Abstract
Enarodustat (JTZ‐951) is an oral hypoxia‐inducible factor prolyl hydroxylase inhibitor developed for treating anemia in chronic kidney disease. Two open‐label, uncontrolled phase 3 studies evaluated the 52‐week safety and efficacy of enarodustat in Japanese anemic patients with chronic kidney disease not on dialysis (n = 132) [SYMPHONY ND‐Long study] or on maintenance hemodialysis (n = 136) [SYMPHONY HD‐Long study]. The most frequent adverse events were viral upper respiratory tract infection (25.8%) followed by chronic kidney disease (8.3%) in the SYMPHONY ND‐Long study, and viral upper respiratory tract infection (49.3%) followed by contusion (16.9%) and diarrhea (16.9%) in the SYMPHONY HD‐Long study. The incidence of any adverse events did not increase over time. Mean hemoglobin levels and 95% confidence intervals were maintained within the target range (10.0–12.0 g/dl) over 52 weeks in both studies. The long‐term safety and efficacy of enarodustat were confirmed in Japanese anemic patients with chronic kidney disease.
Keywords: anemia, chronic kidney disease, enarodustat, hypoxia‐inducible factor prolyl hydroxylase inhibitor, long‐term study
1. INTRODUCTION
Anemia is a common complication of chronic kidney disease (CKD) mainly caused by a reduction in endogenous erythropoietin (EPO) production[ 1 ] in combination with other factors including disordered iron homeostasis,[ 2 ] and shortened erythrocyte survival.[ 3 ] It was reported that renal anemia is a component of cardio‐renal anemia syndrome, which accelerates progressive renal dysfunction and increases the risk of cardiovascular disease.[ 4 , 5 , 6 ] Therefore, it is important to manage the treatment of anemia in CKD patients.
Hypoxia‐inducible factor (HIF), a transcription factor that regulates the expressions of downstream target genes involved in EPO production and iron metabolism, is regulated by a family of oxygen‐sensitive enzymes termed prolyl‐hydroxylases (PH).[ 7 , 8 , 9 ] HIF‐PH inhibitors are a new class of agents for the treatment of anemia in patients with CKD. They are expected to be alternatives to erythropoiesis stimulating agents (ESAs), the standard therapy for renal anemia, because HIF‐PH inhibitors have the advantage of mediating their effects through the HIF pathway leading to endogenous EPO production and improved iron utilization.[ 10 ]
Enarodustat (JTZ‐951) is a new orally available HIF‐PH inhibitor developed by Japan Tobacco Inc.[ 11 ] In late phase 2 studies, enarodustat showed a dose–response relationship and maintenance effect on hemoglobin (Hb) levels over 30 weeks with a well‐tolerated safety profile in Japanese anemic patients with CKD.[ 12 , 13 ] In addition, its non‐inferiority to darbepoetin alpha (DA) for the control of mean Hb levels between 20 and 24 weeks was verified and no new safety concerns compared with DA were observed in phase 3 studies.[ 14 , 15 ]
Here, we report the results of two phase 3 studies that evaluated the long‐term safety and efficacy of enarodustat for up to 52 weeks in Japanese anemic patients with CKD not on dialysis (SYMPHONY ND‐Long study) or on maintenance hemodialysis (SYMPHONY HD‐Long study).
2. PATIENTS AND METHODS
2.1. Study design
SYMPHONY ND‐Long and HD‐Long were multicenter, open‐label, uncontrolled, intra‐individual dose adjustment, phase 3 studies conducted in Japan. The studies consisted of a 2‐ or 4‐week screening (Scr) period, a 52‐week treatment period, and a 2‐week follow‐up (F‐up) period.
The studies were registered with the Japan Pharmaceutical Information Center (JapicCTI‐173699 and JapicCTI‐173700) and conducted in accordance with the ethical principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice of the Japanese Ministerial Ordinance. The studies were approved by the Institutional Review Board of each participating study site. All patients provided written informed consent prior to participation.
2.2. Subject population
In the SYMPHONY ND‐Long study, Japanese anemic patients with CKD not on dialysis who had not received treatment with ESA within 12 weeks before the Scr Visit (ESA‐naïve) or had received treatment with ESA within 8 weeks before the Scr Visit (ESA‐treated) were enrolled. Major inclusion criteria were: (a) aged ≥20 years; (b) estimated glomerular filtration rate based on serum creatinine (eGFRcreat) <60 ml/min/1.73 m2 at Scr Visit; (c) transferrin saturation (TSAT) >20% or ferritin >50 ng/ml at Scr Visit; and (d) Hb level ≥8.0 g/dl and ≤10.5 g/dl in ESA‐naïve patients or Hb level ≥9.0 g/dl and ≤12.0 g/dl in ESA‐treated patients at Scr Visit.
In the SYMPHONY HD‐Long study, Japanese anemic patients with CKD on maintenance hemodialysis who had received ESA treatment for 4 weeks or more before the Scr Visit were enrolled. Major inclusion criteria were: (a) aged ≥20 years; (b) TSAT >20% or ferritin >75 ng/ml at Scr Visit; and (c) a pre‐dialysis Hb level of ≥9.0 g/dl and <12.0 g/dl measured after the maximum interdialytic interval at Scr Visit.
In both studies, patients who had poorly controlled hypertension; severe hepatobiliary disease; congestive heart failure (New York Heart Association [NYHA] Class III or more severe) or unstable angina; and who had developed myocardial infarction, cerebral infarction (excluding asymptomatic cerebral infarction), or venous thromboembolism (pulmonary embolism or deep vein thrombosis) during the period between 24 weeks before the Scr Visit and Week 0 were excluded. Further details of eligibility criteria are provided in Table S1.
