Abstract
Background and purpose
Requiring a walking aid is a fundamental milestone in multiple sclerosis (MS), represented by an Expanded Disability Status Scale (EDSS) score ≥6.0. In the present study, we assess the effect of ocrelizumab (OCR) on time to EDSS score ≥6.0 in relapsing MS.
Methods
Time to EDSS score ≥6.0 confirmed for ≥24 and ≥48 weeks was assessed over the course of 6.5 years (336 weeks) in the double‐blind period (DBP) and open‐label extension (OLE) period of the OPERA I (NCT01247324) and OPERA II (NCT01412333) studies.
Results
Time to reach EDSS score ≥6.0 was significantly delayed in those initially randomized to OCR versus interferon. Over 6.5 years, the risk of requiring a walking aid confirmed for ≥24 weeks was 34% lower among those who initiated OCR earlier versus delayed treatment (average hazard ratio [HR] DBP + OLE 0.66, 95% confidence interval [CI] 0.45–0.95; p = 0.024); the risk of requiring a walking aid confirmed for ≥48 weeks was 46% lower (average HR DBP+OLE 0.54, 95% CI 0.35–0.83; p = 0.004).
Conclusion
The reduced risk of requiring a walking aid in earlier initiators of OCR demonstrates the long‐term implications of earlier highly effective treatment.
Keywords: disease progression, interferon beta 1a, multiple sclerosis, ocrelizumab, walking stick
Data from the OPERA I and OPERA II trials in relapsing multiple sclerosis show that earlier ocrelizumab (OCR) use delays the time to requiring a walking aid (Expanded Disability Status Scale score ≥6.0). Differences in disability progression between OCR–OCR continuers versus interferon–OCR switchers were evident throughout the open‐label extension, indicating that switching to OCR is never as effective as earlier treatment, and does not reverse lost function. These results demonstrate the benefit of early and continuous OCR treatment.
![]()
INTRODUCTION
Relapsing multiple sclerosis (RMS) is typically a chronic progressive disease that, in the majority of patients, eventually leads to increased disability and poor quality of life [1]. In clinical trials, disability is assessed using the Expanded Disability Status Scale (EDSS) [2]. Requiring a walking aid (expressed as an EDSS score ≥6.0) is a key clinical disability milestone highly associated with irreversible disability, decreased employment rates [3, 4], and physical, emotional and financial challenges [1, 5, 6]. Delaying the time to reach EDSS score ≥6.0 is an important goal in the treatment of RMS.
Ocrelizumab (OCR) is the first CD20+ B‐cell‐selective monoclonal antibody approved for the treatment of RMS and primary progressive MS, at a dose of 600 mg intravenously twice‐yearly [7]. OCR showed significant benefits on confirmed disability progression (CDP) in the double‐blind period (DBP) of the OPERA I (NCT01247324) and OPERA II (NCT01412333) trials in RMS [8], and sustained efficacy with continuous therapy over 5 years in the open‐label extension (OLE) [9]. In the present study, we focused on the effect of OCR on time to EDSS score ≥6.0 in patients with RMS after 6.5 years (336 weeks).
METHODS
Trial design and patients
OPERA I and OPERA II were Phase III, multicenter, randomized, double‐blind, double‐dummy, interferon (IFN)β‐1a controlled trials with identical designs, assessing OCR in RMS [8]. Key eligibility criteria included age 18–55 years, diagnosis of multiple sclerosis (2010 revised McDonald criteria [10]) and baseline EDSS score 0–5.5. After completion of the DBP, patients meeting specific criteria were eligible to enter an OLE phase; ineligible patients and those who declined entered safety follow‐up. Eligibility criteria for the OLE phase included: completion of the 96‐week DBP; having the potential, in the opinion of the investigator, to benefit from treatment with OCR; ability and willingness to provide written informed consent for the OLE phase and to comply with the study protocol; willingness to continue to use at least two contraceptive methods; and meeting criteria for re‐treatment with OCR [9].
