Abstract
Aims
Statin liver safety in non‐alcoholic fatty liver disease (NAFLD) patients is not well defined. We analysed differences in liver function tests, including alanine transaminase aminotransferase (ALT), aspartate transaminase (AST) and gamma‐glutamyl transpeptidase (GGT) in NAFLD patients treated or not treated with statins.
Methods
We performed a systematic review of MEDLINE via PubMed and EMBASE databases and metanalysis of clinical studies investigating levels of ALT, AST and GGT in NAFLD according to statin treatment. Mean difference (MD) and percentage MD were calculated between the two groups.
Results
We included 22 studies with 2345 NAFLD patients. Overall, 16 were before‐after interventional, five were cross‐sectional and one was combined cross‐sectional/interventional study. In all interventional studies, except one, patients had raised ALT, AST and GGT at baseline. Interventional studies showed reduced ALT values with an MD reduction of −27.2 U/L (95% CI −35.25/−19.15) and a percentage MD reduction of −35.41% (95% CI −44.78/−26.04). Also, AST values were reduced after statin treatment in interventional studies with an MD of −18.82 U/L (95% CI −25.63/−12.02) (percentage −31.78%, 95% CI −41.45/−22.11). Similarly, GGT levels were reduced after statin treatment with an MD of −19.93 U/L (95% CI −27.10/−12.77) (percentage −25.57%, 95% CI −35.18/−15.97). Cross‐sectional studies showed no difference in AST and GGT values between patients treated with and without statins.
Conclusion
In interventional studies, ALT, AST and GGT were reduced after statin treatment with a percentage mean difference of −35.41%, −31.78% and −25.57%, respectively, while observational studies showed a null effect, suggesting liver safety of statins in NAFLD patients.
Keywords: ALT, AST, GGT, liver, NAFLD, safety, statins
1. INTRODUCTION
The prevalence of non‐alcoholic fatty liver disease (NAFLD) is continuously increasing, especially in Western countries 1 and it is expected to become the leading cause of liver transplantation. 2
Patients with NAFLD are frequently characterized by the presence of cardio‐metabolic disorders, the most frequent including impaired fasting glucose/diabetes, overweight/obesity and dyslipidaemia. These conditions define the so‐called metabolic syndrome (MetS), of which NAFLD has long been regarded as the hepatic manifestation. 3 The presence of MetS increases cardiovascular risk in NAFLD patients. 4 , 5 , 6 In particular, the presence of an atherogenic dyslipidaemia, with elevated levels of triglyceride‐rich lipoproteins and low levels of high‐density lipoprotein cholesterol (HDL‐C), is a strong risk factor for cardiovascular events. 7 , 8
For this reason, NAFLD patients are often prescribed statins, which are associated with a substantial reduction in cardiovascular mortality due to several cardio‐metabolic diseases. 9 , 10 , 11
Statin treatment is usually safe and well tolerated by patients. 12 , 13 However, a minority of patients may experience side effects, such as myalgia rarely associated to rhabdomyolysis, and less frequently, elevation of liver enzymes. 14 , 15 Thus, transient asymptomatic elevations in aminotransferases could occur in 0.1–3% of patients, while fulminant hepatic failure is an extremely rare event (2 in 1 million of treated patients). 16 The discontinuation rate of patients due to these effects is variable, ranging from 1.8% 17 up to 12%, 18 which is notably associated with a subsequent increased risk of cardiovascular complications. 19
The prescription of statins may be challenging in patients with chronic liver disease, especially when an elevation of liver enzymes is present. 20 , 21 Despite a general agreement that statins may be safely prescribed to NAFLD patients, 22 firm data supporting this recommendation are still lacking.
We performed a systematic review and metanalysis of clinical studies investigating the safety of statin treatment in NAFLD patients. In particular, the effect of statin therapy on alanine aminotransferase (ALT), aspartate transaminase (AST) and gamma‐glutamyl transferase (GGT) levels was analysed.
2. METHODS
2.1. Selection of studies for inclusion
We conducted a systematic review of literature searching MEDLINE via PubMed and EMBASE databases for observational, randomized (RCT) and before‐after studies, using a combination of the following MESH terms and keywords for PubMed: “NAFLD”, “hydroxymethylglutaryl‐CoA reductase inhibitors”, “statins”, “pitavastatin”, “lovastatin”, “fluvastatin”, “simvastatin”, “atorvastatin”, “transaminases”, “aspartate aminotransferases”, “alanine transaminase”, “gamma‐glutamyltransferase”, “AST”, “ALT”, “GGT”, “liver enzymes” and “liver function tests”.
The search strategy on EMBASE included the following Emtree and keywords “hydroxymethylglutaryl coenzyme a reductase inhibitor” AND “nonalcoholic fatty liver” AND “human”/de AND (“article”/it OR “article in press”/it) AND “clinical article”/de, resulting in the retrieval of 21 articles.
The last search was run on 24 April 2020. There was no time restriction for the inclusion of articles.
Figure S1 in the Supporting Information reports the study selection process, which was performed according to the PRISMA guidelines.
2.2. Types of studies for inclusion
We included all clinical articles reporting liver transaminase values before and after statin administration and case–control studies reporting liver transaminase values in both case and control groups. We included only journal articles in English with full text available.
