Abstract
We test whether the COVID-19 pandemic has an ethnicity-differentiated (Indigenous vs non-Indigenous) effect on infant health in the Brazilian Amazon. Using vital statistics data we find that Indigenous infants born during the pandemic are 0.5% more likely to have very low birth weights. Access to health care contributes to health gaps. Thirteen percent of mothers travel to deliver their babies. For traveling mothers, having an Indigenous baby during the pandemic increases the probability of very low birth weight by 3%. Indigenous mothers are 7.5% less likely to receive adequate prenatal care. Mothers that travel long distances to deliver their babies and give birth during the pandemic are 35% less likely to receive proper prenatal care. We also find evidence that the pandemic shifts medical resources from rural to urban areas, which disproportionately benefits non-Indigenous mothers. These results highlight the need for policies to reduce health inequalities in the Amazon.
Keywords: Indigenous peoples, Infants, COVID-19 pandemic, Health care access, Brazil, Amazon
1. Introduction
The COVID-19 virus rapidly spread around the world causing a dire health crisis with serious setbacks to standards of living. On one hand, the virus has a devastating impact on societies through its direct health impairment, which led to unprecedented stress on health care systems and high mortality rates. On the other hand, public health restrictions to increase social distancing and slow down contagion cause indirect social impacts due to economic deceleration and the consequent deterioration of earnings and socioeconomic status. While the debate is open regarding the severity of different channels, the pandemic has the potential to disproportionately hurt minorities, either through their lower baseline health status (Rytter et al., 2014), or through their higher vulnerability to economic shocks (Ruhm, 2016).
As Bill and Melinda Gates explain, pandemics exploit pre-existing inequalities.1 An impressive research effort has emerged identifying a variety of socioeconomic pandemic impacts on the well-being of vulnerable individuals. For example, we now have evidence that the COVID-19 pandemic increased minority unemployment gaps (Couch et al., 2020), violence against women (Arenas-Arroyo et al., 2021), food insecurity of seniors and vulnerable individuals in developing countries (Ziliak, 2021; Mahajan and Tomar, 2021), and delayed graduation of low income students (Aucejo et al., 2020). However, while the general press reports on challenges imposed by the pandemic on Indigenous peoples, much is still unknown about the effects of the coronavirus crisis on Indigenous health.2
The paper focuses on the pressing question of whether we can identify in large scale data a COVID-19 pandemic impact on infant health and whether possible effects are differentiated by Indigenous ethnicity. Worldwide, almost 400 million Indigenous individuals have low standards of health (Gracey and King, 2009). Brazil has an Indigenous population of 800 thousand individuals that face strong health inequalities (Victora et al., 2011; Pontes and Santos, 2020). In light of health gaps, we might expect COVID-19 related health impacts to be stronger in Indigenous communities.
To explore these questions, we examine infant health microdata from DATASUS – the database of the Brazilian Public Health Care System (Sistema Único de Saúde – SUS hereafter). The focus on infants is important for two reasons. First, it allows for the early investigation of short-run health effects of the pandemic. The relatively predictable pregnancy length and the sensitivity of the fetus to short-run changes reduces the burden of identification of a possible causal link (Carrillo and Feres, 2019). Second, infant health is not only an important health indicator but also a general welfare indicator as it is positively associated with long-term outcomes such as educational attainment and earnings (Bütikofer et al., 2019).
Our sample consists of all births in the Brazilian state of Amazonas from January 1, 2019 to July 31, 2020.3 Amazonas is the state with the largest concentration of Indigenous Peoples in Brazil. Covered by the Amazon forest, the state consists of a collection of small rural municipalities with difficult geographical access.4 Amazonas ranks 25th (out of 26 states plus the Federal District) in average household earnings.5 This setting reflects the unfortunate reality of vulnerabilities and spatial isolation facing Indigenous communities and thus makes Amazonas a suitable case study for Indigenous health.
In the absence of detailed mother-level information about COVID-19 infection in Brazil, we measure the severity of the pandemic in terms of potential exposure. The identifying variation is the staggered spread of the virus throughout the Brazilian Amazon. Using DATASUS data on hospitalizations, we identify different municipality-level starting dates for the epidemic, which allows us to compare infant health outcomes (birth weights and Apgar scores) before and after the start of the local epidemic. We develop empirical models to examine two main questions. First, we use the universe of vital statistics data to test for a possible effect of COVID-19 exposure on infant health. Second, we test for ethnicity differentiated COVID-19 exposure effects by comparing infant health outcomes before and after the pandemic, between Indigenous and non-Indigenous infants.
Our main empirical model excludes births from the capital city Manaus to focus on the set of mothers that reside in the more homogeneous group of small and rural municipalities. In contrast with Manaus, the private health sector is absent in small municipalities, which leaves public health services as the only feasible option in the countryside of Amazonas (see discussion below). Moreover, while only 0.2% of residents of Manaus are Indigenous, the average share of the Indigenous population across municipalities in the countryside is 10.3%, with a maximum of 76.6%. Focusing on the subsample of small municipalities allows for a cleaner examination of ethnic impacts of the pandemic in a setting that is representative of the realities of Indigenous Peoples, e.g. remoteness and lower supply of healthcare infrastructure (Gracey and King, 2009).6 Our models control for mother and pregnancy characteristics, pre-pandemic municipality trends, and municipality and month-by-year fixed effects.
The results show that Indigenous infants weigh on average 60 g less than non-Indigenous infants, however, we do not find empirical evidence of ethnicity differentiated COVID-19 impacts on mean birth weight. We explore distributional impacts by modeling the probability that an infant's birth weight is below X grams, using X values along the distribution of birth weight. While we do not find evidence of a COVID-19 impact for non-Indigenous infants, we find that Indigenous infants born after the start of the pandemic are 0.5% more likely to have birth weights below 1400 g (p < 0.1). The effect is stable and statistically significant in models with X from 1400 to 1050 g. This suggests that the COVID-19 pandemic had ethnicity differentiated impacts for infants at the very bottom of the birth weight distribution.
We explore three mechanisms potentially related to ethnicity differentiated COVID-19 impacts in the Amazon. First, we examine the issue of access to health care facilities by mothers in small municipalities in the remote countryside of the Amazon. We find that Indigenous mothers who travel to deliver their babies are 3.4% more likely (p < 0.05) to have a baby that weighs less than 1400 g when the baby was delivered after the start of the epidemic. This suggests that, after the start of the pandemic, access to health care has an ethnicity differentiated impact on infant health.
Second, we estimate whether ethnicity and the pandemic affect the probability of mothers having 7 or more prenatal consultations. We find that Indigenous mothers are 7.5% less likely (p < 0.01) to have proper prenatal care (before the pandemic). While we do not find evidence that the pandemic had ethnicity differentiated effects, we find statistical support for a COVID-19 impact on the general population of mothers. Mothers who deliver their babies after the start of the pandemic are 5.7% less likely (p < 0.01) to have 7 or more prenatal consultations. For mothers who travel long distances (400 km or more) for the delivery, the magnitude of this effect increases to −35.2% (p < 0.01).
Finally, we examine whether the supply of health care professionals in small municipalities of the countryside of Amazonas changes after the pandemic. We find that the ratio of Midwives per Indigenous birth decreases by almost 100% (p < 0.05) in the aftermath of the pandemic. We also find that the supply of physicians and nurses after the pandemic increases in the capital city Manaus. This increase is at least partially driven by migration of health care workers from the countryside to the capital city.
The paper contributes to a surprisingly small economics literature that examines Indigenous health. Newbold (1997) finds that individuals of Indigenous ethnicity are less likely to utilize physician services in Canada. Booth and Carroll (2008) study Indigenous health in Australia and find an Indigenous/non-Indigenous gap in self-reported health status. Elder et al. (2016) and Watson (2006) study the Native American-white infant mortality gap in the United States. The paper is also related to a larger empirical literature that examines a variety of health gaps across the world. For instance, previous research identifies rural-urban health gaps in China (e.g. Lin et al., 2021), socioeconomic status health gaps in Sweden (e.g. Jans et al., 2018), and black-white health gaps in the United States (e.g. Lhila and Long, 2012; Alexander and Currie, 2017).
In closing, the paper contributes to an evolving economics literature that uses observational data to examine COVID-19 policies (e.g Davillas and Jones, 2021; Brodeur et al., 2021; Dave et al., 2021; Friedson et al., 2021; Ruffini et al., 2021; Fang et al., 2020). Nevertheless, the economics literature that studies how the pandemic affects Indigenous Peoples is significantly less developed. A few papers discuss socioeconomic implications of the COVID-19 crisis for Indigenous individuals. For example, Hobbs (2020) discusses the potential impacts of supply chain disruption on remote Indigenous communities in northern Canada. Other papers explore the effects of the pandemic on Indigenous labor markets in Australia (Dinku et al., 2020) and Peru (Durán, 2022). When it comes to Indigenous health, the economics literature is very much still evolving. Using US county level data, McLaren (2021) investigates associations between the size of a county's Indigenous population share (and other minorities) and the county's COVID-19 mortality rate.
The remainder of the paper is organized as follows. Section 2 offers background information on the Brazilian health care system and the state of Amazonas. Section 3 describes the data and the construction of key variables. Section 4 discusses the empirical strategy. Results are presented in section 5, followed by the investigation of potential mechanisms in section 6. Robustness checks are presented in section 7. Finally, section 8 offers concluding remarks.
2. Background
2.1. The Brazilian health care system
The Brazilian Public Health Care System (Sistema Único de Saúde – SUS hereafter) was created in 1988 to meet the constitutional right that all citizens must have access to health care (Paim et al., 2011). SUS offers publicly provided health care (free of charge, co-payments, or any fees) to all Brazilians. It is a decentralized health care system where the three spheres of government – federal, state, and municipal govt. – are responsible for financing and managing health care delivery.7
The decentralization of the system was designed to meet health demands in a country of continental dimensions and significant regional diversity (Paim, 2009). As a result, in order to deliver health care that is effective at the community level, the management of primary health care systems is the responsibility of local governments (municipalities). Primary care is typically offered in municipal health clinics while more complex health services are offered in state or regional hospitals. The federal government's role in the system is mainly to offer financial and technical support.
In 2006, a new policy known as Pacto pela Saúde (Health Pact) established guidelines for financial transfers from federal to local governments (Brazilian Ministry of Health, 2006).8 In primary care, there are two types of financial transfers to municipalities. The first is an automatic transfer based on a fixed per resident value. The second type of transfer is for funding of community-specific programs with a maximum limit defined annually based on program background and goals, as well as technical parameters. As a result, while the system is designed to promote equitable health supply, several factors contribute to the persistence of significant health disparities among Brazilian communities, including heterogeneous urbanization rates, socioeconomic development, sanitary conditions, and susceptibility to environmental challenges (Victora et al., 2011).
Finally, in addition to creating the legal principles for a public health system, the Brazilian constitution of 1988 instituted that the private sector was free to offer health services.9 The challenges of financing a large system like SUS, especially in periods of economic recession, can lead to fluctuations in the quality of publicly provided health care (Doniec et al., 2018; Azevedo e Silva et al., 2020). As a result, the private health sector has experienced tremendous growth in urban regions (Lewis et al., 2015). In 2020, approximately 22.5% of Brazilians purchased private health insurance coverage.10 The Brazilian private health insurance market is the second largest in the world, trailing only the American market.11 Below we discuss the impacts of having two healthcare sub-sectors (public and private) on our identification strategy.
2.2. The state of Amazonas
Amazonas is a Brazilian state with 62 municipalities and a population of 3.48 million residents. The spatial distribution of residents is very uneven, and one municipality, Manaus (the state's capital), concentrates more than half of the population. The average size of the other 61 municipalities is 27.5 thousand residents. These small municipalities face serious economic challenges and are among the most impoverished and underdeveloped municipalities of Brazil. Amazonas is also the state with the largest Indigenous population in Brazil. In total, 820 thousand Brazilians self-identify as Indigenous Peoples, and approximately 20% of the Brazilian Indigenous population resides in the state of Amazonas.12
Among Brazilian states, Amazonas was arguably hit the hardest by the pandemic. Amazonas' hospitals were operating near capacity even before the coronavirus crisis. At the very beginning of the pandemic, the virus spread at a fast pace and the Amazonas health system quickly collapsed with severe shortages of ICU beds and respirators. Critics of the government blame a lack of investment in the health care system.13 Amid the health crisis, the state of Amazonas was also overwhelmed by a political crisis fueled by a major corruption scandal related to the procurement of health equipment.14
3. Data
3.1. Outcomes
Our primary data source is the Brazilian vital statistics database DATASUS/SINASC.15 According to Brazilian law, every child born alive must not only obtain civil registration documentation (i.e. birth certificate) but also a declaration of live birth. This information is collected in the SINASC database that keeps records of a variety of (mainly health-related) data about the pregnancy, birth, and characteristics of the newborn and the mother.16 Specifically, the data includes two infant health outcomes: the child's weight at birth (in grams) and Apgar score.17 Both outcomes are widely used in the literature to characterize infant health (e.g. Currie et al., 2009; Sonchak, 2015; Rangel and Vogl, 2019). The focus on these outcomes also allows for a short-run examination of health effects associated with the spread of the virus. Moreover, pregnant women may be at higher risk and experience more adverse outcomes from COVID-19 infection, which in turn can affect infant health (Luo and Yin, 2020; Kotlar et al., 2021). While much is still unknown regarding the effects of COVID-19, empirical evidence shows that in utero exposure to the Asian flu has negative effects on weight at birth and cognitive outcomes at later ages (Kelly, 2011). Another body of work suggests that newborns of different race and ethnicity have varying levels of infant health. It is therefore possible for ethnicity to be correlated with exposure to the virus and, as a result, the pandemic could generate ethnicity differentiated infant health (Elder et al., 2011, 2016; Watson, 2006). Finally, birth weight and Apgar scores are fitness at birth health outcomes that have been shown to be strongly correlated with infant mortality (Almond et al., 2005; Rocha and Soares, 2015).
