Abstract
Background
Previous studies evaluating the prognostic value of computed tomography (CT)‐derived body composition data have included few patients. Thus, we assessed the prevalence and prognostic value of sarcopenic obesity in a large population of gastric cancer patients using preoperative CT, as nutritional status is a predictor of long‐term survival after gastric cancer surgery.
Methods
Preoperative CT images were analyzed for 840 gastric cancer patients who underwent gastrectomy between March 2009 and June 2018. Machine learning algorithms were used to automatically detect the third lumbar (L3) vertebral level and segment the body composition. Visceral fat area and skeletal muscle index at L3 were determined and used to classify patients into obesity, sarcopenia, or sarcopenic obesity groups.
Results
Out of 840 patients (mean age = 60.4 years; 526 [62.6%] men), 534 (63.5%) had visceral obesity, 119 (14.2%) had sarcopenia, and 48 (5.7%) patients had sarcopenic obesity. Patients with sarcopenic obesity had a poorer prognosis than those without sarcopenia (hazard ratio [HR] = 3.325; 95% confidence interval [CI] = 1.698–6.508). Multivariate analysis identified sarcopenic obesity as an independent risk factor for increased mortality (HR = 2.608; 95% CI = 1.313–5.179). Other risk factors were greater extent of gastrectomy (HR = 1.928; 95% CI = 1.260–2.950), lower prognostic nutritional index (HR = 0.934; 95% CI = 0.901–0.969), higher neutrophil count (HR = 1.101; 95% CI = 1.031–1.176), lymph node metastasis (HR = 6.291; 95% CI = 3.498–11.314), and R1/2 resection (HR = 4.817; 95% CI = 1.518–9.179).
Conclusion
Body composition analysis automated by machine learning predicted long‐term survival in patients with gastric cancer.
Keywords: body mass index, gastric cancer, machine learning, nutrition process, sarcopenic obesity, survival
1. INTRODUCTION
The annual number of diagnoses and deaths due to gastric cancer was 1.22 million and 865 000, respectively, in 2017 worldwide. 1 The prognosis of gastric cancer depends on the stage of the disease, 2 but not all patients with the same stage have the same survival. Nutritional status has emerged as an alternative prognostic factor for survival in patients with gastric cancer. 3 Among traditional clinical parameters, body mass index (BMI) is commonly used because of its simplicity. 4 , 5 Derived from body weight and height, BMI can be a surrogate marker for nutritional status. 6 , 7 However, patients with the same BMI may have different body compositions, leading to different clinical outcomes. 8 Thus, direct measurements of muscle and fat content may be a more useful marker for nutritional status.
Computed tomography (CT) is routinely included as part of the preoperative diagnostic investigations that are conducted before gastric cancer surgery. Cross‐sectional CT images provide information regarding body composition, such as skeletal muscle, visceral fat, and subcutaneous fat, which can be used to predict complications and prognosis in surgical patients. 9 , 10 , 11 , 12 , 13 However, manual retrieval of body composition data is labor intensive and heavily dependent on the examiner. Accordingly, previous studies evaluating the prognostic value of CT‐derived body composition data included small numbers of patients, and the use of these data on a routine basis in clinical settings is limited. 9 , 11 , 12 , 14
In the current study, machine learning algorithms were used to automatically detect the level of the third lumbar vertebra (L3) among CT images and to automatically quantify the body composition of patients for the selected cross‐sectional image. We determined the amount of skeletal muscle and visceral fat before gastric cancer surgery and evaluated the ability of obesity, sarcopenia, and sarcopenic obesity to predict survival after gastrectomy.
2. METHODS
2.1. Patients
Patients were selected from a data set of individuals with gastric cancer who underwent gastrectomy between March 2009 and June 2018. A total of 1023 patients with available preoperative CT images were eligible for the study. We excluded 183 patients who met one or more of these criteria: limited surgery (not subtotal or total gastrectomy), treatment with neoadjuvant chemotherapy, history of another cancer, unclear cancer stage, or operative mortality. Thus, 840 patients were included in the final analysis. The surgical procedure, staging, follow‐up assessments, and adjuvant chemotherapy were performed as previously described. 15 The extent of surgery and definitions of radical surgery were in accordance with relevant guidelines. 16 , 17 Tumor staging was based on the eighth American Joint Committee on Cancer guidelines. 2 Laboratory data, such as the albumin level, lymphocyte count, and neutrophil count, were collected. The prognostic nutritional index (PNI) was calculated as previously defined 18 : (10 × serum albumin level [g/dl]] + [0.005 × total lymphocyte count). The patients were followed for a median of 41 months after surgery. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (No. 4‐2019‐0359) and was exempted from the informed consent requirement because of the study design.
