Abstract
Background:
Vitamin D has been found to be associated with the pathogenesis of attention deficit hyperactivity disorder (ADHD). However, the potential role of parathyroid hormone (PTH) is still unclear.
Aim:
We aimed to investigate the association between calcium metabolism and ADHD symptomatology.
Methods:
We included 106 participants aged between 7 and 13 years old (51 ADHD patients, mean age: 9.54 ± 1.77, 55 healthy controls mean age: 9.97 ± 0.94) to this study. K-SADS-PL and Conners’ Parent/Teacher Rating Scales, Stroop Test were performed. Blood samples to measure serum levels of Vitamin D, PTH, calcium (Ca), magnesium (Mg), phosphorus (P), and alkaline phosphatase (ALP) were collected in the spring (March–April–May) to prevent seasonal variability.
Results:
PTH, P, and ALP values were significantly lower and Vitamin D, Ca, and Mg values were significantly higher in the ADHD group (P < 0.05, for all). Both groups had Vitamin D deficiency. Control group has lower Vitamin D levels than the ADHD group (respectively; 17.66 ± 9.07, 21.99 ± 10.99, P < 0.05). There was a negative correlation between PTH and CTRS hyperactivity, CGI-RI and CGI-EL sub-scores, CGI-Total, DSM-IV-Inattention, DSM-IV Hyperactivity/Impulsivity, DSM-IV-Total scores (P < 0.05, for all).
Conclusions:
We found lower PTH levels in ADHD patients and a strong and negative correlation between PTH and symptom severity. Future studies are needed to clarify if these findings are due to the key role of PTH in ADHD pathology or PTH’s function in activating vitamin D.
Keywords: Attention deficit hyperactivity disorder, parathyroid hormone, vitamin D
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD), a neurodevelopmental disorder characterized by inattention (IN), hyperactivity (H), and impulsivity,[1] is affecting approximately 12 to 13% of the children in Turkey.[2] Genetic predisposition, familial transmission, neurobiological disorders, environmental and psychosocial factors have been shown to be associated with ADHD. However, its etiology, despite many studies, is still unclear.[1]
Up to 30% of ADHD children are reported to not to respond to pharmacotherapy.[3,4] Because ADHD is a multifactorial disorder and its definite etiology is unknown, nutrition is thought to play a role in the pathophysiology.[5,6] Vitamin D has many functions in both the developing and adult brain, including maintaining calcium balance and signaling, regulating neurotrophic factors, providing neuroprotection, modulating neurotransmission, and contributing to synaptic plasticity. An increasing number of epidemiological studies indicate that Vitamin D deficiency is associated with a wide range of neuropsychiatric disorders and neurodegenerative diseases.[7] Recently, several studies reported an association between lower 25 (OH) Vitamin D3 concentrations and ADHD.[8,9,10]
In a recent study, patients with ADHD were found to have low parathyroid hormone (PTH) levels among psychiatric out-patients in Sweden.[11] In another research, although the level of PTH was within the normal range, blunted PTH response was reported to be associated with ADHD compared to healthy controls.[12]
Because the Vitamin D level is controlled by PTH and is affected by serum calcium level, serum PTH, calcium (Ca), phosphorus (P), magnesium (Mg), alkaline phosphatase (ALP) levels were also assessed in our study. The aim of this study was to investigate whether Vitamin D and related laboratory variables differ between ADHD and healthy control groups and the association between these differences and clinical features and sociodemographic variables.
METHODS AND MATERIALS
Study population
The study period was 3 months (March–April–May 2014). We included 106 participants aged between 7 and 13 years old (51 ADHD patients, mean age: 9.54 ± 1.77 and 55 healthy controls, mean age: 9.97 ± 0.94). The ADHD group consisted of those who applied to the outpatient clinic and were diagnosed with ADHD. The center where the study was conducted (Cukurova University Hospital/Adana) is the largest university hospital in the south of the country (Turkey). Cukurova University Child and Adolescent Psychiatry clinic serves patients of more than five cities, including Adana. The study was started with 80 patients, and 12 of them were excluded because they had comorbidities, 8 because they did not want to give consent, and 9 because they did not accept blood sampling. Patients with any comorbid psychopathology other than oppositional defiant disorde, patients treated with any psychiatric medication before, intellectual disability, epilepsy, autism spectrum disorder, progressive neurological disease, drug abuse, psychotic disorder, mood disorder, chronic endocrine disease, patients using Vitamin D/calcium supplements, and patients with a history of severe head trauma in the last 12 months were excluded from the study. Patient and control groups were assessed by the first author who is a child and adolescent psychiatrist. The control group consisted of children who applied to the healthy children outpatient clinic of Cukurova University Hospital for control and did not have any active disease, and who were sociodemographically parallel to the patient group and who met the study criteria. We interviewed the teachers of those who agreed to be included in the study in their schools. In total, 60 children were included in the control group, and 5 of them were excluded from the study because they were diagnosed with a psychiatric diagnosis as a result of clinical evaluation. Exclusion criteria for the control group were the presence of any medical or psychiatric disease, use of previous ADHD treatment or Vitamin D treatment, and growth retardation or intellectual disability. After the approval of the study protocol by the institutional ethics committee, written informed consent was obtained from parents of all patients. Blood samples to measure serum Vitamin D, PTH, Ca, P, ALP, and Mg levels were obtained from all participants.
