Abstract
The continuous improvement of life expectancy of patients with chronic lymphocytic leukemia (CLL) has resulted in increased risk of second primary malignancy that potentially may affect survival and quality of life of CLL patients. We performed a systematic review to assess the risk and the clinical‐pathological features and prognosis of cutaneous squamous cell carcinoma (cSCC) in patients with CLL. We searched PubMed, Embase, and Cochrane Central Register of Control Trials databases for articles published from database inception to December 31, 2019. English‐language studies reporting original data on patients with a specific diagnosis of CLL and cSCC were included. Data were extracted using a standardized extraction form, and any discordance was resolved by consensus. Descriptive data were generated by pooling patients from eligible studies. Of the 4588 non‐duplicate records identified, 55 articles met our inclusion criteria. These studies reported that CLL patients have a 3.2% prevalence of cSCC, with an 11.5% cSCC‐related lethality and an overall risk of metastasis of 5.7% (7.3% for regional lymph node involvement and 3.8% for distant metastasis). The quality of evidence was limited by the high heterogeneity in the design, populations, and objectives of the included studies. This systematic review suggests that cSCC in CLL patients tends to behave less aggressively compared with the solid organ transplant recipients but has a higher morbidity and mortality than in the general population. Future prospective studies are needed to increase the quality of evidence and to determine the best treatment modalities and screening intervals for these patients.
Introduction
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder characterized by the progressive accumulation of functionally incompetent monoclonal B lymphocytes in peripheral blood. CLL is the most common form of leukemia in adults in the Western world, with an annual incidence rate of five cases per 100,000 individuals, a male predominance, and a median age at diagnosis of 70 years. 1 Although the spectrum of clinical presentations ranges from indolent form not requiring treatment to rapidly progressing disease, most patients have a favorable prognosis with a 5‐year survival of approximately 85%. 2 Patients with CLL have an immunosuppressed status due to the intrinsic nature of the disease, its treatment, or both. 3
There is a well‐established association between immunosuppression in solid organ transplant recipients (SOTRs) and the increased risk of secondary malignancy, skin cancer above all, with a higher risk of adverse outcome. Unlike the general population, where the most common cutaneous malignancy is basal cell carcinoma (BCC), in SOTRs the incidence of cutaneous squamous cell carcinoma (cSCC) is the highest, and the BCC:SCC ratio is reversed. 4 , 5 , 6 These patients have a 65–250 times increased incidence of cSCC that tends to have an aggressive biologic behavior, with increased rates of recurrence, metastasis, and death compared with immunocompetent patients. 4 , 6 , 7 , 8 Although still very high, the risk of cSCC in OTR seems to be decreasing, which suggests that cSCC risk may be lower in OTR treated with the modern immunosuppressive drugs and that cSCC preventive measures may be effective. 9
The continuous improvement of survival rates of CLL patients, related to more efficient treatment, increases the likelihood of second primary malignancies (SPMs) such as cSCC that potentially may affect survival and quality of life. 10 , 11 , 12 Thus, it is of fundamental importance to understand the impact of SPMs on survival rate of primary cancer with relatively good prognosis, like CLL. The aim of this systematic review is to analyze the prevalence, clinical‐pathological features, and prognosis of cSCC in patients with CLL.
Methods
Search strategy and selection criteria
We conduct this systematic review according with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and the Meta‐analyses of Observational Studies in Epidemiology (MOOSE) reporting guidelines, to investigate the association between CLL and cSCC. 13 , 14 We performed our systematic search in Pubmed, Embase, and Cochrane Central Register of Controlled Trials databases from database inception until Decemeber 31, 2019.
Our search criteria for PubMed were as follows: ("chronic lymphocytic leukaemia" [All Fields] OR "chronic lymphocytic leukemia" [All Fields] OR "leukemia, lymphocytic, chronic, b‐cell" [MeSH Terms] OR leukemia [All Fields] OR leukaemia [All Fields]) AND ("carcinoma, squamous cell" [MeSH Terms] OR squamo* [All Fields] OR spino* [All Fields]).
Our search criteria for Embase and the Cochrane Register were as follows: ("chronic lymphocytic leukaemia" OR "chronic lymphocytic leukemia" OR leukemia OR leukaemia) AND (squamo* OR spino*). Moreover, we manually searched the reference list of the included articles to identify additional articles that met our inclusion criteria.
Eligible studies were English‐language articles, prospective or retrospective studies, clinical trials, case series, and case reports, describing original data on patients of any age with a specific diagnosis of CLL and cSCC.
Data analysis
Three of us (L.C., G.O., and A.P.) independently performed the search, the title/abstract screening, and extracted data using a standardized extraction form. Any discordance or uncertainty was resolved by consensus. For each study, the reviewer recorded the following variables: characteristics of study (type of publication, year, and study design), number of patients enrolled, and demographics (age and gender), first diagnosis (CLL first, cSCC first, or both diseases at onset), mean time from CLL to cSCC diagnosis, mean follow‐up after SCC diagnosis, death from cSCC, death from CLL progression after cSCC diagnosis, characteristics of CLL (age at disease onset, sex, clinical stage, previous treatment), characteristics of cSCC (anatomical site, risk factors, histopathological features, number of lesions, nodal or distant metastasis, local recurrence after therapy, metastasis of SCC to CLL‐infiltrated lymph node, previous treatment). In case of missing information, only complete data were considered for statistical analysis. Standard descriptive statistics were used to summarize the data. Descriptive data, expressed in mean values or percentages, were generated by pooling patients from eligible studies. Statistical analyses were performed using the IBM SPSS 26.0 package (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp).
Moreover, the reviewers performed quality assessment using an arbitrarily modified version of the Newcastle‐Ottawa Scale (NOS) for observational studies in order to assess the quality of case series and case reports. 15
Results
Literature search
The database search identified 6205 publications. After duplicates removal, 4588 titles and abstracts were reviewed; 4509 studies that did not meet our inclusion criteria were excluded. Further 24 publications were excluded after full‐text review. The study selection process is illustrated in Figure 1.
Figure 1.
