Abstract
Conditions for inactivating chromosomal genes of Chlorobium tepidum by natural transformation and homologous recombination were established. As a model, mutants unable to perform nitrogen fixation were constructed by interrupting nifD with various antibiotic resistance markers. Growth of wild-type C. tepidum at 40°C on agar plates could be completely inhibited by 100 μg of gentamicin ml−1, 2 μg of erythromycin ml−1, 30 μg of chloramphenicol ml−1, or 1 μg of tetracycline ml−1 or a combination of 300 μg of streptomycin ml−1 and 150 μg of spectinomycin ml−1. Transformation was performed by spotting cells and DNA on an agar plate for 10 to 20 h. Transformation frequencies on the order of 10−7 were observed with gentamicin and erythromycin markers, and transformation frequencies on the order of 10−3 were observed with a streptomycin-spectinomycin marker. The frequency of spontaneous mutants resistant to gentamicin, erythromycin, or spectinomycin-streptomycin was undetectable or significantly lower than the transformation frequency. Transformation with the gentamicin marker was observed when the transforming DNA contained 1 or 3 kb of total homologous flanking sequence but not when the transforming DNA contained only 0.3 kb of homologous sequence. Linearized plasmids transformed at least an order of magnitude better than circular plasmids. This work forms a foundation for the systematic targeted inactivation of genes in C. tepidum, whose 2.15-Mb genome has recently been completely sequenced.
Green sulfur bacteria are strictly anaerobic phototrophs which occur in sulfide-rich aquatic environments (21). They form a coherent phylogenetic group and are not closely related to other bacteria (13). Current areas of interest in this group of bacteria include photosynthetic electron transport (involving the reaction center, cytochromes, quinones, etc.); organization of and energy transfer in the light-harvesting antennae (the FMO protein and chlorosomes); biosynthesis and function of chlorophylls (bacteriochlorophylls a, c, d, and e and chlorophyll a), carotenoids, and isoprenoid quinones; lithotrophic oxidation of sulfur compounds; CO2 fixation (which occurs via the reverse tricarboxylic acid cycle); and other attributes contributing to their ecological and evolutionary significance (2).
Chlorobium tepidum is a moderately thermophilic green sulfur bacterium. It grows rapidly on a defined medium and makes a suitable model for genetic, biochemical, and physiological studies of the green sulfur bacteria (3, 22). The 2.15-Mb genome of C. tepidum has recently been sequenced and reveals about 2,284 open reading frames of which about 50% have been assigned a known function (J. A. Eisen et al., unpublished data).
A powerful way to investigate the function of the genes of an organism is by targeted gene inactivation by homologous recombination (20). The success of such an approach typically depends on the availability of an antibiotic which effectively inhibits the growth of wild-type cells and to which spontaneously resistant mutants are not easily formed, on a selection marker which confers resistance to this antibiotic, and on a method to introduce DNA into the cells. Transformation of Chlorobium species using natural transformation (5, 12), chemical transformation (10), and electroporation (9) has previously been reported. Spectinomycin and streptomycin were the only antibiotics used for selection in these transformations (5, 9, 12). Ampicillin and chloramphenicol resistance markers have also been reported to be useful for selection in conjugation studies of C. tepidum (24). At present, the only genes in any Chlorobium species that have been reported as targets for inactivation encode chlorosomal proteins CsmC and CsmA (5), reaction center cytochrome c551 PscC (9), and Rubisco subunit RbcL (T. E. Hanson and F. R. Tabita, personal communication). Of these, only the csmC (5) and rbcL mutants (T. E. Hanson and F. R. Tabita, personal communication) fully segregated.
C. tepidum is a nitrogen-fixing organism (23), and in the present study the nifD gene, which encodes a subunit of nitrogenase (15), was studied as a general model for gene inactivation. This allowed facile detection of true transformants as mutants that had a known phenotype, namely, the inability to grow diazotrophically. Several antibiotics and resistance markers were tested, and three markers were found to be suitable for use in C. tepidum: a spectinomycin-streptomycin resistance marker, a gentamicin resistance marker, and an erythromycin resistance marker. Natural transformation of C. tepidum was also characterized as a general method for gene inactivation by homologous recombination.
MATERIALS AND METHODS
Organisms and growth conditions.
The strain of C. tepidum used for transformation was WT2321 (24), which is a plating strain derived from C. tepidum strain ATCC 49652 (22). Growth conditions were essentially as previously described (24). All growth and manipulations of C. tepidum were performed in an anaerobic chamber (Coy Laboratory Products, Grass Lake, Mich.) which had an atmosphere of 10% CO2 and 5% H2 balanced with N2. C. tepidum was grown at 40°C throughout the study.
All cloning was carried out with Escherichia coli DH5α grown in Luria-Bertani medium. Antibiotic selection conditions for E. coli were obtained with 100 μg of ampicillin ml−1 combined with 10 μg of gentamicin, 100 μg of spectinomycin, or 10 μg of chloramphenicol ml−1. Ampicillin, gentamicin, and kanamycin were obtained from U.S. Biochemicals (Cleveland, Ohio), and other antibiotics were obtained from Sigma (St. Louis, Mo).
