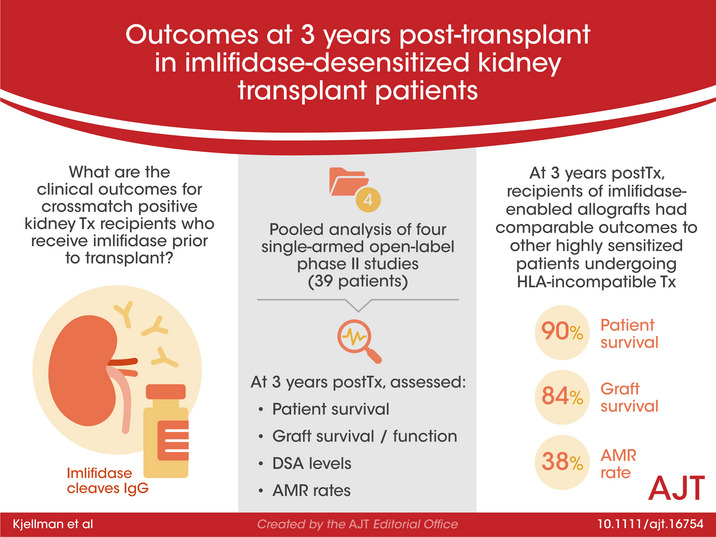
Abstract
Imlifidase is a cysteine proteinase which specifically cleaves IgG, inhibiting Fc‐mediated effector function within hours of administration. Imlifidase converts a positive crossmatch to a potential donor (T cell, B cell, or both), to negative, enabling transplantation to occur between previously HLA incompatible donor‐recipient pairs. To date, 39 crossmatch positive patients received imlifidase prior to a kidney transplant in four single‐arm, open‐label, phase 2 studies. At 3 years, for patients who were AMR+ compared to AMR−, death‐censored allograft survival was 93% vs 77%, patient survival was 85% vs 94%, and mean eGFR was 49 ml/min/1.73 m2 vs 61 ml/min/1.73 m2, respectively. The incidence of AMR was 38% with most episodes occurring within the first month post‐transplantation. Sub‐analysis of patients deemed highly sensitized with cPRA ≥ 99.9%, and unlikely to be transplanted who received crossmatch‐positive, deceased donor transplants had similar rates of patient survival, graft survival, and eGFR but a higher rate of AMR. These data demonstrate that outcomes and safety up to 3 years in recipients of imlifidase‐enabled allografts is comparable to outcomes in other highly sensitized patients undergoing HLA‐incompatible transplantation. Thus, imlifidase is a potent option to facilitate transplantation among patients who have a significant immunologic barrier to successful kidney transplantation.
Clinical Trial: ClinicalTrials.gov (NCT02790437), EudraCT Number: 2016‐002064‐13.
Keywords: alloantibody, clinical research/practice, crossmatch, desensitization, immunosuppressant – other, immunosuppression/immune modulation, kidney transplantation/nephrology, sensitization
Crossmatch positive patients who received imlifidase prior to kidney transplantation enjoy comparable patient survival, graft survival, eGFR and rates of acute antibody mediated rejection to other highly sensitized patients undergoing HLA‐incompatible transplantation. See Schinstock and Tambur's editorial on page 3825.

Abbreviations
- AMR
antibody‐mediated rejection
- CDCXM
complement dependent cytotoxicity crossmatch
- CIT
cold ischemia time
- CPRA
calculated panel reactive antibody
- DD
deceased donor
- DGF
delayed graft function
- DSA
donor specific antibody
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- HLA
human leukocyte antigen
- IdeS
imlifidase
- IEC
independent ethics committee
- IRB
institutional review board
- IVIg
intravenous immune globulin
- KAS
kidney allocation system
- KPD
kidney paired donation
- MDRD
modification of diet in renal disease
- MFI
mean fluorescence intensity
- SAB
single antigen bead
- SCr
serum creatinine
- XM
crossmatch
1. INTRODUCTION
Patients in need of kidney transplantation with preformed donor‐specific antibodies (DSA) face multiple challenges. Reflecting the underlying complexity of the humoral immune response, these patients face lengthy waits for compatible kidneys and an increased risk of antibody‐mediated rejection. 1 , 2 , 3 , 4 , 5 , 6 , 7 Although enhanced allocation algorithms and desensitization protocols attempt to alleviate the former, it is often at the expense of the latter. Clinical practice in handling highly sensitized patients differs between countries, but there is an unmet medical need to increase access to transplantation for the most sensitized patients regardless of territory or allocation system. To expand the donor pool, highly sensitized patients are given priority within kidney allocation systems, but despite a rise in transplant rates, for as many as 35% of the most highly sensitized patients (≥98% cPRA) in the EU and within the United States Kidney Allocation System (US‐KAS), that is, those with cPRA ≥ 99.9%, a compatible donor is rarely found. 3 , 6 , 7 , 8
Imlifidase (Idefirix®, Hansa Biopharma AB), a cysteine proteinase derived from immunoglobulin G (IgG)‐degrading enzyme of Streptococcus pyogenes (IdeS), specifically cleaves IgG into F(ab′)2 and Fc fragments, thereby inhibiting complement‐dependent cytotoxicity (CDC) and antibody‐dependent cellular cytotoxicity (ADCC) within hours of administration. 9 Imlifidase converts a positive crossmatch to a potential donor (T cell, B cell, or both), to negative, enabling transplantation to occur between previously incompatible donor–recipient pairs. The main difference between imlifidase and existing desensitization methods (plasmapheresis and IVIg) is the speed of its action and the extent to which it removes total body IgG. As IgG levels recover and DSA rebound, patients may be subjected to complications expected in this population, including antibody‐mediated rejection (AMR), as with other approaches to desensitization. 1 , 2 , 3 , 4 , 5 Desensitization with imlifidase has now been utilized in 46 patients receiving living‐ or deceased‐donor kidney transplants across four studies conducted in the United States and Europe, with short‐term efficacy and safety in recipients of these otherwise incompatible transplants previously published. 10 , 11 , 12 , 13 This report summarizes clinical and immunologic outcomes up to 3 years after transplantation to allow continued assessment in imlifidase treated patients, with additional sub‐analysis of patients deemed highly sensitized and unlikely to be transplanted in the context of the US KAS—the target population in the United States with the highest unmet need. 14 , 15 , 16 , 17
2. METHODS
2.1. Analysis groups
Between 2014 and 2017, 46 adult (18–70 years) patients at six transplant centers (Cedars‐Sinai Medical Center, Los Angeles, USA; Hôpital Necker, Paris, France; The Johns Hopkins Hospital, Baltimore, USA; Karolinska University Hospital, Stockholm, Sweden; New York University Langone Transplant Institute, New York, USA; and Uppsala University Hospital, Uppsala, Sweden) received imlifidase prior to a kidney transplant in four single‐arm, open‐label, phase 2 studies (13‐HMedIdeS‐02 [n = 1], 13‐HMedIdeS‐03 [n = 10], 14‐HMedIdeS‐04 [n = 17], and 15‐HMedIdeS‐06 [n = 18]) denoted as the feeder studies. Of the 46 patients in the pooled analysis, 30 patients are actively enrolled in the long‐term follow up study (17‐HMedIdeS‐14) with the primary endpoint of evaluating graft survival (Figure S1). Six patients were excluded prior to enrollment (but after the feeder studies) due to graft loss (n = 3) or death with a functioning graft (n = 3); 10 patients were lost to follow‐up (graft loss in feeder study [n = 3], declined to participate [n = 3], or could not be contacted [n = 4]). Patients in the phase 2 trials who had a negative crossmatch prior to imlifidase (n = 7) and in whom outcomes followed the natural course of a compatible transplant were also excluded from this analysis. The number of patients analyzed with permission from local independent ethics committee/institutional review board (IEC/IRB) or analyzed only with data from the feeder studies are depicted in Figure 1.
