Abstract
This 36‐week, open‐label, single‐arm, phase 3 study investigated the safety and efficacy of molidustat in Japanese patients with renal anemia undergoing peritoneal dialysis. Molidustat was titrated every 4 weeks to maintain Hb levels within the target range (≥11.0 and <13.0 g/dL). The primary efficacy outcome was the responder rate, defined as the proportion of patients who met all of the following criteria: (1) mean Hb levels in the target range during the evaluation period (Weeks 30–36); (2) ≥50% of Hb values within the target range during the evaluation period; and (3) no rescue treatment before the end of the evaluation period. Overall, 51 patients received molidustat. The responder rate (95% CI) during the evaluation period was 54.9% (40.3, 68.9). Overall, 98.0% of patients experienced at least 1 adverse event during the study. No deaths were reported. Molidustat maintained Hb levels in the prespecified range in more than half of the patients and was well tolerated.
Keywords: anemia, erythropoiesis, renal dialysis, renal insufficiency
1. INTRODUCTION
CKD is a progressive condition characterized by the permanent partial loss of kidney function (1). People with advanced CKD are at high risk of developing end‐stage kidney disease (ESKD), which requires treatment with dialysis or a kidney transplant (2, 3, 4). In Japan at the end of 2018, 339 841 patients were undergoing dialysis, and 2.8% (9445) received peritoneal dialysis (PD), including PD plus HD combination therapy (5). CKD can reduce the ability of the kidneys to produce erythropoietin (EPO), resulting in decreased levels of red blood cells, and consequently leading to anemia (6).
The risk of anemia increases as glomerular filtration rate declines; approximately 32% of patients with stages 3–5 CKD are affected (6) and the prevalence increases to 50%–80% in individuals with ESKD (7, 8). Anemia has been defined by the Japanese Society for Dialysis Therapy as a Hb level <13.5 g/dL in men younger than 60 years old and <11.5 g/dL in women younger than 60 years old (9). Current standard of care for anemia due to CKD, also known as renal anemia, consists of erythropoiesis‐stimulating agents (ESAs), often accompanied by iron supplementation. Guidelines for the treatment of anemia in Japanese patients with CKD receiving PD state that Hb levels should be maintained in the range 11–13 g/dL, and that ESA doses should be reduced or discontinued if the Hb level exceeds 12 g/dL (9). However, ESAs fail to increase Hb levels in 10%–20% of patients and have been associated with several safety concerns, such as increased risks of cardiovascular events and stroke (1, 10, 11, 12). Consequently, novel treatment strategies for renal anemia are needed.
Molidustat is an orally bioavailable hypoxia‐inducible factor prolyl hydroxylase (HIF–PH) inhibitor that might offer such an alternative. In response to hypoxia, EPO transcription is activated by hypoxia‐inducible factors (HIFs), whereas, in the presence of oxygen, the HIF‐α subunit is hydroxylated by HIF–PH and subsequently degraded, preventing EPO synthesis (13). Inhibition of HIF–PH results in stabilization of HIF, which triggers endogenous production of EPO and, ultimately, erythropoiesis (14). It has been previously demonstrated that molidustat increases circulating levels of EPO close to the normal physiological range (15, 16). Following positive results from the Phase 2 DIALOGUE (DaIly orAL treatment increasing endOGenoUs EPO) clinical program (17, 18), the MIYABI (MolIdustat once dailY improves renal Anemia By Inducing EPO) program consisting of five phase 3 trials was designed to investigate the efficacy and safety of molidustat in Japanese patients with renal anemia with and without dialysis (19, 20, 21). Here, we present the results of the MIYABI PD study, conducted to evaluate the efficacy and safety of molidustat in Japanese patients with renal anemia undergoing PD who were treated or not treated with ESAs.
2. MATERIALS AND METHODS
2.1. Study design
The design of the MIYABI PD study (ClinicalTrials.gov identifier: NCT03418168) has been described previously (19). Briefly, it was a 36‐week, open‐label, single‐arm, multicenter, phase 3 trial investigating the efficacy and safety of molidustat in patients with renal anemia undergoing PD who were treated or not treated with ESAs. Study visits took place every 2 weeks for the first 8 weeks (Weeks 0–8), every 4 weeks until Week 28, and every 2 weeks from Week 28 and during the evaluation period (Weeks 30–36), followed by a 4‐week follow‐up period. Efficacy was assessed after 30, 32, 34, and 36 weeks of treatment (Figure 1).
FIGURE 1.
