Abstract
Introduction
Recent studies have shown the prognostic value of capillary refill time (CRT) and suggested that resuscitation management guided by CRT may reduce morbidity and mortality in patients with septic shock. However, little is known about the current use of CRT in routine clinical practice. This study aimed to assess the modalities of CRT use among French adult and pediatric intensivists.
Methods
A cross-sectional survey exploring CRT practices in acute circulatory failure was performed. The targeted population was French adult and pediatric intensivists (SFAR and GFRUP networks). An individual invitation letter including a survey of 32 questions was emailed twice. Descriptive and analytical statistics were performed.
Results
Among the 6071 physicians who received the letter, 418 (7%) completed the survey. Among all respondents, 82% reported using CRT in routine clinical practice, mainly to diagnose acute circulatory failure, but 45% did not think CRT had any prognostic value. Perfusion goal-directed therapy based on CRT was viewed as likely to improve patient outcome by 37% of respondents. The measurement of CRT was not standardized as the use of a chronometer was rare (3%) and the average of multiple measurements rarely performed (46%). Compared to adult intensivists, pediatric intensivists used CRT more frequently (99% versus 76%) and were more confident in its diagnostic value and its ability to guide treatment.
Conclusion
CRT measurement is widely used by intensivists in patients with acute circulatory failure but most often in a non-standardized way. This may lead to a misunderstanding of CRT reliability and clinical usefulness.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12873-022-00681-x.
Keywords: Capillary refill time, Acute circulatory failure, Survey, Peripheral perfusion, Shock
Introduction
Recent cohort studies have emphasized the prognostic value of capillary refill time (CRT) [1–3] and a recent clinical trial suggested that resuscitation management guided by CRT measurement may reduce morbidity and mortality in patients with septic shock [4, 5]. Subsequently, the clinical benefit has been endorsed by the recent guidelines of the surviving sepsis campaign [6, 7]. When used for research purposes, CRT requires a standardized approach encompassing the number of averaged CRT values, the level of pressure applied to the skin, the duration of skin compression, the localization of the test, and the use of a chronometer. Although CRT has proven to be useful and potentially lifesaving, little is known about its use in real-life conditions and how it is performed in routine clinical practice. It is however likely that CRT is both underused and misused and that healthcare providers do not strictly follow the standard at the bedside, creating a wide variability in practices. Such a sub-optimal use of CRT could lead to a misunderstanding of its reliability and clinical usefulness. To date, no study has focused on assessing the modalities of CRT use in routine clinical practice among adult and pediatric intensivists.
This cross-sectional survey aimed at describing and analyzing the practices in terms of CRT use among French adult and pediatric intensivists, in the setting of acute circulatory failure.
Methods
The networks from the Société Française d’Anesthésie et de Réanimation (SFAR) and the Groupe Francophone de Réanimation et Urgences Pédiatriques (GFRUP) were solicited in September and October 2021 and asked to email, twice, an invitation letter for survey participation at each physician member of the networks, who are mainly adult/pediatric anesthesiologists and/or intensivists. The members of these learned societies are mainly from France, but also from Belgium, Algeria, Tunisia, Morocco, Luxembourg, and Canada. Responses of different physicians from the same institution were allowed. The invitation letter explained the purposes and benefits of the survey and gave a link to the dedicated web page (Survey Monkey, Palo Alto, CA, USA).
The survey included a total of 32 questions, XX of which were multiple choice questions, and is available in Additional files 1 and 2. The entire survey took approximately 10 min to complete. Two authors (CW and MJL) designed the questions and distributed them for comments and modifications within the scientific committee of the study (JLF, ML, FD, MC, RS, FB, EJ). Thereafter, the survey was sent to a test group of 15 physicians to test both its feasibility and overall quality.
The demographic characteristics of the physicians were collected. The reasons why the physicians perform CRT and how they consider and interpret it were explored. The approach used to perform CRT in routine clinical practice was also assessed and the potential leads to improve clinical practice when using CRT were examined.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and continuous variables as mean ± standard deviation. Chi-squared tests were used to perform between-group comparisons. All tests were two-sided, and a p-value < 0.05 was considered statistically significant. The descriptive and analytical statistical analyses were computed using the Free Software Foundation’s CRAN R, R version 4.0.4.
Results
Test feasibility and quality
The 15 physicians who initially answered the survey test reported no issues in terms of feasibility, and the overall quality was rated as good. The comments were used to refine the final version of the survey.
