Abstract
Objective
To assess the care for hypertension in Sierra Leone, by the use of a cascade‐of‐care approach, to identify where the need for healthcare system interventions is greatest.
Methods
Using data from a nationwide household survey on surgical conditions undertaken in 1956 participants ≥18 years from October 2019 to March 2020, a cascade of care for hypertension consisting of four categories – hypertensive population, those diagnosed, those treated and those controlled – was constructed. Hypertension was defined as having a blood pressure ≥140/90 mmHg, or self‐reported use of antihypertensive medication. Logistic regression analysis was used to investigate factors associated with undiagnosed hypertension.
Results
The prevalence of hypertension was 22%. Among those with hypertension, 23% were diagnosed, 11% were treated and 5% had controlled blood pressure. The largest loss to care (77%) was between being hypertensive and receiving a diagnosis. Male sex, age and living in a rural location, were significantly associated with the odds of undiagnosed hypertension. There was no significant difference between men and women in the number of patients with controlled blood pressure. Adults aged 40 or older were observed to be better retained in care compared with those younger than 40 years of age.
Conclusion
There is a significant loss to care in the care cascade for hypertension in Sierra Leone. Our results suggest that increasing awareness of cardiovascular risk and risk factor screening for early diagnosis might have a large impact on hypertension care.
Keywords: care cascade, hypertension, low resource setting, Sierra Leone, sub‐Saharan Africa
INTRODUCTION
Hypertension is an increasing global health problem. According to the WHO, 1.13 billion people had hypertension in 2019 [1] with increasing prevalence in low‐ and middle‐income countries (LMICs) in part exceeding those from high‐income countries [1]. Hypertension is a major risk factor for cardiovascular disease (CVD), which is the number one cause of death globally [2]. An estimated 17.9 million people died from CVDs in 2016, with three out of four CVD deaths in LMICs [2]. The increasing trend of high blood pressure (BP) in LMICs is attributable to population ageing and changes in behavioural risk factors, including smoking, alcohol consumption, physical inactivity and unhealthy diet [3].
Despite multiple studies on the prevalence and determinants of hypertension in sub‐Saharan Africa (SSA) [4], information on awareness, treatment and control of hypertension is limited. Several studies have shown that the increasing incidence of hypertension and the lack of proper care are major problems in SSA. A multi‐country study on the prevalence, awareness and treatment of hypertension in Kenya, Nigeria, Uganda and Tanzania, showed that among African adults ≥18 years, nearly 25% had hypertension. Only 50% of those with hypertension were taking medication and of those treated, less than 50% had their BP under control [5]. A study examining the awareness, management and control of hypertension in Benin showed similarly high rates of unmet need for hypertension care [6].
One way to examine the care of hypertension is through a care cascade. The cascade‐of‐care model can be used to define the proportion of people in various healthcare stages, beginning with screening and diagnosis, followed by treatment and control [7]. This model has been widely used to assess the care for HIV in Africa [8, 9, 10] and has recently been extended to assess the care for hypertension in people living with HIV [11, 12]. To date, only a few studies have used this model solely to assess the care for hypertension [7, 13, 14]. These studies assessed the proportion of patients who reached each stage in the care cascade, allowing them to identify where the loss of patients occurs in linking hypertensive patients to appropriate care, in order to formulate improvements [7, 13, 15].
This study assessed the cascade of care for hypertension in Sierra Leone, one of the poorest countries globally with one of the most fragile healthcare systems in the world [16], partly due to the civil war 1991–2002 impacting negatively on the country's infrastructure, including its healthcare system. The West African Ebola virus disease epidemic (2013–2016) crippled the healthcare system, even more, complicating among others the response to the rising burden of NCD [17]. This study investigated where the need for healthcare system interventions for hypertension is the greatest. We further assessed possible predictors of increased risk for falling out of the care cascade for hypertension.
METHODS
This study used data from a national household‐based survey to estimate surgical and maternal health conditions (PREvalence Study on Surgical COnditions (PRESSCO 2020) study) in Sierra Leone. Data were collected via individual face‐to‐face interviews at the homes of the respondents. Standardised data collection was undertaken by trained nurses and medical staff. Tablets with mobile internet access were used to collect data during field work. Data were subsequently uploaded to and stored in REDCap©, a secure, web‐based software platform hosted at the University Medical Center Utrecht [18, 19]. Data collection took place October to November 2019 and February to March 2020.
Sampling was done through a weighted random cluster design, where the probability of cluster choice is proportional to the population size. Clusters in this context referred to enumeration areas, the smallest administrative units in Sierra Leone as per Statistics Sierra Leone. 1.875 households from 75 nationwide clusters were visited.
Information on demographic, socio‐economic status (SES), medical history and physical condition was collected by questionnaire. In participants ≥18 years, systolic and diastolic BP was measured three times, preferably on the upper left arm (if this was not possible, the right arm was used), with a 3‐min interval between measurements with OMRON M6 comfort machines (OMRON Healthcare, the Netherlands). The average of the second and third BP measurements was used for analysis.
Definitions of hypertension and the cascade of care
Hypertension was defined as having a mean systolic blood pressure (SBP) ≥140 mmHg and/or a mean diastolic blood pressure (DBP) ≥90 mmHg or self‐reported use of antihypertensive drugs [13, 20, 21]. In order to identify gaps in the care cascade, study participants with hypertension were divided into four categories: total number of participants with hypertension based on BP measurements during the survey (Category 1); number of study participants diagnosed with hypertension (Category 2); number of participants treated (Category 3); and the number that had controlled BP (Category 4). Being diagnosed with hypertension was defined as having received a previous diagnosis by a healthcare worker or health professional. Being treated for hypertension was defined as having a diagnosis and self‐reported current use of antihypertensive drugs. Being controlled for hypertension was defined as currently using antihypertensives and a mean SBP <140 mmHg [20, 21].
The proportion of study participants who reached each stage of the cascade of care was determined using the number of participants in the previous step as the denominator.