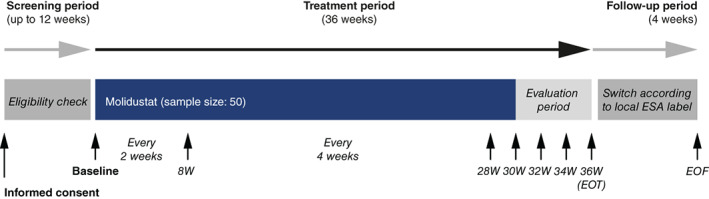
Study design. EOF, end of follow‐up; EOT, end of treatment; ESA, erythropoiesis‐stimulating agent; W, week
The study protocol was approved by the institutional review board and ethics committee for the participating centers (Table S1). The study was conducted according to Good Clinical Practice guidance and the principles detailed in the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from each participant before any study‐related procedures were conducted. Adverse events of special interest (i.e., death, myocardial infarction, unstable angina pectoris, stroke [ischemic, hemorrhagic, and undetermined], transient ischemic attack, pulmonary embolism, or acute limb ischemia) were monitored and outcomes were adjudicated by the relevant independent committees (Table S2).
2.2. Study population
The target population was 50 patients with a prerandomization Hb level ≥8.0 and <11.0 g/dL for patients not treated with ESAs and ≥10.0 and <13.0 g/dL for those receiving ESA therapy. Men and women aged at least 20 years with a diagnosis of renal anemia and ESKD who were undergoing PD and who were not expected to start maintenance dialysis (e.g., HD, hemodiafiltration) were eligible to participate in the study. Key exclusion criteria included previous and concurrent indications for removal of a dialysis catheter and any current condition leading to significant blood loss. A complete list of patient eligibility criteria is presented in Table S3.
2.3. Treatment
Patients eligible for inclusion received molidustat orally once daily at a starting dose of 75 mg. Doses were titrated individually at visits every 4 weeks based on the patient's Hb response to the previous dose to maintain the Hb level within the target range of ≥11.0 and <13.0 g/dL. Oral doses of molidustat were available in multiple doses of 5, 12.5, 25, and 50 mg tablets. Patients with iron, folate, or Vitamin B12 deficiency were treated before study enrollment and during the study. Supplements were administered at the investigator's discretion in line with current guidelines. During the treatment period, iron supplements were administered orally to achieve serum ferritin levels of ≥100 ng/mL or transferrin saturation of ≥20%, in line with Japanese guidelines (22). Rescue treatment (i.e., a red blood cell transfusion to treat renal anemia or any ESA treatment owing to lack of efficacy) could be administered at any time during the study period at the investigator's discretion.
2.4. Outcomes
The primary efficacy outcome was the responder rate, defined as the proportion of patients who met all of the following criteria: (1) mean Hb level within the target range of ≥11.0 and <13.0 g/dL during the evaluation period; (2) ≥50% of Hb levels within the target range during the evaluation period; and (3) no rescue treatment received up to the end of the evaluation period. Other efficacy outcomes included mean Hb level during the evaluation period and change in mean Hb level during the evaluation period from baseline, Hb level at each visit, and the proportion of patients whose Hb level (one valid Hb level was required) was in, above, or below the target range during the evaluation period and at each visit. Parameters of iron metabolism were also investigated.
Primary safety variables assessed by the investigator during the 36 weeks of treatment were treatment‐emergent adverse events (TEAEs), coded using the Medical Dictionary for Regulatory Activities Version 22.0. The incidence of major adverse cardiovascular events (MACEs) adjudicated by a specialist was also assessed. MACEs were defined as cardiovascular or undetermined death, myocardial infarction, unstable angina pectoris, ischemic stroke (ischemic stroke or ischemic stroke with hemorrhagic transformation), pulmonary thromboembolism, and acute limb ischemia.
