Abstract
Objective
To examine the association between prenatal stomach position (SP) grade and stomach volume (SV) and the need for pulmonary hypertension (PH) treatment after birth in prenatally diagnosed left‐sided congenital diaphragmatic hernia (CDH), live born >34 weeks.
Methods
In retrospect, SP grade and SV were determined in fetuses with isolated left‐sided CDH from 19 weeks gestational age (GA) onwards at three different time periods (≤24 weeks' GA: US1, 24–30 weeks' GA: US2; ≥30 weeks' GA: US3). Primary outcome was need for treatment of PH after birth. Secondary analyses included the predictive value of SP and SV for other respiratory outcomes and postnatal defect size.
Results
A total of 101 fetuses were included. SP grade was significantly associated with need for treatment of PH (US1, US2, and US3: p < 0.02). Also, prenatal SP grade was positively associated with defect size and development of chronic lung disease (CLD) in survivors. No association was found between SV and respiratory morbidities or postnatal defect size.
Conclusion
SP grade in left‐sided CDH fetuses is associated with an increased need for PH treatment, a larger postnatal defect size and CLD in survivors. We consider SP determination a valuable contribution to the prenatal assessment of left‐sided CDH.
Key points
What is already known about this topic?
Prenatal stomach position (SP) grade has been proposed as a predictive ultrasound parameter for postnatal survival, patch repair, need for extracorporeal membrane oxygenation and need for prolonged respiratory support in left‐sided congenital diaphragmatic hernia (CDH).
What does this study add?
In children with left‐sided CDH, prenatal SP grade is associated with an increase in need for treatment of pulmonary hypertension and development of chronic lung disease, with the greatest increase in SP Grade 2 and 4.
A higher prenatal SP grade is associated with a larger postnatal defect size.
In the majority of cases SP grade does not vary throughout gestation.
1. INTRODUCTION
Congenital diaphragmatic hernia (CDH) is a life‐threatening condition after birth. Survival rates have increased over the years; yet those who survive have an increased risk of morbidity. 1 , 2 Prediction of longer term morbidity is difficult and prenatal counseling of parents remains challenging. 3 , 4 Currently the most validated predictive ultrasound (US) parameters for postnatal outcome in fetuses with left‐sided CDH include the observed‐to‐expected lung‐to‐head ratio (O/E LHR) and liver position (intra‐abdominal or intra thoracic). 5 , 6 , 7 However, the predictive value of these parameters for (co)morbidity in surviving CDH infants, for example, pulmonary hypertension (PH), gastrointestinal morbidities, or prediction of the actual (postnatal) defect size remains limited. 3 , 4 , 8 , 9 , 10 , 11
Previous studies have explored the predictive value of the prenatal stomach position (SP) for the occurrence of postnatal morbidity, using the grading system proposed by Cordier et al. 12 , 13 , 14 , 15 It was shown that the prenatal SP is a potential marker for postnatal survival, the need for postnatal patch repair, need for extracorporeal membrane oxygenation (ECMO), and need for prolonged respiratory support. 12 , 14 Moderate to almost perfect inter‐ and intraobserver agreement for the determination of prenatal SP has been shown by different groups, which might support its clinical applicability. 12 , 16
We hypothesize that a higher SP grade, ranging from 1 up to 4, is directly correlated to a larger defect size and a greater shift of the abdominal organs to the thorax. If this is true, a larger intrathoracic shift, together with a larger intrathoracic stomach volume (SV), might result in more severe pulmonary hypoplasia. Therefore, the primary aim of the study was to determine whether SP and SV predict the development of respiratory morbidity, primarily the development of PH. Secondarily, we studied the predictive values of SP and SV for the development of chronic lung disease (CLD) in survivors, presence of gastrointestinal disorders, mode of surgery (thoracoscopy or laparotomy) and/or use of patch, and postnatal defect size.
2. METHODS
This observational, retrospective cohort study included children with isolated left‐sided CDH prenatally diagnosed by US, who were live born >34 weeks gestational age (GA) and treated at the Erasmus University Medical Center Rotterdam, The Netherlands, between January 2007 and January 2018. Exclusion criteria were defined as: right‐sided or bilateral CDH, no postnatal confirmation of CDH diagnosis, associated major structural abnormalities which could influence the development of PH or gastrointestinal function, chromosomal anomalies, intrauterine fetal death, termination of pregnancy, premature birth (<34 weeks), intrapartum death, cases with missing data (defined as: poor quality US images and/or loss to follow‐up) and cases with no intention to treat. All prenatal and postnatal parameters described below were extracted from electronic patient records. The medical ethics review board of the Erasmus University Medical Center, Rotterdam, the Netherlands, waived approval for this study (MEC‐2019‐0618).