Variable definition
Age was categorised as 18–34 years, 35–44 years, 45–54 years, 55–64 years, 65+ years, respectively, as 18–39 years and 40+ years. Residential location was divided as urban and rural, following the definition of the 2015 EA census frame of Sierra Leone [22]; ethnicity differentiated between Creole, Limba, Mende, Temne and other ethnicities (including Fullah, Kissi, Kono, Koranko, Loko, Mandingo, Sherbro, Susu, Vai and Yalunka), other African and non‐African. SES encompassed information on education (none, primary school, secondary school and tertiary/higher education) and occupation (employed and unemployed). Lifestyle factors included tobacco use (smoking and non‐smoking) and alcohol consumption (regularly and not regularly). Medical history information included time since diagnosis of hypertension (past year or before); diabetes screening (screened or never been screened); mobility (no problems, some and confined to bed); medical history (heart problems and/or cerebral vascular accident (CVA), other (including leprosy, tuberculosis, Wuchereria bancrofti filariasis), none and unknown).
Statistical analysis
Frequencies and percentages were used to estimate the prevalence of hypertension. To calculate the proportions of study participants diagnosed with hypertension, treated and controlled, we obtained frequencies and percentages of study participants at each step compared with the preceding step. Multivariable logistic regression using backward elimination, with a p‐value of 0.2 for variable selection, was used to investigate demographic, SES and lifestyle predictors of falling out of the care cascade. Logistic regression was conducted on the cascade step with the biggest loss to care. To extrapolate our results to the entire population, we accounted for the weighted random cluster design consisting of 16 clusters and 75 strata. Analyses were done using STATA/IC 15.1 [23].
Ethics
The study was endorsed by the Masanga Medical Research Unit's Scientific Review Committee (MMRU‐SRC‐009–2019). Ethical approval was obtained from the Sierra Leone National Ethics Committee and The Norwegian National Committee for Research Ethics (Ref‐No 31932). Study approval was obtained from the formal head of each enumerator area, following community engagement activities contributing to the development of the study protocol. District medical officers were informed to expect referrals in case of medical diagnoses, such as undiagnosed high BP in participants of this study, depending on severity referral was within 24 hours or a week. As part of the larger PRESSCO 2020 study, referrals were arranged for patients who had serious medical problems identified during the physical examination. All participants or their guardian signed informed consent forms prior to study enrolment. Privacy and confidentiality were assured through the use of password‐protected tablets, secure internet data transfer and use of anonymised data for analyses.
RESULTS
In total, 3618 individuals aged 0–100 years participated in the nationwide survey. Of those, 2007 were ≥18 years. Twenty participants had missing values for either age or sex, and 20 participants had missing values for BP measurements. Participants with implausible BP values (SBP <70 mmHg or >270 mmHg; DBP <40 mmHg or >150 mmHg) (n = 2) [13, 24] and those with a missing response on the question ever been diagnosed with hypertension (n = 9) were excluded, resulting in an analytic sample of 1956 participants (Figure S1). Thirty‐seven (1.9%) participants answered yes to the question ‘having ever received a diagnosis of hypertension’, but did not meet the definition of hypertension, thus were excluded from our care cascade.
Table 1 presents the characteristics of the study population comprising the analytic sample. The study population was predominantly female (56%); the mean age was 39 (SD 16.5) years. Most participants were of the Mende ethnic group (34% vs. ≤28% of other ethnic groups) and lived in a rural location (67%).
TABLE 1.
Characteristics of the PRESSCO 2020 study sample according to hypertension prevalence, diagnosis, treatment and control
| Study Sample | Hypertension | Diagnosed | Treated | Controlled | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | N | % of HT | 95% CI | N | % of HT | 95% CI | N | % of HT | 95% CI | |
