Abstract
β-Galactosidase enzymes were extracted from pure cultures of Bifidobacterium angulatum, B. bifidum BB-12, B. adolescentis ANB-7, B. infantis DSM-20088, and B. pseudolongum DSM-20099 and used in glycosyl transfer reactions to synthesize oligosaccharides from lactose. At a lactose concentration of 30% (wt/wt) oligosaccharide yields of 24.7 to 47.6% occurred within 7 h. Examination of the products by thin-layer chromatography and methylation analysis revealed distinct product derived spectra from each enzyme. These were found to be different to that of Oligomate 55, a commercial prebiotic galacto-oligosaccharide. Fermentation testing of the oligosaccharides showed an increase in growth rate, compared to Oligomate 55, with products derived from B. angulatum, B. bifidum, B. infantis, and B. pseudolongum. However B. adolescentis had a lower growth rates on its oligosaccharide compared with Oligomate 55. Mixed culture testing of the B. bifidum BS-4 oligosaccharide showed that the overall prebiotic effect was equivalent to that of Oligomate 55.
Oligosaccharides are increasingly being recognized as useful dietary tools for the modulation of the colonic microflora toward a healthy balance (8). This usually involves selectively increasing the levels of gut bifidobacteria and lactobacilli at the expense of less-desirable organisms such as Escherichia coli, clostridia, and proteolytic bacteroides. This selective fermentation is known as the prebiotic concept defined by Gibson and Roberfroid (9).
Although many oligosaccharide preparations are used in functional foods in Japan (17), two general classes are widely used in Europe. These are fructans, such as inulin and fructo-oligosaccharides, and β-galacto-oligosaccharides (17, 18, 22). The latter are manufactured from lactose by glycosyl transfer catalyzed by β-galactosidase and occur as complex mixtures with various glycosidic linkages (7). The commercial products are made using β-galactosidases isolated from several sources such as bacteria and fungi (5, 7). The prebiotic properties of these galacto-oligosaccharides have been established in several studies, both in vitro (21) and in vivo (11). The consensus is that the substrates have a selective stimulatory effect on bifidobacteria.
Despite the interest in galacto-oligosaccharides as prebiotics, there has been very little effort made to study the relative effects of products synthesized by different glycosidases. Given that the β-galactosidase enzymes from different micro-organisms display differing rate constants for hydrolysis for specific glycosidic linkages and that synthesis of galacto-oligosaccharides is kinetically controlled, synthetic product mixtures made with different enzymes are likely to contain differing profiles of glycosidic linkages. There is, therefore, potential to see varying selectivities upon fermentation of these products.
To this end, we have tested the hypothesis that β-galactosidases, extracted from various species of Bifidobacterium, can be used to synthesize galacto-oligosaccharides from lactose with distinct product profiles. Moreover, it is anticipated that these will confer some selectivity at species level when fermented by colonic microorganisms. If successful, this would enhance the specificity of prebiotics, since current products operate at the genus level (11, 21).
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Sigma-Aldrich (Poole, Dorset, United Kingdom) except otherwise stated: Oligomate 55 was a gift from Yakult (Acton, London, United Kingdom). Bacteriological growth media were from Oxoid (Basingstoke, United Kingdom).
Bacterial strains and growth conditions.
Bifidobacterium longum B-2, B. angulatum, B. adolescentis ANB-7, Lactobacillus acidophilus ANR-1 and Bacteroides ovatus ANGN-1 were isolated and characterized at the MRC Dunn Clinical Nutrition Centre, Cambridge, United Kingdom, from human faeces as described by Macfarlane et al. (14). B. infantis DSM-20088 and B. pseudolongum subsp. pseudolongum DSM-20099 were obtained from the German culture collection (Deutsche Sammlung von Mikroorganismen und Zelkultren GmbH). B. bifidum BB-12 is a commercial probiotic strain.
Extraction of β-galactosidase.
