Abstract
Aim
To present the 5 years outcomes of a reconstructive surgical protocol for peri‐implantitis defects with different morphologies, by means of deproteinized bovine bone mineral with 10% collagen (DBBMC).
Material and Methods
The original population of this case series consisted of 75 patients with one crater‐like defect and probing depth (PD) ≥6 mm. After flap elevation, defects were assigned to one characteristic class and treated by means of DBBMC. Following healing, patients were enrolled in an individualized supportive periodontal/peri‐implant (SPT) program.
Results
Fifty‐one patients reached the 5 years examination, as 11 patients were lost to follow‐up and 13 implants were removed. Overall treatment success was registered in 29 patients (45.3%). Mean PD and BOP significantly decreased at one year and remained stable for the rest of observation period. No correlation was found between implant survival rate and defect configuration (p = 0.213). Patients, who did not fully adhere to the SPT, experienced more complications and implant loss than those who regularly attended recall appointments (p = 0.009).
Conclusions
The proposed reconstructive treatment resulted in a high 5 years implant survival rate in patients who fully adhered to SPT. The resolution of the peri‐implantitis defect does not seem significantly associated with the defect configuration at the time of treatment.
Keywords: biomaterial, bone substitute, defect fill, peri‐implantitis, surgical treatment
1. INTRODUCTION
Peri‐implantitis is a plaque‐associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri‐implant mucosa and subsequent progressive loss of supporting bone (Berglundh, Armitage, et al., 2018). Its prevalence has been largely evaluated in recent population cross‐sectional studies (Romandini et al., 2019; Romandini et al., 2021). Non‐surgical approaches appear to be ineffective for the resolution of the disease, particularly in the most severe cases (Renvert et al., 2019; Roccuzzo et al., 2020; De Ry et al., 2020). On the other hand, several surgical treatment protocols have been suggested, even though information on long‐term outcomes is limited (Roccuzzo et al., 2021) to a few studies only (Berglundh, Armitage, et al., 2018; Parma Benfenati et al., 2020). Regardless of the treatment performed, the complete removal of the inflamed tissue and the decontamination of the implant surface are the fundamental initial steps to treatment success (Koo et al., 2019). Thereafter, the ideal procedure should aim at a reconstructive technique to recreate the conditions that favor re‐osseointegration and limit the postoperative soft tissue recession. Even though several reconstructive approaches have been presented, “the evidence on the efficacy of the treatment of peri‐implantitis defects by reconstructive procedures seems limited, especially in the long‐term” (Tomasi et al., 2019).
Recent data suggest a potential association between implant surface characteristics and long‐term results of reconstructive procedures (Roccuzzo et al., 2017, 2020), while controversial data are reported in regard to the correlation between defect morphology and the clinical outcomes (Schwarz et al., 2010; Roccuzzo et al., 2016; Aghazadeh et al., 2020).
The aim of this study was to present the 5 years clinical results of a reconstructive surgical procedure of peri‐implantitis infrabony defects and the possible correlation between the outcome of the intervention and the defect configuration at the time of treatment.
2. MATERIALS AND METHODS
2.1. Patient population
The original population consisted of 75 patients with one crater‐like defect, around sandblasted large grit and acid‐etched surface (SLA) dental implants (Straumann Group AG, Basel CH). Details of the treatment protocol have been described in a previous publication reporting on the 1 year treatment outcomes (Roccuzzo et al., 2016). In brief, 75 patients (39 males and 36 females; mean age: 57.8 ± 8.5 years; 11 smokers), who presented a single peri‐implantitis crater‐like lesion with a PD of ≥6 mm and no implant mobility, were consecutively treated from those attending the principle investigator's private office (specialist periodontal practice, northwestern Italy) between January 2010 and September 2014.
Exclusion criteria were as follows:
(1) PD <6 mm;
(2) Class II defects (characterized by consistent horizontal bone loss);
(3) Multiple defects;
(4) Implant mobility;
(5) No interest in participating in the study;
(6) Implants placed by other clinicians.
Patients had been treated, in the previous years, for periodontitis and subsequently had received therapy by means of non‐submerged tissue‐level dental implants. All implants supported either a single crown or a fixed dental prosthesis.
All patients were informed that their data would be used for statistical analysis and gave their informed consent to the treatment. The present case series was performed in accordance with the revised principles stated in the Declaration of Helsinki and the Good Clinical Practice Guidelines. The study protocol was approved by the Institutional Ethics Committee (Nr.00507/2020).
2.2. Surgical procedure, peri‐implant defect clinical assessment, and post‐surgical care
All surgeries were performed by one surgeon (MR) with 25 years of experience in periodontal surgery. Following the elevation of a muco‐periosteal flap, all granulation tissue was completely removed from the defect area, by means of titanium curettes and a titanium brush (Tigran Peri‐brush, Tigran Technologies AB, Malmö; Sweden) under irrigation. Consequently, implant surfaces were covered with EDTA 24% (Prefgel, Straumann AG) for 2 min and chlorhexidine 1% gel (Corsodyl dental gel, GlaxoSmithKline) for 2 min. Thereafter, the infrabony defects were filled with a deproteinized bovine bone mineral with 10% collagen (DBBMC) (Bio‐Oss Collagen, Geistlich). In case of lack of keratinized tissue, a connective tissue graft was excised from the tuberosity area and applied to cover the entire defect to ensure stability of the graft material. Finally, the flap was sutured around the collar of the implant, with a thick cuff seal to ensure an optimal non‐submerged healing (Figure 1a–f).
FIGURE 1.
