [Google Scholar]
- 62.Mele G, Ori N, Sato Y, Hake S. The knotted1-like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes Dev. 2003;17(17):2088–2093. doi: 10.1101/gad.1120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130(3):413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 65.Csorba T, Kontra L, Burgyan J. Viral silencing suppressors: tools forged to fine-tune host-pathogen coexistence. Virology. 2015;479-480:85–103. doi: 10.1016/j.virol.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 66.Wu Y, Lv W, Hu L, Rao W, Zeng Y, Zhu L, He Y, He G. Identification and analysis of brown planthopper-responsive microRNAs in resistant and susceptible rice plants. Sci Rep. 2017;7(1):8712. doi: 10.1038/s41598-017-09143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan J, Wu Y, Guo J, Li H, Zhu L, Chen R, He G, Du B. A combined microRNA and transcriptome analyses illuminates the resistance response of rice against brown planthopper. BMC Genomics. 2020;21(1):144. doi: 10.1186/s12864-020-6556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C, Kulkarni K, Souret FF, MuthuValliappan R, Tej SS, Poethig RS, Henderson IR, Jacobsen SE, Wang W, Green PJ, et al. MicroRNAs and other small RNAs enriched in the Arabidopsis RNA-dependent RNA polymerase-2 mutant. Genome Res. 2006;16(10):1276–1288. doi: 10.1101/gr.5530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B. MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci U S A. 2012;109(5):1790–1795. doi: 10.1073/pnas.1118282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell. 2012;24(3):859–874. doi: 10.1105/tpc.111.095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui C, Wang JJ, Zhao JH, Fang YY, He XF, Guo HS, Duan CG. A Brassica miRNA regulates plant growth and immunity through distinct modes of action. Mol Plant. 2020;13(2):231–245. doi: 10.1016/j.molp.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Jiang G, Liu D, Yin D, Zhou Z, Shi Y, Li C, Zhu L, Zhai W. A Rice NBS-ARC gene conferring quantitative resistance to bacterial blight is regulated by a pathogen effector-inducible miRNA. Mol Plant. 2020;13(12):1752–1767. doi: 10.1016/j.molp.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Galili G. Tuning the orchestra: miRNAs in plant immunity. Trends Plant Sci. 2019;24(3):189–191. doi: 10.1016/j.tplants.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Fei Q, Li P, Teng C, Meyers BC. Secondary siRNAs from Medicago NB-LRRs modulated via miRNA-target interactions and their abundances. Plant J. 2015;83(3):451–465. doi: 10.1111/tpj.12900. [DOI] [PubMed] [Google Scholar]
- 75.Källman T, Chen J, Gyllenstrand N, Lagercrantz U. A significant fraction of 21-nucleotide small RNA originates from phased degradation of resistance genes in several perennial species. Plant Physiol. 2013;162(2):741–754. doi: 10.1104/pp.113.214643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boccara M, Sarazin A, Thiebeauld O, Jay F, Voinnet O, Navarro L, Colot V. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 2014;10(1):e1003883. doi: 10.1371/journal.ppat.1003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fei Q, Xia R, Meyers BC. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell. 2013;25(7):2400–2415. doi: 10.1105/tpc.113.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, Li Y, Gonzalez AJ, Yan Z, Kitto SL, Grusak MA, et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011;25(23):2540–2553. doi: 10.1101/gad.177527.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan P, Ni S, Wang J, Zhang B, Xu R, Wang Y, Chen H, Zhu X, Li Y. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants. 2015;2:15203. doi: 10.1038/nplants.2015.203. [DOI] [PubMed] [Google Scholar]
- 80.Gao F, Wang K, Liu Y, Chen Y, Chen P, Shi Z, Luo J, Jiang D, Fan F, Zhu Y, et al. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat Plants. 2015;2:15196. doi: 10.1038/nplants.2015.196. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Zhou Z, Bai J, Tao X, Wang L, Zhang H, Zhu J-K. Disruption of MIR396e and MIR396f improves rice yield under nitrogen-deficient conditions. Natl Sci Rev. 2020;7(1):102–112. doi: 10.1093/nsr/nwz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet. 2010;42(6):541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 83.Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet. 2010;42(6):545–549. doi: 10.1038/ng.592. [DOI] [PubMed] [Google Scholar]