3.2. COVID-19
We are interested in constructing a measure of COVID-19 exposure. Since mother-level information is not available, we construct a municipality-level variable to capture the start of the local epidemic in each municipality of residence of the mother. This information allows us to construct a measure of potential COVID-19 exposure, i.e. a localized measure of risk of exposure. In the absence of reliable data regarding number of positive COVID-19 cases in small and remote municipalities in the Brazilian Amazon, we identify the starting date of local epidemic in each municipality by using the date in which the first resident of each municipality was hospitalized with severe acute respiratory syndrome (SARS) due to COVID-19. The data comes from the DATASUS/SRAG database and it is summarized in Table A.1 (Online Appendix).18
We identify the impact of potential COVID-19 exposure using variation in the start of the epidemic in the mother's residence municipality (i.e. temporal and spatial variation). We construct two exposure measures. The first is the extensive margin of exposure, which simply distinguishes births before and after the first SARS-CoV-2 hospitalization, i.e. a dummy variable that equals 1 for births after the start of the epidemic in the mother's municipality of residence, zero otherwise. The second measure is the intensive margin of exposure, which counts the number of days between the start of the epidemic and birth (assigning zeros for births before the first SARS-CoV-2 hospitalization).
3.3. Covariates
The paper uses two sets of covariates. The first is a birth-level set that comes from the SINASC database and contains both mother and child information. Mother's characteristics include age, ethnicity, marital status, education, pregnancy history, and prenatal care. Child and pregnancy characteristics include the child's sex and ethnicity, an indicator for congenital anomaly, and an indicator for multiple pregnancy. Throughout the analysis, we consider a child to be of Indigenous ethnicity if either the mother or the child are identified as Indigenous in the database. The main sample includes all births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births in the capital city Manaus).19 The second set of covariates are constructed using municipality level data from IBGE (socioeconomic) and DATASUS/CNES (health infrastructure).20 We use the following variables to capture characteristics of municipalities: number of primary health care clinics per 1000 residents, GDP per capita, and illiteracy rate.
3.4. Descriptive statistics
Table 1 shows summary statistics of our sample. Between Jan 1, 2019 and July 31, 2020, there were 59,381 births in small municipalities in the Amazon, of which 18.4% were Indigenous births. We observe non-Indigenous/Indigenous infant health gaps in our sample. Mean birth weight of non-Indigenous infants is 3237 g, with Indigenous infants weighting 95 g less (p < 0.01). Indigenous babies have slightly lower Apgar scores with the probability P[Apgar <8] being 0.7 percent higher (p < 0.1) than that of non-Indigenous infants.
Table 1.
Summary statistics.
| Non-Indigenous |
Indigenous |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | mean | median | s.d. | N | mean | median | s.d. | |
| Health outcomes | ||||||||
| Birth weight (grams) | 48,459 | 3237.3 | 3250 | 546.8 | 10,922 | 3142.4 | 3150 | 507.9 |
| Apgar score | 46,873 | 8.27 | 8 | 0.99 | 6772 | 8.19 | 8 | 0.96 |
| COVID-19 Exposure | ||||||||
| Extensive margin | 48,500 | 0.16 | 0 | 0.37 | 11,276 | 0.14 | 0 | 0.35 |
| Intensive margin | 48,500 | 8.89 | 0 | 24.04 | 11,276 | 7.37 | 0 | 21.64 |
| Mother's characteristics | ||||||||
| Age (years) | 48,500 | 24.06 | 23 | 6.6 | 11,272 | 24.52 | 23 | 6.97 |
| Married (baseline) | 48,465 | 0.45 | 0 | 0.50 | 11,247 | 0.39 | 0 | 0.49 |
| Single | 48,465 | 0.55 | 1 | 0.5 | 11,247 | 0.61 | 1 | 0.49 |
| Widow | 48,465 | 0 | 0 | 0.03 | 11,247 | 0 | 0 | 0.03 |
| Divorced | 48,465 | 0 | 0 | 0.05 | 11,247 | 0 | 0 | 0.03 |
| No schooling (baseline) | 48,450 | 0.01 | 0 | 0.10 | 11,227 | 0.10 | 0 | 0.30 |
| 1–3 years of schooling | 48,450 | 0.03 | 0 | 0.18 | 11,227 | 0.08 | 0 | 0.28 |
| 4–7 years of schooling | 48,450 | 0.21 | 0 | 0.4 | 11,227 | 0.27 | 0 | 0.44 |
| 8–11 years of schooling | 48,450 | 0.69 | 1 | 0.46 | 11,227 | 0.53 | 1 | 0.5 |
| 12 or more years of schooling | 48,450 | 0.06 | 0 | 0.24 | 11,227 | 0.02 | 0 | 0.14 |
| # of previous live births | 47,616 | 1.56 | 1 | 1.8 | 11,032 | 2.31 | 2 | 2.28 |
| # of previous stillbirths | 46,141 | 0.22 | 0 | 0.53 | 10,615 | 0.23 | 0 | 0.62 |
| 7 or more prenatal consultations | 48,396 | 0.52 | 1 | 0.5 | 11,266 | 0.39 | 0 | 0.49 |
| Infant/pregnancy characteristics | ||||||||
| Girl | 48,496 | 0.49 | 0 | 0.50 | 11,273 | 0.48 | 0 | 0.5 |
| Congenital anomaly | 48,289 | 0.01 | 0 | 0.07 | 11,222 | 0.01 | 0 | 0.07 |
| Multiple pregnancy | 48,494 | 0.01 | 0 | 0.11 | 11,274 | 0.01 | 0 | 0.12 |
| Municipality characteristics | ||||||||
| Clinics (per 10k residents) | 48,500 | 2.56 | 2 | 1.46 | 11,276 | 2.48 | 2 | 1.54 |
| Illiteracy rate | 48,500 | 0.17 | 0 | 0.08 | 11,276 | 0.20 | 0 | 0.07 |
| GDP per capita (BRL) | 48,500 | 6737.7 | 5239 | 4993.9 | 11,276 | 4403.3 | 4359 | 1259.8 |
Notes: Sample includes all births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births of residents of the capital city Manaus). Unless otherwise indicated, all variables are dummy variables reflecting the information available in the original data files without any prior data treatment. BRL: Brazilian Real. Exchange rate (5 year average): 1 USD = 3.83 BRL.
In terms of COVID-19 potential exposure, 16% of the non-Indigenous (14% of Indigenous) births happened after the start of the epidemic in the municipality of residence of the mother. The average number of days of (intensive margin) exposure, i.e. number of days between birth and the start of the epidemic (with zero days assigned for pre-epidemic births), is 8.9 days for non-Indigenous (and 7.4 days for Indigenous) infants. The table also shows that Indigenous mothers are half a year older, are less likely to be married, are less educated – for instance, 9.2% more likely to have no education and 4.2% less likely to have 12 or more years of education – have a history of more pregnancies, and are 13% less likely to have 7 or more prenatal consultations (all with p < 0.01). Finally, the table shows that Indigenous mothers live in municipalities with less health infrastructure, higher illiteracy rates, and significantly lower GDP per capita (all with p < 0.01).
4. Empirical strategy
We estimate infant health effects of potential exposure to COVID-19 exploiting variation over time and across municipalities. The identifying variation is based on the fact that the epidemic reached different municipalities at different moments in time therefore generating variation in the risk of exposure of mothers across the Amazon. Our estimates are consistent under the assumption that the initial (first wave) spread of the virus is orthogonal to fertility decisions and the estimation approach controls for confounding effects from other unobserved health determinants. If, for example, the level of potential exposure is higher in municipalities that have weaker health care infrastructure, higher unemployment, or lower food security, then the variation in infant health may reflect these systematic shortcomings of local health care systems, as opposed to effects of exposure to the virus.
We adopt a number of strategies to address this issue. First, we focus our empirical analysis on the more homogeneous group of small municipalities in the Amazon and exclude data from the capital city Manaus. Manaus is a large urban center while the other municipalities in the Amazon are small and predominantly rural. The characteristics of both health supply and health demand are very different between these two settings. For example, on the supply side, Manaus has large hospitals equipped to offer complex and sophisticated health services while the health care systems in the countryside largely focus on primary health care. Moreover, many determinants of health care demand are systematically different between capital and countryside. For example, while the GDP per capita of Manaus is BRL 27,833, average (population weighted) GDP per capita of all other municipalities in Amazonas is BRL 6380. With health infrastructure concentrated in Manaus, allied to the fact that the countryside is characterized by fragile and underfunded local health systems, where there is a lack of testing and little information about the COVID-19 virus, it is therefore reasonable to treat the COVID-19 pandemic as a ‘shock’ to mothers in small municipalities of the Amazon.
Another aspect that supports identification is the fact that the virus spread through remote regions of the Amazon with little resistance or awareness of the local population. Despite the fact that the state of Amazonas covers an area of more than 1.5 million sq km,21 the epidemic reached all Amazonian municipalities in a period of less than four months. The rapid spread of the virus throughout these small municipalities aligned with a general sense of misinformation that minimized health risks makes it unlikely that, after controlling for mother's characteristics and municipality and month-by-year fixed effects, our measures of COVID exposure is an endogenous variable during the period of our analysis.22
In addition, by studying the first wave of the epidemic (March–July 2020), we avoid concerns regarding endogenous fertility decisions. To understand why, note that ‘treated’ (post-pandemic) births are the result of fertility decisions in pre-pandemic periods. In other words, even for infants born in the last day of our sample (July 31, 2020), it is reasonable to assume that fertility decisions made approximately nine (or more) months earlier (i.e. November of 2019) by individuals in the remote Amazon are not influenced by the risk of a COVID epidemic.
Finally, while the public health care system is effectively the only option available to patients in small rural Amazonian municipalities, patients in the capital city have access to not only public but also private health services.23 As quality and availability of private health care are significantly different from those offered by the public system, in addition to the systematic differences between patients of the two systems, examining the health care systems of homogeneous small rural municipalities minimizes issues related to selectivity.
Our first empirical model focuses on the estimation of potential exposure effects and their contribution to Indigenous health gaps. In the model we compare births before and after the start of the epidemic in the mother's municipality, interacting the epidemic with Indigenous ethnicity status. To identify coronavirus-related Indigenous infant health gaps, we estimate the following model:
| (1) |
where Y ijt is the infant health outcome of birth i in municipality j at day t. COVIDjt is a measure of potential exposure to the epidemic (intensive or extensive margin). I i is a binary indicator for an Indigenous birth. X it is a vector of birth-specific control variables for maternal and child characteristics. Z j is a vector of pre-pandemic municipality characteristics that is interacted with a linear trend.
Maternal characteristics include age, marital status indicators (single, divorced, widow – baseline is married), schooling indicators (1–3 years of education, 4–7 years of education, 8–11 years of education, 12 plus years – baseline is no education), history of previous pregnancies (number of previous live births and number of previous stillbirths), and an indicator for whether the mother had 7 or more prenatal consultations. Child characteristics include gender, an indicator for congenital anomalies, and an indicator for whether the child is part of a multiple pregnancy. The vector of pre-pandemic municipality characteristics includes the number of primary care clinics (per 1000 residents), illiteracy rate, and GDP per capita.
Finally, μ j is a municipality fixed effect to control for unobserved time-invariant determinants of infant health, including initial socioeconomic environment and persistent municipality characteristics such as infrastructure and local prevalence and incidence of health conditions and diseases. γ m is a month-by-year fixed effect to control for common shocks or time trends such as those induced by variations in economic conditions (e.g. food markets) and national policies, and seasonal effects such as those correlated with family background and other unobserved season-of-birth determinants of health outcomes (Welch et al., 2021; Buckles and Hungerman, 2013). The error term is ϵ ijt. We estimate robust standard errors clustered at the municipality level to account for serial correlation (Bertrand et al., 2004).