Characteristics of the respondents
Among the 6071 physicians who received and opened the email in September and October, 549 (9%) clicked on the survey link. The survey was then completed by 418 (7%) practitioners. The mean ± SD age of the respondents was 39 ± 10 years and the mean ± SD time of clinical experience and practice was 11 ± 9 years. Overall, 60% of respondents worked in an adult intensive care unit (ICU), 30% in a pediatric ICU, and 7% worked in an adult or pediatric emergency department. The respondents practiced mainly in tertiary teaching hospitals (63%), public hospitals (27%), and private hospitals (9%). Among all respondents, 55% worked in units with 11 to 20 beds, and 28% worked in units with more than 20 beds. Overall, 48% of respondents managed 1 to 5 patients with acute circulatory failure per week (Table 1).
Table 1.
Characteristics of the survey respondents
| Overall | Adult practice | Pediatric practice | p-value | |
|---|---|---|---|---|
| n | 418 | 308 | 110 | |
| Age, years, mean (SD) | 39 (10) | 39 (11) | 40 (8) | 0.421 |
| Clinical practice, years, mean (SD) | 11 (9) | 11 (10) | 12 (8) | 0.577 |
| Health care facility location, n (%) | ||||
| Africa | 13 (3.1) | 13 (4.2) | 0 (0.0) | 0.029 |
| America | 3 (0.7) | 1 (0.3) | 2 (1.8) | |
| Other European country | 6 (1.4) | 5 (1.6) | 1 (0.9) | |
| France | 366 (87.6) | 272 (88.3) | 94 (85.5) | |
| Overseas France | 19 (4.5) | 10 (3.2) | 9 (8.2) | |
| No answer | 11 (2.6) | 7 (2.3) | 4 (3.6) | |
| Clinical activity, n (%) | ||||
| Adult ICU | 251 (60.0) | 251 (81.5) | 0 (0.0) | < 0.001 |
| Pediatric ICU | 127 (30.4) | 30 (9.7) | 97 (88.2) | |
| Adult high depency unit | 110 (26.3) | 108 (35.1) | 2 (1.8) | |
| Pediatric high depency unit | 73 (17.5) | 12 (3.9) | 61 (55.5) | |
| Adult emergency department | 14 (3.3) | 14 (4.5) | 0 (0.0) | |
| Pediatric emergency department | 14 (3.3) | 2 (0.6) | 12 (10.9) | |
| Other | 57 (13.6) | 40 (13.0) | 17 (14.7) | |
| Medical specialty, n (%) | ||||
| Intensivist and anesthesiologist | 286 (68.4) | 283 (91.9) | 3 (2.7) | < 0.001 |
| Intensivist | 39 (9.3) | 27 (8.8) | 12 (10.9) | |
| Cardiologist or pneumologist | 4 (0.9) | 3 (0.9) | 1 (0.9) | |
| Pediatrician (%) | 110 (26.3) | 0 (0.0) | 110 (100.0) | |
| Other (%) | 29 (6.9) | 20 (6.5) | 9 (8.2) | |
| Health care facility, n (%) | ||||
| Tertiary teaching hospital | 265 (63.4) | 178 (57.8) | 87 (79.1) | < 0.001 |
| Private hospital | 39 (9.3) | 39 (12.7) | 0 (0.0) | |
| Public hospital | 114 (27.3) | 91 (29.5) | 23 (20.9) | |
| Number of beds in health care facility, n (%) | ||||
| 0 to10 | 70 (16.7) | 50 (16.2) | 20 (18.2) | 0.833 |
| 11 to 20 | 230 (55.0) | 172 (55.8) | 58 (52.7) | |
| > 20 | 118 (28.2) | 86 (27.9) | 32 (29.1) | |
| Weekly number of patients with acute circulatory failure in respondent’s care, n (%) | ||||
| < 1 patient a week | 81 (19.4) | 48 (15.6) | 33 (30.0) | < 0.001 |
| 1 to 5 patients a week | 199 (47.6) | 132 (42.9) | 67 (60.9) | |
| 5 to 10 patients a week | 98 (23.4) | 88 (28.6) | 10 (9.1) | |
| > 10 patients a week | 40 (9.6) | 40 (13.0) | 0 (0.0) | |