- 84.Miao C, Wang Z, Zhang L, Yao J, Hua K, Liu X, Shi H, Zhu JK. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat Commun. 2019;10(1):3822. doi: 10.1038/s41467-019-11830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C, et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet. 2015;47(8):949–954. doi: 10.1038/ng.3352. [DOI] [PubMed] [Google Scholar]
- 86.Dai Z, Wang J, Yang X, Lu H, Miao X, Shi Z. Modulation of plant architecture by the miR156f-OsSPL7-OsGH3.8 pathway in rice. J Exp Bot. 2018;69(21):5117–5130. doi: 10.1093/jxb/ery273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Che R, Tong H, Shi B, Liu Y, Fang S, Liu D, Xiao Y, Hu B, Liu L, Wang H, et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat Plants. 2015;2:15195. doi: 10.1038/nplants.2015.195. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Zhang J, Yan J, Gou F, Mao Y, Tang G, Botella J, Zhu J. Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proc Natl Acad Sci U S A. 2017;114(20):5277–5282. doi: 10.1073/pnas.1703752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao J, Chen H, Yang H, He Y, Tian Z, Li J. A brassinosteroid responsive miRNA-target module regulates gibberellin biosynthesis and plant development. New Phytol. 2018;220(2):488–501. doi: 10.1111/nph.15331. [DOI] [PubMed] [Google Scholar]
- 90.Tsuji H, Aya K, Ueguchi-Tanaka M, Shimada Y, Nakazono M, Watanabe R, Nishizawa NK, Gomi K, Shimada A, Kitano H, et al. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 2006;47(3):427–444. doi: 10.1111/j.1365-313X.2006.02795.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Y, Wen H, Teotia S, Du Y, Zhang J, Li J, Sun H, Tang G, Peng T, Zhao Q. Suppression of microRNA159 impacts multiple agronomic traits in rice (Oryza sativa L.) BMC Plant Biol. 2017;17(1):215. doi: 10.1186/s12870-017-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J, Li Z, Zhao D. Deregulation of the OsmiR160 target gene OsARF18 causes growth and developmental defects with an alteration of auxin signaling in Rice. Sci Rep. 2016;6:29938. doi: 10.1038/srep29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M, Wu H-J, Fang J, Chu C, Wang X-J. A long noncoding RNA involved in rice reproductive development by negatively regulating Osa-miR160. Science Bulletin. 2017;62(7):470–475. doi: 10.1016/j.scib.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Liu B, Li P, Li X, Liu C, Cao S, Chu C, Cao X. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol. 2005;139(1):296–305. doi: 10.1104/pp.105.063420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian C, Zuo Z, Qiu JL. Identification and characterization of ABA-responsive MicroRNAs in Rice. J Genet Genomics. 2015;42(7):393–402. doi: 10.1016/j.jgg.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 96.Fang Y, Xie K, Xiong L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J Exp Bot. 2014;65(8):2119–2135. doi: 10.1093/jxb/eru072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang D, Chen W, Dong J, Li J, Yang F, Wu Z, Zhou H, Wang W, Zhuang C. Overexpression of miR164b-resistant OsNAC2 improves plant architecture and grain yield in rice. J Exp Bot. 2018;69(7):1533–1543. doi: 10.1093/jxb/ery017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang D, Zhou L, Chen W, Ye N, Xia J, Zhuang C. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in Rice via ABA-mediated pathways. Rice (N Y) 2019;12(1):76. doi: 10.1186/s12284-019-0334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding Y, Gong S, Wang Y, Wang F, Bao H, Sun J, Cai C, Yi K, Chen Z, Zhu C. MicroRNA166 modulates cadmium tolerance and accumulation in Rice. Plant Physiol. 2018;177(4):1691–1703. doi: 10.1104/pp.18.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J, Zhang H, Srivastava A, Pan Y, Bai J, Fang J, Shi H, Zhu J. Knockdown of Rice MicroRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018;176(3):2082–2094. doi: 10.1104/pp.17.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu H, Jia S, Shen D, Liu J, Li J, Zhao H, Han S, Wang Y. Four AUXIN RESPONSE FACTOR genes downregulated by microRNA167 are associated with growth and development in Oryza sativa. Funct Plant Biol. 2012;39(9):736–744. doi: 10.1071/FP12106. [DOI] [PubMed] [Google Scholar]