The key coefficient of interest is the coefficient β 3, which measures the ethnicity differentiated effect of COVID on infant health outcomes. Another important coefficient is β 1, which measures the effect of potential exposure for non-Indigenous births (i.e. the effect of COVID on Indigenous infants is captured by β 1 + β 3). Finally, β 2 captures the baseline birth weight gap between Indigenous and non-Indigenous infants.
Note that our approach resembles a triple difference design. A specification that imposes β 3 = 0 is similar to a twoway fixed effect model with differential timing due to the staggered spread of the epidemic across municipalities over time (Angrist and Pischke, 2009; Goodman-Bacon, 2021; Kim and Wang, 2019; Cunningham, 2021). The specification of the interaction term β 3 I i × COVIDjt allows us to examine the effect of the pandemic in the targeted sub-population of Indigenous infants, much in the spirit of a typical triple difference model (i.e. treatment is staggered across time, space, and ethnicity).
We also estimate the following event-study specification to gain insights on the timing of effects of potential exposure to the virus:
| (2) |
where indicates that day t is in month k, measured relative to the start of the epidemic (when k is normalized to 0). Note that model (2) aggregates the measurement of treatment to the month level by making k a (relative) month, as opposed to a (relative) day. This makes the baseline period k = −1 be a collection of births in an entire month, not in a single day. The advantage of such an aggregation is that it avoids day-to-day birth weight fluctuations that are very noisy in small municipalities. An event study specification that relies on daily variations would deliver an imprecise series of more than 400 coefficients. This makes results hard to interpret as it would be possible for a single (or few) coefficient(s) to be statistically different from zero (by chance), which would indicate a birth weight deviation from the average of a single specific day k = −1 that precedes the start of the pandemic.
γk is a month fixed effect, which captures the impact of each relative-month k on non-Indigenous birth weights (the baseline). The coefficients of interest are the β ks, which capture additional impacts of month k on Indigenous birth weights. If the pandemic has an ethnicity differentiated negative effect, we would observe β k = 0 for k < − 1 and β k < 0 for k ≥ 0.
Finally, we also examine the impact of potential exposure along the more vulnerable (below mean) range of the birth weight distribution. Following Lindo (2011), we estimate models to capture the impacts on the probability that birth weight is less than X grams. These distributional models employ municipality and month-by-year fixed effects along with the full set of controls. Specifically, we estimate the following equation:
| (3) |
where is an indicator that birth i in municipality j at day t produced a child with birth weight Y less than X grams. We estimate several models that differ by varying X from 1000 to 3200. Similarly to model (1), the coefficient β 3 measures the ethnicity differentiated effect of COVID on the probability that birth weight is below X grams.
5. Results
We divide the presentation of our findings into two parts. First, we discuss a number of results regarding ethnicity differentiated impacts of the COVID-19 pandemic on infant health (sections 5.1, 5.2, 5.3, 5.4). Next, we document pre-pandemic health gaps between Indigenous and non-Indigenous infant health in the Brazilian Amazon (section 5.5).
5.1. Indigenous health gaps
Table 2 presents the estimates of the main parameters of model (1).24 Panel A shows the results for the extensive margin models, i.e. COVID is measured as a dummy variable with 1s for births after the beginning of the epidemic, zeros otherwise. Panel B shows the results for the intensive margin models, i.e. COVID measures the number of days between birth and the start of the epidemic, zeros before the start. The models in columns (1) have birth weight (in grams) as left-hand side variables. The dependent variable in column (2) is an indicator for Apgar scores below 8.
Table 2.
Effects of COVID on infant health outcomes.
| (1) |
(2) |
|
|---|---|---|
| birth weight | ||
| Panel A: extensive margin | ||
| COVID | −1.046 (19.556) |
−0.023 (0.014) |
| Indigenous | −59.908∗∗∗ (10.075) |
−0.003 (0.009) |
| Indigenous × COVID | −10.552 (20.329) |
0.013 (0.015) |
| N | 55,731 | 50,513 |
| R-squared | 0.105 | 0.061 |
| Panel B: intensive margin | ||
| COVID | 0.225 (0.439) |
−0.000 (0.000) |
| Indigenous | −60.833∗∗∗ (9.809) |
−0.002 (0.009) |
| Indigenous × COVID | −0.090 (0.294) |
0.000 (0.000) |
| N | 55,731 | 50,513 |
| R-squared | 0.105 | 0.061 |
Notes: The table shows coefficients from four different regressions. The dependent variable is birth weigh in the two models displayed in column (1), and in the two models of column (2). Panel A: COVID variable captures the extensive margin of potential COVID-19 exposure. Panel B: COVID variable captures the intensive margin of potential COVID-19 exposure. All regressions include mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. All regressions control for municipality and month-by-year fixed effects. Standard errors clustered at the mother's residence municipality are in parentheses. Sample includes all births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births in the capital city Manaus). ∗p < 0.1, ∗∗p < 0.05, ∗∗∗p < 0.01.
Results suggest that, on average, we cannot reject the null of no impacts of the COVID-19 pandemic on infant health (as measured by birth weight and Apgar scores) in the Brazilian Amazon. The coefficient of COVID (β 1) and its interaction with Indigenous (β 3) are not statistically different from zero. That is, using data on all Amazonian births from 2019 up to July 31, 2020, we find no statistical evidence that our two infant health outcomes for both Indigenous and non-Indigenous infants varied in response to the pandemic.
5.2. Heterogeneity
It is possible that the pandemic null effects reported above mask important forms of heterogeneities. To explore whether the virus have heterogeneous effects across infants of different types of mothers, we estimate model (1) using different subgroups of the population. Using vital statistics data from the U.S., Elder et al. (2016) find that maternal marital status, age, education, and prenatal care are primary drivers of the infant mortality rate gaps for blacks, Puerto Ricans, Asians, and Native Americans (all relative to whites). Table 3 presents estimates from different subsamples that split mothers into different groups based on Elder et al. health gap drivers.
Table 3.
Effects of COVID on infant health outcomes – Heterogeneity.
| (1) |
(2) |
(3) |
(4) |
(5) |
(6) |
(7) |
(8) |
|
|---|---|---|---|---|---|---|---|---|
| Marital status |
Age (years) |
Education (years) |
Prenatal visits |
|||||
| Married | Not married | |||||||
| Panel A: birth weight (EM) | ||||||||
| COVID | −9.995 (26.071) |
7.192 (26.500) |
6.949 (30.843) |
232.925 (188.617) |
49.730 (86.370) |
10.532 (20.926) |
29.430 (23.231) |
−31.776 (25.412) |
| Indigenous | −77.717∗∗∗ (14.173) |
−35.655∗∗∗ (12.595) |
−41.751∗∗ (17.734) |
0.685 (75.184) |
−78.818∗∗∗ (29.199) |
−61.058∗∗∗ (11.441) |
−59.460∗∗∗ (10.702) |
−59.549∗∗∗ (15.333) |
| Indigenous × COVID | 16.955 (30.464) |
−31.497 (21.917) |
−30.348 (35.904) |
−154.377 (159.015) |
−61.510 (55.586) |
−14.140 (26.278) |
−29.800 (24.226) |
13.688 (24.214) |
| N | 24,369 | 31,362 | 12,596 | 747 | 3605 | 39,980 | 27,712 | 28,019 |
| R-squared | 0.101 | 0.105 | 0.110 | 0.138 | 0.115 | 0.109 | 0.090 | 0.089 |
| Panel B: birth weight (IM) | ||||||||
| COVID | 0.600 (0.608) |
−0.221 (0.448) |
0.577 (0.640) |
0.346 (3.225) |
−1.332 (1.864) |
0.280 (0.445) |
0.553 (0.588) |
−0.199 (0.474) |
| Indigenous | −75.851∗∗∗ (14.117) |
−39.000∗∗∗ (12.647) |
−41.600∗∗ (17.809) |
−5.635 (68.710) |
−82.898∗∗∗ (29.299) |
−60.920∗∗∗ (11.020) |
−61.615∗∗∗ (10.242) |
−59.721∗∗∗ (15.421) |
| Indigenous × COVID | 0.034 (0.472) |
−0.218 (0.327) |
−0.579 (0.589) |
−1.540 (2.425) |
−0.492 (0.808) |
−0.275 (0.403) |
−0.321 (0.358) |
0.272 (0.371) |
| N | 24,369 | 31,362 | 12,596 | 747 | 3605 | 39,980 | 27,712 | 28,019 |
| R-squared | 0.102 | 0.105 | 0.110 | 0.136 | 0.115 | 0.109 | 0.090 | 0.088 |
| Panel C: (EM) | ||||||||
| COVID | −0.033∗ (0.020) |
−0.016 (0.018) |
−0.014 (0.028) |
−0.076 (0.110) |
−0.066 (0.054) |
−0.016 (0.015) |
−0.009 (0.019) |
−0.037∗ (0.019) |
| Indigenous | 0.001 (0.011) |
−0.007 (0.011) |
−0.014 (0.014) |
0.022 (0.043) |
−0.005 (0.021) |
−0.002 (0.010) |
0.003 (0.009) |
−0.007 (0.011) |
| Indigenous × COVID | −0.027 (0.022) |
0.037∗ (0.021) |
0.033 (0.035) |
0.080 (0.117) |
0.065 (0.048) |
0.010 (0.015) |
0.018 (0.017) |
0.012 (0.023) |
| N | 22,230 | 28,283 | 11,617 | 636 | 2597 | 37,368 | 24,013 | 26,500 |
| R-squared | 0.067 | 0.061 | 0.069 | 0.211 | 0.095 | 0.061 | 0.064 | 0.064 |
| Panel D: (IM) | ||||||||
| COVID | −0.000 (0.000) |
−0.000 (0.000) |
0.000 (0.000) |
0.002 (0.002) |
0.000 (0.001) |
−0.000 (0.000) |
−0.000 (0.000) |
−0.000 (0.000) |
| Indigenous | −0.001 (0.011) |
−0.004 (0.011) |
−0.012 (0.014) |
0.026 (0.046) |
−0.004 (0.022) |
−0.000 (0.011) |
0.005 (0.009) |
−0.007 (0.011) |
| Indigenous × COVID | −0.000 (0.000) |
0.000 (0.000) |
0.000 (0.000) |
0.000 (0.002) |
0.001 (0.001) |
0.000 (0.000) |
0.000 (0.000) |
0.000 (0.000) |
| N | 22,230 | 28,283 | 11,617 | 636 | 2597 | 37,368 | 24,013 | 26,500 |
| R-squared | 0.067 | 0.061 | 0.069 | 0.211 | 0.095 | 0.061 | 0.064 | 0.064 |
Notes: Each panel shows results from 8 regressions (columns). Dependent variable is birth weight (grams) in panels A and B, and a dummy in panels C and D. Panels A and C: COVID variable captures the extensive margin of potential COVID-19 exposure. Panels B and D: COVID variable captures the intensive margin of potential COVID-19 exposure. All regressions include mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. All regressions control for municipality and month-by-year fixed effects. Standard errors clustered at the mother's residence municipality are in parentheses. Sample includes all Indigenous births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births in the capital city Manaus). ∗p < 0.1, ∗∗p < 0.05, ∗∗∗p < 0.01.
In general, these models confirm previous results – we do not find evidence of impacts of the pandemic on mean birth weights and Apgar scores. For the most part, estimates of COVID and its interaction with Indigenous are not statistically significant. One model suggests that the pandemic widens the gap between non-Indigenous and Indigenous health. For not married mothers, the β 3 estimate suggests that Indigenous infants born after the start of the pandemic are 3.7% more likely to have below 8 Apgar scores (column 2, panel C). However, with a p-value of 0.099, this estimate is only marginally significant and therefore offers little statistical support for the hypothesis that Indigenous status matters for single mothers giving birth after the pandemic. Two models suggest the puzzling result that married mothers and mothers with adequate prenatal care that give birth after the beginning of the pandemic have a marginally significant 3% lower probability (p < 0.1) of having a baby with low Apgar score (columns 1 and 8, panel C).
5.3. Event study
Fig. 1 reports estimates of the coefficients β k of model (2) along with their 95% confidence intervals. As the model includes relative month k fixed effects, the coefficients β k represent ethnicity differentiated impacts of month k on birth weights, relative to the baseline period k = −1. If the pandemic has an effect of widening the ethnicity health gap, we would expect to see a statistically significant downward sloping trend for estimates β to the right of the vertical line at k = −1 (beginning of the epidemic).
Fig. 1.
Event study estimates of ethnicity differentiated impacts on birth weights. Notes: Estimates of the coefficient βk from model (2), along with their 95% confidence intervals. The regression includes mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. The regression controls for municipality and (relative) month fixed effects. Standard errors are clustered at the mother's residence municipality. The regression has 55,731 observations corresponding to all births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births in the capital city Manaus).
