Abstract
Osteosarcoma (OS) is the most common malignant bone tumor in children. Despite efforts to develop and implement new therapies, patient outcomes have not measurably improved since the 1980s. Metastasis continues to be the main source of patient mortality, with 30% of cases developing metastatic disease within 5 years of diagnosis. Research models are critical in the advancement of cancer research and include a variety of species. For example, xenograft and patient-derived xenograft (PDX) mouse models provide opportunities to study human tumor cells in vivo while transgenic models have offered significant insight into the molecular mechanisms underlying OS development. A growing recognition of naturally occurring cancers in companion species has led to new insights into how veterinary patients can contribute to studies of cancer biology and drug development. The study of canine cases, including the use of diagnostic tissue archives and clinical trials, offers a potential mechanism to further canine and human cancer research. Advancement in the field of OS research requires continued development and appropriate use of animal models. In this review, animal models of OS are described with a focus on the mouse and tumor-bearing pet dog as parallel and complementary models of human OS.
Keywords: canine, comparative oncology, dogs, experimental animal models, metastasis, mice, murine models, osteosarcoma, review, veterinary clinical trials
Osteosarcoma (OS) is a locally aggressive, highly metastatic malignancy and the most common primary bone tumor of humans.128 As is the case for all pediatric cancers, OS is rare, affecting less than 1,000 people in the United States every year, with most cases being between 10 and 14 years of age. This time frame correlates with peak skeletal growth, and taller children tend to be at a higher risk.110,128,129 Most OS develop in the appendicular skeleton, specifically the metaphyseal region of long bones, adjacent to growth plates.77,128 The distal femur and proximal tibia are the most commonly affected sites in humans. Although the development of OS is considered multifactorial, germline cancer predisposition syndromes111,112,169 and pre-existing skeletal abnormalities52 are known risk factors.129 Familial syndromes and mutations in genes controlling DNA repair and cell cycle progression, such as TP53111 and RB1,169 have been implicated in the development of OS. For example, approximately 4% of OS cases <30 years of age harbor a TP53 mutation that is either known or likely to be associated with Li-Fraumeni syndrome, while 6% carry an exonic variant of TP53 significantly associated with OS metastasis (odds ratio of 4.27).111
Regardless of the cause of OS, the biologic behavior tends to follow a similar course. The primary tumor is locally aggressive, often causing marked bone lysis and new bone proliferation.54,77 Metastatic spread occurs mainly to the lungs, with metastasis to bone, soft tissues, lymph nodes, and other visceral organs noted less commonly.61,65,109 The current standard of care focuses on both local tumor control via surgery and delaying or preventing metastatic spread with multiagent cytotoxic chemotherapy. Neoadjuvant chemotherapy is given 3 to 4 weeks before surgical removal of the tumor. This is most often achieved via a limb-sparing procedure and is followed by combination chemotherapy approximately 2 weeks after surgery. If the percent necrosis of the tumor is >90% following the neoadjuvant chemotherapy, the same chemotherapy protocol will likely be used postoperatively.45,49,115 The most common chemotherapy combinations involve doxorubicin, a platinum agent (cisplatin or carboplatin), and methotrexate, referred to as the MAP protocol.161,181
Although a multimodal approach is used routinely as front-line therapy, metastatic progression continues to be the leading cause of death in patients.54,109,164 The 5-year survival rate is less than 35% in pediatric patients who present with metastatic osteosarcoma at diagnosis.65,109 Critically, no significant improvements in median survival time have occurred in the last 30 years.54,141 As such, there is a critical need to better understand the biologic and molecular underpinnings of the disease process and to develop novel therapies in the hopes of providing more durable tumor control and improved long-term outcomes. In addition to in silico modeling strategies that involve cell lines and other ex vivo approaches, animal models play a crucial role in these studies of OS biology and drug development. Most models are murine including xenograft, allograft, and patient-derived xenografts (PDX) models. There are also transgenic mice that develop OS due to loss of genes frequently mutated in OS including TP53.11 In addition, the development of spontaneous OS in the pet dog may offer a unique animal model to study disease which more closely mimics clinical disease in humans. Using the appropriate animal model in which specific facets of the disease can be studied in a controlled environment is critical to advancing OS research.
Xenograft/Allograft Mouse Models
To understand the complex nature of tumor biology in OS, pre-clinical models are needed that accurately replicate the various aspects of the human disease state. Metastatic progression continues to be a critical determinant of patient outcome: limited event-free survival and overall survival are associated with the number of metastatic sites (>1 site), the presence of bone metastases, and the number or location of lung metastases (>8 or bilaterally affected).71,109 Mouse models have proven to be an important tool for unraveling the complex interactions involved in the metastatic cascade and delineating its many stages. These models also allow researchers to examine the effects of therapeutic agents in vivo at the primary site and in metastases.40,47 Importantly, spontaneous OS development is rare in mice occurring in <1% of mice from most strains.68 As such, the study of OS tumor growth and metastasis in mice is routinely accomplished through orthotopic or heterotopic implantation of OS cell lines or patient-derived tumors in immunocompromised mice. The 4 most commonly used injection sites for OS cell implantation are subcutaneous, para-tibial, intraosseous, and tail vein (Fig. 1).
Figure 1.
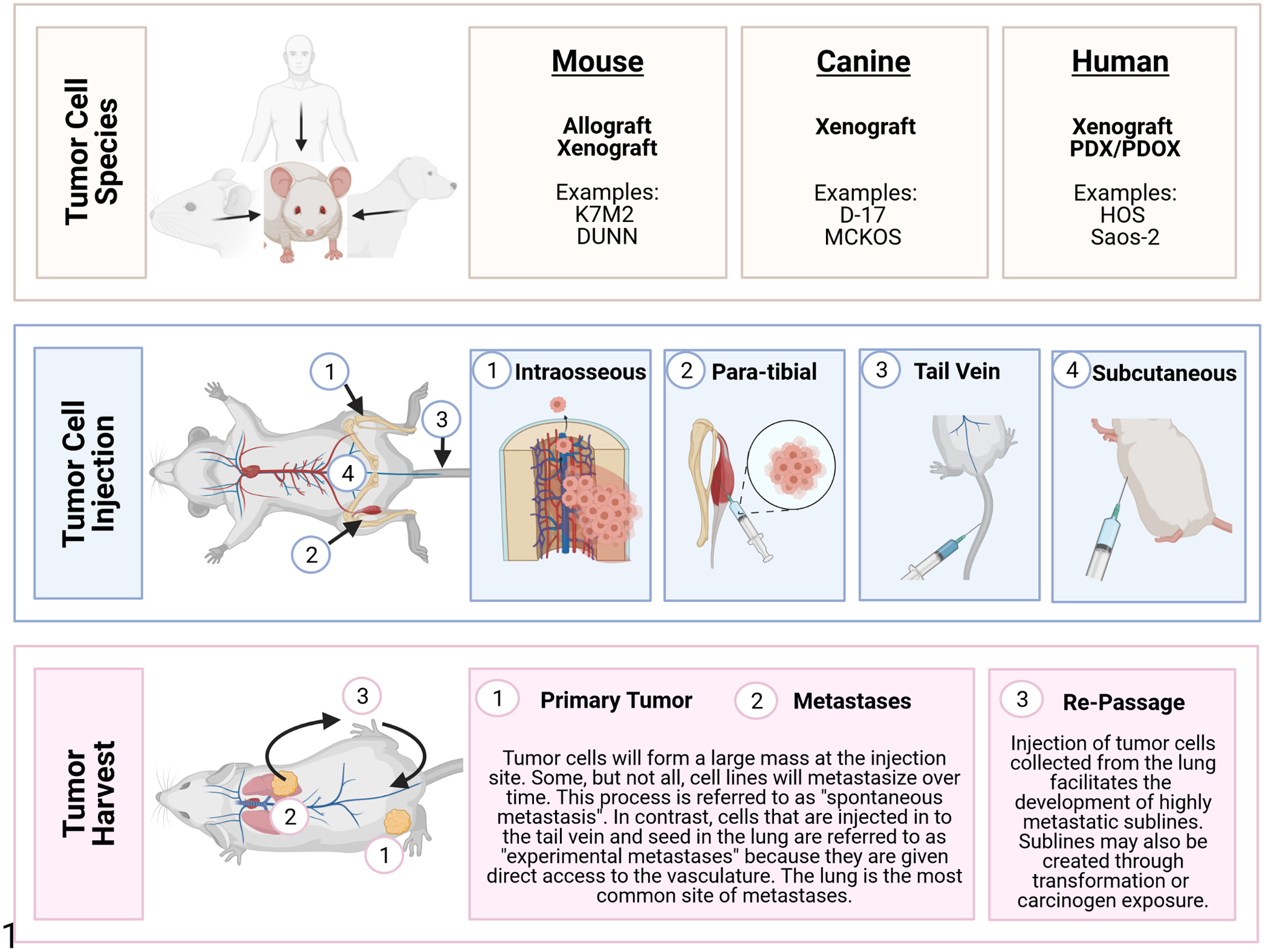
Osteosarcoma (OS) mouse models. OS cell lines may be derived from spontaneous primary OS or metastatic tumors that develop in humans, mice, and dogs (Tables 1 and 3). Once cell lines are established, they can be introduced into mouse models by several injection techniques including intraosseous, para-tibial, tail vein, or subcutaneous. Tail vein injection is associated with production of pulmonary tumors. In contrast, the other 3 methods lead to the development of a large neoplastic nodule at the injection site. These can progress to metastatic disease which primarily affects the lung but may also involve other tissues or organs. Highly metastatic sublines can be developed by collecting and re-passaging tumor cells through the lung.
The subcutaneous model is considered heterotopic because it uses a location in which OS do not naturally form. However, this model has several advantages and is frequently used by cancer researchers.59,116 First, the model is simple, requiring only a subcutaneous injection of tumor cells. Second, tumor growth can be noninvasively and regularly visualized, providing a quick and reliable means of assessing response to a given therapy. Third, the tumor can be easily biopsied before, during, and after therapy for various analyses. However, an important limitation to this model is its inability to fully recapitulate the appropriate tumor microenvironment. The specific site of tumor growth may also influence features of tumor biology including tumor size, metastasis, and chemosensitivity.27,32,63,180 For example, tumors grown subcutaneously have slower growth rates and rarely metastasize compared to orthotopic models. These differences underscore the importance of considering mouse model techniques during the experimental design.
Intraosseous injection of human and murine OS cell lines is most frequently accomplished along the proximal tibia and distal femur. This orthotopic technique permits OS tumor cells to interact with their native microenvironment and potentially develop spontaneous pulmonary metastasis over time. However, there is substantial variation in reported efficiency of bone tumor establishment, tumor volumes, and metastatic efficiency across studies using intratibial injection models. A comprehensive examination of the mouse model of intratibial injection of a metastatic murine OS cell line (K7M2) showed that the maximum volume that can be instilled in the tibial bone marrow cavity is 10 μL.93 When more than this volume is injected, leakage of fluid into the adjacent soft tissue can occur. In studies using intratibial injections of tumor cells between 50 and 100 μL in volume, most tumor cells may seed in the surrounding musculature. In addition, immediate metastasis after intratibial injection of OS cells can occur independent of establishment of a primary tumor because tumor cells are inadvertently deposited within the bone marrow. This allows them to easily migrate into the blood stream and gain access to the lung and other highly vascularized distant sites. Therefore, this model is not recommended as the best model of spontaneous metastasis.