- 102.Yang JH, Han SJ, Yoon EK, Lee WS. Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells. Nucleic Acids Res. 2006;34(6):1892–1899. doi: 10.1093/nar/gkl118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng T, Qiao M, Liu H, Teotia S, Zhang Z, Zhao Y, Wang B, Zhao D, Shi L, Zhang C, et al. A resource for inactivation of MicroRNAs using short tandem target mimic technology in model and crop plants. Mol Plant. 2018;11(11):1400–1417. doi: 10.1016/j.molp.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Zhao B, Ge L, Liang R, Li W, Ruan K, Lin H, Jin Y. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol Biol. 2009;10:29. doi: 10.1186/1471-2199-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu WT, Chen PW, Chen LC, Yang CC, Chen SY, Huang G, Lin TC, Ku HM, Chen JJW. Suppressive effect of microRNA319 expression on rice plant height. Theor Appl Genet. 2017;130(7):1507–1518. doi: 10.1007/s00122-017-2905-5. [DOI] [PubMed] [Google Scholar]
- 106.Wang ST, Sun XL, Hoshino Y, Yu Y, Jia B, Sun ZW, Sun MZ, Duan XB, Zhu YM. MicroRNA319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice (Oryza sativa L.) PLoS One. 2014;9(3):e91357. doi: 10.1371/journal.pone.0091357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang C, Li D, Mao D, Liu X, Ji C, Li X, Zhao X, Cheng Z, Chen C, Zhu L. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.) Plant Cell Environ. 2013;36(12):2207–2218. doi: 10.1111/pce.12130. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Gao F, Xie K, Zeng X, Cao Y, Zeng J, He Z, Ren Y, Li W, Deng Q, et al. The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol J. 2016;14(11):2134–2146. doi: 10.1111/pbi.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao P, Bai X, Yang L, Lv D, Li Y, Cai H, Ji W, Guo D, Zhu Y. Over-expression of Osa-MIR396c decreases salt and alkali stress tolerance. Planta. 2010;231(5):991–1001. doi: 10.1007/s00425-010-1104-2. [DOI] [PubMed] [Google Scholar]
- 110.Liu H, Guo S, Xu Y, Li C, Zhang Z, Zhang D, Xu S, Zhang C, Chong K. OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol. 2014;165(1):160–174. doi: 10.1104/pp.114.235564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu Y, Feng Z, Meng Y, Bian L, Xie H, Mysore KS, Liang J. SLENDER RICE1 and Oryza sativa INDETERMINATE DOMAIN2 regulating OsmiR396 are involved in stem elongation. Plant Physiol. 2020;182(4):2213–2227. doi: 10.1104/pp.19.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miao C, Wang D, He R, Liu S, Zhu JK. Mutations in MIR396e and MIR396f increase grain size and modulate shoot architecture in rice. Plant Biotechnol J. 2020;18(2):491–501. doi: 10.1111/pbi.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu Y, Feng Z, Bian L, Xie H, Liang J. miR398 regulation in rice of the responses to abiotic and biotic stresses depends on CSD1 and CSD2 expression. Funct Plant Biol. 2010;38(1):44–53. doi: 10.1071/FP10178. [DOI] [PubMed] [Google Scholar]
- 114.Jiao X, Wang H, Yan J, Kong X, Liu Y, Chu J, Chen X, Fang R, Yan Y. Promotion of BR biosynthesis by miR444 is required for ammonium-triggered inhibition of root growth. Plant Physiol. 2020;182(3):1454–1466. doi: 10.1104/pp.19.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan Y, Wang H, Hamera S, Chen X, Fang R. miR444a has multiple functions in the rice nitrate-signaling pathway. Plant J. 2014;78(1):44–55. doi: 10.1111/tpj.12446. [DOI] [PubMed] [Google Scholar]
- 116.Guo S, Xu Y, Liu H, Mao Z, Zhang C, Ma Y, Zhang Q, Meng Z, Chong K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat Commun. 2013;4:1566. doi: 10.1038/ncomms2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Q, Hu H, Zhu L, Li R, Feng Y, Zhang L, Yang Y, Liu X, Zhang H. Involvement of miR528 in the regulation of Arsenite tolerance in Rice (Oryza sativa L.) J Agric Food Chem. 2015;63(40):8849–8861. doi: 10.1021/acs.jafc.5b04191. [DOI] [PubMed] [Google Scholar]