How physicians consider and interpret CRT
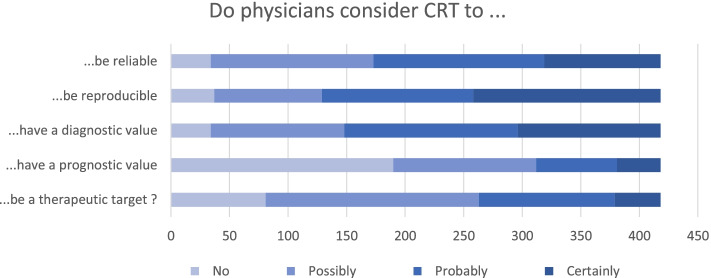
Among all respondents, 82% reported using CRT in routine clinical practice, while others did not consider CRT to be reliable (8%) or reproducible (9%) enough for daily use. Furthermore, 308(74%) respondents declared that 3 seconds was the threshold to define abnormal CRT, 29% were convinced CRT could be used to diagnose acute circulatory failure, and 35% thought it could probably have a diagnostic value (Table 2 and Fig. 1). Conversely, 8% considered that CRT had no diagnostic value and 4% felt it was useless in routine clinical practice. Regarding the prognostic value of CRT, 45% thought it had none, while 55% felt CRT probably or certainly reflects tissue perfusion and 47% thought it does not reflect cardiac output. In the setting of acute coronary failure, 37% of respondents considered that a perfusion goal-directed therapy based on CRT measurement could probably or certainly improve patient outcome. In line with these results, 52% of respondents reported never targeting the normalization of CRT during their resuscitation strategy compared to 6% who always do. Finally, 3% declared that a perfusion-targeted protocol based on CRT was in place in their institution to resuscitate patients with acute circulatory failure.
Table 2.
How respondents use and interpret capillary refill time
| Overall | Adult practice | Pediatric practice | p-value | |
|---|---|---|---|---|
| n = 418 | n = 308 | n = 110 | ||
| Would you say that capillary refill time is a reliable measurement in clinical practice, n (%) | ||||
| No | 34 (8.1) | 31 (10.1) | 3 (2.7) | < 0.001 |
| Possibly | 139 (33.3) | 123 (39.9) | 16 (14.5) | |
| Probably | 146 (34.9) | 96 (31.2) | 50 (45.5) | |
| Certainly | 99 (23.7) | 58 (18.8) | 41 (37.3) | |
| Would you say that capillary refill time is a reproducible measurement in clinical practice, n (%) | ||||
| No | 37 (8.9) | 32 (10.4) | 5 (4.5) | 0.001 |
| Possibly | 92 (22.0) | 79 (25.6) | 13 (11.8) | |
| Probably | 129 (30.9) | 93 (30.2) | 36 (32.7) | |
| Certainly | 160 (38.3) | 104 (33.8) | 56 (50.9) | |
| According to you, what is the pathological threshold of capillary refill time, n (%) | ||||
| It depends on the clinical context; we cannot define a threshold | 39 (9.3) | 34 (11.0) | 5 (4.5) | 0.002 |
| More than 2 seconds | 27 (6.5) | 13 (4.2) | 14 (12.7) | |
| More than 3 seconds | 308 (73.7) | 223 (72.4) | 85 (77.3) | |
| More than 5 seconds | 43 (10.3) | 37 (12.0) | 6 (5.5) | |
| More than 7 seconds | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Do you think that capillary refill time can be used to diagnose acute circulatory failure, n (%) | ||||
| No | 34 (8.1) | 27 (8.8) | 7 (6.4) | 0.012 |
| Possibly | 114 (27.3) | 96 (31.2) | 18 (16.4) | |
| Probably | 148 (35.4) | 103 (33.4) | 45 (40.9) | |
| Certainly | 122 (29.2) | 82 (26.6) | 40 (36.4) | |
| Do you think that capillary refill time has a prognostic value in patients with acute circulatory failure, n (%) | ||||
| No | 190 (45.5) | 122 (39.6) | 68 (61.8) | 0.001 |