| Age (years) | |||||||||||||||
| 18–39 | 1173 | 60.0 | 57.8–62.1 | 104 | 8.9 | 7.3–10.6 | 12 | 11.5 | 6.1–19.3 | 6 | 5.8 | 2.1–12.1 | 2 | 1.9 | 0.2–6.8 |
| 40+ | 783 | 40.0 | 37.9–42.2 | 319 | 40.7 | 37.3–44.3 | 84 | 26.3 | 21.6–31.5 | 41 | 12.9 | 9.4–17.0 | 17 | 5.3 | 3.1–8.4 |
| Sex by age (years) | |||||||||||||||
| Men | |||||||||||||||
| 18–39 | 458 | 52.8 | 49.4‐56.1 | 37 | 8.1 | 5.8–11.0 | 2 | 5.4 | 0.7–18.2 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| 40+ | 410 | 47.2 | 43.9‐50.6 | 142 | 34.6 | 30.0–39.5 | 31 | 21.8 | 15.3– 29.5 | 19 | 13.4 | 8.3– 20.1 | 9 | 6.3 | 2.9–11.7 |
| Women | |||||||||||||||
| 18–39 | 715 | 65.7 | 62.8–68.5 | 67 | 9.4 | 7.3–11.7 | 10 | 14.9 | 7.4–25.7 | 6 | 9.0 | 3.4–18.5 | 2 | 3.0 | 0.4–10.4 |
| 40+ | 373 | 34.3 | 31.5–37.2 | 177 | 47.5 | 42.3–52.7 | 53 | 29.9 | 23.3–37.3 | 22 | 12.4 | 8.0–18.2 | 8 | 4.5 | 2.0–8.7 |
| Sex | |||||||||||||||
| Male | 868 | 44.4 | 42.2‐46.6 | 179 | 20.6 | 18.0–23.5 | 33 | 18.4 | 13.0–24.9 | 19 | 10.6 | 6.5–16.1 | 9 | 5.0 | 2.3–9.3 |
| Female | 1088 | 55.6 | 53.4–57.8 | 244 | 22.4 | 20.0–25.0 | 63 | 25.8 | 20.4–31.8 | 28 | 11.5 | 7.8–16.2 | 10 | 4.1 | 2.0–7.4 |
| Ethnicity | |||||||||||||||
| Creole | 34 | 1.7 | 1.2–2.4 | 9 | 26.5 | 12.9–44.4 | 4 | 44.4 | 13.7–78.8 | 4 | 44.4 | 13.7–78.8 | 2 | 22.2 | 2.8–60.0 |
| Limba | 230 | 11.8 | 10.4–13.3 | 56 | 24.3 | 18.9–30.4 | 19 | 33.9 | 21,8–47.8 | 9 | 16.1 | 7.6–28.3 | 6 | 10.7 | 4.0–21.9 |
| Mende | 662 | 33.8 | 31.7–36.0 | 119 | 18.0 | 15.1–21.1 | 18 | 15.1 | 9.2–22.8 | 9 | 7.6 | 3.5–13.9 | 3 | 2.5 | 0.5–7.2 |
| Temne | 540 | 27.6 | 25.6–29.6 | 123 | 22.8 | 19.3–26.6 | 26 | 21.1 | 14.3–29.4 | 11 | 8.9 | 4.5–15.4 | 3 | 2.4 | 0.5–7.0 |
| Other | 490 | 25.1 | 23.1–27.0 | 116 | 23.7 | 20.0–27.7 | 29 | 25.0 | 17.4–33.9 | 14 | 12.1 | 6.8–19.4 | 5 | 4.3 | 1.4–9.8 |
| Residential location | |||||||||||||||
| Rural | 1312 | 67.1 | 64.9–69.2 | 293 | 22.3 | 20.1–24.7 | 43 | 14.7 | 10.8–19.3 | 23 | 7.8 | 5.0–11.5 | 6 | 2.0 | 0.8–4.4 |
| Urban | 644 | 32.9 | 30.8–35.1 | 130 | 20.2 | 17.2–23.5 | 53 | 40.8 | 32.2–49.7 | 24 | 18.5 | 12.2–26.2 | 13 | 10.0 | 5.4–16.5 |
| Education | |||||||||||||||
| None | 1079 | 55.2 | 52.9–57.4 | 284 | 26.3 | 23.7–29.1 | 53 | 18.7 | 14.3–23.7 | 27 | 9.5 | 6.4–13.5 | 9 | 3.2 | 1.5–5.9 |
| Primary school | 184 | 9.4 | 8.1–10.8 | 36 | 19.6 | 14.1–26.0 | 10 | 27.8 | 14.2–45.2 | 3 | 8.3 | 1.8–22.5 | 1 | 2.8 | 0.1–14.5 |
| Secondary school | 553 | 28.3 | 26.3–30.3 | 68 | 12.3 | 9.7–15.3 | 21 | 30.9 | 20.2–43.3 | 8 | 11.8 | 5.2–21.9 | 4 | 5.9 | 1.6–14.4 |
| Tertiary or higher | 140 | 7.2 | 6.1–8.4 | 35 | 25.0 | 18.1–33.0 | 12 | 34.3 | 19.1–52.3 | 9 | 25.7 | 12.5–43.3 | 5 | 14.3 | 4.8–30.3 |
| Mobility a | |||||||||||||||
| Problems walking | 155 | 8.0 | 6.8–9.3 | 76 | 49.0 | 40.9–57.2 | 31 | 40.8 | 29.6–52.7 | 16 | 21.1 | 12.5–31.9 | 7 | 9.2 | 3.8–18.1 |
| No problems walking | 1789 | 92.0 | 90.7–93.2 | 343 | 19.2 | 17.4–21.1 | 65 | 19.0 | 14.9–23.5 | 31 | 9.0 | 6.2–12.6 | 12 | 3.5 | 1.8–6.0 |
| DM screening | |||||||||||||||
| Yes | 98 | 5.4 | 4.4–6.6 | 34 | 34.7 | 25.4–45.0 | 19 | 55.9 | 37.9–72.8 | 10 | 29.4 | 15.1–47.5 | 5 | 14.7 | 5.0–31.1 |
| No | 1712 | 94.6 | 93.4–95.6 | 350 | 20.4 | 18.6–22.4 | 74 | 21.1 | 17.0–25.8 | 34 | 9.7 | 6.8–13.3 | 12 | 3.4 | 1.8–5.9 |
| Medical history | |||||||||||||||
| CVA/ heart problems | 24 | 1.2 | 0.8–1.8 | 13 | 54.2 | 32.8–74.4 | 8 | 61.5 | 31.6–86.1 | 5 | 38.5 | 13.9–68.4 | 2 | 15.4 | 1.9–45.4 |
| Other | 1929 | 98.8 | 98.2–99.2 | 410 | 21.3 | 19.4– 23.1 | 88 | 21.5 | 17.6–25.8 | 42 | 10.2 | 7.5–13.6 | 17 | 4.1 | 2.4–6.6 |
% of HT = percentage of hypertensive patients, CVA = cerebral vascular accident.
Mobility is defined as problems walking and no problems, redefining 3 participants that were confined to bed as problems walking.
Figure 1 displays the prevalence of hypertension by age groups. Of the 1956 participants, 423 had hypertension, a prevalence of 22% (95% CI: 19.8–23.5). Overall, the prevalence of hypertension was similar between men (21%; 95% CI: 18.0–23.5) and women (22%; 95% CI: 20.0–25.0). For middle‐aged and older women (40+; 48%; 95% CI: 42.3–52.7), a higher prevalence of hypertension was observed than for men (40+; 35%; 95% CI: 30.0–39.5).
FIGURE 1.