To induce β-galactosidase expression, B. angulatum, B. bifidum BB-12, B. infantis, B. pseudolongum, and B. adolescentis were each grown anaerobically (media boiled and sealed under a stream of oxygen-free nitrogen) for 18 h on peptone yeast extract broth with lactose (10 g liter−1) as the carbon source (10). Bacteria were harvested by centrifugation (Heraeus Varifuge 20RS) at 20,000 × g for 30 min at 4°C. The cells were washed twice, resuspended in 0.05 M sodium phosphate buffer (pH 7.5), and subsequently disrupted by two passages through a French pressure cell (1.1 × 105 kPa) to obtain crude cell-associated enzyme fractions. β-Galactosidase activity was determined by monitoring the hydrolysis of lactose at 37°C and pH 7.5, employing the glucose oxidase-peroxidase coupled reaction. The specific enzyme activity was defined as 1 μmol of glucose released min−1 mg of protein−1. Protein estimation was done by the Lowry method with bovine serum albumin as standard (12).
Oligosaccharide synthesis.
Oligosaccharides were synthesized in 0.05 M sodium phosphate buffer (pH 7.5) containing 5 to 30% (wt/wt) lactose, at 55°C with shaking. Samples were taken at hourly intervals, and the reaction was stopped by heating for 2 min at 100°C. Samples were diluted 1:6 in sodium phosphate buffer and analyzed by thin-layer chromatography (TLC).
TLC.
Carbohydrates were separated by TLC with four ascents using butanol-ethanol-water (5:3:2 [vol/vol/vol]) as the mobile phase. Detection was achieved by spraying with 5% ceric sulfate in 15% concentrated H2SO4 and heating for 10 min at 120°C. Oligosaccharides were quantified by scanning the TLC plates in a scanning densitometer.
Methylation analysis.
Linkage positions for the respective galacto-oligosaccharides preparations were determined by methylation analysis. The freeze-dried samples (5 to 6 mg) were dispersed in dry dimethyl sulfoxide at 20°C for 16 h after a flushing with argon. They were methylated by sequential addition of powdered sodium hydroxide (0.5 g) and iodomethane (4 ml) (4, 13). After elution-extraction on a C18-bonded cartridge (Sep-Pak, Waters, Watford, United Kingdom), the methylated carbohydrates were dried, extracted into CHCl3-CH3OH (1:1, [vol/vol]), and evaporated to dryness. The samples were hydrolyzed using trifluoroacetic acid (2) and converted to partially methylated alditol acetates (PMAAs) by NaBD4 reduction and acetylation with acetic anhydride and N-methylimidazole (1). The PMAAs were analyzed by gas chromatography (GC) on a cross-bonded 50% cyanopropyl methyl–50% phenyl methyl polysiloxane column (Thames Chromatography, Maidenhead, United Kingdom) using a flame ionization detector and a temperature program of 55°C (2 min), + 45°C min−1 (1.9 min), 140°C (2min), + 2°C min−1 (35 min), and 210°C (40 min). The PMAAs were identified by measuring their retention times relative to myo-inositol hexaacetate and comparing the relative retention times with those of external standards. A mixture of standards for each sugar was prepared by deliberate undermethylation of methyl glycosides (6). Peak areas were represented as relative molar quantities using effective carbon response factors (20).
Identities of PMAAs were confirmed by their electron-ionization mass spectra (MS) (3). GC-MS analysis was performed on an identical GC column in series with a Fisons Analytical Trio 1S mass spectrometer, using a source temperature of 200°C and an ionization potential of 70eV.
Fermentation of synthetic oligosaccharide mixtures in pure culture.
Fermentation of galacto-oligosaccharide mixtures in peptone yeast extract basal medium (10) was carried out without purification and compared with a commercial galacto-oligosaccharide preparation, Oligomate 55. Specific growth rates (μ) were determined from triplicate batch culture growth curves, monitored spectrophotometrically at 650 nm, by the following equation (19): ln Xt = ln Xo + μt, where Xo and Xt are the original biomass and biomass concentration after the time interval (t). Microorganisms were transferred from agar plates into minimal media containing the respective test oligosaccharide mixture (1% [wt/vol]). These were incubated until comparative cell concentrations were achieved by all microorganisms under investigation (optical density, ca. 1.0), and a constant volume of cells (0.1 ml) was transferred to Hungate tubes, and the growth was monitored at various time intervals.
Fermentation of oligosaccharide mixtures in batch cultures of mixed fecal bacteria.