2.2. Imaging analysis by machine learning
The latest preoperative CT images were used for the analysis (Figure 1). An image slice corresponding to the center of L3 was selected, and from the image, a portion corresponding to fat and muscle was classified according to Hounsfield units (HU): −29 to +150 HU for skeletal muscle 8 , 19 and −190 to −30 HU for adipose tissue. 20 , 21
Figure 1.
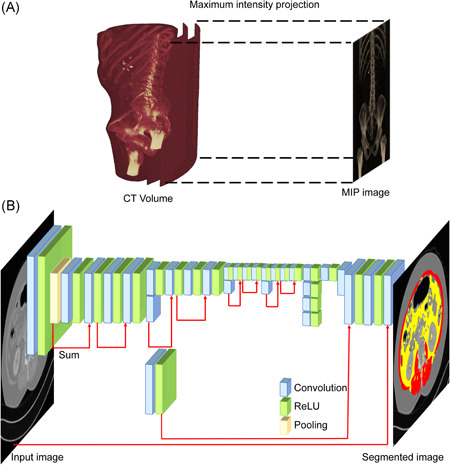
Machine learning algorithm for body composition analysis. (A) Maximum intensity projection (MIP) method for L3 annotation. (B) Machine learning network: layers of the network were based on DeepLab V3+, and Resnet‐18 was used as the base network. CT, computed tomography
2.2.1. L3 annotation
To distinguish the image slice corresponding to the center of L3 in the three‐dimensional (3D) CT volume image, this 3D image was converted to a two‐dimensional (2D) image using the maximum intensity projection method as follows 22 : for each x, y coordinate, only the pixel with the highest HU value along the z‐axis was represented such that in a single 2D image, all dense structures in a given volume were observed. The resultant 2D image showed the shape of bones, including the spine and pelvic bones (Figure 1A). The part corresponding to L3 was masked on the 2D image by an experienced doctor. Based on the original 2D image and the corresponding masked image, we implemented a machine learning algorithm that automatically masked the area corresponding to L3 on the 2D image. The image slice corresponding to the center of the area specified by the machine learning algorithm was considered the L3 center slice.
2.2.2. Fat/muscle segmentation
After specifying the image slice corresponding to the center of L3, the experienced doctor identified pixels corresponding to skeletal muscle, subcutaneous fat, and visceral fat in the selected image slice. Another machine learning algorithm was then implemented to quantify the composition of the selected image. Cross‐sectional skeletal muscle area (SMA), subcutaneous fat area (SFA), and visceral fat area (VFA) were determined. To correct for body height, we subsequently divided these areas by height squared to obtain the skeletal muscle index (SMI), subcutaneous fat index (SFI), and visceral fat index (VFI).
2.2.3. Machine learning algorithms
Two hundred different 3D CT images were used for machine learning. The transfer learning technique based on a pre‐trained convolutional neural network called ResNet‐18 was used for both the L3 annotation and fat/muscle segmentation machine learning algorithms. 23 A semantic segmentation network based on DeepLab V3+ was established. 24 The network structure of the machine learning algorithms was the same for both L3 annotation and fat/muscle segmentation (Figure 1B). The number of layers for each machine learning algorithm was 100. The batch size, initial learning rate, and L2 regularization were 8, 0.001, and 0.005, respectively. The size of the input image and the number of classification classes differed between the algorithms. For the L3 algorithm, the input image size was 512 × 250, whereas for the fat/muscle algorithm, the input image size was 512 × 512. Two classification classes were used for the L3 algorithm: whether they did or did not correspond to L3. Four classification classes were used for the fat/muscle segmentation algorithm: air, visceral fat, subcutaneous fat, and skeletal muscle. Since contrast enhancers such as contrast agents and implants interfere with accurate classification, the HU value of each pixel was converted to background values when the value did not correspond to fat or muscle. In randomly selected 30 patients, manual measurements were performed by an independent expert. Using commercially available workstation (Aquarius 3D workstation, TeraRecon), the acquired values were compared with those retrieved by machine learning. Intraclass correlation coefficient (95% confidence interval [CI]) for SMA, VFA, SFA were 0.604 (0.168–0.811), 0.850 (0.686–0.929), and 0.701 (0.373–0.858), respectively (Figure S1).