| Possibly | 122 (29.2) | 97 (31.5) | 25 (22.7) | |
| Probably | 69 (16.5) | 58 (18.8) | 11 (10.0) | |
| Certainly | 37 (8.9) | 31 (10.1) | 6 (5.5) | |
| Do you think that capillary refill time is a reliable surrogate marker of tissue perfusion, n (%) | ||||
| No | 54 (12.9) | 45 (14.6) | 9 (8.2) | 0.206 |
| Possibly | 135 (32.3) | 102 (33.1) | 33 (30.0) | |
| Probably | 162 (38.8) | 112 (36.4) | 50 (45.5) | |
| Certainly | 67 (16.0) | 49 (15.9) | 18 (16.4) | |
| Do you think that capillary refill time is a surrogate marker of cardiac output, n (%) | ||||
| No | 195 (46.7) | 154 (50.0) | 41 (37.3) | 0.133 |
| Possibly | 150 (35.9) | 105 (34.1) | 45 (40.9) | |
| Probably | 50 (12.0) | 33 (10.7) | 17 (15.5) | |
| Certainly | 23 (5.5) | 16 (5.2) | 7 (6.4) | |
| Do you think that a perfusion goal-directed therapy based on capillary refill time could reduce mortality in patients with acute circulatory failure, n (%) | ||||
| No | 81 (19.4) | 63 (20.5) | 18 (16.4) | 0.027 |
| Possibly | 182 (43.5) | 143 (46.4) | 39 (35.5) | |
| Probably | 116 (27.8) | 79 (25.6) | 37 (33.6) | |
| Certainly | 39 (9.3) | 23 (7.5) | 16 (14.5) | |
| Do you think that capillary refill time is useful in clinical practice, n (%) | ||||
| No | 18 (4.3) | 16 (5.2) | 2 (1.8) | < 0.001 |
| Possibly | 88 (21.1) | 79 (25.6) | 9 (8.2) | |
| Probably | 130 (31.1) | 100 (32.5) | 30 (27.3) | |
| Certainly | 182 (43.5) | 113 (36.7) | 69 (62.7) | |
| Do you follow a resuscitation strategy aiming at normalizing capillary refill time, n (%) | ||||
| Never | 218 (52.2) | 184 (59.7) | 34 (30.9) | < 0.001 |
| Sometimes | 175 (41.9) | 116 (37.7) | 59 (53.6) | |
| Always | 25 (6.0) | 8 (2.6) | 17 (15.5) | |
| Is there a capillary refill time goal directed therapy-based protocol in your institution, n (%) | ||||
| No | 375 (89.7) | 282 (91.6) | 93 (84.5) | 0.093 |
| Yes | 13 (3.1) | 7 (2.3) | 6 (5.5) | |
| I don’t know | 30 (7.2) | 19 (6.2) | 11 (10.0) | |
| Do you report capillary refill time in the medical record (%) | ||||
| Never | 113 (27.0) | 109 (35.4) | 4 (3.6) | < 0.001 |
| Sometimes | 226 (54.1) | 174 (56.5) | 52 (47.3) | |
| Always | 79 (18.9) | 25 (8.1) | 54 (49.1) | |
| Do you personally perform capillary refill time? | ||||
| Yes | 342 (81.8) | 233 (75.6) | 109 (99.1) | < 0.001 |
Fig. 1.
Main considerations regarding CRT according to the 418 survey respondents. CRT: Capillary refill time
How CRT is performed in clinical practice
When performing CRT in patients with acute circulatory failure, 42% of respondents declared assessing CRT 3 to 6 times per day and 50% reported doing it 1 to 2 times per day. CRT is measured at the thorax level for 58% of respondents, at the finger level for 51%, and at the knee level for 40%. To control the pressure applied to the skin, 25% use a specific technique and 75% just apply a firm pressure. The skin is compressed during less than 4 seconds according to 60% of respondents, and 98% assess the compression duration without a chronometer. Forty-six percent averaged at least two consecutive CRT measurements (Table 3).
Table 3.