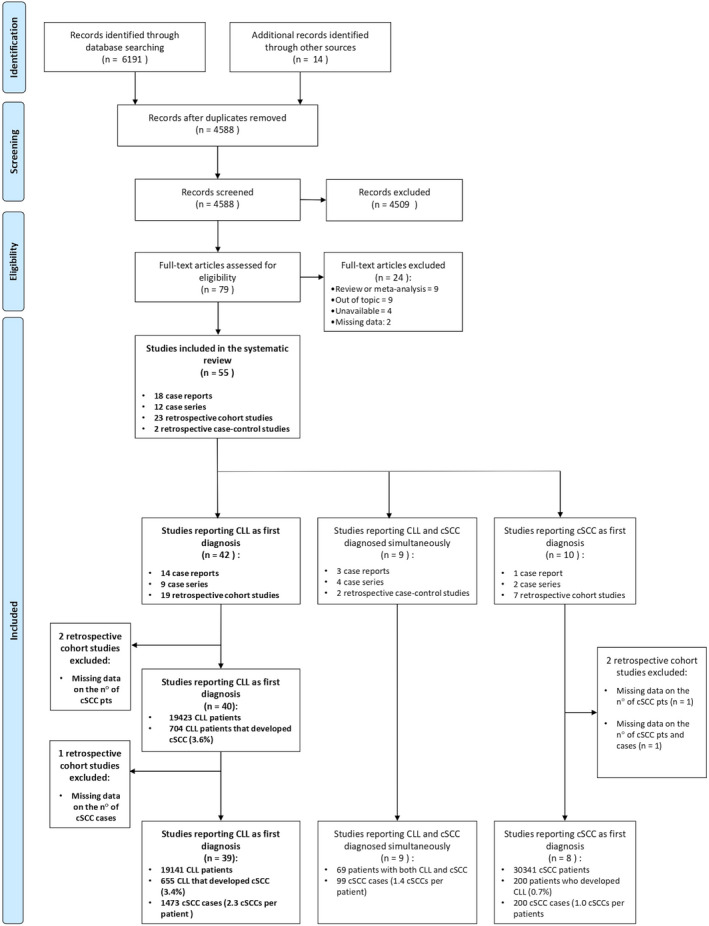
Flow chart of search results and study selection
The included 55 papers were published between 1977 and 2019, 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 of these 25 (45.5%) during the last decade as described in Table 1; most of the included articles were retrospective studies (30, 54.5%), while the remaining were case series or case reports (25, 45.5%) (Table 1).
Table 1.
Summary of the 55 studies included in the systematic review
| Year of study | Country | Study type | First occurrence | Study quality a | |
|---|---|---|---|---|---|
| Villet WT et al. 16 | 1977 | South Africa | Case report | CLL first | 1 |
| Weimar VM et al. 17 | 1979 | USA | Case series | CLL first and simultaneous CLL and cSCC | 1 |
| Mora, RG 28 | 1985 | USA | Case report | CLL first | 1 |
| Bridges N et al. 39 | 1986 | USA | Case report | CLL first | 1 |
| Lishner M et al. 50 | 1987 | Israel | Case series | CLL first | 2 |
| Perez‐Reyes N, Farhi DC. 61 | 1987 | USA | Retrospective cohort study | CLL first | 2 |
| Frierson HF et al. 66 | 1988 | USA | Retrospective cohort study | CLL first | 2 |
| Soares FA et al. 67 | 1992 | Brazil | Case report | Simultaneous CLL and cSCC | 1 |
| Adami J et al. 68 | 1995 | Sweden, Denmark | Retrospective cohort study | CLL first and cSCC first | 2 |
| Hartley BEJ et al. 69 | 1996 | UK | Case series | CLL first and cSCC first | 1 |
| Levi F et al. 18 | 1996 | Switzerland | Retrospective cohort study | CLL first and cSCC first | 2 |
| Davidovitz Y et al. 19 | 1997 | Israel | Case report | CLL first | 1 |
| Dargent JL et al. 20 | 1998 | Belgium | Case report | CLL first | 1 |
| Albregts T et al. 21 | 1998 | USA | Case report | CLL first | 1 |
| Smoller BR et al. 22 | 1998 | USA | Case series | CLL first | 1 |
| Gray Y et al. 23 | 2001 | USA | Case report | CLL first | 1 |
| Smith KJ et al. 70 | 2001 | USA | Case series | Simultaneous CLL and cSCC | 1 |
| Pigeaud‐Klessens ML et al. 24 | 2002 | Netherland | Case report | CLL first | 1 |
| Larsen CR et al. 25 | 2002 | Denmark | Case series | CLL first | 1 |
| Padgett JK et al. 26 | 2003 | USA | Case report | cSCC first | 1 |
| Agnew KL et al. 27 | 2004 | UK | Retrospective cohort study | CLL first | 2 |
| Rashid K et al. 29 | 2005 | USA | Case series | CLL first | 1 |
| Sheahan P et al. 30 | 2005 | Ireland | Retrospective cohort study | SCC first | 2 |
| Mehrany K et al. 31 | 2005 | USA | Retrospective case–control study | Simultaneous CLL and cSCC | 2 |
| Mehrany K et al. 32 | 2005 | USA | Retrospective case–control study | Simultaneous CLL and cSCC | 2 |
| Flezar MS et al. 33 | 2006 | Slovenia | Case series | CLL first | 1 |
| Goh MSY 34 | 2006 | Australia | Case series | CLL first | 1 |
| Wong J. et al. 35 | 2008 | Canada | Case report | CLL first | 1 |
| Peng Y et al. 36 | 2009 | USA | Case report | CLL first | 1 |
| Toro JR et al. 37 | 2009 | USA | Retrospective cohort study | cSCC first | 3 |
| Hodges S. et al. 38 | 2010 | UK | Case report | Simultaneous CLL and cSCC | 1 |
| Wilson ML et al. 40 | 2010 | USA | Case series | Simultaneous CLL and cSCC | 1 |
| Tomaszewski JM et al. 41 | 2014 | Australia | Case series | CLL first | 1 |
| Mansfield AS et al. 42 | 2014 | USA | Retrospective + prospective cohort study | CLL first | 2 |
| Velez NF et al. 43 | 2014 | USA | Retrospective cohort study | CLL first | 3 |
| Brewer JD et al. 44 | 2014 | USA | Retrospective cohort study | CLL first | 3 |
| Tomaszewski JM et al. 45 | 2014 | Australia | Retrospective cohot study | CLL first | 3 |
| Dos Santos HT et al. 46 | 2015 | Brazil | Case report | CLL first | 1 |
| Vyas R et al. 47 | 2015 | USA | Case report | CLL first | 1 |
| Gaide O et al. 48 | 2016 | Switzerland | Case report | CLL first | 1 |
| Wollina U et al. 49 | 2016 | Germany | Case report | Simultaneous CLL and cSCC | 1 |
| Hock BD et al. 51 | 2016 | New Zealand | Retrospective cohort study | CLL first | 2 |
| Kader I et al. 52 | 2016 | Australia | Retrospective cohort study | CLL first | 2 |
| Penne M et al. 53 | 2017 | USA | Retrospective cohort study | CLL first | 3 |
| Hirshoren N et al. 54 | 2017 | Australia | Case series | cSCC first | 3 |
| Hampras S.S et al. 55 | 2017 | USA | Retrospective cohort study | CLL first | 3 |
| Callaghan DJ et al. 56 | 2018 | USA | Case series | cSCC first and simultaneous CLL and cSCC | 1 |