The ability of the synthetic mixture derived from B. angulatum to selectively enrich for bifidobacteria in mixed culture was tested using Oligomate 55 as a control. Two batch culture fermenters (working volume, 50 ml) were each inoculated with 10% (wt/vol) fecal slurries (homogenized samples in anaerobic sodium phosphate buffer at pH 7), and the respective carbohydrate was added (1% [wt/vol]). The fermenters were incubated in an anaerobic chamber under an atmosphere of N2-CO2-H2 (80:10:10 [vol/vol]) at 37°C for 24 h. Samples (1 ml) were removed after 0, 6, 12, and 24 h for bacteriological analysis, in triplicate, on a range of selective plating media used previously to isolate specific microorganisms (15). Subsequently, the bacteria were characterized to the genus level on the basis of colonial appearance, Gram reaction spore production, cell morphology, and fermentation endproduct formation in peptone yeast glucose broth (10).
Bacterial enzyme activities.
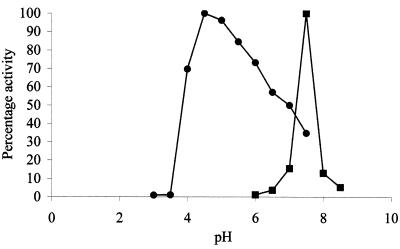
Measurement of cell-associated enzyme activity in the bacteria tested indicated that the bifidobacteria, growing on lactose as sole carbon source, produced a cell-associated β-galactosidase (Table 1). Maximum lactose hydrolysis rates were observed at pH 7.5, whereas activity toward a p-nitrophenyl-β-galactopyranoside substrate was maximal at pH 4.5 (Fig. 1).
TABLE 1.
Cell-associated β-galactosidase activities of the bacteria tested
| Microorganism | Mean activity (U mg of protein−1) ± SD
|
|
|---|---|---|
| Lactose | p-Nitrophenyl-β-galactoside | |
| B. angulatum | 333.47 ± 0.19 | 88.12 ± 0.06 |
| B. bifidum BB-12 | 225.11 ± 0.01 | 71.43 ± 0.07 |
| B. infantis | 254.06 ± 0.02 | 90.91 ± 0.04 |
| B. pseudolongum | 229.28 ± 0.01 | 55.95 ± 0.05 |
| B. adolescentis | 133.01 ± 0.01 | 23.81 ± 0.02 |
The results are mean values for three measurements of specific enzyme activity. One unit is defined as that amount of enzyme that hydrolyzes 1 μmol of substrate per min at pH 7.5 (lactose) or pH 4.5 (p-nitrophenyl-β-galactoside). Lactose hydrolysis was measured by the determination of glucose released, as measured by glucose oxidase-peroxidase assay. The hydrolysis of p-nitrophenyl-β-galactoside was monitored spectrophotometrically at 400 nm.
FIG. 1.
pH optima of cell-associated β-galactosidase and lactase activities of Bifidobacterium angulatum. Cells were harvested after 18 h of growth on 10 g of lactose liter−1; lactase activity (■) was determined using lactose as the substrate (1 μmol of glucose released min−1 mg of protein−1), and β-galactosidase activity (●) was determined using p-nitrophenyl-β-galactoside as the substrate (in nanomoles of p-nitrophenol released minute−1 milligram of protein−1) at 37°C.
Oligosaccharide synthesis.