Study design
In this cross-sectional study, we first compared parameters regarding the calcium metabolism between the patients and the control group. Secondly, we performed correlation analysis between the calcium metabolism parameters and ADHD symptom severity measures in the patient group.
Assessment instruments and procedures
Sociodemographic information form
The semi-structured sociodemographic form was used to determine the past medical and psychiatric histories, sociodemographic characteristics of the children such as age, gender, and body mass index (BMI) to match both groups.
Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL)
K-SADS-PL is a reliable semi-structured diagnostic interview for the assessment of a wide range of psychiatric disorders according to DSM-III and DSM-IV-TR criteria.[13] Turkish validity and reliability of K-SADS-PL were conducted by Gokler.[14]
Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R/L)
The CPRS-R/L comprises 80 items and is used to evaluate the parents’ observations on a child’s behavior over a 1-month time interval. The subscales of CPRS are oppositional, cognitive problems/inattention, hyperactivity, anxious-shy, perfectionism, social problems, psychosomatics, ADHD-index, Conners’ global index-restless/impulsivity (CGI-RI), Conners’ global index-emotional lability (CGI-EL), Conners’ global index total (CGI-Total), DSM-IV-inattention, DSM-IV-hyperactivity/impulsivity, and DSM-IV-total and this Likert-type scale scored between 0 and 3. The scale does not have a cut-off point; however, higher scores reflect greater symptom severity.[15] The reliability and validity study of the Turkish version has been conducted by Kaner et al.[16] CPRS forms were given to parents and asked them to fill together.
Conners’ Teachers Rating Scale-Revised: Long Form (CPRS-R/L)
The CTRS-R/L comprises 59 items that are subdivided into subscales, which are oppositional, cognitive problems/inattention, hyperactivity, anxious-shy, perfectionism, social problems, ADHD-index, Conners’ global index-restless/impulsivity (CGI-RI), Conners’ global index-emotional lability (CGI-EL), Conners’ global index total (CGI-Total), DSM-IV-inattention, DSM-IV-hyperactivity/impulsivity, and DSM-IV-total, and this Likert-type scale scored between 0 and 3. The scale does not have a cut-off point; however, higher scores reflect greater symptom severity.[17] The reliability and validity study of the Turkish version has been conducted by Kaner et al.[18] CPRS forms were given to parents with sealed envelope and asked them to be filled by the core teachers.
Stroop test
The Stroop Color and Word Test assesses the ability to inhibit cognitive interference. There are five different tables; two of them are black and white and the others have four different colors (red, blue, green, and yellow). In the last table, color words are printed in an inconsistent color ink (for instance the word “red” is printed in “blue ink”). Participants are asked to name the color of the ink, not the word (performing a less automated task while inhibiting a more automated task).[19] The reliability and validity study of the Turkish version has been conducted by Karakaş et al.[19]
Laboratory measurements
After overnight fasting, venous blood samples were obtained for 25 (OH) Vitamin D3, calcium, phosphorus, magnesium, PTH, and ALP. In addition, to provide a reliable way to measure 25 (OH) Vitamin D3 levels, all blood samples were collected in the spring (March–April–May) to prevent 25 (OH) Vitamin D3 levels from being affected by seasonal variability. Plasma 25 (OH) Vitamin D3 levels were measured by high-performance liquid chromatography (HPLC) method (ImmuChrom GmbH, Heppenheim) using the Shimadzu HPLC Prominence System (Shimadzu Inc., Kyoto, Japan) and a 25 ng/mL limit was accepted for low levels. Serum total Ca, ionized P, ALP, and Mg levels were measured by an ion-selective electrode, enzymatic and colorimetric methods, using Beckman Coulter kits (CA), Beckman UniCel DXC 800 Synchron fully automatic auto analyzer (Beckman Coulter Inc., CA, USA). Serum PTH hormone levels were measured by the chemiluminescence method using Beckman Coulter kits (CA, USA), Beckman UniCel DXI 800 fully automatic immunoassay autoanalyzer (Beckman Coulter).