One liter of liquid medium for C. tepidum (CL) was made of 20 ml of salts A (0.64 g of Na2 · EDTA · 2H2O, 10 g of MgSO4 · 7H2O, 2.5 g of CaCl2 · 2H2O, and 20 g of NaCl per liter), 20 ml of salts B (25 g of NH4CH3COO, 20 g of NH4Cl, and 115 g of Na2S2O3 · 5H2O per liter), 20 ml of buffers (25 g of KH2PO4 and 105 g of MOPS [3-{N-morpholino}propanesulfonic acid] per liter), 1 ml of trace elements (22), 50 μl of 10-mg ml−1 resazurin, and 20 μl of 1-mg ml−1 vitamin B12. After the medium was autoclaved at 121°C for 20 min, a freshly made, filter-sterilized solution of 0.6 g of Na2S · 9H2O and 2.0 g NaHCO3 in 50 ml of water was added. The pH of the medium was usually within the desired range of 6.9 to 7.0 without further treatment but otherwise was aseptically adjusted with 1 M NaOH or 1 M HCl.
One liter of plating medium for C. tepidum (CP) made about 25 plates and contained 20 ml of salts A, 20 ml of salts B, 20 ml of buffers, 1 ml of trace elements, 50 μl of 10-mg ml−1 resazurin, 20 μl of 1-mg ml−1 vitamin B12, and 0.36 g of l-cysteine. The pH was adjusted to 7.6 with 10 M NaOH, and 15 g of agar (Bacto Agar; Becton Dickinson, Sparks, Md.; used as supplied) per liter was added before autoclaving. The medium was cooled to 50°C in a water bath before addition of antibiotics and the pouring of the plates. The plates were poured in a cold room to allow rapid solidification and moved to the anaerobic chamber in less than 20 min to prevent excessive oxidation of the cysteine. The final pH of the plates was approximately 6.8 to 7.0. Ammonium-free plating medium (CPNF) for diazotrophic growth was made by substituting salts BNF (26.5 g of NaCH3COO and 115 g of Na2S2O3 · 5H2O per liter) for salts B and leaving out cysteine. Once inoculated, the plates were placed in an anaerobic jar (BBL GasPak 100 system; Becton Dickinson) without palladium catalyst but containing one disposable H2-CO2-generating envelope (BBL GasPak; Becton Dickinson) and a small tube with approximately 0.1 g of thioacetamide; H2S generation from the thioacetamide was activated by adding approximately 1 ml of 1 M HCl. The jars were kept in dim light for 1 to 2 h before transfer to the appropriate temperature and illumination conditions. In addition to ambient illumination, a single 100-W incandescent light bulb was placed 0.4 to 0.8 m from the jars. The jars were always kept inside the anaerobic chamber.
Transformation of C. tepidum.
Unless otherwise stated, the standard protocol for agar plate transformation was as follows. Cells from 100 μl of an overnight culture of C. tepidum in the late exponential growth phase (approximately 3 × 109 to 6 × 109 cells ml−1) were harvested in a microcentrifuge tube, resuspended in 20 μl of CL medium containing 1 μg of DNA, and spotted on a nonselective CP plate in an area with a diameter of 6 to 8 mm. The plate was placed in a jar and kept in the dark for 1 to 2 h prior to incubation in the light at 40°C for 18 to 20 h. The cell patch was then scraped off and suspended in 300 μl of CL. This suspension and dilutions thereof were spread on selective and nonselective CP plates and incubated for 5 to 6 days to allow single colonies to appear. The mutation frequency was calculated as the number of antibiotic-resistant mutants counted on the selective plates divided by the total number of viable cells counted on the nonselective plates. All transformation frequencies represented in the figures and tables represent the means of up to four separate experiments in which the standard deviations were less than 50% of the means.
Preparation of plasmid and genomic DNA.
Plasmids were prepared by alkaline lysis (19). Digested plasmids to be used for transformation were extracted with an equal volume of chloroform-isoamyl alcohol (24:1 by volume), precipitated with 0.1 volume of 3 M ammonium acetate and 1 volume of isopropanol at −20°C, washed with 70% (vol/vol) ethanol, and redissolved in sterile water.
Genomic DNA from C. tepidum and Synechococcus sp. strain PCC 7002 was prepared using an unpublished method developed by Dexter Chisholm (DuPont, Wilmington, Del.). Cells from 5 to 20 ml of culture were harvested and incubated in 500 μl of TES buffer (5 mM Tris, 5 mM EDTA, 50 mM NaCl, pH 8.5) containing 10 mg of lysozyme ml−1 for 0.5 to 1 h at 37°C. Sodium Sarkosyl (50 μl of a 10% [wt/vol] stock solution) was added, and the suspension was extracted twice with 600 μl of buffered phenol. RNase was added to a final concentration of 100 μg ml−1, and the mixture was incubated for 30 min at 37°C. To this solution, NaCl (100 μl of a 5 M stock solution) and hexadecyltrimethylammonium bromide (CTAB; 100 μl of a stock solution of 10% [wt/vol] CTAB–0.7 M NaCl) were added; the mixture was then extracted twice with 600 μl of chloroform-isoamyl alcohol (24:1 by volume). DNA in the aqueous phase was precipitated with 600 μl of isopropanol, washed with 70% (vol/vol) ethanol, and redissolved in sterile water. Genomic DNA for PCR analysis was isolated from cells grown on plates. Genomic DNA for Southern hybridization analysis was isolated from cells grown to the late exponential phase in liquid medium because it appeared to be difficult to completely digest DNA isolated from cells grown on plates.
