Abstract
Introduction
The aim of this study was to characterize the associations between sagittal spinopelvic alignment and motor symptoms in patients with Parkinson's disease (PD).
Methods
The study included patients with idiopathic PD (aged <80 years and with abnormal posture). All patients underwent whole‐spine lateral and coronal radiography. Sagittal spinopelvic alignment was evaluated using nine parameters. Motor symptoms were evaluated using the Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) part III score—with bradykinesia and axial motor sub‐scores. Multivariate analysis was used to analyze associations between motor symptoms and sagittal spinopelvic alignment in PD patients according to sex.
Results
The study subjects were 79 PD patients (39 men, 40 women; median age, 70 years). Clear sex‐related differences were noted. In male patients, the MDS‐UPDRS part III score correlated significantly with cervical sagittal vertical axis (SVA), and bradykinesia and axial motor scores correlated significantly with SVA, cervical SVA, and T1 slope. In female patients, the MDS‐UPDRS part III score correlated significantly with thoracic kyphosis, bradykinesia score correlated significantly with cervical SVA and thoracic kyphosis, and the axial motor score correlated significantly with SVA, cervical SVA, T1 slope, sacral slope, and pelvic tilt.
Conclusion
Our results showed clear correlations among various motor symptoms and sagittal global alignment in PD patients and that these correlations are different in female PD patients and their male counterparts.
Keywords: motor symptoms, parkinson disease, postural deformity, sagittal spinopelvic alignment
1. INTRODUCTION
Parkinson's disease (PD) is a progressive neurodegenerative condition that occurs mainly in the middle age or later, and the number of PD patients is increasing as the aging rate increases in many countries, including Japan. 1 In addition to the characteristic motor symptoms, such as bradykinesia, resting tremors, and rigidity, abnormal posture is common in PD patients. 2 Although the typical posture presentation of PD patients is described as stooped appearance with flexion of the hips and knees and rounding of the shoulders, more severe postural abnormalities, such as kyphoscoliosis, camptocormia, Pisa syndrome, and dropped head syndrome, are also observed in some patients. 2 , 3 In this regard, spinopelvic deformities are often associated with these postural abnormalities, and can cause low back pain, walking disability, and social dysfunction in some patients. 4
Assessment of spinopelvic deformity in PD patients is important in considering the characteristics and pathophysiology of postural abnormalities and for prediction and prevention future development of back pain. While abnormal posture in PD has been extensively investigated, studies on spinopelvic deformities in PD patients are limited, and little is known about their pathophysiology. In this regard, studies of the general elderly population have suggested that sagittal spinopelvic alignment differs by sex; nevertheless, only few studies on spinopelvic deformities in PD have analyzed male and female patients separately. 5 , 6
The aim of this study was to characterize abnormalities in the sagittal spinopelvic alignment of PD patients, with particular focus on their association with motor symptoms and clinical phenotypes. We also analyzed the sex‐related differences in the above associations.
2. MATERIALS AND METHODS
2.1. Patients
This retrospective study examined patients with idiopathic PD who presented with abnormal posture at the Outpatient Clinic of the Department of Neurology, Tokyo Medical University Hospital, between October 2018 and March 2020. The inclusion criteria were as follows: 1) age <80 years, 2) modified Hoehn and Yahr (HY) stage ≤4, and 3) availability of whole‐spine lateral and coronal radiographs. We also used the following exclusion criteria in patient selection: 1) presence of other neurodegenerative diseases, 2) history of spinal, hip, or knee surgery, 3) history of stroke, 4) presence of normal pressure hydrocephalus, and 5) lack of patient's interest in voluntary participation in the study. The diagnosis of PD was based on the United Kingdom Parkinson's Disease Society Brain Bank criteria. 7 Abnormal posture was graded as “slight” (“not quite erect, but posterior posture that could be normal for an older person”) or "worse", as described in the “3.13. Posture” section of the Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) part III. 8
At our hospital, we recommend that all consenting PD patients with abnormal posture undergo periodic spine radiography (approximately every 2 years). All patients underwent head magnetic resonance imaging or computed tomography to rule out any comorbidity. Each patient was informed about participation in the clinical study and provided written informed consent. This study was conducted with the approval of Tokyo Medical University Medical Ethics Committee (#T2020‐0110) and was conducted in accordance with principles of the Declarations of Helsinki.