FIGURE 1.
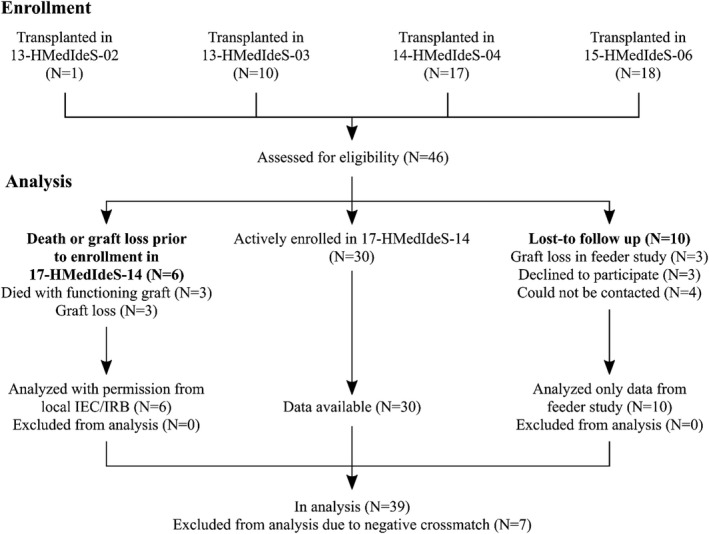
Imlifidase 46 patient enrollment and follow up. Flow chart of the enrolment in feeder studies and in the long‐term follow‐up study (17‐HMedIdeS‐14), and what data is analyzed in the patients
Study 13‐HMedIdeS‐02 assessed the safety, immunogenicity, pharmacokinetics, and efficacy of imlifidase in a single‐center, open‐label ascending‐dose study in highly sensitized patients with chronic kidney disease. Although transplantation was not part of the protocol it was not prohibited, and one patient received a deceased donor kidney offer after imlifidase dosing during the study and was transplanted successfully. 10 Study 13‐HMedIdeS‐03 investigated the safety and efficacy of imlifidase to reduce or remove DSA in order to allow HLA‐incompatible kidney transplantation in a single center with 10 patients transplanted. 11
In the two most recent studies (14‐HMedIdeS‐04 and 15‐HMedIdeS‐06), eligibility for imlifidase was limited to adult transplant candidates who were deemed highly sensitized and unlikely to be transplanted by an investigator, based on a multifactorial judgement with parameters including: low access to transplant, high calculated panel reactive antibody (CPRA), and/or failure to be transplanted within a reasonable timeframe after undergoing traditional desensitization, listing for kidney paired donation (KPD), or those who had a breadth and depth of sensitization making desensitization improbable. 11 , 12 , 13
The overall positive crossmatch group (XM+, n = 39) was additionally analyzed as subgroups depicted as follows: patients with (XM+/AMR+, n = 15) and without antibody‐mediated rejection (XM+/AMR−, n = 24). An additional subset of patients was defined as representing patients highly sensitized and unlikely to receive a transplant in the current US kidney allocation system (US‐KAS and characterized by a CPRA ≥ 99.9% who had positive crossmatch deceased donor transplants [>99.9%/XM+/DD, n = 13]).
All feeder studies were conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki; all ethical and regulatory approvals were available before any patient was exposed to any study‐related procedure. Each study was reviewed and approved by each center's institutional review board before study initiation with all patients providing written informed consent. The ongoing long‐term follow‐up study is registered at ClinicalTrials.gov (NCT03611621).
2.2. Clinical outcomes
Outcomes assessed include patient survival, graft survival, graft function, donor specific antibody (DSA) levels, anti‐drug antibodies (ADA) and occurrence of AMR at up to 3 years after transplantation. To avoid skewing the long‐term projections with more datapoints weighted at the start of the analysis, only patients with at least one data point at 1, 2, and 3 years were included in the analysis of DSA and eGFR.
2.3. HLA analysis
Single antigen bead‐human leukocyte antigen (SAB‐HLA) antibody screens were analyzed for all studies. The sampling timepoints are depicted in Table S1. High‐resolution typing was not conducted on all patients, therefore donor typing and DSA in this analysis was determined at the low‐resolution level (example A*02). In the case of a DSA having multiple single‐antigen bead alleles (ie, A2), the MFI of the highest allele bead was reported.
To reduce or remove prozone, each serum sample was tested with EDTA pretreatment or heat inactivation using the LABScreen SAB kits (LS1A04 for class I and LS2A01 for class II; One Lambda) on the Luminex instrument (Luminex Corporation). The product manual was followed except that serum samples were pretreated with 5 mmol/L of EDTA (Ultrapure™ EDTA 0.5M, Invitrogen) or heat‐inactivated for 30 min at 56℃.
2.4. Statistical methods
For quantitative demographic properties, statistical analyses were performed using the Fisher's exact test for categorical data and analysis of variance (ANOVA) for continuous data. Fisher’ exact tests were used for sex, country of transplant, race, re‐transplants, crossmatch positivity, and the presence of delayed graft function (DGF). ANOVA was used for age, time on dialysis, cold ischemic time, CPRA, pre‐dose DSA, pre‐transplant DSA, and duration of DGF. A prespecified alpha level of 0.05 was set for significance. All statistical analyses were performed using R version 3.5.2 (R Core Team; https://www.R‐project.org/).
Survival analysis was performed using the Kaplan–Meier methodology. Patients are censored at the last confirmed visit. In addition, censoring was performed for lost to follow‐up and death with a functioning graft with death only depicted in the AMR and graft‐loss figures.
DSA and estimated glomerular filtration rate (eGFR, calculated by four variable modification of diet in renal disease [MDRD] equation from local serum creatinine measurements) linear model calculated from 3 months up to 3 years. Anti‐drug antibodies (ADA) were measured in a validated method using customized imlifidase‐ImmunoCAP (Thermo Fisher Scientific, Phadia). Figures are produced using R (v3.5.2) and the libraries survival (v2.43–3), survminer (v0.4.5), and ggplot2 (v3.2.1).
3. RESULTS
3.1. Demographic and baseline characteristics
The study population was predominantly Caucasian (77%), from the US (72%) and received a deceased donor (DD) (82%) (Table 1). The mean age at transplantation was 43.2 years (SD 13), with 69% of patients undergoing retransplantation. Mean time on dialysis prior to the current transplant was 6.4 years (SD 5.6). All patients were sensitized, with a median CPRA of 99.62% (total range 41.67%–100%).
TABLE 1.