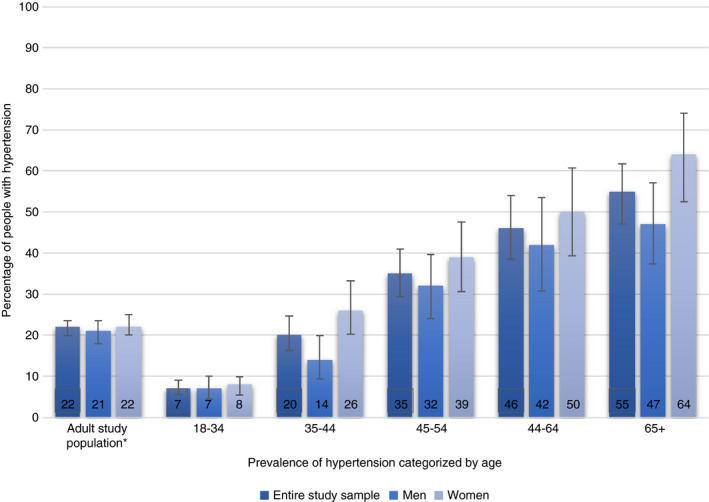
Prevalence of hypertension by age categories for the study population and for men and women separately. * All participants aged 18 and older
The prevalences of hypertension of rural (22%; 95% CI: 20.1– 25.0) and urban (20%; 95% CI: 17.2–23.5 for urban) residence were similar. Overall, the percentages of patients being diagnosed, treated and controlled were higher in urban than rural participants (Table 1). The percentages of participants who were diagnosed, treated and controlled were overall higher in participants who were enrolled in or had completed a tertiary education than in participants with no formal education (Table 1). Among ethnic groups, the percentages of participants diagnosed, treated and controlled were overall higher in participants from the Limba and Creole ethnic groups than in participants from the Mende and Temne groups (Table 1).
Figures 2a–c show results of the hypertension care cascade. Figure 2a depicts the care cascade for the entire study population aged 18 and older. Of the hypertensive patients, 23% reported ever having been diagnosed; 11% of hypertensive patients were treated, and 5% of hypertensive patients were successfully retained in the cascade of care and had their BP under control. Figure 2b on the cascade of care for men and women shows that women had a higher chance of being diagnosed than men (26% vs. 18%). This difference was less pronounced for the proportion of hypertensive patients being treated (12% women vs 11% men) and in the number of hypertensive patients with their BP under control (4% women vs. 5% of men). Figure 2c provides information on the care cascade for young adults (18–39 years) and middle‐aged and older participants (≥40 years). Overall, the ≥40 year age group was better retained in the care cascade, with 26%, 13% and 5% reported ever having received a diagnosis, treatment and having their BP under control, compared to 12%, 6% and 2% for the 18–39 year age group. Differences in the care cascade for men and women categorised by age are presented in Table 1. The cascade step with the largest loss to care was between being hypertensive and receiving a diagnosis (77% for the entire study sample, 74% for women, 82% for men).
FIGURE 2.
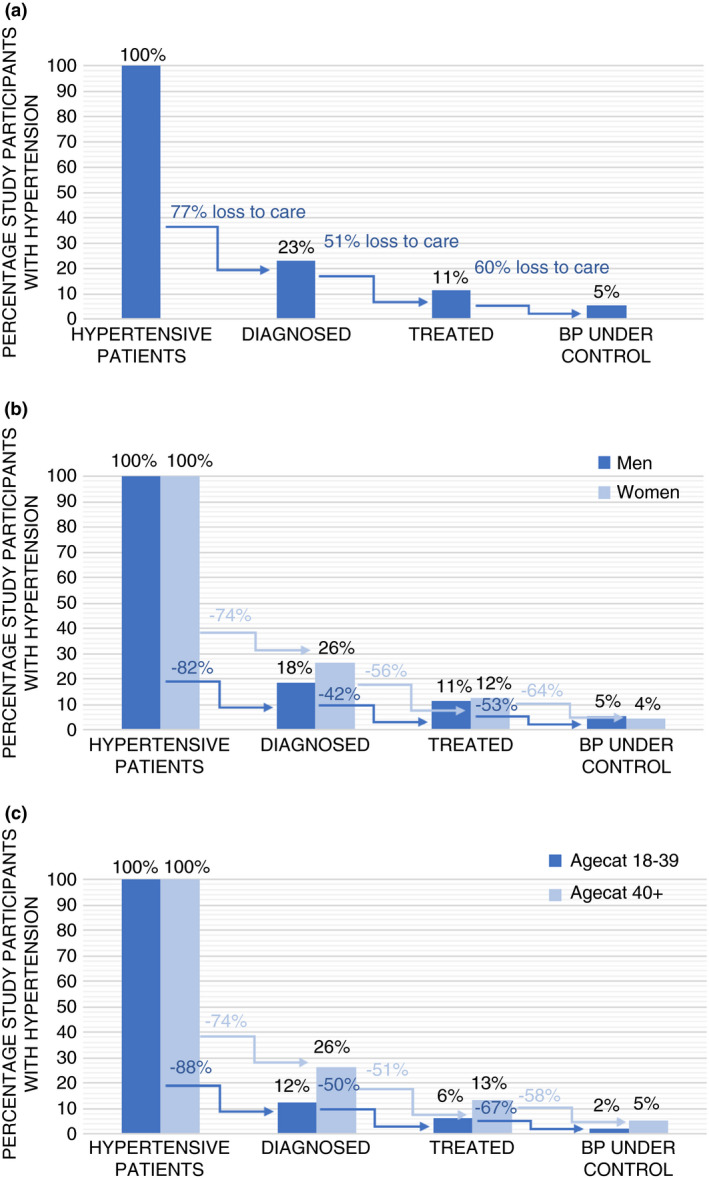
(a) Hypertension care cascade, PRESSCO 2020 study adults aged 18 years and older. The first bar represents the entire hypertensive population, each bar after this represents a percentage of that group. The arrows between bars represent the percentage of patients lost between each step of the care cascade. (b) Hypertension care cascade for men and women, PRESSCO 2020 study. The first bar represents the entire hypertensive population, each bar after this represents a percentage of that group. The arrows between bars represent the percentage of patients lost between each step for men and women separately. (c) Hypertension care cascade by age groups, PRESSCO 2020 study. The first bar represents the entire hypertensive population, each bar after this represents a percentage of that group. The arrows between bars represent the percentage of patients lost between each step for both age groups separately
Male sex (OR = 1.91; 95%CI: 1.03–3.51) and rural residency (OR = 4.84; 95%CI: 2.53–9.25) were significantly associated with undiagnosed hypertension (Table 2). Being aged ≥45 years as compared to being aged <35 years (OR = 0.17–0.26; 95%CI: 0.06–0.70), a medical history of CVA or heart problems (OR = 0.17; 95%CI: 0.03–0.84), having problems with walking considered a proxy for disability (OR = 0.37; 95% CI: 0.19–0.73) and belonging to the ethnic group Limba, versus the Mende ethnic group (OR = 0.27; 95% CI: 0.11–0.66) were inversely associated with undiagnosed hypertension.