Studies including 15 or fewer patients were excluded. We excluded case reports/series, editorials/comments, letters, reviews and metanalyses, and experimental studies. Finally, we excluded interventional studies testing the effect of statins in association with other drugs. When a study reported data derived from both statins alone and statins combined with other drugs, only data from the statins alone arm were included in the metanalysis. No RCTs were found. We included 22 studies (16 before‐after studies, five cross‐sectional studies and one study reporting both cross‐sectional and interventional data).
2.3. Study selection
Two physicians (F.B. and D.P.) independently screened the titles and abstracts of manuscripts identified through the database searches to identify studies potentially eligible for further assessment. A third physician (M.D.B.) reviewed eligible studies for appropriateness and completeness. The study selection was performed in multiple phases. In the first phase, potentially relevant studies were obtained by combined searches of electronic databases using the selected above‐mentioned keywords. Then, studies not in English, not involving humans or not addressing the study question were excluded. In the second phase, studies were reviewed and selected according to the inclusion and exclusion criteria. Finally, F.B. and D.P. independently collected data from the reports.
2.4. Risk of bias
The risk of bias was estimated by “Risk of Bias Assessment tool for Non‐randomized Studies (RoBANS)” (https://abstracts.cochrane.org/2011‐madrid/risk‐bias‐assessment‐tool‐non‐randomized‐studies‐robans‐development‐and‐validation‐new), including six domains: item 1: selection of participants (selection bias); item 2: confounding variables (confounding bias); item 3: measurement of intervention/exposure (performance bias); item 4: blinding of outcome assessment (detection bias); item 5: incomplete outcome data (attrition bias); and item 6: selective outcome reporting (reporting bias).
2.5. Outcomes
The endpoints considered were the mean difference and percentual mean differences in AST, ALT and GGT levels between patients using or not using statins.
2.6. Data analysis
The continuous variables were reported as means with standard deviations (SDs). When data were reported as median and interquartile range (IQR), means and SDs were approximately estimated by means of the method described by Wan et al. 23 Mean differences, percentage mean differences, and their standard error were calculated for cross‐sectional studies. The delta method was used to calculate the asymptotic standard error of the percentage mean difference. 24 In before‐after studies, the pre/post‐intervention means were transformed to a mean difference and percentage mean difference; standard deviations were imputed according to Marinho et al., 25 following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions.
Pooled estimates were obtained by means of a random‐effects model. Results are shown in forest plots, with 95% confidence intervals (Cis). A subgroup analysis was performed after stratifying by study design (interventional vs. observational). Heterogeneity was assessed with the Q‐statistic and Moran's I2 and interpreted qualitatively as low (25–50%), moderate (50–75%) or high (75–100%).
All analyses were performed using the R (R Development Core Team) software version 3.6.1, with “rmeta” and “metafor” packages. All P‐values were two‐tailed, and the statistical significance level was set at .05.
3. RESULTS
3.1. Characteristics of included studies
Table 1 summarizes study characteristics, diagnosis of NAFLD and indication for statin treatment. No RCTs were found. We included 22 studies, 16 before‐after studies, five cross‐sectional and one study, reporting both baseline and interventional data (Table 1).
TABLE 1.
Characteristics of studies included in the metanalysis
| Year/Author | Study design | NAFLD diagnosis | Setting/indication to statin | Type and dose of statin | Age (y) | Women (%) | Total patients |
|---|---|---|---|---|---|---|---|
| Panel A: Observational studies | |||||||
| 2003 Kiyici 26 | Before‐after interventional study | Liver biopsy | NAFLD treatment; ALT elevation | Atorvastatin 10 mg | 50.2 | 55.56 | 27 |
| 2004 Hatzitolios 27 | Before‐after interventional study | Liver biopsy, ultrasound and CT scan | Mixed dyslipidaemia (Fredrickson type IIb); ALT elevation | Atorvastatin 20 mg | 53.0 | 53.57 | 28 |
| 2006 Antonopoulos 28 | Before‐after interventional study | Liver ultrasound | Hyperlipidaemic patients | Rosuvastatin 10 mg | 56.0 | 28.30 | 23 |