2.3. Intervention
Initial doses of 2 mg of enarodustat in the SYMPHONY ND‐Long study and 4 mg of enarodustat in the SYMPHONY HD‐Long study were administered orally once daily during the initial 4 weeks. Thereafter, a dose adjustment was allowed in both studies every 4 weeks in the range of 1 to 8 mg/day of enarodustat to maintain the Hb level within the target range (10.0–12.0 g/dl). The study treatment in both studies was discontinued when the Hb level was <8.0 g/dl at the highest dose or when the Hb level was increased by more than 2.0 g/dl from the previous visit at the lowest dose.
After Week 4, in principle, intravenous or oral iron preparations were prescribed in consideration of the changes in Hb level: if ferritin was ≤100 ng/ml or TSAT was ≤20%.
2.4. Assessments
Safety assessments included adverse events (AEs) occurring after the start of treatment, laboratory tests, vital signs, standard 12‐lead electrocardiogram, chest X‐ray, and fundoscopy.
Other assessments included vascular endothelial growth factor (VEGF), fibroblast growth factor 23 (FGF 23), TSAT, ferritin, hepcidin and eGFRcreat (only in the SYMPHONY ND‐Long study).
Efficacy assessments included the time‐course of Hb levels, mean Hb level and the achievement proportion of the mean Hb level within the target range during the end of treatment (EOT) period and the evaluation period for 24‐ and 52‐week treatments, the mean prescribed dose during the evaluation period for 24‐ and 52‐week treatments, and the mean number of dose adjustments from Week 0 to Week 24 and from Week 24 to 52.
2.5. Statistical analysis
A sample size of 125 subjects was planned for both studies to provide the safety and efficacy data after 1 year of treatment. Analysis of safety assessments was performed using the safety analysis population (SAF), comprising subjects who received the study treatment and who were assessed for safety at least once. AEs reported after the start of the study treatment were coded using the Medical Dictionary for Regulatory Activities/Japanese (MedDRA/J) version 20.0 and summarized. AEs of special interest (“hypertension”, “embolic and thrombotic events”, “malignant or unspecified tumors,” and “retinal disorders”) were categorized with reference to the Standardized MedDRA Queries (SMQs).
Analysis of VEGF, FGF 23, and eGFRcreat was performed using the SAF. Analysis of TSAT, ferritin and hepcidin was performed using the full analysis set (FAS), comprising subjects who received the study treatment and who were assessed for efficacy at Week 4 (including an assessment at discontinuation at the visit corresponding to Week 4).
Analysis of efficacy assessments was performed using the FAS. The mean Hb level during the EOT period was calculated with Hb levels at EOT and the previous visit. If the Hb level was measured once from Week 4 onward, the Hb level during the EOT period was the Hb level at EOT. The mean Hb level during the evaluation period for 24‐ and 52‐week treatments was calculated with Hb levels at Week 20 and Week 24 or at discontinuation corresponding to Week 24, and Week 48 and Week 52 or at discontinuation corresponding to Week 52.
3. RESULTS
3.1. Subject characteristics
Subject dispositions and subject characteristics are shown in Figure 1 and Table 1. In the SYMPHONY ND‐Long study, 132 subjects (42 ESA‐naïve subjects and 90 ESA‐treated subjects) were registered to the treatment period, of whom 39 subjects discontinued treatment. The most frequent reason for discontinuation was AE followed by dialysis introduction. One hundred and thirty subjects were included in the FAS because two subjects discontinued before Week 4. All subjects were included in the SAF.
FIGURE 1.
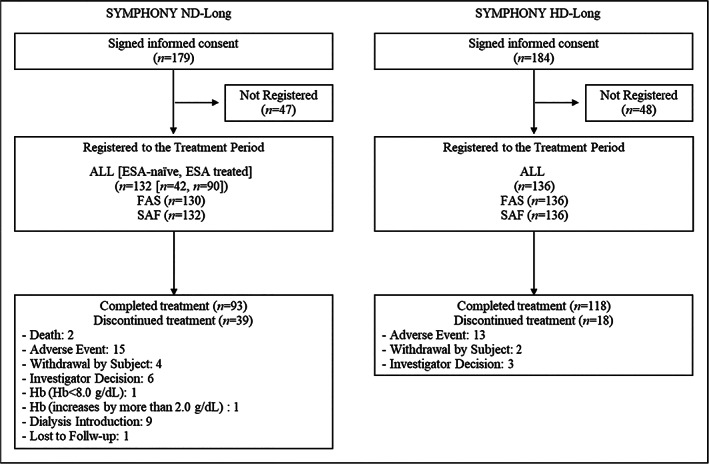
Subject disposition in the SYMPHONY ND‐Long and HD‐Long studies. ESA, erythropoiesis stimulating agent; FAS, full analysis set; Hb, hemoglobin; SAF, safety analysis population
TABLE 1.