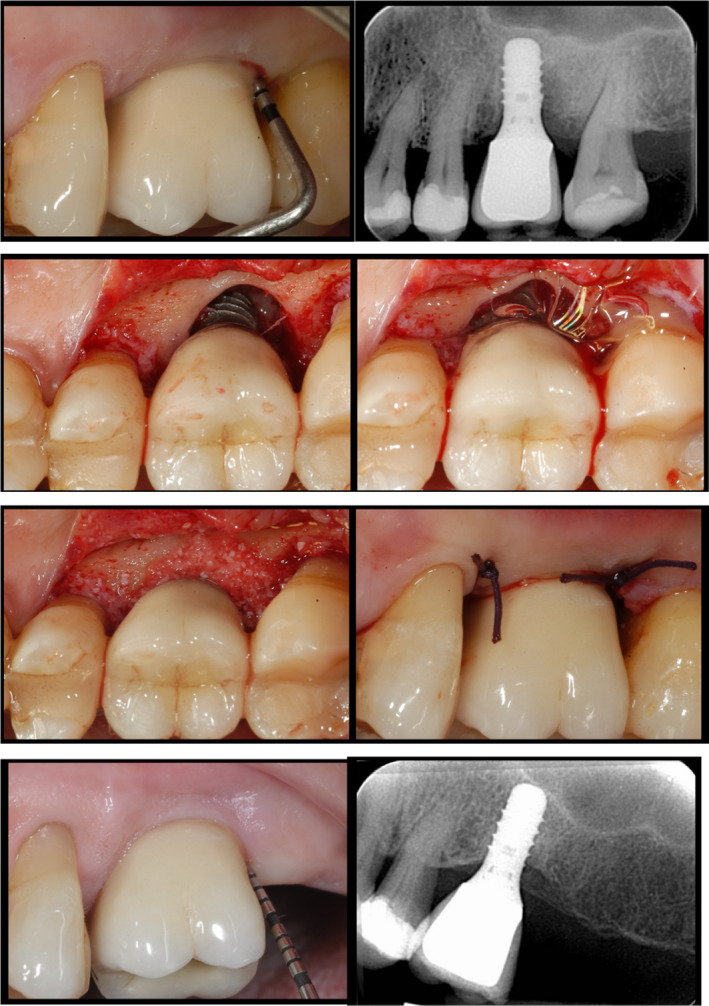
(a) Maxillary left molar implant, showing a PD of 12 mm, bleeding, pus on probing with radiographic (b) and intraoperative clinical signs (c) of an infrabony defect crater‐like defect (d) decontaminated with chlorhexidine 1% gel. (e) Deproteinized bovine bone mineral with 10% collagen is applied in the infrabony defect (f) and the suture ensures an optimal not submerged healing. (g) 5‐year follow‐up: clinical situation around the implant: no signs of inflammation (i.e. BoP, pus), minimal probing pocket depth (PD = 5 mm) (h) and the radiographic image reveals substantial bone fill
Peri‐implant defect class configuration was assessed, after peri‐implant granulation tissue removal, by an independent examiner, on the basis of the circumferential and intra‐bony components of the lesion according to the classification proposed by Schwarz et al. (2007).
Postoperative care included 1 g of amoxicillin and clavulanic acid twice a day for 6 days and 0.2% chlorhexidine digluconate rinse for 1 min three times a day for 3 weeks. After the healing phase, patients were placed on an individually tailored SPT program.
2.3. Supportive peri‐implant/periodontal therapy (SPT)
All patients were asked to follow an individualized supportive care program depending on the initial diagnosis, their risk profile, and the results of the therapy. Patients were recalled at various intervals for oral hygiene measures, biofilm removal, monitoring oral health, and reduction in modifiable risks related to peri‐implantitis. Every effort was made to motivate the patient and facilitate their ability to maintain optimal plaque control both at implants and at teeth, aiming for a low full mouth plaque score (Heitz‐Mayfield et al., 2018a). Patients, who fully complied with the recall program for the 5‐year period, were categorized as “adherent” to SPT. Patients, who were not able to completely follow the strict and individualized maintenance program, including all the suggested additional treatments, were classified as “not‐adherent” to SPT.
2.4. Clinical examinations
At the 1 and 5 years follow‐up, examination implant survival (i.e., presence of the implant in the oral cavity) and success rates (i.e., no PD>5 mm, no BOP, no PUS, no further radiographic bone loss) were calculated and reported in percentages. Moreover, an examiner (SG) with more than 15 years of experience as dental hygienist, blinded to the defect morphology, recorded, for each treated implant, PD measured at four sites (mesial, buccal, distal, and lingual) by means of a periodontal probe (XP23/UNC 15; Hu‐Friedy). At the same time and sites, the presence of dental plaque (Pl), bleeding on probing (BOP), of pus was recorded (Figure 1g,h). Figures were rounded off to the nearest millimeter. Data are reported in accordance with the STROBE checklist.
2.5. Radiographic examinations
Digital peri‐apical radiographs were taken at baseline, at 1, and at 5 years follow‐up, using a long‐cone technique. Film holders, with no individualized bite blocks, were used. The baseline and follow‐up images were displayed on a computer monitor and inserted in a commercially available software (ImageJ, U.S. National Institutes of Health). Consequently, based on the fact that all implants were Straumann tissue‐level implants, the known distance of 1.0 and 1.25 mm between implant threads was used to calibrate the radiographs. One of the authors (D.P), not involved in patients’ treatment, assigned each image to either the group of “no bone loss/bone gain” or “further bone loss,” for the evaluation of the variable “treatment success.”
2.6. Statistical analysis
Each patient contributed with one peri‐implantitis defect and was, therefore, considered as the statistical unit. The clinical parameters (PD, KT, REC, PI, and BOP) were expressed as mean values or percentages (%) ± SD. The presence or absence of suppuration (PUS) was reported as a dichotomous variable. Since quantitative variables did not follow normal distribution according to Kolmogorov–Smirnov test, non‐parametric tests were applied. Kruskal–Wallis tests were used to investigate between‐group differences and Wilcoxon test for intra‐group ones, including Bonferroni's correction in case of multiple pairwise comparisons. McNemar test was used to assess changes of the variable PUS, as a binary outcome. Odds ratio was estimated to assess the likelihood of survival depending on the adherence to SPT using a simple binary logistic regression. All the tests were two‐tailed. Significance level of reference was set at p < 0.05.