2.5. Statistical analysis
Analyses of efficacy and safety were performed in the full analysis set (FAS) and safety analysis set (SAF), respectively. The FAS comprised all patients who had at least one baseline Hb level measurement before the first dose of study drug. The SAF included all patients who received at least one dose of study drug. For sensitivity analyses, the responder rate was also analyzed in the per protocol set (PPS), which included all patients from the FAS who had at least 2 central Hb levels collected at Visits 11, 12, 13, or at the end of treatment (EOT) visit/premature discontinuation visit and showed no validity findings that may have affected efficacy. The baseline Hb level was defined as the mean of the last two Hb values obtained during the screening period and the Hb level at Week 0 (baseline visit). The mean Hb level during the evaluation period was calculated using central Hb values recorded at scheduled visits during the evaluation period from Week 30 to Week 36 (i.e., at Visits 11, 12, and 13, and at the EOT). If rescue treatment was started before Week 34, the most recent Hb value (central or local) recorded before the start of rescue treatment was used as the mean Hb level during the evaluation period. If fewer than two valid central Hb levels were obtained during the evaluation period, postbaseline Hb levels were used for imputation until one valid determination was available at each visit; postbaseline Hb levels were used in the following order of priority: (1) the local Hb level at the scheduled visit; (2) the latest unscheduled central Hb level in the week prior to the scheduled visit; and (3) the latest unscheduled local Hb level in the week prior to the scheduled visit. If fewer than two Hb levels during the evaluation period were obtained after imputation, criterion (1) or (2) was considered unmet (i.e., the patient was a nonresponder) according to the definition. All variables were analyzed descriptively. For the proportion of responders, two‐sided 95% CIs were estimated by the Clopper–Pearson exact method. The statistical evaluation was performed using SAS software release 9.2 or higher (SAS Institute Inc., Cary, NC).
3. RESULTS
3.1. Patient disposition and baseline characteristics
In total, 68 Japanese patients were enrolled in the study, 51 of whom received molidustat. Of these, 36 (70.6%) completed treatment (Figure 2). Of the 15 patients who did not complete treatment, 9 (17.6%) discontinued because of a TEAE, 4 (7.8%) discontinued following the investigator's decision to initiate rescue treatment, and 2 (3.9%) progressed to maintenance dialysis other than PD. Most patients (49/51; 96.1%) had previously received ESAs. The mean (SD) patient age was 63.3 (11.3) years, the mean (SD) body weight of participants was 62.4 (11.5) kg, and 62.7% were men (Table 1). The mean (SD) baseline central Hb level was 11.19 (0.82) g/dL and the mean (SD) duration of PD was 2.8 (2.2) years.
FIGURE 2.
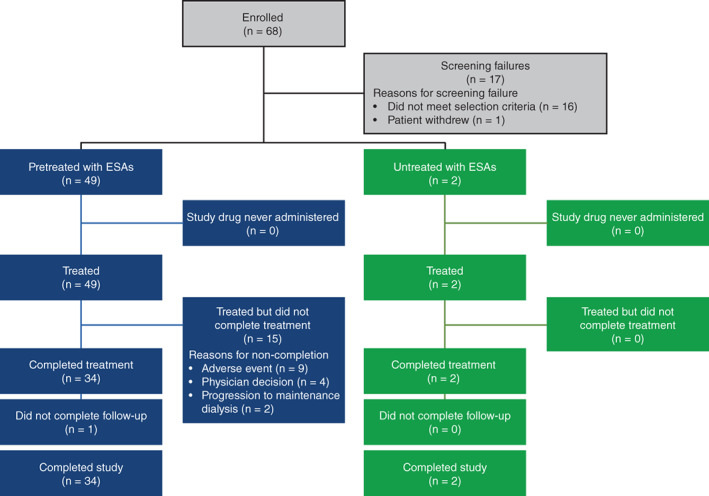
Patient disposition. Completed study: completed both, treatment and follow‐up. ESA, erythropoiesis‐stimulating agent
TABLE 1.