Subject characteristics in the SYMPHONY ND‐Long and HD‐Long studies (full analysis set)
| Characteristics | SYMPHONY ND‐Long (N = 130) | SYMPHONY HD‐Long (N = 136) |
|---|---|---|
| Age (years), mean (SD) | 70.5 (8.9) | 64.3 (10.3) |
| Sex, n (%) | ||
| Male | 67 (51.5) | 82 (60.3) |
| Female | 63 (48.5) | 54 (39.7) |
| Body weight (kg), mean (SD) | 59.27 (12.78) | 61.14 (12.73) |
| Primary disease of CKD, n (%) | ||
| Chronic glomerulonephritis | 27 (20.8) | 49 (36.0) |
| Diabetic nephropathy | 37 (28.5) | 43 (31.6) |
| Nephrosclerosis | 32 (24.6) | 17 (12.5) |
| Other | 34 (26.2) | 27 (19.9) |
| eGFRcreat, ml/min/1.73 m2, mean (SD) | 18.16 (8.62) | – |
| <15, n (%) | 55 (42.3) | – |
| 15≤ to <30, n (%) | 65 (50.0) | – |
| 30≤, n (%) | 10 (7.7) | – |
| History of hemodialysis (year), mean (SD) | – | 7.76 (7.62) |
| Use of Prior ESA, n (%) | ||
| ESA naïve | 42 (32.3) | – |
| ESA treated | 88 (67.7) | 136 (100.0) |
| Types of prior ESA, n (%) | ||
| rHuEPO | 3 (2.3) | 65 (47.8) |
| Darbepoetin alfa | 42 (32.3) | 48 (35.3) |
| Epoetin beta pegol | 43 (33.1) | 23 (16.9) |
| Prior ESA dose, mean (SD) a | ||
| rHuEPO, (ND‐Long IU/2 weeks, HD‐Long IU/week) | 4000 (1732.1) | 3403.8 (2154.5) |
| Darbepoetin alfa, (ND‐Long μg/4 weeks, HD‐Long μg/week) | 68.8 (41.9) | 16.20 (13.41) |
| Epoetin beta pegol, (μg/4 weeks) | 79.1 (55.1) | 105.4 (65.3) |
| Use of intravenous iron preparations during the screening period, n (%) | 0 (0.0) | 33 (24.3) |
| Use of oral iron preparations including iron‐containing phosphate binders during the screening period, n (%) | 29 (22.3) | 48 (35.3) |
Abbreviations: CKD, chronic kidney disease; eGFRcreat, estimated glomerular filtration rate based on serum creatinine; ESA, erythropoiesis‐stimulating agent; rHuEPO, recombinant human erythropoietin.
Mean (SD) was calculated based on the number of ESA‐treated subjects.
In the SYMPHONY HD‐Long study, 136 subjects were registered to the treatment period, of whom 18 subjects discontinued treatment. The most frequent reason for discontinuation was AE. All subjects were included in the FAS and SAF.
3.2. Safety assessments
Four deaths were reported during the SYMPHONY ND‐Long study, three of which (subdural haematoma, chronic kidney disease, and plasma cell myeloma) were judged as not related to enarodustat. The cause of the other death was unknown because the subject died at home. Although every effort was made to obtain the cause of death, no further information was available. AEs leading to study discontinuation occurred in 11.4% (n = 15/132) of the subjects. AEs leading to study discontinuation reported in ≥2 subjects were chronic kidney disease (2.3%; n = 3/132), renal impairment (1.5%; n = 2/132), cardiac failure congestive (1.5%; n = 2/132), and diabetic retinopathy (1.5%; n = 2/132). The summary of AEs and adverse drug reactions (ADRs) is shown in Table 2. AEs and serious AEs (SAEs) except for deaths occurred in 87.1% (n = 115/132) and 25.8% (n = 34/132) of the subjects, respectively. The most frequent AE was viral upper respiratory tract infection (25.8%), followed by chronic kidney disease (8.3%). SAEs that occurred in ≥2 subjects were chronic kidney disease (5.3%), renal impairment (2.3%), atrial fibrillation (1.5%), and cardiac failure congestive (1.5%). ADRs and serious ADRs (SADRs) except for deaths occurred in 13.6% (n = 18/132) and 1.5% (n = 2/132) of the subjects, respectively. ADRs that occurred in ≥2 subjects were hypertension (3.0%), blood pressure increased (1.5%), and fibrin D dimer increased (1.5%). Two SADRs occurred, which were renal impairment and anti‐neutrophil cytoplasmic antibody‐positive vasculitis. There were no severe ADRs or SADRs.
TABLE 2.
Summary of adverse events, adverse drug reactions, and adverse events of special interest in the SYMPHONY ND‐Long study (safety analysis population)
| SYMPHONY ND‐Long (N = 132) | ||
|---|---|---|
| n | % | |
| Adverse events (≥5% subjects) | ||
| Any adverse events | 115 | 87.1 |
| Viral upper respiratory tract infection | 34 | 25.8 |
| Chronic kidney disease | 11 | 8.3 |
| Hypertension | 10 | 7.6 |
| Constipation | 8 | 6.1 |
| Contusion | 8 | 6.1 |
| Diarrhea | 8 | 6.1 |
| Serious adverse events a (≥2 subjects) | ||
| Any serious adverse events | 34 | 25.8 |
| Chronic kidney disease | 7 | 5.3 |
| Renal impairment | 3 | 2.3 |
| Atrial fibrillation | 2 | 1.5 |
| Cardiac failure congestive | 2 | 1.5 |
| Adverse drug reactions (≥2 subjects) | ||
| Any adverse drug reactions | 18 | 13.6 |
| Hypertension | 4 | 3.0 |
| Blood pressure increased | 2 | 1.5 |
| Fibrin D dimer increased | 2 | 1.5 |
| Serious adverse drug reactionsa | ||
| Any serious adverse drug reactions | 2 | 1.5 |
| Renal impairment | 1 | 0.8 |
| Anti‐neutrophil cytoplasmic antibody positive vasculitis | 1 | 0.8 |
| Hypertension | 15 | 11.4 |
| Hypertension | 10 | 7.6 |
| Blood pressure increased | 4 | 3.0 |
| Essential hypertension | 1 | 0.8 |
| Embolic and thrombotic events | 1 | 0.8 |
| Deep vein thrombosis | 1 | 0.8 |
| Malignant or unspecified tumors | 1 | 0.8 |
| Plasma cell myeloma | 1 | 0.8 |
| Retinal disorders | 11 | 8.3 |
| Retinal hemorrhage | 4 b | 3.0 |
| Diabetic retinopathy | 2 | 1.5 |
| Retinal tear | 2 c | 1.5 |
| Vitreous hemorrhage | 2 c | 1.5 |
| Macular degeneration | 1 | 0.8 |
| Retinal detachment | 1 c | 0.8 |
| Retinal exudates | 1 b | 0.8 |
| Vitreous floaters | 1 | 0.8 |
Deaths were excluded.