TABLE 2.
Odds ratio for undiagnosed hypertension, PRESSCO 2020 study
| Predictors | OR | 95% CI | p value |
|---|---|---|---|
| Age categories (years) | |||
| 18–34 | 1 | ||
| 35–44 | 0.49 | 0.17–1.38 | 0.176 |
| 45–54 | 0.26 | 0.10–0.70 | 0.008 |
| 55–64 | 0.22 | 0.08–0.63 | 0.004 |
| 65+ | 0.17 | 0.06–0.47 | 0.001 |
| Sex | |||
| Female | 1 | ||
| Male | 1.91 | 1.03–3.51 | 0.039 |
| Ethnicity | |||
| Mende | 1 | ||
| Creole | 0.58 | 0.11–3.11 | 0.527 |
| Limba | 0.27 | 0.11–0.66 | 0.004 |
| Temne | 0.74 | 0.33–1.65 | 0.462 |
| Other | 0.53 | 0.24–1.18 | 0.122 |
| Education | |||
| None | 1 | ||
| Primary school | 0.63 | 0.24–1.68 | 0.356 |
| Secondary school | 0.44 | 0.20–0.98 | 0.044 |
| Tertiary or higher | 1.09 | 0.39–3.05 | 0.877 |
| Residential location | |||
| Urban | 1 | ||
| Rural | 4.84 | 2.53–9.25 | <0.001 |
| Mobility | |||
| No problems | 1 | ||
| Problems walking | 0.37 | 0.19–0.73 | 0.004 |
| DM screening | |||
| Yes | 1 | ||
| No | 2.25 | 0.95–5.34 | 0.066 |
| Medical history | |||
| Other | 1 | ||
| CVA/ heart problems | 0.17 | 0.03–0.84 | 0.030 |
Abbreviations: 95% CI, 95% confidence interval; DM screening, diabetes mellitus screening; OR, odds ratio.
DISCUSSION
In this study, we report on a care cascade for hypertension in adults ≥18 years in Sierra Leone, observing high rates of unmet need for hypertension care. Only 23% of hypertensive patients were diagnosed, 11% were treated and only 5% had their BP under control. Our findings on low levels of diagnosis, treatment and control of hypertension corroborate findings from a recent study from Sierra Leone [14] and pooled individual‐level population data analysis from 44 LMIC including from SSA [7].
The observed hypertension prevalence rate of 22% in the national PRESSCO household survey is considerably lower than previously reported figures for Sierra Leone with prevalences up to 50% from healthcare facility‐based studies [25, 26], and a population‐based study undertaken in the southern province of the country [14].
In our study, only 5% of hypertensive patients were successfully retained in the care cascade and had their BP under control, similar to the results reported from the study undertaken in the Southern Province of Sierra Leone [14]. Blood pressure control for men and women was similar in our study, even though a higher percentage of women than men were diagnosed patients. Patients aged ≥40 years were decisively better retained than patients aged 18–39 years. This aligns well with the 2019 NCD prevention and treatment protocols of the Ministry of Health, which state that BP should be measured in adults aged 40 years and older when they visit a healthcare facility [27].
Across all cascade steps, the largest loss to care was found between being hypertensive and receiving a diagnosis. We observed that males had a higher risk of being undiagnosed as previously reported in several other studies [7, 13, 14]. This could be due to several factors, such as a focus on maternal and child healthcare services and gender norms concerning care‐seeking and health facility opening hours [7, 14, 26, 28].
Rural participants were observed to have a higher chance of being undiagnosed compared with urban participants. A likely explanation for this difference is that people who live in rural areas encounter more care‐seeking barriers [29, 30, 31]. Physical access to NCD services, principally due to distance to healthcare facilities, was reported to be a key barrier for care‐seeking in Sierra Leone, [32]. The 2017 national Service Availability and Readiness Assessment (SARA) survey analysed the health facility density in Sierra Leone, an indicator of service access to outpatient consultation, observing a wide gap between rural and urban facility densities, 0.7 and 2.6 health facilities/10,000 population, respectively [33]. Not only do rural areas have fewer health facilities, but the infrastructure and transportation system in the country is also poor. Patients considering seeking care at a lower‐level facility may be discouraged to access a higher level of care associated with longer travel distance and high costs for transportation [32, 33].
In addition to the poor rate of diagnosis, there was a large loss between being treated and having BP under control, which suggests a lack of effective treatment. Among those who reported the use of antihypertensive medication, only 40% had their BP under control. This might be due to poor adherence to the prescribed medication, poor monitoring by healthcare workers or ineffective medication [13]. Low adherence levels for several long‐term treatment regiments have been documented in South Africa [34, 35]. Low adherence rates may in turn be caused by unavailability of medicines and the inability of patients to afford them [32]. While the free healthcare initiative in Sierra Leone has focused on providing free medicines for pregnant and lactating women and children under 5 years of age, NCD medicines for all other groups fall within the cost recovery schemes of pharmacies at health centres and hospitals, and may thus not be affordable to patients and their families [32, 36]. In 2020, the costs for a visit to one of the health facilities was about 40,000 Leones (US$4.75) and costs for antihypertensive medication for a month 50,000 Leones (US$6.00). According to recent 2021 estimates from UNDP 60% of Sierra Leoneans live on less than US$1.25 a day, illustrating how very few people can afford treatment for their hypertension [37, 38, 39]. The shortage of medicines in public facilities drives patients to purchase from private pharmacies or informal sources associated more often with poor quality medicines, which may also result in ineffective treatment [32]. The SARA report indicated that antihypertensives were available in only 5% of healthcare facilities [40], highlighting the scarcity of essential NCD medicine medication in Sierra Leone. In addition, they reported that 19% of health facilities do not have BP machines [40] indicating that a large part of patients cannot be properly treated or monitored.