Demographics and baseline characteristics of the patients transplanted after imlifidase treatment
| Characteristics | XM+, n = 39 | AMR & XM+, n = 15 | No AMR & XM+, n = 24 | XM+, DD and cPRA ≥ 99.9%, n = 13 | p‐value f , g |
|---|---|---|---|---|---|
| Patient age (years); mean (SD) | 43.2 (13.0) | 44.5 (14.3) | 42.3 (12.3) | 45.3 (12.6) | 0.904 f |
| Female; n (%) | 18 (46%) | 6 (40%) | 12 (50%) | 5 (38%) | 0.883 g |
| Region, US; n (%) | 28 (72%) | 9 (60%) | 19 (79%) | 11 (85%) | 0.468 g |
| Race; n (%) | |||||
| White | 30 (77%) | 11 (73%) | 19 (79%) | 9 (69%) | 0.998 g |
| Black | 4 (10%) | 2 (13%) | 2 (8%) | 2 (15%) | |
| Asian | 3 (8%) | 1 (7%) | 2 (8%) | 1 (8%) | |
| Other | 2 (5%) | 1 (7%) | 1 (4%) | 1 (8%) | |
| Time on dialysis prior to imlifidase transplantation (years); mean (SD) | 6.4 (5.6) | 7.4 (6.1) | 5.9 (5.4) | 9.3 (7.2) | 0.370 f |
| Deceased Donor; n (%) | 32 (82%) | 13 (87%) | 19 (79%) | 13 (100%) | 0.399 g |
| Total CIT; mean (SD) | 21.0 (10.0) | 23.8 (11.5) | 19 (8.5) | 22.7 (9.6) | 0.554 f |
| Re‐transplants; n (%) | 27 (69%) | 10 (67%) | 17 (71%) | 9 (69%) | 1.000 g |
| cPRA a (%); median (1st & 3rd quartile) | 99.62 (94.92, 99.96) | 99.80 (93.70, 99.99) | 99.53 (96.55, 99.91) | 99.99 (99.97, 100) | 0.345 f |
| Crossmatch positive; n (%) | 39 (100%) | 15 (100%) | 24 (100%) | 13 (100%) | NA |
| Pre‐dose DSA b (MFI); median (1st & 3rd quartile) | 7791 (4108, 16 320) | 13009 (6515, 21 580) | 5727 (2699, 9470) | 16292 (7133, 21 824) | 0.027 f |
| Pre‐transplant DSA c (MFI); median (1st & 3rd quartile) | 774 (292, 1754) | 1584 (904–3303) | 576 (193–1387) | 1292 (774, 2600) | 0.032 f |
| DGF d ; n (%) | 17 (44%) | 7 (47%) | 10 (42%) | 6 (46%) | 1.000 g |
| DGF duration e (days); median (1st & 3rd quartile) | 10 (6, 26) | 24 (8, 28) | 9 (4, 14) | 12 (9, 23) | 0.332 f |
Patents with positive crossmatch (XM+) prior to imlifidase dosing, patents with or without an AMR and a subgroup with the highest unmet medical need in the US (XM+, DD and ≥99.9% cPRA).
Central analysis, cut‐off set to 3000 MFI.
Immunodominant DSA, central analysis.
Closest timepoint prior to transplantation, immunodominant DSA, central analysis.
DGF defined as the need for dialysis first week posttransplant (primary non‐functioning grafts included).
non‐functioning grafts counted as infinite.
For continuous data ANOVA was used for p‐value determination.
For categorical data Fisher’s exact test was used for p‐value determination.
3.2. Patient and death‐censored allograft survival
At the 6‐month completion of the feeder studies, all subjects were alive (Figure 2). Three deaths occurred between 6 months and 1 year, no deaths occurred between 1 and 3 years, resulting in 90% patient survival at 3 years (Figure 2A). In the AMR+ group, patient survival at 3 years was 85% and 94% in the AMR− group. One death was attributed to influenza, one due to cardiac arrest; while the cause of the third death was unknown.
FIGURE 2.
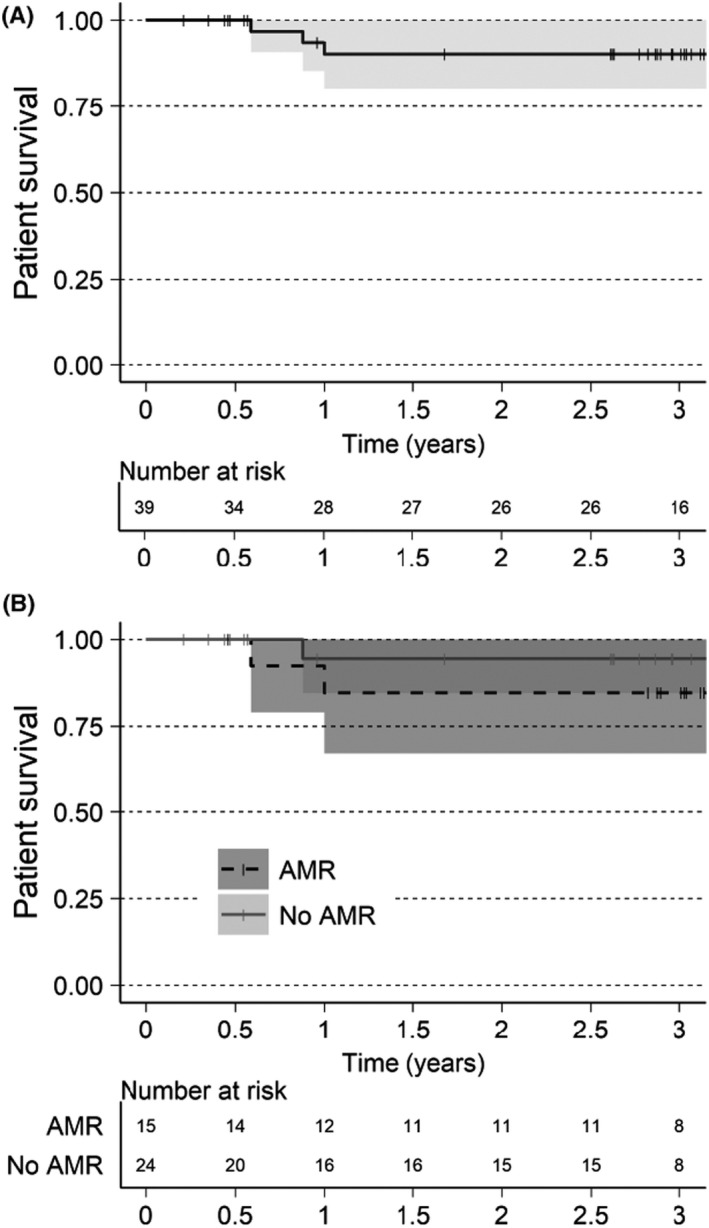
Patient survival estimated with Kaplan‐Meier. Alive patents are censored at last known visit, indicated in graphics as a thin vertical line. (A) XM+ patients. (B) XM+ patients separated by AMR or No AMR
The 3‐year allograft survival was 84% (Figure 3A). Three allograft losses occurred in the feeder studies, one non‐IgG mediated hyperacute rejection and two due to primary non‐functioning grafts. 3 , 5 Two allograft losses occurred between 2 and 3 years, attributed to the reduction of immunosuppression secondary to an infection in one patient and immunosuppression medication non‐adherence in another. Four allograft losses occurred in the AMR− group resulting in allograft survival at 3 years of 77% (Figure 3B), while graft survival in the AMR+ group was 93% at 3 years.
FIGURE 3.