PCR conditions.
Oligonucleotide primers were designed based on the genomic sequence (J. A. Eisen et al., unpublished data) using MacVector software, version 6.5 (Genetics Computer Group, Madison, Wis.) and synthesized at the Nucleic Acid Facility, The Pennsylvania State University. The PCR conditions were as follows: initially 5 min at 95°C, and then 35 cycles of 1 min at 95°C, 1 min at 58°C, and 2 min at 72°C for PCR products <2 kb, and ultimately 10 min at 72°C. For PCR products of 2 to 3 kb, the elongation time at 72°C was increased to 3 min.
Construction of plasmids.
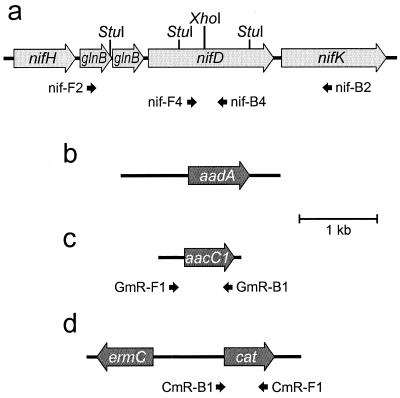
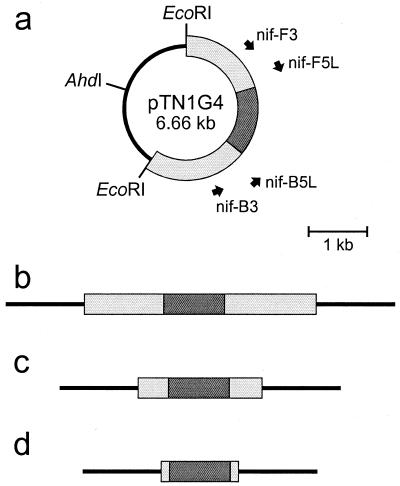
Figure 1a shows a map of a portion of the nif region of C. tepidum based on the complete genomic sequence (J. A. Eisen et al., unpublished data). A 2.93-kb fragment was amplified from genomic DNA using primers nif-F2, 5′-GGAATTCGCGTCGGCGATGTGGTCTAT, and nif-B2, 5′-GGAATTCGTCGGAGGTGTCTGGGAA. (In the primer sequences, heterologous bases are italicized and EcoRI recognition sites are underlined.) Plasmid pTN1 was produced by digesting this PCR product with EcoRI and cloning the product into the EcoRI site of pUC19 (Table 1). Plasmid pTN1G4 was made by inserting the aacC1 cassette from pMS266 (Table 1 and Fig. 1c) into the XhoI site of nifD in pTN1 (Fig. 2a). Similar plasmid constructs with nifD::aacC1 but with shorter flanking regions of C. tepidum DNA were also made: pTN2G1 (primers nif-F3, 5′-GGAATTCAGGGCGTGGTTCTTGGTCC, and nif-B3, 5′-GGAATTCGAGTTCGGCTTTGCTCTTT) and pTN3G11 (primers nif-F5L, 5′-GGGAATTCGCTGGTCACCACATCGCAA, and nif-B5L, 5′-CCATGGAATTCCGTACTTGGTCTC) were made by PCR with pTN1G4 and by cloning the PCR products into the EcoRI site of pUC19 (Fig. 2a and Table 1). pTN1G4, pTN2G1, and pTN3G11 linearized with AhdI were used for transformation and are depicted in Fig. 2b to d. pTN1S3 was made by inserting the aadA cassette from pHP45Ω (Fig. 1b) into the XhoI site of pTN1, and pTN1CE1 was made by inserting the ermC-cat cassette from pRL409 (Fig. 1d) into the XhoI site of pTN1 (Table 1).
FIG. 1.
(a) Map of part of the nif region in C. tepidum; (b) map of the aadA streptomycin-spectinomycin resistance cassette from pHP45Ω; (c) map of the aacC1 gentamicin resistance cassette from pMS266; (d) map of the ermC-cat erythromycin and chloramphenicol resistance cassette from pRL409. The positions of some of the primers discussed in the text are also shown.
TABLE 1.