The figure shows that β estimates oscillate between 0 and -50 for months before and after the epidemic. All β coefficients are statistically insignificant and the figure shows that confidence intervals of all estimates include zero. These event-study results indicate that we cannot reject the null that the pandemic did not generate ethnicity differentiated impacts on mean birth weights.25
5.4. Distributional effects
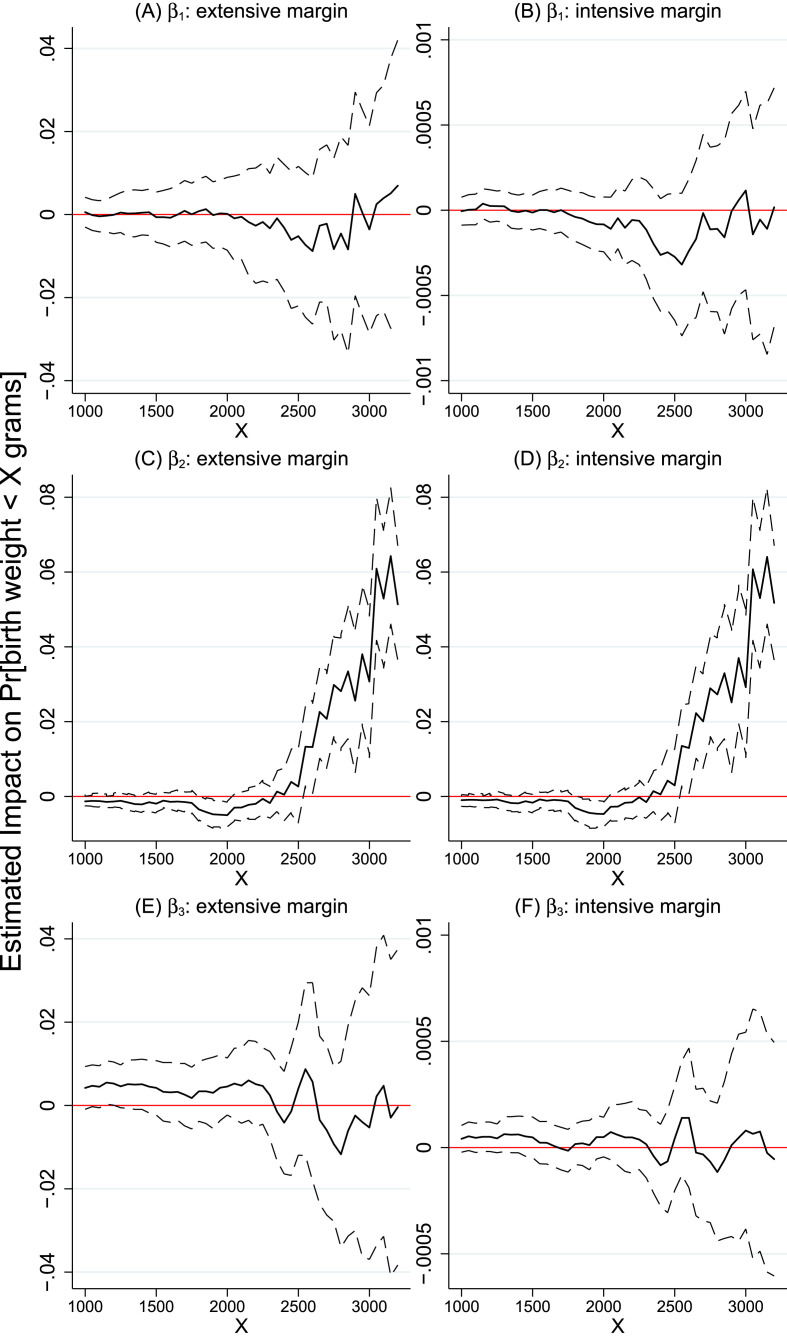
Fig. 2 summarizes distributional impacts by collecting multiple estimates of model (3). Specifically, the impact of each potential exposure margin (extensive and intensive) was estimated using 45 regressions that vary the level of X in equation (3) from 1000 to 3200. The left-hand side graphs plot the coefficients of COVID (β 1, plot A), Indigenous (β 2, plot C), their interaction (β 3, plot E) of the 45 extensive margin regressions, along with their 95% confidence intervals. Similarly, the right-hand side graphs (plots B, D, and F) collect the estimates of the intensive margin regressions.
Fig. 2.
Distributional impacts. Notes: The graphs report estimates of β coefficients from model (3) where the left-hand-side variable is a dummy that takes value of 1 if a child's birth weight is less than X grams (with X plotted in the x-axis). Each figure summarizes estimates from 45 regressions where X varies from X = 1000 to X = 3200, in intervals of 50. The left column plots estimates where COVID is measured by the extensive margin exposure variable. The right column shows results for the intensive margin regressions. All regressions include mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. All regressions control for municipality and month-by-year fixed effects. Standard errors are clustered at the mother's residence municipality. Each regression has 55,731 observations corresponding to all births in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding births in the capital city Manaus).
Graphs A and B (top row of the figure) show that the we cannot reject the null that the pandemic did not affect the probability of non-Indigenous infants having birth weights below X grams. For all ranges of X, the 95% confidence intervals of the estimates of the marginal impact of COVID on P[Y ijt < X] include zero, both in the extensive and intensive margin models. This suggests that, for non-Indigenous infants, the null COVID results reported in previous sections for the mean also hold for other ranges of the birth weight distribution.
Graphs E and F (bottom row of the figure) show the estimates of β 3; ethnicity differentiated marginal impacts of COVID. The β 3 estimates of the intensive margin models are statistically insignificant in all 45 regressions (i.e. for all values of X). However, we estimate statistically significant ethnicity differentiated effects in the extensive margin models at the lower range of the birth weight distribution. In general, we find that Indigenous infants that were born after the start of the epidemic are 0.5% more likely to have birth weights below X, for X ≤ 1400 g. We report these estimates in the Online Appendix (Table A.7, left-hand side, main sample). The strongest effect is detected at X = 1150. Exclusively for Indigenous infants, we find that COVID increases the probability of being born with weight below 1150 g (P[Y < 1150]) by approx 0.6% (p < 0.05). These results suggest that, for vulnerable babies, the pandemic has an impact on infant health that varies by Indigenous ethnicity. This is in line with recent findings that the COVID-19 pandemic has a disproportionate impact on minorities (e.g. Couch et al., 2020).
5.5. Pre-pandemic health gaps
Our models allow us to test whether pre-pandemic (or baseline) health gaps between Indigenous and non-Indigenous infants exist in the Brazilian Amazon. First, we use model (1) to test the null of no baseline mean gaps (H 0: β 2 = 0). Results in Table 2 (column 1) show that we reject the hull of no baseline birth weight gap between Indigenous and non-Indigenous infants. We find that, after conditioning for mother and infant/pregnancy characteristics, municipality and months fixed effects, and socioeconomic municipal trends, pre-pandemic birth weights of Indigenous infants are on average approximately 60 g lower than those of non-Indigenous infants (p < 0.01). While the economics literature has overlooked the Indigenous and non-Indigenous birth weight gap, these results are in line with observations documented in the medical literature (e.g. Roberts and Lancaster, 1999). We do not find evidence of a baseline ethnicity gap in the probability of having Apgar scores below 8 (column 2).26
Second, we use the split sample models (see section 5.2) to further examine baseline infant health gaps. The estimates in Table 3 show significant heterogeneity in pre-pandemic Indigenous birth weight gaps.27 The first two columns of Table 3 show that the Indigenous/non-Indigenous birth weight gap is twice as large for married mothers (−78 g, p < 0.01) than for not-married mothers (−36 g, p < 0.01). Note that these results do not suggest that birth weight levels of children of not-married mothers are higher. In fact, estimates of model (1) show that single mothers have infants with lower birth weights (see Table A.2, Online Appendix). Instead, the analysis suggests that the ethnicity disadvantage in a sample of more vulnerable not-married mothers is smaller.
With respect to age, we find that the Indigenous birth weight gap among young mothers is approximately −42 g (p < 0.05, see column 3). However, column 4 shows that the Indigenous gap disappears in the sample of mothers over 40 years of age. This result contrasts with the finding that the black-white gap in infant mortality increases with mother's age (Elder et al., 2011; Rich-Edwards et al., 2003; Geronimus, 1996). We also find that the gap of Indigenous to non-Indigenous birth weights is larger for infants from mothers with less than 4 years of education than that associated with mothers with 8 or more years of education. Specifically, we estimate ethnicity gaps of −79 g (p < 0.01) and −61 g (p < 0.01) in the samples with less and more educated mothers, respectively. Finally, we find that the estimated difference between Indigenous and non-Indigenous birth weights does not vary between the high and low prenatal visits samples. In fact, these estimates are around −60 g (p < 0.01), which is similar to the level of those from our main model (see Table 2).
Finally, we discuss distributional impacts on the baseline ethnicity gap (see section 5.4). Fig. 2 (graphs C and D) offers new insights about the baseline Indigenous/non-Indigenous birth weight gap. While the estimates of model (1) show that, on average, Indigenous infants have birth weight 60 g lower than non-Indigenous infants (see Table 2), Fig. 2 suggests the mean gap is driven by infants with birth weight greater than 2500 g. The impact of Indigenous on P[Y ijt < 2550] is equal to 0.013 (p < 0.05) indicating that Indigenous infants are 1.3% more likely to have a birth weight less than 2550 g.28 In general, the estimates of β 2 increase with X until a maximum of 0.064 (p < 0.01) at 3150 g, i.e. Indigenous infants are 6.4% more likely to have birth weights below 3150 g.
6. Potential mechanisms
6.1. Access to health care facilities
In addition to the municipality of residency of mothers, the SINASC data set has information on the municipality of the health care facility where delivery happened. We construct an indicator for mothers who deliver their babies in municipalities that are not those of residence. In our main sample of mothers who live in small municipalities, i.e. all municipalities in Amazonas except Manaus, out of the 55,731 births approximately 13.3% of the deliveries were not in the municipality of residence of the mother.29 This statistics reflects the reality that access to health care is a challenge for many mothers in small and remote municipalities in the Amazon. This is in line with previous research that identifies distance to treatment sources as an important deterrent to health care treatment in remote communities of the Brazilian Amazon (de Bartolome and Vosti, 1995).
We revisit our extensive margin analysis of distributional impacts by re-estimating model (3) using the subsample of traveling mothers. The model specification is the same as the one above with an additional explanatory variable: the travel distance between the mother's municipality of residency and the municipality of birth (measured in 100 km).30 In this context, the travel distance is a proxy for remoteness in terms of access to health care as it captures how far a mother has to travel to deliver her baby.
The results (Table 4 ) corroborate our previous findings. First, we note that we do not find an effect of COVID on the probability of low birth weights for non-Indigenous infants born to traveling mothers. However, again we find evidence that the pandemic has an ethnicity differentiated effect on the left tail of the birth weight distribution.31 The estimated impacts for traveling mothers are stronger than those from the main sample. For example, column 2 shows that Indigenous infants born to traveling mothers after the start of the epidemic in the mother's municipality of residence are 3.4% more likely (p < 0.05) than non-Indigenous infants to have birth weights below 1100 g.
Table 4.
Distributional impacts for traveling mothers.
| (1) |
(2) |
(3) |
(4) |
(5) |
(6) |
|
|---|---|---|---|---|---|---|
| COVID | 0.002 (0.007) |
−0.000 (0.008) |
0.000 (0.008) |
−0.006 (0.013) |
−0.009 (0.013) |
−0.002 (0.014) |
| Indigenous | −0.001 (0.003) |
−0.004 (0.004) |
−0.003 (0.003) |
−0.009∗ (0.004) |
−0.013∗∗∗ (0.004) |
−0.012∗∗ (0.005) |
| Indigenous × COVID | 0.022 (0.015) |
0.034∗∗ (0.014) |
0.033∗∗ (0.013) |
0.031∗∗ (0.013) |
0.034∗∗ (0.015) |
0.023 (0.016) |
| Travel distance (100 km) | 0.008∗∗∗ (0.002) |
0.011∗∗∗ (0.002) |
0.013∗∗∗ (0.004) |
0.017∗∗∗ (0.004) |
0.018∗∗∗ (0.005) |
0.019∗∗∗ (0.005) |
| N | 6538 | 6538 | 6538 | 6538 | 6538 | 6538 |
| R-squared | 0.045 | 0.053 | 0.053 | 0.065 | 0.073 | 0.072 |
Notes: Y denotes birth weight (in grams). COVID represents the extensive margin of exposure. All regressions include mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. All regressions control for municipality and month-by-year fixed effects. Standard errors clustered at the mother's residence municipality are in parentheses. Sample includes births from mothers that delivered their babies in a municipality different from that of their residency. The sample does not include mothers residing in the capital city Manaus. Sample period is from Jan 1, 2019 to July 31, 2020. ∗p < 0.1, ∗∗p < 0.05, ∗∗∗p < 0.01.
We also find that the probability of low birth weight depends on the travel distance. For every 100 km that mothers have to travel to deliver their babies, the probability of having an infant that weighs less than 1100 g increases by 1.1% (column 2, p < 0.01), and the probability of having an infant that weighs that weighs less than 1500 g increases by 1.9% (column 6, p < 0.01).