3. RESULTS
Of the initial 75 patients, 51 (68%) reached the 5 years examination and 11 patients (15%) were lost to follow‐up. Reasons for drop‐out are listed in Table 1. The overall 5 years implant survival rate was 80% (n = 51) as 13 implants had to be removed. Successful therapy, defined as absence of PD>5 mm, BOP, PUS, and radiographic bone loss, was found in 37 patients (52.1%) at 1 year, and 29 patients (45.3%) at the 5 years examination (Table 2a).
TABLE 1.
Patient (implant) sample during the study period
| Patients | Implant loss | Lost to follow‐up | |
|---|---|---|---|
| Baseline | 75 | – | – |
| 1 year | 71 | 4 | 0 |
| 5 years | 51 | 13 | 11 |
| List of reasons for drop‐out | |||
| Death | 1 | ||
| Severe health problems | 3 | ||
| Moved | 1 | ||
| Refused to accept a visit | 6 | ||
| TOTAL | 11 | ||
TABLE 2.
Overall results of treatment at 5 years and in relation to the defect configuration
| n | % | |
|---|---|---|
| Success | 29 | 39 |
| Partial resolution | 22 | 29 |
| Lost to follow‐up | 11 | 15 |
| Implant loss | 13 | 17 |
| Defect configuration | n | % | Survival rate | Implant loss | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Ia | 9 | 14.0 | 9 | 14.0 | 0 | 0 |
| Ib | 21 | 33.0 | 17 | 81.0 | 4 | 19.0 |
| Ic | 13 | 20.0 | 11 | 85.0 | 2 | 15.0 |
| Id | 12 | 19.0 | 9 | 75.0 | 3 | 25.0 |
| Ie | 9 | 14.0 | 5 | 56.0 | 4 | 44.0 |
| Total | 64 | 100 | 51 | 80.0 | 13 | 20.0 |
More in details, considering the 51 implants still in function at 5 years, the mean PD statistically decreased from 6.89 ± 1.58 to 3.82 ± 1.07 mm at 1 year (p < 0.001), and to 4.06 ± 1.12 mm at 5 years (p < 0.001).
The number of sites with PD>6 mm changed from 2.80 ± 0.96 to 0.45 ± 1.05 (p < 0.001) 1 year after treatment and remained stable through time (p = 0.661), as well as the mean deepest pocket which decreased from 8.92 ± 1.89 to 4.65 ± 1.40 (p < 0.001) and to 5.02 ± 1.44 at the last follow‐up visit (p = 0.177). Through time, the overall BOP decreased from 70.6 ± 34.9% to 9.3 ± 18. 7% at 1 year (p < 0.001), and 17.2 ± 22.1% at 5 years (p = 0.054). At baseline, plaque was detected around 13.2 ± 24.2% of all implants which reached the 5 years visit and changed to 5.9 ± 13.8% at 1 year, and to 15.7 ± 23.4% at 5 years. Pus was detected around 15 implants (29.4%) before surgical treatment, while it was present only in one (2%) of them at the 1 year follow‐up (p < 0.001) and in 3 (5.9%) of them at the final examination (p = 0.013). The overall clinical parameters are summarized in Table 3.
TABLE 3.
Clinical parameters in the 51 patients which reached the 5 years examination (means ± SD)
| Baseline | 1 year | 5 years | p value | |||
|---|---|---|---|---|---|---|
| Baseline vs 1 year | 1 year vs 5 years | Baseline vs 5 years | ||||
| PD (mm)∋ | 6.89 ± 1.58 | 3.82 ± 1.07 | 4.06 ± 1.12 | <0.001 | 0.332 | <0.001 |
| PD≥6 (mm)∋ | 2.80 ± 0.96 | 0.45 ± 1.05 | 0.63 ± 1.13 | <0.001 | 0.661 | <0.001 |
| Deepest PD (mm)∋ | 8.92 ± 1.89 | 4.65 ± 1.40 | 5.02 ± 1.44 | <0.001 | 0.177 | <0.001 |
| KT (mm)∋ | 3.37 ± 1.41 | 2.76 ± 1.31 | 2.78 ± 1.19 | 0.008 | 1.000 | 0.007 |
| REC (mm)∋ | – | 0.69 ± 0.79 | 0.69 ± 0.79 | – | 1.000 | – |
| BOP at the implant site (%)∋ | 70.6 ± 34.9 | 9.3 ± 18.7 | 17.2 ± 22.1 | <0.001 | 0.054 | <0.001 |
| Pl at the implant site (%)∋ | 13.2 ± 24.2 | 5.9 ± 13.8 | 15.7 ± 23.4 | 0.090 | 0.020 | 1.000 |
| Pus (%)# | 15 (29.4) | 1 (2.0) | 3 (5.9) | <0.001 | 1.000 | 0.013 |
Abbreviations: BOP, Bleeding on probing at the implant site; Pl, Plaque at the implant site.
∋Wilcoxon test with Bonferroni´s correction.
#McNemar test with Bonferroni´s correction.
When considering the differences in the percentages of implant survival rates among the different peri‐implant defect configuration (Table 2b), no statistically significant difference was detected (p = 0.123). All differences between and intra‐groups are listed in details in Tables 4and 5. A statistically significant correlation was found between patients’ adhesion to SPT and the 5 years implant survival rate (OR 0.17; p = 0.009; CI 95% 0.05–0.64) (Table 6).
TABLE 4.