| Wee E et al. 57 | 2018 | Australia | Retrospective cohort study | cSCC first | 2 |
| Walz JS et al. 58 | 2018 | Germany | Retrospective cohort study | CLL first | 3 |
| Rausch CR, Kontoyiannis DP 59 | 2019 | USA | Case report | CLL first | 1 |
| Purcell RV et al. 60 | 2019 | New Zealand | Retrospective cohort study | CLL first | 3 |
| Zheng G et al. 62 | 2019 | Sweden | Retrospective cohort study | CLL first and cSCC first | 2 |
| Inda JJ et al. 63 | 2019 | USA | Retrospective cohort study | CLL first | 2 |
| Thiesen I et al. 64 | 2019 | Germany | Retrospective cohort study | CLL first | 2 |
| Wu PA et al. 65 | 2019 | USA | Retrospective cohort study | CLL first | 2 |
CLL, chronic lymphocytic leukemia; cSCC, cutaneous squamous cell carcinoma; UK, United Kingdom; USA, United States of America.
Based on Newcastle–Ottawa scale modified for case series and case reports: 1(worse) to 4 (better).
Forty‐two articles 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 33 , 34 , 35 , 36 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 , 55 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 68 , 69 reported cases of cSCC developed after the diagnosis of CLL, nine studies 17 , 31 , 32 , 38 , 40 , 49 , 56 , 67 , 70 described the simultaneous diagnosis of both diseases, and 10 papers 18 , 26 , 30 , 37 , 54 , 56 , 57 , 62 , 68 , 69 reported cSCC diagnoses preceding CLL onset. Of note, 13/55 articles reported cases belonging to more than one of the aforementioned categories (Table 1). 17 , 18 , 31 , 32 , 38 , 40 , 49 , 56 , 62 , 67 , 68 , 69 , 70 Since in the vast majority of patients included in this systematic review the diagnosis of CLL preceded the development of cSCC, our analysis focuses predominantly on this subset, albeit for the sake of completeness, we report the finding of all three categories. Due to the heterogeneity and poor quality of studies, we did not do a quantitative synthesis of data.
CLL as the first diagnosis
Forty‐two articles, including 38807 patients affected by CLL, reported subsequent onset of cSCC. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 33 , 34 , 35 , 36 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 , 55 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 68 , 69
To calculate the prevalence of cSCC in this population, we further excluded two studies (including a total of 19384 CLL patients), 55 , 62 since only the number of cSCC was reported and not the number of patients coaffected by CLL and cSCC.
In these 40 studies, a total of 704 out of 19,423 (3.6%) CLL patients developed at least one cSCC.
To calculate the total number of cSCC cases and the mean number of cSCC per patient, another article was then excluded, accounting for 282 CLL patients, in which only the number of patients developing cSCC was provided (n: 49) but not the total number of cSCCs. 44
Thirty‐nine articles were thus finally considered, including 655/19141 patients developing cSCC after CLL diagnosis for a total number of 1473 cSCCs (average number of cSCC per patient 2.3) (Fig. 1). 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 33 , 34 , 35 , 36 , 39 , 41 , 42 , 43 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 , 58 , 59 , 60 , 61 , 63 , 64 , 65 , 66 , 68 , 69 Moreover, to calculate the prevalence of cSCC after CLL diagnosis, we excluded case reports and case series in order to avoid overestimation and 16 articles including 612/19,015 patients developing cSCC after CLL diagnosis were finally considered. 18 , 27 , 42 , 43 , 45 , 51 , 52 , 53 , 58 , 60 , 61 , 63 , 64 , 65 , 66 , 68 The prevalence of cSCC after CLL diagnosis was 3.2%, and the average number of cSCC per patient was 2.3.
cSCC‐related deaths for patients first diagnosed for CLL were 41/356 (lethality rate: 11.5%) and were reported in 18 studies. 17 , 21 , 23 , 24 , 25 , 28 , 29 , 33 , 35 , 39 , 43 , 45 , 47 , 59 , 61 , 63 , 66 , 69
The population characteristics of the 39 finally included studies are summarized in Table 2. The mean age of CLL patients who developed a cSCC was 72.9 years (SD ± 2.5, range 44–83), with a male predominance (84.3%). The mean time from CLL onset to cSCC diagnosis was 6.1 years, and 52% of patients developed more than one lesion. In 416 of 713 (58.3%) patients, the cSCC was located on the head and neck region.
Table 2.