Patient demography and baseline characteristics (full analysis set)
| Parameter | Previously treated with ESA (n = 49) | Not previously treated with ESA (n = 2) | Total (N = 51) |
|---|---|---|---|
| Men, n (%) | 31 (63.3) | 1 (50.0) | 32 (62.7) |
| Age, years | 63.0 (11.4) | 71.0 (7.1) | 63.3 (11.3) |
| Weight, kg | 62.5 (11.4) | 60.6 (19.1) | 62.4 (11.5) |
| BMI, kg/m2 | 24.09 (3.73) | 23.05 (3.89) | 24.05 (3.71) |
| Baseline central Hb level, g/dL | 11.21 (0.83) | 10.55 (0.21) | 11.19 (0.82) |
| Prior thromboembolic event, n (%) | 5 (10.2) | 0 | 5 (9.8) |
| Smoking history, n (%) | |||
| Never | 20 (40.8) | 2 (100.0) | 22 (43.1) |
| Former | 25 (51.0) | 0 | 25 (49.0) |
| Current | 4 (8.2) | 0 | 4 (7.8) |
| SBP, mmHg | 140.6 (17.8) | 131.0 (0.0) | 140.2 (17.6) |
| DBP, mmHg | 82.0 (14.1) | 75.5 (0.7) | 81.7 (13.9) |
| Total iron, μg/dL | 90.2 (32.4) | 103.5 (58.7) | 90.7 (32.9) |
| Ferritin level, ng/mL | 167.8 (122.5) | 209.4 (225.7) | 169.5 (124.4) |
| Transferrin saturation, % | 36.7 (14.2) | 41.1 (28.0) | 36.9 (14.5) |
| Vitamin B12 level, pmol/L | 372.0 (164.2) | 320.6 (143.5) | 370.0 (162.4) |
| Folate level, nmol/L | 219.7 (642.5) | 14.0 (0.3) | 211.7 (630.9) |
| Serum CRP level, mg/dL | 0.190 (0.334) | 0.030 (0.014) | 0.184 (0.329) |
| Main cause of CKD, n (%) | |||
| Immunoglobulin A nephropathy | 3 (6.1) | 0 | 3 (5.9) |
| Chronic glomerulonephritis | 14 (28.6) | 0 | 14 (27.5) |
| Diabetic nephropathy | 14 (28.6) | 0 | 14 (27.5) |
| Nephrosclerosis | 11 (22.4) | 1 (50.0) | 12 (23.5) |
| Polycystic kidney disease | 2 (4.1) | 0 | 2 (3.9) |
| Unknown | 5 (10.2) | 1 (50.0) | 6 (11.8) |
| Duration of CKD, years | 10.7 (8.2) | 3.7 (0.9) | 10.4 (8.1) |
| Duration of peritoneal dialysis, years | 2.8 (2.2) | 2.2 (0.8) | 2.8 (2.2) |
Note: Calculated percentages are subject to rounding. Data presented are mean (SD) unless otherwise stated.
Abbreviations: DBP, diastolic blood pressure; ESA, erythropoiesis‐stimulating agents; SBP, systolic blood pressure.
3.2. Treatment exposure
Over the 36 weeks of treatment, the mean (SD) treatment duration was 200.8 (81.0) days; most patients (68.6%) were treated for ≥224 days. Treatment exposure ended before the evaluation period in 31.4% of participants. The starting dose of molidustat was 75 mg in all patients and the mean (SD) dosage was 93.82 (41.55) mg/day. Figure S1 summarizes the mean actual dosage of molidustat at each study visit. The most common maximum dose was 200 mg (16 patients [31.4%]), followed by 75 mg (13 patients [25.5%]), 100 mg (12 patients [23.5%]), and 150 mg (10 patients [19.6%]). Dose adjustments were required in 50 patients (98.0%).
3.3. Efficacy outcomes
The responder rate (95% CI) in the FAS during the evaluation period was 54.9% (40.3, 68.9) (Figure 3). The proportion (95% CI) of patients with a mean Hb level during the evaluation period within the target range of ≥11.0 and <13.0 g/dL (Criterion 1) was 54.9% (40.3, 68.9), and for 58.8% (44.2, 72.4) of patients, ≥50% of Hb values were within the target range during the evaluation period (Criterion 2). Most patients (92.2% [95% CI: 81.1, 97.8]) did not receive rescue treatment (Criterion 3). The responder rate in the PPS during the evaluation period was 75.7% (56.7, 87.5) (Table S4).
FIGURE 3.