2.2. Measurements of spinopelvic alignment
Spinopelvic alignment was assessed by a single examiner based on a single whole‐spine lateral and coronal radiographic examination. Patients with motor fluctuation underwent the examination during the “on” phase. Before the examination, the patient was instructed to relax, while assuming the posture as directed. Following a standardized protocol, lateral standing radiographs were obtained using vertical films and a radio‐opaque calibration tool, with a constant distance between the patient and the radiation source; the patient stood in a fist‐on‐clavicle position and was instructed to look straight ahead with the knees locked. 9 The following spinal parameters were assessed: 1) the sagittal vertical axis (SVA; the distance between the C7 plumb line and the S1 superio‐posterior corner), interpreted as global spinal alignment; 2) cervical SVA (the distance between the plumb line from the center of the C2 vertebral body and the posterior superior corner of C7) and cervical lordosis (the lordotic angle between the C2 and C7 inferior endplates), interpreted as cervical alignment; 3) T1 slope (the angle between the horizontal plane and T1 superior endplate) and thoracic kyphosis (the kyphotic angle between the T5 superior endplate and T12 inferior endplate), interpreted as thoracic alignment; 4) lumbar lordosis (the lordotic angle between the L1 superior endplate and sacral plate), interpreted as lumbar alignment; 5) sacral slope (the angle between the horizontal plane and sacral plate), pelvic tilt (the angle between the line connecting the midpoint of the sacral plate to the bicoxofemoral axis and the vertical line from the bicoxofemoral axis), and pelvic incidence (the angle between the line perpendicular to sacral plate and the line connecting the midpoint of the sacral plate to the bicoxofemoral axis), interpreted as pelvic alignment. 5 , 10 , 11 Pelvic incidence was calculated as the sum of the sacral slope and pelvic tilt. These are positional and related to pelvic orientation 10 (Figure 1). The Cobb angle was measured based on full‐length standing spine radiographs (anteroposterior views) and was defined as the angle between the line parallel to the superior end plate of the superior‐most vertebra of the curve and the line parallel to the inferior end plate of the inferior‐most vertebra of the curve. 11 , 12
FIGURE 1.
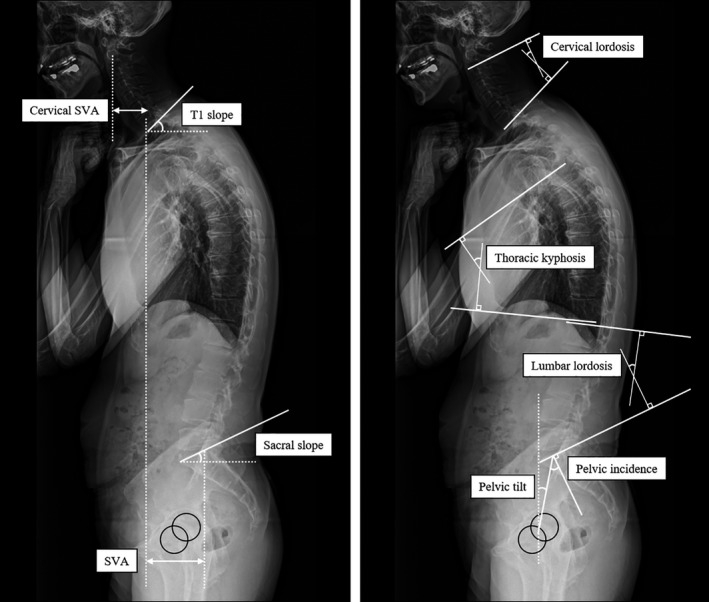
Radiographic parameters of sagittal alignment. SVA, sagittal vertical axis
2.3. Clinical assessment
Motor symptoms were evaluated using the modified HY stage and MDS‐UPDRS Part III score. 8 When measuring the MDS‐UPDRS Part III score, the axial motor sub‐score (the sum of the scores of the items in the MDS‐UPDRS Part III on “axial symptoms”; items 1, 9, 10, 12 and 13) and the bradykinesia sub‐score (the sum of the scores of the items of the MDS‐UPDRS on “bradykinesia”; items 4–8 and 14) were also evaluated. 8 , 13 , 14 With respect to the clinical phenotype of PD, were compared the postural instability/gait difficulty (PIGD) and tremor dominant (TD) phenotypes. 15 The clinical phenotypes were classified using the PIGD (the sum of 5 items) and tremor scores (the sum of 11 items) from the MDS‐UPDRS: “TD phenotype” represented TD/PIGD ratio ≥1.15 while “PIGD phenotype” represented TD/PIGD ratio ≤0.9. 15 Antiparkinsonian medications used by the patients at the time of the study are presented at their L‐dopa equivalent daily doses (LEDD). 16 Dopamine agonists are also presented at their dopamine agonist daily doses (DADD), in addition to the LEDD.
2.4. Statistical analysis
All clinical parameters used in this study were subjected to data distribution analysis using the Kolmogorov‐Smirnov test, and normally distributed parameters are expressed as mean ±standard deviation (SD), while parameters with skewed distribution are expressed as median and interquartile range (IQR). The Student's t‐test and Mann‐Whitney U test were used to compare the clinical and spinopelvic alignment parameters between the sexes. We analyzed the correlation between spinopelvic alignment parameters with the Spearman's rank correlation coefficient. Associations between each spinopelvic alignment parameter and motor symptoms were analyzed using multiple linear regression analysis adjusted for age and body mass index (BMI). Statistical significance was set at P‐value of <0.05. All statistical analyses were performed using IBM SPSS Statistics software (version 22.0, IBM Corp, Armonk, NY).
3. RESULTS
3.1. Patient demographics and sex differences in sagittal spinopelvic alignment
A total of 79 PD patients (39 men and 40 women; median age, 70.0 years) were included in this study. The male patients were older with longer disease duration than their female counterparts (Table 1). The modified HY stage and MDS‐UPDRS part III, bradykinesia, and axial motor scores did not differ significantly between the sexes. This was also true for LEDD and DADD (Table 1). In the assessment of coronal spinal alignment, 4 patients (all women) were found to have scoliosis (Cobb angle ≥30°) of the lumbar curve type. 12 The Cobb angle was significantly larger in females than males (Table 1). Assessment of sagittal spinopelvic alignment showed significantly longer cervical SVA, in male patients than the females, whereas pelvic tilt and pelvic incidence were significantly larger in the latter than male patients (Table 1). There were no significant differences in SVA, cervical lordosis, T1 slope, thoracic kyphosis, lumbar lordosis, and sacral slope between the sexes (Table 1).