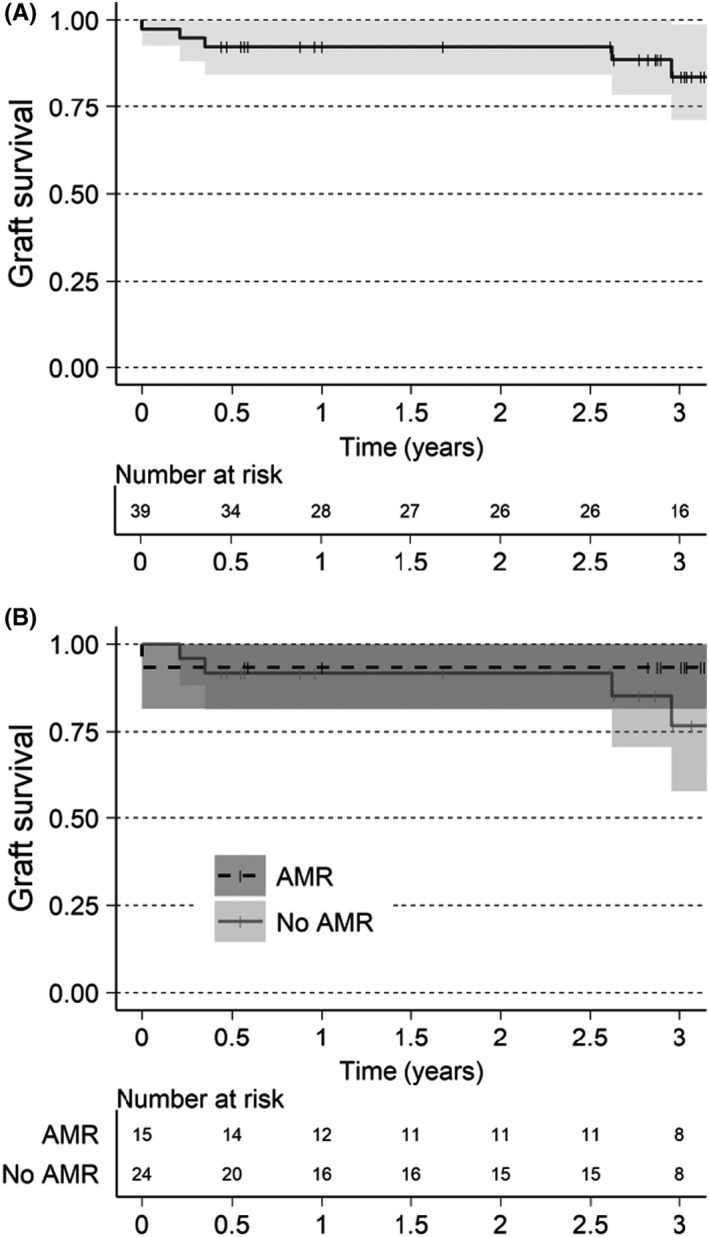
Graft survival estimated with Kaplan–Meier. Patients without graft loss are censored last known visit or death, indicated in graphics as a thin vertical line. (A) XM+ patients. (B) XM+ patients separated by AMR or No AMR
3.3. Kidney function
The eGFR for the patients are presented in Figure 4 and Table 2. Of the 21 patients with a functioning graft and with available eGFR estimation at 3 years, the mean eGFR was 55 ml/min/1.73 m2 and 14% (n = 3) had an eGFR < 30 ml/min/1.73 m2. The eGFR increased gradually over time, which held true for both AMR+ and AMR−. There was a 12 ml/min/m2 difference in eGFR between the AMR+ group compared to the AMR− group (49 vs 61 ml/min/m2).
FIGURE 4.
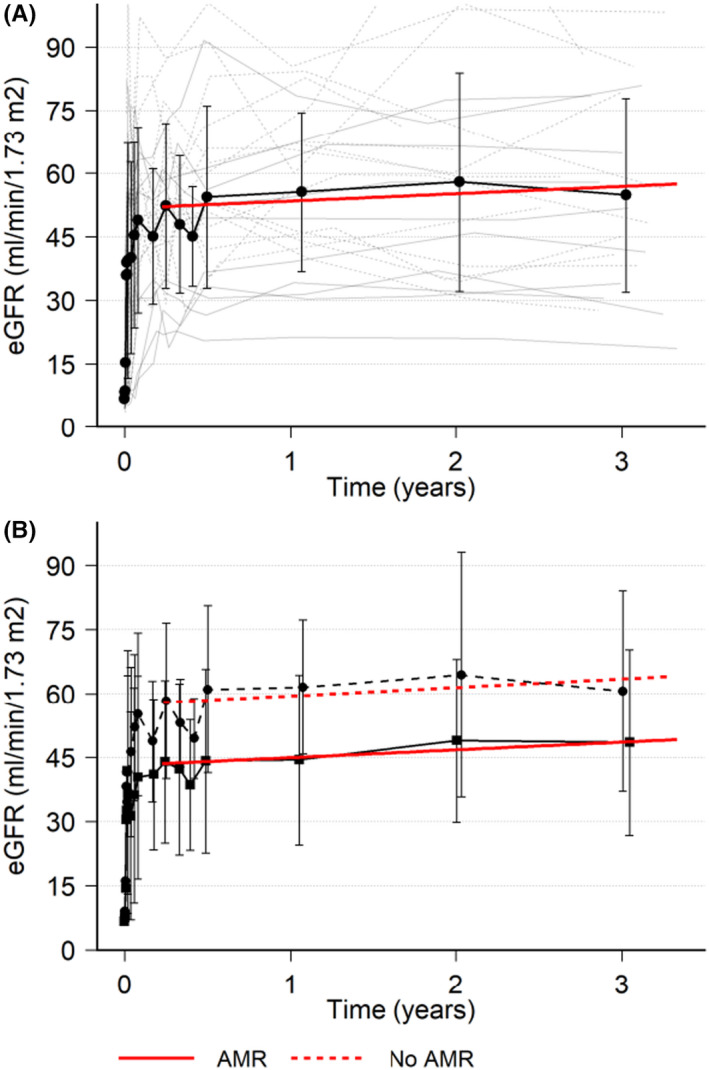
Estimated GFR with MDRD. eGFR with individual patient data (thin gray lines), mean (black line), standard deviation (error bars), and red line is a linear regression from 30 days up to 3 years (A) XM+ patients. (B) XM+ patients separated by AMR (solid lines) or No AMR (dashed lines)
TABLE 2.
Mean eGFR by MDRD
| Visit | XM+ (eGFR [SD], n = 39) | AMR & XM+ (eGFR [SD], n = 15) | No AMR & XM+ (eGFR [SD], n = 24) | XM+, DD and cPRA ≥ 99.9% (eGFR [SD], n = 13) |
|---|---|---|---|---|
| Predose | 7.3 (4.1), n = 36 | 6.3 (3.6), n = 15 | 8.0 (4.5), n = 21 | 7.5 (4.3), n = 12 |
| Month 6 | 52.7 (23.4), n = 31 | 41.2 (21.3), n = 14 | 62.1 (21.2), n = 17 | 53.8 (20.4), n = 10 |
| Year 1 | 55.6 (18.8), n = 23 | 44.4 (19.9), n = 8 | 61.5 (15.7), n = 15 | 59.8 (19.7), n = 6 |
| Year 2 | 58.0 (25.9), n = 24 | 48.9 (19.1), n = 10 | 64.4 (28.7), n = 14 | 68.4 (32.9), n = 6 |
| Year 3 | 54.8 (23.0), n = 21 | 48.5 (21.8), n = 10 | 60.5 (23.4), n = 11 | 60.2 (23.2), n = 6 |
Patents with positive crossmatch (XM+) prior to imlifidase dosing, patents with or without an AMR and a subgroup with the highest unmet medical need in the US (XM+, DD and ≥99.9% cPRA).