Differences pre‐ and 5 years treatment between‐groups and intra‐groups (means ± SD)
| Defect configuration | Ia (n = 9) | Ib (n = 17) | Ic (n = 11) | Id (n = 9) | Ie (n = 5) | p (between) |
|---|---|---|---|---|---|---|
| PUS elimination (%) | 3/3 (100) | 2/2 (100)Ø | 4/4 (100) | 4/4 (100) | 1/2 (50) | 0.219 |
| p (intra) | p = 0.083 | p = 1.000 | p = 0.046 | p = 0.046 | p = 0.317 | |
| PD (mm) | 1.67 ± 0.94 | 2.41 ± 1.30 | 3.32 ± 1.82 | 3.22 ± 1.61 | 4.60 ± 2.75 | 0.042 |
| p (intra) | p = 0.012 | p < 0.001 | p = 0.003 | p = 0.008 | p = 0.042 | |
| PD≥6 mm§ | 1.67 ± 1.00 | 1.82 ± 1.33 | 2.45 ± 1.69 | 2.56 ± 1.42 | 3.00 ± 1.22 | 0.182 |
| p (intra) | p = 0.011 | p = 0.001 | p = 0.006 | p = 0.011 | p = 0.041 | |
| Deepest PD (mm) | 2.89 ± 1.90 | 3.41 ± 2.00 | 4.36 ± 2.16 | 4.67 ± 2.35 | 5.00 ± 2.35 | 0.411 |
| p (intra) | p = 0.012 | p < 0.001 | p = 0.003 | p = 0.007 | p = 0.042 | |
| KT mm) | 1.11 ± 0.93 | 0.35 ± 1.37 | 0.64 ± 1.29 | 0.22 ± 1.48 | 1.00 ± 0.71 | 0.471 |
| p (intra) | p = 0.014 | p = 0.227 | p = 0.143 | p = 0.726 | p = 0.059 | |
| BOP (%) | 38.9 ± 43.5 | 57.4 ± 26.2 | 56.8 ± 46.2 | 52.8 ± 49.1 | 60.0 ± 41.8 | 0.836 |
| p (intra) | p = 0.044 | p < 0.001 | p = 0.010 | p = 0.020 | p = 0.063 | |
| PI (%) | −5.6 ± 32.5 | −1.5 ± 33.6 | −4.6 ± 38.4 | −5.6 ± 39.1 | 10.0 ± 22.4 | 0.219 |
| p (intra) | p = 0.581 | p = 0.952 | p = 0.914 | p = 0.595 | p = 0.317 |
Kruskal‐Wallis test (between‐groups comparisons), Wilcoxon test (intra‐group comparisons).
Abbreviations: Bop, Bleeding on probing at the implant site; Pl, Plaque at the implant site.
§Number of sites per patient with PD ≥ 6mm.
TABLE 5.
Differences 1 year and 5 years treatment between groups and intra‐groups (means ± SD)
| Defect configuration | Ia (n = 9) | Ib (n = 17) | Ic (n = 11) | Id (n = 9) | Ie (n = 5) | p (between) |
|---|---|---|---|---|---|---|
| PUS elimination (%) | 0/0 | 0/0Ø | 0/0 | 0/0 | 0/1 (0.0) | 0.395 |
| p (intra) | p = 1.000 | p = 0.157 | p = 1.000 | p = 1.000 | p = 1.000 | |
| PD (mm) | −0.61 ± 0.98 | −0.22 ± 1.06 | −0.20 ± 1.23 | −0.06 ± 0.89 | 0.00 ± 0.66 | 0.746 |
| p (intra) | p = 0.107 | p = 0.345 | p = 0.575 | p = 1.000 | p = 0.891 | |
| PD≥6 mm§ | −0.56 ± 0.88 | 0.06 ± 1.09 | −0.27 ± 1.56 | −0.33 ± 0.50 | 0.20 ± 0.45 | 0.390 |
| p (intra) | p = 0.102 | p = 0.942 | p = 0.581 | p = 0.083 | p = 0.317 | |
| Deepest PD (mm) | −0.78 ± 1.39 | −0.53 ± 1.59 | −0.09 ± 1.51 | −0.22 ± 1.20 | 0.00 ± 1.22 | 0.787 |
| p (intra) | p = 0.121 | p = 0.163 | p = 0.832 | p = 0.516 | p = 1.000 | |
| KT (mm) | 0.11 ± 0.33 | −0.24 ± 1.03 | 0.36 ± 0.50 | −0.22 ± 0.44 | 0.00 ± 0.71 | 0.188 |
| p (intra) | p = 0.317 | p = 0.380 | p = 0.046 | p = 0.157 | p = 1.000 | |
| BOP (%) | −11.1 ± 28.3 | −7.4 ± 29.0 | −11.4 ± 13.1 | −11.1 ± 25.3 | 10.0 ± 13.7 | 0.315 |
| p (intra) | p = 0.234 | p = 0.339 | p = 0.025 | p = 0.194 | p = 0.157 | |
| PI (%) | −2.8 ± 31.7 | −8.8 ± 17.6 | −15.9 ± 30.2 | −16.7 ± 25.0 | 0.0 ± 0.0 | 0.557 |
| p (intra) | p = 0.705 | p = 0.058 | p = 0.059 | p = 0.083 | p = 1.000 | |
| REC (mm) | 0.11 ± 0.33 | 0.06 ± 0.90 | −0.18 ± 0.60 | −0.33 ± 0.71 | 0.60 ± 1.14 | 0.305 |
| p (intra) | p = 0.317 | p = 0.782 | p = 0.317 | p = 0.180 | p = 0.257 |
Kruskal–Wallis test (between‐groups comparisons), Wilcoxon test (intra‐group comparisons)
Abbreviations: BOP, Bleeding on probing at the implant site; Pl, Plaque at the implant site.
§Number of sites per patient with PD ≥6 mm.
TABLE 6.
Five‐year implant survival rate in relation to the adhesion to SPT
| Adhesion to SPT |
NO (n = 23) |
YES (n = 41) |
OR (95% CI) |
p |
|---|---|---|---|---|
| Survival rate | 14 (60.9%) | 37 (90.2%) |
0.17 (0.05–0.64) |
0.009 |
| Implants removed | 9/23 | 4/41 |
4. DISCUSSION
The aim of the present study was to present the 5 years clinical results of a reconstructive surgical procedure to treat peri‐implantitis defects and the possible correlation between the outcome of the intervention and the defect configuration at the time of treatment.