2.3. Definitions and patient groups
We defined obesity as a VFA > 100 cm2, as previously reported. 25 Sarcopenia was defined as an SMI ≤ 49 cm2/m2 for men and ≤31 cm2/m2 for women. 26 According to these criteria, patients were classified into four groups: (1) control (control), no sarcopenia or obesity; (2) sarcopenic (S), sarcopenia but no obesity; (3) obese (O), obesity but no sarcopenia; and (4) sarcopenic obesity (SO), sarcopenia and obesity (Figure 2). Patients who did not satisfy the criteria for the S, O, or SO group were used as the counter group (i.e., non‐sarcopenic [non‐S], nonobese [non‐O], and non‐sarcopenic obese [non‐SO] groups, respectively) in statistical analyses.
Figure 2.

Representative computed tomography images of groups based on sarcopenia and obesity criteria. Red color stands for the skeletal muscle area, yellow color stands for the visceral fat area
2.4. Statistical analysis
Categorical data were compared using the chi‐square test. Continuous data are presented as the mean and standard deviation, and the means were compared using an analysis of variance or the Kruskal–Wallis H test (if the Kolmogorov–Smirnov test of normality was significant [p < 0.05]). All tests were two‐sided, and the level of significance was set at p < 0.05, with Bonferroni correction. Survival was defined as the number of months from surgery to death from any cause. The Kaplan–Meier method was used to generate survival curves, and the log‐rank test was used to compare survival between groups. Univariate and multivariate Cox proportional‐hazards models with forward conditional selection were used to identify independent risk factors and estimate hazard ratios (HRs) with corresponding 95% CIs. Clinically relevant variables were selected for the univariate analysis. All analyses were performed with SPSS v23.0 (IBM).
3. RESULTS
Among the 840 included patients, 119 (14.2%) met the criteria for sarcopenia (Figure 3A), approximately two‐thirds (534; 63.5%) were obese (Figure 3B), and 48 (5.7%) had both sarcopenia and obesity (Figure 3C). The control, S, O, and SO groups consisted of 235, 71, 486, and 48 patients, respectively. As shown in Table 1, patients in the SO group were significantly older than were those in the control and O groups (p < 0.001 and p = 0.013, respectively), and the SO group included only men. The mean BMI of the SO group was significantly higher than that of the control group (22.9 vs. 21.4 kg/m2, p = 0.006; Figure 3D). BMI was the highest in the O group (25.0 kg/m2) and the lowest in the S group (20.9 kg/m2). As for the PNI and all body composition parameters (SFA, VFA, SMA, SFI, VFI, and SMI), the O group had the highest values, and the S group had the lowest values.
Figure 3.
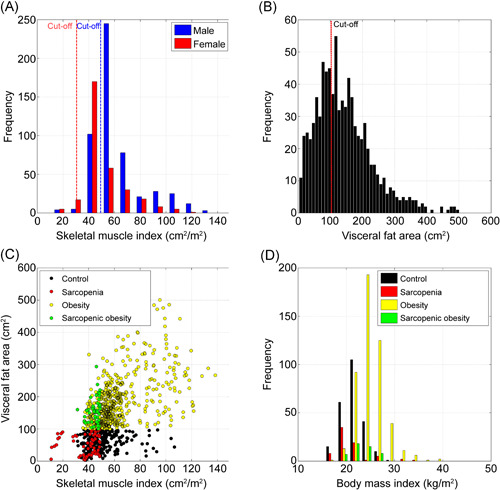
Segmentation and patient groups. (A) Sarcopenia according to skeletal mass index (SMI). (B) Obesity according to visceral fat area (VFA). (C) Scatter plots of VFA and SMI. (D) Distribution of body mass index according to patient group
Table 1.