Plasmids used in this study
| Name | Size (kb) | Relevant characteristics | Phenotypea | Source or reference |
|---|---|---|---|---|
| pMS266 | 3.7 | Contains a 1.1-kb aacC1 gentamicin resistance cassette | Apr Gmr | 1 |
| pHP45Ω | 4.4 | Contains a 2.1-kb aadA streptomycin and spectinomycin resistance cassette | Apr Smr Spr | 16 |
| pRL409 | 5.4 | Contains a 2.8-kb ermC-cat erythromycin and chloramphenicol resistance cassette | Apr Emr Cmr | 7 |
| pTN1 | 5.6 | Derived from pUC19; contains a 2.93-kb fraction of the C. tepidum nif region | Apr | This study |
| pTN1G4 | 6.7 | Similar to pTN1, but nifD is interrupted by the aacC1 cassette from pMS266 | Apr Gmr | This study |
| pTN2G1 | 4.8 | Similar to pTN1G4 but contains only a 1.08-kb fraction of the C. tepidum nif region | Apr Gmr | This study |
| pTN3G11 | 4.0 | Similar to pTN1G4 but contains only a 0.29-kb fraction of the C. tepidum nif region | Apr Gmr | This study |
| pTN1S3 | 7.7 | Similar to pTN1, but nifD is interrupted by the aadA cassette from pHP45Ω | Apr Smr Spr | This study |
| pTN1CE1 | 8.4 | Similar to pTN1, but nifD is interrupted by the ermC-cat cassette from pRL409 | Apr Emr Cmr | This study |
Apr, ampicillin resistant; Gmr, gentamicin resistant; Smr, streptomycin resistant; Spr, spectinomycin resistant; Emr, erythromycin resistant; Cmr, chloramphenicol resistant.
FIG. 2.
(a) Map of plasmid pTN1G4 also showing the positions of some of the primers discussed in the text; (b) AhdI-digested pTN1G4; (c) AhdI-digested pTN2G1; (d) AhdI-digested pTN3G11. Line, pUC19 DNA; light grey, C. tepidum DNA; dark grey, gentamicin resistance marker.
Genomic DNA analysis of C. tepidum transformants.
Detection of wild-type nifD and cassette-interrupted nifD was performed by PCR with primers nif-F4, 5′-CACCACATCGCAAACAAC, and nif-B4 5′-GCAGGAACCTCTTCGGCAATC (Fig. 1a). PCR detection of the aacC1 cassette was performed with primers GmR-F1, 5′-GTGACGCACACCGTGGAAAC, and GmR-B1, 5′-TCCCGTATGCCCAACTTTGTA (Fig. 1c). Southern hybridization was carried out as described previously (19) with [α-32P]dATP-labeled probes (Random Primed DNA labeling kit; Boehringer Mannheim, Indianapolis, Ind.). Hybridizing DNA fragments were detected with a PhosphorImager 445-SI (Molecular Dynamics, Sunnyvale, Calif.). A 413-bp probe for nifD was made by PCR with primers nif-F4 and nif-B4 (Fig. 1a). A 559-bp probe for the ermC-cat cassette was made by PCR with primers CmR-F1, 5′-ACGGGGGCGAAGAAGTTGTC and CmR-B1, 5′-CGGGCGTATTTTTTGAGTTATCG (Fig. 1d). A 1.1-kb probe for aadA was excised from plasmid pSRA2, which contains an aadA cassette derived from pHP45Ω (N.-U. Frigaard and D. A. Bryant, unpublished data).
RESULTS AND DISCUSSION
Antibiotic sensitivity.
Because the antibiotic resistance markers used in this study originate from mesophiles, the temperature used in the transformation experiments was lower than the optimum 47 to 48°C growth temperature of C. tepidum. To test the antibiotic sensitivity of wild-type C. tepidum, cells from 100 μl of a late-exponential culture were plated on CP plates with increasing concentrations of antibiotics and incubated at 40°C. The following concentrations were found to inhibit growth completely, and these concentrations were used throughout the present study: gentamicin, 100 μg ml−1; erythromycin, 2 μg ml−1; chloramphenicol, 30 μg ml−1; tetracycline, 1 μg ml−1. Streptomycin and spectinomycin only efficiently inhibited growth when combined. Therefore, streptomycin (300 μg ml−1) and spectinomycin (150 μg ml−1) were used in combination, since the aadA cassette from pHP45Ω confers resistance to both antibiotics. Spontaneous mutants resistant to gentamicin, erythromycin, chloramphenicol, tetracycline, or streptomycin-spectinomycin were not observed when the wild-type culture was plated on plates with the stated concentration of antibiotic. Kanamycin and ampicillin did not completely inhibit growth on plates at concentrations below 100 μg ml−1.
Previous work (11) with other Chlorobium species suggests that amoxicillin, nalidixic acid, vancomycin, mitomycin C, and colistin might also efficiently inhibit C. tepidum. However, these antibiotics were not tested in this study.
Optimization of transformation.
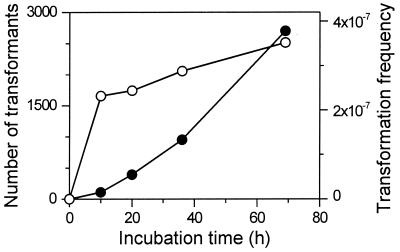
Figure 3 shows the number of transformants and the transformation frequency when C. tepidum cells from a 100-μl late-exponential-phase culture were incubated with 1 μg of AhdI-digested pTN1G4 on a CP plate for various times. The transformation frequency reached about 2 × 10−7 to 3 × 10−7 at 10 h and increased only slightly thereafter. (This corresponds to a yield of approximately 102 transformants per μg of DNA at 10 h.) This suggests that most of the transformation events occurred at the beginning of the experiment (before 10 h) and that the transformation events were stable.
FIG. 3.
Transformation of C. tepidum incubated for various periods. Solid circles, numbers of gentamicin-resistant transformants; open circles, transformation frequencies. See text for details.