This is, to the best of authors’ knowledge, the first study that reports on the treatment of a large number of implants of identical macro‐design and surface characteristics.
The described surgical approach was able to re‐established healthy clinical conditions around many of the treated implants and, with an appropriate SPT, the conditions were maintained for a 5 years period. More specifically, PD and BOP values significantly decreased after treatment and remained low throughout time. Nevertheless, during the observation period, 13 implants (20%) had to be removed due to recurrent infections, the majority of which in patients who did not fully adhere to the proposed SPT. Overall, treatment success (i.e., no PD>5 mm, no BOP, no PUS, no further radiographic bone loss) was obtained in 29 of the 64 subjects who reached the 5 years examination. These results are similar to those recently published by different groups which presented similar reconstructive procedures (Mercado et al., 2018, Lo Monaca et al., 2018; Isehed et al., 2018).
In comparison with other studies (Schwarz et al., 2010), several aspects may explain the success of treatment even in cases where the morphology of the defect seemed not favorable. First of all, DBBMC has better handling properties, adhering well to the site, tailoring to the morphology of the defect, and remaining stable for long term, due to the low resorption rate, compared with other material granules (Araújo et al., 2010; Mordenfeld et al., 2010; Sculean et al., 2005).
Secondly, if the area presented no keratinized mucosa, a connective tissue graft was excised from the tuberosity, and adapted around the collar of the implant and over the entire defect so as to cover 2–3 mm of the surrounding alveolar bone to ensure a greater stability of the graft. Third, the type of implants, treated in this study, presented low thread pitch and thread depth values, which appear to be the most favorable condition for the optimal removal of the biofilm from the surface with mechanical instrumentation (Sanz‐Martín et al., 2020). Implant surface decontamination is considered a fundamental step in the treatment of peri‐implantitis defects (Claffey et al., 2008). For this purpose, a titanium brush was employed for mechanical decontamination, after tissue debridement by means of titanium curettes. The efficacy of this tool has been recently confirmed in an RCT by de Tapia et al. (2019) who reported statistically significant benefits in terms of PPD reduction compared with controls (i.e., no use of titanium brush).
Implant‐related characteristics, such as thread depth, thread pitch, or thread design, can influence the outcome of decontamination procedures (Steiger‐Ronay et al., 2017). Knowing that thread geometry influences significantly the access of the decontamination devices, the positive results of this research cannot be completely generalized and new studies are necessary to assess whether similar outcomes can be obtained, using the same protocols, on implants with different designs.
A tendency to disease recurrence after more years of observation following surgical treatment of peri‐implantitis defects, irrespective of the chosen approach (i.e., reconstructive vs. resective), has been recently reported by two 5 years studies (Lo Carcuac et al., 2020; Monaca et al., 2018): In particular, 32% of the implants defined as “success” at the 1 year follow‐up examination displayed clinical and/or radiographic signs of recurrence leading to an overall success rate of this reconstructive procedure of 59%. Similar results have been published by Carcuac et al. (2020) who reported that 44% (n = 57) of the implants previously treated with an OFD procedure displayed recurrence/progression. These authors also correlated the increased risk for disease progression with the residual deep probing pocket depth (PPD), a reduced marginal bone level, and modified peri‐implant surface (Carcuac et al., 2020). Furthermore, the increasing evidence on the long‐term (i.e., ≥5 years follow‐up) efficacy of peri‐implantitis surgical interventions whether by resective (Berglundh et al., 2018; Heitz‐Mayfield et al., 2018) or by reconstructive (Roccuzzo et al., 2017; Isehed et al., 2018) approaches stressed the importance of patients’ enrollment and adhesion to a tailored SPT program to maintain the positive short‐term results (Roccuzzo et al., 2018). The present data support these findings: Indeed, patients who did not completely adhere to the SPT (n = 9) experienced more implant loss (39.1%) than those who regularly attended recall appointments (n = 4) (9.8%).
One still open question is whether after surgical reconstructive interventions, a submerged healing should be preferable. This topic has been recently investigated in a 12 months prospective case series on 15 patients rehabilitated with 27 dental implants by Monje and workers (Monje et al., 2020). The advantage of this approach would be to achieve primary wound closure and to promote an aseptic healing. On the other hand, this protocol increases the postoperative discomfort and the overall complexity and treatment time. Irrespective of the healing modalities, the importance of the creation of a firm peri‐implant soft tissue seal has been underlined by both authors (Monje et al., 2020; Roccuzzo et al., 2011). Therefore, it is authors’ suggestion to carefully evaluate the quality of the peri‐implant soft tissues before surgical reconstructive interventions.
The arbitrary definition of “adhesion to SPT” makes comparison with other similar recent studies difficult (Carcuac et al., 2017; Heitz‐Mayfield et al., 2018; Echeverría et al., 2019). Overall, studies which consider patients’ adherence to the maintenance program are difficult to compare due to different definitions. For example, Agrawal categorized erratic compliers, as patients who did not attend all but >50% of the scheduled visits and non‐compliers, as patients with <50% of the visits (Agrawal et al., 2015), while Costa and co‐workers differentiated regular compliers who attended all SPT visits from erratic compliers who missed any of the SPT visits (Costa et al., 2012). For Hu and coworkers “defined maintenance program” group consisted of patients who have been active with SPT program with at least yearly reviews after implant placement (Hu et al., 2020). Recently, Sonnenschein and co‐workers defined four degrees of adherence of patients (fully/partially/ insufficiently/non‐adherent) for a more detailed view on adherence behavior (Sonnenschein et al., 2020). In the present study, in order to reduce the number of variables, and to increase the number of patients in each group, only two degrees of adherence were defined. Nevertheless, it has to be pointed out that most of patients were asked to be visited 3–4 times per year, based on their risk profile at the time of the visit. The same frequency of the SPT interval has been reported by other authors (Carcuac et al., 2017; Heitz‐Mayfield et al., 2018; Roccuzzo, Marchese, et al., 2018). Furthermore, it must be pointed out that regardless of the number of visits per year, not every patient accepted the proposed additional treatment. Therefore, patients who came to the appointment, but did not accept the proposed additional treatment, were classified as a “not‐adherent” (Roccuzzo, Marchese, et al., 2018).