Statistical analysis
Statistical analyses were conducted using SPSS, version 16.0 (SPSS Inc. Chicago, Illinois). Data are expressed as mean ± standard deviation (SD) for continuous variables and percentage for categorical variables. The Shapiro–Wilk test was used to test the normality and a P value >0.05 was defined as normally distributed data. Continuous variables that showed normal distribution were compared using Student’s t-test and analysis of variance (ANOVA), whereas the Mann–Whitney U test and Kruskal–Wallis test were used for non-normally distributed samples. Associations of categorical variables between groups were tested using the Chi-square test. Wilcoxon test was used to analyze the dependent variables such as the mean scores of psychometric scales obtained by applying the same measurement tool more than once. Pearson’s and Spearman’s correlations were used to examine the relationship between continuous variables. Statistical significance was defined as a P value <0.05 for all comparisons.
RESULTS
When we compared baseline demographics of the two groups, we found higher rates of male participants in the ADHD group. There were no significant differences between the groups in terms of age and BMI [Table 1].
Table 1.
Baseline demographics and laboratory values of study groups
| ADHD group | Healthy Control | P | |
|---|---|---|---|
| ADHD subgroup (ADHD-C%/ADHD-IN%) | 80.4/19.6 | --- | |
| ODD comorbidity, n (%) | 30 (58.8) | --- | |
| Gender, male (%) | 32 (62.7) | 24 (43.6) | 0.049 |
| BMI | 17.61±3.99 | 19.67±4.82 | 0.055 |
| Age (years) | 9.54±1.77 | 9.97±0.94 | 0.061 |
| PTH (12-88 pg/mL) | 29.50±9.58 | 39.90±19.87 | 0.004 |
| 25-OH Vitamin D (10-60 ng/mL) | 21.99±10.99 | 17.66±9.07 | 0.033 |
| Ca (8.8-10.2 mg/dL) | 9.68±0.88 | 9.54±0.29 | 0.004 |
| P (2.7-4.5 mg/dL) | 4.66±0.51 | 4.99±0.55 | 0.004 |
| Mg (1.8-2.5 mg/dL) | 2.19±1.28 | 2.12±0.19 | 0.025 |
| ALP (28-126 U/L) | 151.9±53.2 | 202.0±55.7 | 0.000 |
ADHD-C: Attention deficit hyperactivity disorder combine subgroup, ADHD-IN: Attention deficit hyperactivity disorder inattentive subgroup, ODD: Oppositional defiant disorder, BMI: Body mass index, PTH: Parathyroid hormone, Vitamin D: 25 dihydroxycholecalciferol, Ca: Calcium, P: Phosphorus, Mg: Magnesium, ALP: Alkaline phosphates
In the comparison of laboratory values, PTH, P, and ALP values were significantly lower and Vitamin D, Ca and Mg values were significantly higher in the ADHD group than in the control group [Table 1]. The study revealed that both groups had Vitamin D deficiency. Nevertheless, the control group was suffering from Vitamin D deficiency more than the ADHD group (respectively; 17.66 ± 9.07, 21.99 ± 10.99, P < 0.05). Moreover, when we compared the PTH levels between ADHD subtypes (ADHD-C and ADHD-IA), we found no significant difference.
The correlation analysis between the CPRS, CTRS, and the biochemistry values were shown in Table 2. There was a negative correlation between serum PTH and CTRS hyperactivity sub-score, CGI-RI sub-score, CGI-EL sub-score, CGI-Total, DSM-IV-Inattention, DSM-IV Hyperactivity/Impulsivity, DSM-IV-Total scores (P < 0.05). We were not able to find correlations between other markers of calcium phosphorus metabolism and symptom severity of ADHD. Scatter plot diagrams of the negative correlations of PTH with Conners Teachers Rating Scale global index-restless/impulsivity score and Conners Teachers Rating Scale DSM-IV-total score are shown in Figures 1 and 2, respectively.
Table 2.
