Abstract
To revise the FIGO staging for carcinoma of the vulva using a new approach that involves analyses of prospectively collected data. The FIGO Committee for Gynecologic Oncology reviewed the recent literature to gain an insight into the impact of the 2009 vulvar cancer staging revision. The Committee resolved to revise the staging with a goal of simplification and actively collaborated with the United States National Cancer Database to analyze prospectively collected data on carcinoma of the vulva. Many tumor characteristics were collected for all stages of vulvar cancer treated between 2010 and 2017. Statistical analysis was performed with SAS software. Overall survival was estimated based on tumor characteristics. Log‐rank and Wilcoxon tests were used to analyze overall survival similarities between and within groups of tumor characteristics. Characteristics with similar survivals were then grouped into the same stages and substages. Kaplan–Meier overall survival curves were generated for the resulting stages and substages. There were 12 063 cases with available data. The resulting new staging for carcinoma of the vulva has two substages in Stage I, no substage in Stage II, three substages in Stage III, and two substages in Stage IV. The Kaplan–Meier overall survival curves showed clear separation between stages and substages. The 2021 vulvar cancer staging is the first from the FIGO Committee for Gynecologic Oncology to be derived from data analyses. This revision has a new definition for depth of invasion, uses the same definition for lymph node metastases utilized in cervical cancer, and allows findings from cross‐sectional imaging to be incorporated into vulvar cancer staging. The 2021 FIGO staging for carcinoma of the vulva is data‐derived, validated, and much simpler than earlier revisions.
Keywords: carcinoma, staging, vulva
Synopsis
The FIGO Committee for Gynecologic Oncology's last revision of vulvar cancer staging was published over a decade ago. This 2021 revision is data‐derived, simplified, and user‐friendly.
1. INTRODUCTION
Vulvar cancer is rare, accounting for 4% of all gynecologic cancers globally with approximately 65% of all cases occurring in higher‐income regions. 1 The GLOBOCAN 2018 worldwide estimates were 44 235 new cases and 15 222 deaths. 2 Squamous cell carcinoma accounts for more than 90% of the malignant tumors of the vulva and several morphologic variants have been described, including keratinizing, basaloid, warty, and verrucous carcinoma. Basaloid and warty variants, representing about one‐third of cases, are more common in younger women and are often associated with HPV DNA detection. These tumors share many risk factors with cervical cancer. In contrast, keratinizing variants arise from chronic vulvar dermatosis such as lichen sclerosus, are not associated with HPV, and tend to occur in older women. 3
In 1988, FIGO (International Federation of Gynecology and Obstetrics) modified vulvar cancer staging by incorporating surgical pathologic factors. The 1988 surgical staging was shown to be more accurate in assigning patients to their corresponding stage and, more importantly, prognostic ability was improved compared with the preceding clinical staging. 4 The most recent revision to vulvar cancer staging by the FIGO Committee for Gynecologic Oncology was made in 2009. 5 That revision made substantial changes to stage assignment, including: (1) disease involvement of the lower urethra, vagina, and anus was assigned to Stage II; (2) all nonmetastatic tumors were collectively assigned to Stage I; and (3) the number and extent of lymph node involvement was extensively substaged within Stage III. 6
The data informing the 2009 FIGO staging of carcinoma of the vulva 5 originated from a careful review of the published literature but mostly comprised single and multi‐institution retrospective studies. As such, subsequent studies assessing the prognostic capability of the staging yielded inconsistent findings. For instance, a retrospective study of 468 patients that used the 2009 FIGO staging to assign stage and correlated assigned stage with outcome indicated that 10‐year cause‐specific survival was similar for Stages I and II. Stages IIIA, IIIB, and IIIC were not significantly different in terms of cause‐specific survival. 7 This lack of prognostic capability was the conclusion of another study of 76 cases. 8 In contrast, another retrospective study of 269 patients who were restaged with the 2009 FIGO staging for carcinoma of the vulva concluded that the staging system provided a better reflection of prognosis for patients with vulvar squamous cell cancer. 9 The introduction sections of all of the referenced studies clearly stated that the performance of the 2009 staging with regard to prognostic capability had not been tested. The FIGO Committee for Gynecologic Oncology acknowledged this and resolved to change the Committee's approach in future staging revisions.
Over 10 years have now elapsed since the publication of the 2009 revisions to the FIGO vulvar cancer staging, which represents enough time to evaluate the impact of the revisions made on the ease of utilization and prognostication. In addition, enough data have accumulated based on the 2009 revision to enable the FIGO Committee for Gynecologic Oncology to re‐evaluate stage assignments and make data‐driven revisions. In this 2021 revision, prospectively collected data were analyzed to determine the best cut‐offs between stages and substages, making this the most evidence‐driven staging revision ever undertaken by the FIGO Committee for Gynecologic Oncology.