A further reason for poor adherence to medication is personal and cultural beliefs. A study on community understanding of NCD and healthcare‐seeking behaviour in Sierra Leone observed that participants hardly spoke of diseases as being chronic and generally approached healthcare services when they experienced symptoms, hoping for a quick cure and permanent solution [17]. Chronic conditions are sometimes attributed to spiritual causes such as evil spirits or witchcraft, resulting in care seeking from spiritual and traditional sources [17]. People also seek advice from family and friends with regard to which medical help should be sought, possibly resulting in seeking care from informal and traditional healers, using the formal healthcare system as a last resort [17] possibly in part due to the financial vulnerability [41].
Our results suggest that people who do not frequently visit formal healthcare facilities, either because they have no obvious medical reason to or because of care‐seeking barriers, have a higher risk of being undiagnosed hypertensive. The opposite effect can be seen in our results as well; people who visit healthcare centres more often, for example people with a medical history of CVA, heart problems or problems with walking considered as a proxy of physical disability, have higher odds of being diagnosed with hypertension. This suggests that outreach care programmes focusing on cardiovascular risk factor screening and management might have the most impact on hypertension care, especially in increasing diagnosis. In some LMIC mobile health platforms show promise in promoting diagnosis, follow‐up and medication adherence. However, in Sierra Leone connectivity is a problem, especially in rural areas, making this a less promising solution [42]. The use of broadcast media such as television or radio, as well as outdoor media, including print media which have been shown to disseminate health messages, could be employed in promoting NCD screening and management in these settings [43, 44]. In addition, it is important to invest in the training of community health officers (CHO’s) for provision of care. This has been shown to be effective in the Bombali district in Sierra Leone, where training of CHO’s on patient communication, diagnosis and management of hypertension for three months improved SBP and DBP [38]. A systematic review published in 2017 also showed that training community healthcare workers (CHW’s) in prevention and control of NCDs increases favourable behaviour and impacts positively on biochemical risk factors for NCDs [45]. CHW’s are present in almost every village and are, therefore, closer to the community in both distance and trust than CHO’s in health facilities [45]. Other studies have shown that health workers can be effectively trained in cardiovascular risk prevention and management and that it is cost‐effective for chronic disease care in LMIC [46, 47]. The sustainability of CHW programmes is a challenge [48]. Appropriate supervision, good relationship with health system management and securing adequate, and continuous funding have been suggested as ways to sustain the CHW programme [48, 49].
The implementation of CHW programmes can only improve the care for hypertension if people seek formal care. In order to increase retainment in the care cascade, improvements are needed in Sierra Leone's healthcare infrastructure and the provision of adequate equipment and antihypertensive medication at low costs in public care facilities. If patients cannot obtain or afford their medication, it becomes very difficult for them to control their hypertension, making education on prevention and lifestyle modification all the more important. Prevention in the form of population‐based approaches such as health information and communication campaigns in combination with fiscal measures such as taxes on unhealthy foods have proven effective in reducing the burden of NCD in LMIC [50].
The primary strength of this analysis is the use of a care cascade approach in order to analyse the proportion of patients who reached each stage of hypertension in a national population‐based survey in Sierra Leone.
Limitations of the current study relate to its cross‐sectional nature and that the data, in part, relies on self‐reported questions. We can, therefore, not exclude the possibility of recall bias and social acceptability bias. As we could not account for lifestyle treatment of hypertension [7, 11], underestimation of the performance of our study sample in the care cascade might have occurred. For example, there were 37 participants in our study who answered yes to the question ‘having ever received a diagnosis of hypertension’ but were excluded from our care cascade because they did not meet our definition of hypertension. Although this could have been pregnancy‐induced hypertension or another form of temporary hypertension or a misdiagnosis, they could also have had hypertension and brought their BP under control with lifestyle modification. Another limitation of the study is that BP was measured on a single point in time, whereas hypertension is normally diagnosed based on several separate measurements [51]. Furthermore, the study did not properly account for informal urban settlements, as in our data, only three participants indicated to live in an urban slum, whereas only Freetown already has between 27 and 61 informal settlements [52]. Better consideration of this population might have narrowed the observed difference between the rural and urban population with regard to hypertension prevalence, diagnosis, treatment and control. Considering the limitations of this study, it is important that its findings are confirmed in other national cohorts including detailed information on health facility visits for screening and management of high blood pressure.
CONCLUSION
SSA countries in comparison with other LMICs have the lowest number of patients reaching each stage of the hypertension care cascade [7]. Sierra Leone is no exception; results in this study show that only 5% of hypertensive patients had their BP under control. Groups most at risk seem to be people who do not frequent a care facility, either because they have no need to or because of healthcare‐seeking barriers. This suggests that increasing awareness of cardiovascular risk and risk factor screening for early diagnosis might have a large impact on hypertension care. Mass media campaigns in combination with training CHWs in prevention, diagnosis and control have proven to be effective in increasing diagnosis, follow‐up and medication adherence and could assist to improve hypertension care; future studies to analyse the effect of these measures on the hypertension care cascade in Sierra Leone are recommended.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We greatly appreciate the contributions of study participants, research assistants, Statistics Sierra Leone and the PRESSCO 2020 study group, funding from the Norwegian University of Science and Technology (Norway), CapaCare (Norway), the Center of Tropical Medicine and Travel Medicine, Amsterdam University Medical Center (the Netherlands), the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (the Netherlands) and the Masanga Research Unit, Masanga Hospital (Sierra Leone).