| 2006 Athyros 29 | Before‐after interventional study | Liver ultrasound | Non‐diabetic | Atorvastatin 20 mg | 60.0 | 35.00 | 63 |
| 2008 Hyogo 30 | Before‐after interventional study | Liver biopsy | ‐ | Atorvastatin 10 mg | 52.5 | 35.48 | 31 |
| 2009Abel 31 | Before‐after interventional study | ALT >40 U/L or AST > 37 U/L in men; ALT or AST > 31 U/L in women | Well‐controlled diabetes | Simvastatin 20 mg | 58.6 | 46.15 | 26 |
| 2010 Kimura 32 | Before‐after interventional study | Liver biopsy | Dyslipidaemia | Atorvastatin 10 mg | 50.2 | 32.56 | 43 |
| 2010 GREACE 33 | Post‐hoc analysis RCT | ALT >45 and <135 U/L or AST > 37 and <101 U/L | Secondary prevention | Atorvastatin 10 to 80 mg | 60 | 21 | 227 |
| 2011 Hyogo 34 | Before‐after interventional study | Liver biopsy | ‐ | Pitavastatin 2 mg | 50.6 | 55.00 | 20 |
| 2011 Maroni 35 | Retrospective | Liver ultrasound and abnormal liver enzyme values (AST ≥ 33 U/L and/or ALT ≥33 U/L; and/or GGT ≥ 49 U/L). | Dyslipidaemia |
Atorvastatin (n = 19) Simvastatin (n = 11) Rosuvastatin (n = 10) Fluvastatin (n = 2) Lovastatin (n = 1) |
54.5 | 30.23 | 43 |
| 2012 Han‐1 36 | Before‐after interventional study | ALT elevation ≥1.25 times and ≤2.5 times 40 IU/L | Hyperlipidaemic patients | Atorvastatin 10–20 mg | 54.9 | 46.03 | 85 |
| 2012 Han‐2 36 | Before‐after interventional study | ALT elevation ≥1.25 times and ≤2.5 times 40 IU/L | Hyperlipidaemic patients | Pitavastatin 2–4 mg | 55.8 | 46.97 | 88 |
| 2012 Hyogo 37 | Before‐after interventional study | Liver biopsy | Hyperlipidaemic patients | Atorvastatin 10 mg | 50.0 | 33.30 | 42 |
| 2012 Nakahara 38 | Before‐after interventional study | Liver biopsy | Hyperlipidaemic patients | Rosuvastatin 2.5 mg | 46.3 | 57.89 | 19 |
| 2015 Derosa 39 | Before‐after interventional study | Liver ultrasound | Hypertensive normo‐cholesterolemic patients | Simvastatin 20 mg | ‐ | 49.6 | 139 |
| 2017 Bril 40 | Before‐after interventional study | Liver biopsy | Prediabetes/diabetes | Simvastatin (67%) Rosuvastatin (21%) Atorvastatin and Pravastatin (12%) | ‐ | ‐ | 19 |
| 2017 Cioboatǎ 41 | Before‐after interventional study | Liver biopsy | Hyperlipidaemic patients | Atorvastatin 20 mg | ‐ | ‐ | 57 |
| 2018 Hadzi‐Petrushev 42 | Before‐after interventional study | Liver ultrasound | ‐ | Atorvastatin 20 mg | 43.0 | 0.00 | 20 |
| Panel B: Cross‐sectional studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year/Author | NAFLD diagnosis | Setting/indication to statin | Type and dose of statin | Age (y) | Women (%) | Total patients | On statin (n) | Not on statin (n) |
| 2006 Dallas Heart Study 43 | MR spectroscopy | General population screened for the presence of liver steatosis | Simvastatin (48%) Atorvastatin (32%) Pravastatin (12%) Fluvastatin (6%) Cerivastatin (1%) Lovastatin (1%) | 45.7 | ‐ | 638 | 54 | 584 |
| 2007 Ekstedt 44 | Liver biopsy | Chronic elevation of ALT and AST | Simvastatin (n = 11) Atorvastatin (n = 5) Pravastatin (n = 1) | 60.7 | 29.41 | 68 | 17 | 51 |
| 2016 Nascimbeni 45 | Liver biopsy | Diabetes |
Atorvastatin (n = 82) Rosuvastatin (n = 24) Simvastatin (n = 23) Pravastatin (n = 10) Fluvastatin (n = 3) Statin + ezetimibe (n = 10) |
53.0 | 60.00 | 343 | 154 | 192 |
| 2017 Bril 40 | Liver biopsy | Prediabetes/diabetes | 8% on high‐intensity and 79% on moderate intensity therapy. | 50.6 | 29.70 | 101 | 38 | 63 |
| 2017 Del Ben 21 | Liver ultrasound | ASCVD | Not reported | 59.9 | 35.29 | 442 | 230 | 212 |
| 2020 Khoo 46 | Liver ultrasound/CT/MR | NAFLD |
Simvastatin (68.6%) Atorvastatin (21.6%) Rosuvastatin (8.6%) Lovastatin (0.5%) Pravastatin (0.5%) |
54.3 | 47.90 | 428 | 185 | 243 |
ALT: alanine aminotransferase; ASCVD: atherosclerotic cardiovascular disease; AST: aspartate transaminase; CT: computed tomography; GGT: gamma‐glutamyl transferase; MR: magnetic resonance; NAFLD: non‐alcoholic fatty liver disease.
A total of 2345 NAFLD patients was included, 1000 from interventional and 1345 from observational studies (Table 1). In eight out of 16 interventional studies, diagnosis of NAFLD was biopsy‐proven, in four studies it was based on imaging findings, in four studies by raised serum liver enzymes, and in two studies by combined liver biopsy, ultrasound and imaging or liver enzymes (Table 1).
The mean age of patients ranged from 43 to 60 years, and the proportion of women ranged from 0.0% to ~60% (Table 1). The type of statin was atorvastatin 10–20 mg in ten studies, pitavastatin 2–4 mg in two studies, rosuvastatin 2.5 and 10 mg in two studies, two studies used simvastatin 20 mg, and two studies more than one statin. The duration of intervention ranged from 3 to 24 months.