One subject experienced both adverse events (AEs).
One subject experienced both AEs.
There were no deaths in the SYMPHONY HD‐Long study. AEs leading to study discontinuation occurred in 9.6% (n = 13/136) of the subjects; however, none of these AEs occurred in ≥2 subjects. The summary of AEs and ADRs is shown in Table 3. AEs and serious SAEs occurred in 97.8% (n = 133/136) and 22.8% (n = 31/136) of the subjects, respectively. The most frequent AE was viral upper respiratory tract infection (49.3%), followed by contusion (16.9%), and diarrhea (16.9%). SAEs that occurred in ≥2 subjects were shunt occlusion (4.4%), pneumonia (2.9%), and cholangitis (2.2%). ADRs and SADRs occurred in 8.8% (n = 12/136) and 1.5% (n = 2/136) of the subjects. ADRs that occurred in ≥2 subjects were hypertension (2.9%) and eczema (1.5%). Two SADRs occurred, which were peripheral arterial occlusive disease and brain stem infarction. There were no severe ADRs and SADRs.
TABLE 3.
Summary of adverse events, adverse drug reactions, and adverse events of special interest in the SYMPHONY HD‐Long study (safety analysis population)
| SYMPHONY HD‐Long (N = 136) | ||
|---|---|---|
| n | % | |
| Adverse events (≥5% subjects) | ||
| Any adverse events | 133 | 97.8 |
| Viral upper respiratory tract infection | 67 | 49.3 |
| Contusion | 23 | 16.9 |
| Diarrhea | 23 | 16.9 |
| Shunt stenosis | 20 | 14.7 |
| Upper respiratory tract inflammation | 17 | 12.5 |
| Excoriation | 12 | 8.8 |
| Vomiting | 12 | 8.8 |
| Muscle spasms | 11 | 8.1 |
| Back pain | 10 | 7.4 |
| Eczema | 10 | 7.4 |
| Shunt occlusion | 10 | 7.4 |
| Influenza | 9 | 6.6 |
| Pharyngitis | 9 | 6.6 |
| Skin exfoliation | 9 | 6.6 |
| Gastroenteritis | 8 | 5.9 |
| Constipation | 7 | 5.1 |
| Dermatitis contact | 7 | 5.1 |
| Dry eye | 7 | 5.1 |
| Hypertension | 7 | 5.1 |
| Myalgia | 7 | 5.1 |
| Pain in extremity | 7 | 5.1 |
| Serious adverse events (≥2 subjects) | ||
| Any serious adverse events | 31 | 22.8 |
| Shunt occlusion | 6 | 4.4 |
| Pneumonia | 4 | 2.9 |
| Cholangitis | 3 | 2.2 |
| Adverse drug reactions (≥2 subjects) | ||
| Any adverse drug reactions | 12 | 8.8 |
| Hypertension | 4 | 2.9 |
| Eczema | 2 | 1.5 |
| Serious adverse drug reactions | ||
| Any serious adverse drug reactions | 2 | 1.5 |
| Peripheral arterial occlusive disease | 1 | 0.7 |
| Brain stem infarction | 1 | 0.7 |
| Hypertension | 7 | 5.1 |
| Hypertension | 7 | 5.1 |
| Embolic and thrombotic events | 17 | 12.5 |
| Shunt occlusion | 10 | 7.4 |
| Cerebral infarction | 3 a | 2.2 |
| Peripheral arterial occlusive disease | 2 | 1.5 |
| Brain stem infarction | 1 a | 0.7 |
| Transient ischaemic attack | 1 | 0.7 |
| Venous occlusion | 1 | 0.7 |
| Malignant or unspecified tumors | 3 | 2.2 |
| Colon cancer | 1 | 0.7 |
| Gastric cancer | 1 | 0.7 |
| Glottis carcinoma | 1 | 0.7 |
| Retinal disorders | 11 | 8.1 |
| Retinal hemorrhage | 3 b | 2.2 |
| Diabetic retinopathy | 3 | 2.2 |
| Retinal exudates | 2 c | 1.5 |
| Diabetic retinal oedema | 1 | 0.7 |
| Macular oedema | 1 c | 0.7 |
| Retinal detachment | 1 | 0.7 |
| Vitreous hemorrhage | 1 | 0.7 |
| Macular hole | 1 b | 0.7 |
One subject experienced both adverse events (AEs).
One subject experienced both AEs.
One subject experienced both AEs.
Summaries of AEs of special interest (“hypertension”, “embolic and thrombotic events”, “malignant or unspecified tumors”, and “retinal disorders”) in the SYMPHONY ND‐Long and HD‐Long studies are shown in Tables 2 and 3.
In both studies, there were no obvious changes in laboratory tests, VEGF, vital signs, standard 12‐lead electrocardiogram, chest X‐ray, and fundoscopy.
3.3. Fibroblast growth factor 23 and iron related parameters
The time courses of C‐terminal FGF 23 (cFGF 23) and intact FGF 23 (iFGF 23) are shown in Figure 2. The descriptive statistics of cFGF 23, iFGF 23, ferritin, TSAT and hepcidin is shown in Table S2. No obvious changes in the median cFGF 23 and iFGF 23 values were observed in either study. The median TSAT values slightly decreased but not continuously decreased in the SYMPHONY ND‐Long study, and no obvious changes in the median TSAT values were observed in the SYMPHONY HD‐Long study. The median ferritin and hepcidin values decreased in the SYMPHONY ND‐Long study and had a tendency to decrease in the SYMPHONY HD‐Long study.