Transformation was also attempted in a liquid suspension with the same amount of cells and DNA as described above for transformation on an agar plate. The cells were washed and incubated in 100 μl of fresh CL medium containing 1 μg of DNA for various periods between 1 and 25 h and then plated on selective CP plates. The highest transformation frequency obtained was about an order of magnitude lower than the transformation frequencies obtained on agar plates (data not shown). When the cells and DNA are spotted on a solid agar surface and allowed to dry, the cells and DNA may interact differently than in a liquid suspension. This may allow an increased uptake of DNA by the cells and thus increase the transformation frequency.
Previous work with Chlorobium limicola strain 8327 showed that these cells are competent in both the exponential and stationary growth phases (12). The exact growth state of the cells used for transformation was also not critical in our transformation protocol for C. tepidum. When equal volumes of an overnight culture in the late exponential growth phase and a 4-day-old culture in the stationary growth phase were used in a 20-h transformation, the stationary cells gave about one-half as many transformants as the late-exponential-phase cells (data not shown). In both cases, the spotted cells exhibited visible growth and sulfur formation during the 20-h incubation on nonselective plates.
Transformation with increasing amounts of linearized DNA resulted in an increased transformation frequency (Table 2). Increasing the DNA amount 100-fold from 0.1 to 10 μg only increased the transformation frequency about 3-fold; this suggests that 10 μg of DNA is close to a saturating amount of DNA for the number of cells used. The transformation frequency with 1 μg of linearized plasmid (EcoRI- or AhdI-digested pTN1G4) was about an order of magnitude higher than that for the same amount of circular plasmid (undigested pTN1G4) (data not shown). The reason for this difference is not clear but probably results from the DNA binding and uptake mechanisms of the cells. These observations suggest that at least 1 μg of linearized plasmid is suitable for routine transformation of C. tepidum.
TABLE 2.
Transformation with AhdI-digested pTN1G4 in various amounts
| DNA amt (μg) | Transformation frequencya |
|---|---|
| 0 | 0 |
| 0.1 | (4 ± 1) × 10−8 |
| 1 | (1.0 ± 0.4) × 10−7 |
| 10 | (1.4 ± 0.1) × 10−7 |
Values are means ± standard deviations of two independent experiments.
Effect of variation in length of homologous flanking DNA.
When a plasmid construct is made for gene inactivation by homologous recombination, it is usually advantageous to include a large region of homologous DNA to increase the probability of homologous recombination. However, restriction endonuclease sites and toxic gene products may impose practical restrictions on the length of homologous DNA that can easily be cloned. To determine the effect of the length of homologous flanking DNA on transformation of C. tepidum, three constructs for nifD inactivation were made; these contained a total of 2.93, 1.08, and 0.29 kb of flanking homologous DNA in which the aacC1 gentamicin resistance marker was inserted approximately in the middle (Table 1 and Fig. 2). These constructs were digested with either AhdI, which cuts the plasmids only once and which leaves flanks of pUC19 DNA (Fig. 2), or with EcoRI, which cuts twice and which excises all of the pUC19 DNA (Fig. 2a). The transformation frequencies with 2.93 kb of homologous DNA were similar regardless of whether the plasmid was digested with EcoRI or AhdI (Table 3). But with 1.08 kb of homologous DNA, the transformation frequency was an order of magnitude lower when the plasmid was digested with EcoRI than when the plasmid was digested with AhdI. No transformation was observed with only 0.29 kb of homologous DNA regardless of the enzyme used for linearization. Some bacteria partially degrade absorbed DNA via exonuclease activity (14), and this may be the case for C. tepidum as well. Therefore, it may be advantageous to include dispensable DNA at the ends of the linearized DNA used for transformation. Such exonuclease activity might explain the difference in transformation frequency with pTN2G11 depending on the enzyme used for linearization (Table 3).
TABLE 3.
Transformation with 10 μg of DNA with various lengths of homologous regions
| DNA | Total length of homologous DNA (kb) | Transformation frequencya |
|---|---|---|
| AhdI-digested pTN1G4 | 2.93 | (1.3 ± 0.4) × 10−7 |
| EcoRI-digested pTN1G4 | 2.93 | (4 ± 1) × 10−7 |
| AhdI-digested pTN2G1 | 1.08 | (1.1 ± 0.3) × 10−7 |
| EcoRI-digested pTN2G1 | 1.08 | (7 ± 3) × 10−9 |
| AhdI-digested pTN3G11 | 0.29 | 0 |
| EcoRI-digested pTN3G11 | 0.29 | 0 |
Values are means ± standard deviations of four independent experiments.
These observations suggest that a region of homologous flanking DNA of about 1 kb should be suitable for routine transformation experiments and that it may be advantageous to linearize plasmids with a restriction enzyme that leaves dispensable flanking DNA at the ends of the fragment. Separate fragments of nontransforming DNA produced by an enzyme digest (e.g., the pUC19 vector residue excised by EcoRI from pTN1G4) may be disadvantageous because this nontransforming DNA may compete with the transforming DNA for uptake into the cells. Inhibition of transformation by competing DNA was demonstrated by another observation. Addition of 20 μg of sonicated chromosomal DNA from Synechococcus to a transformation mixture of C. tepidum containing 1 μg of linearized DNA (AhdI-digested pTN1G4) decreased the transformation frequency an order of magnitude (data not shown).