TABLE 1.
Clinical features and spinopelvic alignment in patients with Parkinson's disease
| Patients (n=79) | Males (n=39) | Females (n=40) | p‐value | |
|---|---|---|---|---|
| Age (years) | 70.0 (66.0–73.5) | 71.2±4.6 | 68.4±6.0 | 0.021 a |
| Height (m) | 1.59±0.08 | 164.6±6.3 | 154.4±5.5 | <0.001 a |
| Body mass index | 22.0±3.2 | 22.6±3.2 | 21.4±3.0 | 0.088 a |
| Disease duration (years) | 2.8 (1.1–6.5) | 3.5 (1.8–7.8) | 2.1 (0.7–5.6) | 0.048 b |
| MMSE score | 28.0 (27.0–29.0) | 28.0 (27.0–29.0) | 29.0 (27.0–29.5) | 0.222 b |
| Modified HY stage | 2.0 (2.0–3.0) | 2.0 (2.0–2.8) | 2.5 (2.0–3.0) | 0.362 b |
| MDS‐UPDRS part III score | 16.0 (11.0–26.0) | 19.0 (13.0–26.0) | 15.0 (11.0–25.0) | 0.410 b |
| Bradykinesia score | 10.0 (6.5–15.5) | 10.6±5.7 | 11.7±8.1 | 0.527 a |
| Axial motor score | 3.0 (2.0–6.0) | 3.0 (2.0–5.5) | 4.0 (1.0–7.0) | 0.859 b |
| LEDD (mg) | 300.0 (25.0–517.1) | 340.0 (100.0–563.2) | 150.0 (0.0–500.0) | 0.084 b |
| DADD (mg) | 0.0 (0.0–141.7) | 0.0 (0.0–150.0) | 0.0 (0.0–67.4) | 0.524 b |
| Spinopelvic alignment | ||||
| Cobb angle (°) | 10.0 (8.0–15.5) | 10.0 (7.5–11.5) | 12.0 (8.0–24.0) | 0.019 b |
| SVA (mm) | 54.0 (27.5–95.5) | 59.0 (32.0–92.0) | 48.5 (19.5–96.0) | 0.573 b |
| Cervical SVA (mm) | 29.0 (23.5–40.0) | 37.0 (27.0–50.0) | 26.0 (13.0–32.5) | <0.001 b |
| Cervical lordosis (°) | 12.0 (3.0–21.0) | 14.0 (6.0–20.0) | 12.0 (−3.0–24.5) | 0.356 b |
| T1 slope (°) | 35.0 (29.0–43.5) | 37.0 (30.5–44.5) | 32.5 (26.0–42.0) | 0.115 b |
| Thoracic kyphosis (°) | 33.0 (24.5–41.0) | 36.0 (29.0–41.5) | 29.0 (20.5–41.0) | 0.069 b |
| Lumbar lordosis (°) | 40.0 (31.5–50.5) | 39.0 (31.0–50.0) | 41.5 (35.5–55.5) | 0.322 b |
| Sacral slope (°) | 31.0 (25.0–39.0) | 29.0 (24.0–35.0) | 32.5 (26.0–40.0) | 0.220 b |
| Pelvic tilt (°) | 18.0 (13.0–23.5) | 17.0 (11.0–21.0) | 20.5 (14.5–24.5) | 0.034 b |
| Pelvic incidence (°) | 49.0 (42.0–57.0) | 46.0 (42.0–50.5) | 52.5 (42.0–62.0) | 0.028 b |
Data are mean±SD or median with interquartile ranges.
Abbreviations: axial motor scores, the sum of the scores of the items of MDS‐UPDRS part III on “axial symptoms” items 3.1, 3.9, 3.10, 3.12, and 3.13.; bradykinesia scores, the sum of the scores of the items of MDS‐UPDRS part III on “bradykinesia” items 3.4–8, and 3.14; DADD, dopamine agonist daily dose; LEDD, levodopa equivalent daily dose; MDS‐UPDRS part III score, Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale part III score; MMSE, Mini‐Mental State Examination; modified HY stage, modified Hoehn and Yahr stage;SVA, sagittal vertical axis.
Student's t‐test,
Mann‐Whitney U test.
3.2. Relationship between spinopelvic parameters in patients with PD
In patients with PD, SVA correlated significantly with cervical SVA, T1 slope, lumbar lordosis, and pelvic tilt, and lumbar lordosis with sacral slope and pelvic incidence, irrespective of sex (Tables 2, 3). In male PD patients, cervical SVA correlated significantly with cervical lordosis, T1 slope, and thoracic kyphosis, and T1 slope correlated significantly with lumbar lordosis (Table 2). In female PD patients, cervical SVA correlated significantly with T1 slope and pelvic tilt, and T1 slope correlated significantly with thoracic kyphosis. There was also a significant correlation between thoracic kyphosis and lumbar lordosis (Table 3).
TABLE 2.