FIGURE 2.
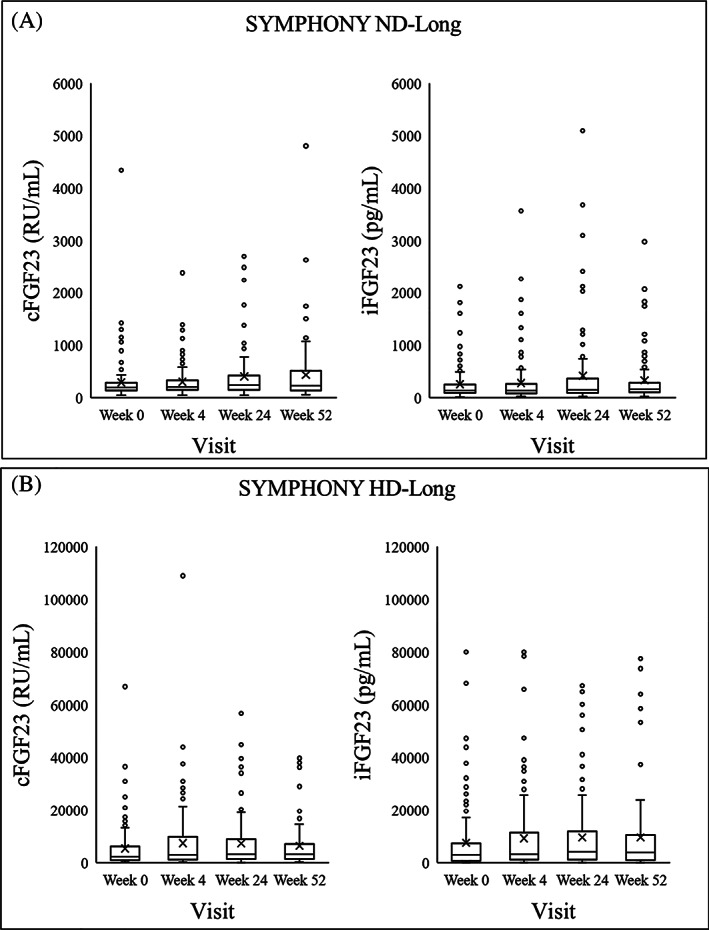
Box plot of C‐terminal fibroblast growth factor 23 and intact fibroblast growth factor 23 in the (A) SYMPHONY ND‐Long and (B) SYMPHONY HD‐Long studies (safety analysis population). cFGF 23, C‐terminal fibroblast growth factor 23; iFGF 23, intact fibroblast growth factor 23. The bottom and top edges of the box were located at the 25th and 75th percentiles of the data. Within the box, the median (50th percentile) is displayed as a line and the mean is displayed as a marker. Outliers were observations that were more extreme than the upper and lower fences (±1.5 interquartile range)
3.4. Estimated glomerular filtration rate based on serum creatinine
In the SYMPHONY ND‐Long study, eGFRcreat values were measured at Week 0, Week 24, and Week 52 (Table 4). The mean eGFRcreat gradually decreased over the treatment period. The mean (SD) change in eGFRcreat from Week 0 was −1.39 (2.79) ml/min/1.73 m2 at Week 24 and −2.21 (3.83) ml/min/1.73 m2 at Week 52.
TABLE 4.
Estimated glomerular filtration rate based on serum creatinine in the SYMPHONY ND‐Long study (safety analysis population)
| Parameter | SYMPHONY ND‐Long | ||
|---|---|---|---|
| Week 0 | Week 24 | Week 52 | |
| n | 132 | 113 | 93 |
|
eGFRcreat ml/min/1.73 m2, mean (SD) |
18.11 (8.60) | 16.58 (8.59) | 16.87 (9.18) |
|
Change from Week 0 ml/min/1.73 m2, mean (SD) |
– | −1.39 (2.79) | −2.21 (3.83) |
Abbreviation: eGFRcreat, estimated glomerular filtration rate based on serum creatinine.
3.5. Efficacy assessments
The time‐courses of Hb levels are shown in Figure 3. In both studies, the mean Hb levels and 95% confidence interval (CI) of each visit were maintained within the target range (10.0–12.0 g/dl) over the treatment period. The efficacy variables are summarized in Table 5. The mean [95% CI] Hb levels and the achievement proportions of the mean Hb level within the target range during the EOT period were 10.74 g/dl [10.58, 10.91] and 79.2% (n = 103/130), respectively, in the SYMPHONY ND‐Long study, and 10.72 g/dl [10.56, 10.88] and 76.5% (n = 104/136), respectively, in the SYMPHONY HD‐Long study.
FIGURE 3.