In the para-tibial model, tumor cells are injected within the caudal gastrocnemius muscle along the tibial periosteum. The studies using this approach have shown consistent results in primary tumor growth and lung metastasis.139 In contrast to the direct seeding observed in the intraosseous model,93 para-tibial injection allows OS cells to expand into the adjacent bone during progression where they may gain access to the vasculature and metastasize to distant sites including the lung.139 This process is referred to as “spontaneous metastasis.”138 The advantage of this model is that it more accurately recapitulates the metastatic cascade observed in clinical disease. However, depending on the tumor cell line, metastases may be uncommon or develop slowly.139 In this case, metastatic lesions may be better studied by injecting tumor cells directly into the tail vein to induce “experimental metastasis.”138 This tail vein model of metastasis is quicker and often produces higher numbers of metastases in the lung; however, it does not allow researchers to study the prerequisite steps of metastasis prior to invasion of the intravascular space by tumor cells. Instead, the experimental metastasis model can be used to evaluate the capability of cancer cells to extravasate from vessels and proliferate in the lung. In addition to omitting the first steps of metastasis, this model involves the introduction of many tumor cells at one time into the circulation which does not accurately represent the multistep process of natural metastasis.
Choosing the appropriate model is also critically dependent on the experimental questions. For example, in the para-tibial model, the primary tumor initially develops along the periosteum and may not fully recapitulate the contributions of bone marrow cells to early tumor development. In contrast, the intraosseous model develops within the bone; however, the injection of tumor cells within vessel-rich regions of the bone may allow immediate vascular access and subsequent metastasis of OS cells.93 If the goal is to model these earlier stages of metastasis, the para-tibial model may be used to study spontaneous metastasis from the primary tumor growth at the injection site. Understanding the benefits and limitations of each model is critical to designing the appropriate experiment to address the research questions.
Many mouse and human OS cell lines are available through commercial vendors or research institutes. The biologic behavior of each cell line varies widely including different histologic patterns, tumor matrix, and metastatic potential. This can also be affected by experimental factors such as the mouse strain or by the method in which cells are introduced into the model. In addition to parental cell lines, multiple sublines are also available (Table 1). These sublines may be highly metastatic sub-clones or be transformed via genetic modulation. For example, the HOS cell line17,100 (ATCC CRL-1543) was derived from a fibroblast/epithelial-like primary OS (13 year, female). This cell line was further transformed by the carcinogenic nitrosamine MNNG and by v-Ki-ras-oncogene to produce the MNNG/HOS140 (ATCC CRL-1547) and KRIB10 cell lines. The SaOS-2142 (ATCC HTB-85), MG6312 (ATCC CRL-1427), and HuO969 cell lines were similarly derived from OS in 3 different pediatric patients. These parental strains were re-passaged using mouse models to isolate metastases and create highly metastatic sublines including LM7182 (AKA SaOS-LM7), HuO9-M112,73 and HuO9-M132.73 Mouse cell lines have been similarly developed. For example, the murine DUNN cell line was originally derived from a spontaneous OS in the tail of C3H mouse. DUNN cells injected into a BALB/c mouse form a primary tumor at the site of injection. If allowed to grow, tumor cells invade the vasculature and form metastases in the lung (Figs. 2–7). Tumor cells collected from lung metastases can be reinjected into mouse models. This experimental method of re-passaging lung metastases can be used to enhance the metastatic potential of tumor cells. For example, the DLM8 cell line is a highly metastatic subline of the DUNN cell line derived from lung metastasis after 8 in vivo passages.5
Table 1.
Examples of mouse and human osteosarcoma cell lines and sublines.
| General description | References | |
|---|---|---|
| Human | ||
| SaOS-2 (ATCC HTB-85) | Epithelial OS from 11-year-old female Sublines: LM6 (SaOS-LM6), LM7 (SaOS-LM7) |
62,142,182 |
| MG63 (ATCC CRL-1427) | Fibroblastic OS from 14-year-old male Sublines: MG63.2, MG63.3 |
12,139,167 |
| HuO9 | OS from a 13-year-old female Sublines: HuO9-M112, HuO9-M132, HuO9-H3, HuO9-L6, HuO9-L12, HuO9-L13 |
69,73,122 |
| HOS (ATCC CRL-1543) | Fibroblast/epithelial-like OS from 13-year-old female Sublines: 143B, MNNG/HOS, KRIB |
10,17,18,20,100,140 |
| Mouse | ||
| DUNN | Spontaneous OS from C3H mouse Sublines: DLM8 (LM8) |
5,17 |
| K7 | Spontaneous OS from distal femur of BALB/c mouse Subline: K7M2 |
72,148 |
Abbreviations: OS, osteosarcoma; ATCC, American Type Culture Collection.
Figures 2–7.
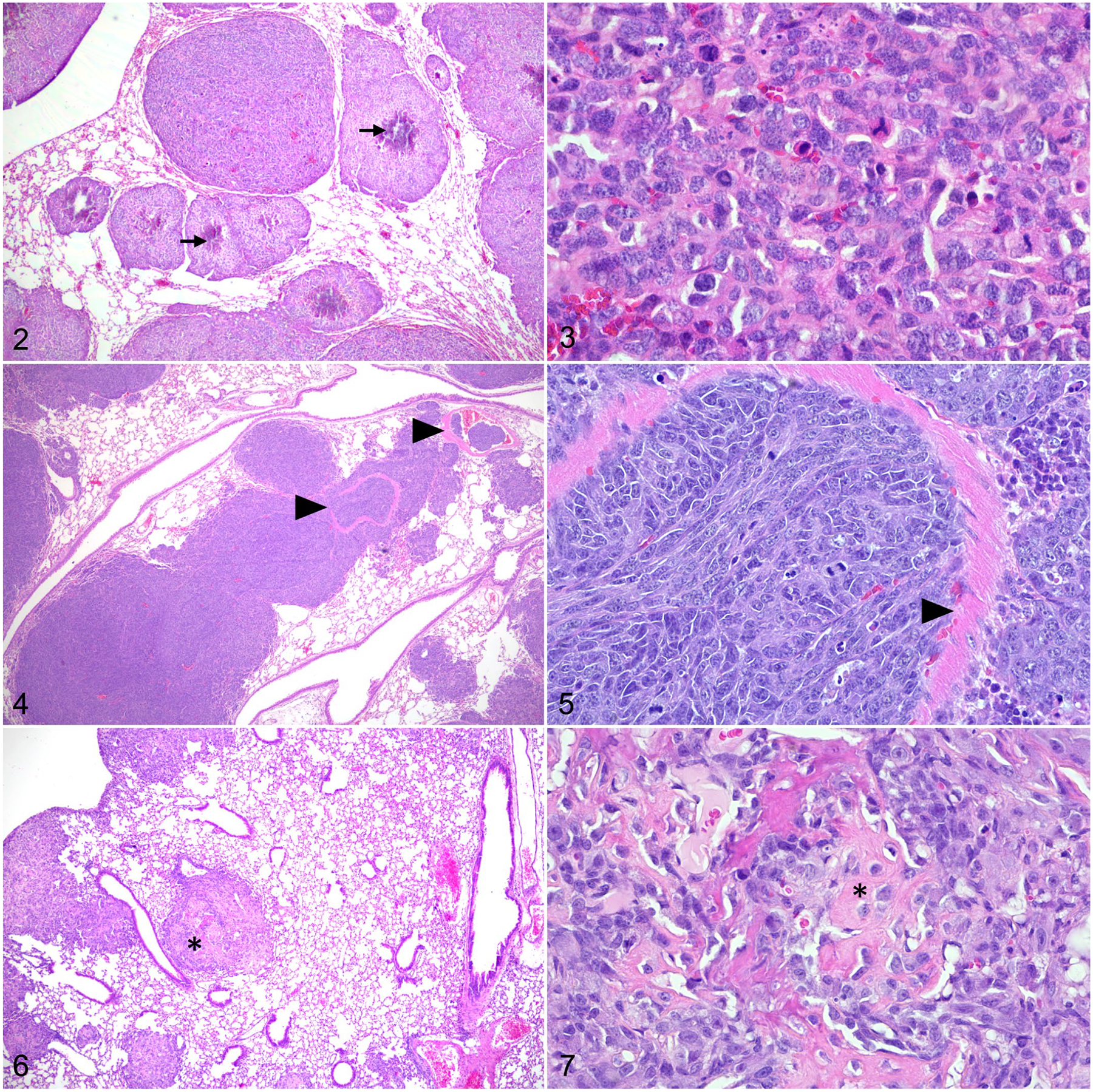
Osteosarcoma, lung, mouse. Hematoxylin and eosin. Figure 2. Following para-tibial injection, SaOS-2 cells form pulmonary nodules with central mineralization (arrows). Figure 3. Lung metastasis of SaOS-2 cells with mitotic figures. Figure 4. Following tail vein injection, DLM8 murine OS cells form numerous pulmonary nodules. Tumor emboli are observed within pulmonary vessels (arrowheads). Figure 5. The remnants of a vessel wall (arrowhead) within a DLM8 cell tumor nodule. Figure 6. Para-tibial injection of MC-KOS canine OS cells forms multiple pulmonary tumors. Few tumor nodules contain central deposits of eosinophilic matrix (asterisk). Figure 7. Lung metastasis of MC-KOS cells with production of variably mineralized osteoid (asterisk).
PDX Models
Patient-derived xenografts have been increasingly used in OS research, including some canine studies,21,23 and are particularly useful for preclinical testing of new therapeutic anticancer agents or drug repurposing. Generation of a PDX model begins with expansion of a tumor sample collected from a patient within 1 to 2 hrs of surgical resection.117,166 Most studies engraft a small piece of fresh tumor tissue subcutaneously into the flank of an immune-compromised mouse59,143 or dissociate the tumor tissue enzymatically to allow injection of a single-cell suspension into an anatomically relevant location.165,166 After sufficient growth, the tumor is harvested, fragmented, and either transplanted for further engraftment or collected for further analysis. Potential host animals include athymic nude mice,60,183 SCID mice,43,66,70,89 BRG mice,94,132 and NSG mice.88,117,124,126 Cell lines can also be generated from PDXs and are easy to cryopreserve and store for later applications.9,43,56,57,66,94,160 Interestingly, some cell lines are reported to carry specific drug resistance to the treatment to which the original tumor was exposed, suggesting that this may provide an important model to study treatment resistance, test novel therapeutic agents, or investigate combination therapies.