This study has several limitations: First, the relative high number of drop‐outs (i.e., 15%) might have had an impact on the final analysis, even though it was in the same range of other recent publications (Lo Carcuac et al., 2020; Monaca et al., 2018), and other studies have demonstrated that over time, the majority of patients demonstrate only partial compliance (Zeza et al., 2017).
Second, the clinical measurements did not follow a calibration session, even though they were collected by an experienced dental hygienist, blinded to the defect morphology, as it is usually carried out in a private clinic. The benefit, in accordance with the Consensus Report of 6th European Workshop on Periodontology (Lindhe & Meyle, 2008), is that the simpler approach provides information on the “effectiveness” rather than “efficacy” of the therapy.
Third, due to the lack of standardized radiographic analysis, the radiographic findings were not reported in numeric measurements. Nevertheless, precise radiographic diagnosis is often very difficult in class Ie (circumferential only) defects, and it is virtually impossible in class Ia (buccal dehiscence) defects.
It is worth to mention that the classification of the peri‐implant defects was the first ever published more than a decade ago (Schwarz et al., 2007). More recently, the morphology of the peri‐implant defects has been studied in a large clinical trial which failed to prove specific morphological patterns (Monje et al., 2019). Consequently, some questions are still open on the exact description of peri‐implant pathologic bone defects.
Within the limitations described, the proposed reconstructive surgical approach was able to recreate and maintain peri‐implant healthy conditions around most of the treated implants for the 5 years period, regardless of the initial defect configuration. Nevertheless, patients who did not completely adhere to the SPT experienced a high implant failure rate. Therefore, the decision whether to treat or remove an implant affected by peri‐implantitis should be taken after a careful evaluation of several factors, starting from the motivation and the compliance of the patient.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests with respect to this study. M.R. received grants and travel funds from Institut Straumann AG, Sunstar, and Geistlich Pharma AG outside the submitted work. A.R. is the recipient of a 3‐year scholarship from the Clinical Research Foundation (CFR) for the Promotion of Oral Health, Brienz, Switzerland.
AUTHOR CONTRIBUTIONS
M.R. conceived the idea, performed the surgeries, and critically revised the manuscript. D.M and D.P. collected and analyzed the data, and contributed to the writing. G.R. critically revised the manuscript. A.R. collected and analyzed the data, and led to the writing.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ms. Silvia Lissona for her valuable help in data collection, and Mr. Juan Luis Gómez Martínez (stHalley Statistics) for his valuable help in statistical analysis. Open Access funding provided by Universitat Bern.
Roccuzzo, M. , Mirra, D. , Pittoni, D. , Ramieri, G. , & Roccuzzo, A. (2021). Reconstructive treatment of peri‐implantitis infrabony defects of various configurations: 5‐year survival and success. Clinical Oral Implants Research, 32, 1209–1217. 10.1111/clr.13818
Funding information
The study was self‐funded; no external funding was available for this research.
REFERENCES
- Aghazadeh, A. , Persson, R. G. , & Renvert, S. (2020). Impact of bone defect morphology on the outcome of reconstructive treatment of peri‐implantitis. International Journal of Implant Dentistry, 6(1):33, 10.1186/s40729-020-00219-5j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal, N. , Jain, R. , Jain, M. , Agarwal, K. , & Dubey, A. (2015). Compliance with supportive periodontal therapy among patients with aggressive and chronic periodontitis. Journal of Oral Science, 57, 249–254. 10.2334/josnusd.57.249 [DOI] [PubMed] [Google Scholar]
- Araújo, M. G. , Liljenberg, B. , & Lindhe, J. (2010). Dynamics of Bio‐Oss collagen incorporation in fresh extraction wounds: An experimental study in the dog. Clinical Oral Implants Research, 21(1), 55–64. 10.1111/j.1600-0501.2009.01854.x [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , Chen, S. , Cochran, D. , Derks, J. , Figuero, E. , Hämmerle, C. H. F. , Heitz‐Mayfield, L. J. A. , Huynh‐Ba, G. , Iacono, V. , Koo, K. T. , Lambert, F. , McCauley, L. , Quirynen, M. , Renvert, S. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl. 20), S286–S291. 10.1111/jcpe.12957. PMID: 29926491. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Wennström, J. L. , & Lindhe, J. (2018). Long‐term outcome of surgical treatment of peri‐implantitis. A 2–11‐year retrospective study. Clinical Oral Implants Research, 29(4), 404–410. 10.1186/s40729-017-0106-210.1111/clr.13138 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Derks, J. , Abrahamsson, I. , Wennström, J. L. , & Berglundh, T. (2020). Risk for recurrence of disease following surgical therapy of peri‐implantitis: A prospective longitudinal study. Clinical Oral Implants Research, 31(11), 1072–1077. 10.1111/clr.13653 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Derks, J. , Abrahamsson, I. , Wennström, J. L. , Petzold, M. , & Berglundh, T. (2017). Surgical treatment of peri‐implantitis: 3‐year results from a randomized controlled clinical trial. Journal of Clinical Periodontology, 44(12), 1294–1303. 10.1111/jcpe.12813 [DOI] [PubMed] [Google Scholar]