Relationship between spinopelvic alignment parameters in male patients with Parkinson disease
| SVA | cervical SVA | Cervical lordosis | T1 slope | Thoracic kyphosis | Lumbar lordosis | Sacral slope | Pelvic tilt | Pelvic incidence | |
|---|---|---|---|---|---|---|---|---|---|
| SVA | |||||||||
| Cervical SVA | 0.355 a | ||||||||
| Cervical lordosis | −0.059 | −0.357 a | |||||||
| T1 slope | 0.464 b | 0.509 b | 0.267 | ||||||
| Thoracic kyphosis | 0.067 | 0.379 a | −0.194 | 0.242 | |||||
| Lumbar lordosis | −0.521 b | −0.249 | 0.192 | −0.326 a | 0.238 | ||||
| Sacral slope | −0.267 | −0.225 | 0.295 | −0.166 | 0.079 | 0.868 b | |||
| Pelvic tilt | 0.339 a | 0.190 | −0.132 | 0.182 | 0.017 | −0.239 | −0.276 | ||
| Pelvic incidence | −0.030 | −0.052 | 0.101 | −0.065 | 0.212 | 0.562 b | 0.635 b | 0.458 b |
Data are Spearman's rank correlation coefficients (ρ).
Abbreviation: SVA, sagittal vertical axis.
p<0.05,
p<0.01.
TABLE 3.
Relationship between spinopelvic alignment parameters in female patients with Parkinson disease
| SVA | cervical SVA | Cervical lordosis | T1 slope | Thoracic kyphosis | Lumbar lordosis | Sacral slope | Pelvic tilt | Pelvic incidence | |
|---|---|---|---|---|---|---|---|---|---|
| SVA | |||||||||
| Cervical SVA | 0.343 a | ||||||||
| Cervical lordosis | 0.210 | −0.230 | |||||||
| T1 slope | 0.586 b | 0.354 a | 0.612 b | ||||||
| Thoracic kyphosis | 0.130 | 0.173 | 0.545 b | 0.497 b | |||||
| Lumbar lordosis | −0.429 b | −0.237 | 0.227 | −0.065 | 0.341 a | ||||
| Sacral slope | −0.033 | −0.129 | 0.279 | 0.052 | 0.249 | 0.788 b | |||
| Pelvic tilt | 0.418 b | 0.351 a | −0.067 | 0.216 | 0.078 | −0.179 | 0.036 | ||
| Pelvic incidence | 0.240 | 0.092 | 0.184 | 0.174 | 0.230 | 0.492 b | 0.774 b | 0.608 b |
Data are Spearman's rank correlation coefficients (ρ).
Abbreviation: SVA, sagittal vertical axis.
p<0.05,
p<0.01.
3.3. Sagittal spinopelvic alignment and motor symptoms in male PD patients
None of the alignment parameters correlated with the modified HY stage. However, there was a significant association between the MDS‐UPDRS part III score and cervical SVA. In addition, the bradykinesia and axial motor scores correlated significantly associated with SVA, cervical SVA, and T1 slope (Table 4). SVA was significantly longer in patients with the PIGD phenotype than the TD phenotype (p = 0.030), but no significant differences in other parameters were observed between the two phenotypes (Appendix 1).
TABLE 4.
Associations between spinopelvic alignment and clinical parameters in male patients with Parkinson's disease
| Modified HY stage | MDS‐UPDRS part III score | Bradykinesia score | Axial motor score | |||||
|---|---|---|---|---|---|---|---|---|
| β | p‐value | β | p‐value | β | p‐value | β | p‐value | |
| SVA | 0.231 | 0.190 | 0.232 | 0.183 | 0.386 | 0.035 | 0.412 | 0.018 |
| Cervical SVA | 0.144 | 0.418 | 0.429 | 0.011 | 0.576 | 0.001 | 0.353 | 0.045 |
| Cervical lordosis | 0.239 | 0.178 | 0.084 | 0.635 | 0.050 | 0.794 | 0.151 | 0.405 |
| T1 slope | 0.313 | 0.070 | 0.274 | 0.112 | 0.419 | 0.020 | 0.358 | 0.040 |
| Thoracic kyphosis | 0.077 | 0.640 | <0.001 | 0.999 | 0.165 | 0.338 | 0.160 | 0.331 |
| Lumbar lordosis | −0.004 | 0.983 | −0.107 | 0.533 | −0.184 | 0.314 | −0.106 | 0.547 |
| Sacral slope | 0.031 | 0.862 | −0.067 | 0.704 | −0.129 | 0.492 | −0.042 | 0.814 |
| Pelvic tilt | −0.066 | 0.714 | −0.040 | 0.824 | 0.061 | 0.747 | −0.056 | 0.757 |
| Pelvic incidence | −0.015 | 0.933 | −0.093 | 0.595 | −0.084 | 0.651 | −0.080 | 0.654 |
Data are standardized regression coefficients (β weights).
Abbreviations: axial motor score, the sum of the scores of the items of MDS‐UPDRS part III on “axial symptoms” items 3.1, 3.9, 3.10, 3.12, and 3.13.; bradykinesia score, the sum of the scores of the items of MDS‐UPDRS part III on “bradykinesia” items 3.4–8, and 3.14; MDS‐UPDRS part III score, Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale part III score; modified HY stage, modified Hoehn and Yahr stage; SVA, sagittal vertical axis.