Demographics and cutaneous squamous cell carcinoma (cSCC)‐related features of patients with both chronic cell leukemia (CLL) and cSCC
| Variables | CLL diagnosis first | CLL & cSCC simultaneous | cSCC diagnosis first | ||||
|---|---|---|---|---|---|---|---|
| 655 patients with 1473 cSCCs (39 studies) | 69 patients with 99 cSCCs (9 studies) | 200 patients with 200 cSCCs (8 studies) | |||||
| value | % | value | % | value | % | ||
| Mean age ± SD | 72·9 ± 2·5 | on 343 patients | 73·3 ± 5·4 | on 69 patients | 69·0 ± 0·0 | on 1 patient | |
| Sex | Female | 77/490 | 15·7 | 9/69 | 13·0 | 0/3 | 0·0 |
| cSCC location | Head and Neck | 416/713 | 58·3 | 99/99 | 100·0 | 14/14 | 100·0 |
| cSCC histology | Invasive | 25/41 | 61·0 | 0/5 | 0·0 | 1/1 | 100·0 |
| Grade 1 (well differentiated) | 268/398 | 67·3 | 22/41 | 53·7 | 1/1 | 100·0 | |
| Grade 2 (moderately differentiated) | 70/398 | 17·6 | 16/41 | 39·0 | 0/1 | 0·0 | |
| Grade 3 (poorly differentiated) | 60/398 | 15·1 | 3/41 | 7·3 | 0/1 | 0·0 | |
| Keratoacanthoma | 1/41 | 2·4 | 0/5 | 0·0 | 0/1 | 0·0 | |
| Perineural invasion | 24/177 | 13·6 | 1/4 | 25·0 | 0/1 | 0·0 | |
| Cutaneous CLL infiltrates around cSCC | 33/97 | 34·0 | 5/6 | 83·3 | 3/53 | 5·7 | |
| cSCC features | Patients with more than 1 cSCC | 75/144 | 52·1 | 29/37 | 78·4 | 0/1 | 0·0 |
| Locally advanced | 17/572 | 3·0 | 0/6 | 0·0 | 0/1 | 0·0 | |
| Regional lymph node metastasis | 77/1056 | 7·3 | 5/13 | 38·5 | 1/1 | 100·0 | |
| Distant metastasis | 28/783 | 3·6 | 5/39 | 12·8 | 2/2 | 100·0 | |
| Recurrence after therapy | 61/1041 | 5·9 | 9/64 | 14·1 | 1/2 | 50·0 | |
| Metastasis of cSCC to CLL‐infiltrated lymph node | 8/22 | 36·4 | 3/7 | 42·9 | 0/1 | 0·0 | |
| cSCC therapy | Cryotherapy | 1/83 | 1·2 | 0/6 | 0·0 | 0/2 | 0·0 |
| Laser therapy | 1/83 | 1·2 | 0/6 | 0·0 | 0/2 | 0·0 | |
| Diathermocoagulation | 2/83 | 2·4 | 0/6 | 0·0 | 0/2 | 0·0 | |
| 5‐fluorouracil | 1/83 | 1·2 | 0/6 | 0·0 | 0/1 | 0·0 | |
| Imiquimod | 0/83 | 0·0 | 5/6 | 83·3 | 0/1 | 0·0 | |
| Photodynamic therapy | 0/83 | 0·0 | 0/6 | 0·0 | 0/1 | 0·0 | |
| Radiotherapy | 31/228 | 13·6 | 0/6 | 0·0 | 1/2 | 50·0 | |
| Chemotherapy | 2/19 | 10·5 | 0/6 | 0·0 | 0/0 | 0·0 | |
| Surgery | 133/281 | 47·3 | 5/11 | 45·5 | 13/14 | 92·9 | |
| Mohs Micrographic Surgery | 12/90 | 13·3 | 58/93 | 62·4 | 4/54 | 7·4 | |
| Post‐surgery radiotherapy | 30/209 | 14·4 | 1/7 | 14·3 | 13/14 | 92·9 | |
| Elective node irradiation | 10/164 | 6·1 | 1/6 | 16·7 | 1/1 | 100·0 | |
| Nodal dissection | 36/182 | 19·8 | 2/8 | 25·0 | 1/1 | 100·0 | |
| Other skin cancer associated with cSCC in CLL patients | Melanoma with cSCC | 14/198 | 7·1 | 0/0 | 0·0 | 0/0 | 0·0 |
| BCC with cSCC | 9/26 | 34·6 | 9/29 | 31·0 | 0/0 | 0·0 | |
| Merkel cell carcinoma with cSCC | 2/184 | 1·1 | 0/0 | 0·0 | 0/0 | 0·0 | |
| Sebaceous carcinoma with cSCC | 2/69 | 2·9 | 0/0 | 0·0 | 0/0 | 0·0 | |
| Deaths | from cSCC‐related | 41/356 | 11·5 | 4/34 | 11·8 | 1/2 | 50·0 |
| CLL‐related after cSCC diagnosis | 3/22 | 13·6 | 1/6 | 16·7 | 0/1 | 0·0 | |
BCC, basal cell carcinoma; CLL, chronic lymphocytic leukemia; cSCC, cutaneous squamous cell carcinoma; SD, standard deviation.
Concerning histopathological features of cSCC in CLL patients, 25 of 41 (61%) lesions were invasive and 16 (39%) were in situ; 268 of 398 (67.3%) cSCC were well‐differentiated, 70 of 398 (17.6%) moderately differentiated, and 60 of 398 (15.1%) poorly differentiated. Perineural invasion was reported in 24/177 (13.6%) cSCC. Seventeen (3%) locally advanced cSCC were reported in 14 studies including 572 lesions; nodal metastasis was detected in 77 of 1056 (7.3%) cases, and distant metastasis developed in 28 of 783 (3.6%) cases. Local recurrence of cSCC after treatment occurred in 61 of 1041 (5.9%) cases. In eight of 22 (36.4%) cases, metastatic cells of cSCC were detected in CLL‐infiltrated lymph nodes.
Due to the poor quality and incompleteness of data, it was impossible to evaluate the correlation between cSCC features and CLL clinical stage or previous/ongoing specific treatment.
Regarding the diagnosis of other cutaneous malignancies in association with cSCC in CLL patients, the included articles reported 14 melanomas, nine BCC, two Merkel cell carcinomas, and two sebaceous carcinomas.
CLL and cSCC diagnosed simultaneously
A total of nine articles reported the simultaneous diagnosis of CLL and cSCC in 69 patients, with a total number of 99 cSCC lesions (average number of cSCC per patient: 1.4), all located on the head and neck region. 17 , 31 , 32 , 38 , 40 , 49 , 56 , 67 , 70 The mean age of patients was 73.3 years (SD 5.4; range 60–88); 60 were males (87%) and nine females (13%). The CLL and cSCC characteristics are reported in Table 2.
cSCC as the first diagnosis
Concerning patients first diagnosed with cSCC who subsequently developed CLL, 10 studies were first identified. 18 , 26 , 30 , 37 , 54 , 56 , 57 , 62 , 68 , 69 However, one study was excluded because neither the total number of cSCC lesions nor the number of cSCC patients were provided (including 414 patients with both CLL and cSCC). 62 Another study was also excluded (including 202 SCC patients, of these two with CLL) 30 because the total number of cSCC lesions was not reported.