Baseline patient and tumor characteristics
| Control group (n = 235) | Sarcopenic group (n = 71) | Obese group (n = 486) | Sarcopenic obese group (n = 48) | p a | |
|---|---|---|---|---|---|
| Age (years) | 56.1 (12.6) | 61.9 (12) | 61.7 (10.3) | 66.9 (10) | <0.001 |
| Sex | <0.001 | ||||
| Male | 93 (39.6%) | 62 (87.3%) | 323 (66.5%) | 48 (100%) | |
| Female | 142 (60.4%) | 9 (12.7%) | 163 (33.5%) | 0 (0%) | |
| ASA physical status class | <0.001 | ||||
| I/II | 202 (86.0%) | 46 (64.8%) | 349 (71.8%) | 32 (66.7%) | |
| III/IV | 33 (14.0%) | 25 (35.2%) | 137 (28.2%) | 16 (33.3%) | |
| Body mass index (kg/m2) | 21.4 (2.3) | 20.9 (3.4) | 25 (2.9) | 22.9 (2.1) | <0.001 |
| Prognostic nutritional index | 53.2 (5.7) | 52.3 (5.7) | 54.4 (5.9) | 53.5 (6.5) | 0.006 |
| Neutrophil count | 3607 (1636) | 4151 (1930) | 3996 (2171) | 4005 (992) | 0.054 |
| Extent of gastrectomy | 0.099 | ||||
| Subtotal gastrectomy | 192 (81.7%) | 51 (71.8%) | 403 (82.9%) | 36 (75%) | |
| Total gastrectomy | 43 (18.3%) | 20 (28.2%) | 83 (17.1%) | 12 (25.0%) | |
| R0 resection b | 0.139 | ||||
| R0 | 225 (95.7%) | 67 (94.4%) | 477 (98.1%) | 47 (97.1%) | |
| R1–2 | 10 (4.3%) | 4 (5.6%) | 9 (1.9%) | 1 (2.1%) | |
| TNM stage | 0.087 | ||||
| I | 162 (68.9%) | 45 (63.4%) | 362 (74.5%) | 27 (56.3%) | |
| II | 34 (14.5%) | 9 (12.7%) | 49 (10.1%) | 8 (16.7%) | |
| III | 34 (14.5%) | 14 (19.7%) | 71 (14.6%) | 13 (27.1%) | |
| IV | 5 (2.1%) | 3 (4.2%) | 4 (0.8%) | 0 (0%) | |
| Subcutaneous fat area (cm2) | 117.9 (52.4) | 64 (37.8) | 183.3 (85.0) | 117.4 (34.7) | <0.001 |
| Visceral fat area (cm2) | 63.2 (25.1) | 52.7 (29.3) | 192.3 (77.6) | 156.7 (38.9) | <0.001 |
| Skeletal muscle area (cm2) | 132.6 (38.1) | 107.6 (30.8) | 174.3 (59.4) | 125.4 (12.2) | <0.001 |
| Subcutaneous fat index (cm2/m2) | 46.6 (22.0) | 23.7 (15.1) | 70 (35.7) | 42 (12.5) | <0.001 |
| Visceral fat index (cm2/m2) | 24.7 (10.0) | 19.3 (10.9) | 71.9 (28.3) | 56.2 (14.8) | <0.001 |
| Skeletal muscle index (cm2/m2) | 51.2 (12.8) | 38.8 (10.4) | 64.5 (20.0) | 44.8 (3.9) | <0.001 |
Note: Data are presented as the mean (standard deviation) or number (percentage).
Abbreviations: ASA, American Society of Anesthesiologists; TNM, tumor, nodes, and metastasis.
Analysis of variance for continuous variables; chi‐square test for categorical data.
R0 = curative resection, R1 = microscopic residual cancer, R2 = macroscopic residual cancer.
During a median follow‐up of 41 months, the S group (Figure 4A–C) had significantly worse survival than did the non‐S group for all patients (Figure 4A, p = 0.018) and for patients with stage I/II disease (Figure 4B, p = 0.030). The O group (Figure 4D–F) had significantly poorer survival than did the non‐O group only for patients with stage I/II disease (Figure 4E, p = 0.046). Very few patients died in the non‐O group. The SO group (Figure 4G–I) had significantly poorer survival than did the non‐SO group for all patients (Figure 4G, p < 0.001) and for patients with stage I/II disease (Figure 4H, p < 0.001).
Figure 4.