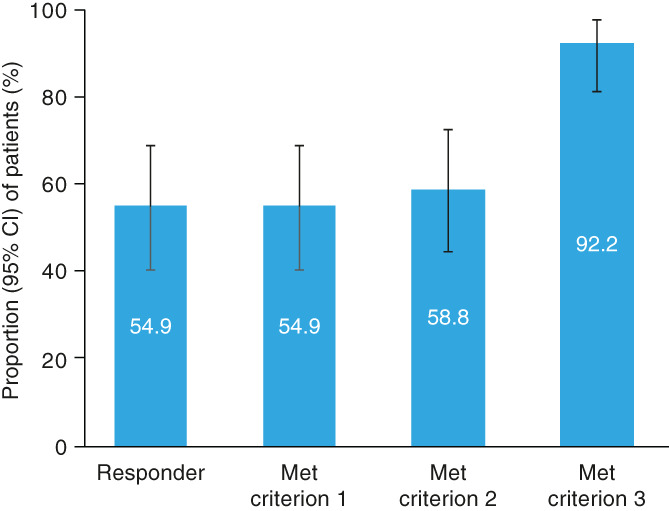
Responder rate (full analysis set). Criterion 1: Mean Hb level within the target range of ≥11.0 and <13.0 g/dL during the evaluation period. Criterion 2: ≥50% of Hb levels in the target range during the evaluation period. Criterion 3: No rescue treatment received up to the end of the evaluation period
The mean (95% CI) for mean Hb level during the evaluation period was 11.18 (10.83, 11.54) g/dL and the mean (95% CI) for change in mean Hb level during the evaluation period from baseline was 0.00 (−0.41, 0.41) g/dL (Figure 4). The mean Hb level was maintained in the target range of ≥11.0 and <13.0 g/dL during the evaluation period and more broadly from Week 12 to Week 36 (Figure 4). The proportions of patients whose mean Hb level during the evaluation period was in, above, or below the target range were 66.7%, 2.0%, and 31.4%, respectively. Overall, the proportion of patients whose mean Hb level was in the target range of ≥11.0 and <13.0 g/dL increased from baseline to Week 36 (Figure S2). After treatment assignment, the proportion of patients who received rescue treatment was 7.8% (4/51 patients). The mean (SD) change in total iron from baseline to Week 36 was −7.7 (36.9) μg/dL (Figure 5). Other parameters of iron metabolism over the treatment period are presented in Figure 5.
FIGURE 4.
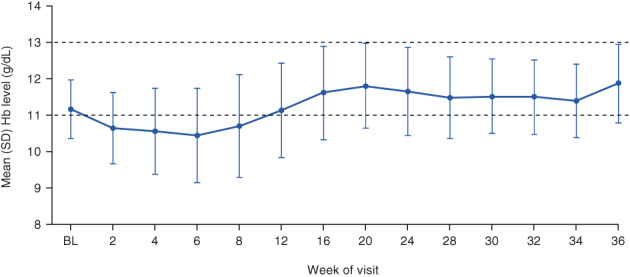
Mean (SD) Hb levels at study visits (full analysis set). Baseline Hb level was defined as the mean of the last two Hb levels during the screening period and the Hb level at Week 0. Dashed lines represent the boundaries of the target range. BL, baseline
FIGURE 5.
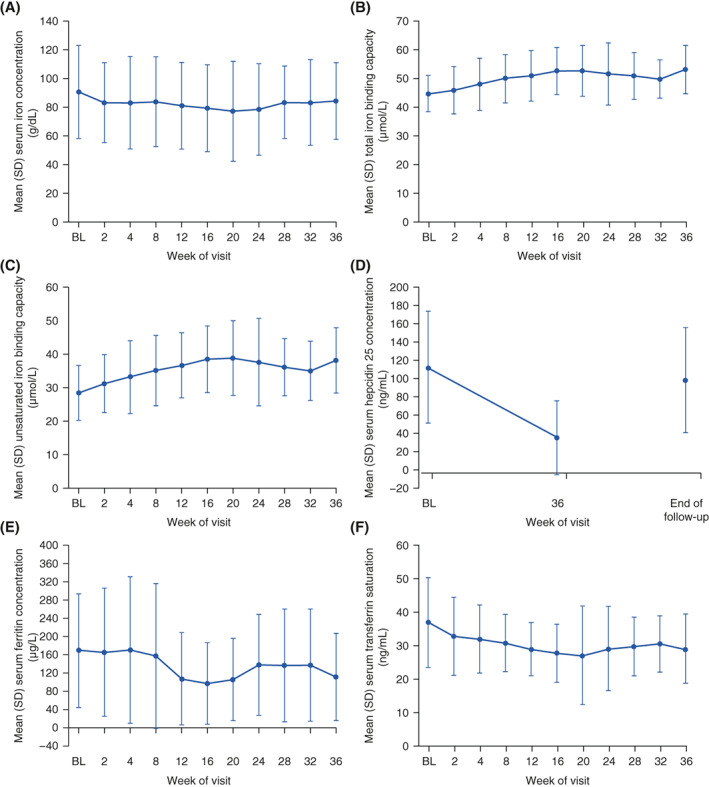
Mean (SD) (A) serum iron concentration, (B) total iron binding capacity, (C) unsaturated iron binding capacity, (D) serum hepcidin 25 concentration, (E) serum ferritin concentration, and (F) serum transferrin saturation
3.4. Safety
Overall, 98.0% of patients experienced at least one TEAE during the study; most TEAEs were mild (49.0%) or moderate (37.3%) in intensity (Table 2). The most commonly reported TEAEs were nasopharyngitis (35.3%), constipation (11.8%), and medical device site infection (11.8%) (Table 2). No deaths were reported during the study. MACEs occurring after the start of study drug administration were reported in 2.0% of patients (Table S5). No clinically meaningful changes from baseline were observed in laboratory parameters, vital signs, or electrocardiogram parameters.