3.4. Immunology (DSA and ADA)
The median MFI for the immunodominant DSA levels pre‐treatment was 7791 MFI, with 1st and 3rd quartiles 4108 and 16 320 MFI, respectively (Table 1). The median MFI for the immunodominant DSA levels pre‐treatment was higher for the patients who experienced an AMR, 13 009 vs 5727 MFI for the XM+ patients that did not experience an AMR. The reduction between pre‐treatment and pre‐transplantation values after imlifidase treatment was similar in both groups. After imlifidase treatment the MFI remained low for approximately 1 week (Figure S2), then rebounded to approximately 80% of pre‐treatment levels, with the peak occurring 14 days post‐treatment (Figure 5). Immunodominant Class II DSA were more prevalent than Class I. In general, the Class II DSA strength was higher pre‐dose and with rebound after imlifidase dosing (Figure 5). After the initial peak at 14 days post‐treatment, the levels of DSA progressively decreased over time.
FIGURE 5.
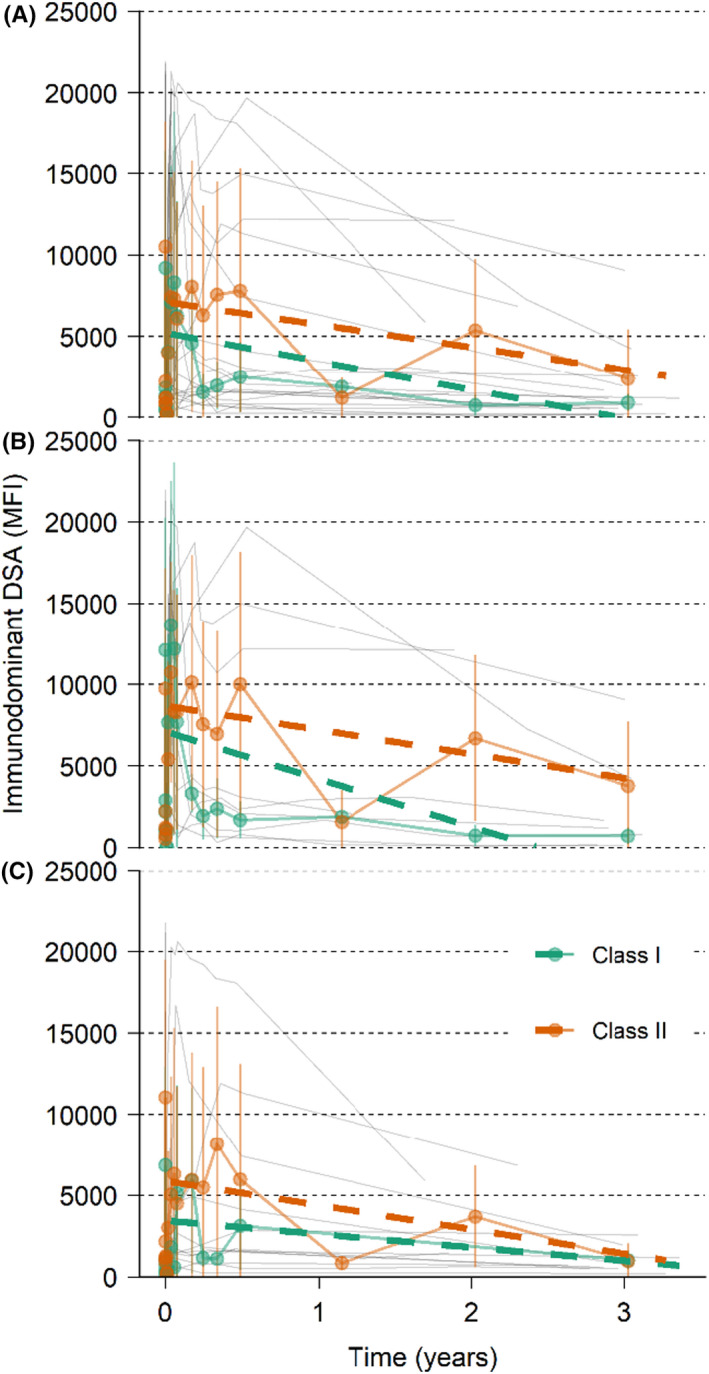
Immunodominant DSA with individual patient data. Immunodominant DSA with individual patient data (thin gray lines), mean (colored lines with points and SD as error bars), and linear regression (dashed colored lines) from 14 days up to 3 years for HLA class I and II. (A) XM+ patients. (B) XM+ patients with AMR. (C) XM+ patients without AMR
The median anti‐imlifidase IgG (ADA) levels pre‐treatment was 8 mg/L (range ˂2–35 mg/L). All subjects dosed with imlifidase responded with an ADA response reaching a peak 2–8 weeks after dosing (Figure S3) with a median of 163 mg/L (range 19–2600 mg/L). Thereafter the ADA level decreased in all subjects and at 3 years the median was 31 mg/L (range 26–79 mg/L).
3.5. AMR
The rate of AMR for the XM+ population was 28% within the first month (n = 11, Figure 6). Four additional AMRs occurred between 2 and 6 months from transplantation, bringing the AMR rate to 38%. Few AMR were reported beyond the first 6 months perhaps due to lack of protocol biopsy in the long term follow up, and only one patient recorded with an early AMR had an AMR during the follow‐up period, which may have been deemed a persistent AMR.
FIGURE 6.
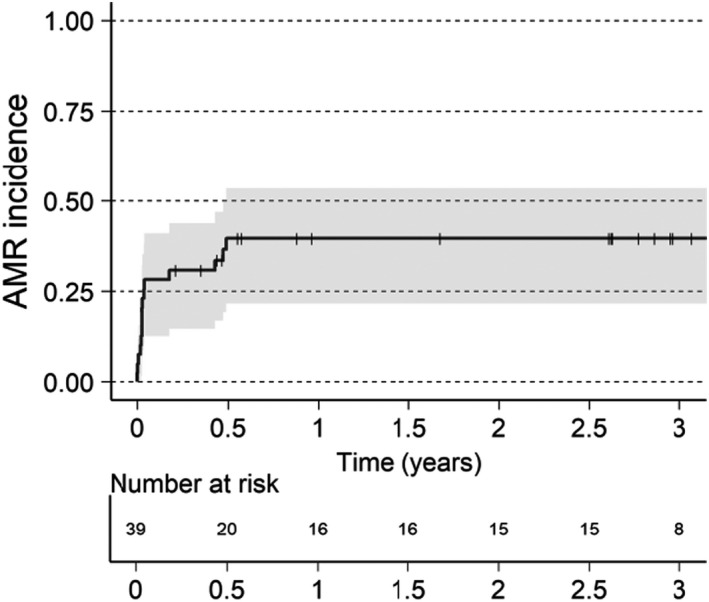
Time to first AMR. Visualized using reverse Kaplan–Meier estimate for cumulative incidence of AMR for XM+ patients. Patients without AMR are censored last known visit or death, indicated in graphics as a thin vertical line
All AMRs were treated (defined as clinical or histological [when available] resolution as judged by the investigator) with standard therapies, most commonly plasmapheresis with or without the addition of intravenous immune globulin (IVIg), optimization of maintenance immunosuppression, and corticosteroids. Other therapies including anti‐CD20, complement inhibition, proteasome inhibition, spleen embolization or splenectomy were added in two patients. Overall, there was a correlation between the magnitude of DSA levels pre‐ and post‐imlifidase and the incidence of AMR. The MFI of the pre‐imlifidase treatment DSA differed significantly (p < 0.05) between the AMR+ (median ~13 000, interquartile range [IQR] 6500–22 000) and the AMR− (median ~6000, IQR 3000–9000) patients (Table 1).