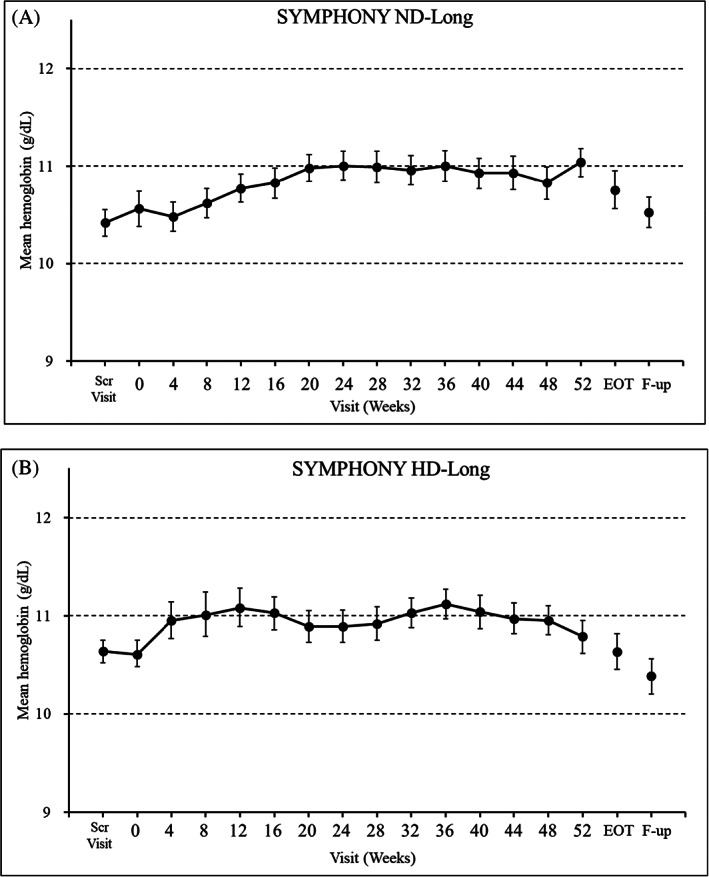
The mean (95% confidence interval) hemoglobin levels in the (A) SYMPHONY ND‐Long and (B) SYMPHONY HD‐Long studies (full analysis set). EOT, end of treatment; F‐up, Follow‐up Visit; Scr Visit, Screening Visit
TABLE 5.
The mean Hb levels, proportion achieving the mean Hb level within the target range, mean prescribed dose, and mean number of dose adjustments in the SYMPHONY ND‐Long and HD‐Long studies (full analysis set)
| Evaluation period | SYMPHONY ND‐Long | SYMPHONY HD‐Long | ||||
|---|---|---|---|---|---|---|
| EOT period | 24‐week treatment | 52‐week treatment | EOT period | 24‐week treatment | 52‐week treatment | |
|
Mean Hb level (g/dl), mean [95% CI] |
10.74 [10.58, 10.91] (n = 130) |
10.98 [10.85, 11.11] (n = 113) |
10.95 [10.82, 11.08] (n = 93) |
10.72 [10.56, 10.88] (n = 136) |
10.91 [10.76, 11.06] (n = 121) |
10.87 [10.73, 11.01] (n = 118) |
| Achievement proportion of the mean Hb level within the target range, % [95% CI] |
79.2 [71.2, 85.8] (n = 103/130) |
89.4 [82.2, 94.4] (n = 101/113) |
89.2 [81.1, 94.7] (n = 83/93) |
76.5 [68.4, 83.3] (n = 104/136) |
77.7 [69.2, 84.8] (n = 94/121) |
81.4 [73.1, 87.9] (n = 96/118) |
| Mean prescribed dose a (mg/day), mean (SD) | – |
2.84 (1.74) (n = 116) |
3.02 (2.08) (n = 96) |
– |
3.73 (2.46) (n = 124) |
3.29 (2.36) (n = 118) |
| Mean number of dose adjustments b , mean (SD) | – |
1.1 (1.0) (n = 130) |
1.5 (1.5) (n = 113) |
– |
1.7 (1.2) (n = 136) |
1.5 (1.5) (n = 121) |
Abbreviation: EOT, end of treatment; Hb, hemoglobin; 95% CI, 95% confidence interval.
The mean prescribed dose during the evaluation period for 24‐ and 52‐week treatments was calculated using the prescribed dose from Week 20 to 24 and from Week 48 to 52.
The mean number of dose adjustments during the evaluation period for 24‐ and 52‐week treatment was calculated using the number of dose adjustments from Week 0 to 24 and from Week 24 to 52.
4. DISCUSSION
The SYMPHONY ND‐Long and HD‐Long studies were the first to evaluate the safety and efficacy of enarodustat for up to 52 weeks in anemic patients with CKD not on dialysis or on maintenance hemodialysis. The study results showed that enarodustat was well tolerated and was effective at maintaining Hb levels within the target range (10.0–12.0 g/dl) for up to 52 weeks.
Regarding safety assessments in the SYMPHONY ND‐Long and HD‐Long studies, AEs occurred in 87.1% and 97.8% of the subjects, respectively; however, no AEs had an increased incidence as the treatment period progressed. We previously reported the results of two 24‐week comparative studies (SYMPHONY ND and HD studies) that indicated no new safety concerns of enarodustat compared with DA. Furthermore, only two AEs (vomiting [10.3%] and gastroenteritis [5.7%] in the enarodustat arm) were observed more frequently in the SYMPHONY HD study than in the DA arm.[ 14 , 15 ] The frequencies of vomiting (8.8%) and gastroenteritis (5.9%) in the SYMPHONY HD‐Long study did not increase compared with those in the SYMPHONY HD study and all the events were mild in severity and judged to not be related to enarodustat.
Regarding AEs of special interest, we summarized the AEs as “hypertension” and “embolic and thrombotic event” because they were related to events during ESA therapy.[ 16 ] In previous comparative studies, no apparent difference in the frequencies of “hypertension” and “embolic and thrombotic event” were observed between the treatment arms.[ 14 , 15 ] The incidences of “hypertension” (SYMPHONY ND‐Long; 11.4% and SYMPHONY HD‐Long; 5.1%) and “embolic and thrombotic event” (SYMPHONY ND‐Long; 0.8% and SYMPHONY HD‐Long; 12.5%) in the present studies were not obviously increased compared with that of “hypertension” (SYMPHONY ND; 4.7% and SYMPHONY HD; 4.6%) and “embolic and thrombotic event” (SYMPHONY ND; 0% and SYMPHONY HD; 6.9%) in the enarodustat arm of the previous comparative studies, considering the treatment periods in the present studies were more than twice as long as those in the previous comparative studies.