TABLE 2.
Summary of TEAEs up to Week 36 (safety analysis set)
| TEAEs | Molidustat (n = 51) |
|---|---|
| Any TEAE, n (%) | 50 (98.0) |
| Mild | 25 (49.0) |
| Moderate | 19 (37.3) |
| Severe | 6 (11.8) |
| Any study drug‐related TEAE, n (%) | 12 (23.5) |
| Mild | 7 (13.7) |
| Moderate | 3 (5.9) |
| Severe | 2 (3.9) |
| Any TEAE resulting in molidustat discontinuation, n (%) | 10 (19.6) |
| Any serious TEAE, n (%) | 17 (33.3) |
| Any study drug‐related serious TEAE, n(%) | 2 (3.9) |
| Any serious TEAE resulting in molidustat discontinuation, n (%) | 6 (11.8) |
| TEAE leading to death, n (%) | 0 |
| Common (≥5%) TEAEs by primary system organ class and preferred term | |
| Blood and lymphatic system disorders, n (%) | |
| Iron deficiency anemia | 3 (5.9) |
| Cardiac disorders, n (%) | |
| Congestive heart failure | 3 (5.9) |
| Gastrointestinal disorders, n (%) | |
| Constipation | 6 (11.8) |
| Diarrhea | 3 (5.9) |
| Nausea | 4 (7.8) |
| Hepatobiliary disorders, n (%) | |
| Gallbladder polyp | 4 (7.8) |
| Infections and infestations, n (%) | |
| Bronchitis | 4 (7.8) |
| Gastroenteritis | 3 (5.9) |
| Medical device site infection | 6 (11.8) |
| Nasopharyngitis | 18 (35.3) |
| Peritonitis | 5 (9.8) |
| Metabolism and nutrition disorders, n (%) | |
| Hyperkalemia | 3 (5.9) |
| Hyperphosphatemia | 4 (7.8) |
| Hypoglycemia | 3 (5.9) |
| Psychiatric disorders, n (%) | |
| Insomnia | 3 (5.9) |
| Renal and urinary disorders, n (%) | |
| CKD worsening a | 3 (5.9) |
| Respiratory, thoracic, and mediastinal disorders, n (%) | |
| Rhinitis allergic | 3 (5.9) |
| Skin and subcutaneous tissue disorders, n (%) | |
| Asteatosis | 3 (5.9) |
| Eczema asteatosis | 3 (5.9) |
| Vascular disorders, n (%) | |
| Hypertension | 3 (5.9) |
Note: A patient was counted only once within each preferred term or any primary system organ class.
Abbreviations: TEAE, treatment‐emergent adverse event.
The term “CKD worsening” is a collection of a variety of reported terms in the Medical Dictionary for Regulatory Activities (MedDRA). Specifically, “CKD worsening” includes the following terms: chronic kidney disease exacerbation, chronic kidney disease progression, deterioration of CKD, exacerbation of CKD, progression of chronic kidney disease, worsening of CKD, worsening of chronic kidney disease, worsening of chronic renal failure, progression of chronic kidney disease, renal failure chronic aggravated, worsening of CKD, and worsening of renal failure. According to the study protocol, the worsening of kidney function as a natural course of CKD was not recorded as a TEAE.
4. DISCUSSION
The MIYABI PD study is part of the phase 3 MIYABI program and is the first assessment of the efficacy and safety of molidustat in patients with renal anemia undergoing PD. In this phase 3, single‐arm, open‐label study, more than half of the patients met the responder criteria and over 70% completed the study. The mean Hb level during the evaluation period was within the prespecified target range. In addition, over the 36‐week treatment period, molidustat was well tolerated, with no new safety signals observed.