Example patients are described in Figure 7 and Figure S4. Panel A patient had 3 DSAs (class I and class II HLA), all of which responded to imlifidase treatment. HLA A*24 and HLA DQB1*04 rebounded at much higher levels than pre‐imlifidase; however, the patient did not develop AMR and at 3 years, was doing well with an eGFR of 79 ml/min/1.73 m2 (SCr 0.94 mg/dl). Panel B patient had a high number of HLA mismatches with some pre‐dose MFI above 10 000–16 000. After imlifidase treatment, DSAs continued to stay at a low level, without incidence of AMR and at the 3‐year visit had an eGFR of 59 ml/min/1.73 m2 (SCr 1.0 mg/dl). Panel C patient received a KDPI 63%, standard criteria DD kidney transplant with a CIT of ~43.5 h with cold storage during transport and machine perfusion on site. The patient had DGF which resolved after ~3 weeks. The patient had two class II antibodies which rebounded intensely, and the patient experienced a mixed ACR/AMR at 2 weeks, which was treated successfully. Although DSA persisted at levels above 5000 MFI, the patient continued to have graft function throughout the study with eGFR in the 20 ml/min/1.73 m2 range, up to, and including the last study visit (SCr of 3.75 mg/dl) at 3 years. Panel D patient had two HLA‐DP dominant DSAs which rebounded intensely after imlifidase treatment and the patient experienced an AMR on day 9 (with concomitant DGF), which was treated successfully. After the AMR event, DSAs stayed at a high level up to and including at 2 years with a 3‐year eGFR of 27 ml/min/1.73 m2 (SCr 3.1 mg/dl). Panel E patient received a standard criteria deceased donor transplant with <24 h CIT and had a Class I immunodominant DSA with MFI > 20 000 which rebounded to near original levels after imlifidase dosing and transplantation. AMR was diagnosed on day 10 and despite early and repeated intervention with depleting antibody therapy, plasmapheresis and complement inhibition, the HLA B*44 MFI level continued to rise, necessitating a splenectomy to decrease the humoral response. Despite early and intense AMR, the patient had good graft function throughout the study and reported an eGFR of 81 ml/min/1.73 m2 (SCr of 0.98 mg/dl) at 3 years. Lastly, Panel F patient received a living donor kidney 48 h after imlifidase dosing. Prior to imlifidase, the patient had low DSA MFI with the highest recorded below 5000 MFI. After transplantation, the patient had very intense rebound of HLA DRB1*13 above 15 000 MFI and was diagnosed with severe AMR on day 3 necessitating treatment with multimodal therapies including high dose corticosteroids, proteosome inhibition, complement inhibition, anti‐CD20 therapy and IVIg. After the AMR event, DSAs continue to remain low and at 3 years, the patient reported an eGFR of 34 ml/min/1.73 m2 (SCr of 2.2 mg/dl).
FIGURE 7.
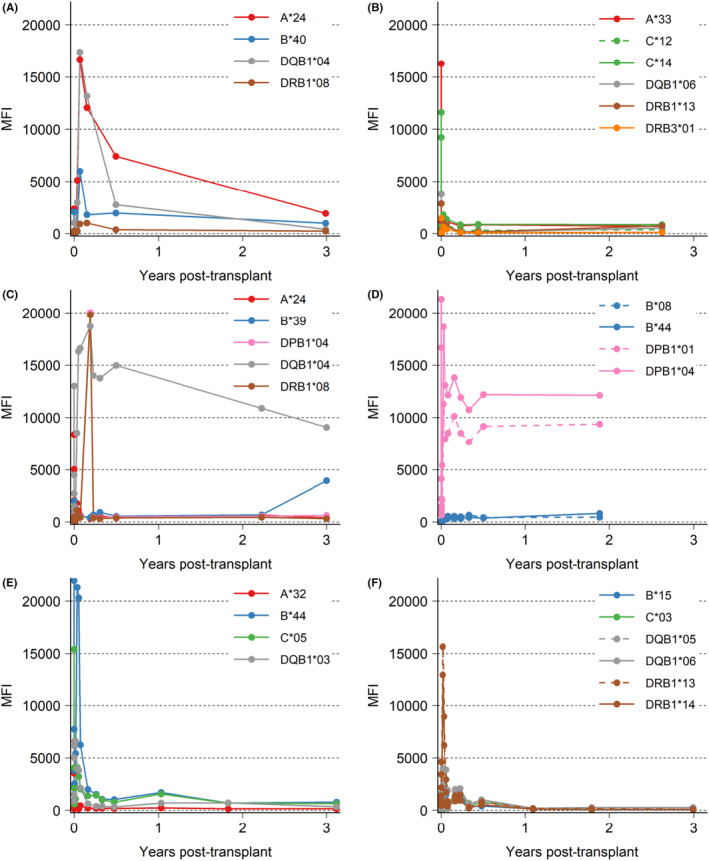
Intensity of 6 patient's individual DSA over time. DSA (MFI ≥ 1000 at any time) for each patient visualized as a line, color depending on HLA‐type, if multiple DSA for one type one line is dashed. (A,B) Patients without AMR, (C–F) patients with AMR
3.6. Subanalysis of patients with CPRA ≥ 99.9%, and XM+ to a deceased donor
The subset of patients deemed highly sensitized and unlikely to be transplanted with CPRA ≥ 99.9% who received XM+ deceased donor transplants (n = 13) had a longer mean time on dialysis (9.3 years) in comparison to the overall group (Table 1). The pre‐imlifidase DSA levels were among the highest in the XM+ group, with median MFI of 16 292, 1st and 3rd quartiles as 7133 and 21 824, respectively. This subset of patients observed similar graft survival (92%, Figures 3A and 8B) as the XM+ population and demonstrated an increase in the eGFR over time with a mean eGFR of 60 ml/min/1.73 m2 at 3 years (Table 2, Figure 8D). As expected, there was a high rate of AMR, 38% (n = 5) within the first 14 days (Figure 8C) with two additional AMRs occurring between 5 and 6 months from transplantation. However, all these AMRs were treated with no graft losses attributed to the AMRs. These patients represent the extremely highly sensitized population in both the breadth of antibodies and the strength of the antibodies, with DSA rebound to approximately 80% of pre‐dose levels and with the majority of the AMRs in this group coinciding with the peak of the DSAs. As with the other patients, the peak of ~16 000 MFI DSA in this group decreased over time and at 3 years post‐transplantation, the intensity was less than 50% of the pre‐dose values (Figure 8E). However, this observation must be taken with a degree of caution due to the relatively fewer samples for centralized SAB‐HLA collected at the 2‐ and 3‐year visits.
FIGURE 8.