3.2. Risk of bias of the included studies
Risk of bias for before‐after and cross‐sectional studies is reported in Figure 1. All observational studies were deemed to have an overall high risk of bias, primarily driven by lack of blinding of outcome assessment (detection bias), and bias in selection of the reported result (reporting bias).
FIGURE 1.
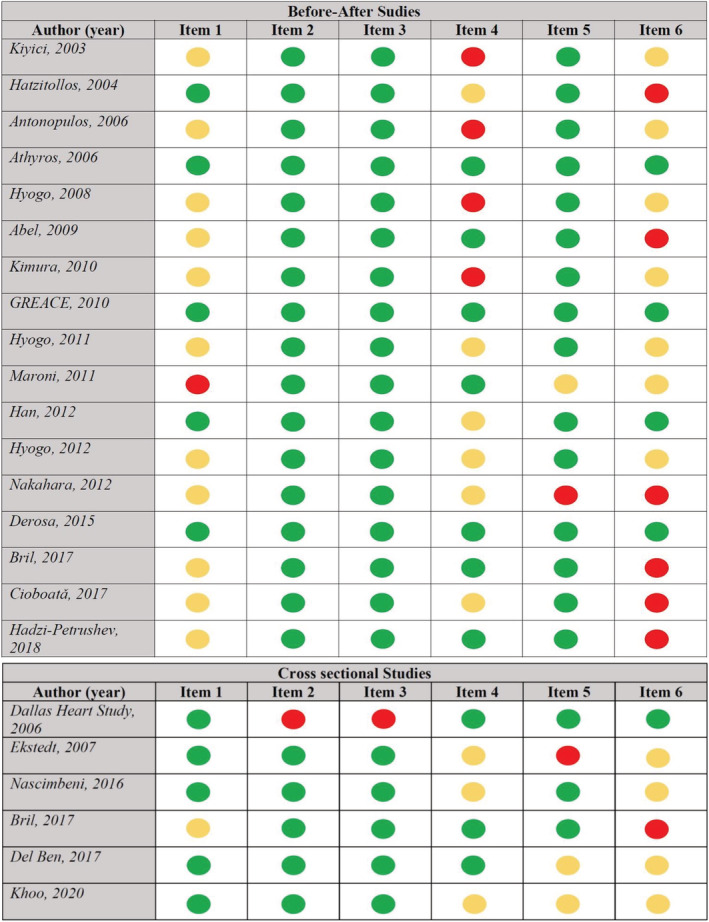
The Risk of Bias Assessment tool for Non‐randomized Studies (RoBANS) (red: high risk; yellow: unclear risk; green: low risk)
3.3. ALT, AST and GGT
All patients included in the interventional studies had a mean value of ALT >40 U/L, AST > 35 U/L and GGT > 50 U/L, except for Maroni 35 (see Table 2).
TABLE 2.
Liver function tests before and after statin treatment in interventional studies (Panel A) and in patients taking or not taking statins in cross‐sectional studies (Panel B)
| Year/Author | ALT before statin | ALT after statin | AST before statin | AST after statin | GGT before statin | GGT after statin |
|---|---|---|---|---|---|---|
| Panel A. Interventional studies | ||||||
| 2003 Kiyici 26 | 81.8 | 44.8 | 45.4 | 32.1 | 64.2 | 37.2 |
| 2004 Hatzitolios 27 | 115.0 | 76.6 | 68.0 | 46.0 | 98.0 | 33.0 |
| 2006 Antonopoulos 28 | 90.9 | 28.30 | 38.0 | 30.4 | 52.0 | 39.7 |
| 2006 Athyros 29 | 54.0 | 32.0 | 38.0 | 25.0 | 52.0 | 33.0 |
| 2008 Hyogo 30 | 89.4 | 35.9 | 51.1 | 25.8 | 87.0 | 51.0 |
| 2009 Abel 31 | 66.6 | 29.5 | 51.1 | 25.8 | 87.0 | 51.0 |
| 2010 Kimura 32 | 61.8 | 56.2 | 48.9 | 33.1 | 90.3 | 65.0 |
| 2010 GREACE 33 | 57.0 | 37.0 | 49.0 | 26.0 | 70.0 | 38.0 |
| 2011 Hyogo 34 | 102.1 | 68.2 | 62.6 | 41.8 | 94.5 | 59.6 |
| 2011 Maroni 35 | 37.6 | 44.7 | 26.3 | 34.3 | 59.0 | 86.5 |
| 2012 Han‐1 36 | 58.7 | 53.3 | 44.5 | 41.5 | 79.9 | 68.8 |
| 2012 Han‐2 36 | 56.4 | 51.3 | 39.5 | 39.0 | 75.7 | 64.8 |
| 2012 Hyogo 37 | 89.0 | 56.6 | 48.0 | 33.0 | 90.4 | 65.1 |
| 2012 Nakahara 38 | 68.7 | 50.3 | 40.1 | 33.8 | 78.7 | 61.4 |
| 2015 Derosa‐1 39 | 58.0 | 36.0 | 36.0 | 38.0 | ‐ | ‐ |
| 2015 Derosa‐2 39 | 55.0 | 38.0 | 58.0 | 39.0 | ‐ | ‐ |
| 2017 Bril 40 | 66.0 | 38.0 | 48.0 | 31.00 | ‐ | ‐ |
| 2017 Cioboatǎ 41 | 82.6 | 43.6 | 83.1 | 43.3 | 53.0 | 43.1 |
| 2018 Hadzi‐Petrushev 42 | 41.9 | 30.8 | 42.7 | 24.2 | ‐ | ‐ |
| Panel B. Cross‐sectional studies | ||||||
|---|---|---|---|---|---|---|
| ALT on statin | ALT not on statin | AST on statin | AST not on statin | GGT on statin | GGT not on statin | |
| 2006 Dallas Heart Study 43 | 30.0 | 25.0 | ‐ | ‐ | ‐ | ‐ |
| 2007 Ekstedt 44 | 61.0 | 63.0 | 35.0 | 36.0 | ‐ | ‐ |
| 2016 Nascimbeni 45 | 42.0 | 39.0 | 32.0 | 31.0 | 50.0 | 54.0 |
| 2017 Bril 40 | 75.0 | 57.0 | 53.0 | 43.0 | ‐ | ‐ |
| 2017 Del Ben 21 | 34.5 | 29.0 | 25.8 | 23.9 | 44.5 | 43.8 |
| 2020 Khoo 46 | 44.3 | 46.6 | 31.2 | 36.8 | 45.8 | 54.5 |
All values expressed as U/L.