The responder rate among Japanese patients with renal anemia undergoing PD was similar to rates reported in Japanese ESA‐naive patients with renal anemia undergoing HD (56.0%) or not undergoing HD (59.8%), and lower than that reported in ESA‐experienced patients not undergoing dialysis (72%) (21, 23, 24). The responder rate was driven by Criterion 1 (mean Hb levels in the target range during the evaluation period). Almost two‐thirds of patients had more than 50% of their Hb levels in the target range during the evaluation period (Criterion 2), and the vast majority did not require rescue treatment (Criterion 3). Interestingly, the responder rate in the PPS, which accounted for the patients who discontinued treatment, increased to just over 75%. The mean Hb level was in the target range during the evaluation period; however, it decreased from baseline to Week 8 to just under the lower limit of the target range. This was followed by an increase into the target range until Week 20, and then stabilization until Week 36. The proportion of patients with Hb levels in the target range decreased from baseline to Week 6, with a corresponding rise in the proportion of patients with Hb level below the target range. These observations are consistent with the onset of effect of molidustat of 6–8 weeks observed in the MIYABI ND‐M and HD‐M studies, as well as in phases 1 and 2 studies conducted in white and Asian patients (REF to MIAYBI ND‐M and HD‐M) (16, 18,24,25). These adjustment phases observed for Hb level and for the proportion of patients with Hb levels in the target range are most likely a reflection of the time needed to reach an optimal molidustat dosage.
Roxadustat, another HIF–PH inhibitor, has been investigated in a phase 3 clinical trial in Japanese patients with renal anemia undergoing PD (26). Mean Hb levels reported in the present study were similar to those reported for roxadustat (11.05 g/dL in ESA‐naive patients and 10.93 g/dL in ESA‐experienced patients) (26).
The proportion of patients reporting at least one TEAE in the present study was also in line with previously published data for molidustat (17, 18). As previously reported for the phase 2 extension studies, the most common TEAE was nasopharyngitis, and most TEAEs were moderate or mild in intensity.
The results presented here should be interpreted in the context of several limitations. The open‐label design may have introduced bias and is a limitation of this study. The single‐arm design, the low number of patients (especially, only two were ESA‐naive), and the short duration of the study were also limitations. Larger, long‐term studies would provide significant information. Finally, investigating the effect of residual kidney function on the Hb response would be of interest. Despite these limitations, the data presented here are extremely valuable owing to the low number of articles reporting the efficacy and safety of HIF–PH inhibitors in patients undergoing PD.
5. CONCLUSIONS
Molidustat maintained Hb levels in the prespecified target range of ≥11.0 and <13 g/dL in Japanese patients with renal anemia undergoing PD. Overall, 36 weeks of treatment with dose‐titrated molidustat was well tolerated in these individuals, and no new safety signals were observed. The results of the present study demonstrate that molidustat could be an effective and well‐tolerated alternative to current standards of care in patients receiving PD.
CONFLICT OF INTEREST
Tadao Akizawa received consulting and lecture fees from Bayer Yakuhin Ltd during the study. He also received consulting, lecture, or manuscript fees outside the submitted work from Astellas, GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical Co. Ltd, Kyowa Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd, Torii Pharmaceutical Co. Ltd, Sanwa Chemical Co. Ltd, Ono Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Tanabe Mitsubishi Co. Ltd, and Chugai Pharmaceutical Co. Ltd. Hiroyasu Yamamoto received consulting and lecture fees from Bayer Yakuhin Ltd during the study. Kiyoshi Nobori, Yoshimi Matsuda, Kentaro Taki, Yasuhiro Hayashi, and Takanori Hayasaki are employees of Bayer Yakuhin Ltd.
Supporting information
TABLE S1 Investigators and participating centers
TABLE S2. Coordinating investigator, central adjudication, and data monitoring committee members
TABLE S3. Inclusion and exclusion criteria
TABLE S4. Responder rate (per protocol set)
TABLE S5. MACEs including undetermined death evaluated by a specialist after the start of study drug treatment and up to Week 36 (safety analysis set)
FIGURE S1. Mean (SD) actual dosage of molidustat (safety analysis set)
FIGURE S2. Proportion of patients whose Hb level was in, above, or below the target range ≥11.0 to <13.0 g/dL by visit (full analysis set)
ACKNOWLEDGMENTS
The authors would like to thank Eriko Ogura for leading protocol development and managing the trial and Ken Miyazaki for his contribution to this study. Medical writing support was provided by Nicolas Bertheleme, PhD, of Oxford PharmaGenesis, Oxford, UK, with funding from Bayer Yakuhin Ltd.
Akizawa T, Nobori K, Matsuda Y, Taki K, Hayashi Y, Hayasaki T, et al. Molidustat for the treatment of anemia in Japanese patients undergoing peritoneal dialysis: a single‐arm, open‐label, phase 3 study. Ther Apher Dial. 2022;26:368–77. 10.1111/1744-9987.13713
DATA AVAILABILITY STATEMENT
Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient‐level clinical trial data, study‐level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after January 1, 2014.