Geraedts TJM, Boateng D, Lindenbergh KC, van Delft D, Mathéron HM, Mönnink GLE, Martens JPJ, et al. Evaluating the cascade of care for hypertension in Sierra Leone. Trop Med Int Health. 2021;26:1470–1480. 10.1111/tmi.13664
Sustainable Development Goals: Good Health and Well‐being, Reduced Inequality
REFERENCES
- 1. WHO . Hypertension fact sheets. WHO. Published 2019. Accessed February 26, 2020. https://www.who.int/news‐room/fact‐sheets/detail/hypertension
- 2. WHO . Cardiovascular diseases (CVDs) fact sheets. WHO. Published 2017. Accessed February 26, 2020. https://www.WHO.int/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds)
- 3. Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS One. 2014;9:e104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leon DA, Addo J, Smeeth L. Hypertension in sub‐Saharan Africa: a systematic review. Hypertension. 2007;50(6):1012–8. 10.1161/HYPERTENSIONAHA.107.093336 [DOI] [PubMed] [Google Scholar]
- 5. Samson O, Muhihi A, Mohamed S, Ameh S, Ochimana C, Oluwasanu B, et al. P1946Hypertension prevalence, awareness, treatment and control and 10‐year estimated CVD risk in East and West Africa: pooled analysis of data from 4 African countries. Eur Heart J. 2019;40(Suppl_1). 10.1093/eurheartj/ehz748.0693 [DOI] [Google Scholar]
- 6. Desormais I, Amidou SA, Houehanou YC, Houinato SD, Gbagouidi GN, Preux PM, et al. The prevalence, awareness, management and control of hypertension in men and women in Benin, West Africa: The TAHES study. BMC Cardiovasc Dis. 2019;19(1). 10.1186/s12872-019-01273-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geldsetzer P, Manne‐Goehler J, Marcus ME, Ebert C, Zhumadilov Z, Wesseh CS, et al. The state of hypertension care in 44 low‐income and middle‐income countries: a cross‐sectional study of nationally representative individual‐level data from 1·1 million adults. Lancet. 2019;394(10199):652–62. 10.1016/S0140-6736(19)30955-9 [DOI] [PubMed] [Google Scholar]
- 8. McNairy ML, Lamb MR, Abrams EJ, Elul B, Sahabo R, Hawken MP, et al. Use of a comprehensive HIV care cascade for evaluating HIV program performance: Findings from 4 sub‐Saharan African countries. J Acquir Immune Def Syndr. 2015;70(2):e44–51. 10.1097/QAI.0000000000000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haber N, Tanser F, Bor J, Naidu K, Mutevedzi T, Herbst K, et al. From HIV infection to therapeutic response: a population‐based longitudinal HIV cascade‐of‐care study in KwaZulu‐Natal, South Africa. Lancet HIV. 2017;4(5):e223–30. 10.1016/S2352-3018(16)30224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallett TB, Eaton JW. A side door into care cascade for HIV‐infected patients? J Acquir Immune Defic Syndr. 2013;63(Suppl. 2):S228–32. 10.1097/QAI.0b013e318298721b [DOI] [PubMed] [Google Scholar]
- 11. Muddu M, Tusubira AK, Sharma SK, Akiteng AR, Ssinabulya I, Schwartz JI. Integrated hypertension and HIV care cascades in an HIV treatment program in Eastern Uganda: a retrospective cohort study. J Acq Immune Defic Synd. 2019;81(5):552–61. 10.1097/QAI.0000000000002067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manne‐Goehler J, Siedner MJ, Montana L, Harling G, Geldsetzer P, Rohr J, et al. Hypertension and diabetes control along the HIV care cascade in rural South Africa. J Int AIDS Soc. 2019;22(3):e25213. 10.1002/jia2.25213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berry KM, Parker WA, McHiza ZJ, Sewpaul R, Labadarios D, Rosen S, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Global Health. 2017;2(3):2011–2. 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odland ML, Bockarie T, Wurie H, Ansumana R, Lamin J, Nugent R, et al. Prevalence and access to care for cardiovascular risk factors in older people in Sierra Leone: a cross‐sectional survey. BMJ open. 2020;10(9):e038520. 10.1136/bmjopen-2020-038520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nosyk B, Montaner JSG, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: A population‐based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–9. 10.1016/S1473-3099(13)70254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ventura L. Poorest Countries in the World 2020. Global finance. Published 2020. Accessed March 28, 2021. https://www.gfmag.com/global‐data/economic‐data/the‐poorest‐countries‐in‐the‐world
- 17. Idriss A, Diaconu K, Zou G, Senesi RGB, Wurie H, Witter S. Rural‐urban health‐seeking behaviours for non‐communicable diseases in Sierra Leone. BMJ Global Health. 2020;5(2):e002024. 10.1136/bmjgh-2019-002024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Minor BL, Elliot V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Informatics. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Heart Association . Understanding Blood Pressure Readings. Aha. Published 2017. Accessed April 20, 2020. https://www.heart.org/en/health‐topics/high‐blood‐pressure/understanding‐blood‐pressure‐readings
- 21. Banga JD,Dijk JL, Dis I, Giepmans L, Goudswaard AN, Grobbee DE, et al. Multidisciplinaire richtlijn Cardiovasculair risicomanagement. Published2011.
- 22. Statistics Sierra Leone . Sierra Leone 2015 Population and Housing Census, National Analytical Report. Sierra Leone Official. Published online 2017.
- 23. StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC
- 24. Gnatiuc L, Alegre‐Díaz J, Halsey J, Herrington WG, López‐Cervantes M, Lewington S, et al. Adiposity and Blood Pressure in 110 000 Mexican Adults. Hypertension. 2017;69(4):608–14. 10.1161/HYPERTENSIONAHA.116.08791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Awad M, Ruzza A, Mirocha J, Setareh‐Shenas S, Pixton JR, Soliman C, et al. Prevalence of hypertension in the Gambia and Sierra Leone, western Africa: A cross‐sectional study. Cardiovasc J Africa. 2014;25(6):269‐78. 10.5830/CVJA-2014-058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benya H, Ikoona E, Gudina E. Prevalence of hypertension and its risk factors in western are urban, Freetown Sierra Leone. Sierra Leone J Biomed Res. 2020;12(1). [Google Scholar]
- 27. Sesay S. Non‐communicable Diseases: Diagnosis and Treatment Deskguide. Ministry of Health NCD‐MH Department Sierra Leone. 2019.