During the DBP, patients were randomized (1:1) to receive OCR or IFNβ‐1a for 96 weeks [8]. On completing the DBP, patients entered the OLE via a 4‐week screening phase. At OLE initiation, patients who received OCR in the DBP continued OCR (OCR–OCR group) and patients from the IFNβ‐1a group switched to OCR (IFN–OCR group), given every 24 weeks; in order to maintain blinding and in accordance with the start of the DBP, all patients received the first dose of OCR in the OLE as 2 × 300 mg infusions, 2 weeks apart. Treatment allocation was unblinded after the last data point from the last patient from the DBP was received. The first patient completing the DBP entered the OLE in August 2013 and the last patient entered the OLE in February 2015. The clinical cut‐off date for this analysis was January 3, 2020.
The relevant institutional review boards/ethics committees approved the trial protocols (NCT01247324/NCT01412333) [8]. All patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki.
Time to walking aid requirement assessments
Post hoc analyses were carried out to assess time to walking aid requirement (EDSS score ≥6.0) confirmed for ≥24 or ≥48 weeks in patients with baseline EDSS scores ≤5.5, using Kaplan–Meier and Cox survival analysis in the intention‐to‐treat population. Hazard ratios (HRs) were estimated by Cox regression, stratified by study, geographical region (United States vs. rest of world), and baseline EDSS score (<4.0 vs. ≥4.0); average HRs over the whole DBP + OLE period are reported. A comparison of the survival distributions between patients randomized to OCR versus IFN using the log‐rank test was carried out. p values for difference in event rates were calculated using a t‐test on the survival curve estimates at the indicated time points and associated standard deviation was derived by the Greenwood formula. Patients with post‐baseline EDSS scores ≥6.0 sustained for ≥24 or ≥48 weeks were considered to have experienced an event. Patients with an initial EDSS score ≥6.0 at the time of treatment discontinuation and those with no follow‐up visit after ≥24 or ≥48 weeks were censored.
Trial registration
The OPERA I and OPERA II trials were registered in the ClinicalTrials.gov registry (NCT01247324/NCT01412333).
RESULTS
Patient demographics and disposition
Baseline demographics and disease characteristics at DBP and OLE baseline have been presented previously [8, 9] (summarized in Table 1); these were well balanced between treatment arms. In total, 96% of patients who completed the DBP entered the OLE (n = 1,325/1,386), representing 80% of those initially randomized (n = 1,325/1,656).
TABLE 1.
Patient demographics and disease characteristics at double‐blind period baseline and open‐label extension baseline
| DBP baseline | OLE baseline | |||
|---|---|---|---|---|
|
IFNβ‐1a 44 µg (n = 829) |
OCR 600 mg (n = 827) |
IFNβ‐1a 44 µg/OCR 600 mg (n = 623) |
OCR 600 mg/OCR 600 mg (n = 702) |
|
| Age, mean (SD), years | 37.2 (9.2) | 37.1 (9.2) | 39.3 (9.2) | 39.2 (9.1) |
| Female, n (%) | 552 (66.6) | 541 (65.4) | 408 (65.5) | 454 (64.7) |
| EDSS score, mean (SD) | 2.8 (1.3) | 2.8 (1.3) | 2.7 (1.5) | 2.6 (1.3) |
|
Patients with T1 Gd‐enhancing lesions, n (%)† |
327 (39.8)¶ | 333 (40.7)†† | 106 (17.3)†† | 5 (0.7)§§ |
|
Number of T1 Gd‐enhancing lesions, mean (SD)† |
1.9 (5.0)¶ | 1.8 (4.6)†† | 0.5 (2.1)†† | 0.02 (0.2)§§ |
| Number of T1 hypointense lesions (SD)‡ | 32.9 (35.1)¶ | 32.4 (35.2)†† | 35.6 (36.8)¶¶ | 33.9 (36.1)¶¶ |
| T2 hyperintense lesion volume, cm3, mean (SD)§ | 10.2 (11.8)‡‡ | 10.8 (14.1)‡‡ | 9.4 (11.5)††† | 10.1 (13.8)‡‡‡ |
| Number of T2 lesions mean (SD)‡ | 51.0 (37.8)‡‡ | 50.1 (38.8)‡‡ | 55.5 (41.3) | 50.9 (39.4) |
For magnetic resonance imaging measurements: †OLE baseline is the assessment at Week 96; ‡OLE baseline is the sum of lesion counts at baseline, Week 24, Week 48 and Week 96; §OLE baseline is the last assessment prior to or at the start of OLE treatment.