- 118.Tang W, Thompson WA. OsmiR528 enhances cold stress tolerance by repressing expression of stress response-related transcription factor genes in plant cells. Curr Genomics. 2019;20(2):100–114. doi: 10.2174/1389202920666190129145439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang R, Li P, Mei H, Wang D, Sun J, Yang C, Hao L, Cao S, Chu C, Hu S, et al. Fine-tuning of MiR528 accumulation modulates flowering time in Rice. Mol Plant. 2019;12(8):1103–1113. doi: 10.1016/j.molp.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, He RR, Lian JP, Zhou YF, Zhang F, Li QF, Yu Y, Feng YZ, Yang YW, Lei MQ, et al. OsmiR528 regulates rice-pollen intine formation by targeting an uclacyanin to influence flavonoid metabolism. Proc Natl Acad Sci U S A. 2020;117(1):727–732. doi: 10.1073/pnas.1810968117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inoue H, Hayashi N, Matsushita A, Xinqiong L, Nakayama A, Sugano S, Jiang CJ, Takatsuji H. Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction. Proc Natl Acad Sci U S A. 2013;110(23):9577–9582. doi: 10.1073/pnas.1222155110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Si L, Chen J, Huang X, Gong H, Luo J, Hou Q, Zhou T, Lu T, Zhu J, Shangguan Y, et al. OsSPL13 controls grain size in cultivated rice. Nat Genet. 2016;48(4):447–456. doi: 10.1038/ng.3518. [DOI] [PubMed] [Google Scholar]
- 123.Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q, et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44(8):950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- 124.Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet. 2009;41(4):494–497. doi: 10.1038/ng.352. [DOI] [PubMed] [Google Scholar]
- 125.Yuan H, Qin P, Hu L, Zhan S, Wang S, Gao P, Li J, Jin M, Xu Z, Gao Q, et al. OsSPL18 controls grain weight and grain number in rice. J Genet Genomics. 2019;46(1):41–51. doi: 10.1016/j.jgg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 126.Yuan S, Li Z, Li D, Yuan N, Hu Q, Luo H. Constitutive expression of Rice MicroRNA528 alters plant development and enhances tolerance to salinity stress and nitrogen starvation in creeping Bentgrass. Plant Physiol. 2015;169(1):576–593. doi: 10.1104/pp.15.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iwamoto M, Tagiri A. MicroRNA-targeted transcription factor gene RDD1 promotes nutrient ion uptake and accumulation in rice. Plant J. 2016;85(4):466–477. doi: 10.1111/tpj.13117. [DOI] [PubMed] [Google Scholar]
- 128.Chen J, Teotia S, Lan T, Tang G. MicroRNA techniques: valuable tools for agronomic trait analyses and breeding in Rice. Front Plant Sci. 2021;12:744357. doi: 10.3389/fpls.2021.744357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abbas A, Shah AN, Tanveer M, Ahmed W, Shah AA, Fiaz S, et al. MiRNA fine tuning for crop improvement: using advance computational models and biotechnological tools. Mol Biol Rep. 2022;49:5437-5450. [DOI] [PubMed]
- 130.Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, Cao F, Zhu S, Zhang F, Mao Y, et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23(10):1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23(R1):R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 133.Tang X, Lowder LG, Zhang T, Malzahn AA, Zheng X, Voytas DF, Zhong Z, Chen Y, Ren Q, Li Q, et al. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants. 2017;3:17018. doi: 10.1038/nplants.2017.18. [DOI] [PubMed] [Google Scholar]
- 134.Zhou J, Deng K, Cheng Y, Zhong Z, Tian L, Tang X, Tang A, Zheng X, Zhang T, Qi Y, et al. CRISPR-Cas9 based genome editing reveals new insights into MicroRNA function and regulation in Rice. Front Plant Sci. 2017;8:1598. doi: 10.3389/fpls.2017.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou J, Zhong Z, Chen H, Li Q, Zheng X, Qi Y, Zhang Y. Knocking out MicroRNA genes in Rice with CRISPR-Cas9. Methods Mol Biol. 2019;1917:109–119. doi: 10.1007/978-1-4939-8991-1_9. [DOI] [PubMed] [Google Scholar]
- 136.Bi H, Fei Q, Li R, Liu B, Xia R, Char SN, Meyers BC, Yang B. Disruption of miRNA sequences by TALENs and CRISPR/Cas9 induces varied lengths of miRNA production. Plant Biotechnol J. 2020;18(7):1526–1536. doi: 10.1111/pbi.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.