Correlation analysis between CPRS, CTRS, and biochemistry values
| PTH | Vitamin D | P | Ca | Mg | ALP | |
|---|---|---|---|---|---|---|
| CPRS | ||||||
| Oppositional | 0.097 | -0.056 | 0.185 | 0.101 | -0.132 | 0.106 |
| Cognitive problems/inattention | -0.132 | -0.689 | 0.014 | -0.025 | -0.067 | 0.087 |
| Hyperactivity | 0.089 | -0.011 | 0.102 | 0.163 | -0.133 | 0.126 |
| Anxious-Shy | 0.067 | 0.057 | -0.114 | -0.004 | -0.108 | 0.088 |
| Perfectionism | -0.129 | -0.049 | -0.137 | 0.332* | -0.265 | -0.106 |
| Social problems | 0.106 | -0.192 | 0.024 | 0.141 | -0.179 | 0.159 |
| Psychosomatic | -0.023 | -0.130 | 0.075 | 0.181 | -0.189 | -0.070 |
| ADHD-Index | -0.033 | -0.118 | -0.024 | 0.131 | -0.113 | 0.017 |
| CGI-RI | 0.143 | -0.144 | 0.027 | 0.199 | -0.205 | -0.013 |
| CGI-EL | 0.163 | -0.067 | 0.020 | 0.184 | -0.242 | 0.207 |
| CGI-Total | 0.161 | -0.129 | 0.027 | 0.209 | -0.233 | 0.060 |
| DSM-IV-Inattention | -0.156 | -0.150 | 0.086 | 0.077 | -0.097 | 0.025 |
| DSM-IV-Hyperactivity/Impulsivity | 0.108 | -0.021 | 0.064 | 0.159 | -0.067 | 0.125 |
| DSM-total | -0.022 | -0.0091 | 0.082 | 0.131 | -0.289 | 0.084 |
| CTRS | ||||||
| Oppositional | -0.094 | -0.063 | 0.007 | -0.150 | 0.162 | 0.097 |
| Cognitive problems/inattention | -0.254 | -0.138 | 0.056 | 0.118 | -0.109 | -0.263 |
| Hyperactivity | -0.394* | -0.045 | 0.025 | -0.035 | 0.175 | 0.108 |
| Anxious-Shy | 0.000 | 0.142 | -0.143 | -0.014 | -0.124 | -0.103 |
| Perfectionism | -0.066 | 0.043 | -0.163 | 0.134 | -0.143 | -0.073 |
| Social problems | -0.292 | -0.015 | 0.093 | -0.125 | 0.081 | -0.121 |
| CGI-RI | -0.491** | -0.087 | 0.047 | -0.152 | 0.236 | -0.150 |
| CGI-EL | -0.382* | 0.054 | 0.131 | 0.003 | 0.091 | -0.104 |
| CGI-Total | -0.481** | -0.034 | 0.086 | -0.098 | 0.192 | -0.141 |
| DSM-IV-Inattention | -0.455** | -0.148 | -0.024 | -0.063 | 0.086 | -0.169 |
| DSM-IV-Hyperactivity/Impulsivity | -0.330* | -0.003 | 0.075 | -0.074 | 0.208 | -0.083 |
| DSM-IV-Total | -0.448** | -0.072 | 0.046 | -0.079 | 0.177 | -0.140 |
| Stroop Test-Duration | -0.062 | 0.039 | 0.140 | 0.137 | -0.211 | -0.112 |
| Stroop Test-Errors | -0.124 | -0.122- | -0.116 | 0.057 | -0.121 | -0.097 |
| Stroop Test-Corrected | -0.260 | -0.016 | -0.060 | 0.269 | -0.105 | -0.036 |
PTH: parathyroid hormone, Vitamin D: 25 dihydroxycholecalciferol, Ca: calcium, P: phosphorus, Mg: magnesium, ALP: alkaline phosphates, CPRS: Conners’ Parent Rating Scale, CTRS: Conners’ Teacher Rating Scale, CGI-RI: Conners’ global index-restless/impulsivity, CGI-EL: Conners’ global index-emotional lability, CGI-Total: Conners’ global index total. *P<0.05, **P<0.01,
Figure 1.
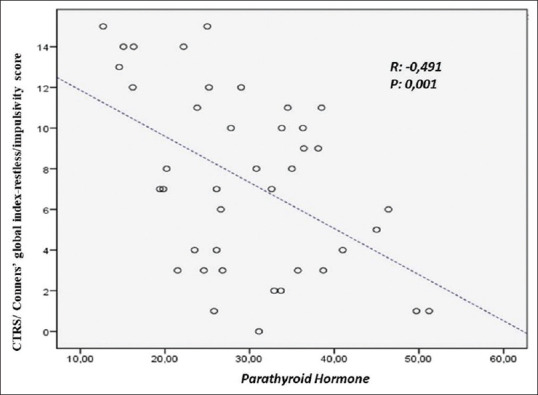
Scatter plot diagram of the correlation of PTH with Conners Teachers Rating Scale global index-restless/impulsivity score
Figure 2.
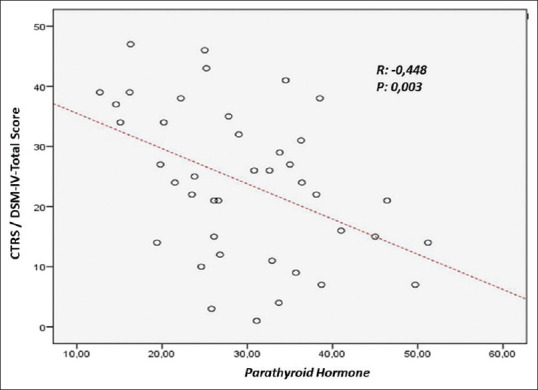
Scatter plot diagram of the correlation of PTH with Conners Teachers Rating Scale DSM-IV-total score
When we calculated the posthoc power analysis for vitamin D, the power was found to be 71.6% with a 95% confidence interval (CI). When we calculated it for PTH, the power was found to be 93.3% with 95% CI.