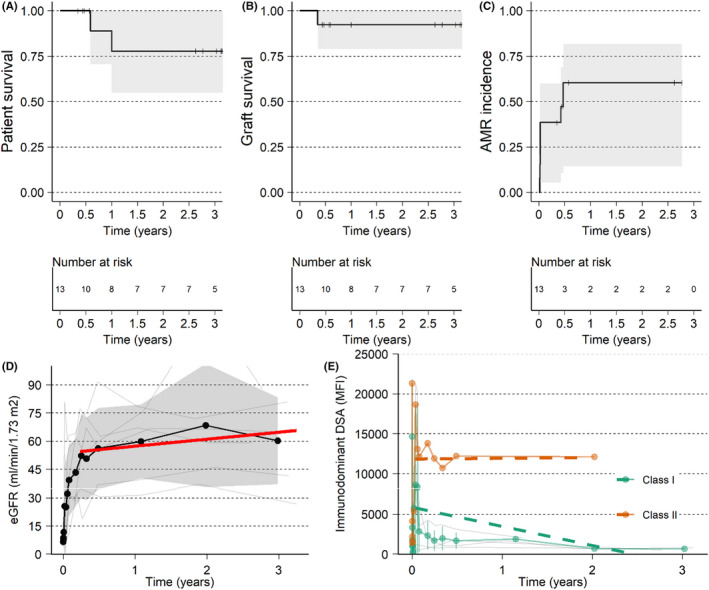
Outcome of the group with XM+, DD, and cPRA ≥ 99.9%. (A) Patient survival. (B) Graft survival, death censored. (C) Time to first AMR. (D) eGFR with individual patient data (thin gray lines), mean (black line with points), standard deviation (gray ribbon) and red line is a linear regression from 30 days up to 3 years. (E) Immunodominant DSA with individual patient data (thin gray lines), mean (colored lines with points and SD as error bars) and linear regression (dashed colored lines) from 14 days up to 2 years for HLA class I and II
3.7. Safety
Adverse events assessed as related to imlifidase have been described previously. 9 , 10 , 11 , 12 At 3 years, two borderline biopsy proven cell‐mediated rejections were reported. The incidence and pattern (including infectious agent) of serious or severe infections were not different from those observed in kidney‐transplanted patients in general and included mainly upper respiratory and urinary tract infections (n = 15). There have been no reported malignancies in the patients in the long‐term follow‐up.
4. DISCUSSION
Imlifidase effectively cleaves preformed IgG anti‐HLA antibodies, via a novel mechanism of action and without additional preconditioning, when administered at the time of organ availability. In contradistinction to other desensitization methods, imlifidase provides an IgG antibody‐free window to enable HLA‐incompatible transplantation from both living and deceased donors. Three years after imlifidase‐enabled desensitization and transplantation, the death‐censored allograft survival was 84%, patient survival 90%, and mean eGFR was 55 ml/min/1.73 m2 (49 ml/min/1.73 m2 for those with AMR and 61 ml/min/m2 for those without AMR).
Recent data on impact of the US‐KAS shows enhanced access for patients with CPRA 90%–99.9%. 11 However, among kidney candidates with CPRA > 80%, as many as 55% of listed patients are not transplanted within 3 years of waitlisting, under the current KAS. For those with CPRA values above 99.9%, benefit has been less evident, with the overwhelming majority of candidates either dead, delisted or still waiting after 3 years compared to those transplanted. 3 Despite a short‐lived “bolus” effect after the implementation of the current KAS, patients with a CPRA > 99.9% continue to have transplant rates far below other highly sensitized patients. 14 , 15 , 16 , 17 In this analysis, 13 XM+ imlifidase‐treated patients with CPRA ≥ 99.9% were considered those with the highest unmet medical need, unlikely to be transplanted, and despite these immunologic challenges, demonstrated outcomes comparable to the overall group. Thus, imlifidase may represent an essential intervention for transplant candidates with CPRA approaching 100%, and be of potential benefit for those in whom other approaches have not resulted in successful transplantation.
Posttransplant management of highly sensitized patients is clinically challenging. The sensitized transplant candidates have typically lived for years with end‐stage renal disease (ESRD), accumulating substantial frailty and/or comorbidity. Mean time on dialysis for the overall group in the current data was 6.4 years (SD: 5.6 years), substantially longer than the mean time on dialysis (3.5–3.9 years) among other highly sensitized (CPRA ≥ 80%) transplant recipients in other groups and comparable to dialysis time among those with CPRA of 100%. 3 , 6 , 7 , 14 , 15 , 16 , 17 In addition, the group with the highest unmet need analyzed had an even longer time on dialysis with a mean of 9.3 (SD: 7.2) years. The extended dialysis time places the recipients at greater risk of infection, malignancy, cardiovascular events, and mandates addressing these comorbidities in clinical management. 18 , 19 Although the graft losses and deaths in this pooled analysis were not deemed to be directly related to lack of imlifidase efficacy, decreases in immunosuppression and patient frailty are universal risks for poor outcomes in the general population which are even more heightened in this highly sensitized population.
In addition to the morbidity associated with prolonged duration of dialysis, transplantation of highly sensitized patients, whether via allocation of a compatible kidney or transplantation with desensitization, is associated with a greater risk of acute rejection, particularly antibody‐mediated rejection. Even in the absence of desensitization, AMR risk in highly sensitized patients can be substantial. 20 The rate of antibody rebound that follows imlifidase treatment is comparable to that described with more traditional approaches (e.g., IVIg and plasmapheresis), although lessened in the imlifidase studies that included rituximab in post‐transplantation treatment regimens. 5 The data described in this report clearly indicate that the highest risk for AMR is during the first weeks following imlifidase treatment with the incidence diminishing over time. Both early and late AMR in this pooled analysis responded to treatment with no attributable graft losses.
The clinical impact of comorbidity and high immunologic risk is interrelated; preexisting comorbidities may preclude the administration of added immunosuppression necessary to control rejection in this population at greater risk. The current data, with follow‐up up to 3 years, indicate these challenges are manageable, and no greater with imlifidase desensitization than with other approaches to transplantation in this at‐risk group.
The onset of AMR remains a concern throughout the posttransplant course in highly sensitized recipients. Nonetheless, it is encouraging to note that, in general, the pre‐transplant DSA, diminished via imlifidase administration, and reoccurred as IgG titers repopulate, does not (1) increase in strength during rebound relative to pre‐transplant levels or (2) increase over time. In fact, as noted in Figure 5, the MFI of the immunodominant DSA declines over time in most patients. The frequency or severity of early AMR was not substantially different from what is expected and reported in highly sensitized candidates receiving incompatible kidneys. 4 , 5 , 6 , 21 The current data indicate that the majority of the grafts continue to function well up to 3 years.
As illustrated in Figure 7, there were some intense and severe AMRs occurring very early post‐transplantation, which required multimodal interventions for treatment. Upon review of these cases, it may be prudent to reflect on which patient‐specific antigens should be considered unacceptable antigens and which to delist to facilitate an incompatible organ offer. Although a standardized method for removing lower risk unacceptable antigens does not exist, there are strategies which may mitigate the immunological risk. 3 , 5 , 16 , 23 , 24 , 25 The rate of AMR in the CPRA ≥ 99.9%, DD, XM+ subgroup indicates that along with recipient immunologic risk, donor selection is also critical to mitigate the known outcomes. As more experience with imlifidase desensitization becomes available, data regarding strategic delisting of antigens, organ offer evaluation and acceptance may shed light on the right combination of donor and recipient to balance the dual needs of equity and utility in this population. The difference, or lack of, in magnitude between pre‐dose and pre‐transplant immunodominant MFI between those who went on to develop AMR vs those that did not elaborate thresholds for future studies with imlifidase use.
Many of the advantages of transplantation over dialysis are linked to the adequacy of kidney allograft function and are lost with compromised eGFR. 18 , 22 Among patients completing the original 6 months of posttransplant follow‐up in the Phase 2 studies, renal function was acceptable and relatively stable. Findings on surveillance biopsies were generally favorable, with little evidence of acute or chronic active AMR beyond the early initial period. 13 Although additional protocol biopsies are not available, the eGFR in most patients remains stable up to 3 years. Gaston et al, reporting recent data from the DeKAF study, noted that late allograft failure was preceded by a downward inflection in eGFR 6–8 months earlier, a pattern not evident in those who either retained or died with a functioning allograft. 26 In the current data, there is no evidence of a decline in eGFR up to 3 years, consistent with a low likelihood of impending graft failure.