3.4. Sagittal spinopelvic alignment and motor symptoms in female PD patients
The modified HY stage correlated significantly with cervical SVA, T1 slope, and sacral slope. There was also a significant correlation between the MDS‐UPDRS part III score and thoracic kyphosis (Table 5). In addition, the bradykinesia score correlated significantly with cervical SVA and thoracic kyphosis, while the axial motor score correlated significantly with SVA, cervical SVA, T1 slope, sacral slope, and pelvic tilt (Table 5). Lumbar lordosis and the sacral slope were significantly greater in patients with the PIGD phenotype than the TD phenotype (p = 0.034 for lumbar lordosis; p = 0.030 for sacral slope), but there were no significant differences in other parameters between the two phenotypes (Appendix 1).
TABLE 5.
Associations between spinopelvic alignment and clinical parameters in female patients with Parkinson's disease
| Modified HY stage | MDS‐UPDRS part III score | Bradykinesia score | Axial motor score | |||||
|---|---|---|---|---|---|---|---|---|
| β | p‐value | β | p‐value | β | p‐value | β | p‐value | |
| SVA | 0.284 | 0.080 | 0.293 | 0.110 | 0.344 | 0.051 | 0.399 | 0.013 |
| Cervical SVA | 0.506 | 0.002 | 0.316 | 0.093 | 0.429 | 0.016 | 0.489 | 0.003 |
| Cervical lordosis | −0.183 | 0.258 | −0.005 | 0.978 | −0.067 | 0.705 | −0.182 | 0.265 |
| T1 slope | 0.366 | 0.021 | 0.243 | 0.185 | 0.331 | 0.058 | 0.344 | 0.032 |
| Thoracic kyphosis | 0.265 | 0.118 | 0.442 | 0.018 | 0.385 | 0.035 | 0.250 | 0.145 |
| Lumbar lordosis | −0.327 | 0.059 | −0.047 | 0.814 | −0.231 | 0.228 | −0.342 | 0.050 |
| Sacral slope | −0.482 | 0.004 | −0.122 | 0.540 | −0.304 | 0.110 | −0.418 | 0.015 |
| Pelvic tilt | 0.304 | 0.065 | 0.126 | 0.507 | 0.151 | 0.411 | 0.361 | 0.029 |
| Pelvic incidence | −0.165 | 0.326 | −0.007 | 0.971 | −0.133 | 0.469 | −0.074 | 0.665 |
Data are standardized regression coefficients (β weights).
Abbreviations: axial motor score, the sum of the scores of the items of MDS‐UPDRS part III on “axial symptoms” items 3.1, 3.9, 3.10, 3.12, and 3.13.; bradykinesia score, the sum of the scores of the items of MDS‐UPDRS part III on “bradykinesia” items 3.4–8, and 3.14; MDS‐UPDRS part III score, Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale part III score; modified HY stage, modified Hoehn and Yahr stage; SVA, sagittal vertical axis.
4. DISCUSSION
Spinal deformity is highly prevalent in individuals aged 65 years and older, affecting between 32% and 68% of that population. 17 A previous Japanese study indicated that the global spinal alignment deviates linearly with age in both men and women of the general population aged 50–80 years of age. 5 Specifically, SVA protrusion was significantly higher in men in their 80s and in women in their 70s and 80s, compared to those in their 50s. 5 On the other hand, cervicothoracic alignment was sex‐dependent: among men, cervical SVA was significantly greater in the 80s age group than in the 50s age group, whereas age had no influence in women. In contrast, cervical lordosis was significantly greater in women in their 80s than other age groups, whereas age had no significant effect on this parameter in men. 5 As for thoracic alignment, T1 slope was significantly greater in the 80s age group than in the 50s group in both sexes, whereas thoracic kyphosis was significantly greater in the ≥70s groups, though only in men. 5 These results point to age‐related changes in various sagittal spinopelvic alignment parameters, in addition to sex‐related differences.
Abnormal posture as well as age‐related postural changes characterized the PD patients of the present study. We compared our spinopelvic alignment data with the corresponding data of the elderly general population matched for age (men in their 70s and women in their 60s) reported in a Japanese cohort survey randomly sampled from a basic resident registry (here considered as the control group) (Appendix 2). 5 The median values of SVA of our PD patients, and cervical SVA and T1 slope of the female PD patients were higher (by more than 1 SD) than the mean values of the control. Conversely, the median values of the other parameters were within ±1 SD range of the mean values of the control (Appendix 2). 5 Since our study included a relatively large number of patients with early‐stage PD, it is possible that patients with mild abnormal posture formed a large proportion of the study population.
A study of adult patients with spinal deformity showed that the SVA is strongly associated with health‐related quality of life (HRQOL). 10 In the present study of PD patients, SVA correlated significantly with cervical SVA, T1 slope, and pelvic tilt, and had negatively and significantly with lumbar lordosis. There was also a significant correlation between cervical SVA and T1 slope. In male patients, the T1 slope correlated significantly with lumbar lordosis. In female patients, the T1 slope correlated significantly with thoracic kyphosis. The increase in T1 slope can be related to thoracic hyperkyphosis or lumbopelvic sagittal malalignment, which can cause a compensatory anterior tilt of the cervical spine and a forward head posture. 18 Our results suggest that observed increases in SVA and cervical SVA could be due to thoracic hyperkyphosis and sagittal lumbopelvic malalignment in patients with PD irrespective of sex. In this regard, pelvic parameters are reported to be associated with lumbar lordosis. 10 , 19 In our study, lumbar lordosis correlated significantly with the sacral slope and pelvic incidence in both male and female PD patients.