Various selection markers.
Three constructs for nifD inactivation were made with different antibiotic resistance markers, pTN1G4, pTN1S3, and pTN1CE1 (Table 1). The transformation frequencies were about the same when the aacC1 gentamicin resistance marker and the ermC-cat erythromycin-chloramphenicol resistance marker were used (Table 4.) Antibiotic-resistant mutants were only obtained with the ermC-cat marker when erythromycin was used as the selective agent and not when chloramphenicol was used. The obtained Emr mutants were not Cmr even though the marker contains both the cat and ermC genes (Fig. 1d). Southern hybridization analysis confirmed that the cat marker was present in the Emr mutants (see below; Fig. 4). Thus, this cat marker did not function in C. tepidum, probably either because the expressed Cat protein is not functional in C. tepidum or because the cat promoter is too weak in C. tepidum. Our failure with the cat marker is in contrast to the conjugation studies by Wahlund and Madigan (24), who successfully used a cat marker similar to ours for selection in C. tepidum. Previous work with the tetracycline resistance marker (tet) from pBR325 suggested that this marker does not work in C. tepidum (24), and this marker was not investigated further in this study. It is possible that both the tet and cat markers may work in C. tepidum if their promoters are replaced, e.g., with the promoter from ermC in pRL409 or from aacC1 in pMS266 or with a strong indigenous promoter such as that for csmCA (4, 5, 8).
TABLE 4.
Transformation with 10 μg of DNA with different antibiotic resistance markers
| DNA | Drug(s) used for selectionb | Transformation frequencya |
|---|---|---|
| AhdI-digested pTN1G4 | GM | (3 ± 1) × 10−7 |
| AhdI-digested pTN1CE1 | EM | (6 ± 3) × 10−7 |
| AhdI-digested pTN1S3 | SP and SM | (3 ± 1) × 10−3 |
Values are means ± standard deviations of three independent experiments.
GM, gentamicin; EM, erythromycin; SP, spectinomycin; SM, streptomycin.
FIG. 4.
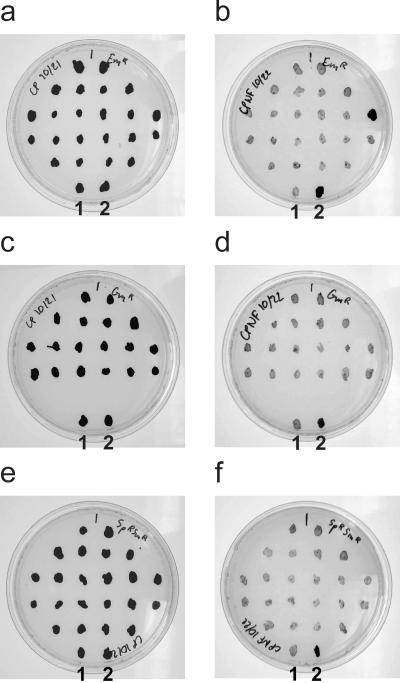
Growth of wild-type C. tepidum and antibiotic-resistant transformants spotted on nonselective CP plates (a, c, and e) and on nonselective CPNF plates (b, d, and f). (a and b) 22 Emr transformants; (c and d) 18 Gmr transformants; (e and f) 22 Smr/Spr transformants. As controls, wild-type C. tepidum (labeled 2) and a confirmed Nif− transformant (labeled 1) were also spotted on all plates.
A transformation frequency 4 orders of magnitude higher than that obtained with the aacC1 and ermC markers was observed with the aadA marker (Table 4). Analysis of the obtained mutants resistant to the combination of streptomycin and spectinomycin (Smr/Spr mutants) showed that all of 22 mutants analyzed were incapable of diazotrophic growth and that nifD therefore was inactivated (see below). The reason for this increased transformation frequency is not clear, but the genomic sequence of C. tepidum may hold some clues. In contrast to the aacC1 and ermC-cat markers, the aadA marker from pHP45Ω contains a 59-bp recombinational hot spot immediately downstream of aadA (17). This hot spot is recognized by IntI-like integrases (6, 18), and the C. tepidum genome contains an integrase (CT0176) with high homology to this class of enzymes (J. A. Eisen et al., unpublished data). The hot spot in the aadA marker and the indigenous integrase CT0176 in C. tepidum may be related to the high transformation frequency of C. tepidum with aadA-containing plasmid pTN1S3. However, regions of C. tepidum DNA were necessary to obtain high transformation frequencies with the aadA marker. Transformation with a linear DNA fragment encoding only the aadA marker (PstI-digested pHP45Ω) resulted in a transformation frequency of only approximately 3 × 10−8, which is 5 orders of magnitude lower than the transformation frequency obtained with linearized pTN1S3 (Table 4). The C. tepidum genome also contains a gene (encoding CT1017) with a downstream 59-bp recombinational hot spot (J. A. Eisen et al., unpublished data). CT1017 has no significant sequence similarity with any protein in GenBank, but the gene seems to encode a cytoplasmic protein and could be a novel antibiotic resistance-encoding gene (18).
Test of transformants.