Studies have shown that the OS PDXs can recapitulate the gross, histologic, and radiographic features of primary OS tumors and that their genomic profiles maintain similarity to the original tumors even over multiple passages.14,76,79,88,108,166 Several research groups have developed patient-derived orthotopic xenografts, known as PDOX56 or O-PDX.166 Instead of subcutaneous implantation, cells or tissues are engrafted into or around bone,46,56,166 which has the advantage of growing in the same anatomic microenvironment in which the original tumor developed. When compared to other pediatric O-PDX models, OS had the best preservation of the primary tumor’s clonal complexity.166 Potential reasons for favoring orthotopic implantation include the presence of the tumor site-specific microenvironment, more relevant pharmacokinetics, and improved engraftment and subsequent dissociation of tumor cells from orthotopic sites as compared to tissue pieces engrafted in the flank.166 In addition, unlike the traditional subcutaneous implantation in which metastasis is rare, local recurrence and metastatic potential is enhanced by orthotopic implantation.46 Critically, the use of the orthotopic xenograft may lead to rapid pulmonary dissemination after injection, if tumor cells injected in the highly vascularized bone marrow cavity are able to gain direct access to the vasculature. In this case, PDXs form lung metastasis with moderate capacity.88,93
One resource for identifying PDX models is the PDX Finder (https://www.pdxfinder.org/). This comprehensive catalog of PDX models identifies relevant repositories for a variety of OS PDX models from the Pediatric Preclinical Tumor Consortium (PPTC), St Jude Children’s Research Hospital (Childhood Solid Tumor Network, https://cstn.stjude.cloud), The Jackson Lab, Charles River Lab, Patient-Derived Models Repository, and Washington University in St Louis. Most of the human OS tissues in this catalog were collected from primary or recurrent lesions at the primary site; few were from metastases. Although no differences were observed between metastatic and primary or pretreatment and posttreatment samples, PDXs derived from recurrent tumors were more successfully engrafted as compared to those obtained from the diagnostic samples.166
Transgenic Mouse Models
Spontaneous OS are rare in mice,68 but transgenic mouse models have been developed and are an important resource used to study the mechanisms underlying OS initiation and progression. Features that separate transgenic models include differences in tumor latency and penetrance, tumor location, tumor types, and metastatic potential. Several transgenic models have a long latency period. For example, in Prx1-cre p53lox/+ mice, heterozygous deletion of p53 in primitive mesenchymal cells of the limb bud leads to development of OS at approximately 2 years of age.85 This tumor latency can be reduced by homozygous p53 deletion (Prx1-cre p53lox/lox) which leads to formation of OS by approximately 1 year. Although researchers may pursue methods to accelerate tumor development, it is important to consider that efforts to reduce tumor latency may be accompanied by altered biologic behavior. For example, compared to targeting p53 with shRNA, p53 loss in osteoblasts by Cre:lox results in shorter tumor latency (6 vs 14 months) but is associated with altered tumor cell morphology (fibroblastic vs osteoblastic), fewer long bone tumors (29% vs 60%), and reduced metastasis (29% and 70%).119
Interestingly, several transgenic OS mouse models develop tumors in uncommon locations such as along the jaw or snout.11,173 For example, expression of the intracellular domain of Notch1 (NICD) induces the formation of osteomas and OS primarily in the skull and femur, respectively.168 The development of OS in bones of the skull has been associated with reduced metastatic potential in some models.173 As metastasis is a major cause of cancer-related death in humans,164 it is important to consider the ability of transgenic models to recapitulate the metastatic cascade. For example, while H2-c-fosLTR mice develop primary OS with 100% penetrance, no macroscopic lesions are described in nonbone tissues, suggesting that this model is best used for primary tumor research.50 In Osx-Cre mice deficient in p53 and/or Rb, metastatic potential varies by genotype.173 For example, 32% to 40% of Osx-Cre+p53−/− mice develop metastatic tumors primarily in the lung and liver.11,173 Reduced incidence of metastasis in some genotypes, such as Osx-Cre+p53−/−Rb−/−, may be due to short tumor latency, rapid tumor growth, and poor survival.11,173 This further illustrates the importance of weighing different model features such as tumor latency or penetrance and the development of metastatic disease.
Finally, transgenic mouse models continue to provide critical insight into the genetic alterations that promote the initiation and progression of OS. For example, human OS, particularly those that recur or metastasize, overexpress c-fos, a proto-oncogene that regulates cell functions and is thought to be critical to tumor growth.41,137 Consistent with this hypothesis, elevated expression of Fos protein in osteoblasts promotes the formation of OS with 100% penetrance in H2-c-fosLTR mice.50 In addition, familial syndromes and mutations in genes controlling DNA repair and cell cycle progression, such as TP53111 and RB1,169 have been implicated in the development of human OS. For example, in an Osx-Cre mouse model targeting osteoblast progenitors, formation of OS is dependent on p53 loss but can be potentiated by loss of Rb.173 In the Prx1-cre model, targeting p53 in primitive mesenchymal cells of the limb bud results in a tumor latency of approximately 1 year, while co-deletion of Rb and p53 (Prx1-cre p53lox/lox Rblox/lox) dramatically reduces this period to less than 5 months.85 In contrast, targeted deletion of Rb alone (Prx1-cre Rblox/lox) fails to produce OS, further suggesting that loss of p53 is critical to OS initiation while co-deletion of Rb promotes progression.
Importantly, because different cancer types share overlapping genetic mutations, some transgenic mouse models develop multiple tumor types during their lifespan. For example, most mice deficient in p53 develop tumors by 6 months of age including lymphomas (77%) and hemangiosarcomas (27%).29 Approximately 35% of these mice develop multiple tumors types.29 In addition, double knockout of Rb and p53 in osteoblast progenitor cells (Osx-Cre) is associated with the development of OS (75%−100%),11,173 adipogenic tumors (e.g., hibernomas, 20%−44%),11,173 and neuroendocrine tumors (60%).11 Histologic examination of transgenic mice is critical to model development. High incidence of multiple tumor types may preclude the use of certain mouse models, particularly if the tumor of interest is not the most commonly observed tumor type.
Although this review focuses primarily on murine and canine models of OS, additional species are also used and may offer advantages in specific research contexts. For example, pigs deficient in p53 develop OS in the long bones and skull144 and may offer a unique opportunity to study cancer biomarkers, diagnostic imaging, and surgical intervention.145 In addition, zebrafish are frequently used in OS research, particularly to evaluate large drug libraries for novel anticancer therapeutics or to examine certain aspects of metastasis.113,114,133 Finally, virus-induced OS have also been described in rodents infected with retroviruses including Finkel Biskis Jenkins (FBJ) murine osteosarcoma virus and Moloney murine sarcoma virus (MSV).28,37,68 For example, inoculation of MSV directly into the tibia induces OS development in >90% of rats with metastases described in the lungs and regional lymph nodes.28 Following Injection of FBJ virus, mice develop chondroosseous tumors that do not metastasize but resemble primary parosteal OS in humans.175 These animal models are less frequently used but garnered great interest in human OS research. However, while an association has been suggested, the functional role of viruses in the development of human OS is less clear.25,99,103 As most animal models recapitulate a subset of OS biology or a critical component of clinical disease in humans, choosing the appropriate model is dependent on its ability to address the experimental questions.
The Pet Dog With Spontaneous OS: A Complementary Patient-Based Model
Mouse models offer a homogeneous population in which specific molecular processes can be manipulated and interrogated. In addition, the development of primary tumors and metastatic disease is rapid and relatively predictable in mouse models. However, using multiple and complementary animal models is an important and frequently used method to strengthen OS research. Similarities between canine and human OS have supported the ongoing characterization of the dog as a potential model of pediatric and adolescent OS. Several advantages in rodent models—for example, homogeneity and rapid tumor engraftment and growth—are not reflective of what occurs in the human cancer patient. Pet dogs with OS represent a heterogenous group of patients, more accurately representing outbred human patient populations. Osteosarcoma in dogs develops spontaneously but also in the context of an intact immune system and complex tumor microenvironment, both of which are increasingly recognized as important facets of the disease.26,34,36,64,97,180
Many of the first canine OS studies examined the impact of radiation on tumor development as a model for accidental human exposure.163 In humans, exposure to environmental radiation or radiotherapy contributes to the development of several different sarcoma types including OS.96,84,129 Similar radiation-induced malignancy is observed in animals.19,101 In dogs, OS can be induced through a variety of mechanisms163 including external beam radiation101 or ingestion of bone-seeking radionuclides.176 Although radiation-induced OS has clear relevance to environmental or therapeutic radiation exposure in people and animals, these tumors likely differ from their spontaneous counterparts.102 For example, most of the spontaneous OS in humans and dogs occur in long bones and involve the femur and radius, respectively.31,77,128,179 However, studies of radiation-induced tumors demonstrate an increase in incidence of pelvic OS, a location rarely affected by spontaneous tumors in humans (23.1% vs 9.7%).170 Interestingly, radiation is also associated with an increased incidence of pelvic OS in the dog (12.2% vs 1.0%).170
Critically, pet dogs are also known to naturally develop OS within their lifespan, with an estimated 10,000 or more cases in the United States each year.51,147 Most canine OS occur in the distal radius and proximal humerus.155 These sites correlate with the maximal skeletal load which is hypothesized to increase the risk for strain, microtrauma, and bone remodeling.33 In addition, large and giant breed dogs are predisposed to OS suggesting that, similar to humans,110 height appears to play a role in tumor development.30 The gross appearance of canine OS depends partly on whether the tumor is predominantly lytic, productive, or a mixture of both.98,106,158 Lytic OS are soft with red and yellow regions of hemorrhage and necrosis. In contrast, productive tumors are gray and firm or gritty due to the presence of OS-derived osteoid or cartilage. The gross appearance also varies with histologic subtype. For example, while chondroblastic OS with abundant chondroid matrix may be light gray and glistening, telangiectatic OS may contain multiple blood-filled cavities appearing grossly similar to hemangiosarcoma.98,106
There are 6 histologic subtypes of canine OS: osteoblastic, chondroblastic, fibroblastic, telangiectatic, giant cell-rich, and poorly differentiated.98,106,121 Osteoblastic is the most common subtype in the dog; however, similar to human tumors,77 many canine OS are heterogenous and contain multiple histologic patterns (Figs. 8–15).121 This tumor heterogeneity may complicate the prognostic value of histopathology in canine osteosarcomas. Histologic subtype and features used to grade OS including mitotic index, degree of necrosis, and cellular pleomorphism, have demonstrated variable success in predicting clinical outcome in canine cases.2,15,74,80,121,150 In human cases, treatment-induced necrosis is a critical prognostic factor.45 Following neoadjuvant multiagent chemotherapy, tumors with less than 90% necrosis were twice as likely to recur.6
Figures 8–15.