Eight studies were thus finally considered, including 30,341 cSCC patients, 200 of which subsequently diagnosed for CLL. 18 , 26 , 37 , 54 , 56 , 57 , 68 , 69 The total number of cSCC in patients co‐affected by CLL was 200 (average number of cSCC per patient: 1.0). After the exclusion of case reports/series (four studies including 67 cSCC patients, 14 of these with a subsequent diagnosis of CLL), 26 , 54 , 56 , 69 the prevalence of CLL in cSCC patients was 0.72%. The time from cSCC to CLL diagnosis was only reported for one patient and was 5 years. 56 More data on patients developing CLL after cSCC diagnosis are reported in Table 2.
Discussion
The results of our systematic review suggest that CLL patients have a high prevalence of cSCC that tends to behave more aggressively compared with the general population. 10 , 31 , 45 , 51 , 71 Thus, the development of a second cancer in this subset of patients represents a considerable clinical burden. Based on our data, 3.2% of CLL patients developed a subsequent cSCC. Several epidemiologic studies have reported a 1.86 to 8.6 increased risk of cSCC in patients with CLL. 37 , 51 , 62 , 68 Studies using cancer registries data from Denmark and Sweden have reported an overall relative risk of cSCC of 8.6 (95% CI 7.2–10.3), increasing with time during the first decade after CLL diagnosis 68 ; a more recent study has identified a relative risk of 24.58 for in situ and 7.63 for invasive cSCC. 62 It might be speculated that this high rate of cSCC could result from the higher level of surveillance and diagnosis in this subset of patients. However, one study performed at Mayo Clinic contradicted this hypothesis documenting a low compliance with skin cancer screening in patients with CLL. 42 Nevertheless, it is likely that the true incidence and prevalence of cSCC are underestimated since many countries’ cancer registries do not document cSCC.
Regarding patient and tumor characteristics, most of the CLL patients who developed a subsequent cSCC were male with a mean age of 72.9 years, and the most common location of the skin cancer was the head and neck region. These features are in line with data reported in the general population. 72
Interestingly, SOTRs with cSCC have different clinico‐pathological traits compared with CLL patients. In contrast to SOTRs, CLL patients developing cSCC seem to be older (72.9, range 44‐83 vs. 62, range 36–77), data expected due to overall older age of CLL patients compared with SOTRs, and tend to have cSCC with a lower T stage (39% vs 2% in situ), a higher grade of differentiation of the lesions (67.3% vs 2% well‐differentiated; 15.1% vs 41% poorly differentiated), a lower rate of perineural invasion (13.6% vs 39%), and a lower rate of local recurrence (5.9% vs 45%). 73
Noteworthy, on histopathological examination more than one‐third of cSCC in CLL patients are associated with a dense leukemic infiltrate that can complicate histopathologic interpretation especially on frozen section during Mohs micrographic surgery (MMS). 32 This finding may explain why CLL patients are by far more likely to develop recurrence after MMS compared with the general population (local recurrence rate of 13% in CLL patients vs. 3% rate observed in the general population). 44
Notably, our data show an 11.5% cSCC‐related lethality in patients with CLL and an overall risk of metastasis of 5.7% (7.3% for regional lymph node involvement and 3.8% for distant metastasis). To the best of our knowledge, only one study evaluated the impact of cSCC to the overall mortality of CLL patients. 43 More specifically, the authors found that CLL patients had 12–13% risk of death from skin cancer, and the predictors of poor outcome were the following: a high Rai stage at the time of the first skin cancer diagnosis and the occurrence of cSCC with high T stage.
Due to the poor quality and incompleteness of data, it was impossible to evaluate a potential correlation between CLL clinical stage and the development and features of cSCC. A study from the Boston group evaluating the impact of Rai stage on the outcome of skin cancer found that, in a multivariate analysis, advanced Rai stage (III or IV) and high skin cancer tumor (T) stage are significantly associated with poor skin cancer outcome. Thus, the authors suggest that, even though high‐T‐stage cSCC is associated with a poor outcome regardless of the CLL stage, the Rai stage may be useful in stratifying the risk of patients with low T stage skin cancer. 43 Furthermore, a recent study evaluating the effectiveness of tumor risk stratification of four different tumor staging systems for cSCC (American Joint Committee on Cancer seventh edition and eighth edition, Union for International Cancer Control eighth edition, and Brigham and Women’s Hospital staging systems) has suggested that the Brigham and Women’s Hospital system provides a superior risk stratification of cSCC tumors in patients with CLL. 63
Our results do not shed light on the impact of chemoimmunotherapy and irradiation used to treat CLL on the development of secondary malignancy. Existing data suggest that chemoimmunotherapeutic agents, such as fludarabine, chlorambucil, cyclophosphamide, and rituximab, increase the susceptibility to second cancer. 12 , 19 , 29 , 53 , 71 Further studies are needed to evaluate the possible oncogenic role of classical CLL treatments and recent drugs such as BTK inhibitors, PI3K inhibitors, and BH3‐mimetics. 74
In this systematic review, we also included articles reporting the simultaneous diagnosis of cSCC and CLL. 17 , 31 , 32 , 38 , 40 , 49 , 56 , 67 , 70 In these studies, the presence of a dense tumor‐associated lymphocytic infiltrate in patients with no history of leukemia represented a clue to the prompt evaluation for the underlying hematologic malignancy. Few studies analyzed the bi‐directional relationship between CLL and cSCC. 18 , 62 , 68 Further studies are needed to evaluate the impact of non‐melanoma skin cancer on the subsequent onset of CLL as the possibility that subclinical CLL may be present at the time of SCC diagnosis should be addressed.