Technical details on how CRT is performed in clinical practice
| Overall | Adult practice | Pediatric practice | p-value | |
|---|---|---|---|---|
| n = 418 | n = 308 | n = 110 | ||
| If you think of the last patient with acute circulatory failure, how often did you assess capillary refill time, n (%) | ||||
| 0 /24 h | 26 (6.2) | 25 (8.1) | 1 (0.9) | < 0.001 |
| 1 to 2 /24 h | 165 (39.5) | 141 (45.8) | 24 (21.8) | |
| 3 to 6/24 h | 137 (32.8) | 56 (18.2) | 81 (73.6) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
| On what part of the body do you assess capillary refill time, n (%) | ||||
| On the fingertip (%) | 169 (40.4) | 138 (44.8) | 31 (28.2) | < 0.001 |
| On the thorax (%) | 191 (45.7) | 86 (27.9) | 105 (95.5) | |
| On the knee (%) | 131 (31.3) | 107 (34.7) | 24 (21.8) | |
| On the gingiva (%) | 5 (1.2) | 4 (1.3) | 1 (0.9) | |
| How do you control the pressure during the compression of the skin, n (%) | ||||
| You apply a firm pressure | 247 (59.1) | 164 (53.2) | 83 (75.5) | < 0.001 |
| You use the blanching of your finger nail to assess the pressure | 74 (17.7) | 51 (16.6) | 23 (20.9) | |
| You use a glass microscope slide to assess the blanching of the patient’s skin | 7 (1.7) | 7 (2.3) | 0 (0.0) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
| How long does the compression last, n (%) | ||||
| 0 to 3 seconds | 192 (45.9) | 145 (47.1) | 47 (42.7) | < 0.001 |
| 4 to 7 seconds | 119 (28.5) | 62 (20.1) | 57 (51.8) | |
| 7 to 10 seconds | 12 (2.9) | 10 (3.2) | 2 (1.8) | |
| > 10 seconds | 5 (1.2) | 5 (1.6) | 0 (0.0) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
| How do you measure the duration of the compression, n (%) | ||||
| Using a chronometer | 8 (1.9) | 8 (2.6) | 0 (0.0) | < 0.001 |
| Counting in your head | 320 (76.6) | 214 (69.5) | 106 (96.4) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
| How do you measure capillary refill time, n (%) | ||||
| Using a chronometer | 12 (2.9) | 12 (3.9) | 0 (0.0) | < 0.001 |
| Counting in your head | 316 (75.6) | 210 (68.2) | 106 (96.4) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
| To evaluate the capillary refill time of a patient at a given moment, how many CRT do you average, n (%) | ||||
| 1 measurement | 102 (24.4) | 81 (26.3) | 21 (19.1) | < 0.001 |
| 2 measurement | 147 (35.2) | 99 (32.1) | 48 (43.6) | |
| 3 measurement | 47 (11.2) | 28 (9.1) | 19 (17.3) | |
| the longest of the measurements performed | 32 (7.7) | 14 (4.5) | 18 (16.4) | |
| No answer | 90 (21.5) | 86 (27.9) | 4 (3.6) | |
Leads to improve clinical practice
Among the obstacles that limit the widespread use of CRT during routine practice, 51% of respondents pointed out the difficulty in obtaining a reliable measurement, and 42 and 57% reported the lack of medical staff and non-medical staff training, respectively (Table 4). A large majority (90%) considered that CRT measurements could be performed by the non-medical staff. The main leads for improving the daily clinical use of CRT were to delegate measurements to a specifically trained non-medical staff (64%) and to use a dedicated device (54%). In the last open question, which aimed to collect further comments, respondents insisted on the fact that CRT could not be taken as a standalone variable, but should rather be integrated in a multivariable approach (Fig. 2).
Table 4.
Leads to improve capillary refill time in clinical practice
| What are according to you the obstacles for a more widespread use of capillary refill time, n (%) | ||||
| The lack of clinical benefit | 78 (18.7) | 67 (21.8) | 11 (10.0) | 0.004 |
| The difficulty to obtain a reliable measurement | 197 (47.1) | 146 (47.4) | 51 (46.4) | |
| The fact that obtaining a reliable measurement is time consuming | 18 (4.3) | 16 (5.2) | 2 (1.8) | |
| The lack of training of non-medical staff | 220 (52.6) | 154 (50.0) | 66 (60.0) | |
| The lack of training of medical staff | 161 (38.5) | 134 (43.5) | 27 (24.5) | |
| According to you, can non-medical staff, following a dedicated training, handle the measurement of capillary refill time, n (%). | ||||
| No | 9 2.2) | 8 (2.6) | 1 (0.9) | 0.148 |
| Yes | 375 (89.7) | 271 (88.0) | 104 (94.5) | |
| No answer | 34 (8.1) | 29 (9.4) | 5 (4.5) | |
| What could, according to you, improve the reliability of capillary refill time in clinical practice, n (%)e | ||||
| We should abandon this useless measurement | 20 (4.8) | 20 (6.5) | 0 (0.0) | 0.011 |
| CRT should be performed by a trained medical staff | 116 (27.8) | 89 (28.9) | 27 (24.5) | |
| CRT should be performed by a trained non-medical staff | 245 (58.6) | 165 (53.6) | 80 (72.7) | |
| CRT should be performed by non-medical staff without specific training | 21 (5.0) | 17 (5.5) | 4 (3.6) | |
| A specific device that measures CRT should be used | 208 (49.8) | 155 (50.3) | 53 (48.2) | |
Fig. 2.

Word cloud obtained from the responses to the open question “Do you have a comment to make about CRT?”