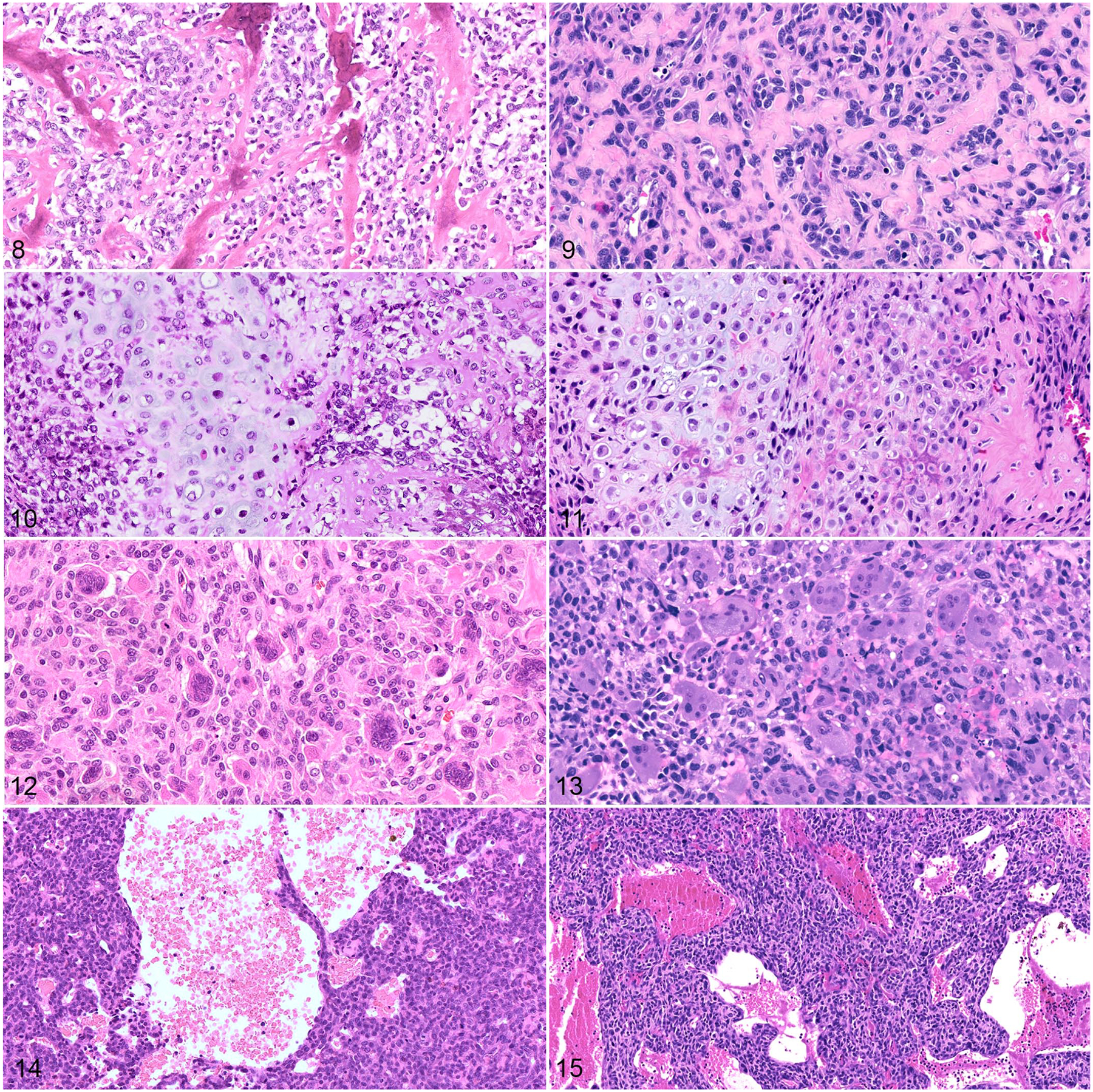
Osteosarcoma, dog (left column) and human (right column). Hematoxylin and eosin. Figures 8 and 9. Osteoid is a distinguishing tumor feature observed in canine (Figure 8) and human (Figure 9) osteosarcomas. Figures 10 and 11. Some canine (Figure 10) and human (Figure 11) osteosarcomas also contain chondroid matrix (chondroblastic subtype). Figures 12 and 13. Giant cell-rich osteosarcomas are an uncommon histologic variant reported in both dogs (Figure 12) and humans (Figure 13). Figures 14 and 15. Telangiectatic osteosarcomas are histologically characterized by blood-filled spaces lined by tumor cells (dog, Figure 14; human, Figure 15).
The histologic diagnosis of OS is dependent on the demonstration of tumor-derived osteoid.98,106 However, the amount of osteoid can vary dramatically between tumors or in different regions of the same tumor. Diagnosis can be hindered either by poor osteoid production or the inability to differentiate osteoid from collagenous fibrous tissue. For example, identification of osteoid distinguishes chondroblastic and fibroblastic OS from chondrosarcomas and fibrosarcomas, respectively. Compared to collagen, osteoid is less fibrillar, may be mineralized, and is more likely to surround tumor cells forming lacunae. In addition, care should be taken not to confuse tumor-derived bone with reactive non-neoplastic new bone which is typically more organized (interconnected) and is lined by a single layer of osteoblasts. Additional histologic considerations, including tumor cell morphology and differentiation from amyloid-producing tumors or early fracture calluses, are well-described in the veterinary literature.98,106
Fortunately, the histologic diagnosis is often supported by other factors including the patient’s clinical history and tumor imaging features. In addition, elevated serum alkaline phosphatase (ALP) or ALP staining of cytologic specimens may help to differentiate OS from other sarcomas.7,106 Immunohistochemistry can also eliminate differential diagnoses. For example, endothelial markers, such as von Willebrand factor (vWF), can be used to distinguish hemangiosarcoma from telangiectatic OS.44 Once diagnosed, the standard of care involves surgical removal of the tumor, followed by chemotherapy. In contrast to humans, canine patients are more likely to undergo limb amputation rather than limb-sparing procedures. Dogs also receive adjunctive chemotherapy, although no significant benefit to a multiagent approach over carboplatin has been observed.155,158,159 With surgery and adjuvant chemotherapy, the median survival time in dogs is approximately 1 year.82,155
The etiopathogenesis of OS in dogs, as in humans, is not fully characterized, but likely is a combination of physical, genetic, and environmental factors.33 Overlapping risk factors for OS development in human and canine cases have been reviewed previously33,92 and include comparative environmental exposures, gene mutations, and familial or breed-specific predisposition. In people with Li-Fraumeni Syndrome, germline mutations in TP53 increases the risk for developing OS.111 Similarly, patients harboring an oncogenic variant of RB1169 may develop secondary OS after radiation or chemotherapy.169 These studies underscore the importance of tumor suppressor genes in oncogenesis in humans. While there are no well-recognized familial disorders leading to a heightened risk for OS in the dog, TP53 mutations are frequent in both human and canine OS.75,130,172 Compared to mice, dogs with OS represent a relatively outbred population, with the unique addition of subpopulations (breeds) carrying described genetic predispositions to OS (Table 2). Genomic analysis in Greyhounds, Rottweilers, and Irish Wolfhounds led to the identification of 33 inherited risk loci including one locus upstream of CDKN2A/B that may contribute to tumorigenesis by disrupting the critical balance between cellular proliferation and senescence.67 Interestingly, CDKN2A deletion and subsequent loss of p16 expression is also observed in human OS and may be particularly important in OS that lack Rb mutations.125 In other breeds such as the Scottish Deerhound, Great Dane, and St. Bernard, hereditary predisposition is evident, but specific genes have not yet been implicated.16,127,136
Table 2.
Genetic alterations in canine osteosarcoma development.
| Breed | Genes |
|---|---|
| Greyhound | COLI IA2, POSTN, CDKN2A/B |
| Rottweiler | FOS, RUNX2 |
| Irish Wolfhound | RBI, CCNBI, POSTN |
Comparative Canine OS Research
The mouse and canine models offer distinct and complementary benefits for modeling OS in people (Fig. 16). To support comparative OS studies in the dog, canine OS cell lines have been developed by several research groups. These cell lines are derived from primary and metastatic canine OS. Ongoing research aims to fully characterize the biologic activities and drug responses of these cell lines in a variety of models (Table 3). In addition, osteoblasts can be derived from normal canine bone (e.g., TNO178 or CnOb42) and are often used as a nontumor control in experiments. Efforts to study and compare these and other canine OS cell lines may help identify important features underlying the phenotypic heterogeneity of canine OS. For example, c-Fos expression has been associated with chondroblastic differentiation in human tissues.1,55 Critically, these types of comprehensive studies require a cooperative effort between scientific institutions because many canine cell lines are not commercially available.
Figure 16.
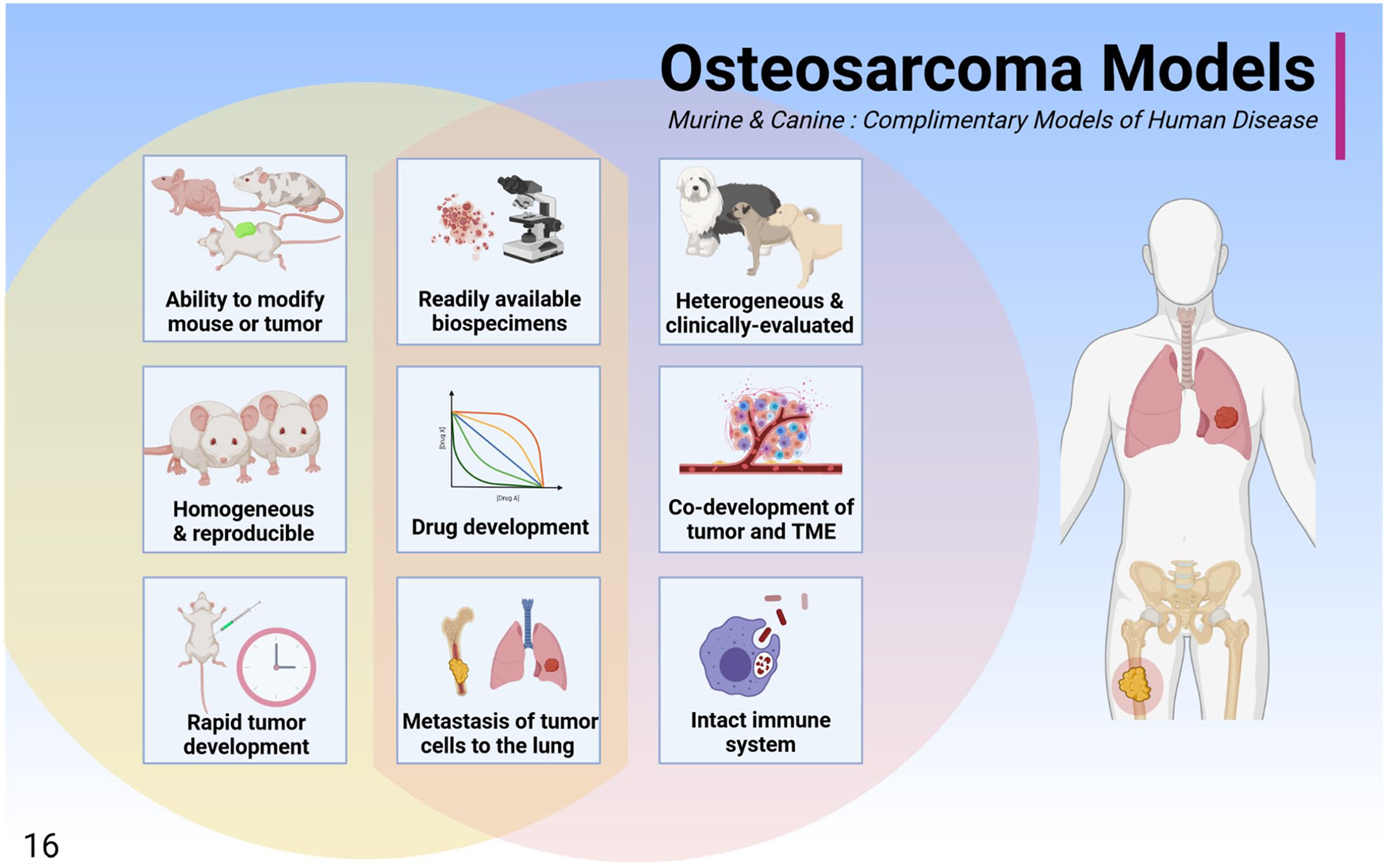
The murine and canine osteosarcoma models are complimentary models of human disease that can be used separately or in parallel to interrogate osteosarcoma biology. These models share several benefits including readily available biospecimens, the ability to develop drugs, and tumor biology that is similar to that observed in humans, including the development of pulmonary metastases. While the mouse model is rapid, reproducible, and can be experimentally modulated, the dog may be more representative of human clinical disease due to natural co-development of the tumor and tumor microenvironment, shared environmental exposures, a diverse genetic background, and the presence of an intact immune system.