- Claffey, N. , Clarke, E. , Polyzois, I. , & Renvert, S. (2008). Surgical treatment of peri‐implantitis. Journal of Clinical Periodontology, 35, 316–332. 10.1111/j.1600-051X.2008.01277.x [DOI] [PubMed] [Google Scholar]
- Costa, F. O. , Takenaka‐Martinez, S. , Cota, L. O. , Ferreira, S. D. , Silva, G. L. , & Costa, J. E. (2012). Peri‐implant disease in subjects with and without preventive maintenance: A 5‐year follow‐up. Journal of Clinical Periodontology, 39(2), 173–181. 10.1111/j.1600-051X.2011.01819.x [DOI] [PubMed] [Google Scholar]
- De Ry, S. P. L. , Roccuzzo, A. , Sculean, A. , & Salvi, G. E. (2020). Current approaches for the non‐surgical management of peri‐implantitis. Implantologie, 28(2), 117–127. [Google Scholar]
- de Tapia, B. , Valles, C. , Ribeiro‐Amaral, T. , Mor, C. , Herrera, D. , Sanz, M. , & Nart, J. (2019). The adjunctive effect of a titanium brush in implant surface decontamination at peri‐implantitis surgical regenerative interventions: A randomized controlled clinical trial. Journal of Clinical Periodontology, 46(5), 586–596. 10.1111/jcpe.13095 [DOI] [PubMed] [Google Scholar]
- Echeverría, J. J. , Echeverría, A. , & Caffesse, R. G. (2019). Adherence to supportive periodontal treatment. Periodontology 2000, 79(1), 200–209. 10.1111/prd.12256 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , Aaboe, M. , Araujo, M. , Carrión, J. B. , Cavalcanti, R. , Cionca, N. , Cochran, D. , Darby, I. , Funakoshi, E. , Gierthmuehlen, P. C. , Hashim, D. , Jahangiri, L. , Kwon, Y. , Lambert, F. , Layton, D. M. , Lorenzana, E. R. , McKenna, G. , Mombelli, A. , Müller, F. , … Yeo, A. (2018). Group 4 ITI consensus report: Risks and biologic complications associated with implant dentistry. Clinical Oral Implants Research, 29(16), 351–358. 10.1111/clr.13307 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. A. , Salvi, G. E. , Mombelli, A. , Loup, P. J. , Heitz, F. , Kruger, E. , & Lang, N. P. (2018). Supportive peri‐implant therapy following anti‐infective surgical peri‐implantitis treatment: 5‐Year survival and success. Clinical Oral Implants Research, 29(1), 1–6. 10.1111/clr.12910 [DOI] [PubMed] [Google Scholar]
- Hu, C. , Lang, N. P. , Ong, M. M. , Lim, L. P. , & Tan, W. C. (2020). Influence of periodontal maintenance and periodontitis susceptibility on implant success: A 5‐year retrospective cohort on moderately rough surfaced implants. Clinical Oral Implants Research, 31(8), 727–736. 10.1111/clr.13621 [DOI] [PubMed] [Google Scholar]
- Isehed, C. , Svenson, B. , Lundberg, P. , & Holmlund, A. (2018). Surgical treatment of peri‐implantitis using enamel matrix derivative, an RCT: 3‐And 5‐year follow‐up. Journal of Clinical Periodontology, 45, 744–753. 10.1111/jcpe.12894 [DOI] [PubMed] [Google Scholar]
- Koo, K. T. , Khoury, F. , Keeve, P. L. , Schwarz, F. , Ramanauskaite, A. , Sculean, A. , & Romanos, G. (2019). Implant surface decontamination by surgical treatment of periimplantitis: A literature review. International Journal of Implant Dentistry, 28(2), 173–176. 10.1097/ID.0000000000000840 [DOI] [PubMed] [Google Scholar]
- La Monaca, G. , Pranno, N. , Annibali, S. , Cristalli, M. P. , & Polimeni, A. (2018). Clinical and radiographic outcomes of a surgical reconstructive approach in the treatment of peri‐implantitis lesions: A 5‐year prospective case series. Clinical Oral Implants Research, 29(10), 1025–1037. 10.1111/clr.13369 [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , & Meyle, J. (2008). Group D of European Workshop on Periodontology . Peri‐implant diseases: Consensus report of the sixth European workshop on periodontology. Journal of Clinical Periodontology, 35(8), 282–285. 10.1111/j.1600-051X.2008.01283.x [DOI] [PubMed] [Google Scholar]
- Mercado, F. , Hamlet, S. , & Ivanovski, S. (2018). Regenerative surgical therapy for peri‐implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and doxycycline‐A prospective 3‐year cohort study. Clinical Oral Implants Research, 29(6), 583–591. 10.1111/clr.13256 [DOI] [PubMed] [Google Scholar]
- Monje, A. , Pons, R. , Insua, A. , Nart, J. , Wang, H. L. , & Schwarz, F. (2019). Morphology and severity of peri‐implantitis bone defects. Clinical Implant Dentistry and Related Research, 21(4), 635–643. 10.1111/cid.12791 [DOI] [PubMed] [Google Scholar]
- Monje, A. , Pons, R. , Roccuzzo, A. , Salvi, G. E. , & Nart, J. (2020). Reconstructive therapy for the management of peri‐implantitis via submerged guided bone regeneration: A prospective case series. Clinical Implant Dentistry and Related Research, 22(3), 342–350. 10.1111/cid.12913 [DOI] [PubMed] [Google Scholar]
- Mordenfeld, A. , Hallman, M. , Johansson, C. B. , & Albrektsson, T. (2010). Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clinical Oral Implants Research, 21(9), 961–970. 10.1111/j.1600-0501.2010.01939.x [DOI] [PubMed] [Google Scholar]
- Parma‐Benfenati, S. , Tinti, C. , Romano, F. , Roncati, M. , & Aimetti, M. (2020). Long‐Term outcome of surgical regenerative treatment of peri‐implantitis: A 2‐ to 21‐Year retrospective evaluation. International Journal of Periodontics and Restorative Dentistry, 40(4), 487–496. 10.11607/prd.4647 [DOI] [PubMed] [Google Scholar]
- Renvert, S. , Hirooka, H. , Polyzois, I. , Kelekis‐Cholakis, A. , & Wang, H. L. (2019). Diagnosis and non‐surgical treatment of peri‐implant diseases and maintenance care of patients with dental implants–Consensus report of working group 3. International Dental Journal, 69(2), 12–17. 10.1111/idj.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccuzzo, A. , De Ry, S. P. , Sculean, A. , Roccuzzo, M. , & Salvi, G. E. (2020). Current approaches for the non‐surgical management of peri‐implant diseases. Current Oral Health Reports, 7(3), 274–282. 10.1007/s40496-020-00279-x [DOI] [Google Scholar]