Patients missing and excluded for calculating percentages: ¶ n = 7; †† n = 9; ‡‡ n = 5; §§ n = 6; ¶¶ n = 1; ††† n = 33; ‡‡‡ n = 44.
Demographics and disease characteristics at Week 96 of the DBP (clinical cut‐off dates: OPERA I, April 2, 2015; OPERA II, May 12, 2015) are considered baseline for the OLE phase.
Abbreviations: DBP, double‐blind period; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; OCR, ocrelizumab; OLE, open‐label extension; SD, standard deviation.
Time to walking aid requirement confirmed for ≥24 weeks
The proportion of patients who required a walking aid confirmed for ≥24 weeks was lower in OCR–OCR continuers versus IFN–OCR switchers at the end of the DBP (1.4% vs. 4.0%; p = 0.003) and at OLE Year 4.5 (7.2% vs. 10.0%; p = 0.07 [Figure 1a]). At the end of the DBP, the overall risk of requiring a walking aid confirmed for ≥24 weeks was 68% lower among those who initiated OCR earlier versus delayed treatment (HR 0.32, 95% confidence interval [CI] 0.16–0.64; p < 0.001); over 6.5 years, the risk was 34% lower (average HR DBP + OLE 0.66, 95% CI 0.45–0.95; p = 0.024).
FIGURE 1.
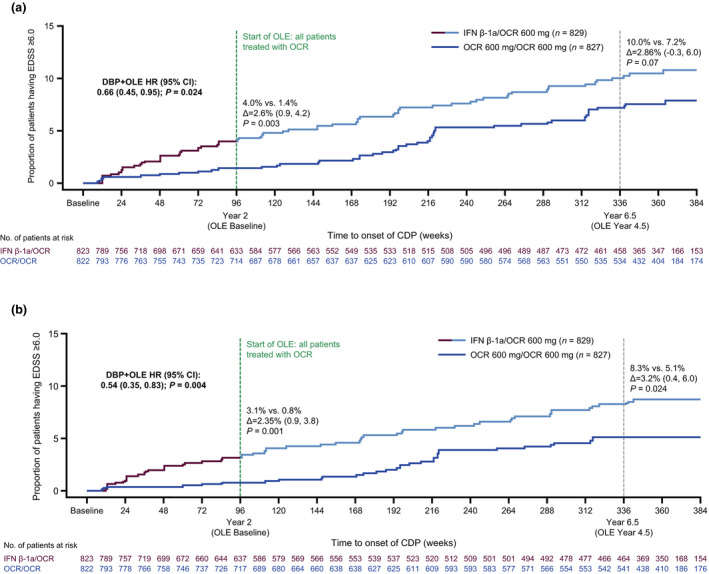
(a) Time to walking aid (EDSS score ≥6.0) confirmed for ≥24 weeks in the OLE. (b) Time to walking aid (EDSS score ≥6.0) confirmed for ≥48 weeks in the OLE. Hazard ratios were estimated by Cox regression stratified by study, geographical region (United States vs. ROW), and baseline EDSS score (<4.0 vs. ≥4.0). Comparison of the survival distributions used the log‐rank test. Patients with a post‐baseline EDSS score ≥6 sustained for ≥24 or ≥48 weeks were considered as having an event. Patients with an EDSS score ≥6 at the time of treatment discontinuation with no further EDSS score were censored. Patients with missing baseline EDSS score were excluded from the analysis. CDP, confirmed disability progression; DBP, double‐blind period; HR, hazard ratio; IFN, interferon; OCR, ocrelizumab; OLE, open‐label extension; ROW, rest of world
Time to walking aid requirement confirmed for ≥48 weeks
The proportion of patients who required a walking aid confirmed for ≥48 weeks was significantly lower in OCR–OCR continuers versus IFN–OCR switchers at the end of the DBP (0.8% vs. 3.1%; p = 0.001) and at OLE Year 4.5 (5.1% vs. 8.3%; p = 0.024 [Figure 1b]). At the end of the DBP, the overall risk of requiring a walking aid confirmed for ≥48 weeks was 78% lower in those who initiated OCR earlier versus delayed treatment (HR 0.22, 95% CI 0.09–0.55; p < 0.001); over 6.5 years, the risk was 46% lower (average HR DBP + OLE: 0.54, 95% CI 0.35–0.83; p = 0.004).