The FIGO Committee for Gynecologic Oncology wishes to thank the United States National Cancer Database (NCDB) and the American College of Surgeons for allowing access to their data and for helping with data analyses.
2. MATERIALS AND METHODS
Data from the NCDB collected between January 1, 2010 and December 31, 2017 were utilized for the analyses. A total of 12 063 cases were identified during this period (median age 64.3 ± 14.4 years). Patients were predominantly non‐Hispanic white (85.9%), non‐Hispanic black (8.0%), and Hispanic (4.3%). These patients received therapy for vulvar cancer at American College of Surgeons’ Commission on Cancer (CoC) accredited hospitals and were treated per the prevailing standard of care. It is therefore safe to assume that the disease course was determined by stage at diagnosis and not significantly impacted by variations in therapy. Data selection criteria are shown in Table 1. Stage distribution of included cases is shown in Table 2.
TABLE 1.
United States National Cancer Database (NCDB) selection criteria
| Criteria | Description |
|---|---|
| Primary site codes | C51.0, C51.1, C51.2, C51.8, C51.9 |
| Histology | 8000–8246, 8248–8576, 8940–8950, 8980–8981 |
| Months survived | If greater or equal to 0 and not missing months survived |
| Diagnosis years | 2010–2017 |
| Class of case | Analytic cases |
| Age at diagnosis | 18+ years |
| Sequence number | First or only cancer diagnosis |
| Clinical stage group | Clinical stage group analysis only, unknown clinical stage group was excluded |
| Pathologic stage group | Pathologic stage group analysis only, unknown pathologic stage group was excluded |
TABLE 2.
Stage distribution of all cases (12 063) included in analyses
| Pathologic stage group | Number of patients (n = 12 063) | 1‐year survival, % | 2‐year survival, % | 3‐year survival, % | 4‐year survival, % | 5‐year survival, % | Median survival (months) |
Median survival lower 95% CI |
Median survival upper 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| IA | 653 | 98.0 | 97.0 | 93.7 | 92.0 | 86.3 | . | . | . |
| IB | 7604 | 96.6 | 91.1 | 86.4 | 81.7 | 77.1 | . | . | . |
| II | 500 | 93.4 | 83.2 | 74.2 | 68.3 | 64.5 | 100.0 | 90.4 | . |
| IIIA | 676 | 90.1 | 76.9 | 69.4 | 63.6 | 60.3 | 86.7 | 71.1 | 109.6 |
| IIIB | 1043 | 84.5 | 67.4 | 58.2 | 52.4 | 49.6 | 57.8 | 45.3 | 74.6 |
| IIIC | 707 | 68.4 | 47.0 | 40.4 | 37.2 | 31.3 | 20.8 | 18.3 | 24.9 |
| IVA | 70 | 55.6 | 40.7 | 34.5 | 25.9 | 25.9 | 16.8 | 10.7 | 30.8 |
| IVB | 810 | 44.7 | 29.4 | 23.5 | 20.4 | 18.3 | 9.2 | 8.0 | 10.9 |
Statistical analysis was performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). For each stage category, many possible tumor characteristics were included. Tumor characteristics that were evaluated included tumor size, depth of invasion, extent of local spread, lymph node status, and regional and distant spread of disease. Log‐rank and Wilcoxon tests were used to analyze overall survival similarities between and within groups of tumor characteristics resulting in the new (2021) FIGO staging for carcinoma of the vulva (Table 3).
TABLE 3.
New (2021) FIGO staging for carcinoma of the vulva
| Stage | Description | |
|---|---|---|
| I | Tumor confined to the vulva | |
| IA | Tumor size ≤2 cm and stromal invasion ≤1 mm a | |
| IB | Tumor size >2 cm or stromal invasion >1 mm a | |
| II | Tumor of any size with extension to lower one‐third of the urethra, lower one‐third of the vagina, lower one‐third of the anus with negative nodes | |
| III | Tumor of any size with extension to upper part of adjacent perineal structures, or with any number of nonfixed, nonulcerated lymph node | |
| IIIA | Tumor of any size with disease extension to upper two‐thirds of the urethra, upper two‐thirds of the vagina, bladder mucosa, rectal mucosa, or regional lymph node metastases ≤5 mm | |
| IIIB | Regional b lymph node metastases >5 mm | |
| IIIC | Regional b lymph node metastases with extracapsular spread | |
| IV | Tumor of any size fixed to bone, or fixed, ulcerated lymph node metastases, or distant metastases | |
| IVA | Disease fixed to pelvic bone, or fixed or ulcerated regional b lymph node metastases | |
| IVB | Distant metastases | |
Groups with overlapping survivals were then combined into individual stages and substages. These analyses were repeated multiple times. A secondary analysis was then performed to check whether each higher stage carries a worse prognosis than the preceding stage or substage. Starting from Stage IA the analysis confirmed that each higher stage or substage indeed has a worse prognosis than the preceding stage or substage; furthermore, that the associated prognosis with each stage or substage is unchanged by including tumor characteristics from any or all of the preceding stages or substages. As an example, Stage IIIB is defined by lymph node metastasis >5 mm, and combining this with disease extension to upper two‐thirds of perineal structures did not change the prognosis. Similarly, Stage IIIC is defined by lymph node metastasis with extracapsular extension, and combining this with lymph node metastases that are >5 mm without extracapsular extension and tumor extension to upper two‐thirds of adjacent perineal structures did not alter the prognosis.