DISCUSSION
To the best of our knowledge, this is the first study that demonstrates the correlation of the markers of calcium metabolism with symptom severity of ADHD. Furthermore, our study is also the first to measure 25 (OH) Vitamin D3 levels in the spring (March–April–May) to avoid seasonal variability. Our patients were drug naïve. Therefore, we were able to perform analysis regarding the association of Ca metabolism and ADHD symptom severity without the effects of ADHD treatment. The main findings of the current study are that (1) compared with the control group, PTH, P, and ALP values are significantly lower and Vitamin D, Ca and Mg values are significantly higher in patients with ADHD, and (2) there is a strong and negative correlation between serum PTH and symptom severity scores of ADHD such as CTRS hyperactivity sub-score, CGI-RI sub-score, CGI-EL sub-score, CGI-Total, DSM-IV-Inattention, DSM-IV Hyperactivity/Impulsivity, and DSM-IV-Total scores.
There are several forms of Vitamin D, and its most active form of 1,25 dihydroxycholecalciferol (1,25-OH2 vitamin D3) is approximately a thousand times stronger than other forms. PTH is required for the formation of 1,25-OH2 vitamin D3, and if there is no PTH, 1,25-OH2 vitamin D3 never develops. Therefore, PTH runs a strong role in the functional effects of Vitamin D in the body.[20] To date, numerous studies have focused on the effects of Vitamin D on ADHD pathogenesis, whereas hypotheses of these previous studies were lacking the potential role of PTH. In previous studies, rather than the most potent form of Vitamin D (1,25-OH2 vitamin D3), 25 (OH) Vitamin D3 was used due to the fact that 1,25-OH2 vitamin D3 is an unreliable indicator of vitamin D because it is kept in the reference range for as long as possible with hormonal mechanisms.[21] The use of 25 (OH) Vitamin D3 instead of 1,25-OH2 vitamin D3 highlights the importance of PTH. In our study, PTH levels were lower in the ADHD group than those in the control group (respectively 29.50 ± 9.58; 39.90 ± 19.87; P = 0.004). There was a negative correlation between serum PTH levels and CTRS-Hyperactivity subscale, Conners’ global index-restless/impulsivity (CGI-RI), Conners’ global index-emotional lability (CGI-EL), Conners’ global index total (CGI-Total), DSM-IV-inattention, DSM-IV-hyperactivity/impulsivity, and DSM-IV-total subscales (P < 0.05, for all). Hence, these results are remarkable to demonstrate that the ADHD symptom severity increases as PTH levels decrease. There are limited studies that compare serum PTH levels in ADHD and healthy groups. Avcil et al.[12] detected blunted PTH response in the ADHD population. The fact that we found lower PTH levels in the ADHD group supports the findings of Humble et al.[11] who reported higher PTH levels in almost all psychiatric diseases, except ADHD, in which PTH levels were lower than the healthy population. Similarly, in another study conducted in Germany, lower levels of PTH (although not statistically significant) were detected in the ADHD group compared with healthy children.[22] Humble et al.[11] suggested low levels of PTH in the ADHD population may be a result of Mg deficiency. However, we find Mg levels to be higher in ADHD patients. Therefore, this explanation is not appropriate for our results. PTH and P levels were lower in ADHD patients and only low PTH was correlated with ADHD symptom severity. Thus, we suggest that P or more likely PTH plays the key role in the ADHD pathology rather than vitamin D or Mg. Reverse causality may also play a role in the association between PTH and ADHD symptom severity. As PTH is involved in activating Vitamin D, low Vitamin D and hypocalcemia will stimulate the release of PTH to rectify hypocalcemia by activating osteoclasts. The relationship is bidirectional. ADHD patients with higher symptom severity may be thought to be more likely to be outdoors due to severe hyperactivity (explaining their higher Vitamin D levels) may itself explain their lower PTH levels. Randomized controlled trials with a larger sample size are needed to clarify the potential mechanism regarding the role of PTH in ADHD pathology. To our knowledge, this is the first study that evaluates the relationship between serum levels of Vitamin D and PTH and the severity of ADHD. We found strong and negative correlations between serum PTH and ADHD symptom severity scales. However, similar correlations were not found with Vitamin D.