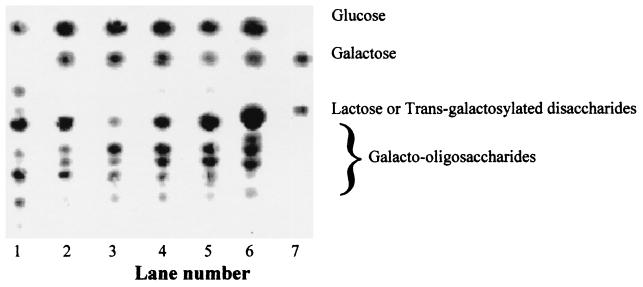
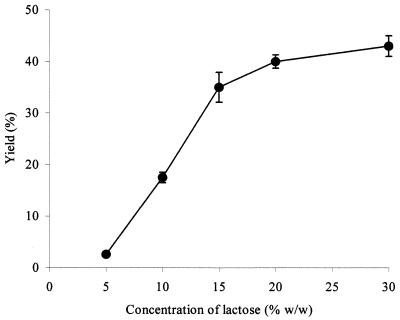
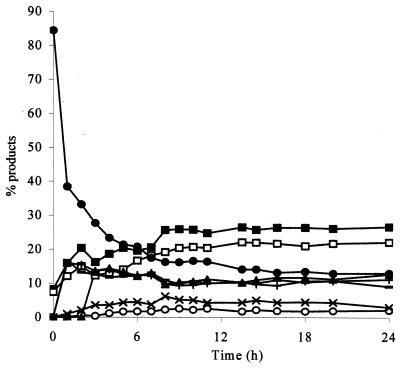
Incubation of cell-associated β-galactosidases with high concentrations of lactose resulted in the production of a range of oligosaccharides, together with glucose and galactose (Fig. 2). Oligosaccharide synthesis was studied in more detail, using the enzyme extracted from B. angulatum. A maximal yield of total oligosaccharides was seen at 30% (wt/wt) lactose (Fig. 3). Production of individual oligosaccharides as a function of time was determined (Fig. 4). Based upon these data, a lactose concentration of 30% (wt/wt) and a reaction time of 6 to 7 h were chosen for further synthesis reactions. Syntheses were subsequently carried out with enzymes extracted from a selection of other bifidobacteria. Yields for bifidobacterial enzymes were comparable to those seen with B. angulatum (Table 2).
FIG. 2.
Synthesis of oligosaccharides with enzymes extracted from selected probiotics. Oligosaccharide mixtures are identified as follows: lane 1, Oligomate 55; lane 2, B. bifidum BB-12 oligosaccharide; lane 3, B. infantis DSM-20088 oligosaccharide; lane 4, B. pseudolongum DSM-20099 oligosaccharide; lane 5, B. adolescentis B-7 oligosaccharide; lane 6, B. angulatum oligosaccharide; lane 7, lactose-galactose mix. Synthesis was performed at 30% (wt/wt) lactose at 55°C for 7 h.
FIG. 3.
Synthesis of oligosaccharides by B. angulatum β-galactosidase as a function of lactose concentration. Values are the means of duplicate determinations ± the standard deviation, obtained at 55°C for 7 h.
FIG. 4.
Synthesis of oligosaccharides by B. angulatum β-galactosidase as a function of time. Components (lactose, ●; glucose, ■; other disaccharides, ○; galactose, □; and the oligosaccharides (+, ×, −, ▴) were identified by TLC after incubation at 55°C with 30% (wt/wt) lactose over 24 h.
TABLE 2.
Oligosaccharide yields using enzymes from various probiotics
| Microorganism | Mean % yield ± SDa |
|---|---|
| B. angulatum | 43.8 ± 2.0 |
| B. bifidum BB-12 | 37.6 ± 1.1 |
| B. infantis | 47.6 ± 2.3 |
| B. pseudolongum | 26.8 ± 2.6 |
| B. adolescentis | 43.1 ± 4.7 |
The results are mean values for two measurements. Yields are expressed as the percent (wt/wt) oligosaccharide content of the total carbohydrate as determined by densitometry of TLC plates.
Linkages for the oligosaccharides synthesized were determined by methylation analysis and linkage molar ratios are summarized in Table 3. The predominant linkage in Oligomate 55 was 1,4-Galp and differed from all the novel preparations in this respect. The B. bifidum BB-12, B. infantis, and B. pseudolongum products had similar bond profiles. B. angulatum oligosaccharides resembled the B. adolescentis product with a predominance of 1,6-Galp linkages.
TABLE 3.
Linkage molar ratios of synthetic oligosaccharides mixtures determined by methylation analysis
| Residue and linkage | Molar ratio for mixturea:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| t-Glcp | 0.7 | 6.2 | 4.5 | 4.6 | 2.3 | 0.5 |
| t-Galf | ND | ND | ND | ND | ND | 0.1 |
| t-Galp | 2.4 | 15.5 | 7.9 | 0.9 | 17.4 | 1.6 |
| 1,2-Gal plus 1,6-Glc | ND | 1.3 | 1.7 | 1.9 | ND | ND |
| 1,3-Glcp | 0.4 | ND | ND | ND | ND | ND |
| 1,2-Glcp | 0.1 | ND | ND | ND | ND | ND |
| 1,3-Galp | ND | 1.0 | 1.0 | 1.0 | ND | 0.1 |
| 1,4-Galp | 1.0 | ND | ND | ND | ND | ND |
| 1,6-Galp | ND | 2.7 | 0.9 | 0.6 | 1.0 | 0.2 |
| 1,2-Glc plus 1,3-Glc | ND | ND | 0.6 | 0.4 | ND | ND |
| 1,4-Glcp | 1.0 | 8.8 | 2.1 | 3.4 | 12.3 | 1.0 |
| 1,6-Glcp | ND | ND | ND | ND | ND | ND |
Oligosaccharide mixtures were as follows: 1, Oligomate 55; 2, B. bifidum BB-12 oligosaccharide; 3, B. infantis DSM-20088 oligosaccharide; 4, B. pseudolongum DSM-20099 oligosaccharide; 5, B. adolescentis B-7 oligosaccharide; 6, B. angulatum oligosaccharide; ND, not detected.