ALT: alanine aminotransferase; AST: aspartate transaminase; GGT: gamma‐glutamyl transferase.
In patients treated with statins, the global mean difference of ALT values was −21.82 U/L (95% CI −29.06/−14.58) with a percentage mean difference reduction of −29.38% (95% CI −37.81/−20.95). This difference was most evident in interventional studies showing an ALT mean reduction of −27.2 U/L (95% CI −35.25/−19.15) and a percentage mean difference reduction of −35.41% (95% CI −44.78/−26.04), while it was less significant in cross‐sectional ones −5.85 U/L (95% CI −12.19/0.49) (percentage −12.18 U/L, 95% CI −21.60/−2.75) (Figure 2).
FIGURE 2.
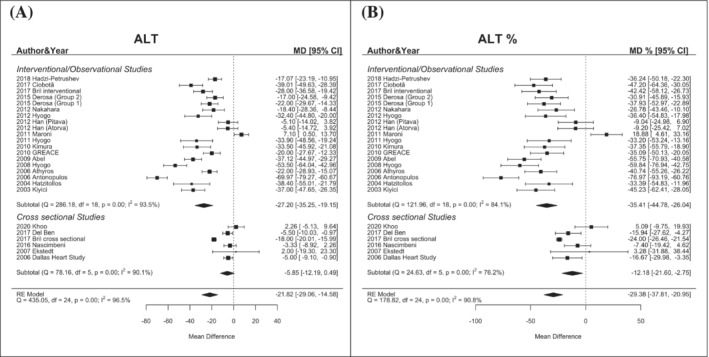
Changes in ALT levels (mean difference, Panel A; percentual difference, Panel B) according to statin treatment
The global mean difference of AST values in patients on statins was −15.25 U/L (95% CI −21.40/−9.11) with a percentual reduction of −25.91% (95% CI −35.17/−16.65). This difference was significant in interventional studies −18.82 U/L (95% CI −25.63/−12.02) (percentage −31.78%, 95% CI −41.45/−22.11) but not in cross‐sectional ones (Figure 3).
FIGURE 3.
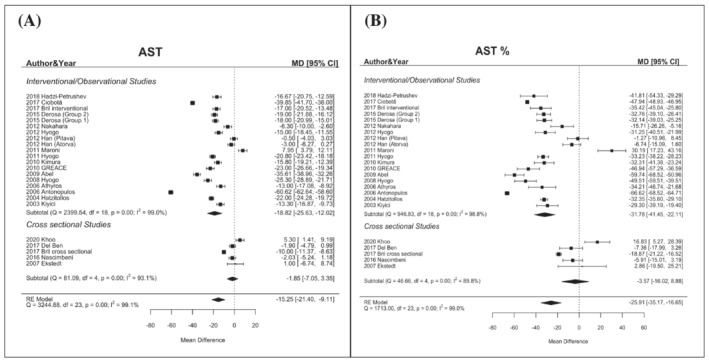
Changes in AST levels (mean difference, Panel A; percentual difference, Panel B) according to statin treatment
Regarding GGT, in patients treated with statins, the global mean difference was −15.78 U/L (95% CI −23.16/−8.40) with a percentual reduction of −20.29% (95% CI −30.24/−10.35). This difference was most evident in interventional studies −19.93 U/L (95% CI −27.10/−12.77) (percentage −25.57%, 95% CI −35.18/−15.97) while it was non‐significant in observational ones (Figure 4).
FIGURE 4.