Differences between adult and pediatric physicians
In routine clinical practice, CRT is used more often by pediatric intensivists (99%) compared to adult ones (76%). The former more frequently reported that CRT is a reliable and reproducible measure and were more confident in the diagnostic value of CRT in the setting of acute circulatory failure. They also more strongly believed in the effectiveness of CRT-based perfusion goal-directed therapy, 63% of them being convinced that CRT is useful at the bedside compared to 37% of adult intensivists. In terms of the technical approach used for CRT measurement, pediatric intensivists used longer compression times and more often averaged multiple measurements (Tables 2 and 3).
Discussion
This survey found that the majority of physicians use CRT, more likely for its diagnostic than its prognostic value. Less than half of them think that a CRT-based perfusion goal-directed therapy can reduce mortality during acute circulatory failure. The standardized approach used in clinical studies does not seem to be applied during routine practice, as almost none of the respondents reported using a chronometer to measure CRT. Pediatric intensivists were more trustful of CRT in terms of reliability, reproducibility, and diagnostic ability, and their approach was closer to the required standards when compared to adult intensivists.
CRT has been correlated to microcirculatory and perfusion variables such as NIRS [8], video microscopy, and lactate, and has been shown to be a strong prognostic factor in different settings [9–11], including acute circulatory failure [1]. Moreover, CRT has the advantage of being faster, cheaper, and easier to use than specific devices assessing microcirculation and has been used as a triage method [12]. Furthermore, clinical trials suggest a benefit of a CRT-targeted treatment strategy in acute circulatory failure [4, 13, 14] or during the deresuscitation phase [15, 16]. However, the results of the trial by Hernandez et al. [4]were strongly debated and led to ancillary Bayesian analyses [5] which confirmed the potential benefit of such strategies targeting CRT [17]. Ongoing studies may confirm the encouraging results in coming years [18, 19]. Although most of the respondents herein recognized the clinical usefulness of CRT they did not trust its prognostic value and most of them were not convinced that CRT-based perfusion-goal directed therapy could improve the outcome of patients with acute circulatory failure. In the pediatric setting, although the prognostic value of CRT is clear [20] there is less data from interventional studies concerning CRT-targeted therapy than in the adult setting. Nevertheless, the pediatric respondents of the present survey were more confident in the ability to improve outcome if CRT-targeted therapy is applied. This could be due to the fact that CRT-related literature is less recent and more abundant in the pediatric than the adult setting. Moreover, in textbooks considering perfusion assessment in children, CRT is described as one of the signs of circulatory failure and pediatric septic shock [21] . CRT is also used to define septic shock in pediatric randomized clinical trial [22]. This could also be explained by the fact that the pediatric approach is more focalized on noninvasive assessments of circulation as well as the higher need to spare blood in children. For instance, recent guidelines recommend titrating fluid load based on clinical surrogates of cardiac output, which include CRT [23].
In the Hernandez et al. clinical trial, CRT was measured by applying firm pressure to the ventral surface of distal phalanx of the right index finger with a glass microscope slide. Compression time was 10 seconds and the time for normal skin color to return was registered using a chronometer [4]. In the study by Ait-oufella et al. [1], the compression time was 15 seconds and the pressure applied “was just enough to remove the blood at the tip of the physician’s nail illustrated by appearance of a thin white distal crescent (blanching) under the nail”. A chronometer was also used and two measurements were averaged [1]. Others have shown that a syringe filled with air can also be used as a piston to control the pressure applied [24, 25].
Although the absence of link between cardiac output and CRT has been shown [26], its link to tissue perfusion is more obvious [12, 27] and consistent with the loss of hemodynamic coherence in septic shock and acute circulatory failure [28, 29]. Respondents appeared in accordance with these data as they trusted more CRT as a perfusion surrogate than a cardiac output surrogate marker.
The present study has some limitations. With only a 7% response rate, one could argue the results are not representative of the French intensivist population. This rate is however comparable to other studies using the SFAR [30, 31]or the GFRUP networks [32]. Nevertheless, it cannot be excluded that the physicians who decided not to participate were more likely to not use CRT regularly resulting in a possible overestimation of CRT use. This could be due to the networks used to dispatch the survey, as among the anesthesiologist and intensivist members of the SFAR network, only a minority work in ICUs, although the latter are the most likely to manage patients with acute circulatory failure. Given that in France there are 775 anesthesiologists and intensivists working in adult ICUs and 214 of these answered the survey, this represents a 28% response rate from those most likely to manage acute circulatory failure. As the survey was written in French language, this likely impedes the generalizability of the results, as does the fact that a majority of respondents worked in public hospitals. Finally, a selection bias was presumably present, as intensivists with a special interest in CRT were more likely to respond.