Kaplan–Meier curves were generated for all of the stages and substages (Figure 1). The method used for computing confidence intervals requires an estimate of the survival distribution at the value nth percentile (n = 50). This means that if the survival function does not reach n–5 (0.45), a standard error or confidence interval bounds for the median will not be estimated. This is while the median overall survival for each stage or substage is not given.
FIGURE 1.
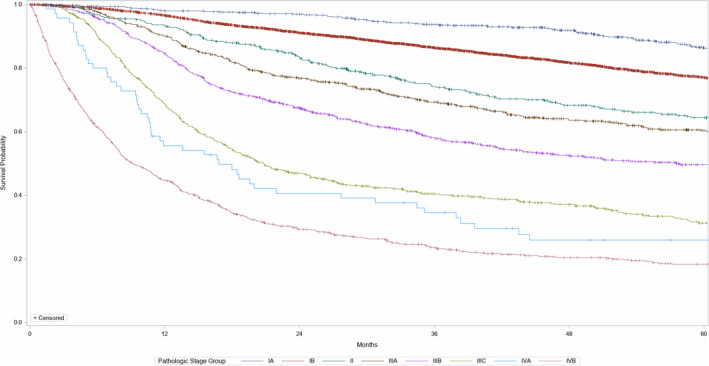
New (2021) FIGO staging for carcinoma of the vulva
The NCDB data utilized for these analyses may not cover the entire global experience, but the large size of the data compensates for this shortcoming and makes the findings generalizable.
3. IMPORTANT NOTATIONS AND CHANGES
Depth of invasion is measured from the basement membrane of the deepest, adjacent, dysplastic, tumor‐free rete ridge (or nearest dysplastic rete peg) to the deepest point of invasion. 10 , 11
Lymph node positivity should reflect the consensus utilized in cervical cancer staging, which is micrometastasis and macrometastasis. 12
Individual tumor cells (ITC) will not count toward lymph node metastasis.
Allow incorporation of cross‐sectional imaging findings into vulvar cancer staging similar to cervical cancer. 12
This staging is to be used for all morphological types of vulvar cancer and not just the most common squamous cell carcinoma. The only exception to this is vulvar melanoma.
Documentation regarding the HPV status of the carcinoma of the vulva (HPV‐associated or HPV‐independent) is strongly recommended. This is assessed by p16 block‐type immunoreactivity and/or positive molecular testing for HPV. 13
As shown in this new FIGO staging for carcinoma of the vulva, depth of invasion is an important prognostic factor in Stage I disease. The previous definition of depth of invasion, from the epithelial junction of the most superficial adjacent dermal papilla to the deepest point of invasion was proposed and adopted in 1984 by the International Society for the Study of Vulvovaginal Disease (ISSVD) and the International Society of Gynecological Pathologists (ISGYP). Reasons for choosing this method include: (1) adjacent dermal papilla can be found in all sites of the vulva; (2) it is not altered by variations in the depth of rete ridges; and (3) the measurement is not significantly influenced by hyperkeratosis, tumor surface ulceration, or adjacent epithelial hyperplasia. 14 , 15 These reasons are practical but not scientific. 10 In one study, 11 gynecologic pathologists failed to agree on the presence of invasion and only had moderate agreement regarding noninvasive or superficially invasive disease and invasion more than 1 mm. 16 Two studies subsequently investigated the value of measuring the depth of invasion from the basement membrane of the deepest, adjacent, dysplastic rete ridge or deepest dysplastic rete peg to the deepest point of invasion. 10 , 11 Both studies concluded that the alternative method of measurement correlates more with treatment outcome and deserves to be explored, hence the decision of the FIGO Committee for Gynecologic Oncology to adopt the alternative depth of invasion measurement in the 2021 staging. We realize that this depth of invasion definition is different from what was used during the collection of the NCDB data utilized to define this staging. However, we sincerely believe that the new definition is reasonable for many reasons: depth of invasion is only relevant in Stage I vulvar cancer; applying the method to the utilized data may result in stage migration of some cases (up to 22% in the studies used)—this proportion is unlikely to affect the overall findings from the statistical analyses; and lastly this represents an opportunity to collect large volume data with the new method of measurement for future analyses. The FIGO Committee for Gynecologic Oncology will assess the prognostic capability of this new depth of invasion measurement in the next revision of carcinoma of the vulva, which is expected in 10 years.