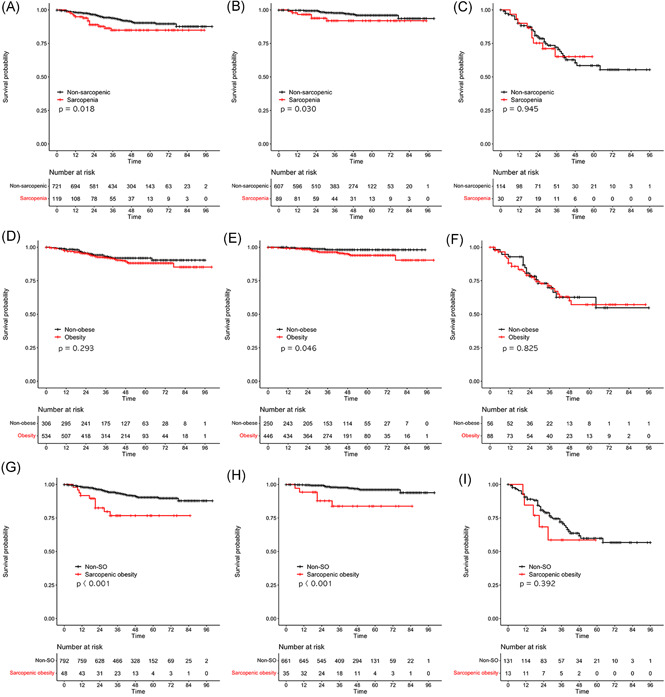
Overall survival according to the sarcopenia, obesity, and sarcopenic obesity groups. (A) Sarcopenia versus non‐sarcopenia: all patients (p = 0.018). (B) Sarcopenia versus non‐sarcopenia: patients with stage I/II disease (p = 0.030). (C) Sarcopenia versus non‐sarcopenia: patients with stage III/IV disease (p = 0.945). (D) Obesity versus non‐obesity: all patients (p = 0.293). (E) Obesity versus non‐obesity: patients with stage I/II disease (p = 0.046). (F) Obesity versus non‐obesity: patients with stage III/IV disease (p = 0.825). (G) Sarcopenic obesity versus non‐sarcopenic obesity: all patients (p < 0.001). (H) Sarcopenic obesity versus non‐sarcopenic obesity: patients with stage I/II disease (p < 0.001). (I) Sarcopenic obesity versus non‐sarcopenic obesity: patients with stage III/IV disease (p = 0.392)
As shown in Table 2, Cox proportional‐hazards multivariate analyses identified the following as independent prognostic factors for increased mortality: greater extent of gastrectomy (HR = 1.928; 95% CI = 1.260–2.950), lower PNI (HR = 0.934; 95% CI = 0.901–0.969), higher neutrophil count (HR = 1.101; 95% CI=1.031–1.176), presence of lymph node metastasis (HR = 6.291; 95% CI = 3.498–11.314), R1/2 resection (HR = 4.817; 95% CI=1.518–9.179), and presence of sarcopenic obesity (HR = 2.608; 95% CI = 1.313–5.179).
Table 2.
Univariate and multivariate analyses of variables associated with overall survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Coefficient (SE) | Hazard ratio (95% CI) | Coefficient (SE) | Hazard ratio (95% CI) | |
| Age | 0.039 (0.012) | 1.040 (1.016–1.064) | ||
| Sex | −0.579 (0.274) | 0.561 (0.327–0.960) | ||
| ASA physical status class (III/IV vs. I/II) | 0.687 (0.254) | 1.988 (1.208–3.272) | ||
| Body mass index | −0.037 (0.039) | 0.964 (0.893–1.041) | ||
| Prognostic nutritional index | −0.110 (0.017) | 0.896 (0.866–0.927) | −0.067 (0.019) | 0.935 (0.901–0.970) |
| Neutrophil count | 0.145 (0.035) | 1.156 (1.079–1.239) | 0.096 (0.034) | 1.100 (1.030–1.175) |
| Extent of gastrectomy (total vs. subtotal) | 1.213 (0.247) | 2.519 (1.776–3.575) | 0.669 (0.254) | 1.952 (1.186–3.212) |
| Resection (R1/2 vs. R0) | 2.606 (0.314) | 13.542 (7.320–25.054) | 1.560 (0.329) | 4.758 (2.496–9.070) |
| Depth of invasion (T3/4 vs. T1/2) | 2.177 (0.266) | 8.821 (5.242–14.845) | ||
| Lymph node metastasis (+ vs. −) | 2.334 (0.286) | 10.318 (5.896–18.056) | 1.826 (0.303) | 6.212 (3.433–11.241) |
| Sarcopenic obesity (SO vs. non‐SO) | 1.201 (0.343) | 3.325 (1.698–6.508) | 0.973 (0.35) | 2.645 (1.333–5.249) |
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; non‐SO, non‐sarcopenic obesity counter group; SE, standard error; SO, sarcopenic obesity group.
4. DISCUSSION
In this study, we assessed the body composition of patients with gastric cancer using preoperative CT. Through algorithms implementing semantic segmentation using transfer learning, the image within the entire CT volume image corresponding to the L3 center was selected, and visceral fat/muscle segmentation for body composition calculation was performed. The quantified VFA and SMA were used to predict patient prognosis. Defining obesity as a VFA > 100 cm2 and sarcopenia as an SMI ≤ 49 cm2/m2 for men and ≤31 cm2/m2 for women identified 48 out of 840 patients with sarcopenic obesity. Patients with sarcopenic obesity had a poorer prognosis than did patients without sarcopenic obesity. This poorer prognosis was primarily observed in patients with stage I/II disease.