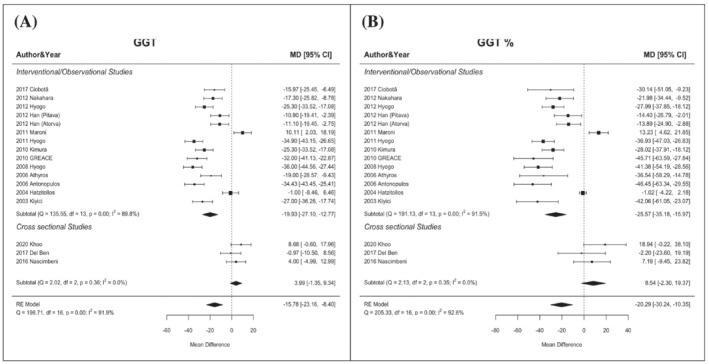
Changes in GGT levels (mean difference, Panel A; percentual difference, Panel B) according to statin treatment
4. DISCUSSION
This metanalysis provides pooled data on the safety of statin treatment in patients with NAFLD. Our results show that NAFLD patients prescribed statins in before‐after interventional studies had a reduction of baseline values for all safety outcomes analysed, such as ALT, AST and GGT. This evidence is even more important considering that almost all patients treated with statins had baseline raised levels of liver enzymes, reinforcing the evidence that statin therapy may be safe in NAFLD patients also when liver damage is almost clinically evident.
Our results add to previous evidence from a retrospective study including 4024 hyperlipidaemic patients which showed no excess in the risk of hepatotoxicity in statin‐treated patients with elevated liver enzymes. 47 Furthermore, data on more than 11 000 patients from randomized clinical trials showed that statins, in particular atorvastatin, improved NAFLD/NASH and reduced cardiovascular events twice as much as in those with normal liver function. 48 Finally, in 2013, a Cochrane meta‐analysis, including only two small RCTs, showed a reduction in liver enzymes by statin use. 20
Our data indicate that clinicians should not be discouraged from prescribing statins to NAFLD patients, even when a mild elevation of serum liver enzymes is present. Indeed, a clinically significant drug‐induced liver injury (DILI) by statins is very rare. Björnsson et al., 49 extracting data from the Swedish Adverse Reactions Advisory Committee, found that, in the period 1988–2010, only one patient prescribed with statin required a liver transplantation and two patients died as a consequence of a DILI. In addition, the estimated incidence of DILI (defined as aminotransferases more than five times the upper limit normal value [ULN], and/or alkaline phosphatase more than twice the ULN or bilirubin more than twice the ULN) was 1.6 × 10−4 person‐years. The majority of DILIs induced by statins are a consequence of idiosyncratic effect. However, as most of the statins are metabolized by CYP3A4, the concomitant use of a drug inhibiting CYP3A4 could elevate serum concentration of atorvastatin, simvastatin and lovastatin and may favour liver enzyme elevation. 50 , 51 Furthermore, physicians should perform a laboratory and instrumental work‐up to exclude other possible causes responsible for raised serum liver enzymes. 52
The mechanisms responsible for the beneficial association between statin use and reduced liver enzymes may be several. The reduction of hepatic lipid content induced by statins might result in less hepatic inflammation and oxidative stress, with lower lipidic peroxidation and ox‐LDL formation, which are increased in NAFLD patients and potentially contributing to disease progression. 53 In an experimental study, atorvastatin was associated with an increased activity of some antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase. 54 In addition, NAFLD patients with dyslipidaemia prescribed atorvastatin showed increased levels of the anti‐inflammatory adiponectin with a reduction of tumour necrosis factor‐alpha. 30
Statins may also exert a direct effect on the liver, as suggested by experimental studies showing a modulation of liver fibrosis and inflammation by statins. 55 All these mechanisms may account for the lower risk of hepatic decompensation and hepatocellular carcinoma described in liver disease patients on statins. 56 Finally, discontinuation of statin treatment resulted in an increased cardiovascular risk in several clinical settings, 57 suggesting that the benefits of continuing statin treatment outweigh putative risks.
Unfortunately, there was too little available data to investigate whether liver enzyme reduction paralleled cholesterol lowering or if this effect was related to a direct/pleiotropic effect of statins. Furthermore, we also hypothesized that liver enzyme improvement might be related to body weight loss. However, available data do not support this hypothesis, as in only one interventional study did BMI improve after statin therapy, 29 and in all other studies no BMI change after statin therapy was reported.
4.1. Strengths and limitations
In the majority of interventional studies, NAFLD was diagnosed by liver biopsy or second‐level imaging techniques such as CT or MR spectroscopy, giving robustness to their results. Conversely, the diagnosis of NAFLD was more heterogeneous in observational studies, ranging from liver ultrasound to biochemical variables (liver enzymes, FLI). This is mainly due to the impossibility of performing liver biopsy on large populations and may account for the different result between the two types of studies. A limitation of this analysis lies in the lack of data from RCTs, as all interventional studies were unblinded single‐arm interventions or post‐hoc analysis of RCTs. Furthermore, despite most studies used atorvastatin, we cannot draw any conclusions on the effect of different statins on liver enzymes. In addition, no study investigated the effect of very high‐intensity statins (i.e., atorvastatin 40 mg or rosuvastatin 20 mg) or directly compared the effect of different statins. Finally, our data should be interpreted with caution given the high heterogeneity found among studies.
5. CONCLUSIONS
Our analysis shows that NAFLD patients prescribed statins have a significant reduction in liver enzymes. These data support the safe use of statins in these patients.
ACKNOWLEDGEMENT
The work reported in this article did not receive any special funding. Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement. [Correction added on 20 May 2022, after first online publication: CRUI funding statement has been added.]