Fermentation of oligosaccharide mixtures in pure culture.
The growth of selected probiotic micro-organisms on the synthetic products was tested and maximal specific growth rates were compared to those on Oligomate 55. Products synthesized with glycosidases from B. angulatum, B. bifidum BB-12, B. infantis, and B. pseudolongum supported higher growth rates for the respective producing species than did Oligomate (Table 4).
TABLE 4.
Growth rates of selected gut bacteria on novel galacto-oligosaccharides
| Microorganism | Mean growth rate (μ h−1) ± SD on oligosaccharide prepna:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| L. acidophilus ANR-1 | 0.69 ± 0.03 | 1.16 ± 0.06 | 0.93 ± 0.10 | 0.57 ± 0.04 | 1.30 ± 0.03 | 0.72 ± 0.05 |
| B. ovatus ANGNIb | 0.50 ± 0.04 | 0.26 ± 0.04 | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.35 ± 0.05 | 0.22 ± 0.02 |
| B. pseudolongum DSM-20099 | 0.56 ± 0.02 | 0.69 ± 0.02 | 0.66 ± 0.01 | 0.99 ± 0.05 | 0.64 ± 0.02 | 0.69 ± 0.03 |
| B. longum ANB-2 | 0.52 ± 0.05 | 0.58 ± 0.00 | 0.67 ± 0.03 | 0.64 ± 0.01 | 0.52 ± 0.01 | 0.84 ± 0.02 |
| B. infantis DSM-20088 | 0.95 ± 0.01 | 0.98 ± 0.00 | 1.20 ± 0.04 | 0.73 ± 0.03 | 0.99 ± 0.05 | 0.95 ± 0.01 |
| B. bifidum BB-12 | 0.95 ± 0.03 | 1.05 ± 0.01 | 0.76 ± 0.02 | 0.79 ± 0.03 | 0.73 ± 0.02 | 1.05 ± 0.01 |
| B. angulatum | 0.87 ± 0.05 | 0.89 ± 0.00 | 0.96 ± 0.02 | 0.71 ± 0.00 | 0.91 ± 0.04 | 1.27 ± 0.09 |
| B. adolescentis ANB-7 | 1.02 ± 0.06 | 0.48 ± 0.02 | 0.48 ± 0.01 | 0.81 ± 0.05 | 0.83 ± 0.01 | 1.01 ± 0.03 |
Values are specific growth rates and are means of triplicate determinations. Oligosaccharide mixtures are identified as follows: 1, Oligomate 55; 2, B. bifidum BB-12 oligosaccharide; 3, B. infantis DSM-20088 oligosaccharide; 4, B. pseudolongum DSM-20099 oligosaccharide; 5, B. adolescentis ANB-7 oligosaccharide; 6, B. angulatum oligosaccharide. Values in boldface represent better growth on homologous oligosaccharide.
That is, Bacteroides ovatus. All other organisms, except L. acidophilus, belong to the genus Bifidobacterium.
Fermentation of oligosaccharide mixtures by mixed populations of gut bacteria.
Populations of anaerobic bacteria increased over the 24 h of oligosaccharide fermentation (Table 5). This effect was most marked for lactobacilli and bifidobacteria. When Oligomate was used as substrate, lactobacilli increased by 2.5-log values and bifidobacteria increased by 1.5-log values in the first 6 h of growth. Similarly, the use of the B. angulatum synthetic oligosaccharides corresponded to 1.5- and 1.2-log increases for lactobacilli and bifidobacteria, respectively. These increases were accompanied by decreases in the populations of enterobacteriaceae and clostridia. Oligomate was a good substrate for bacteroides, supporting a 1.3-log increase that was sustained for 24 h, in contrast to fermentation of the derived oligosaccharides.