- Roccuzzo, A. , Stähli, A. , Monje, A. , Sculean, A. , & Salvi, G. E. (2021). Peri‐Implantitis: A clinical update on prevalence and surgical treatment outcomes. Journal of Clinical Medicine, 10, 1107. 10.3390/jcm10051107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccuzzo, M. , Bonino, F. , Bonino, L. , & Dalmasso, P. (2011). Surgical therapy of peri‐implantitis lesions by means of a bovine‐derived xenograft: Comparative results of a prospective study on two different implant surfaces. Journal of Clinical Periodontology, 38, 738–745. 10.1111/j.1600-051X.2011.01742.x [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Fierravanti, L. , Pittoni, D. , Dalmasso, P. , & Roccuzzo, A. (2020). Implant survival after surgical treatment of peri‐implantitis lesions by means of deproteinized bovine bone mineral with 10% collagen: 10‐Year results from a prospective study. Clinical Oral Implant Research, 31, 768–776. 10.1111/clr.13628 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Gaudioso, L. , Lungo, M. , & Dalmasso, P. (2016). Surgical therapy of single peri‐implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. Journal of Clinical Periodontology, 43, 311–318. 10.1111/jcpe.12516 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Layton, D. M. , Roccuzzo, A. , & Heitz‐Mayfield, L. J. (2018). Clinical outcomes of peri‐implantitis treatment and supportive care: A systematic review. Clinical Oral Implants Research, 29(16), 331–350. 10.1111/clr.13287 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Marchese, S. , Dalmasso, P. , & Roccuzzo, A. (2018). Periodontal regeneration and orthodontic treatment of severely periodontally compromised teeth: 10‐Year results of a prospective study. International Journal of Periodontics and Restorative Dentistry, 38(6), 801–809. 10.11607/prd.3756 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Pittoni, D. , Roccuzzo, A. , Charrier, L. , & Dalmasso, P. (2017). Surgical treatment of peri‐implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7‐Year‐results. Clinical Oral Implants Research, 28(12), 1577–1583. 10.1111/clr.13028 [DOI] [PubMed] [Google Scholar]
- Romandini, M. , Cordaro, M. , Donno, S. , & Cordaro, L. (2019). Discrepancy between patient satisfaction and biologic complication rate in patients rehabilitated with overdentures and not participating in a structured maintenance program after 7 to 12 years of loading. The International Journal of Oral & Maxillofacial Implants, 34(5), 1143–1151. 10.11607/jomi.7465 [DOI] [PubMed] [Google Scholar]
- Romandini, M. , Lima, C. , Pedrinaci, I. , Araoz, A. , Soldini, M. C. , & Sanz, M. (2021). Prevalence and risk/protective indicators of peri‐implant diseases: A university‐representative cross‐sectional study. Clinical Oral Implants Research, 32(1), 112–122. 10.1111/clr.13684 [DOI] [PubMed] [Google Scholar]
- Sanz‐Martín, I. , Paeng, K. , Park, H. , Cha, J. K. , Jung, U. W. , & Sanz, M. (2021). Significance of implant design on the efficacy of different peri‐implantitis decontamination protocols. Clinical Oral Investigations, 25(6), 3589–3597. 10.1007/s00784-020-03681-y [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Herten, M. , Sager, M. , Bieling, K. , Sculean, A. , & Becker, J. (2007). Comparison of naturally occurring and ligature‐induced peri‐implantitis bone defects in humans and dogs. Clinical Oral Implants Research, 18(2), 161–170. 10.1111/j.1600-0501.2006.01320.x [DOI] [PubMed] [Google Scholar]
- Schwarz, F , Sahm, N , Schwarz, K , & Becker, J. (2010). Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri‐implantitis. Journal of Clinical Periodontology, 37(5), 449–455. 10.1111/j.1600-051X.2010.01540.x [DOI] [PubMed] [Google Scholar]
- Sculean, A. , Chiantella, G. C. , Windisch, P. , Arweiler, N. B. , Brecx, M. , & Gera, I. (2005). Healing of intra‐bony defects following treatment with a composite bovine‐derived xenograft (Bio‐Oss Collagen) in combination with a collagen membrane (Bio‐Gide PERIO). Journal of Clinical Periodontology, 32(7), 720–724. 10.1111/j.1600-051X.2005.00758.x [DOI] [PubMed] [Google Scholar]
- Sonnenschein, S. K. , Kohnen, R. , Ruetters, M. , Krisam, J. , & Kim, T. S. (2020). Adherence to long‐term supportive periodontal therapy in groups with different periodontal risk profiles. Journal of Clinical Periodontology, 47(3), 351–361. 10.1111/jcpe.13252 [DOI] [PubMed] [Google Scholar]
- Steiger‐Ronay, V. , Merlini, A. , Wiedemeier, D. B. , Schmidlin, P. R. , Attin, T. , & Sahrmann, P. (2017). Location of unaccessible implant surface areas during debridement in simulated peri‐implantitis therapy. BMC Oral Health, 17(1), 137. 10.1186/s12903-017-0428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, C. , Regidor, E. , Ortiz‐Vigón, A. , & Derks, J. (2019). Efficacy of reconstructive surgical therapy at peri‐implantitis‐related bone defects. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 46(21), 340–356. 10.1111/jcpe.13070 [DOI] [PubMed] [Google Scholar]
- Zeza, B. , Pilloni, A. , Tatakis, D. N. , Mariotti, A. , Di Tanna, G. L. , & Mongardini, C. (2017). Implant patient compliance varies by periodontal treatment history. Journal of Periodontology, 88(9), 846–853. 10.1902/jop.2017.160528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