Finally, we note a negative baseline gap on the probability of the birth weight being less than 1400 or 1500 g (columns 5 and 6). This implies that, when examining the subset of traveling mothers, Indigenous children are approximately one percentage point more likely to be above 1400 or 1500 g (relative to non-Indigenous). While it might seem puzzling, this result is in line with findings discussed in section 5.5. First, as discussed above, we find that the ethnicity disadvantage is smaller in the sample of more vulnerable not-married mothers. Given that mothers likely travel to deliver their babies because they need to, probably because there is no acceptable alternative health care facility in the municipality of residence, the baseline finding in Table 4 again shows that, for vulnerable and socially disadvantaged mothers, the Indigenous disadvantage is attenuated. In addition, we note that for the general population, the results in Fig. 2 (graphs C and D) show that the mean Indigenous birth weight gap is driven by infants with birth weight greater than 2500 g. That is, the Indigenous baseline birth weight disadvantage is not driven by the few infants on the left side of the birth weight distribution, but by those in the middle and right side (see discussion in section 5.5).
6.2. Prenatal care
We examine whether the start of the pandemic affects health care utilization by pregnant women. We test this hypothesis by estimating an empirical model for the probability that the mother had proper prenatal care (i.e. at least 7 prenatal visits). Here, it is important to highlight a limitation of our data. While COVID indicates births after the start of the pandemic, we do not have information about when prenatal visits happened or the exact number of visits for all mothers (due to data censoring). Therefore, our analysis focuses on the binary indicator .
Table 5 shows the results of the prenatal visits model. While we do not find ethnicity differentiated COVID impacts, mothers who delivered their babies after the start of their local epidemic are 5.7% less likely to have had adequate prenatal care (column 1, p < 0.01). This result suggests that the pandemic affected prenatal care of mothers of all ethnicities.
Table 5.
Effects of COVID on prenatal care.
| (1) |
(2) |
(3) |
(4) |
|
|---|---|---|---|---|
| All | Non Traveling | Traveling | Traveling ( 400 km) | |
| COVID | −0.057∗∗∗ (0.020) |
−0.051∗∗ (0.022) |
−0.087 (0.056) |
−0.352∗∗∗ (0.059) |
| Indigenous | −0.075∗∗∗ (0.018) |
−0.077∗∗∗ (0.019) |
−0.046 (0.028) |
−0.052 (0.103) |
| Indigenous × COVID | 0.051 (0.031) |
0.052 (0.032) |
0.031 (0.085) |
−0.126 (0.193) |
| N | 56,117 | 48,699 | 7418 | 1040 |
| R-squared | 0.121 | 0.130 | 0.100 | 0.154 |
Notes: Dependent variable is an indicator for 7 (or more) prenatal visits. COVID represents the extensive margin of exposure. All regressions include mother and child covariates in addition to pre-pandemic municipality characteristics interacted with a linear trend. All regressions control for municipality and month-by-year fixed effects. Standard errors clustered at the mother's residence municipality are in parentheses. Sample includes all mothers who delivered a baby in the state of Amazonas from Jan 1, 2019 to July 31, 2020 (excluding mothers that reside in the capital city Manaus). ∗p < 0.1, ∗∗p < 0.05, ∗∗∗p < 0.01.
While we cannot reject the null that the pandemic did not cause ethnicity differentiated gaps in prenatal care, results show a baseline (pre-pandemic) Indigenous/non-Indigenous health gap in prenatal care. The estimates are large and reflect a situation of persistent inequalities in the Brazilian Amazon. Indigenous mothers are 7.5% less likely to have 7 (or more) prenatal consultations (column 1, p < 0.01).
Next, we split the sample into two groups; non-traveling and traveling mothers. The results from the non traveling subsample (column 2) are very similar to those of the main sample. While we find some discrepancy in the point estimates of coefficients between the main sample (column 1) and the subsample of traveling mothers (column 3), the latter coefficients are imprecisely estimated and we cannot reject the null of no associations between COVID or Indigenous ethnicity with prenatal care. However, when we examine the subsample of traveling mothers who reside in the most remote locations, defined as those who travel at least 400 km (250 miles) to deliver their babies, we find a large and precisely estimated negative effect of COVID on the probability of proper care. The mothers who live in the most remote locations and delivered a baby after the pandemic were 35.2% less likely (p < 0.01) to have had adequate prenatal care. These estimates reflect important shortcomings of the Brazilian health care system in rural communities in the Amazon.
6.3. Supply of physicians, nurses, and midwives
This section examines the impact of the COVID-19 pandemic on the supply of health care professionals, namely: physicians, nurses, and midwives. We match the health care facility number of the health unit where delivery took place (from the SINASC data set) with monthly data on the number of health care professionals in each facility (available in the CNES databse).32 As our focus is infant health, we consider the facility's supply of workers relative to fertility of mothers that deliver their babies in such a facility. Therefore, for each facility, we aggregate births to the monthly level to calculate the supply of health care workers per birth.
We estimate the following equation:
| (4) |
where S it is the supply of health care workers (per birth) of facility i in month t, and COVIDit is an indicator for the start of the epidemic in the municipality of facility i, i.e. 1s for months t after the first resident of the municipality of facility i is hospitalized due to COVID-19, 0s before the epidemic. μ i and γ t are facility and month-by-year fixed effects, respectively. The error term is ϵ it.
Note that we cannot match mothers/births to health care professionals. Instead, we match total number of births and health care workers to facilities. As a result, to examine the possibility of ethnicity differentiated effects of the pandemic on the supply of health care workers, we estimate model (4) separately for Non-Indigenous and Indigenous births by dividing the facility-level supply of workers by the number of Non-Indigenous and Indigenous births in each facility, respectively.
Estimates of model (4) are particularly relevant for policy because fertility is stable over the sample period.33 As a result, S measures the supply of professionals relative to the (on average constant) demand from pregnant women and changes in S over time largely reflect changes on the public health system supply of doctors, nurses, and midwives.
The results are shown in Table 6 . Estimates of α capture the baseline (pre-pandemic) supply of workers per birth, while β captures supply changes induced by the pandemic. To be able to compare impacts across Non-indigenous and Indigenous models, and between the three types of health care workers (physicians, nurses, and midwives), we normalize the estimates of the COVID impact and report effects relative to the pre-pandemic supply (per birth), i.e. we report β/α. The table shows models that use data on facilities located in small municipalities (panel A), as well as models that use data from facilities located in the capital city Manaus (panel B).
Table 6.
Effects of COVID on the supply of health care professionals.
| (1) |
(2) |
(3) |
(4) |
(5) |
(6) |
|
|---|---|---|---|---|---|---|
| Non-Indigenous |
Indigenous |
|||||
| Physicians | Nurses | Midwives | Physicians | Nurses | Midwives | |
| Panel A: Small Municipalities | ||||||
| COVID (β) | −0.230 (0.196) |
−0.219 (0.192) |
−0.038 (0.028) |
−0.739 (0.553) |
−1.091 (0.757) |
−0.286∗ (0.149) |
| Constant (α) | 0.865∗∗∗ (0.027) |
0.702∗∗∗ (0.026) |
0.050∗∗∗ (0.004) |
2.988∗∗∗ (0.078) |
3.112∗∗∗ (0.107) |
0.288∗∗∗ (0.021) |
| β/α | −0.266 (0.218) |
−0.311 (0.261) |
−0.757 (0.500) |
−0.247 (0.179) |
−0.351 (0.231) |
−0.995∗∗ (0.444) |
| N | 1219 | 1219 | 1219 | 741 | 741 | 741 |
| R-squared | 0.616 | 0.646 | 0.751 | 0.781 | 0.808 | 0.931 |
| Panel B: Manaus | ||||||
| COVID (β) | 0.102∗∗ (0.042) |
0.047∗ (0.026) |
−0.000 (0.000) |
7.333 (10.239) |
9.160 (8.477) |
−0.001 (0.001) |
| Constant (α) | 0.512∗∗∗ (0.009) |
0.158∗∗∗ (0.005) |
0.000∗∗∗ (0.000) |
66.316∗∗∗ (1.807) |
20.782∗∗∗ (1.496) |
0.087∗∗∗ (0.000) |
| β/α | 0.199∗∗ (0.085) |
0.300∗ (0.176) |
−0.091 (0.089) |
0.111 (0.157) |
0.441 (0.440) |
−0.013 (0.011) |
| N | 307 | 307 | 307 | 102 | 102 | 102 |
| R-squared | 0.892 | 0.936 | 0.994 | 0.678 | 0.422 | 0.644 |
Notes: Unit of observation is a health care facility by month. Dependent variable is number of health care professionals per birth. COVID is an indicator for municipality specific epidemic months. Panel A uses health care facilities outside Manaus. Panel B uses health care facilities in Manaus. Panel A controls for facility and month-by-year fixed effects. Panel B controls for facility fixed effect. Standard errors of α and β are clustered at the health care facility level. Standard errors of β/α obtained through the delta method. ∗p < 0.1, ∗∗p < 0.05, ∗∗∗p < 0.01.
We estimate that the supply of health care professionals in facilities in the countryside decreases after the pandemic, however, in general, the estimates are not statistically significant. The exception is the estimate of the supply of midwives in the Indigenous model (panel A, column 6). The results indicate that, controlling for facility and time effects, the supply of midwives per Indigenous birth significantly decreases after the pandemic. Our estimate of the (pre-pandemic) intercept is (p < 0.01) and the (post-pandemic) coefficient is (p < 0.1), which almost completely offsets pre-pandemic levels. These estimates imply that the pandemic decreased the supply of midwives per Indigenous birth by 99.5% (p < 0.05).34
In contrast, when examining the data from Manaus, we find that the supply of physicians and nurses (per non-Indigenous births) increased by 20% (p < 0.05) and 30% (p < 0.1), respectively (panel B, columns 1 and 2). We find evidence that this is at least partially driven by a migration of public physicians and nurses from the countryside to the metropolitan region after the start of the epidemic in Manaus.35 Specifically, we find that physicians that worked all 12 months of 2019 exclusively in the countryside are 5.9% more likely (p < 0.01) to work in Manaus after March 2020 (see Table A.8, Online Appendix). For nurses that worked exclusively in the countryside in 2019, the pandemic increases the probability of working in a Manaus' health care facility by 1.6% (p < 0.01). Models' details are available in the Online Appendix (see notes of Table A.8).
7. Robustness checks
7.1. Alternative measures for the start of the pandemic
Measuring the start of the pandemic with the date of the first hospitalization is an approach that is sensitive to noise in the data and extreme values. As a robustness check, we use different thresholds to determine municipality-level epidemic start dates. Specifically, we re-define both the extensive and intensive measurements of COVID by using two new thresholds. First, we use the 50th hospitalization of a resident in the mother's municipality as the time cut-off for the local epidemic. Second, we use as cut-off the date of the first confirmed COVID-19 death of an individual that resides in the mother's municipality. We use the alternative COVID variables to re-estimate model (1). The results of both checks are very similar to our original estimates and we do not find COVID impacts on birth weights or the probability of having Apgar scores below 8 (see Table A.3, Online Appendix).
7.2. Matched sample
King and Nielsen (2019) suggest the use of matching models to prune data and enhance strength of identification of empirical analysis by estimating regression models on matched samples. The idea is to use matching to find ‘hidden experiments’ in the data and estimate models using treatment/control observations that are ‘similar’ based on meaningful observables. They also show that data pruning procedures reduce the sensitivity of results to model specification (i.e. lower model dependence).
In this spirit, we use matching methods to balance characteristics over Indigenous ethnicity of mothers in our sample. Specifically, we use nearest neighbor matching to construct a matched sample by requiring exact matches between Indigenous and non-Indigenous observations on the following mother's characteristics: married, schooling levels (based on the categories of Table 1), age, and municipality of residence. We also require at least 3 matches in each ethnicity group. This resulted in a matched sample with 9431 observations.
We use the matched sample to perform two robustness checks. First, we re-estimate model (1) (Table A.4, Online Appendix). Second, we re-estimate the distributional impacts in Fig. 2 (Table A.7, Online Appendix). The results of model (1) in the matched sample are similar to those from the main sample (Table 2). The COVID variable and its interaction with Indigenous remain statistically insignificant suggesting that potential exposure did not affect birth weights and Apgar scores. Moreover, the matched sample results corroborate the previous finding of a baseline ethnicity birth weight gap, however the estimate of the size of the gap is smaller in the matched sample. Specifically, in the matched sample, Indigenous birth weights are 48 g lower (p < 0.01) than non-Indigenous birth weights (in contrast to −60 g in the main sample).
The results of the distributional analysis related to ethnicity differentiated COVID impacts are again similar to those of the main specification. Using the matched sample, we again find evidence that the extensive margin of potential exposure has higher impact on Indigenous infants at the lower tail of the birth weight distribution. The results in Table A.7 show that, compared to the main sample, while the range of statistically significant effects shrinks to X1100 g, the point estimates increase (and in some cases more than double). The largest estimate comes at the very bottom of the X range. The table shows that Indigenous infants born after the start of the epidemic are 1% more likely to have birth weight below 1000 g (p < 0.05). These results are additional evidence that indeed the COVID-19 pandemic had ethnicity differentiated impacts at the lower end of the birth weight distribution in the Brazilian Amazon.