Nevertheless, in the absence of a previous evaluation of CRT use in routine practice, these results provide a relevant picture of current practices in France.
Conclusion
CRT is widely used by physicians managing acute circulatory failure, but most often in a sub-optimal way. Moreover, a gap remains between available data and the degree of confidence in CRT to predict patient outcome or to help clinical decision-making and resuscitation strategies. This may lead to a misunderstanding of the reliability and clinical usefulness of CRT. A simple way to increase the reliability of CRT would be the use of a chronometer, reducing the variation in compression level, and averaging several measurements. Nurse training programs and/or the use of specific devices should help standardize CRT measurements and render CRT-targeted therapy effective in routine clinical practice.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Study concept and design: M.J-L., CW., R.S., L.D., M.R., M.C., M.L., F.B., E.J., F.D., J-L.F. Acquisition and interpretation of data: M.J-L., CW., Interpretation of data: R.S., M.J-L. Drafting of manuscript: M.J-L. R.S., J-L.F. Statistical analysis: M.J-L. R.S., J-L.F. Study supervision: M.J-L. R.S., J-L.F. Critical revision of the manuscript for important intellectual content: M.J-L., CW., R.S., L.D., M.R., M.C., M.L., F.B., E.J., F.D., J-L.F. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work. All authors ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
No funding sources.
Availability of data and materials
Data set generated/analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed with the Ethical standards outlined in the 1964 Declaration of Helsinki. Data were handled following the national commission for data protection guidelines [20]. Ethic committee was deemed unnecessary according to national legislation: the survey design was not about the collection of data related to patients or animals. The design was purely observational and in agreement with the French law (Loi Jardé, n° 2012–300, March 5, 2012) for research concerning data. We followed the data management methodology MR004 (Délibération n° 2018–155 du 3 mai 2018) in accordance with the national commission for data protection guidelines.
Consent for publication
Not applicable.
Competing interests
MJL is cofounder and shareholder of the DiCARTECH Company that has been created to build and sell a device that measure Capillary refill time. This can be viewed as competing interest with the data presented in this manuscript.
All the other authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med. 2014;40:958–964. doi: 10.1007/s00134-014-3326-4. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez G, Bruhn A, Castro R, Pedreros C, Rovegno M, Kattan E, et al. Persistent Sepsis-Induced Hypotension without Hyperlactatemia: A Distinct Clinical and Physiological Profile within the Spectrum of Septic Shock. Crit Care Res Pract. 2012;2012:536852. 10.1155/2012/536852. [DOI] [PMC free article] [PubMed]
- 3.Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opin Crit Care. 2012;18(3):280-6. 10.1097/MCC.0b013e3283532c08. [DOI] [PubMed]
- 4.Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654–64. [DOI] [PMC free article] [PubMed]
- 5.Zampieri FG, Damiani LP, Bakker J, et al. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med. 2020;201:423–9. [DOI] [PubMed]
- 6.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87. [DOI] [PMC free article] [PubMed]
- 7.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247. [DOI] [PMC free article] [PubMed]
- 8.Lima A, van Bommel J, Sikorska K, et al. The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Crit Care Med. 2011;39:1649–54. [DOI] [PubMed]
- 9.Lima A, Jansen TC, van Bommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37:934–8. [DOI] [PubMed]
- 10.van Genderen ME, Lima A, Akkerhuis M, Bakker J, van Bommel J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit Care Med. 2012;40:2287–94. [DOI] [PubMed]
- 11.van Genderen ME, Paauwe J, de Jonge J, et al. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: a prospective observational study in adults. Crit Care. 2014;18:R114. [DOI] [PMC free article] [PubMed]
- 12.Lara B, Enberg L, Ortega M, et al. Capillary refill time during fluid resuscitation in patients with sepsis-related hyperlactatemia at the emergency department is related to mortality. PLoS One. 2017;12:e0188548. [DOI] [PMC free article] [PubMed]