TABLE 5.
Growth of selected bacterial groups on Oligomate or B. angulatum synthetic oligosaccharide product in batch culturea
| Bacterial group | Mean bacterial populations (log10 ml−1) ± SD on:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BS-4
|
Oligomate-55
|
|||||||
| 0 h | 6 h | 12 h | 24 h | 0 h | 6 h | 12 h | 24 h | |
| Bifidobacteria | 6.9 ± 0.4 | 8.1 ± 0.2 | 8.0 ± 0.2 | 8.1 ± 0.1 | 7.0 ± 0.6 | 8.5 ± 0.1 | 7.5 ± 0.5 | 7.8 ± 0.4 |
| Lactobacilli | 4.1 ± 0.1 | 5.6 ± 0.1 | 6.6 ± 0.1 | 7.9 ± 0.0 | 4.1 ± 0.0 | 6.7 ± 0.1 | 8.6 ± 0.2 | 8.2 ± 0.6 |
| Bacteroides | 7.6 ± 0.4 | 7.7 ± 0.5 | 7.1 ± 0.4 | 7.9 ± 0.3 | 7.4 ± 0.2 | 8.7 ± 0.1 | 8.7 ± 0.1 | 8.7 ± 0.1 |
| Clostridia | 4.8 ± 0.1 | 4.5 ± 0.6 | <102 | <102 | 4.8 ± 0.1 | 4.4 ± 0.6 | <102 | <102 |
| Enterobacteria | 5.9 ± 0.2 | 4.9 ± 0.1 | 4.0 ± 0.2 | 3.5 ± 0.5 | 5.7 ± 0.6 | 4.8 ± 0.5 | 4.8 ± 0.3 | 3.5 ± 0.6 |
| Total anaerobes | 8.2 ± 0.5 | 8.7 ± 0.1 | 8.3 ± 0.3 | 8.2 ± 0.1 | 8.2 ± 0.2 | 8.7 ± 0.1 | 8.7 ± 0.3 | 8.8 ± 0.1 |
The results are mean values of triplicate determinations from two separate batch culture experiments.
DISCUSSION
Novel oligosaccharide mixtures were synthesized using β-galactosidases from probiotic bacteria. Distinct product spectra were obtained for each reaction mixture and these were different to that seen for Oligomate 55, a commercial galacto-oligosaccharide product. Differences seen by TLC (Fig. 2) were supported by methylation analysis of the oligosaccharide mixtures (Table 3). In this work, the crude reaction mixtures including lactose, galactose, and glucose were analyzed. Further work will seek to separate the individual oligomers for the promising products and characterize these in more detail. Any commercial product based upon these synthetic reactions will, however, be used in an unpurified or semipurified state, as is the case with Oligomate 55, and characterization of growth rates on these crude mixtures was judged to be appropriate.
Increases in growth rate on homologous galacto-oligosaccharide (compared with Oligomate 55) were seen for B. angulatum, B. bifidum BB-12, B. infantis, and B. pseudolongum. In the cases of B. angulatum, B. infantis, and B. pseudolongum, these organisms displayed the highest growth rates of all the probiotics tested on their homologous oligosaccharide mixture. B. adolescentis had a decreased growth rate compared with Oligomate 55.
Testing of the B. angulatum product in the presence of mixed populations of intestinal bacteria suggested that this oligosaccharide had an equivalent prebiotic activity to that of Oligomate 55. With regard to selectivity, the synthesized galacto-oligosaccharide exhibited lower stimulatory effects on bacteroides and lactobacilli than did Oligomate 55.
At the present time, however, it is not possible to ascertain whether the novel oligosaccharides generated here can select for the homologous probiotic in mixed culture. Such data can only be obtained through the use of species-specific oligonucleotide probes, which are unavailable at the present time. The ability to selectively enrich a particular species would facilitate the rational design of synbiotics, combining a probiotic and a prebiotic in a single product, improving survival and colonization by the probiotic (16).
ACKNOWLEDGMENTS
Bodun Rabiu was supported by the Medical Research Council (grant G78/5480) for this work.