AEs categorized as “malignant or unspecified tumors” and “retinal disorders” were also summarized because activating the HIF pathway theoretically involves tumorigenesis and proangiogenesis mediated by VEGF signaling.[ 17 , 18 , 19 ] Four events (one in SYMPHONY ND‐Long and three in SYMPHONY HD‐Long) were categorized as “malignant or unspecified tumors”. All events were judged to not be related to enarodustat. Of the AEs categorized as “retinal disorders”, retinal hemorrhage was most frequently observed in the SYMPHONY ND‐Long and HD‐Long studies, similar to the previous comparative studies. However, the incidences of retinal hemorrhage (SYMPHONY ND‐Long; 3.0% and SYMPHONY HD‐Long; 2.2%) in the present studies were not increased compared with those (SYMPHONY ND; 1.9% and SYMPHONY HD; 3.4%) in the enarodustat arm of the previous comparative studies even though the treatment period was longer. All the events were mild in severity and judged to not be related to enarodustat. In addition, no apparent changes in VEGF level were observed in the SYMPHONY ND‐Long and HD‐Long studies, and no subjects with ADRs categorized as “retinal disorders” had increased VEGF levels (data not shown). Thus, potential safety concerns of enarodustat related to AEs induced by VEGF expression were not confirmed.
Recent findings showed that Inflammation and iron deficiency which are common occurrences in CKD induced FGF 23 production.[ 20 ] FGF 23 is associated with an increased risk of mortality and cardiovascular events in patients with CKD.[ 21 ] It was also reported that exogenous EPO administration or endogenous EPO production through the HIF pathway were associated with increased FGF 23 levels in rodents.[ 22 , 23 ] The changes of iron related parameters in the present studies were almost the same as those in the previous comparative studies,[ 14 , 15 ] suggesting that the improvement of iron utilization continued even though the treatment period was longer. The present studies are the first reports in which no apparent changes in FGF 23 were observed during HIF‐PH inhibitor treatment in patients with CKD. Thus, enarodustat is unlikely to increase the safety risk associated with elevated FGF 23.
In the SYMPHONY ND‐Long study, eGFRcreat was measured to evaluate the effect of enarodustat on renal function. After dosing with enarodustat, eGFRcreat was gradually decreased; however, the change seemed to be within the natural course of CKD progression.[ 24 ] Therefore, the possibility that enarodustat has a negative effect on renal function is considered low.
These findings indicate that enarodustat was well tolerated and that there were no new safety concerns compared with previously reported studies even though the treatment period was longer (up to 52 weeks).
Regarding efficacy assessments, the mean Hb level and 95% CI during the EOT period were within the target range (10.0–12.0 g/dl). The achievement proportion of the mean Hb level within the target range during the evaluation period for 24‐week treatment was comparable to that during the evaluation period for 52‐week treatment. In addition, the mean prescribed dose during the evaluation period for 52‐week treatment and the mean number of dose adjustments from Week 24 to Week 52 did not increase compared with those during the evaluation period for 24‐week treatment or from Week 0 to Week 24. These findings indicate that enarodustat stably controlled Hb levels within the target range without increasing the mean prescribed dose and frequency of dose adjustments even though the treatment period was longer.
As a limitation of both studies, the relative safety and efficacy of enarodustat were not directly compared with other renal anemia treatments because of the open‐label and non‐comparative study design.
5. CONCLUSIONS
Overall, the results of the SYMPHONY ND‐Long and HD‐Long studies demonstrate that enarodustat was well tolerated and that there were no new safety concerns compared with previously reported studies. In addition, enarodustat was effective at maintaining Hb levels within the target range (10.0–12.0 g/dl) for up to 52 weeks in Japanese anemic patients with CKD not on dialysis or on maintenance hemodialysis.
CONFLICT OF INTEREST
Tadao Akizawa reports personal fees from Japan Tobacco Inc., during the study, and personal fees from Astellas, Bayer Yakuhin Ltd., Kyowa Kirin Co. Ltd., Kissei Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Fuso Pharmaceutical Industries Ltd., Torii Pharmaceutical Co. Ltd., GlaxoSmithKline, Nipro Corporation, Otsuka Pharmaceutical, Sanwa Chemical, Chugai Pharmaceutical Co. Ltd. and Mitsubishi Tanabe Pharma Corporation outside the submitted work. Masaomi Nangaku reports grants and personal fees from Japan Tobacco Inc., during the study, and personal fees from Kyowa Kirin Co. Ltd, Astellas, Astra Zeneca, GlaxoSmithKline, Mitsubishi Tanabe Pharma Corporation, Akebia Therapeutics Inc., Bayer Yakuhin Ltd., and Torii Pharmaceutical Co. Ltd. and grants from Kyowa Kirin Co. Ltd., Astellas, Mitsubishi Tanabe Pharma Corporation, Bayer Yakuhin Ltd., and Torii Pharmaceutical Co. Ltd. outside of the submitted work. Takuhiro Yamaguchi reports personal fees from Japan Tobacco Inc., during the study, and personal fees from Ono Pharmaceutical Co. Ltd., Kowa, Chugai Pharmaceutical Co. Ltd, TSUMURA&Co., CAC Croit Corporation, Kyowa Kirin Co. Ltd., Daiichi Sankyo, ASAHI INTECC, Asahi Kasei Corporation, Kaken Pharmaceutical, 3H Clinical Trial Co. Ltd., Welby, 3H Medi Solution, and Nipro Corporation, and grants from Ono Pharmaceutical Co. Ltd., CAC Croit Corporation, Kyowa Kirin Co. Ltd., Daiichi Sankyo, 3H Clinical Trial Co. Ltd., AC Medical, A2 Healthcare, Facet Biotech, Japan Media Corporation, Luminary Medical, Medidata Solutions Inc., Senju Pharmaceutical, Otsuka Pharmaceutical, Eisai, FMD K&L Japan, Intellim, Welby, 3H Medi Solution, Nipro Corporation, Hemp Kitchen, NOBORI, Puravida Technologies LLC., and Medrio Inc. outside the submitted work. Hideki Hirakata reports personal fees from Japan Tobacco Inc., during the study, and personal fees from Kyowa Kirin Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Torii Pharmaceutical Co. Ltd. outside the submitted work. Ryosuke Koretomo, Kazuo Maeda, and Osamu Yamada are employees of Japan Tobacco Inc. and have stock or stock options in Japan Tobacco Inc.