The expected phenotype of the C. tepidum nifD transformants is the inability to reduce dinitrogen. Several mutants obtained by transformation with the three different markers (Table 4) were transferred to selective CP plates three times and then transferred to nonselective CP and nonselective CPNF plates to check for diazotrophic growth (Fig. 4). Wild-type C. tepidum grew on CPNF plates although slightly slower than on CP plates. All Gmr mutants (23 tested), all Smr/Spr mutants (22 tested), and nearly all Emr mutants (23 out of 24 tested) failed to grow on the CPNF plates. These results confirm that the mutants had lost the ability to perform nitrogen fixation and that the mutations had segregated completely.
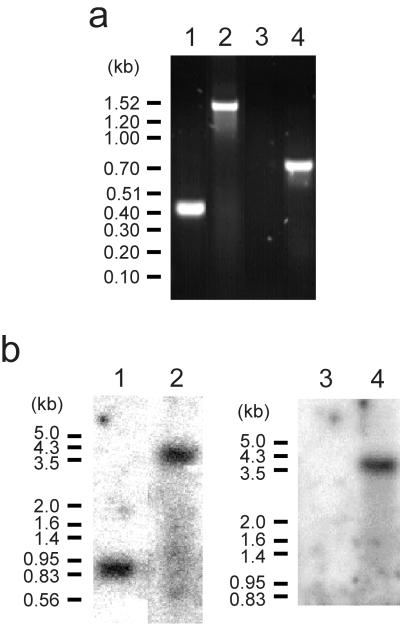
Genomic DNA was isolated from five Gmr mutants and analyzed by PCR. The results from one of the mutants are shown in Fig. 5a. As expected, PCR with primers specific for nifD (nif-F4 and nif-B4) amplified a 0.41-kb fragment in the wild type and a 1.46-kb fragment in the mutants. PCR with primers specific for aacC1 (GmR-F1 and GmR-B1) did not produce a PCR product in the wild type but amplified a 0.75-kb fragment in the mutants.
FIG. 5.
(a) PCR analysis of wild-type C. tepidum (lanes 1 and 3) and a Gmr mutant (lanes 2 and 4). Primers specific for nifD, nif-F4 and nif-B4, were used in lanes 1 and 2, and primers specific for the gentamicin resistance cassette (accC1), GmR-F1 and GmR-B1, were used in lanes 3 and 4. (b) Southern hybridization analysis of wild-type C. tepidum (lanes 1 and 3) and an Emr mutant (lanes 2 and 4). A probe specific for nifD was used in lanes 1 and 2, and a probe specific for the erythromycin-chloramphenicol resistance cassette (ermC-cat) was used in lanes 3 and 4.
Genomic DNA was also isolated from three Emr Nif− mutants and analyzed by PCR and Southern hybridization. As expected, PCR with primers nif-F4 and nif-B4 did not produce a 0.41-kb fragment in the mutants. PCR with primers specific for cat (CmR-F1 and CmR-B1) did not produce a PCR product in the wild type but amplified a 0.56-kb fragment in the mutants. PCR with primers CmR-B1 and nif-B4 did not produce a PCR product in the wild type but amplified a 1.3-kb fragment in the mutants, which confirms the insertion of the ermC-cat marker in nifD in the expected orientation. Figure 5b shows a Southern hybridization analysis of StuI-digested genomic DNA from an Emr Nif− mutant. As expected, a nifD probe hybridized with a 0.9-kb fragment in the wild type and with a 3.6-kb fragment in the mutant. A cat probe did not hybridize with wild-type genomic DNA but hybridized with a 3.6-kb fragment in the mutant. PCR analysis of genomic DNA isolated from the only Emr mutant that exhibited diazotrophic growth showed the presence of wild-type nifD using primers nif-F4 and nif-B4 and the absence of cat using primers CmR-F1 and CmR-B1. This Emr Nif+ mutant could therefore represent a spontaneously resistant mutant or an Emr transformant produced by an illegitimate recombination event.
Six Smr/Spr transformants were likewise analyzed by digesting genomic DNA with StuI and performing a Southern hybridization analysis (data not shown). As expected, the nifD probe hybridized with a 0.9-kb fragment in the wild type and with a 2.9-kb fragment in the mutants. The aadA probe did not hybridize with wild-type genomic DNA but hybridized with a 2.9-kb fragment in all six transformants.
Conclusion.
Genes in C. tepidum can be insertionally inactivated by natural transformation and homologous recombination. Markers for resistance to gentamicin (aacC1 from pMS266), erythromycin (ermC from pRL409), and streptomycin-spectinomycin (aadA from pHP45Ω) were successfully used in the present study to inactivate nifD. The aadA marker gave a significantly higher transformation yield than the two other markers. We suggest the following general guidelines for routine gene inactivation by natural transformation: (i) cells from at least 100 μl of a late-exponential liquid culture should be used; (ii) linearized DNA (1 to 10 μg) with sequences of at least 0.5 kb of homologous DNA flanking each side of the selection marker should be used; (iii) transforming cells should be spotted on an agar surface and incubated for 10 to 20 h at 40°C; shorter incubation times can probably be used, especially if the incubation temperature is higher. Finally, in its simplest form, transformation may be performed by scraping cells off a plate and incubating a mixture of these cells and transforming DNA on a nonselective plate overnight. The cells should then be restreaked on selective plates the next day.