Table 3.
Canine OS cell lines.
| Sources | Name | References |
|---|---|---|
| ATCC | D-17 (ATCC CCL-183) | 39,83,91,149 |
| CSU | Gracie, MacKinley, Vogel, Yamane | 39,91 |
| HU/Tokyo | POS, HMPOS | 8,39 |
| NCI | BWKOS (KOS-001), CSKOS (KOS-002), MC-KOS (KOS-003), SK-KOS (KOS-004) | 13,58,178 |
| OVC | OVC-cOSA-31, OVC-cOSA-75, OVC-cOSA-106 | 149 |
| TAMU | TOB, TOK, TOL, TOM, TOT | 178 |
| UC Davis | Parental cell line and tumor-forming clone (48–4) | 154 |
| UMN/Kerafasta | OSCA-8, OSCA-16, OSCA-29, OSCA-32, OSCA-36, OSCA-40, OSCA-71, OSCA-78 | 39,104,152 |
| UVM-V | COS_1033, COS_1186w/h,b COS_1189, COS_1220 | 107 |
| UWM | Abrams, Grey, Hughes, Ingles, Jarques, Marisco/Moresco,c UWOS-1, UWOS-2 | 13,39,83,91,177,178 |
| WSU | OS2.4 | 39 |
Abbreviations: OS, osteosarcoma; ATCC, American Type Culture Collection; CSU, Colorado State University; HU, Hokkaido University; NCI, National Cancer Institute; OVC, Ontario Veterinary College; TAMU, Texas A&M University; Tokyo, University of Tokyo; UC Davis, University of California Davis; UMN, University of Minnesota; UVM-V, University of Veterinary Medicine in Vienna; UMW, University of Wisconsin-Madison; WSU, Washington State University.
These cell lines may be referred to as either OSCA or OSA.
Two cells line derived from soft (COS_1186w) and hard (COS_1186h) regions of the same tumor.
This cell line has been referred to by 2 similar names.
In addition to basic research, clinical trials in pet dogs with OS offer valuable scientific and clinical insight. In general, canine OS trials fall into 1 of 4 major categories: cancer biology, drug development, diagnostics, and imaging (Table 4). Trials may also be designed to answer important questions in multiple categories. For example, collecting appropriate pretreatment and posttreatment biopsies may allow clinical researchers to evaluate the efficacy of a novel treatment while examining cancer biology or diagnostic features associated with treatment resistance. Enrollment in trials is frequently multicenter, may include academic institutions and veterinary referral centers, and is supported by veterinary clinical trial consortia such as the NCI-sponsored PRECINCT (PRE-medical Cancer Immunotherapy Network Canine Trials) or COTC (Comparative Oncology Trials Consortium).48,81 Trials can be structured to mirror their pediatric counterparts or designed to answer a veterinary-specific question or goal. Data generated from such trials can be used to advance a diagnostic or therapeutic procedure to directly benefit dogs, humans, or both species, if the hypothesis and generated data set have translational relevance.
Table 4.
Canine clinical trials.
| Category | Description |
|---|---|
| Cancer biology | Aimed at a specific aspect of OS tumor biology in the dog, with potential for translational relevance to human OS. Biology trials may be designed to understand the shared molecular landscape across species, particularly when iteratively moving between models (e.g., mouse to dog to human). E.g.,Comparing gene expression in tumor/nontumor tissues |
| Drug development | Trials carried out in pet dogs with OS to advance a novel agent and/or combination therapeutic strategy to improve disease-free interval (DFI) and survival. E.g., Investigating combination of standard of care (SOC) with a novel immunotherapeutic or small molecule linked to a specific target or biological process |
| Diagnostics | Assessment of new technologies to improve the diagnosis and enhance the ability of clinicians to monitor disease using noninvasive surrogates for tissue biopsy in pet dogs with OS. E.g., Sampling circulating tumor-derived DNA or proteins to detect disease or monitor treatment response |
| Imaging | Clinical trials designed to advance an imaging method or agent for disease detection or monitoring that is either novel or is a human clinical imaging modality that is not yet widely available for veterinary patients. E.g., Positron emission tomography/computed tomography (PET/CT) staging in canine patients |
Abbreviation: OS, osteosarcoma.
Although clinical trials are valuable for advancing OS research, there are several important considerations and challenges specific to canine-focused research. For example, it is important to maintain a reasonable quality of life for veterinary patients. As such, the need to collect biologic samples, such as tumor biopsies, must be considered in the context of ethical patient care. Serial bone tumor biopsies may introduce additional pain and may predispose to infection or pathologic fracture. Trials must be designed to maximize sample collection while minimizing pain and stress to the canine patient. For example, in a Phase 0 study of pharmacodynamics (PD), clinicians collect a single pretreatment biopsy. This is followed by drug treatment and limb amputation as part of the standard of care therapy. Posttreatment tumor tissue can then be harvested from the amputated limb. These samples are used to examine PD but can also be useful to study other features of OS biology such as histopathology or tumor-associated gene expression prior to and after treatment. During the design phase of clinical trials, thorough consideration of which assays are to be pursued is critical. For example, in situ hybridization and nucleic acid isolation from formalin-fixed paraffin-embedded (FFPE) tissues is dramatically improved if patient tissues are decalcified using EDTA.151 In addition, the ability to collect, store or ship RNA from a variety of tissues, including bone, may be improved by modifying tissue homogenization methods or using reagents such as RNAlater (Thermo Fis her).22,120,123,134
In addition to sample considerations, it is important to consider the applicability of clinical studies to human or canine disease. For example, effective treatment for metastatic OS continues to be a critical unmet need in human research. Similar to human OS, canine OS can metastasize to multiple locations. Most metastasize to the lung (50%−85%); other reported locations include distant bone, regional lymph nodes, liver, and skin (dermis/subcutis).24,35,105,131 Many canine patients develop metastases to multiple tissue sites (Figs. 17–19).
Figures 17–19.
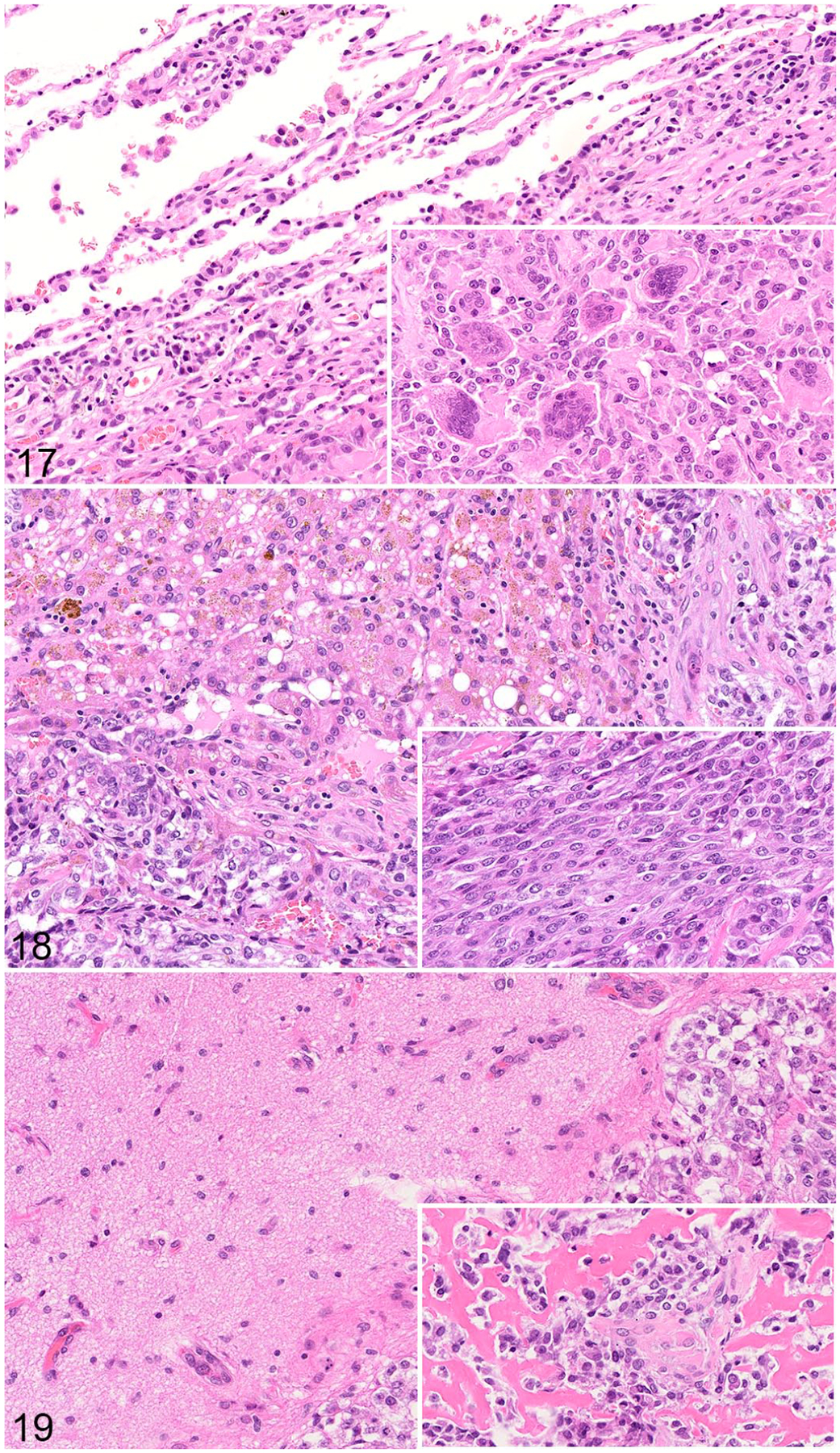
Metastatic osteosarcoma, dog. Hematoxylin and eosin. Figure 17. Lung. Alveoli along the edge of the metastasis are compressed; many contain erythrocytes. Inset: multinucleated cells are observed throughout the mass. Figure 18. Liver. There is mild lymphocytic infiltration of the hepatic parenchyma adjacent to the tumor. Many hepatocytes are vacuolated and contain brown pigment. Inset: The metastasis is comprised of polygonal to spindle-shaped cells. Figure 19. Brain. There is a well-demarcated OS metastasis with compression of the adjacent parenchyma. Inset: Osteoid is abundant within the tumor.