The pathogenesis of leukemia‐associated skin cancer is still unknown, but several causative factors have been considered including immunosuppression associated with CLL, ultraviolet (UV) light exposure, and the mutagenic effect of chemotherapy or radiotherapy. CLL is characterized by an impaired functioning of the immune system with dysfunctional lymphocytes unable to elicit an antitumor response. 11 , 75 , 76 Both exposure to UV radiation and immunosuppression are pivotal risk factors for the development of cSCC. 72 , 77 In our systematic review, elderly males are in a high‐risk group, and cSCC are preferentially located on the head and neck region. These findings suggest the critical role of years of cumulative UV radiation exposure in the development of keratinocyte carcinomas, reinforcing the need of awareness and educational program of CLL patients on primary prevention strategies for skin cancer (e.g. sun avoidance, sun protection measures). Genetic susceptibility, environmental factor, and human papillomavirus infection have been reported to play a role in the oncogenesis of cSCC in CLL patients as well as in other immunosuppressed subsets of patients. 5 , 7 , 11
Our review has some limitations. There is a high heterogeneity in the design, populations, and objectives of the included studies. Most of the included articles are case reports or case series involving a small number of patients. Moreover, we included three studies based on data from the Swedish Cancer Registry 37 , 62 , 68 and five studies from the Mayo Clinic, 31 , 32 , 42 , 44 , 63 and we are not able to exclude that some duplicate cases were encompassed. As a result of the generally high risk of bias across the included studies, we could present only a narrative synthesis of the evidence, and it was not possible to perform a meta‐analysis. The generally poor quality of the evidence base implies that caution is needed in the interpretation of our findings since there is significant uncertainty regarding the included data.
Our systematic review specifically examines the risk of cSCC in patients with CLL and provides a comprehensive picture of available epidemiological evidence on this topic. In addition, it confirms the relevant morbidity and mortality of cSCC in patients with CLL and strengthens the importance of prompt treatment and close dermatologic surveillance for this high‐risk subset of patients. Future prospective studies with larger population are needed to increase the quality of evidence regarding the occurrence of cSCC in patients with CLL and to determine the best treatment modalities and screening intervals for these patients.
Acknowledgment
Open access funding enabled and organized by CRUI.
Conflict of interest: None.
Funding sources: None.
[Correction added on May 15, 2022, after first online publication: CRUI funding statement has been added.]
References
- 1. Campo E, Ghia P, Monserrat E, Harris NL. Chronic lymphocytic leukaemia / small lymphocytic lymphoma. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4t. Lyon: IARC, 2017: 216–221. [Google Scholar]
- 2. American Cancer Society . Cancer Facts & Figures 2019. 2019; Available from: https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/annual‐cancer‐facts‐and‐figures/2019/cancer‐facts‐and‐figures‐2019.pdf (accessed June 6, 2020).
- 3. Tam S, Gross ND. Cutaneous squamous cell carcinoma in immunosuppressed patients. Curr Oncol Rep 2019; 21: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Greenberg JN, Zwald FO. Management of skin cancer in solid‐organ transplant recipients: a multidisciplinary approach. Dermatol Clin 2011; 29: 231–241. [DOI] [PubMed] [Google Scholar]
- 5. Lindelöf B, Sigurgeirsson B, Gäbel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000; 143: 513–519. [PubMed] [Google Scholar]
- 6. Park CK, Fung K, Austin PC, et al. Incidence and risk factors of keratinocyte carcinoma after first solid organ transplant in Ontario, Canada. JAMA Dermatol 2019; 155: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003; 348: 1681–1691. [DOI] [PubMed] [Google Scholar]
- 8. Cheng JY, Li FY, Ko CJ, Colegio OR. Cutaneous squamous cell carcinomas in solid organ transplant recipients compared with immunocompetent patients. JAMA Dermatol 2018; 154: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plasmeijer EI, Sachse MM, Gebhardt C, et al. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance – the impact of immunosuppression on frequency of cSCC. J Eur Acad Dermatology Venereol 2019; 33(S8): 33–37. [DOI] [PubMed] [Google Scholar]
- 10. Royle JA, Baade PD, Joske D, et al. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population‐based study. Br J Cancer 2011; 105: 1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onajin O, Brewer JD. Skin cancer in patients with chronic lymphocytic leukemia and non‐Hodgkin lymphoma. Clin Adv Hematol Oncol 2012; 10: 571–576. [PubMed] [Google Scholar]
- 12. Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma 2007; 48: 855–865. [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2020 (accessed June 10).
- 16. Villet WT, Staples WG, Gétaz EP. Squamous carcinoma metastasis to lymph nodes in chronic lymphatic leukaemia. S Afr Med J 1977; 51: 738. [PubMed] [Google Scholar]
- 17. Weimar VM, Ceilley RI, Goeken JA. Aggressive biologic behavior of basal‐ and squamous‐cell cancers in patients with chronic lymphocytic leukemia or chronic lymphocytic lymphoma. J Dermatol Surg Oncol 1979; 5: 609–614. [DOI] [PubMed] [Google Scholar]
- 18. Levi F, Randimbison L, Te VC, La Vecchia C. Non‐Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer 1996; 74: 1847–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davidovitz Y, Baltin A, Meytes D. Flare‐up of squamous cell carcinoma of the skin following fludarabine therapy for chronic lymphocytic leukemia. Acta Haematol 1997; 98: 44–46. [DOI] [PubMed] [Google Scholar]
- 20. Dargent JL, Kornreich A, Andre L, Lespagnard L. Cutaneous infiltrate of chronic lymphocytic leukemia surrounding a primary squamous cell carcinoma of the skin. Report of an additional case and reflection on its pathogenesis [1]. J Cutan Pathol 1998; 25: 479–480. [DOI] [PubMed] [Google Scholar]