Studies on the sagittal spinopelvic alignment of PD patients are limited, and there is no consensus regarding its relationship with motor symptoms. In a study of 48 PD patients with chronic low back pain (mean disease duration, 10.6 ± 10.0 years), HY stage correlated significantly with SVA, thoracolumbar kyphosis, and the lumbar range of motion, but not with thoracic kyphosis, lumbar lordosis, or pelvic tilt. 4 Furthermore, the UPDRS score tended to correlate with thoracic kyphosis and thoracolumbar kyphosis. 4 In a cross‐sectional study of 175 PD patients (mean disease duration, 6.77 ± 6.1 years), the HY stage and UPDRS motor scores correlated weakly and positively with plumb line‐C7, and moderately and negatively with the spinosacral angle. In contrast, neither HY stage nor UPDRS motor score correlated with thoracic kyphosis, lumbar lordosis, sacral slope, pelvic incidence, or pelvic tilt. 20 Our study showed that motor symptoms correlated significantly with certain spinopelvic alignment parameters in PD patients, and such correlation was sex‐dependent. In male patients, the bradykinesia and axial motor scores correlated significantly with SVA, cervical SVA, and T1 slope, suggesting the association of bradykinesia and axial symptoms with forward‐bent posture in PD. In female patients, the MDS‐UPDRS part III and bradykinesia scores correlated significantly with thoracic kyphosis, suggesting the association of bradykinesia with thoracic hyperkyphosis. In addition, the axial motor score correlated significantly with the SVA, cervical SVA, T1 slope, sacral slope, and pelvic tilt, and negatively with lumbar lordosis although such correlations were not statistically significant. These results suggest the association of axial symptoms with forward‐bent posture and lumbopelvic sagittal malalignment. They also point to important differences between male and female PD patients with regard to the association between sagittal spinopelvic deformities and motor symptoms, with a stronger association in women than in men.
Comparison of the clinical phenotypes showed that the SVA was greater in male patients with the PIGD phenotype than in those with the TD phenotype, and that the lumbopelvic alignment abnormality was greater in the former than the latter among female patients, indicating differing characteristics between sexes. Further large‐scale studies are needed to define the characteristics of each phenotype and the reasons for the observed sex‐related differences.
Abnormal posture is a common finding in PD patients, with a prevalence of approximately 1 in 3. 2 Previous studies have shown that posture abnormalities in PD correlate with the severity motor symptoms. 21 , 22 , 23 In addition to motor symptoms, abnormal posture is associated with several conditions and factors, including dystonia, impaired proprioception and kinanesthesia, cognitive impairment, myopathy, spina and soft tissue changes, in addition to the use of medications. 2 , 24 , 25 Although the pathogenic mechanism of spinopelvic deformities in PD has not been fully investigated, the possible factors involved in their development can be divided into primary factors (direct mechanical effects), such as degenerative spinal conditions, trauma, and back surgery (eg, laminectomy), and secondary factors (indirect mechanical effects) related to complications of PD, especially abnormal posture. With regard to the primary factors, a recent systematic review and meta‐analysis study showed that PD patients are at higher risk of both osteoporosis and osteopenia than healthy controls, and that female patients are at greater risk than male patients. 26 The present study showed sex differences in the relationship between parameters of sagittal spinopelvic alignment and motor symptoms in PD patients. A possible explanation for such relationship is that female patients, compared with male patients, are more likely to develop vertebral degeneration earlier in life due to osteoporosis and osteopenia and thus are more susceptible to its effect on motor function. Our results imply the need to consider the patient's sex in the overall clinical management of spinopelvic deformities and abnormal posture. Studies of the elderly general population have shown that sagittal spinal alignment is associated with physical function and HRQOL, suggesting the importance of evaluating this parameter in this population. 6 , 12 Only a few studies have investigated sagittal spinopelvic alignment in PD patients. Future studies should investigate its association with factors other than motor symptoms, especially osteoporosis and osteopenia, as well as explore the mechanisms of the effects of sagittal spinopelvic deformities on physical function and HRQOL in PD patients.
This study has several limitations. First, although our cross‐sectional study included PD patients with abnormal posture, the population also included those with early‐stage PD, resulting in a relatively high proportion of patients with mild abnormal posture. Second, since no healthy controls were included in this study, no direct comparison of spinopelvic alignment between PD patients and healthy individuals was performed. Third, PD posture abnormalities are known to be influenced by antiparkinsonian medications, especially with the use of dopamine agonists. However, our study did not investigate this issue. Future large‐scale studies on the effects of antiparkinsonian drugs on spinopelvic alignment are warranted. In addition, although several of our patients underwent general (non‐specialized) rehabilitation therapies at the time, no information regarding the frequency, duration, or content of these therapies was available. We therefore did not investigate the possible effects of rehabilitation therapy on spinopelvic alignment. Fourth, prior to radiographic examination, patients were instructed to relax as much as possible during imaging, while assuming the posture as directed. However, we cannot rule out the possibility that the patient consciously modified his or her posture during the process of imaging, resulting in an underestimation of spinopelvic deformities. Finally, male PD patients were older and had longer disease duration than female PD patients. It is possible that this demographic difference affected the observed association between spinopelvic sagittal alignment and motor symptoms.