4. CONCLUSION
The 2021 carcinoma of the vulva revision by the FIGO Committee for Gynecologic Oncology represents a bold step as it is the first time that the Committee has utilized analyses of prospectively collected data and validated the prognostic capability of the staging at publication. In addition, the Committee's goal of simplification of vulvar cancer staging is also accomplished.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
ABO: literature review, data analyses, and manuscript writing. JC: data analysis. MAC, NB, AO, SW, CNP, GL, JSB: literature review and manuscript writing. SK: literature review, work coordination, and manuscript writing.
MEMBERS OF THE FIGO COMMITTEE FOR GYNECOLOGIC ONCOLOGY, 2018–2021
Sean Kehoe (Chair), Alexander B. Olawaiye, Mauricio A. Cuello, Neerja Bhatla, Aikou Okamoto, Sarikapan Wilailak, Gerhard Lindeque, Jonathan S. Berek, Chittaranjan N. Purandare (ex‐officio), Joanne Cain (co‐opted).
ACKNOWLEDGMENTS
The FIGO Committee for Gynecologic Oncology sincerely appreciates the help and support of The American College of Surgeons’ (ACoS) Commission on Cancer (CoC) and National Cancer Database (NCDB) programs, Timothy Mullett, MD, FACS, Chair of the Commission on Cancer, and Bryan Palis, Senior Statistician of the National Cancer Data Base for data access and data analyses.
Olawaiye AB, Cotler J, Cuello MA, et al. FIGO staging for carcinoma of the vulva: 2021 revision. Int J Gynecol Obstet. 2021;155:43–47. 10.1002/ijgo.13880
Contributor Information
Alexander B. Olawaiye, Email: olawaiyea@mail.magee.edu.
Sean Kehoe, Email: olawaiyea@mail.magee.edu.
REFERENCES
- 1. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607‐615. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 3. de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450‐3461. [DOI] [PubMed] [Google Scholar]
- 4. Shanbour KA, Mannel RS, Morris PC, Yadack A, Walker JL. Comparison of clinical versus surgical staging systems in vulvar cancer. Obstet Gynecol. 1992;80:927‐930. [PubMed] [Google Scholar]
- 5. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103‐104. [DOI] [PubMed] [Google Scholar]
- 6. Hacker NF. Revised FIGO staging for carcinoma of the vulva. Int J Gynecol Obstet. 2009;105:105‐106. [DOI] [PubMed] [Google Scholar]
- 7. Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, Cliby WA. Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecol Oncol. 2012;127:147‐152. [DOI] [PubMed] [Google Scholar]
- 8. Sznurkowski JJ, Milczek T, Emerich J. Prognostic factors and a value of 2009 FIGO staging system in vulvar cancer. Arch Gynecol Obstet. 2013;287:1211‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Steen S, de Nieuwenhof HP, Massuger L, Bulten J, de Hullu JA. New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis. Gynecol Oncol. 2010;119:520‐525. [DOI] [PubMed] [Google Scholar]
- 10. van den Einden LC, Massuger LF, Jonkman JK, Bult P, de Hullu JA, Bulten J. An alternative way to measure the depth of invasion of vulvar squamous cell carcinoma in relation to prognosis. Mod Pathol. 2015;28:295‐302. [DOI] [PubMed] [Google Scholar]
- 11. Skala SL, Ebott JA, Zhao L, Lieberman RW. Predictive value of an alternative strategy for measuring depth and size of Stage 1 vulvar squamous cell carcinoma. J Low Genit Tract Dis. 2020;24:265‐271. [DOI] [PubMed] [Google Scholar]
- 12. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145:129‐135. [DOI] [PubMed] [Google Scholar]
- 13. WHO Classification of Tumours Editorial Board . Female Genital Tumours. WHO Classification of Tumours, 5th edn. IARC; 2020:419‐449. [Google Scholar]
- 14. Wilkinson EJ. Superficial invasive carcinoma of the vulva. Clin Obstet Gynecol. 1985;28:188‐195. [DOI] [PubMed] [Google Scholar]
- 15. Preti M, Rouzier R, Mariani L, Wilkinson EJ. Superficially invasive carcinoma of the vulva: diagnosis and treatment. Clin Obstet Gynecol. 2005;48:862‐868. [DOI] [PubMed] [Google Scholar]
- 16. Abdel‐Mesih A, Daya D, Onuma K, et al. Interobserver agreement for assessing invasion in stage 1A vulvar squamous cell carcinoma. Am J Surg Pathol. 2013;37:1336‐1341. [DOI] [PubMed] [Google Scholar]