DISCUSSION
Over 6.5 years, patients with RMS initiating OCR 2 years earlier had a reduced risk of requiring a walking aid, confirmed for ≥24 and ≥48 weeks, versus those initially receiving IFNβ‐1a. Due to modern treatment practice, identifying a contemporary real‐world cohort who were treated for a comparable duration with IFNβ‐1a alone, in order to better contextualize this analysis, proved challenging.
Requiring a walking aid can lead to significant physical, emotional and financial challenges and an overall reduced quality of life [1, 5, 6], along with increased healthcare costs and burdens [3]. Amongst other impairments, a walking aid is often cited as a specific fear or concern at diagnosis. Earlier OCR use delays the time to such meaningful disability milestones. Differences in disability progression between OCR–OCR continuers and IFN–OCR switchers were evident throughout the OLE, indicating that switching to OCR is never as effective as earlier treatment, and does not reverse lost function. However, this difference was more evident at the end of the DBP compared to the OLE Year 4.5. This is likely explained by the fact that all patients were receiving OCR in the OLE. At OLE Year 4.5, the proportion of patients who required a walking aid confirmed for ≥24 weeks was numerically lower in OCR–OCR continuers versus IFN–OCR switchers, but this did not reach statistical significance. This could be because shorter confirmation periods may result in identification of temporary disability changes, leading to an overestimation of the proportion of patients with long‐term accumulation of irreversible disability [11]. When confirmed for ≥48 weeks, the difference in the proportion of patients who required a walking aid at OLE Year 4.5 was shown to be significant. Furthermore, the overall risk of requiring a walking aid throughout the whole DBP + OLE period was significantly lower in OCR–OCR continuers versus IFN–OCR switchers when confirmed for both ≥24 and ≥48 weeks.
Together with the previously reported sustained, long‐term benefit of OCR on other measures of disability progression, including CDP and time to wheelchair need (EDSS score ≥7.0), in both RMS and primary progressive MS [9, 12], these results demonstrate the benefit of early and continuous OCR treatment in a disease leading to progressive disability in the majority.
CONFLICT OF INTEREST
G. Giovannoni has received personal compensation for serving as a consultant for F. Hoffmann‐La Roche Ltd, AbbVie, Actelion, Atara Biotherapeutics, Biogen, Celgene, Sanofi Genzyme, Genentech, Inc., GlaxoSmithKline, Merck Serono, Novartis and Teva, has received personal compensation from Elsevier for serving as an editor on MSARDs, and has received financial support for research activities from F. Hoffmann‐La Roche Ltd, Biogen, Merck, Merck Serono, Novartis, Sanofi Genzyme and Takeda. L. Kappos's institution (University Hospital Basel) received in the last 3 years and used exclusively for research support at the Department: steering committee, advisory board, consultancy fees and support of educational activities from Actelion, Allergan, Almirall, Baxalta, Bayer, Biogen, Celgene/Receptos, CSL‐Behring, Desitin, Excemed, Eisai, Genzyme, Japan Tobacco, Merck, Minoryx, Novartis, Pfizer, F. Hoffmann‐La Roche Ltd, Sanofi Aventis, Santhera and Teva, and license fees for Neurostatus‐UHB products. The Research of the MS Center in Basel has been supported by grants from Bayer, Biogen, Novartis, the Swiss MS Society, the Swiss National Research Foundation, Innosuisse, the European Union and Roche Research Foundations. J. de Seze has received consultancy fees and served as an expert for advisory boards for Alexion, Allergan, Almirall, Bayer, Biogen, Chugai, CSL Behring, F. Hoffmann‐La Roche Ltd, Genzyme, LFB, Merck, Novartis, Sanofi and Teva. S. L. Hauser serves on the board of trustees for Neurona and on scientific advisory boards for Alector, Annexon, Bionure and Molecular Stethoscope, and has received travel reimbursement and writing assistance from F. Hoffmann‐La Roche Ltd and Novartis for CD20‐related meetings and presentations. J. Overell is currently an employee and shareholder of F. Hoffmann‐La Roche Ltd. During his previous employment he received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Teva, Biogen, Celgene, EMD Serono, MedDay, Novartis, Roche, Sanofi Genzyme, WebMD Global and Allergan. His research and department were supported by grants from Sanofi Genzyme, Biogen, Novartis, and Roche. H. Koendgen is an employee and shareholder of F. Hoffmann‐La Roche Ltd. M. Manfrini is an employee and shareholder of F. Hoffmann‐La Roche Ltd. Q. Wang is an employee of F. Hoffmann‐La Roche Ltd. J. S. Wolinsky has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Acorda Therapeutics, Actelion, Alkermes, Brainstorm Cell Therapeutics, Celgene, EMD Serono, GeNeuro, GW Pharma Ltd, MedDay Pharmaceuticals, NervGen Pharma Corp, Novartis, Otsuka, PTC Therapeutics, Roche/Genentech and Sanofi Genzyme; royalties are received for out‐licensed monoclonal antibodies through UTHealth from Millipore Corporation.