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
D.P. was responsible for the conceptualization and design of the study, data curation, writing, review manuscript, and is the guarantor of the manuscript. F.B., A.P., D.M. and G.G. curated the data and wrote and reviewed the manuscript. A.D.R. and A.F. conducted the formal analysis and reviewed the manuscript. F.A., L.D.E. and M.D.B. wrote and reviewed the manuscript.
Supporting information
FIGURE S1 Flow diagram for study selection
Pastori D, Pani A, Di Rocco A, et al. Statin liver safety in non‐alcoholic fatty liver disease: A systematic review and metanalysis. Br J Clin Pharmacol. 2022;88(2):441–451. 10.1111/bcp.14943
Maria Del Ben and Francesco Baratta are joint senior authors of this article.
REFERENCES
- 1. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 2. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547‐555. [DOI] [PubMed] [Google Scholar]
- 3. Angelico F, Del Ben M, Conti R, et al. Non‐alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18(5):588‐594. [DOI] [PubMed] [Google Scholar]
- 4. Käräjämäki AJ, Bloigu R, Kauma H, et al. Non‐alcoholic fatty liver disease with and without metabolic syndrome: different long‐term outcomes. Metabolism. 2017;66:55‐63. [DOI] [PubMed] [Google Scholar]
- 5. Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73(8):948‐963. [DOI] [PubMed] [Google Scholar]
- 6. Pastori D, Sciacqua A, Marcucci R, et al. Non‐alcoholic fatty liver disease (NAFLD), metabolic syndrome and cardiovascular events in atrial fibrillation. A prospective multicenter cohort study. Intern Emerg Med. 2021. 10.1007/s11739-021-02682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pastori D, Baratta F, Novo M, et al. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non‐alcoholic fatty liver disease. J Clin Med. 2018;7(11):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride‐rich lipoproteins and high‐density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267‐1278. [DOI] [PubMed] [Google Scholar]
- 10. Pastori D, Farcomeni A, Milanese A, et al. Statins and major adverse limb events in patients with peripheral artery disease: a systematic review and meta‐analysis. Thromb Haemost. 2020;120(5):866‐875. [DOI] [PubMed] [Google Scholar]
- 11. Pastori D, Baratta F, Di Rocco A, et al. Statin use and mortality in atrial fibrillation: a systematic review and meta‐analysis of 100,287 patients. Pharmacol Res. 2021;165:105418. [DOI] [PubMed] [Google Scholar]
- 12. Mach F, Ray KK, Wiklund O, et al. Adverse effects of statin therapy: perception vs. the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526‐2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol. 2013;10(8):453‐464. [DOI] [PubMed] [Google Scholar]
- 14. Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757‐769. [DOI] [PubMed] [Google Scholar]
- 15. Šimić I, Reiner Ž. Adverse effects of statins—myths and reality. Curr Pharm Des. 2015;21(9):1220‐1226. [DOI] [PubMed] [Google Scholar]
- 16. Bhardwaj SS, Chalasani N. Lipid‐lowering agents that cause drug‐induced hepatotoxicity. Clin Liver Dis. 2007;11(3):597‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newman C, Tsai J, Szarek M, Luo D, Gibson E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97(1):61‐67. [DOI] [PubMed] [Google Scholar]
- 18. Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472‐483. [DOI] [PubMed] [Google Scholar]
- 19. Marrs JC, Kostoff MD. Discontinuation of statins: what are the risks? Curr Atheroscler Rep. 2016;18(7):41. [DOI] [PubMed] [Google Scholar]
- 20. Eslami L, Merat S, Malekzadeh R, Nasseri‐Moghaddam S, Aramin H. Statins for non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623. [DOI] [PubMed] [Google Scholar]
- 21. Del Ben M, Baratta F, Polimeni L, et al. Under‐prescription of statins in patients with non‐alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2017;27:161‐167. [DOI] [PubMed] [Google Scholar]
- 22. Pastori D, Polimeni L, Baratta F, Pani A, Del Ben M, Angelico F. The efficacy and safety of statins for the treatment of non‐alcoholic fatty liver disease. Digest Liver Dis. 2015;47(1):4‐11. [DOI] [PubMed] [Google Scholar]
- 23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolter KM. Introduction to Variance Estimation. New York: Springer; 1985. [Google Scholar]
- 25. Marinho VC, Higgins JP, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003;(1):CD002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol/J Canadien de Gastroenterologie. 2003;17(12):713‐718. [DOI] [PubMed] [Google Scholar]
- 27. Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega‐3 fatty acids, atorvastatin and orlistat in non‐alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23(4):131‐134. [PubMed] [Google Scholar]
- 28. Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non‐alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184(1):233‐234. [DOI] [PubMed] [Google Scholar]
- 29. Athyros VG, Mikhailidis DP, Didangelos TP, et al. Effect of multifactorial treatment on non‐alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22(5):873‐883. [DOI] [PubMed] [Google Scholar]
- 30. Hyogo H, Tazuma S, Arihiro K, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism: Clin Exp. 2008;57(12):1711‐1718. [DOI] [PubMed] [Google Scholar]