7.3. Including the capital city Manaus
As discussed above, our main specification excludes births in the large urban region of Manaus. By focusing on small municipalities we work with a homogeneous sample and alleviate concerns regarding selectivity between the private and public health systems. Nevertheless, as a robustness check, we estimate model (1) using the entire data set (Table A.5, Online Appendix). In general, results obtained using the sample that includes births in Manaus are similar to those in our main specification. This suggests that our fixed effects strategy is able to capture much of the structural differences between urban and rural settings in Amazonas.
7.4. Excluding municipalities characteristics
To strengthen the internal validity of our estimates, our main specification includes interactions of pre-treatment municipality characteristics with a linear time trend. Arguably, the time horizon of 19 months may not be long enough to warrant municipal controls beyond municipality and month-by-year fixed effects. We test whether failure to reject the null of no COVID-19 health impacts is a function of the inclusion of municipality-specific trends. It is encouraging to learn that our results are not affected by the inclusion of these trends (Table A.6, Online Appendix). This suggests that our estimates are not driven by other differential trends related to the epidemic variation between municipalities, alleviating municipality-level selectivity concerns such as those driven by a possible correlation between unobserved determinants of health status and the strength of COVID-19 exposure.
7.5. Nonlinearities
We use nonparametric tools to test whether the null effects from model (1) mask important nonlinearities. We test for the possibility of nonlinear effects by estimating a partially linear model of birth weights (Robinson, 1988; Stock, 1989). In the model, mother and infant/pregnancy characteristics enter the conditional expectation function in a (linear) parametric fashion, while the key variable, the intensive margin of potential COVID exposure, enters the model nonparametrically. We estimate the model separately for Indigenous and non-Indigenous infants.36 Fig. 3 plots the gradient of COVID against its own values, i.e. the number of days between birth and the start of the pandemic. For both Indigenous and non-Indigenous models, we see that estimates fluctuate around zero, with the confidence interval including zero over the entire range of COVID. In short, we do not find significant changes in birth weights as each municipality's epidemic progresses over time. We also do not find strong nonlinearities suggesting that the main parametric model should produce acceptable results.
Fig. 3.
Nonlinear impacts – gradient estimates with respect to COVID (IM). Notes: Results of the partially linear model. The figure reports gradient estimates with respect to the intensive margin measure of potential COVID-19 exposure: number of days between birth and the start of the pandemic, with zeros for births before the pandemic. Panel A shows results for the model estimated on non-Indigenous births. Panel B shows results of the Indigenous model. The dashed lines represent 95% confidence intervals based on 400 replications of wild bootstraps (see Henderson and Parmeter, 2015).
7.6. Endogeneity
Consistency of our estimates is based on municipality and month-by-year fixed effects, municipality-specific socioeconomic trends, and the fact that the pandemic was a universal and unanticipated shock to mothers living in a homogeneous region of the Amazon, i.e. rural and small municipalities. The identification assumptions of our models are comparable to those from empirical work in the economics literature aiming to evaluate impacts of the COVID-19 pandemic in the sense that the onset of the pandemic is, after controlling for observables and fixed effects, uncorrelated with unobservable drivers of the outcome of interest (e.g. Couch et al., 2020; McLaren, 2021; Liu and Su, 2021; Couch et al., 2021). Some papers in this literature argue that the pandemic is an exogenous shock to their outcomes (e.g. Altig et al., 2020; Arenas-Arroyo et al., 2021; Ftiti et al., 2021).
Nevertheless, as a robustness test, we offer an additional contribution to the literature by conducting endogeneity tests and instrumental variable (IV) estimations (Baum and Lewbel, 2019). One challenge about performing such tests is the availability of a valid instrument. However, Lewbel (2012) proposes an instrumental variable approach that can be used when an appropriate instrument is not available. Lewbel's method is part of an emerging literature that proposes restrictions on higher order moments as means to identification. Specifically, identification is achieved when regressors are uncorrelated with the product of heteroskedastic errors.37 As Lewbel discusses, the estimator can be used to address a variety of sources of endogeneity, including measurement errors and omitted variables/confounding effects.38
We use Lewbel's heteroskedasticity-based instrument to perform Hausman specification tests for the endogeneity of COVID in model (1). The null hypothesis is that the estimates are efficient and consistent (i.e., no endogeneity). Under the null, there should be no systematic difference between the OLS and IV estimators. For all models, the results of the Hausman tests show that we cannot reject the null of no endogeneity.39
Underidentification and weak identification tests support the use of heteroskedasticity-based instruments, especially in the extensive margin models. IV estimates of model (1) (Table A.9, Online Appendix) are very similar to our main models (Table 2), which is in line with the findings of the Hausman tests. We again do not find empirical evidence of an impact of COVID on mean infant health measures. Corroborating results above, we find statistically significant pre-pandemic birth weight gaps. Specifically, results show that indigenous infants have birth weight, on average, 60.4 g (extensive margin model, p < 0.01) or 61.32 g (intensive margin model, p < 0.01) lower than their non-Indigenous counterparts.
8. Conclusion
8.1. Discussion and policy implications
In March of 2020, the World Health Organization characterized COVID-19 as a pandemic. Back then, it was hard to have a good grasp of the magnitude of all different impacts the COVID-19 global crisis would generate. The demand for information about the virus, including not only technical medical information but also information that could inform policy, increased together with our knowledge about how easily the virus spreads and mutates, exposing weaknesses of health care systems. Scholars around the world accepted the challenge and COVID-19 became one of the most extensively researched topics across many disciplines. One of the most important outcomes of this research was the development of vaccines and creation of randomized clinical trial protocols to facilitate vaccine evaluation (Ogburn et al., 2020; Ledford et al., 2020). While clinical trials play a critical role in our knowledge framework, our understanding of the pandemic would be very limited if we solely relied on the high internal validity results from clinical trials. It is also important to use observational data to examine general impacts through large and representative data sets.
The paper contributes to this literature by using vital statistics data from the Brazilian state of Amazonas to estimate impacts of the pandemic on Indigenous infant health. In our first analysis, we use the staggered variation on the start of the pandemic across small municipalities in the Amazon to examine the impacts of the spread of the virus on mean birth weight and Apgar scores. We fail to find statistical support for a mean pandemic effect. This result is robust to a number of checks, including alternative measures for the start of the pandemic, evidence from subsamples (based on mother characteristics, matched sample, geographical variation), nonparametric investigations of nonlinearities, alternative specifications, and instrumental variable estimation. Our findings of no effects are similar to that from Bach et al. (2021), who are unable to detect any causal effect of active participation in the 2020 elections on mortality of French politicians.
Moving beyond the mean, we turn to distributional effects and we are able to detect ethnicity differentiated impacts of the pandemic on infant health. Most importantly, these effects were detected when exploring the left tail of the birth weight distribution by modeling Pr[birth weightX], for X lower than 1400 g. Our models find evidence that Indigenous infants born after the start of the pandemic are at greater risk of having these very low birth weights. Using our main sample, effects are more precisely estimated when X = 1150, in which case the additional risk for Indigenous infant is 0.6% (p < 0.05). While at first this may appear to be a small effect, the economic significance of such an estimate should not be understated. To see this significance, note that the Indigenous baseline probability of having a live infant weighing less than 1150 g is 0.43% (which interestingly is lower than the baseline of 0.57% for non-Indigenous infants). While no additional impacts are found for non-Indigenous infants, our estimates suggest that COVID-19 more than doubles the risk of very low birth weights for Indigenous infants.
We conclude the paper by investigating possible reasons for these ethnicity differentiated pandemic effects. Access to health care facilities is especially important for Indigenous mothers. When examining the subsample of mothers who travel to another municipality to deliver their babies, we find that the risk of being born with very low ( 1100 g) birth weight is 3.4% higher (p < 0.05) after the pandemic for Indigenous infants. We also find different channels at play related to prenatal care. The pandemic reduced the probability of proper prenatal care by 5.7% for all mothers, i.e. we do not find ethnicity-related effects of the pandemic. However, the pre-pandemic (baseline) probability of adequate prenatal care is 7.5% lower for Indigenous mothers. Finally, we examine how the pandemic affected the facility-level supply of health care professionals. First, we find that the supply of midwives per non-Indigenous births was not altered by the pandemic. However, our results indicate that the pandemic almost completely offsets the pre-pandemic level of midwives per Indigenous birth, which can be an important factor for the health of Indigenous communities in remote regions of the Amazon. Putting all the evidence together, it seems like Indigenous mothers in small, rural, and remote regions of the Amazon are too far away from the health care system, and the pandemic exploited these deficiencies.
Brazil has historically struggled to prevent ethnic-based health care gaps and develop health policy to decrease disadvantages faced by Indigenous Peoples (Victora et al., 2011; Pontes and Santos, 2020). As a notable effort in this direction, Brazil introduces in 2013 the Mais Médicos (More Physicians) program that focus on improving the national supply of physicians with a target at health supply in Indigenous communities.40 While a few papers focus on evaluating impacts of the Mais Médicos (Fontes et al., 2018; Carrillo and Feres, 2019), the debate is open as to whether the program was successful in reducing ethnic health gaps. Our empirical analysis (using data from 2019 to 2020) shows that important gaps exist in infant health, suggesting persistent health inequalities in the Brazilian Amazon. Our findings give direction regarding mechanisms at play that contribute to health gaps. Health policy in Brazil should play close attention to the links between Indigenous communities and access to health care facilities, prenatal care, and the supply of medical personnel, and how these features of local health care systems fulfill the needs of Indigenous individuals in moments of system stress and critical crises.
8.2. Limitations and future research
The paper focuses on developing empirical models to test for ethnicity differentiated pre- and post-pandemic health gaps. We explore heterogeneities and potential mechanisms through which impacts may operate through. In doing so, we find a number of interesting and puzzling results that meet the limitations of our data and should be the focus of future research. For example, there are both advantages and disadvantages related to the timing of our data, i.e. birth records up to 4.5 months after the start of the pandemic in the Brazilian Amazon. On the positive side, the data allow us to obtain early insights into the impact of the pandemic on Indigenous health, and has the identification advantage of avoiding the possibility of endogenous fertility decisions. However, the literature on in utero impacts often emphasizes effects by the trimester of negative shocks. All births in our sample had no first trimester exposure to COVID-19. Therefore, it is possible that our empirical approach underestimates the impact of potential exposure on infant health. Future work should address this limitation, examining, for instance, whether or not the hypothesis of no mean effects of the pandemic on birth weight is rejected using other large scale data sets with longer timelines of potential exposure.
Another limitation of our data is the reduced number of observations related to the Apgar score of Indigenous infants (see Table 1). While infant records in SINASC are mandated by law, the missing Apgar score data could be an indication of a possible correlation between health care facility characteristics and Apgar data availability.41 The Hausman tests suggest this is not the case (see section 7.6). However, if the Apgar score sample is a selected sample of infants born in better health care facilities, then our null results from Apgar models may be driven by this superior quality of health services which can mask the effects of the pandemic. Again, this is another data limitation that may bias estimates against the identification of negative effects from the COVID-19. In this sense, the point estimates of these models can be considered a lower bound of possible pandemic effects. Nevertheless, abstracting away from selectivity issues, the finding that pandemic impacts depend on the infant health outcome warrants additional research.42
We find ethnicity-differentiated effects detected at the very low birth weight portion of the birth weight distribution. A possible interpretation of this result is that the pandemic disproportionately impacted the rate of preterm births of Indigenous infants born in the Brazilian Amazon. However, empirical evidence on the impact of the interaction between the pandemic and race/ethnicity on the probability of preterm birth is not clear (Karasek et al., 2021; Janevic et al., 2021). Future research is needed to examine more carefully this mechanism.
Finally, we find that pre-pandemic gaps decrease with certain parameters of vulnerability, e.g. the baseline disadvantage is smaller (or disappears) for not married mothers, for mothers who travel to deliver their babies, and for infants at lower ranges of the birth weight distribution. One explanation might be that some subsets of vulnerable mothers form a more homogenous group. Social networks may also help explain these results. Munshi and Rosenzweig (2016) find that rural families in caste-based networks use loans and gifts as substitutes for formal insurance and state-sponsored safety nets. It is therefore possible for the Indigenous/non-Indigenous health gap in rural Amazon to be attenuated for vulnerable mothers in Indigenous communities due to strong social networks that offer social support for Indigenous individuals (Waterworth et al., 2014). Further investigations of these hypotheses should be addressed by future research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Disclaimer Statement: The findings, interpretations, and conclusions expressed in this paper are entirely those of the authors. They do not necessarily represent the views of the International Bank for Reconstruction and Development/World Bank and its affiliated organizations, or those of the Executive Directors of the World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. We thank Sandeep Mohapatra, Rudi Rocha, and seminar participants at the Statistics Department of the State University of Paraiba and Graduate Program in Economics at Federal University of Rio Grande do Sul for helpful comments and suggestions. We also would like to thank the editor and two anonymous referees for constructive comments which made the paper more valuable. All errors and omissions are our own.