- 28. Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey‐Bassler K. The influence of gender and other patient characteristics on health care‐seeking behaviour: A QUALICOPC study. BMC Family Practice. Published online. 2016;17(1). 10.1186/s12875-016-0440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, et al. Inequities in access to health care in South Africa. J Public Health Policy. 2011;32(S1):S102–23. 10.1057/jphp.2011.35 [DOI] [PubMed] [Google Scholar]
- 30. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. Journal of Rural Health. 2006;22(2):140–6. 10.1111/j.1748-0361.2006.00022.x [DOI] [PubMed] [Google Scholar]
- 31. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206–13. 10.1111/j.1748-0361.2005.tb00084.x [DOI] [PubMed] [Google Scholar]
- 32. Witter S, Zou G, Diaconu K, Senesi RGB, Idriss A, Walley J, et al. Opportunities and challenges for delivering non‐communicable disease management and services in fragile and post‐conflict settings: Perceptions of policy‐makers and health providers in Sierra Leone. Conflict and Health. 2020;14(1). 10.1186/s13031-019-0248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oyerinde K, Harding Y, Amara P, Garbrah‐Aidoo N, Kanu R, Oulare M. Barriers to uptake of emergency obstetric and newborn care services in Sierra Leone: a qualitative study. J Community Med Health Educ. 2012;2:149. 10.4172/2161-0711.1000149 [DOI] [Google Scholar]
- 34. Miller CM, Ketlhapile M, Rybasack‐Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow‐up? A qualitative study from South Africa. Trop Med Int Health. 2010;15:48–54. 10.1111/j.1365-3156.2010.02514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV‐positive patients in rural South Africa: Implications for antiretroviral delivery in resource‐poor settings? Int J Tuberculosis Lung Dis. 2005;9(3):263–9. [PubMed] [Google Scholar]
- 36. Witter S, Brikci N, Harris T, Williams R, Keen S, Mujica A, et al. The free healthcare initiative in sierra leone: Evaluating a health system reform, 2010–2015. International Journal of Health Plan Manage. 2018;33(2):434–48. 10.1002/hpm.2484 [DOI] [PubMed] [Google Scholar]
- 37. Herskind J, Zelasko J, Bacher K, Holmes D. The outpatient management of hypertension at two Sierra Leonean health centres: a mixed‐method investigation of follow‐up compliance and patient‐reported barriers to care. Afr J Primary Health Care Fam Med. 2020;12(1):e1–7. 10.4102/phcfm.v12i1.2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou G, Witter S, Caperon L, Walley J, Cheedella K, Senesi RGB, et al. Adapting and implementing training, guidelines and treatment cards to improve primary care‐based hypertension and diabetes management in a fragile context: results of a feasibility study in Sierra Leone. BMC Public Health. 2020;20(1). 10.1186/s12889-020-09263-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. United Nations Development Programme, Sierra Leone . About Sierra Leone. Accessed July 27, 2021. https://www.sl.undp.org/content/sierraleone/en/home/countryinfo.html
- 40. Ministry of Health and Sanitation, Sierra Leone . Summary report of the 2017 SARA Plus in Sierra Leone : service availability and readiness assessment (SARA), quality of care survey, and data quality review. Freetown: Government of Sierra Leone Ministry of Health and Sanitation, 2017.
- 41. Margolis D, Rosas N, Turay A, Turay S. Findings from the 2014 Labor Force Survey in Sierra Leone. 2016. 10.1596/978-1-4648-0742-8 [DOI]
- 42. Vedanthan R, Bernabe‐Ortiz A, Herasme OI, Joshi R, Lopez‐Jaramillo P, Thrift AG, et al. Innovative approaches to hypertension control in low‐ and middle‐income countries. Cardiol Clin. 2017;35(1):99. 10.1016/J.CCL.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeet G, Thakur JS, Prinja S, Singh M, Nangia R, Sharma D, et al. Protocol for a systematic review of reviews evaluating effectiveness of mass media interventions for prevention and control of non‐communicable diseases. BMJ Open. 2020;10(6):e032611. 10.1136/bmjopen-2019-032611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabassum R, Froeschl G, Cruz JP, Colet PC, Dey S, Islam SMS. Untapped aspects of mass media campaigns for changing health behaviour towards non‐communicable diseases in Bangladesh. Globalization Health. 2018;14(1):7. 10.1186/s12992-018-0325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeet G, Thakur JS, Prinja S, Singh M. Community health workers for noncommunicable diseases prevention and control in developing countries: Evidence and implications. PLoS One. 2017;12(7):e0180640. 10.1371/journal.pone.0180640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gaziano TA, Abrahams‐Gessel S, Denman CA, Montano CM, Khanam M, Puoane T, et al. An assessment of community health workers’ ability to screen for cardiovascular disease risk with a simple, non‐invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: An observational study. Lancet Global Health. 2015;3(9):e556–63. 10.1016/S2214-109X(15)00143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaziano T, Abrahams‐Gessel S, Surka S, Sy S, Pandya A, Denman CA, et al. Cardiovascular disease screening by community health workers can be cost‐effective in low‐ resource countries. Health Aff. 2015;34(9):1538–45. 10.1377/hlthaff.2015.0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jobson G, Naidoo N, Matlakala N, Marincowitz G, Railton J, McIntyre JA, et al. Contextual factors affecting the integration of community health workers into the health system in Limpopo Province, South Africa. 10.1093/inthealth/ihz082 [DOI] [PMC free article] [PubMed]
- 49. Mehra R, Boyd LM, Lewis JB, Cunningham SD. Considerations for Building Sustainable Community Health Worker Programs to Improve Maternal Health. J Primary Care Community Health. 2020;11:215013272095367. 10.1177/2150132720953673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ronto R, Wu JH, Singh GM. Commentary The global nutrition transition: trends, disease burdens and policy interventions. Public Health Nutrition. 2018;21(12):2267–70. 10.1017/S1368980018000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. How to Diagnose High Blood Pressure . Accessed July 27, 2021. https://www.webmd.com/hypertension‐high‐blood‐pressure/guide/whypertension‐diagnosing‐high‐blood‐pressure
- 52. Koroma B, Rigon A, Walker J & Sellu SA. Urban Livelihoods in Freetown’s Informal Settlements. The Sierra Leone Urban Research Centre (SLURC). 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