AUTHOR CONTRIBUTIONS
Ludwig Kappos: Conceptualization (lead); Investigation (equal); Methodology (equal); Supervision (lead); Writing – review and editing (equal). Jerome De Seze: Investigation (equal); Validation (supporting); Writing – review and editing (supporting). Stephen L Hauser: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (equal); Methodology (equal); Project administration (supporting); Supervision (equal); Validation (equal); Visualization (supporting); Writing – original draft (supporting); Writing – review and editing (supporting). James Overell: Conceptualization (equal); Methodology (equal); Validation (supporting); Writing – original draft (lead); Writing – review and editing (supporting). Harold Koendgen: Conceptualization (supporting); Supervision (supporting); Writing – review and editing (equal). Marianna Manfrini: Data curation (lead); Methodology (supporting); Writing – review and editing (supporting). Qing Wang: Conceptualization (equal); Formal analysis (lead); Methodology (lead); Validation (lead); Writing – original draft (lead); Writing – review and editing (lead). Jerry S Wolinsky: Conceptualization (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Writing – review and editing (supporting).
ACKNOWLEDGMENTS
Eleanor Foy (Articulate Science, UK) drafted the manuscript based on author input (funded by F. Hoffmann‐La Roche Ltd). Authors had full editorial control and provided final approval. We thank Gisèle von Büren (F. Hoffmann‐La Roche Ltd) for critical review and technical advice, and everyone who participated in the OPERA trials.
Funding information
This research was funded by F. Hoffmann‐La Roche Ltd, Basel, Switzerland.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
REFERENCES
- 1. Jones E, Pike J, Marshall T, Ye X. Quantifying the relationship between increased disability and health care resource utilization, quality of life, work productivity, health care costs in patients with multiple sclerosis in the US. BMC Health Serv Res. 2016;16:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444‐1452. [DOI] [PubMed] [Google Scholar]
- 3. Tomassini V, Fanelli F, Prosperini L, Cerqua R, Cavalla P, Pozzilli C. Predicting the profile of increasing disability in multiple sclerosis. Mult Scler. 2019;25(9):1306‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones KH, Jones PA, Middleton RM, et al. Physical disability, anxiety and depression in people with MS: an internet‐based survey via the UK MS Register. PLoS ONE. 2014;9(8):e104604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26(1):109‐119. [DOI] [PubMed] [Google Scholar]
- 7. Genentech . Ocrevus (Ocrelizumab) [Full Prescribing Information]. https://www.gene.com/download/pdf/ocrevus_prescribing.pdf. Accessed January 20, 2020.
- 8. Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus Interferon Beta‐1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221‐234. [DOI] [PubMed] [Google Scholar]
- 9. Hauser SL, Arnold DL, Bar‐or A, et al. Five‐years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open‐label extension. Neurology. 2020;95(13):e1854‐e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138:3287‐3298. [DOI] [PubMed] [Google Scholar]
- 12. Wolinsky JS, Arnold DL, Brochet B, et al. Long‐term follow‐up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post‐hoc analysis from the ongoing open‐label extension of the randomised, placebo‐controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998‐1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).