The technical assistance of John Eagles of the Institute of Food Research, Norwich, United Kingdom, is gratefully acknowledged for producing MS of derivatized samples.
REFERENCES
- 1.Albersheim P D, Nevins D J, English P D, Karr A. A method for the analysis of sugars on plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr Res. 1967;5:340–345. [Google Scholar]
- 2.Blakeney A B, Harris P J, Henry R J, Stone B A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res. 1983;113:291–299. [Google Scholar]
- 3.Carpita N C, Shea E M. Linkage structure of carbohydrates by gas chromatography-mass spectroscopy (GC-MS) of partially methylated alditol acetates. In: Biermann C J, McGinnis G D, editors. Analysis of carbohydrates by gas-liquid chromatography and mass spectroscopy. Boca Raton, Fla: CRC Press; 1989. pp. 157–216. [Google Scholar]
- 4.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- 5.Crittenden R G, Playne M J. Production, properties and applications of food grade oligosaccharides. Trends Food Sci Technol. 1996;7:353–361. [Google Scholar]
- 6.Doares S H, Albersheim P, Darvill A G. An improved method for the preparation of standards for glycosyl-linkage analysis of complex carbohydrates. Carbohydr Res. 1991;210:311–317. [Google Scholar]
- 7.Ekhart P F, Timmermans E. Techniques for the production of transgalactosylated oligosaccharides (TOS) Bull IDF. 1996;313:59–64. [Google Scholar]
- 8.Fuller R, Gibson G R. Probiotics and prebiotics: microflora management for improved gut health. Clin Microbiol Infect. 1998;4:477–480. [Google Scholar]
- 9.Gibson G R, Roberfroid M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 10.Holdeman L V, Cato E P, Moore W E C. Anaerobic laboratory manual. 4th ed. Blacksburg: Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- 11.Ito M, Deguchi Y, Miyamori A, Matsumoto K, Kikuchi H, Matsumoto K, Kobayashi Y, Yajima T, Kan T. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microbiol Ecol Health Dis. 1990;3:285–292. [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.MacCormick C A, Harris J E, Gunning A P, Morris V J. Characterisation of a variant of the polysaccharide acetan produced by a mutan of Acetobacter xylinum strain CR1/4. J Appl Bacteriol. 1993;74:196–199. doi: 10.1111/j.1365-2672.1993.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane G T, Cummings J H, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132:1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- 15.Newton D F, Cummings J H, Macfarlane S, Macfarlane G T. Growth of a human intestinal Desulfovibrio desulfuricans in continuous cultures containing defined populations of saccharolytic and amino acid fermenting bacteria. J Appl Microbiol. 1998;85:372–380. doi: 10.1046/j.1365-2672.1998.00522.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberfroid M B. Prebiotics and synbiotics: concepts and nutritional properties. Br J Nutr. 1998;80:S197–S202. [PubMed] [Google Scholar]
- 17.Sako T, Matsumoto K, Tanaka R. Recent progress on research and applications of non-digestible galactooligosaccharides. Int Dairy J. 1999;9:69–80. [Google Scholar]
- 18.Salminen S, Bouley C, Boutron-Ruault M-C, Cummings J H, Franck A, Gibson G R, Isolauri E, Moreau M-C, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80:S147–S171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- 19.Stanbury P F, Whitaker A, Hall S J, editors. Principles of Fermentation Technology. Oxford, Great Britain: Butterworth Heinemann; 1995. Microbial growth kinetics; pp. 13–33. [Google Scholar]
- 20.Sweet D P, Shapiro R, Albersheim P. Quantitative analysis by various GLC response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr Res. 1975;40:217–225. [Google Scholar]
- 21.Tanaka R, Takayama H, Morotomi M, Kuroshima T. Effects of administration of transgalactosylated oligosaccharides and Bifidobacterium breve 4006 on human faecal flora. Bifid Microflora. 1983;2:17–24. [Google Scholar]
- 22.Van Loo J, Cummings J, Delzenne N, Englyst H, Franck A, Hopkins M, Kok N, Macfarlane G, Newton D, Quigley M, Roberfroid M, Van Vliet T, Van den Heuvel E. Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94–1095) Br J Nutr. 1999;81:121–132. doi: 10.1017/s0007114599000252. [DOI] [PubMed] [Google Scholar]