- 21. Albregts T, Orengo I, Salasche S, et al. Squamous cell carcinoma in a patient with chronic lymphocytic leukemia. Dermatologic Surg 1998; 24: 269–272. [DOI] [PubMed] [Google Scholar]
- 22. Smoller BR, Warnke RA. Cutaneous infiltrate of chronic lymphocytic leukemia and relationship to primary cutaneous epithelial neoplasms. J Cutan Pathol 1998; 25: 160–164. [DOI] [PubMed] [Google Scholar]
- 23. Gray Y, Robidoux HJ, Farrell DS, Robinson‐Bostom L. Squamous cell carcinoma detected by high‐molecular‐weight cytokeratin immunostaining mimicking atypical fibroxanthoma. Arch Pathol Lab Med 2001; 125: 799–802. [DOI] [PubMed] [Google Scholar]
- 24. Pigeaud‐Klessens MLE, Van der Valk P. Multiple neoplasms: a case report. Orbit 2002; 21: 145–148. [DOI] [PubMed] [Google Scholar]
- 25. Larsen CR, Hansen PB, Clausen NT. Aggressive growth of epithelial carcinomas following treatment with nucleoside analogues. Am J Hematol 2002; 70: 48–50. [DOI] [PubMed] [Google Scholar]
- 26. Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in Mohs micrographic surgery frozen sections. Dermatologic Surg 2003; 29: 769–771. [DOI] [PubMed] [Google Scholar]
- 27. Agnew KL, Ruchlemer R, Catovsky R, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol 2004; 150: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 28. Mora RG. Metastatic squamous cell carcinoma of the skin occurring in a lymphomatous lymph node. J Am Acad Dermatol 1985; 12: 571–575. [DOI] [PubMed] [Google Scholar]
- 29. Rashid K, Ng R, Mastan A, et al. Accelerated growth of skin carcinoma following fludarabine therapy for chronic lymphocytic leukemia. Leuk Lymphoma 2005; 46: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 30. Sheahan P, Hafidh M, Toner M, Timon C. Unexpected findings in neck dissection for squamous cell carcinoma: incidence and implications. Head Neck 2005; 27: 28–35. [DOI] [PubMed] [Google Scholar]
- 31. Mehrany K, Weenig RH, Lee KK, et al. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol 2005; 53: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 32. Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol 2005; 140: 985–988. [DOI] [PubMed] [Google Scholar]
- 33. Fležar MS, Prevodnik VK, Kirbiš IS, Strojan P. Cutaneous squamous cell carcinoma metastatic to chronic lymphocytic leukaemia: diagnostic potential of fine needle aspiration cytology. Cytopathology 2006; 17: 288–294. [DOI] [PubMed] [Google Scholar]
- 34. Goh MSY. Invasive squamous cell carcinoma after treatment of carcinoma in situ with 5% imiquimod cream. Australas J Dermatol 2006; 47: 186–188. [DOI] [PubMed] [Google Scholar]
- 35. Wong J, Breen D, Balogh J, et al. Treating recurrent cases of squamous cell carcinoma with radiotherapy. Curr Oncol 2008; 15: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng Y, Wang HY, Molberg KH. Cutaneous colocalized invasive poorly differentiated carcinoma and chronic lymphocytic leukemia/small lymphocytic lymphoma of the head and neck region: a case report and review of the literature. Arch Otolaryngol ‐ Head Neck Surg 2009; 135: 606–610. [DOI] [PubMed] [Google Scholar]
- 37. Toro JR, Blake PW, Björkholm M, et al. Prior history of non‐melanoma skin cancer is associated with increased mortality in patients with chronic lymphocytic leukemia. Haematologica 2009; 94: 1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hodges S, Williams MD, Moody AB, et al. Ultrasound‐guided core biopsy for investigation of cervical lymph node: chronic lymphocytic leukaemia and metastatic squamous cell carcinoma. Br J Oral Maxillofac Surg 2010; 48: 46–47. [DOI] [PubMed] [Google Scholar]
- 39. Bridges N, Steinberg JJ. Aggressive squamous cell carcinoma of the skin after chronic lymphocytic leukemia. J Surg Oncol 1986; 33: 27–30. [DOI] [PubMed] [Google Scholar]
- 40. Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non‐melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J 2010; 16: 4. [PubMed] [Google Scholar]
- 41. Tomaszewski JM, Lau E, Corry J. Utility of positron emission tomography/computed tomography for nodal staging of cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. Am J Otolaryngol ‐ Head Neck Med Surg 2014; 35: 66–69. [DOI] [PubMed] [Google Scholar]
- 42. Mansfield AS, Rabe KG, Slager SL, et al. Skin cancer surveillance and malignancies of the skin in a community‐dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia. J Oncol Pract 2014; 10: e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Velez NF, Karia PS, Vartanov AR, et al. Association of advanced leukemic stage and skin cancer tumor stage with poor skin cancer outcomes in patients with chronic lymphocytic leukemia. JAMA Dermatol 2014; 150: 280–287. [DOI] [PubMed] [Google Scholar]
- 44. Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non‐Hodgkin lymphoma: a Rochester epidemiology project population‐based study in Minnesota. J Am Acad Dermatol 2015; 72: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomaszewski JM, Gavriel H, Link E, et al. Aggressive behavior of Cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. Laryngoscope 2014; 124: 2043–2048. [DOI] [PubMed] [Google Scholar]
- 46. dos Santos HT, Benevenuto BA, Filho ERC, Altemani A. Synchronous metastatic cutaneous squamous cell carcinoma and chronic lymphocytic leukaemia/small lymphocytic lymphoma in a cervical lymph node: case report of an unusual event. J Clin Exp Dent 2015; 7: e660–e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vyas R, Gotow EK, Depry JL, et al. Multiple skin cancers in a patient within 1 year of allogeneic haematopoietic cell transplant for chronic lymphocytic leukaemia. Clin Exp Dermatol 2015; 40: 927–929. [DOI] [PubMed] [Google Scholar]
- 48. Gaide O, Clayton H, Girardin M, Kuonen F. Ingenol mebutate 500 μg for treatment of the scalp in refractory field cancerization. Dermatol 2016; 232: 7–8. [DOI] [PubMed] [Google Scholar]
- 49. Wollina U, Schönlebe J, Heinig B, Nowak A, et al. Rare association of cystic squamous cell carcinoma and small lymphocytic B cell lymphoma: successful surgical approach. Wien Med Wochenschr 2017; 167(5–6): 104–109. [DOI] [PubMed] [Google Scholar]