This study shows the potential clinical impact of introducing machine learning in patient care by enabling body composition analysis in a large number of patients, which would have been notably difficult using manually derived composition analysis. Segmentation of patients with similar BMI values into more appropriate groups reflecting body composition/nutritional status was possible. As shown in the univariate and multivariate analyses, sarcopenic obesity was a significant prognostic factor for mortality after gastrectomy, independent of other nutritional and clinical parameters, PNI, neutrophil count, or American Society of Anesthesiologists physical status class. Body composition has been used as a reliable and detailed nutritional status indicator in patients with gastric cancer and other types of cancer. 8 , 27 Information obtained from CT images is notably different from laboratory data and body weight information. Weight and laboratory values can change quickly depending on the patient's status, as infection and other acute disorders can produce changes within days or even hours. In contrast, body composition reflects body status over several months or even years and is therefore more resistant to acute changes.
Herein, our criteria successfully identified the SO group as a high‐risk group. More than 90% of non‐SO patients with stage I/II disease survived for over 100 months. Our results suggest that preoperative sarcopenic obesity could be a target for intervention. For this reason, we evaluated various cutoff values for diagnosing sarcopenia and obesity. Obesity defined 25 , 28 as a VFA > 100 cm2 showed the best discriminating power, compared with other cutoff values, such as a VFA > 130 cm2 in men and >90 cm2 in women 29 or >163.8 cm2 in men and >90 cm2 in women. 12 Likewise, the sarcopenia cutoff values of an SMI ≤ 49 cm2/m2 for men and ≤31 cm2/m2 for women yielded optimal results. 26 Other cutoff values, such as an SMI < 52.4 cm2/m2 for men and <38.5 cm2/m2 for women 19 , 27 or <43 cm2/m2 for men with a BMI < 25 kg/m2 and <53 cm2/m2 for men with a BMI ≥ 25 kg/m2 or <41 cm2/m2 for women, 12 also showed survival differences between the SO and non‐SO groups.
The present study sheds light on the underestimated value of CT information in patient care. Preoperatively, body composition determination can be used to predict short‐term surgical outcomes, including operation times, 29 bleeding, 30 number of retrieved lymph nodes, 31 surgical site infections, 32 , 33 and major complications, 28 , 34 which are associated with body shape. 35 , 36 This information may be used to select the type of operative approach, such as open, laparoscopic, or robot‐assisted, during subsequent procedures. 37 , 38 We are currently exploring the usefulness of CT images for predicting postoperative complications, especially pancreatic fistulas, to independently validate the results of a previous study. 39 Postoperatively, the objective monitoring of muscle and fat loss after gastrectomy using follow‐up CT scans may allow appropriate interventions to potentially improve quality of life and survival. 40 , 41
Although this study showed the value of body composition analysis using machine learning based on preoperative CT images, it has some limitations. First, the surgery was performed by a single surgeon. This feature might increase the homogeneity of the surgical technique but it should be considered for the interpretation of the results and generalization. Second, the accuracy and reliability of body composition data require verification in a subsequent study. We plan to assess the variability of quantified areas in serial follow‐up CT scans according to body weight variability. Third, not all of the automatically derived segmentation data could be used. When a contrast enhancer (e.g., contrast media) was used, image contrast was reduced during the image normalization process and segmentation accuracy was decreased; however, we eliminated this problem by processing pixels with HU values other than fat or muscle as background. Nevertheless, when contrast was poor in the original image or when patients had minimal body fat, segmentation accuracy was decreased and could not be corrected by image processing or the algorithm alone. In addition to those limitations, unidentified limitations might have resulted in systematic measurement error, as presented in Figure S1, higher mean values having a higher positive difference. Thus, final assessment by a clinician was necessary to determine whether segmentation was appropriate for predicting long‐term outcomes. These technical limitations should reduce as more investigations are conducted in this field.
5. CONCLUSION
Machine learning algorithms successfully detected preoperative sarcopenic obesity, which was predictive of poorer long‐term survival in patients with gastric cancer. This prognostic value was primarily observed in patients with stage I/II disease. Machine learning‐enabled body composition analysis in a large number of patients (840). Integration of body composition data into clinical practice can be facilitated using machine learning to potentially improve patient care.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SYNOPSIS
We analyzed the preoperative computed tomography (CT) images of 840 gastric cancer patients to assess the impact of sarcopenic obesity on survival. We assumed that sarcopenia—as muscle volume loss—and obesity—as visceral fat accumulation—could be indicators of the nutritional status of patients. The machine learning algorithm automatically selected CT image section of L3 level and retrieved information regarding muscle and visceral fat. Out of 840 patients, 48 (5.7%) were sarcopenic obese and these patients showed poorer survival compared to patients without sarcopenic obesity.