The developmental Vitamin D-deficient rat model has shown decreased dopamine turnover.[23] However, the adult female developmental Vitamin D-deficient rat model has demonstrated significantly increased dopamine transporter density compared with controls and reported more sensitivity to amphetamine, a dopamine-releasing agent.[24] Interestingly, dopamine can induce Vitamin D receptor-mediated signaling in the deficiency of the active form of Vitamin D.[25] Moreover, in animal studies, increased basal striatal dopamine levels were demonstrated after high doses of active Vitamin D treatment.[26] This implies that there could be a complex relationship between catecholamines and Vitamin D. Additionally, in humans, the cortex, cerebellum, and limbic system, which are associated with behavior, have Vitamin D receptors.[27,28] Symptom severity of patients with ADHD and Vitamin D deficiency who received Vitamin D in addition to methylphenidate therapy has improved; however, it is difficult to attribute these improvements to Vitamin D precisely because it was not given as monotherapy.[29] Before evaluating previous studies regarding the association of Vitamin D and ADHD, we should consider the fact that they used 25 (OH) Vitamin D3, which is not the active form and needs PTH to be activated. Lower levels of Vitamin D in ADHD populations have been reported in several previous studies.[8,9,10] In our study, in contrast to previous studies, although Vitamin D levels of both groups were low, Vitamin D level was higher in the ADHD group compared with that in the control group (P < 0.05). Similar to our findings, in the study by Reinehr et al.,[22] higher levels of vitamin D (although not statistically significant) were detected in the ADHD group compared with healthy children. Moreover, they confirmed that there was also a significant variability in Vitamin D levels according to the seasons.[22] Higher Vitamin D levels in the ADHD population may be a consequence of ADHD rather than playing a role in ADHD pathogenesis. The reason for this may be hidden in the cultural structure of our country. Parents allow their hyperactive children to play outside, as they often have trouble keeping their children at home. Considering the high solar intensity of our city, the increased sunlight exposure of the ADHD patients may be associated with higher levels of Vitamin D. In previous studies, lower levels of Vitamin D have been reported in girls than boys.[30] Poopedi et al.[30] suggested that male participants may spend more time outdoors. In our study, male dominance in the ADHD group may reflect the possibility that male participants may spend more time outdoors. However, we did not measure the data regarding the time spent outdoors. Moreover, these studies indicate that children from low socioeconomic status suffer Vitamin D deficiency more often. We did not analyze the socioeconomic status of the participants; however, it could be another reason this difference. The effect of different cut-off points and techniques to measure Vitamin D levels should also be considered. High-pressure liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (HPLC-APCI-MS) method, which is used in our study, is more valid than the other techniques such as radioimmunoassay, chemiluminescent immunoassay, enzyme-linked immunoassay to detect serum 25 (OH) Vitamin D3 levels.[31] None of the previous studies used standardized sample collection time to avoid seasonal variability regarding Vitamin D measurement.[8,9,10] Our study is the first to measure Vitamin D3 levels in the spring (March–April–May). We found lower levels of PTH in ADHD patients. Considering the fact that PTH is essential in the conversion of 25 (OH) Vitamin D3 to 1,25-OH2-D3, which is the active form of Vitamin D, accumulation of 25-OH-D3 due to decreased conversion rates may explain higher 25 (OH) Vitamin D3 levels in ADHD patients than in the control group. Although we did not measure 1,25-OH2-D3 levels, considering the low levels of PTH in ADHD patients, 1,25-OH2-D3 levels may also be thought to be lower in the ADHD group. Therefore, the effects of Vitamin D on the nervous system, such as brain development and function, neurogenesis, neuroimmunomodulatory effects, may be disrupted in the ADHD group.[7,23] A 22-year follow-up study suggested no association between serum levels of Vitamin D at the 30th week of pregnancy and ADHD symptoms of children.[32] In a recent study, there was no association between parental Vitamin D levels and behavioral problems in ADHD children from 5 to 18 years old.[33] Tolppanen et al. (2012)[34] have found that higher Vitamin D concentrations were associated with worse academic performance and showed no relation between Vitamin D levels and behavioral problems. Similar to previous studies, we found no correlation between 25-OH-D3 levels and ADHD symptom severity.
There is an association between serum Mg and Vitamin D levels bilaterally.[35] Moreover, Mg and Vitamin D affect similar behavior-related areas in the brain.[27,36] Subjects with ADHD had lower serum magnesium levels compared with the control group in a meta-analysis.[37] Moreover, the addition of Ca, Mg, and zinc to the diet together with pharmacotherapy increased the success of ADHD treatment.[38] In contrast to the above-mentioned previous studies, ADHD patients have higher levels of Mg compared with the control group in our study. We also found no correlation between Mg and ADHD symptom severity. These findings suggest that Mg plays a minor role or the role of Mg is dependent on other important factors such as PTH in the pathogenesis of ADHD.
There are very limited studies that have examined the serum Ca and P levels in ADHD patients.[8,9,12] Goksugur et al. (2014)[9] found no significance difference between ADHD patients and healthy subjects in terms of serum Ca and P levels. However, Kamal et al. (2014)[8] and Avcil et al.[12] reported lower levels of Ca and P in children with ADHD. We also found lower levels of P, but higher levels of Ca in ADHD patients. There is an obvious gap in the literature regarding the potential associations of these electrolytes with ADHD pathogenesis. Therefore, large-scaled randomized controlled studies are needed to clarify these associations.