4.1. Conclusions
We have demonstrated in the present study the presence of a close association between axial motor symptoms and sagittal global alignment in PD patients. The severity of bradykinesia correlated with thoracic kyphosis in female PD patients. The association between spinopelvic sagittal alignment and motor symptoms in PD patients seems to be different between male and female patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest in relation to this study.
ETHICAL APPROVAL
This study was conducted with the approval of the Human Ethics Committee of Tokyo Medical University Medical (#T2020‐0110).
ACKNOWLEDGMENTS
None
APPENDIX 1.
DIFFERENCES IN SPINOPELVIC ALIGNMENT AMONG CLINICAL PHENOTYPES OF PARKINSON'S DISEASE
| Male patients | Female patients | |||||
|---|---|---|---|---|---|---|
| PIGD phenotype (n=23) | TD phenotype (n=16) | p‐value | PIGD phenotype (n=26) | TD phenotype (n=11) | p‐value | |
| Age (years) | 71.0±4.4 | 71.5±4.9 | 0.764 a | 69.2±6.3 | 66.2±5.4 | 0.180 a |
| Disease duration (years) | 5.5 (2.0–10.7) | 2.5 (1.6–3.7) | 0.030 b | 4.3±4.9 | 2.5±3.3 | 0.268 a |
| SVA (mm) | 73.4±46.5 | 45.1±28.8 | 0.037 a | 68.2±63.3 | 41.6±49.8 | 0.222 a |
| Cervical SVA (mm) | 39.7±17.6 | 37.2±15.3 | 0.648 a | 28.5 (20.0–35.0) | 17.0 (7.0–28.0) | 0.051 b |
| Cervical lordosis (°) | 14.0 (9.0–17.5) | 11.0 (1.5–21.5) | 0.343 b | 6.8±24.9 | 8.8±17.1 | 0.805 a |
| T1 slope (°) | 39.9±9.3 | 34.6±10.9 | 0.110 a | 36.2±11.4 | 28.6±13.6 | 0.092 a |
| Thoracic kyphosis (°) | 36.1±10.1 | 35.1±12.4 | 0.769 a | 31.4±12.0 | 27.8±12.0 | 0.415 a |
| Lumbar lordosis (°) | 39.7±12.3 | 43.1±19.6 | 0.509 a | 39.4±16.6 | 51.3±9.4 | 0.034 a |
| Sacral slope (°) | 29.0 (25.5–34.0) | 31.0 (21.5–37.0) | 0.855 b | 30.8±13.7 | 40.6±5.8 | 0.030 a |
| Pelvic tilt (°) | 17.1±6.9 | 15.6±8.2 | 0.551 a | 22.5 (14.0–27.0) | 20.0 (15.5–21.0) | 0.270 b |
| Pelvic incidence (°) | 47.0 (45.0–50.5) | 44.0 (37.5–52.5) | 0.343 b | 53.9±15.7 | 61.2±16.1 | 0.206 a |
Data are mean±SD or median values and interquartile ranges.
Abbreviation: SVA, sagittal vertical axis.
Student's t‐test,
Mann‐Whitney U test.
APPENDIX 2.
Measurements of sagittal spinopelvic alignment parameters in patients with Parkinson's disease (PD) (box and whisker plots; the center line indicates the median value, the upper end of the box indicates the third quartile, and the lower end indicates the first quartile) and those of the elderly general population (men in their 70s and women in their 60s) (thick solid lines; expressed as mean±1 SD). Data for the elderly general population were adapted from a Japanese cohort survey randomly sampled from a basic resident registry. 5 SVA, sagittal vertical axis.
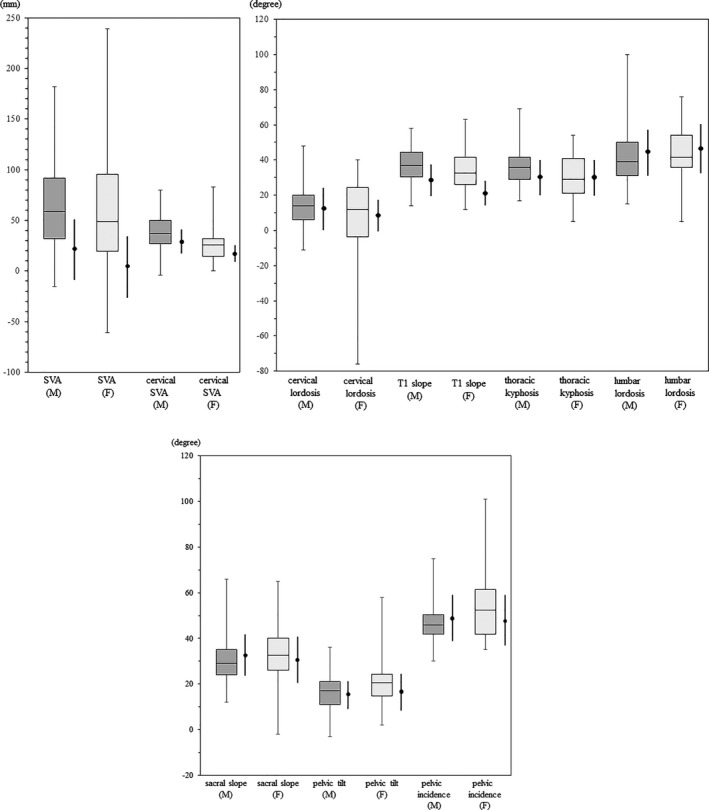
Terashi H, Endo K, Kato H, Ido N, Aizawa H. Characteristics of sagittal spinopelvic alignment in patients with Parkinson's disease. Acta Neurol Scand. 2021;145:53–62. 10.1111/ane.13521
Funding information
This research did not receive any financial support from public, private, or not‐for‐profit organizations.