ACKNOWLEDGMENT
This study was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean Government (MSIT) (Nos. 2016R1A2B4014984 and 2019R1H1A2079953).
Kim J, Han SH, Kim H‐I. Detection of sarcopenic obesity and prediction of long‐term survival in patients with gastric cancer using preoperative computed tomography and machine learning. J Surg Oncol. 2021;124:1347‐1355. 10.1002/jso.26668
Jaehyuk Kim and Seung Hee Han contributed equally as co‐first authors of this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Hyoung‐Il Kim, upon reasonable request.
REFERENCES
- 1. GBD 2017 Stomach Cancer Collaborators . The global, regional, and national burden of stomach cancer in 195 countries, 1990‐2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):42‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, MK W. AJCC Cancer Staging Manual. 8th ed. 2‐New York: Springer International Publishing; 2017. [Google Scholar]
- 3. Kiuchi J, Komatsu S, Kosuga T, et al. Long‐term postoperative nutritional status affects prognosis even after infectious complications in gastric cancer. Anticancer Res. 2018;38(5):3133‐3138. [DOI] [PubMed] [Google Scholar]
- 4. Minami Y, Kawai M, Fujiya T, et al. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer. 2015;136(2):411‐424. [DOI] [PubMed] [Google Scholar]
- 5. Feng F, Zheng G, Guo X, et al. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer. 2018;18(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S, Nie RC, OuYang LY, et al. Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jun DH, Kim BJ, Park JH, et al. Preoperative body mass index may determine the prognosis of advanced gastric cancer. Nutr Cancer. 2016;68(8):1295‐1300. [DOI] [PubMed] [Google Scholar]
- 8. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539‐1547. [DOI] [PubMed] [Google Scholar]
- 9. Hu CL, Jin XH, Yuan ZD, et al. Prognostic significance of preoperative skeletal muscle status in patients with gastric cancer after radical gastrectomy. Asia Pac J Clin Nutr. 2019;28(3):442‐449. [DOI] [PubMed] [Google Scholar]
- 10. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997‐1006. [DOI] [PubMed] [Google Scholar]
- 11. Park HS, Kim HS, Beom SH, et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: single‐center study from the CLASSIC trial. Ann Surg Oncol. 2018;25(11):3222‐3230. [DOI] [PubMed] [Google Scholar]
- 12. Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112(4):403‐407. [DOI] [PubMed] [Google Scholar]
- 13. Huang DD, Zhou CJ, Wang SL, et al. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161(3):680‐693. [DOI] [PubMed] [Google Scholar]
- 14. Feng W, Huang M, Zhao X, et al. Severe loss of visceral fat and skeletal muscle after chemotherapy predicts poor prognosis in metastatic gastric cancer patients without gastrectomy. J Cancer. 2020;11(11):3310‐3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang SY, Roh KH, Kim YN, et al. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24(7):1770‐1777. [DOI] [PubMed] [Google Scholar]
- 16. Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence‐based approach. J Gastric Cancer. 2014;14(2):87‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JY, Kim HI, Kim YN, et al. Clinical significance of the prognostic nutritional index for predicting short‐ and long‐term surgical outcomes after gastrectomy: a retrospective analysis of 7781 gastric cancer patients. Medicine. 2016;95(18):e3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Wang WB, Jiang HG, et al. Predictive value of pancreatic dose‐volume metrics on sarcopenia rate in gastric cancer patients treated with adjuvant chemoradiotherapy. Clin Nutr. 2019;38(4):1713‐1720. [DOI] [PubMed] [Google Scholar]
- 20. Yoshikawa K, Shimada M, Kurita N, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy‐assisted gastrectomy for gastric cancer. Surg Endosc. 2011;25(12):3825‐3830. [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Chin HM, Hwang SS, Jun KH. Impact of intra‐abdominal fat on surgical outcome and overall survival of patients with gastric cancer. Int J Surg. 2014;12(4):346‐352. [DOI] [PubMed] [Google Scholar]
- 22. Cody DD. AAPM/RSNA physics tutorial for residents: topics in CT. Image processing in CT. Radiographics. 2002;22(5):1255‐1268. [DOI] [PubMed] [Google Scholar]
- 23. He K, Zhang X, Ren S & Sun J Deep residual learning for image recognition. Paper presented at: IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 2016:770‐778.