- 31. Abel T, Feher J, Dinya E, Eldin MG, Kovacs A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with non‐alcoholic fatty liver disease. Med Sci Monit. 2009;15:MS6‐MS11. [PubMed] [Google Scholar]
- 32. Kimura Y, Hyogo H, Yamagishi S, et al. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J Gastroenterol. 2010;45(7):750‐757. [DOI] [PubMed] [Google Scholar]
- 33. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post‐hoc analysis. Lancet. 2010;376(9756):1916‐1922. [DOI] [PubMed] [Google Scholar]
- 34. Hyogo H, Ikegami T, Tokushige K, et al. Efficacy of pitavastatin for the treatment of non‐alcoholic steatohepatitis with dyslipidemia: an open‐label, pilot study. Hepatol Res. 2011;41(11):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 35. Maroni L, Guasti L, Castiglioni L, et al. Lipid targets during statin treatment in dyslipidemic patients affected by nonalcoholic fatty liver disease. Am J Med Sci. 2011;342(5):383‐387. [DOI] [PubMed] [Google Scholar]
- 36. Han KH, Rha SW, Kang HJ, et al. Evaluation of short‐term safety and efficacy of HMG‐CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol. 2012;6(4):340‐351. [DOI] [PubMed] [Google Scholar]
- 37. Hyogo H, Yamagishi S, Maeda S, Kimura Y, Ishitobi T, Chayama K. Atorvastatin improves disease activity of nonalcoholic steatohepatitis partly through its tumour necrosis factor‐alpha‐lowering property. Digest Liver Dis. 2012;44(6):492‐496. [DOI] [PubMed] [Google Scholar]
- 38. Nakahara T, Hyogo H, Kimura Y, et al. Efficacy of rosuvastatin for the treatment of non‐alcoholic steatohepatitis with dyslipidemia: an open‐label, pilot study. Hepatol Res. 2012;42(11):1065‐1072. [DOI] [PubMed] [Google Scholar]
- 39. Derosa G, Mugellini A, Pesce RM, D'Angelo A, Maffioli P. Perindopril and barnidipine alone or combined with simvastatin on hepatic steatosis and inflammatory parameters in hypertensive patients. Eur J Pharmacol. 2015;766:31‐36. [DOI] [PubMed] [Google Scholar]
- 40. Bril F, Portillo Sanchez P, Lomonaco R, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102(8):2950‐2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cioboată R, Găman A, Traşcă D, et al. Pharmacological management of non‐alcoholic fatty liver disease: atorvastatin versus pentoxifylline. Exp Ther Med. 2017;13(5):2375‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hadzi‐Petrushev N, Dimovska K, Jankulovski N, Mitrov D, Mladenov M. Supplementation with alpha‐tocopherol and ascorbic acid to nonalcoholic fatty liver disease's statin therapy in men. Adv Pharm Sci. 2018;2018:4673061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44(2):466‐471. [DOI] [PubMed] [Google Scholar]
- 44. Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non‐alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow‐up study. J Hepatol. 2007;47(1):135‐141. [DOI] [PubMed] [Google Scholar]
- 45. Nascimbeni F, Aron‐Wisnewsky J, Pais R, et al. Statins, antidiabetic medications and liver histology in patients with diabetes with non‐alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khoo S, Wong VW, Goh GB, et al. Suboptimal treatment of dyslipidemia in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(2):320‐325. [DOI] [PubMed] [Google Scholar]
- 47. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287‐1292. [DOI] [PubMed] [Google Scholar]
- 48. Doumas M, Imprialos K, Dimakopoulou A, Stavropoulos K, Binas A, Athyros VG. The role of statins in the management of nonalcoholic fatty liver disease. Curr Pharm Des. 2018;24(38):4587‐4592. [DOI] [PubMed] [Google Scholar]
- 49. Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post‐marketing. J Hepatol. 2012;56(2):374‐380. [DOI] [PubMed] [Google Scholar]
- 50. Karahalil B, Hare E, Koç G, Uslu İ, Şentürk K, Özkan Y. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68(4):254‐260. [DOI] [PubMed] [Google Scholar]
- 51. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565‐581. [DOI] [PubMed] [Google Scholar]
- 52. Bays H, Cohen DE, Chalasani N, Harrison SA, The National Lipid Association's Statin Safety Task Force . An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3):S47‐S57. [DOI] [PubMed] [Google Scholar]
- 53. Ferro D, Baratta F, Pastori D, et al. New insights into the pathogenesis of non‐alcoholic fatty liver disease: gut‐derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12(9):2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goodarzi Z, Karami E, Yousefi S, Dehdashti A, Bandegi AR, Ghanbari A. Hepatoprotective effect of atorvastatin on cadmium chloride induced hepatotoxicity in rats. Life Sci. 2020;254:117770. [DOI] [PubMed] [Google Scholar]
- 55. Vargas JI, Arrese M, Shah VH, Arab JP. Use of statins in patients with chronic liver disease and cirrhosis: current views and prospects. Curr Gastroenterol Rep. 2017;19(9):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamal S, Khan MA, Seth A, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta‐analysis. Am J Gastroenterol. 2017;112(10):1495‐1505. [DOI] [PubMed] [Google Scholar]
- 57. Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population‐based cohort study in France. Eur Heart J. 2019;40(43):3516‐3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Flow diagram for study selection