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. GBD 2016 Parkinson's Disease Collaborators . Global, regional, and national burden of Parkinson's disease, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538‐549. [DOI] [PubMed] [Google Scholar]
- 3. Rabin ML, Earnhardt MC, Patel A, Ganihong I, Kurlan R. Postural, bone, and joint disorders in Parkinson's disease. Mov Disord Clin Pract. 2016;3:538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe K, Hirano T, Katsumi K, et al. Characteristics of spinopelvic alignment in Parkinson's disease: Comparison with adult spinal deformity. J Orthop Sci. 2017;22:16‐21. [DOI] [PubMed] [Google Scholar]
- 5. Uehara M, Takahashi J, Ikegami S, et al. Sagittal spinal alignment deviation in the general elderly population: A Japanese cohort survey randomly sampled from a basic resident registry. Spine J. 2019;19:349‐356. [DOI] [PubMed] [Google Scholar]
- 6. Tokida R, Uehara M, Ikegami S, et al. Association between sagittal spinal alignment and physical function in the Japanese general elderly population: A Japanese cohort survey randomly sampled from a basic resident registry. J Bone Joint Surg Am. 2019;101:1698‐1706. [DOI] [PubMed] [Google Scholar]
- 7. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129‐2170. [DOI] [PubMed] [Google Scholar]
- 9. Murata K, Endo K, Aihara T, et al. Relationship between cervical and global sagittal balance in patients with dropped head syndrome. Eur Spine J. 2020;29:413‐419. [DOI] [PubMed] [Google Scholar]
- 10. Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J. 2019;28:1889‐1905. [DOI] [PubMed] [Google Scholar]
- 11. Dagdia L, Kokabu T, Ito M. Classification of adult spinal deformity: Review of current concepts and future directions. Spine Surg Relat Res. 2019;3:17–26. 10.22603/ssrr.2017-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwab F, Ungar B, Blondel B, Buchowski J, Coe J, Deinlein D, DeWald C, Mehdian H, Shaffrey C, Tribus C, Lafage V, Scoliosis Research Society‐Schwab adult spinal deformity classification. Spine. 2012;37(12):1077–1082. 10.1097/brs.0b013e31823e15e2 [DOI] [PubMed] [Google Scholar]
- 13. Kotagal V, Albin RL, Müller ML, Koeppe RA, Frey KA, Bohnen NI. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology. 2014;82:1514‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buck PO, Wilson RE, Seeberger LC, Conner JB, Castelli‐Haley J. Examination of the UPDRS bradykinesia subscale: Equivalence, reliability and validity. J Parkinson's Dis. 2011;1:253‐258. [DOI] [PubMed] [Google Scholar]
- 15. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: Comparison with the unified Parkinson's disease rating scale. Mov Disord. 2013;28:668‐670. [DOI] [PubMed] [Google Scholar]
- 16. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649‐2653. [DOI] [PubMed] [Google Scholar]
- 17. Diebo BG, Shah NV, Boachie‐Adjei O, et al. Adult spinal deformity. Lancet. 2019;394:160‐172. [DOI] [PubMed] [Google Scholar]
- 18. Ye IB, Tang R, Cheung ZB, White SJW, Cho SK. Can C7 slope be used as a substitute for T1 slope? a radiographic analysis. Global Spine J. 2020;10:148‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87:260‐267. [DOI] [PubMed] [Google Scholar]
- 20. Bissolotti L, Berjano P, Zuccher P, et al. Sagittal balance is correlated with Parkinson's disease clinical parameters: An overview of spinopelvic alignment on 175 consecutive cases. Eur Spine J. 2017;26(Suppl 4):471‐478. [DOI] [PubMed] [Google Scholar]
- 21. Yoshii F, Moriya Y, Ohnuki T, Ryo M, Takahashi W. Postural deformities in Parkinson's disease ‐Mutual relationships among neck flexion, fore‐bent, knee‐bent and lateral‐bent angles and correlations with clinical predictors. J Clin Mov Disord. 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ando Y, Fujimoto KI, Ikeda K, et al. Postural abnormality in Parkinson's disease: A large comparative study with general population. Mov Disord Clin Pract. 2019;6:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tinazzi M, Gandolfi M, Ceravolo R, et al. Postural abnormalities in Parkinson's disease: An epidemiological and clinical multicenter Study. Mov Disord Clin Pract. 2019;6:576‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ninomiya S, Morita A, Teramoto H, Akimoto T, Shiota H, Kamei S. Relationship between postural deformities and frontal function in Parkinson's disease. Parkinsons Dis. 2015;2015:462143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Artusi CA, Montanaro E, Tuttobene S, Romagnolo A, Zibetti M, Lopiano L. Pisa syndrome in Parkinson's disease is associated with specific cognitive alterations. Front Neurol. 2019;10:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torsney KM, Noyce AJ, Doherty KM, Bestwick JP, Dobson R, Lees AJ. Bone health in Parkinson's disease: A systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2014;85:1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