ACKNOWLEDGMENTS
N.-U.F. was supported by The Danish Natural Science Research Council. This work was supported by U.S. Department of Energy grant DE-FG02–97ER20137 to D.A.B.
We thank the Institute of Genomic Research for prepublication access to the genome sequence of C. tepidum.
REFERENCES
- 1.Becker A, Schmidt M, Jäger W, Pühler A. New gentamicin-resistance and lacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Gene. 1995;162:37–39. doi: 10.1016/0378-1119(95)00313-u. [DOI] [PubMed] [Google Scholar]
- 2.Blankenship R E, Madigan M T, Bauer C E. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. [Google Scholar]
- 3.Castenholz R W, Bauld J, Jørgensen B B. Anoxygenic microbial mats of hot springs: thermophilic Chlorobium sp. FEMS Microbiol Ecol. 1990;74:325–336. [Google Scholar]
- 4.Chung S, Frank G, Zuber H, Bryant D A. Genes encoding two chlorosome components from the green sulfur bacteria Chlorobium vibrioforme strain 8327D and Chlorobium tepidum. Photosynth Res. 1994;41:261–275. doi: 10.1007/BF02184167. [DOI] [PubMed] [Google Scholar]
- 5.Chung S, Shen G, Ormerod J, Bryant D A. Insertional inactivation studies of the csmA and csmC genes of the green sulfur bacterium Chlorobium vibrioforme 8327: the chlorosome protein CsmA is required for viability but CsmC is dispensable. FEMS Microbiol Lett. 1998;164:353–361. doi: 10.1111/j.1574-6968.1998.tb13109.x. [DOI] [PubMed] [Google Scholar]
- 6.Collis C M, Kim M-J, Stokes H W, Hall R M. Binding of the purified integron DNA integrase IntI1 to integron- and cassette-associated recombination sites. Mol Microbiol. 1998;29:477–490. doi: 10.1046/j.1365-2958.1998.00936.x. [DOI] [PubMed] [Google Scholar]
- 7.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 8.Gruber T M, Bryant D A. Characterization of the group 1 and group 2 sigma factors of the green sulfur bacterium Chlorobium tepidum and the green nonsulfur bacterium Chloroflexus aurantiacus. Arch Microbiol. 1998;170:285–296. doi: 10.1007/s002030050644. [DOI] [PubMed] [Google Scholar]
- 9.Kjærulff S, Diep D B, Okkels J S, Scheller H V, Ormerod J G. Highly efficient integration of foreign DNA into the genome of the green sulfur bacterium Chlorobium vibrioforme by homologous recombination. Photosynth Res. 1994;41:277–283. doi: 10.1007/BF02184168. [DOI] [PubMed] [Google Scholar]
- 10.Méndez-Alvarez S, Pavón V, Esteve I, Guerrero R, Gaju N. Transformation of Chlorobium limicola by a plasmid that confers the ability to utilize thiosulfate. J Bacteriol. 1994;176:7395–7397. doi: 10.1128/jb.176.23.7395-7397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogales B, Guerrero R, Esteve I. Susceptibility of various purple and green sulfur bacteria to different antimicrobial agents. FEMS Microbiol Lett. 1994;123:37–42. doi: 10.1111/j.1574-6968.1994.tb07198.x. [DOI] [PubMed] [Google Scholar]
- 12.Ormerod J G. Natural genetic transformation in Chlorobium. In: Olson J M, Ormerod J G, Amesz J, Stackebrandt E, Trüper H G, editors. Green photosynthetic bacteria. New York, N.Y: Plenum Press; 1988. pp. 315–319. [Google Scholar]
- 13.Overmann J, Tuschak C. Phylogeny and molecular fingerprinting of green sulfur bacteria. Arch Microbiol. 1997;167:302–309. doi: 10.1007/s002030050448. [DOI] [PubMed] [Google Scholar]
- 14.Palmen R, Hellingwerf K J. Uptake and processing of DNA by Acinetobacter calcoaceticus—a review. Gene. 1997;192:179–190. doi: 10.1016/s0378-1119(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 15.Postgate J. Nitrogen fixation. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1998. [Google Scholar]
- 16.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 17.Prentki P, Binda A, Epstein A. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene. 1991;103:17–23. doi: 10.1016/0378-1119(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 18.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Thiel T. Genetic analysis of cyanobacteria. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 581–611. [Google Scholar]
- 21.Trüper H G, Pfennig N. The family Chlorobiaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3583–3592. [Google Scholar]
- 22.Wahlund T M, Woese C R, Castenholz R W, Madigan M T. A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch Microbiol. 1991;156:81–90. [Google Scholar]
- 23.Wahlund T M, Madigan M T. Nitrogen fixation by the thermophilic green sulfur bacterium Chlorobium tepidum. J Bacteriol. 1993;175:474–478. doi: 10.1128/jb.175.2.474-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlund T M, Madigan M T. Genetic transfer by conjugation in the thermophilic green sulfur bacterium Chlorobium tepidum. J Bacteriol. 1995;177:2583–2588. doi: 10.1128/jb.177.9.2583-2588.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]