- 24. Liang‐Chieh C, Yukun Z, George P, Florian S, Hartwig A. Encoder‐Decoder with Atrous Separable Convolution for Semantic Image Segmentation. Springer; 2018:833‐851. [Google Scholar]
- 25. Ueda J, Ichimiya H, Okido M, Kato M. The impact of visceral fat accumulation on laparoscopy‐assisted distal gastrectomy for early gastric cancer. J Laparoendosc Adv Surg Tech A. 2009;19(2):157‐162. [DOI] [PubMed] [Google Scholar]
- 26. Lee JS, Kim YS, Kim EY, Jin W. Prognostic significance of CT‐determined sarcopenia in patients with advanced gastric cancer. PLOS One. 2018;13(8):e0202700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9(7):629‐635. [DOI] [PubMed] [Google Scholar]
- 28. Takeuchi M, Ishii K, Seki H, et al. Excessive visceral fat area as a risk factor for early postoperative complications of total gastrectomy for gastric cancer: a retrospective cohort study. BMC Surg. 2016;16(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahakyan MA, Shahbazyan SS, Martirosyan A, Gabrielyan A, Petrosyan H, Sahakyan AM. Gastrectomy for gastric cancer in patients with BMI ≥ 30 kg/m². Am Surg. 2020;86(2):158‐163. [PubMed] [Google Scholar]
- 30. Lv T, Beeharry MK, Zhu ZL. Impact of intra‐peritoneal fat distribution on intra‐operative bleeding volume with D2 lymphadenectomy in Chinese patients with gastric cancer. Asian J Surg. 2019;42(7):768‐774. [DOI] [PubMed] [Google Scholar]
- 31. Go JE, Kim MC, Kim KH, Oh JY, Kim YM. Effect of visceral fat area on outcomes of laparoscopyassisted distal gastrectomy for gastric cancer: subgroup analysis by gender and parameters of obesity. Ann Surg Treat Res. 2015;88(6):318‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishigori T, Tsunoda S, Okabe H, et al. Impact of sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann Surg Oncol. 2016;23(Suppl 4):S524‐S531. [DOI] [PubMed] [Google Scholar]
- 33. Olmez T, Gulmez S, Karakose E, et al. Relation between sarcopenia and surgical site infection in patients undergoing gastric cancer surgery. Surg Infect. 2020;22:551‐555. [DOI] [PubMed] [Google Scholar]
- 34. Zhang WT, Lin J, Chen WS, et al. Sarcopenic obesity is associated with severe postoperative complications in gastric cancer patients undergoing gastrectomy: a prospective study. J Gastrointest Surg. 2018;22(11):1861‐1869. [DOI] [PubMed] [Google Scholar]
- 35. Lee JH, Paik YH, Lee JS, et al. Abdominal shape of gastric cancer patients influences short‐term surgical outcomes. Ann Surg Oncol. 2007;14(4):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 36. Ojima T, Iwahashi M, Nakamori M, et al. The impact of abdominal shape index of patients on laparoscopy‐assisted distal gastrectomy for early gastric cancer. Langenbecks Arch Surg. 2012;397(3):437‐445. [DOI] [PubMed] [Google Scholar]
- 37. Makino H, Kunisaki C, Izumisawa Y, et al. Effect of obesity on laparoscopy‐assisted distal gastrectomy compared with open distal gastrectomy for gastric cancer. J Surg Oncol. 2010;102(2):141‐147. [DOI] [PubMed] [Google Scholar]
- 38. Hiki N, Fukunaga T, Yamaguchi T, et al. Increased fat content and body shape have little effect on the accuracy of lymph node retrieval and blood loss in laparoscopic distal gastrectomy for gastric cancer. J Gastrointest Surg. 2009;13(4):626‐633. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka K, Miyashiro I, Yano M, et al. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol. 2009;16(6):1520‐1525. [DOI] [PubMed] [Google Scholar]
- 40. Lim HS, Lee B, Cho I, Cho GS. Nutritional and clinical factors affecting weight and fat‐free mass loss after gastrectomy in patients with gastric cancer. Nutrients. 2020;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gharagozlian S, Mala T, Brekke HK, Kolbjørnsen LC, Ullerud ÅA, Johnson E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer – a cross‐sectional pilot study. Clin Nutr ESPEN. 2020;37:195‐201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Hyoung‐Il Kim, upon reasonable request.