There are several limitations in this pooled analysis which merit discussion. The limitations and heterogeneity of the data presented are acknowledged. The feeder studies were not originally designed for robust long‐term follow up beyond the original 6‐month study endpoint and the long‐term follow‐up study was not introduced and consented for until after the completion of the original phase 2 studies. This led to a proportion of patients who declined or were unable to participate in the long‐term observational study. That being said, the data in this paper represent the best available long‐term data for all patients who have received an imlifidase‐enabled transplantation. Lastly, although early AMRs were treated without associated graft loss, the lack of long‐term biopsy data to shed light on the delta change over time in terms of transplant glomerulopathy, chronic AMR, and vasculopathy remain to be elucidated.
In conclusion, these data demonstrate that outcomes up to 3 years in recipients of imlifidase‐enabled allografts resemble outcomes in other highly sensitized patients fortunate enough to undergo transplantation. Incidence of antibody‐mediated injury is comparable to other desensitization protocols and manageable in this high‐risk population, with the relative stability of allograft function and the long‐term safety profile has indicated no increase in the rates of infection or malignancy. Though further studies may define optimal utilization of this novel agent, imlifidase is a potent option to enable transplantation among those patients for whom lifetime dialysis, and its accompanying morbidity may be the only alternative.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Christian Kjellman, Angela Q. Maldonado, Kristoffer Sjöholm, Håkan Olsson, Anna Runström, and Tomas Lorant receive employment funds from and have a personal financial interest in Hansa Biopharma AB. Bonnie E. Lonze, Robert A. Montgomery, Tomas Lorant, Niraj M. Desai, Christophe Legendre, Torbjörn Lundgren, Bengt von Zur Mühlen, Ashley A. Vo, and Stanley C. Jordan receive research funds from Hansa Biopharma AB.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
ACKNOWLEDGMENTS
This study was funded by Hansa Biopharma AB, Lund, Sweden. The authors would like to acknowledge the contributions of Annika Sundberg, Jonas Tuvesson, Gunilla Eckerwall, and CTI Clinical Trial & Consulting on this manuscript.
Kjellman C, Maldonado AQ, Sjöholm K, et al. Outcomes at 3 years posttransplant in imlifidase‐desensitized kidney transplant patients. Am J Transplant. 2021;21:3907–3918. 10.1111/ajt.16754
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Habal MV, Farr M, Restaino S, Chong A. Desensitization in the era of precision medicine: moving from the bench to bedside. Transplantation. 2019;103(8):1574‐1581. [DOI] [PubMed] [Google Scholar]
- 2. Jackson KR, Holscher C, Motter JD, et al. Posttransplant outcomes for CPRA‐100% recipients under the new kidney allocation system. Transplantation. 2020;104(7):1456‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schinstock CA, Smith BH, Montgomery RA, et al. Managing highly sensitized renal transplant candidates in the era of kidney paired donation and the new kidney allocation system: is there still a role for desensitization? Clin Transplant. 2019;33(12):e13751. [DOI] [PubMed] [Google Scholar]
- 4. Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost‐effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95(6):852‐858. [DOI] [PubMed] [Google Scholar]
- 5. Jackson AM, Kraus ES, Orandi BJ, Segev DL, Montgomery RA, Zachary AA. A closer look at rituximab induction on HLA antibody rebound following HLA‐incompatible kidney transplantation. Kidney Int. 2015;87(2):409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson KR, Covarrubias K, Holscher CM, et al. The national landscape of deceased donor kidney transplantation for the highly sensitized: transplant rates, waitlist mortality, and posttransplant survival under KAS. Am J Transplant. 2018;4:1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson KR, Motter JD, Kernodle A, et al. How do highly sensitized patients get kidney transplants in the United States? Trends over the last decade. Am J Transplant. 2020;20:2101‐2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. EUROSTAM . Final Report Summary ‐ EUROSTAM (A Europe‐wide Strategy to enhance Transplantation of highly sensitized patients on basis of Acceptable HLA Mismatches.). 2018. https://cordis.europa.eu/project/rcn/106298/reporting/en. Accessed 10 January 2018.
- 9. Ge S, Chu M, Choi J, et al. Imlifidase inhibits HLA antibody‐mediated NK cell activation and antibody‐dependent cell‐mediated cytotoxicity (ADCC) in vitro. Transplantation. 2020;104(8):1574‐1579. [DOI] [PubMed] [Google Scholar]
- 10. Lorant T, Bengtsson M, Eich T, et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti‐HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am J Transplant. 2018;18(11):2752‐2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377(5):442‐453. [DOI] [PubMed] [Google Scholar]
- 12. Lonze BE, Tatapudi VS, Weldon EP, et al. IdeS (Imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high‐strength donor‐specific antibody. Ann Surg. 2018;268:488‐496. [DOI] [PubMed] [Google Scholar]
- 13. Jordan SC, Legendre C, Desai N, et al. Imlifidase desensitization in crossmatch‐positive, highly‐sensitized kidney transplant recipients: results of an international phase 2 trial (Highdes). Transplantation. 2020;268:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart DE, Kucheryavaya AY, Klassen DR, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16:1834‐1847. [DOI] [PubMed] [Google Scholar]
- 15. Hahn AB, Mackey M, Constantino D, et al. The new kidney allocation system does not equally advantage all very high CPRA candidates – a single center analysis. Hum Immunol. 2017;78:37‐40. [DOI] [PubMed] [Google Scholar]
- 16. Stewart DE, Wilk AR, Toll AE, et al. Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant. 2018;18:1924‐1935. [DOI] [PubMed] [Google Scholar]
- 17. Stewart DE, Wilk AR, Klassen DK. KAS turns four: the state of deceased donor kidney allocation in the U.S. OBM Transplantation. 2019;3:1. 10.21926/obm.transplant.1901041. [DOI] [Google Scholar]
- 18. Meier‐Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end‐stage renal disease. Am J Transplant. 2004;4(10):1662‐1668. [DOI] [PubMed] [Google Scholar]
- 19. Meier‐Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291‐1295. [DOI] [PubMed] [Google Scholar]
- 20. Heidt S, Haasnoot GW, Witvliet MD, et al. Allocation to highly sensitized patients based on acceptable mismatches results in low rejection rates comparable to nonsensitized patients. Am J Transplant. 2019;19(10):2926‐2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orandi BJ, Luo X, Massie AB, et al. Surival benefit of kidney transplants from HLA‐incomptable live donors. N Engl J Med. 2016;374:940‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasiske BL, Israni AK, Snyder JJ, Skeans MA. Patient outcomes in renal transplantation I. The relationship between kidney function and long‐term graft survival after kidney transplant. Am J Kidney Dis. 2011;57(3):466‐475. [DOI] [PubMed] [Google Scholar]
- 23. Duquesnoy R, Marrari M. Detection of antibodies against HLA‐C epitopes in patients with rejected kidney transplants. Transpl Immunol. 2011;24(3):164‐171. [DOI] [PubMed] [Google Scholar]
- 24. Tinckam K, Rose C, Hariharan S, Gill J. Re‐examining risk of repeated HLA mismatch in kidney transplantation. J Am Soc Nephrol. 2016;27(9):2833‐2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heidt S, Roelen D, de Vaal Y, et al. A novel ELISPOT assay to quantify HLA‐specific B cells in HLA‐immunized individuals. Am J Transplant. 2012;12(6):1469‐1478. [DOI] [PubMed] [Google Scholar]
- 26. Gaston RS, Fieberg A, Helgeson ES, et al. Late graft loss after kidney transplantation: is "death with function" really death with a functioning allograft? Transplantation. 2020;104(7):1483‐1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Data Availability Statement
Research data are not shared.