In pediatric OS management, metastectomies are commonly carried out to rid patients of pulmonary nodules.87 In dogs, metastectomies are challenging. Many dogs are diagnosed with too many rapidly progressive metastatic lesions to reasonably undergo this procedure. However, survival benefit has been reported in dogs with less than 3 pulmonary lesions and a prior disease-free interval of over 275 days.171 The development of cost-effective, noninvasive techniques to aid in metastectomy, such as video-assisted thoracoscopy (VAT),174 could improve the ability of veterinary oncologists and surgeons to manage metastatic disease in canine patients. Advancing metastectomy techniques in dogs would allow for parallel clinical trials that use the time-to-first-event endpoint alongside their pediatric counterparts. This would facilitate comparative studies of OS biology in the context of metastatic OS progression and would represent a major advance in how dogs with OS could inform pediatric OS drug development.
Finally, another critical challenge is the relative paucity of canine-specific reagents and assays. For example, a lack of monoclonal antibodies validated in canine tissues inhibits the advancement of immunotherapies. This can also complicate canine clinical trial design such as the development of PD assays which often rely on diagnostic reagents validated solely in human cases. Importantly, resources for canine research continue to improve. For example, a multi-institutional effort through PRECINCT and NanoString has produced a canine immuno-oncology panel for transcriptional profiling of canine tissues. In addition, efforts by the Division of Cancer Treatment and Diagnosis (DCTD) led to the launch of an NCI-sponsored publicly available data repository, the Integrated Canine Data Commons (ICDC; https://caninecommons.cancer.gov/), in 2020. This platform facilitates the comparative analysis of human and canine cancers by allowing researchers to query canine-focused data sets including RNA-seq and whole genome sequencing. These and similar efforts will help to improve canine genome annotation, expand studies of cancer-associated genes, and advance comparative cancer research.
Future Directions
The development of microarray technology in 1995,146 completion of the Human Genome Project, and numerous “-omics” tools available to researchers today have revolutionized the biological sciences and allowed an unprecedented understanding of the molecular underpinnings of biological systems. The impact of these technologies in cancer is no better illustrated than the genotypic classification of breast cancer135,162 which has had a considerable clinical impact on treatment strategies.90 The complete characterization of the human genome was closely followed by characterization of the canine genome published in 2005,86 enabling the use of “-omics” to study canine cancer. Since then, a number of genomic biomarkers have been proposed in several different canine cancers53 including OS. Copy number aberrations have been reported for several genes including ADAM15, CDC5L, MYC, CTC1, MEN1, CDKY,4 RUNX2,3,4 TUSC3, and PTEN.3 In addition, deletion of bone tumor suppressor gene DGL2 has been reported,157 and mutations have been discovered in the genes DMD and SETD2.42
Several studies have highlighted multiple gene transcriptomic signatures, focusing on the comparative aspects between human and canine OS. For example, several gene profiles pertaining to cell cycle and immune response were common between canine, human, and mouse OS tumors and cell lines; furthermore, a cell cycle multigene signature was significantly associated with patient outcome in both canine and human OS.153 Another analysis looked at 32 canine OS cases and identified 51 genes differentially expressed between long- and short-term survivors, and gene enrichment analysis revealed overlapping pathway activity in 7 different pathways between canine and human studies.156 Comparative genomic approaches are essential for understanding the fundamental biology of cancer that is conserved across species.
In addition, statistical modeling and machine learning models that use genomic input to predict drug response both in in vitro and in vivo settings have become an important topic in the field of precision medicine.78,95,118 These methods have been extended to canine models using gene co-expression between NCI60 cancer cell lines and canine OS to build a predictive classifier of response to carboplatin and doxorubicin adjuvant chemotherapy.38 Computational genomic and machine learning techniques are promising areas that will lead to better understanding and treatment options for canines and humans alike.
Canine OS shares clinical, histologic, and molecular features with humans, making it a highly advantageous species in which to study and develop therapeutic and diagnostic strategies with translational benefit. Novel treatment options such as immunotherapy are being investigated in canine patients and could prove to have significant implications for human patients in the future. Dogs provide an exciting model to see how these and other novel therapies may affect long-term survival, but it is also important to remember that there are still some limitations to using dogs as a comparative model. Differences in the age of onset, site predilection, and response to combination therapies must be considered. Whether these differences will impact the translation of treatments between species remains to be seen.
Acknowledgments
The authors would like to acknowledge our colleague, Dr. Troy McEachron, for providing scanned images of FFPE specimens from pediatric OS patients (Department of Pathology, Children’s Hospital Los Angeles, IRB Protocol HS-16-588). Figures 1 and 16 were created using BioRender.com.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Intramural Program of the National Cancer Institute, NIH (Z01-BC006161). This project has been funded (E. Berger) in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261201800001I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Abarrategi A, Gambera S, Alfranca A, et al. c-Fos induces chondrogenic tumor formation in immortalized human mesenchymal progenitor cells. Sci Rep. 2018;8:15615–15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khan AA, Nimmo JS, Day MJ, et al. Fibroblastic subtype has a favourable prognosis in appendicular osteosarcoma of dogs. J Comp Pathol. 2020;176:133–144. [DOI] [PubMed] [Google Scholar]
- 3.Angstadt AY, Motsinger-Reif A, Thomas R, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosom Cancer. 2011;50:859–874. [DOI] [PubMed] [Google Scholar]
- 4.Angstadt AY, Thayanithy V, Subramanian S, et al. A genome-wide approach to comparative oncology: high-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet. 2012;205:572–587. [DOI] [PubMed] [Google Scholar]
- 5.Asai T, Ueda T, Itoh K, et al. Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int J Cancer. 1998;76:418–422. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. [DOI] [PubMed] [Google Scholar]
- 7.Barger A, Graca R, Bailey K, et al. Use of alkaline phosphatase staining to differentiate canine osteosarcoma from other vimentin-positive tumors. Vet Pathol. 2005;42:161–165. [DOI] [PubMed] [Google Scholar]
- 8.Barroga EF, Kadosawa T, Okumura M, et al. Establishment and characterization of the growth and pulmonary metastasis of a highly lung metastasizing cell line from canine osteosarcoma in nude mice. J Vet Med Sci. 1999;61:361–367. [DOI] [PubMed] [Google Scholar]
- 9.Batth IS, Meng Q, Wang Q, et al. Rare osteosarcoma cell subpopulation protein array and profiling using imaging mass cytometry and bioinformatics analysis. BMC Cancer. 2020;20:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin O, Samid D, Donthineni-Rao R, et al. Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer Res. 1993;53:4890–4895. [PubMed] [Google Scholar]
- 11.Berman SD, Calo E, Landman AS, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A. 2008;105:11851–11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billiau A, Edy VG, Heremans H, et al. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittner ML, Lopes R, Hua J, et al. Comprehensive live-cell imaging analysis of cryptotanshinone and synergistic drug-screening effects in various human and canine cancer cell lines. PLoS One. 2021;16:e0236074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blattmann C, Thiemann M, Stenzinger A, et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J Transl Med. 2015;13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boerman I, Selvarajah GT, Nielen M, et al. Prognostic factors in canine appendicular osteosarcoma—a meta-analysis. BMC Vet Res. 2012;8:56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnett BN, Egenvall A, Olson P, et al. Mortality in insured Swedish dogs: rates and causes of death in various breeds. Vet Rec. 1997;141:40–44. [DOI] [PubMed] [Google Scholar]
- 17.Botter SM, Arlt MJE, Born W, et al. Mammalian models of bone sarcomas. In: Heymann D, ed. Bone Cancer. 2nd ed. Academic Press; 2015. [Google Scholar]
- 18.Boyle DB, Coupar BE. Identification and cloning of the fowlpox virus thymidine kinase gene using vaccinia virus. J Gen Virol. 1986;67(pt 8):1591–1600. [DOI] [PubMed] [Google Scholar]
- 19.Buchholz TA, McCabe K, Cobb J, et al. TP53 overexpression in radiation-induced osteosarcoma of the rabbit mandible. Radiat Res. 1999;151:278–282. [PubMed] [Google Scholar]
- 20.Campione-Piccardo J, Rawls WE, Bacchetti S. Selective assay for herpes simplex viruses expressing thymidine kinase. J Virol. 1979;31:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canter RJ, Grossenbacher SK, Foltz JA, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer. 2017;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter LE, Kilroy G, Gimble JM, et al. An improved method for isolation of RNA from bone. BMC Biotechnol. 2012;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo-Tandazo W, Mutsaers AJ, Walkley CR. Osteosarcoma in the post genome era: preclinical models and approaches to identify tractable therapeutic targets. Curr Osteoporos Rep. 2019;17:343–352. [DOI] [PubMed] [Google Scholar]
- 24.Cesario L, Garrett LD, Barger AM, et al. Diagnosis and ultrasonographic appearance of hepatic metastasis in six cases of canine appendicular osteosarcoma (2005–2013). Aust Vet J. 2016;94:160–165. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, Chen J, Li Y, et al. Kaposi’s sarcoma herpesvirus is associated with osteosarcoma in Xinjiang populations. Proc Natl Acad Sci U S A. 2021;118:e2016653118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corre I, Verrecchia F, Crenn V, et al. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020;9:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crenn V, Biteau K, Amiaud J, et al. Bone microenvironment has an influence on the histological response of osteosarcoma to chemotherapy: retrospective analysis and preclinical modeling. Am J Cancer Res. 2017;7:2333–2349. [PMC free article] [PubMed] [Google Scholar]
- 28.Czitrom AA, Pritzker KP, Langer F, et al. Virus-induced osteosarcoma in rats. J Bone Jt Surg Am. 1976;58:303–308. [PubMed] [Google Scholar]
- 29.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. [DOI] [PubMed] [Google Scholar]
- 30.Edmunds GL, Smalley MJ, Beck S, et al. Dog breeds and body conformations with predisposition to osteosarcoma in the UK: a case-control study. Canine Med Genet. 2021;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egenvall A, Nodtvedt A, von Euler H. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: incidence and survival. Can J Vet Res. 2007;71:292–299. [PMC free article] [PubMed] [Google Scholar]
- 32.Erstad DJ, Sojoodi M, Taylor MS, et al. Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy. Dis Model Mech. 2018;11:dmm034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan TM, Khanna C. Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci. 2015;2:210–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan TM, Roberts RD, Lizardo MM. Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Front Oncol. 2020;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fares J, Fares MY, Khachfe HH, et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. 2014;55:69–85. [DOI] [PubMed] [Google Scholar]