Source: Bill and Melinda Gates. 2021 Annual Letter. Available online at https://www.gatesnotes.com/2021-Annual-Letter.
For example, see The Economist article from Sep 25, 2020 entitled “Abandoned in the Amazon: how indigenous Brazilians fought covid-19”. Available online at https://www.economist.com/1843/2020/09/25/abandoned-in-the-amazon-how-indigenous-brazilians-fought-covid-19; or the BBC article from July 29, 2020 entitled “How Covid-19 could destroy indigenous communities”. Available online at https://www.bbc.com/future/article/20200727-how-covid-19-could-destroy-indigenous-communities.
The Amazonas first wave crisis was arguably the worst crisis in the beginning of the pandemic in Brazil. The first COVID-19 case was confirmed on March 13, 2020. The patient was a resident of Manaus that had recently returned from a trip to London. Since then, cases in Amazonas rapidly spread reaching a peak around mid-May. Refer to the Online Appendix for a comparison of confirmed cases between Amazonas and selected countries (Fig. A.2).
For example, in Amazonas, vaccination efforts involve using small boats to reach Indigenous communities (see https://g1.globo.com/am/amazonas/noticia/2021/02/06/barcos-transportam-vacinas-ate-aldeias-indigenas-isoladas-no-am.ghtml).
Source: Brazilian Institute of Geography & Statistics (IBGE). Available online at https://cidades.ibge.gov.br/brasil/am/panorama.
We perform robustness checks where we estimate models using the entire sample (i.e. including Manaus) and obtain similar results.
According to National Council of Health Secretariats, in 2013, the federal government financed 42.6% of the total public health spending, with 26.7% from states and 30.7% from municipalities (CONASS, 2015).
The policy created specific rules for six blocks of public health financing: i) primary care; ii) medium and complex care; iii) epidemiological and sanitary; iv) medication; v) SUS management; and vi) investments.
Full text made available by the Brazilian Federal Senate at https://www.senado.leg.br/atividade/const/con1988/con1988_06.06.2017/art_199_.asp.
Source: Agência Nacional de Saúde. Available online at http://www.ans.gov.br/perfil-do-setor/dados-gerais. Statistics includes plans with and without dental coverage.
Source: KPMG. Available online at https://home.kpmg/xx/en/home/insights/2019/04/meeting-healthcare-challenges-in-brazil.html.
Source: IBGE, 2010 Brazilian census. Available online at https://censo2010.ibge.gov.br/.
For example, refer to ABC News 20 April 2020. Available online at https://abcnews.go.com/Health/wireStory/virus-crisis-ravages-brazilian-amazon-citys-health-system-70244102.
In June 2020, the head of Amazonas' health secretariat was arrested due to corruption charges associated with the purchase of respirators (source: G1 Globo 30 June 2020. Available online at https://g1.globo.com/am/amazonas/noticia/2020/06/30/secretaria-de-saude-do-am-e-presa-em-operacao-da-pf-que-apura-desvio-na-compra-de-respiradores.ghtml. The governor of the state of Amazonas was also under severe scrutiny of the Brazilian Federal Police (source: G1 Globo 30 June 2020. Available online at https://g1.globo.com/politica/noticia/2020/06/30/stj-ve-indicios-de-envolvimento-do-governador-do-amazonas-em-irregularidades-na-compra-de-respiradores.ghtml).
Available online at http://svs.aids.gov.br/dantps/cgiae/sinasc/apresentacao/.
The law that regulates data collection (PORTARIA 116/2009) states that the declaration of live birth is mandatory for children that die soon after the delivery, regardless of pregnancy length, weight, or stature of the newborn. Therefore, stillbirths are not recorded in SINASC, however, all live births are part of the data set, regardless of the duration of life.
A newborn's Apgar score is based on five characteristics: heart rate, respiratory effort, muscle tone, reflex irritability, and color. Each characteristic is assessed (one and 5 min after delivery) and assigned a value from 0 to 2. The total score is the sum of the five components, i.e. scale from 0 to 10, and a score of 7 or higher indicates that the infant's condition is good to excellent (Casey et al., 2001). Apgar scores used in this paper are determined 1 min after delivery.
Source: DATASUS, SRAG 2020. Available online at https://opendatasus.saude.gov.br/dataset/bd-srag-2020.
Section 4 offers a discussion about the reasons for restricting the sample to health care systems of small municipalities.
Source: IBGE, 2010 Brazilian census. Available online at http://censo2010.ibge.gov.br. DATASUS/CNES - Cadastro Nacional de Estabelecimentos de Saúde. Available online at http://cnes.datasus.gov.br.
Amazonas has an area more than twice as large as that of Texas and larger than Spain, France, and Germany combined.
Source: Bolsonaro calls coronavirus a ‘little flu’. CNN, May 25, 2020. Available online at https://www.cnn.com/2020/05/23/americas/brazil-coronavirus-hospitals-intl/index.html.
For example, our data show that Manaus has 557 private clinics (or 3.1 clinics per 10,000 residents) while the other 61 municipalities of the state of Amazonas have collectively 33 private clinics (or 0.1 clinics per 10,000 residents).
Full model estimates are available in the Online Appendix, Table A.2.
We also estimate an event study specification that does not interact relative months with the Indigenous indicator (hence does not have the fixed effect γk). This model represents a more standard version of event study where β estimates capture epidemic effects (as opposed to ethnicity differentiated epidemic effects). We again do not find evidence that the epidemic decreased birth weights. These results are available upon request.
We revisit this result in section 8.2 where we discuss some limitations of the model.
It is worthwhile noting the reassuring result that estimates of the Indigenous coefficient β2 are similar between the extensive and intensive margin models (within column comparison of Panels A and B).
As discussed above, it is again reassuring that the estimates of β2 from the extensive margin model are very similar to those from the intensive margin model.
Fig. A.1 in the Online Appendix shows, for the 61 small municipalities in our main sample, the distribution of shares of births delivered outside the municipality of residence of the mother.
We construct a municipality distance matrix using the linear distances between the municipalities' head offices. Source: World Bank. Available online at https://datacatalog.worldbank.org/dataset/2010-brazil-municipalities-location.
As discussed above, we did not find statistically significant effects of COVID and/or Indigenous × COVID on Pr(birth weight < X) for X > 1500 g.
The CNES databse, under the module Professionals (in Portuguese, Profissionais), contains monthly information on the supply of SUS (public health system) health care professionals by health care facility. Both the worker identifier and the number of hours worked are available monthly. The discussion that follows uses data on the stock of physicians, nurses, and midwives that worked at least 1 h in a given month. We also estimated models using the total number of hours worked (as opposed to number of workers). Results are similar and available upon request.
Fig. A.3 (Online Appendix) shows that the time trend of the number of births per facility is generally flat.
The average number of midwives per indigenous birth (in small municipalities) is 0.270 before COVID, and 0.165 after COVID. A regression of midwives per birth on COVID (without accounting for fixed effects) yields α = 0.270 and β = −0.105. However, the estimate Δ = 0.165–0.270 = −0.105 (or proportionally β/α = −0.39) cannot be interpreted as the causal impact of COVID on the labor supply due to the likely correlations between the local pandemic and unobserved facility effects (e.g. management style or quality standards) or unobserved time-specific (common) shocks (e.g. regional drought, variation in state-wide policy or macroeconomic conditions). Another way to interpret our result is that a two-way fixed effect ratio β/α = −0.995 implies that the reasons why the post-pandemic stock of midwives (per birth) is not approaching zero in the sample are the facility-specific and time-specific effects.
Doctors of the Brazilian public health care system are allowed to work in multiple municipalities. Therefore, we investigate whether the pandemic produced a migration of physicians from the countryside to the capital city. In our Amazonas data (here all months of 2019–2020), out of the 103,935 physician-month observations, 75,568 are observations of physicians that worked exclusively in Manaus (72.7% of the physician-by-month data), 22,087 are physicians that work exclusively in small municipalities (21.2%), and the remainder 6% represent doctors that, in a given month, worked both in the countryside and in Manaus.
Estimations of the parametric and nonparametric portions of the conditional mean of birth weights are done separately using a combination of OLS and nonparametric local-linear least-squares. Estimation relies on a Gaussian kernel. To avoid computationally demanding bandwidth selection methods, we use rule-of-thumb bandwidth 1.06σn−1/5, where σ is the standard deviation of COVID (refer to Henderson and Parmeter (2015) for further discussion).
Breusch-Pagan heteroskedasticity tests reject the null hypothesis of constant variance in model (1) (p < 0.01).
The model can be conceptualized by assuming our potentially endogenous regressor takes the form COVID = X′α + ν, where X includes all regressors from equation (1) and the error ν is correlated with the error ε of the outcome model. Lewbel (2012) shows that, under heteroskedasticity, the set can be used as valid instruments in the outcome equation, where W is equal to (or a subset) of X.
P-values of Hausman tests for each model are as follows. Birth weight: extensive margin (Probchi2 = 0.955), intensive margin (Probchi2 = 0.871). Apgar: extensive margin (Probchi2 = 0.207), intensive margin (Probchi2 = 0.598).
The program allocated more than 18,000 full time physicians (of which the majority were imported from other countries such as Cuba). One of the pillars of the program was to improve Indigenous health via the allocation of doctors in Indigenous Special Sanitary Districts – ISSDs (Carrillo and Feres, 2019). Given the scarcity of Brazilian doctors willing to practice in remote Indigenous communities, foreign doctor were heavily allocated in the Amazon region. Specifically, 99% of the doctors working in ISSDs were imported from Cuba as part of a large cooperation agreement with the Pan American Health Organization (United Nations, 2016).
According to Brazilian Federal Law Number 12.662/2012, the declaration of live birth must be filled out by the medical professional that was responsible for either prenatal care, delivery, or pediatric care of the newborn.