- 13.van Genderen ME, Engels N, van der Valk RJP, et al. Early peripheral perfusion-guided fluid therapy in patients with septic shock. Am J Respir Crit Care Med. 2015;191:477-80. [DOI] [PubMed]
- 14.Castro R, Kattan E, Ferri G, et al. Effects of capillary refill time-vs. lactate-targeted fluid resuscitation on regional, microcirculatory and hypoxia-related perfusion parameters in septic shock: a randomized controlled trial. Ann Intensive Care. 2020;10:150. [DOI] [PMC free article] [PubMed]
- 15.Bigé N, Lavillegrand J-R, Dang J, et al. Bedside prediction of intradialytic hemodynamic instability in critically ill patients: the SOCRATE study. Ann Intensive Care. 2020;10:47. [DOI] [PMC free article] [PubMed]
- 16.Ruste M, Sghaier R, Chesnel D, Didier L, Fellahi J-L, Jacquet-Lagrèze M. Deresuscitation during continuous renal replacement therapy: A before-after pilot study (The EARLY DRY COHORT). 2022. Available from: https://www.researchsquare.com/article/rs-1416993/v1. 10.21203/rs.3.rs-1416993/v1. [DOI] [PubMed]
- 17.Dépret F, Coutrot M. Interpretation or misinterpretation of clinical trials on septic shock: about the ANDROMEDA-SHOCK trial. Ann Transl Med. 2020;8:800. [DOI] [PMC free article] [PubMed]
- 18.Pettilä V, Merz T, Wilkman E, et al. Targeted tissue perfusion versus macrocirculation-guided standard care in patients with septic shock (TARTARE-2S): study protocol and statistical analysis plan for a randomized controlled trial. Trials. 2016;17:384. [DOI] [PMC free article] [PubMed]
- 19.Pontificia Universidad Catolica de Chile. Hemodynamic Phenotype-Based, Capillary Refill Time-Targeted Resuscitation In Early Septic Shock: The ANDROMEDA-SHOCK-2 Randomized Clinical Trial (A2) [Internet]. clinicaltrials.gov; 2021 Sep Report No.: NCT05057611. Available from: https://clinicaltrials.gov/ct2/show/NCT05057611.
- 20.Fleming S, Gill P, Jones C, et al. The Diagnostic Value of Capillary Refill Time for Detecting Serious Illness in Children: A Systematic Review and Meta-Analysis. PLoS One. [Internet] 2015 [cited 2017 Sep 26]; 10 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4573516/. [DOI] [PMC free article] [PubMed]
- 21.Christopher W. Mastropietro, Kevin M. Valentine. Pediatric Critical Care, Current Controversies. Springer; 2019. 10.1007/978-3-319-96499-7.
- 22.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. [DOI] [PubMed]
- 23.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21:e52. [DOI] [PubMed]
- 24.Jacquet-Lagrèze M, Bouhamri N, Portran P, et al. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit Care. 2019;23:281. [DOI] [PMC free article] [PubMed]
- 25.Maurin C, Portran P, Schweizer R, et al. Effects of methylene blue on microcirculatory alterations following cardiac surgery: A prospective cohort study. Eur J Anaesthesiol. 2021. [DOI] [PubMed]
- 26.Hiemstra B, Koster G, Wiersema R, et al. The diagnostic accuracy of clinical examination for estimating cardiac index in critically ill patients: the Simple Intensive Care Studies-I. Intensive Care Med. 2019;45:190–200. [DOI] [PubMed]
- 27.Hernandez G, Boerma E, Dubin A, et al. The relationship between microcirculatory flow abnormalities and systemic hemodynamic variables in septic shock patients. A multi-centre cross-sectional study [abstract]. Intensive Care Med. 2011;37:0341.
- 28.De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. [DOI] [PubMed]
- 29.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19 Suppl 3:S8. [DOI] [PMC free article] [PubMed]
- 30.Champigneulle B, Neuschwander A, Bronchard R, et al. Intraoperative management of brain-dead organ donors by anesthesiologists during an organ procurement procedure: results from a French survey. BMC Anesthesiol. 2019;19:108. [DOI] [PMC free article] [PubMed]
- 31.Binhas M, Egbeola-Martial J, Kluger MD, Roudot-Thoraval F, Grimbert P. Opioids and nonopioids for postoperative pain control in patients with chronic kidney disease. J Opioid Manag. 2017;13:17–25. [DOI] [PubMed]
- 32.Berne C, Evain JN, Bouzat P, Mortamet G. Organization of trauma management in French level-1 pediatric trauma centers: A cross-sectional survey. Arch Pediatr. 2022;S0929-693X(22):00070-7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data set generated/analyzed during the current study are available from the corresponding author on reasonable request.