- 37.Finkel MP, Biskis BO, Jinkins PB. Virus induction of osteosarcomas in mice. Science. 1966;151:698–701. [DOI] [PubMed] [Google Scholar]
- 38.Fowles JS, Brown KC, Hess AM, et al. Intra- and interspecies gene expression models for predicting drug response in canine osteosarcoma. BMC Bioinform. 2016;17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowles JS, Dailey DD, Gustafson DL, et al. The Flint Animal Cancer Center (FACC) Canine Tumour Cell Line Panel: a resource for veterinary drug discovery, comparative oncology and translational medicine. Vet Comp Oncol. 2017;15:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francia G, Cruz-Munoz W, Man S, et al. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamberi G, Benassi MS, Bohling T, et al. C-myc and c-fos in human osteosarcoma: prognostic value of mRNA and protein expression. Oncology. 1998;55:556–563. [DOI] [PubMed] [Google Scholar]
- 42.Gardner HL, Sivaprakasam K, Briones N, et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun Biol. 2019;2:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill J, Zhang W, Zhang Z, et al. Dose-response effect of eribulin in preclinical models of osteosarcoma by the pediatric preclinical testing consortium. Pediatr Blood Cancer. 2020;67:e28606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuffrida MA, Bacon NJ, Kamstock DA. Use of routine histopathology and factor VIII-related antigen/von Willebrand factor immunohistochemistry to differentiate primary hemangiosarcoma of bone from telangiectatic osteosarcoma in 54 dogs. Vet Comp Oncol. 2017;15:1232–1239. [DOI] [PubMed] [Google Scholar]
- 45.Glasser DB, Lane JM, Huvos AG, et al. Survival, prognosis, and therapeutic response in osteogenic sarcoma. the Memorial Hospital experience. Cancer. 1992;69:698–708. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein SD, Hayashi M, Albert CM, et al. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin Exp Metastasis. 2015;32:703–715. [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Cuadrado L, Tracey N, Ma R, et al. Mouse models of metastasis: progress and prospects. Dis Model Mech. 2017;10:1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon I, Paoloni M, Mazcko C, et al. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorlick R, Meyers PA. Osteosarcoma necrosis following chemotherapy: innate biology versus treatment-specific. J Pediatr Hematol Oncol. 2003;25:840–841. [DOI] [PubMed] [Google Scholar]
- 50.Grigoriadis AE, Schellander K, Wang ZQ, et al. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gustafson DL, Duval DL, Regan DP, et al. Canine sarcomas as a surrogate for the human disease. Pharmacol Ther. 2018;188:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58–P63. [DOI] [PubMed] [Google Scholar]
- 53.Harrison BM, Loukopoulos P. Genomics and transcriptomics in veterinary oncology (Review). Oncol Lett. 2021;21:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18:39–50. [DOI] [PubMed] [Google Scholar]
- 55.He Y, Zhu W, Shin MH, et al. cFOS-SOX9 axis reprograms bone marrow-derived mesenchymal stem cells into chondroblastic osteosarcoma. Stem Cell Rep. 2017;8:1630–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higuchi T, Miyake K, Oshiro H, et al. Trabectedin and irinotecan combination regresses a cisplatinum-resistant osteosarcoma in a patient-derived orthotopic xenograft nude-mouse model. Biochem Biophys Res Commun. 2019;513:326–331. [DOI] [PubMed] [Google Scholar]
- 57.Hingorani P, Roth ME, Wang Y, et al. ABBV-085, antibody-drug conjugate targeting LRRC15, is effective in osteosarcoma: a report by the pediatric pre-clinical testing consortium. Mol Cancer Ther. 2021;20:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong SH, Osborne T, Ren L, et al. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Vet Comp Oncol. 2011;9:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. [DOI] [PubMed] [Google Scholar]
- 60.Iniguez AB, Stolte B, Wang EJ, et al. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell. 2018;33:202–216. e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–506. [DOI] [PubMed] [Google Scholar]
- 63.Jun E, Hong S-M, Yoo HJ, et al. Genetic and metabolic comparison of orthotopic and heterotopic patient-derived pancreatic-cancer xenografts to the original patient tumors. Oncotarget. 2018;9:7867–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13:357–368. [DOI] [PubMed] [Google Scholar]
- 65.Kager L, Zoubek A, Pötschger U, et al. Cooperative German-Austrian-Swiss Osteosarcoma Study Group. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. [DOI] [PubMed] [Google Scholar]
- 66.Karkare S, Allen KJH, Jiao R, et al. Detection and targeting insulin growth factor receptor type 2 (IGF2R) in osteosarcoma PDX in mouse models and in canine osteosarcoma tumors. Sci Rep. 2019;9:11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karlsson EK, Sigurdsson S, Ivansson E, et al. Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B. Genome Biol. 2013;14:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kavirayani AM, Sundberg JP, Foreman O. Primary neoplasms of bones in mice: retrospective study and review of literature. Vet Pathol. 2012;49:182–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawai A, Ozaki T, Ikeda S, et al. Two distinct cell lines derived from a human osteosarcoma. J Cancer Res Clin Oncol. 1989;115:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kendsersky NM, Lindsay J, Kolb EA, et al. The B7-H3-targeting antibody-drug conjugate m276-SL-PBD is potently effective against pediatric cancer preclinical solid tumor models. Clin Cancer Res. 2021;27:2938–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khanna C, Fan TM, Gorlick R, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20:4200–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khanna C, Prehn J, Yeung C, et al. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–271. [DOI] [PubMed] [Google Scholar]
- 73.Kimura K, Nakano T, Park YB, et al. Establishment of human osteosarcoma cell lines with high metastatic potential to lungs and their utilities for therapeutic studies on metastatic osteosarcoma. Clin Exp Metastasis. 2002;19:477–485. [DOI] [PubMed] [Google Scholar]
- 74.Kirpensteijn J, Kik M, Rutteman GR, et al. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol. 2002;39:240–246. [DOI] [PubMed] [Google Scholar]
- 75.Kirpensteijn J, Kik M, Teske E, et al. TP53 gene mutations in canine osteosarcoma. Vet Surg. 2008;37:454–460. [DOI] [PubMed] [Google Scholar]
- 76.Kito F, Oyama R, Sakumoto M, et al. Establishment and characterization of novel patient-derived osteosarcoma xenograft and cell line. In Vitro Cell Dev Biol Anim. 2018;54:528–536. [DOI] [PubMed] [Google Scholar]
- 77.Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–581. [DOI] [PubMed] [Google Scholar]
- 78.Kourou K, Exarchos TP, Exarchos KP, et al. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kresse SH, Meza-Zepeda LA, Machado I, et al. Preclinical xenograft models of human sarcoma show nonrandom loss of aberrations. Cancer. 2012;118:558–570. [DOI] [PubMed] [Google Scholar]
- 80.Kruse MA, Holmes ES, Balko JA, et al. Evaluation of clinical and histopathologic prognostic factors for survival in canine osteosarcoma of the extracranial flat and irregular bones. Vet Pathol. 2013;50:704–708. [DOI] [PubMed] [Google Scholar]
- 81.LeBlanc AK, Mazcko CN. Improving human cancer therapy through the evaluation of pet dogs. Nat Rev Cancer. 2020;20:727–742. [DOI] [PubMed] [Google Scholar]
- 82.LeBlanc AK, Mazcko CN, Cherukuri A, et al. Adjuvant sirolimus does not improve outcome in pet dogs receiving standard-of-care therapy for appendicular osteosarcoma: a prospective, randomized trial of 324 dogs. Clin Cancer Res. 2021;27:3005–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legare ME, Bush J, Ashley AK, et al. Cellular and phenotypic characterization of canine osteosarcoma cell lines. J Cancer. 2011;2:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Vu B, de Vathaire F, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumours during childhood. Int J Cancer. 1998;77:370–377. [DOI] [PubMed] [Google Scholar]
- 85.Lin PP, Pandey MK, Jin F, et al. Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis. 2009;30:1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. [DOI] [PubMed] [Google Scholar]
- 87.Liu Z, Yin J, Zhou Q, et al. Survival after pulmonary metastasectomy for relapsed osteosarcoma. J Thorac Cardiovasc Surg. 2022;163:469–479. e8. [DOI] [PubMed] [Google Scholar]
- 88.Loh AHP, Stewart E, Bradley CL, et al. Combinatorial screening using orthotopic patient derived xenograft-expanded early phase cultures of osteosarcoma identify novel therapeutic drug combinations. Cancer Lett. 2019;442:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lowery CD, Dowless M, Renschler M, et al. Broad spectrum activity of the checkpoint kinase 1 inhibitor prexasertib as a single agent or chemopotentiator across a range of preclinical pediatric tumor models. Clin Cancer Res. 2019;25:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lukong KE. Understanding breast cancer—the long and winding road. BBA Clin. 2017;7:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maeda J, Yurkon CR, Fujisawa H, et al. Genomic instability and telomere fusion of canine osteosarcoma cells. PLoS ONE. 2012;7:e43355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makielski KM, Mills LJ, Sarver AL, et al. Risk factors for development of canine and human osteosarcoma: a comparative review. Vet Sci. 2019;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maloney C, Edelman MC, Kallis MP, et al. Intratibial injection causes direct pulmonary seeding of osteosarcoma cells and is not a spontaneous model of metastasis: a mouse osteosarcoma model. Clin Orthop Relat Res. 2018;476:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manara MC, Valente S, Cristalli C, et al. A quinoline-based DNA methyltransferase inhibitor as a possible adjuvant in osteosarcoma therapy. Mol Cancer Ther. 2018;17:1881–1892. [DOI] [PubMed] [Google Scholar]
- 95.Mannheimer JD, Duval DL, Prasad A, et al. A systematic analysis of genomics-based modeling approaches for prediction of drug response to cytotoxic chemotherapies. BMC Med Genom. 2019;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martland HS, Humphries RE. Osteogenic sarcoma in dial painters using luminous paint. CA Cancer J Clin. 1973;23:368–374. [Google Scholar]
- 97.Mason NJ. Comparative immunology and immunotherapy of canine osteosarcoma. Adv Exp Med Biol. 2020;1258:199–221. [DOI] [PubMed] [Google Scholar]
- 98.Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie G, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. W.B. Saunders; 2015. [Google Scholar]
- 99.Mazzoni E, Benassi MS, Corallini A, et al. Significant association between human osteosarcoma and simian virus 40. Cancer. 2015;121:708–715. [DOI] [PubMed] [Google Scholar]
- 100.McAllister RM, Gardner MB, Greene AE, et al. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971;27:397–402. [DOI] [PubMed] [Google Scholar]
- 101.McChesney Gillette S, Gillette EL, Powers BE, et al. Radiation-induced osteosarcoma in dogs after external beam or intraoperative radiation therapy. Cancer Res. 1990;50:54–57. [PubMed] [Google Scholar]
- 102.McHugh JB, Thomas DG, Herman JM, et al. Primary versus radiation-associated craniofacial osteosarcoma: biologic and clinicopathologic comparisons. Cancer. 2006;107:554–562. [DOI] [PubMed] [Google Scholar]
- 103.McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochim Biophys Acta. 2008;1782:127–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McMahon MB, Bear MD, Kulp SK, et al. Biological activity of gemcitabine against canine osteosarcoma cell lines in vitro. Am J Vet Res. 2010;71:799–808. [DOI] [PubMed] [Google Scholar]
- 105.McNeill CJ, Overley B, Shofer FS, et al. Characterization of the biological behaviour of appendicular osteosarcoma in Rottweilers and a comparison with other breeds: a review of 258 dogs. Vet Comp Oncol. 2007;5:90–98. [DOI] [PubMed] [Google Scholar]
- 106.Meuten DJ. Tumors in Domestic Animals. Somerset, England: John Wiley; 2020. [Google Scholar]
- 107.Meyer FRL, Walter I. Establishment and characterization of new canine and feline osteosarcoma primary cell lines. Vet Sci. 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meyer WH, Houghton JA, Houghton PJ, et al. Development and characterization of pediatric osteosarcoma xenografts. Cancer Res. 1990;50:2781–2785. [PubMed] [Google Scholar]