Another notable finding of this study was the lower ALP levels in the ADHD group. In the literature, ALP levels were found to be slightly lower in the ADHD group although not statistically significant.[9,39] As low ALP indicates reduced bone turnover, low ALP levels are coherent with our data because the osteoclastic activity is reduced due to low PTH.[20]
Limitations
Our research has several limitations. First, the cross-sectional design of this study is not appropriate to demonstrate the causal relationship and evaluate the effect of markers of Ca metabolism on treatment response. Our sample size is relatively small. As a single-center study, our cohort may be different from other centers.
CONCLUSION
In conclusion, we found low levels of PTH in patients with ADHD and a strong and negative correlation between serum PTH and symptom severity of ADHD. Future studies with a larger sample size are needed to clarify if these findings are due to the key role of PTH in the ADHD pathology rather than Vitamin D or PTH’s important function in activating Vitamin D by converting 25 (OH) vitamin D3 to 1,25-OH2 vitamin D3.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was funded by the local resources of Cukurova University (TSA-20143107).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Biederman J. Attention-deficit/hyperactivity disorder:A selective overview. Biol Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Ercan ES, Kandulu R, Uslu E, Ardic UA, Yazici KU, Basay BK, et al. Prevalence and diagnostic stability of ADHD and ODD in Turkish children:A 4-year longitudinal study. Child Adolesc Psychiatry Ment Health. 2013;7:30. doi: 10.1186/1753-2000-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroux JR, Turgay A, Quinn D. Advances in ADHD treatment. Can J Diagn. 2009;26:49–52. [Google Scholar]
- 4.Mcgough JJ, Biederman J, Wigal SB, Lopez FA, Mccracken JT, Spencer T, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:530–8. doi: 10.1097/01.chi.0000157550.94702.a2. [DOI] [PubMed] [Google Scholar]
- 5.Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics. 2012;129:330–7. doi: 10.1542/peds.2011-2199. [DOI] [PubMed] [Google Scholar]
- 6.Konikowska K, Regulska-Ilow B, Rozanska D. The influence of components of diet on the symptoms of ADHD in children. Rocz Państw ZakłHig. 2012;63:127–34. [PubMed] [Google Scholar]
- 7.Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nut. 2014;34:117–41. doi: 10.1146/annurev-nutr-071813-105557. [DOI] [PubMed] [Google Scholar]
- 8.Kamal M, Bener A, Ehlayel MS. Is high prevalence of vitamin D deficiency a correlate for attention deficit hyperactivity disorder? Atten Defic Hyperact Disord. 2014;6:73–8. doi: 10.1007/s12402-014-0130-5. [DOI] [PubMed] [Google Scholar]
- 9.Goksugur SB, Tufan AE, Semiz M, Gunes C, Bekdas M, Tosun M, et al. Vitamin D status in children with attention-deficit–hyperactivity disorder. Pediatr Int. 2014;56:515–9. doi: 10.1111/ped.12286. [DOI] [PubMed] [Google Scholar]
- 10.Sharif MR, Madani M, Tabatabaei F, Tabatabaee Z. The relationship between serum vitamin D level and attention deficit hyperactivity disorder. Iran J Child Neurol. 2015;9:48. [PMC free article] [PubMed] [Google Scholar]
- 11.Humble MB, Gustafsson S, Bejerot S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden:Relations with season, age, ethnic origin and psychiatric diagnosis. J Steroid Biochem Mol Biol. 2010;121:467–70. doi: 10.1016/j.jsbmb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Avcil S, Uysal P, Yilmaz M, Erge D, Demirkaya SK, Eren E. Vitamin D deficiency and a blunted parathyroid hormone response in children with attention-deficit/hyperactivity disorder. Clin Lab. 2017;63:435–43. doi: 10.7754/Clin.Lab.2016.160629. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman J, Birmaher B, Brent D, Rao UM, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL):Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Gokler B. Reliability and validity of schedule for affective disorders and Schizophrenia for school age children-present and lifetime version-Turkish version (K-SADS-PL-T) [in Turkish] Turk J Child Adolesc Mental Health. 2004;11:109–16. [Google Scholar]
- 15.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners'Parent Rating Scale (CPRS-R):Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 16.Kaner S, Büyüköztürk Ş, İşeri E, Ak A, Özaydın L. Conners'parent rating scale long form-revised:Factor structure, reliability and validity studies. Turk J Child Adolesc Ment Health. 2011;18:45–58. [Google Scholar]