Supporting information
TABLE S1 Eligibility criteria for the SYMPHONY ND‐Long and HD‐Long studies
TABLE S2. Descriptive statistics of C‐terminal fibroblast growth factor 23 and intact fibroblast growth factor 23 (safety analysis population), and transferrin saturation, ferritin and hepcidin (full analysis set) in the SYMPHONY ND‐Long and HD‐Long studies
ACKNOWLEDGMENTS
These studies were funded by Japan Tobacco Inc. and Torii Pharmaceutical Co. Ltd. We would like to thank all the physicians, nurses, and patients at the participating centers for their support. We acknowledge the editorial support of ASCA Corporation (http://www.asca-co.com/english_site/) for proofreading a draft of this manuscript.
Akizawa T, Nangaku M, Yamaguchi T, Koretomo R, Maeda K, Yamada O, et al. Two long‐term phase 3 studies of enarodustat (JTZ‐951) in Japanese anemic patients with chronic kidney disease not on dialysis or on maintenance hemodialysis: SYMPHONY ND‐Long and HD‐Long studies. Ther Apher Dial. 2022;26:345–56. 10.1111/1744-9987.13724
Funding information Japan Tobacco Inc.; Torii Pharmaceutical Co. Ltd.
REFERENCES
- 1. Artunc F, Risler T. Serum erythropoietin concentrations and responses to anaemia in patients with or without chronic kidney disease. Nephrol Dial Transplant. 2007;22:2900–8. [DOI] [PubMed] [Google Scholar]
- 2. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long‐term dialysis patients. Am J Kidney Dis. 2011;58:591–8. [DOI] [PubMed] [Google Scholar]
- 4. Scrutinio D, Passantino A, Santoro D, Catanzaro R. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long‐term survival. Eur J Heart Fail. 2011;13:61–7. [DOI] [PubMed] [Google Scholar]
- 5. Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–44. [DOI] [PubMed] [Google Scholar]
- 6. Silverberg D, Wexler D, Blum M, Wollman Y, Iaina A. The cardio‐renal anemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18:viii7–12. [DOI] [PubMed] [Google Scholar]
- 7. Minamishima YA, Kaelin WG Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science. 2010;329:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med. 2007;85:1325–30. [DOI] [PubMed] [Google Scholar]
- 9. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakashita M, Tanaka T, Nangaku M. Hypoxia‐inducible factor‐prolyl hydroxylase domain inhibitors to treat anemia in chronic kidney disease. Contrib Nephrol. 2019;198:112–23. [DOI] [PubMed] [Google Scholar]
- 11. Ogoshi Y, Matsui T, Mitani I, Yokota M, Terashita M, Motoda D, et al. Discovery of JTZ‐951: a HIF prolyl hydroxylase inhibitor for the treatment of renal anemia. ACS Med Chem Lett. 2017;8:1320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Matsui A, et al. A placebo‐controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long‐term trial. Am J Nephrol. 2019;49:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Maeda K, et al. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo‐controlled phase 2b trial followed by long‐term trial. Nephron. 2019;143:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akizawa T, Nangaku M, Yamaguchi T, Koretomo R, Maeda K, Miyazawa Y, et al. A phase 3 study of enarodustat in anemic patients with CKD not requiring dialysis, the SYMPHONY ND study. Kidney Int Rep. 2021;6:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akizawa T, Nangaku M, Yamaguchi T, Koretomo R, Maeda K, Miyazawa Y, et al. A phase 3 study of enarodustat (JTZ‐951) in Japanese hemodialysis patients for treatment of anemia in chronic kidney disease: SYMPHONY HD study. Kidney Dis. 2021;1–9. 10.1159/000517053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3:36. [Google Scholar]
- 17. Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–43. [DOI] [PubMed] [Google Scholar]
- 18. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krock BL, Skuli N, Simon MC. Hypoxia‐induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanudel MR, Laster M, Salusky IB. Non‐renal‐related mechanisms of FGF23 pathophysiology. Curr Osteoporos Rep. 2018;16:724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovesdy CP, Quarles LD. The role of fibroblast growth factor‐23 in cardiorenal syndrome. Nephron Clin Pract. 2013;123:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2019;34:2057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flamme I, Ellinghaus P, Urrego D, Krüger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12:e0186979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inaguma D, Imai E, Takeuchi A, et al. Risk factors for CKD progression in Japanese patients: findings from the chronic kidney disease Japan cohort (CKD‐JAC) study. Clin Exp Nephrol. 2017;21:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Eligibility criteria for the SYMPHONY ND‐Long and HD‐Long studies
TABLE S2. Descriptive statistics of C‐terminal fibroblast growth factor 23 and intact fibroblast growth factor 23 (safety analysis population), and transferrin saturation, ferritin and hepcidin (full analysis set) in the SYMPHONY ND‐Long and HD‐Long studies