- 109.Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104:1100–1109. [DOI] [PubMed] [Google Scholar]
- 110.Mirabello L, Pfeiffer R, Murphy G, et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Cont. 2011;22:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mirabello L, Yeager M, Mai PL, et al. Germline TP53 variants and susceptibility to osteosarcoma. J Natl Cancer Inst. 2015;107:djv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mirabello L, Zhu B, Koster R, et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 2020;6:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohseny AB, Hogendoorn PC. Zebrafish as a model for human osteosarcoma. Adv Exp Med Biol. 2014;804:221–236. [DOI] [PubMed] [Google Scholar]
- 114.Mohseny AB, Xiao W, Carvalho R, et al. An osteosarcoma zebrafish model implicates Mmp-19 and Ets-1 as well as reduced host immune response in angiogenesis and migration. J Pathol. 2012;227:245–253. [DOI] [PubMed] [Google Scholar]
- 115.Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J. 2011;189:268–277. [DOI] [PubMed] [Google Scholar]
- 116.Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247–250. [DOI] [PubMed] [Google Scholar]
- 117.Moyer AM, Yu J, Sinnwell JP, et al. Spontaneous murine tumors in the development of patient-derived xenografts: a potential pitfall. Oncotarget. 2019;10:3924–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murali N, Kucukkaya A, Petukhova A, et al. Supervised machine learning in oncology: a clinician’s guide. Dig Dis Interv. 2020;4:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mutsaers AJ, Ng AJ, Baker EK, et al. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone. 2013;55:166–178. [DOI] [PubMed] [Google Scholar]
- 120.Mutter GL, Zahrieh D, Liu C, et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genom. 2004;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagamine E, Hirayama K, Matsuda K, et al. Diversity of histologic patterns and expression of cytoskeletal proteins in canine skeletal osteosarcoma. Vet Pathol. 2015;52:977–984. [DOI] [PubMed] [Google Scholar]
- 122.Nakano T, Tani M, Ishibashi Y, et al. Biological properties and gene expression associated with metastatic potential of human osteosarcoma. Clin Exp Metastasis. 2003;20:665–674. [DOI] [PubMed] [Google Scholar]
- 123.Nance R, Agarwal P, Sandey M, et al. A method for isolating RNA from canine bone. Biotechniques. 2020;68:311–317. [DOI] [PubMed] [Google Scholar]
- 124.Nanni P, Landuzzi L, Manara MC, et al. Bone sarcoma patient-derived xenografts are faithful and stable preclinical models for molecular and therapeutic investigations. Sci Rep. 2019;9:12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nielsen GP, Burns KL, Rosenberg AE, et al. CDKN2A gene deletions and loss of p16 expression occur in osteosarcomas that lack RB alterations. Am J Pathol. 1998;153:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nomura M, Rainusso N, Lee YC, et al. Tegavivint and the beta-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. J Natl Cancer Inst. 2019;111:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ostrander EA, Dreger DL, Evans JM. Canine cancer genomics: lessons for canine and human health. Annu Rev Anim Biosci. 2019;7:449–472. [DOI] [PubMed] [Google Scholar]
- 128.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- 129.Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009;152:15–32. [DOI] [PubMed] [Google Scholar]
- 130.Overholtzer M, Rao PH, Favis R, et al. The presence of p53 mutations in human osteosarcomas correlates with high levels of genomic instability. Proc Natl Acad Sci U S A. 2003;100:11547–11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parachini-Winter C, Curran KM, Pellin M, et al. Cutaneous and subcutaneous metastasis of appendicular osteosarcoma in dogs: 20 cases. J Vet Intern Med. 2019;33:2200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park JA, Cheung NV. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J Hematol Oncol. 2020;13:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Paul CD, Bishop K, Devine A, et al. Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 2019;9:187–206. e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pedersen KB, Williams A, Watt J, et al. Improved method for isolating high-quality RNA from mouse bone with RNAlater at room temperature. Bone Rep. 2019;11:100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 136.Phillips JC, Stephenson B, Hauck M, et al. Heritability and segregation analysis of osteosarcoma in the Scottish deerhound. Genomics. 2007;90:354–363. [DOI] [PubMed] [Google Scholar]
- 137.Piechaczyk M, Blanchard JM. c-fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 1994;17:93–131. [DOI] [PubMed] [Google Scholar]
- 138.Price JE. Spontaneous and experimental metastasis models: nude mice. Methods Mol Biol. 2014;1070:223–233. [DOI] [PubMed] [Google Scholar]
- 139.Ren L, Mendoza A, Zhu J, et al. Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget. 2015;6:29469–29481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rhim JS, Park DK, Arnstein P, et al. Transformation of human cells in culture by N-methyl-N’-nitro-N-nitrosoguanidine. Nature. 1975;256:751–753. [DOI] [PubMed] [Google Scholar]
- 141.Roberts RD, Lizardo MM, Reed DR, et al. Provocative questions in osteosarcoma basic and translational biology: a report from the Children’s Oncology Group. Cancer. 2019;125:3514–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodan SB, Imai Y, Thiede MA, et al. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–4966. [PubMed] [Google Scholar]
- 143.Rokita JL, Rathi KS, Cardenas MF, et al. Genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep. 2019;29:1675–1689. e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saalfrank A, Janssen KP, Ravon M, et al. A porcine model of osteosarcoma. Oncogenesis. 2016;5:e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schachtschneider KM, Schwind RM, Newson J, et al. The oncopig cancer model: an innovative large animal translational oncology platform. Front Oncol. 2017;7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schena M, Shalon D, Davis RW, et al. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. [DOI] [PubMed] [Google Scholar]
- 147.Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmidt J, Strauss GP, Schon A, et al. Establishment and characterization of osteogenic cell lines from a spontaneous murine osteosarcoma. Differentiation. 1988;39:151–160. [DOI] [PubMed] [Google Scholar]
- 149.Schott CR, Ludwig L, Mutsaers AJ, et al. The autophagy inhibitor spautin-1, either alone or combined with doxorubicin, decreases cell survival and colony formation in canine appendicular osteosarcoma cells. PLoS ONE. 2018;13:e0206427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schott CR, Tatiersky LJ, Foster RA, et al. Histologic grade does not predict outcome in dogs with appendicular osteosarcoma receiving the standard of care. Vet Pathol. 2018;55:202–211. [DOI] [PubMed] [Google Scholar]
- 151.Schrijver WA, van der Groep P, Hoefnagel LD, et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol. 2016;29:1460–1470. [DOI] [PubMed] [Google Scholar]
- 152.Scott MC, Sarver AL, Gavin KJ, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49:356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scott MC, Temiz NA, Sarver AE, et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res. 2018;78:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seguin B, Zwerdling T, McCallan JL, et al. Development of a new canine osteosarcoma cell line. Vet Comp Oncol. 2006;4:232–240. [DOI] [PubMed] [Google Scholar]
- 155.Selmic LE, Burton JH, Thamm DH, et al. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Selvarajah GT, Kirpensteijn J, van Wolferen ME, et al. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. 2009;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shao YW, Wood GA, Lu J, et al. Cross-species genomics identifies DLG2 as a tumor suppressor in osteosarcoma. Oncogene. 2019;38:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simpson S, Dunning MD, de Brot S, et al. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Skorupski KA, Uhl JM, Szivek A, et al. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol. 2016;14:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smeester BA, Slipek NJ, Pomeroy EJ, et al. PLX3397 treatment inhibits constitutive CSF1R-induced oncogenic ERK signaling, reduces tumor growth, and metastatic burden in osteosarcoma. Bone. 2020;136:115353. [DOI] [PubMed] [Google Scholar]
- 161.Smeland S, Bruland OS, Hjorth L, et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop. 2011;82:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spatola GJ, Ostrander EA, Mousseau TA. The effects of ionizing radiation on domestic dogs: a review of the atomic bomb testing era. Biol Rev Camb Philos Soc. 2021;96:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stewart E Ewing Sarcoma. New York, NY: Springer; 2020. [Google Scholar]
- 166.Stewart E, Federico SM, Chen X, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. 2017;549:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Su Y, Luo X, He BC, et al. Establishment and characterization of a new highly metastatic human osteosarcoma cell line. Clin Exp Metastasis. 2009;26:599–610. [DOI] [PubMed] [Google Scholar]
- 168.Tao J, Jiang MM, Jiang L, et al. Notch activation as a driver of osteogenic sarcoma. Cancer Cell. 2014;26:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Temming P, Arendt M, Viehmann A, et al. Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: a report from the German reference center. Pediatr Blood Cancer. 2017;64:71–80. [DOI] [PubMed] [Google Scholar]
- 170.Thurman GB, Mays CW, Taylor GN, et al. Skeletal location of radiation-induced and naturally occurring osteosarcomas in man and dog. Cancer Res. 1973;33:1604–1607. [PubMed] [Google Scholar]
- 171.Turner H, Seguin B, Worley DR, et al. Prognosis for dogs with stage III osteosarcoma following treatment with amputation and chemotherapy with and without metastasectomy. J Am Vet Med Assoc. 2017;251:1293–1305. [DOI] [PubMed] [Google Scholar]
- 172.van Leeuwen IS, Cornelisse CJ, Misdorp W, et al. P53 gene mutations in osteosarcomas in the dog. Cancer Lett. 1997;111:173–178. [DOI] [PubMed] [Google Scholar]
- 173.Walkley CR, Qudsi R, Sankaran VG, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walton RS. Video-assisted thoracoscopy. Vet Clin North Am Small Anim Pract. 2001;31:729–759, ix. [DOI] [PubMed] [Google Scholar]
- 175.Ward JM, Young DM. Histogenesis and morphology of periosteal sarcomas induced by FBJ virus in NIH Swiss mice. Cancer Res. 1976;36:3985–3992. [PubMed] [Google Scholar]
- 176.White RG, Raabe OG, Culbertson MR, et al. Bone sarcoma characteristics and distribution in beagles fed strontium-90. Radiat Res. 1993;136:178–189. [PubMed] [Google Scholar]
- 177.Wilson H, Chadalapaka G, Jutooru I, et al. Effect of tolfenamic acid on canine cancer cell proliferation, specificity protein (sp) transcription factors, and sp-regulated proteins in canine osteosarcoma, mammary carcinoma, and melanoma cells. J Vet Intern Med. 2012;26:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wilson-Robles H, Franks K, Pool R, et al. Characterization of five newly derived canine osteosarcoma cell lines. BMC Vet Res. 2019;15:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wolke RE, Nielsen SW. Site incidence of canine osteosarcoma. J Small Anim Pract. 1966;7:489–492. [DOI] [PubMed] [Google Scholar]
- 180.Yang C, Tian Y, Zhao F, et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21:6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu D, Zhang S, Feng A, et al. Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: a meta-analysis and clinical observation. Medicine (Baltimore). 2019;98:e15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yuan J, Ossendorf C, Szatkowski JP, et al. Osteoblastic and osteolytic human osteosarcomas can be studied with a new xenograft mouse model producing spontaneous metastases. Cancer Invest. 2009;27:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou C, Zhang Z, Zhu X, et al. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine. 2020;59:102955. [DOI] [PMC free article] [PubMed] [Google Scholar]