Our results are similar to that of other health economics papers that find that interventions impact some but not all examined infant health outcomes (e.g. Hill, 2018; Rangel and Vogl, 2019).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.econmod.2022.105962.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alexander D., Currie J. Is it who you are or where you live? residential segregation and racial gaps in childhood asthma. J. Health Econ. 2017;55:186–200. doi: 10.1016/j.jhealeco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond D., Chay K.Y., Lee D.S. The costs of low birth weight. Q. J. Econ. 2005;120(3):1031–1083. [Google Scholar]
- Altig D., Baker S., Barrero J.M., Bloom N., Bunn P., Chen S., Davis S.J., Leather J., Meyer B., Mihaylov E., et al. Economic uncertainty before and during the covid-19 pandemic. J. Publ. Econ. 2020;191 doi: 10.1016/j.jpubeco.2020.104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrist J.D., Pischke J.-S. Princeton university press; 2009. Mostly Harmless Econometrics: an Empiricist's Companion. [Google Scholar]
- Arenas-Arroyo E., Fernandez-Kranz D., Nollenberger N. Intimate partner violence under forced cohabitation and economic stress: evidence from the COVID-19 pandemic. J. Publ. Econ. 2021;194 doi: 10.1016/j.jpubeco.2020.104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucejo E.M., French J., Araya M.P.U., Zafar B. The impact of COVID-19 on student experiences and expectations: evidence from a survey. J. Publ. Econ. 2020;191 doi: 10.1016/j.jpubeco.2020.104271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo e Silva G., Giovanella L., de Camargo K.R. Brazil's national health care system at risk for losing its universal character. Am. J. Publ. Health. 2020;110(6):811–812. doi: 10.2105/AJPH.2020.305649. PMID: 32374681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach L., Guillouzouic A., Malgouyres C. Does holding elections during a Covid-19 pandemic put the lives of politicians at risk? J. Health Econ. 2021 doi: 10.1016/j.jhealeco.2021.102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C.F., Lewbel A. Advice on using heteroskedasticity-based identification. STATA J. 2019;19(4):757–767. [Google Scholar]
- Bertrand M., Duflo E., Mullainathan S. How much should we trust differences-in-differences estimates? Q. J. Econ. 2004;119(1):249–275. [Google Scholar]
- Booth A.L., Carroll N. Economic status and the indigenous/non-indigenous health gap. Econ. Lett. 2008;99(3):604–606. [Google Scholar]
- Brazilian Ministry of Health . 2006. Diretrizes Operacionais. Pactos pela Vida, em Defesa do SUS e de Gestão.http://bvsms.saude.gov.br/bvs/publicacoes/PactosPelaVida_Vol1DiretOperDefesaSUSeGestao.pdf Available online at: [Google Scholar]
- Brodeur A., Clark A.E., Fleche S., Powdthavee N. Covid-19, lockdowns and well-being: evidence from google trends. J. Publ. Econ. 2021;193 doi: 10.1016/j.jpubeco.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles K.S., Hungerman D.M. Season of birth and later outcomes: old questions, new answers. Rev. Econ. Stat. 2013;95(3):711–724. doi: 10.1162/REST_a_00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütikofer A., Løken K.V., Salvanes K.G. Infant health care and long-term outcomes. Rev. Econ. Stat. 2019;101(2):341–354. [Google Scholar]
- Carrillo B., Feres J. Provider supply, utilization, and infant health: evidence from a physician distribution policy. Am. Econ. J. Econ. Pol. 2019;11(3):156–196. [Google Scholar]
- Casey B.M., McIntire D.D., Leveno K.J. The continuing value of the apgar score for the assessment of newborn infants. N. Engl. J. Med. 2001;344(7):467–471. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- CONASS . 2015. Conselho Nacional de Secretarios de Saude. A Gestao do SUS.https://www.conass.org.br/biblioteca/pdf/A-GESTAO-DO-SUS.pdf Available online at: [Google Scholar]
- Couch K.A., Fairlie R.W., Xu H. Early evidence of the impacts of covid-19 on minority unemployment. J. Publ. Econ. 2020;192 doi: 10.1016/j.jpubeco.2020.104287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch K.A., Fairlie R.W., Xu H. Economic Inquiry; 2021. The Evolving Impacts of the COVID-19 Pandemic on Gender Inequality in the US Labor Market: the COVID Motherhood Penalty. [Google Scholar]
- Cunningham S. Yale University Press; 2021. Causal Inference: the Mixtape. [Google Scholar]
- Currie J., Neidell M., Schmieder J.F. Air pollution and infant health: lessons from New Jersey. J. Health Econ. 2009;28(3):688–703. doi: 10.1016/j.jhealeco.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave D., Friedson A.I., Matsuzawa K., Sabia J.J. When do shelter-in-place orders fight COVID-19 best? Policy heterogeneity across states and adoption time. Econ. Inq. 2021;59(1):29–52. doi: 10.1111/ecin.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davillas A., Jones A.M. Health Economics; 2021. The First Wave of the Covid-19 Pandemic and its Impact on Socioeconomic Inequality in Psychological Distress in the UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolome C.A., Vosti S.A. Choosing between public and private health-care: a case study of malaria treatment in Brazil. J. Health Econ. 1995;14(2):191–205. doi: 10.1016/0167-6296(94)00045-6. [DOI] [PubMed] [Google Scholar]
- Dinku Y., Hunter B., Markham F. How might COVID-19 affect the Indigenous labour market? Aust. J. Lab. Econ. 2020;23(2):189–209. [Google Scholar]
- Doniec K., Dall'Alba R., King L. Brazil's health catastrophe in the making. Lancet. 2018;392(10149):731–732. doi: 10.1016/S0140-6736(18)30853-5. [DOI] [PubMed] [Google Scholar]
- Durán R.L. COVID-19 and heterogeneous vulnerabilities in the Peruvian labor market: implications for social inequalities and for gender gaps. Econ. Politic. 2022;39(1):129–156. doi: 10.1007/s40888-021-00245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder T.E., Goddeeris J.H., Haider S.J. A deadly disparity: a unified assessment of the black-white infant mortality gap. B E J. Econ. Anal. Pol. 2011;11(1) [Google Scholar]
- Elder T.E., Goddeeris J.H., Haider S.J. Racial and ethnic infant mortality gaps and the role of socio-economic status. Lab. Econ. 2016;43:42–54. doi: 10.1016/j.labeco.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Wang L., Yang Y. Human mobility restrictions and the spread of the novel coronavirus (2019-ncov) in China. J. Publ. Econ. 2020;191 doi: 10.1016/j.jpubeco.2020.104272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes L.F.C., Conceição O.C., Jacinto P.d.A. Evaluating the impact of physicians' provision on primary healthcare: evidence from Brazil's more doctors program. Health Econ. 2018;27(8):1284–1299. doi: 10.1002/hec.3775. [DOI] [PubMed] [Google Scholar]
- Friedson A.I., McNichols D., Sabia J.J., Dave D. Shelter-in-place orders and public health: evidence from California during the covid-19 pandemic. J. Pol. Anal. Manag. 2021;40(1):258–283. [Google Scholar]
- Ftiti Z., Ameur H.B., Louhichi W. Does non-fundamental news related to COVID-19 matter for stock returns? evidence from Shanghai stock market. Econ. Modell. 2021;99 doi: 10.1016/j.econmod.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc. Sci. Med. 1996;42(4):589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Goodman-Bacon A. Difference-in-differences with variation in treatment timing. J. Econom. 2021;225(2):254–277. [Google Scholar]
- Gracey M., King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374(9683):65–75. doi: 10.1016/S0140-6736(09)60914-4. [DOI] [PubMed] [Google Scholar]
- Henderson D.J., Parmeter C.F. Cambridge University Press; 2015. Applied Nonparametric Econometrics. [Google Scholar]
- Hill E.L. Shale gas development and infant health: evidence from Pennsylvania. J. Health Econ. 2018;61:134–150. doi: 10.1016/j.jhealeco.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J.E. Food supply chains during the covid-19 pandemic. Can. J. Agric. Econ. 2020;68(2):171–176. [Google Scholar]
- Janevic T., Glazer K.B., Vieira L., Weber E., Stone J., Stern T., Bianco A., Wagner B., Dolan S.M., Howell E.A. Racial/ethnic disparities in very preterm birth and preterm birth before and during the covid-19 pandemic. JAMA Netw. Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans J., Johansson P., Nilsson J.P. Economic status, air quality, and child health: evidence from inversion episodes. J. Health Econ. 2018;61:220–232. doi: 10.1016/j.jhealeco.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Karasek D., Baer R.J., McLemore M.R., Bell A.J., Blebu B.E., Casey J.A., Coleman-Phox K., Costello J.M., Felder J.N., Flowers E., et al. The association of covid-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg. Health-Am. 2021;2 doi: 10.1016/j.lana.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. The scourge of asian flu in utero exposure to pandemic influenza and the development of a cohort of british children. J. Hum. Resour. 2011;46(4):669–694. [Google Scholar]
- Kim K.I.I.S., Wang E. Working Paper. University of California; Los Angeles: 2019. Matching Methods for Causal Inference with Time-Series Cross-Sectional Data. [Google Scholar]
- King G., Nielsen R. Why propensity scores should not be used for matching. Polit. Anal. 2019;27(4):435–454. [Google Scholar]
- Kotlar B., Gerson E., Petrillo S., Langer A., Tiemeier H. The impact of the covid-19 pandemic on maternal and perinatal health: a scoping review. Reprod. Health. 2021;18(1):1–39. doi: 10.1186/s12978-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H., Cyranoski D., Van Noorden R. The UK has approved a covid vaccine-here’s what scientists now want to know. Nature. 2020;588(7837):205–206. doi: 10.1038/d41586-020-03441-8. [DOI] [PubMed] [Google Scholar]
- Lewbel A. Using heteroscedasticity to identify and estimate mismeasured and endogenous regressor models. J. Bus. Econ. Stat. 2012;30(1):67–80. [Google Scholar]
- Lewis M., Penteado E., Malik A.M. Brazil's mixed public and private hospital system. World Hosp. Health Serv. 2015;51(2):22–26. [PubMed] [Google Scholar]
- Lhila A., Long S. What is driving the black–white difference in low birthweight in the us? Health Econ. 2012;21(3):301–315. doi: 10.1002/hec.1715. [DOI] [PubMed] [Google Scholar]
- Lin Y., Liu F., Xu P. Effects of drought on infant mortality in China. Health Econ. 2021;30(2):248–269. doi: 10.1002/hec.4191. [DOI] [PubMed] [Google Scholar]
- Lindo J.M. Parental job loss and infant health. J. Health Econ. 2011;30(5):869–879. doi: 10.1016/j.jhealeco.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Liu S., Su Y. The impact of the covid-19 pandemic on the demand for density: evidence from the US housing market. Econ. Lett. 2021;207 doi: 10.1016/j.econlet.2021.110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yin K. Management of pregnant women infected with covid-19. Lancet Infect. Dis. 2020;20(5):513–514. doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan K., Tomar S. Covid-19 and supply chain disruption: evidence from food markets in India. Am. J. Agric. Econ. 2021;103(1):35–52. doi: 10.1111/ajae.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren J. Racial disparity in covid-19 deaths: seeking economic roots in Census data. B.E. J. Econ. Anal. Pol. 2021;21(3):897–919. Published Online: April 30, 2021. [Google Scholar]
- Munshi K., Rosenzweig M. Networks and misallocation: insurance, migration, and the rural-urban wage gap. Am. Econ. Rev. 2016;106(1):46–98. [Google Scholar]
- Newbold K.B. Aboriginal physician use in Canada: location, orientation and identity. Health Econ. 1997;6(2):197–207. doi: 10.1002/(sici)1099-1050(199703)6:2<197::aid-hec260>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ogburn E.L., Bierer B.E., Brookmeyer R., Choirat C., Dean N.E., De Gruttola V., Ellenberg S.S., Halloran M.E., Hanley D.F., Jr., Lee J.K., et al. Aggregating data from covid-19 trials. Science. 2020;368(6496):1198–1199. doi: 10.1126/science.abc8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paim J. 2009. O Que É O SUS. SciELO-Editora FIOCRUZ.https://www.jstor.org/stable/10.7476/9788575413425 Available online at: [Google Scholar]
- Paim J., Travassos C., Almeida C., Bahia L., Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011;377(9779):1778–1797. doi: 10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
- Pontes A.L.d.M., Santos R.V. Health reform and Indigenous health policy in Brazil: contexts, actors and discourses. Health Pol. Plann. 2020;35(Supplement_1):i107–i114. doi: 10.1093/heapol/czaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel M.A., Vogl T.S. Agricultural fires and health at birth. Rev. Econ. Stat. 2019;101(4):616–630. [Google Scholar]
- Rich-Edwards J.W., Buka S.L., Brennan R.T., Earls F. Diverging associations of maternal age with low birthweight for black and white mothers. Int. J. Epidemiol. 2003;32(1):83–90. doi: 10.1093/ije/dyg008. [DOI] [PubMed] [Google Scholar]
- Roberts C.L., Lancaster P.A. Australian national birthweight percentiles by gestational age. Med. J. Aust. 1999;170(3):114–118. doi: 10.5694/j.1326-5377.1999.tb127678.x. [DOI] [PubMed] [Google Scholar]
- Robinson P.M. Root-n-consistent semiparametric regression. Econometrica. 1988:931–954. [Google Scholar]
- Rocha R., Soares R.R. Water scarcity and birth outcomes in the Brazilian semiarid. J. Dev. Econ. 2015;112:72–91. [Google Scholar]
- Ruffini K., Sojourner A., Wozniak A. Who's in and who's out under workplace COVID symptom screening? J. Pol. Anal. Manag. 2021;40(2):614–641. doi: 10.1002/pam.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhm C.J. Health effects of economic crises. Health Econ. 2016;25:6–24. doi: 10.1002/hec.3373. [DOI] [PubMed] [Google Scholar]
- Rytter M.J.H., Kolte L., Briend A., Friis H., Christensen V.B. The immune system in children with malnutrition—a systematic review. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonchak L. Medicaid reimbursement, prenatal care and infant health. J. Health Econ. 2015;44:10–24. doi: 10.1016/j.jhealeco.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Stock J.H. Nonparametric policy analysis. J. Am. Stat. Assoc. 1989;84(406):567–575. [Google Scholar]
- United Nations . vol. 1. United Nations Office for South-South Cooperation; 2016. (Good Practices in South-South and Triangular Cooperationfor Sustainable Development). [Google Scholar]
- Victora C.G., Barreto M.L., do Carmo Leal M., Monteiro C.A., Schmidt M.I., Paim J., Bastos F.I., Almeida C., Bahia L., Travassos C., et al. Health conditions and health-policy innovations in Brazil: the way forward. Lancet. 2011;377(9782):2042–2053. doi: 10.1016/S0140-6736(11)60055-X. [DOI] [PubMed] [Google Scholar]
- Waterworth P., Rosenberg M., Braham R., Pescud M., Dimmock J. The effect of social support on the health of indigenous Australians in a metropolitan community. Soc. Sci. Med. 2014;119:139–146. doi: 10.1016/j.socscimed.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Watson T. Public health investments and the infant mortality gap: evidence from federal sanitation interventions on US Indian reservations. J. Publ. Econ. 2006;90(8–9):1537–1560. [Google Scholar]
- Welch J.R., Ferreira A.A., Souza M.C.D., Coimbra C.E., Jr. Food profiles of indigenous households in Brazil: results of the first national survey of Indigenous peoples' health and nutrition. Ecol. Food Nutr. 2021;60(1):4–24. doi: 10.1080/03670244.2020.1781105. [DOI] [PubMed] [Google Scholar]
- Ziliak J.P. Food hardship during the covid-19 pandemic and great recession. Appl. Econ. Perspect. Pol. 2021;43(1):132–152. doi: 10.1002/aepp.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.