- 17.Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R):Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–91. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 18.Kaner S, Büyüköztürk Ş, İşeri E, Ak A, Özaydın L. Conners'teacher rating scale long form-revised:Factor structure, reliability and validity studies. Turk J Child Adolesc Ment Health. 2011;18:45–58. [Google Scholar]
- 19.Karakaş S, Erdoğan E, Sak L, Soysal AŞ, Ulusoy T, Ulusoy İY, et al. Stroop Test TBAG Form:Standardisation for Turkish Culture, Reliability and Validity. J Clin Psy. 1999;2:75–88. [Google Scholar]
- 20.Hall JE, Hall ME. Guyton and Hall Textbook of Medical Physiology e-Book. 14th ed. Elsevier Health Sciences; 2020. pp. 991–1011. [Google Scholar]
- 21.Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56:692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, Langrock C, Hamelmann E, Lücke T, Koerner-Rettberg C, Holtmann M, et al. 25-Hydroxvitamin D concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attention-deficient/hyperactivity disorder than in healthy children. Nutr Res. 2018;52:39–47. doi: 10.1016/j.nutres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461:155–8. doi: 10.1016/j.neulet.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 24.Kesby JP, Cui X, O’Loan J, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology. 2010;208:159–68. doi: 10.1007/s00213-009-1717-y. [DOI] [PubMed] [Google Scholar]
- 25.Matkovits T, Christakos S. Ligand occupancy is not required for vitamin D receptor and retinoid receptor-mediated transcriptional activation. J Mol Endocrinol. 1995;9:232–42. doi: 10.1210/mend.9.2.7776973. [DOI] [PubMed] [Google Scholar]
- 26.Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533–9. doi: 10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- 27.Annweiler C, Schott AM, Berrut G, Chauviré V, Le Gall D, Inzitari M, et al. Vitamin D and ageing:Neurological issues. Neuropsychobiology. 2010;62:139–50. doi: 10.1159/000318570. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 29.Gan J, Galer P, Ma D, Chen C, Xiong T. The effect of vitamin D supplementation on attention-deficit/hyperactivity disorder:A systematic review and meta-analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. 2019;29:670–87. doi: 10.1089/cap.2019.0059. [DOI] [PubMed] [Google Scholar]
- 30.Poopedi MA, Norris SA, Pettifor JM. Factors influencing the vitamin D status of 10-year-old urban South African children. Public Health Nutr. 2011;14:334–9. doi: 10.1017/S136898001000234X. [DOI] [PubMed] [Google Scholar]
- 31.Snellman G, Melhus H, Gedeborg R, Byberg L, Berglund L, Wernroth L, et al. Determining vitamin D status:A comparison between commercially available assays. PLoS One. 2010;5:e11555. doi: 10.1371/journal.pone.0011555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strøm M, Halldorsson TI, Hansen S, Granström C, Maslova E, Petersen SB, et al. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes:A prospective study with long-term follow-up. Ann Nutr Metab. 2014;64:254–61. doi: 10.1159/000365030. [DOI] [PubMed] [Google Scholar]
- 33.López-Vicente M, Sunyer J, Lertxundi N, González L, Rodríguez-Dehli C, Sáenz-Torre ME, et al. Maternal circulating Vitamin D 3 levels during pregnancy and behaviour across childhood. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-51325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolppanen AM, Sayers A, Fraser WD, Lawlor DA. Association of serum 25-hydroxyvitamin D3 and D2 with academic performance in childhood:Findings from a prospective birth cohort. J Epidemiol Community Health. 2012;66:1137–42. doi: 10.1136/jech-2011-200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelishadi R, Ataei E, Ardalan G, Nazemian M, Tajadini M, Heshmat R, et al. Relationship of serum magnesium and vitamin D levels in a nationally-representative sample of Iranian adolescents:The CASPIAN-III study. Int J Prev Med. 2014;5:99–103. [PMC free article] [PubMed] [Google Scholar]
- 36.Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Effatpanah M, Rezaei M, Effatpanah H, Effatpanah Z, Varkaneh HK, Mousavi SM, et al. Magnesium status and attention deficit hyperactivity disorder (ADHD):A meta-analysis. Psychiatry Res. 2019;274:228–34. doi: 10.1016/j.psychres.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Firouzkouhi Moghaddam M, Rakhshani T, Khosravi M. Effectiveness of methylphenidate supplemented by zinc, calcium, and magnesium for treatment of ADHD patients in the city of Zahedan. Shiraz E-Med J. 2016:17. [Google Scholar]
- 39.Lahat E, Weiss M, Ben-Shlomo A, Evans S, Bistritzer T. Bone mineral density and turnover in children with attention-deficit hyperactivity disorder receiving methylphenidate. J Child Neurol. 2000;15:436–9. doi: 10.1177/088307380001500702. [DOI] [PubMed] [Google Scholar]