- 50. Lishner M, Prokocimer M, Ron E, Shaklai M. Primary malignant neoplasms associated with chronic lymphocytic leukaemia. Postgrad Med J 1987; 63: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hock BD, McIntosh ND, McKenzie JL, et al. Incidence of cutaneous squamous cell carcinoma in a New Zealand population of chronic lymphocytic leukaemia patients. Intern Med J 2016; 46: 1414–1421. [DOI] [PubMed] [Google Scholar]
- 52. Kader I, Leavers B, Shashinder S, et al. Synchronous or metachronous lymphoma and metastatic cutaneous squamous cell carcinoma in the head and neck region: a diagnostic and management dilemma. J Laryngol Otol 2016; 130(S4): S45–S49. [DOI] [PubMed] [Google Scholar]
- 53. Penne M, Sarraf Yazdy M, Nair KS, Cheson BD. Extended follow‐up of patients treated with bendamustine for lymphoid malignancies. Clin Lymphoma, Myeloma Leuk 2017; 17: 637–644. [DOI] [PubMed] [Google Scholar]
- 54. Hirshoren N, Olayos E, Herschtal A, et al. Preoperative positron emission tomography for node‐positive head and neck cutaneous squamous cell carcinoma. Otolaryngol ‐ Head Neck Surg (United States) 2018; 158: 122–126. [DOI] [PubMed] [Google Scholar]
- 55. Hampras SS, Locke FL, Chavez JC, et al. Prevalence of cutaneous viral infections in incident cutaneous squamous cell carcinoma detected among chronic lymphocytic leukemia and hematopoietic stem cell transplant patients. Leuk Lymphoma 2018; 59: 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Callaghan DJ, Waldman A. Aggressive squamous cell carcinoma as a harbinger of non‐Hodgkin lymphoma. JAAD Case Rep 2018; 4: 869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wee E, Goh MS, Estall V, et al. Retrospective audit of patients referred for further treatment following Mohs surgery for non‐melanoma skin cancer. Australas J Dermatol 2018; 59: 302–308. [DOI] [PubMed] [Google Scholar]
- 58. Walz JS, Kowalewski DJ, Backert L, et al. Favorable immune signature in CLL patients, defined by antigen‐specific T‐cell responses, might prevent second skin cancers. Leuk Lymphoma 2018; 59: 1949–1958. [DOI] [PubMed] [Google Scholar]
- 59. Rausch CR, Kontoyiannis DP. Prolonged voriconazole treatment in a patient with chronic lymphocytic leukemia resulting in a litany of chronic overlapping toxicities. J Oncol Pharm Pract 2019; 25: 747–753. [DOI] [PubMed] [Google Scholar]
- 60. Purcell RV, Hock BD, Gardner J, et al. Analysis of human papillomavirus infection and leukaemic infiltrate in cutaneous squamous cell carcinoma from patients with chronic lymphocytic leukaemia. Br J Haematol 2019; 186: e67–71. [DOI] [PubMed] [Google Scholar]
- 61. Perez‐Reyes N, Farhi DC. Squamous cell carcinoma of head and neck in patients with well‐differentiated lymphocytic lymphoma. Cancer 1987; 59: 540–544. [DOI] [PubMed] [Google Scholar]
- 62. Zheng G, Chattopadhyay S, Sud A, et al. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br J Haematol 2019; 185: 232–239. [DOI] [PubMed] [Google Scholar]
- 63. Inda JJ, Kabat BF, Larson MC, et al. Comparison of tumor staging systems for cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol 2019; 80: 639–645. [DOI] [PubMed] [Google Scholar]
- 64. Thiesen I, Wehkamp U, Brüggemann M, et al. Skin involvement by chronic lymphocytic leukaemia is frequently associated with unrelated neoplastic or inflammatory cutaneous disease and is not indicative of general disease progression. Br J Dermatol 2019; 180: 227–228. [DOI] [PubMed] [Google Scholar]
- 65. Wu PA, Stern RS, Huang V, et al. Reduced‐intensity conditioning regimens, prior chronic lymphocytic leukemia, and graft‐versus‐host disease are associated with higher rates of skin cancer after allogeneic hematopoietic stem cell transplantation. J Invest Dermatol 2019; 139: 591–599. [DOI] [PubMed] [Google Scholar]
- 66. Frierson HF, Deutsch BD, Levine PA. Clinicopathologic features of cutaneous squamous cell carcinomas of the head and neck in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum Pathol 1988; 19: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 67. Soares FA, Potenciano O, Saldanha JC, Laemmel A. Fine needle aspiration of squamous cell carcinoma of the skin metastatic to the site of leukemic lymphadenopathy. A case report. Acta Cytol 1992; 36: 407–409. [PubMed] [Google Scholar]
- 68. Adami J, Frisch M, Yuen J, et al. Evidence of an association between non‐Hodgkin’s lymphoma and skin cancer. BMJ 1995; 310: 1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hartley BEJ, Searle AE, Breach NM, et al. Aggressive cutaneous squamous cell carcinoma of the head and neck in patients with chronic lymphocytic leukaemia. J Laryngol Otol 1996; 110: 694–695. [DOI] [PubMed] [Google Scholar]
- 70. Smith KJ, Germain M, Skelton H. Bowen’s disease (squamous cell carcinoma in situ) in immunosuppressed patients treated with imiquimod 5% cream and a cox inhibitor, sulindac: potential applications for this combination of immunotherapy. Dermatologic Surg 2001; 27: 143–146. [DOI] [PubMed] [Google Scholar]
- 71. Wiernik PH. Second neoplasms in patients with chronic lymphocytic leukemia. Curr Treat Options Oncol 2004; 5: 215–223. [DOI] [PubMed] [Google Scholar]
- 72. Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 1. epidemiology, diagnostics and prevention. Eur J Cancer 2020; 128: 60–82. [DOI] [PubMed] [Google Scholar]
- 73. Lanz J, Bouwes Bavinck JN, Westhuis M, et al. Aggressive squamous cell carcinoma in organ transplant recipients. JAMA Dermatol 2019; 155: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Farooqui AA, Ashraf A, Bin FT, et al. Novel targeted therapies for chronic lymphocytic leukemia in elderly patients: a systematic review. Clin Lymphoma Myeloma Leuk 2020; 20(7): e414–e426. [DOI] [PubMed] [Google Scholar]
- 75. Brewer JD, Habermann TM, Shanafelt TD. Lymphoma‐associated skin cancer: incidence, natural history, and clinical management. Int J Dermatol 2014; 53: 267–274. [DOI] [PubMed] [Google Scholar]
- 76. Otley CC. Non‐Hodgkin lymphoma and skin cancer: a dangerous combination. Australas J Dermatol 2006; 47: 231–236. [DOI] [PubMed] [Google Scholar]
- 77. Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol 2017; 177: 373–381. [DOI] [PubMed] [Google Scholar]