REFERENCES
- 1. Kidney Disease . Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279–335. [Google Scholar]
- 2. Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end‐stage renal disease. A collaborative meta‐analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 4. Grams ME, Yang W, Rebholz CM, Wang X, Porter AC, Inker LA, et al. Risks of adverse events in advanced CKD: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2017;70(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nitta K, Goto S, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: survey methods, facility data, incidence, prevalence, and mortality. Renal Replace Ther. 2020;6(1):41. [Google Scholar]
- 6. Akizawa T, Okumura H, Alexandre AF, Fukushima A, Kiyabu G, Dorey J. Burden of anemia in chronic kidney disease patients in Japan: a literature review. Ther Apher Dial. 2018;22(5):444–56. [DOI] [PubMed] [Google Scholar]
- 7. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohagura K, Tomiyama N, Kinjo K, Takishita S, Iseki K. Prevalence of anemia according to stage of chronic kidney disease in a large screening cohort of Japanese. Clin Exp Nephrol. 2009;13(6):614–20. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3(1):36. [Google Scholar]
- 10. Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis‐stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2016;15(8):1021–30. [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–32. [DOI] [PubMed] [Google Scholar]
- 12. Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet. 2007;369(9559):381–8. [DOI] [PubMed] [Google Scholar]
- 13. Wenger RH, Hoogewijs D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin‐producing cells. Am J Physiol Renal Physiol. 2010;298(6):F1287–96. [DOI] [PubMed] [Google Scholar]
- 14. Gupta N, Wish JB. Hypoxia‐inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815–26. [DOI] [PubMed] [Google Scholar]
- 15. Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U. Mimicking hypoxia to treat anemia: HIF‐stabilizer BAY 85‐3934 (molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014;9(11):e111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bottcher M, Lentini S, Arens ER, Kaiser A, van der Mey D, Thuss U, et al. First‐in‐man/proof of concept study with molidustat—a novel selective oral HIF‐prolyl hydroxylase inhibitor for the treatment of renal anaemia. Br J Clin Pharmacol. 2018;84(7):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akizawa T, Macdougall IC, Berns JS, Bernhardt T, Staedtler G, Taguchi M, et al. Long‐term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am J Nephrol. 2019;49(4):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14(1):28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akizawa T, Taguchi M, Matsuda Y, Iekushi K, Yamada T, Yamamoto H. Molidustat for the treatment of renal anaemia in patients with dialysis‐dependent chronic kidney disease: design and rationale of three phase III studies. BMJ Open. 2019;9(6):e026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto H, Taguchi M, Matsuda Y, Iekushi K, Yamada T, Akizawa T. Molidustat for the treatment of renal anaemia in patients with non‐dialysis‐dependent chronic kidney disease: design and rationale of two phase III studies. BMJ Open. 2019;9(6):e026704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akizawa T, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, Yamamoto H. Molidustat for anemia correction in Japanese patients undergoing hemodialysis: a single‐arm, phase 3 study. Ther Apher Dial. 2021;1–9. 10.1111/1744-9987.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14(3):240–75. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto H, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, Akizawa T. Efficacy and safety of molidustat for anemia in ESA‐naive non‐dialysis patients: a randomized, phase 3 trial. Am J Nephrol. 2021; In Press. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto H, Nobori K, Matsuda Y, Hayashi Y. Molidustat for renal anemia in non dialysis patients previously treated with erythropoiesis‐stimulating agents: a randomized, open‐label, phase 3 study. Am J Nephrol. 2021; In Press. [DOI] [PubMed] [Google Scholar]
- 25. Akizawa T, Yamada T, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, et al. Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int. Rep. 2021. 10.1016/j.ekir.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open‐label study. Ther Apher Dial. 2020;24(2):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Investigators and participating centers
TABLE S2. Coordinating investigator, central adjudication, and data monitoring committee members
TABLE S3. Inclusion and exclusion criteria
TABLE S4. Responder rate (per protocol set)
TABLE S5. MACEs including undetermined death evaluated by a specialist after the start of study drug treatment and up to Week 36 (safety analysis set)
FIGURE S1. Mean (SD) actual dosage of molidustat (safety analysis set)
FIGURE S2. Proportion of patients whose Hb level was in, above, or below the target range ≥11.0 to <13.0 g/dL by visit (full analysis set)
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient‐level clinical trial data, study‐level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after January 1, 2014.