2.1. Prenatal parameters
Prenatal parameters were assessed from US data obtained from 19 weeks GA onwards, in the following time periods (in weeks GA): US1 (19+0–24+0); US2 (24+1–29+6); US3 (≥30+0). 7
Two physicians in fetal medicine (K. Weller and N. C. J. Peters) graded SP with the use of the grading system of Cordier et al. 13 (Figure 1). If US images were not of sufficient quality to determine the grade, SP was classified as “poor quality.” The intrathoracic SV was measured in available three‐dimensional (3D)‐sweeps of the thoracic cavity. With the use of VOCAL software, the inner SV was traced with a rotation step of six degrees (4D‐view, GE Medical System) and expressed in mm3. If more than one volume had been obtained in one US study, the mean volume was used in the analysis. Volumes were made as part of an ongoing study on lung development in CDH patients (MEC 2004‐227 P04.1325L).
FIGURE 1.
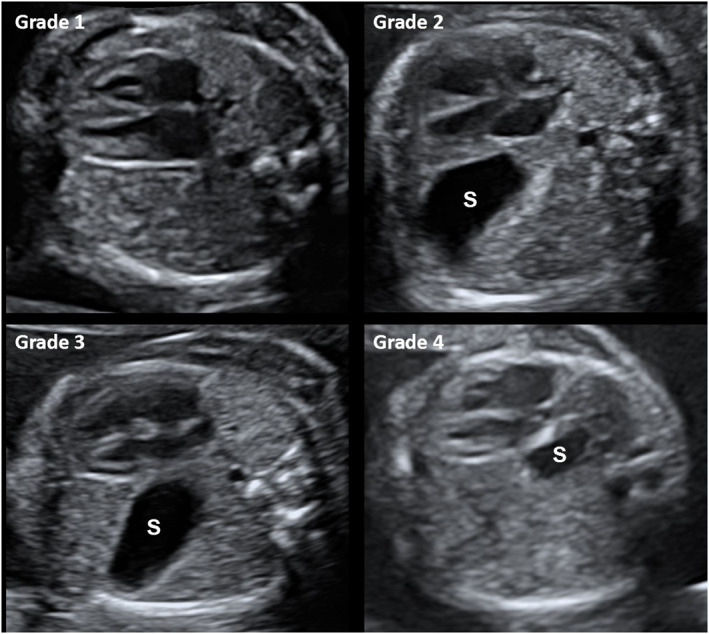
Ultrasound images. Prenatal stomach (S) position Grades 1–4, according to the grading system of Cordier et al. 14 at 20–24 weeks GA. Grade 1: abdominal, stomach not visualized in the chest. Grade 2: or anterior left in the chest. Grade 3: mid to posterior left chest. Grade 4: retrocardiac. Ultrasound images from the Erasmus University Medical Center
For the estimation of pulmonary hypoplasia severity, we created three sub groups according to Jani et al.—based on the combination of O/E LHR and liver position. 17 The O/E LHR was determined using the tracing method. 7 , 17 Liver position was defined as either in the thorax: “up” or in the abdomen: “down.” The subgroups were classified as either mild (O/E LHR >46% or O/E LHR 36%–45% + liver down), moderate (O/E LHR 36%–45% + liver up or O/E LHR 26%–35%) or severe (O/E LHR ≤25%). The presence of polyhydramnios was defined as an amniotic fluid index greater than 24 cm. 18
2.2. Perinatal and postnatal parameters
The following perinatal and postnatal parameters were collected up to the age of 1 year: GA at birth, birthweight, need for treatment of PH, PH on echocardiogram, presence of CLD, need for ECMO, mode of surgery, need for prosthetic patch, defect size, position of the liver as reported during surgery (intrathoracic or abdominal), time elapsed until full enteral feeding, duration of tube feeding, length of stay at the intensive care unit (ICU), total hospital stay, survival, height and weight at 6 and 12 months.
Need for treatment of PH was defined by treatment with either inhaled nitric oxide or intravenous sildenafil during initial hospitalization, which are the first choice of treatment for PH in our center. 19 , 20 PH on echocardiogram was defined as pulmonary pressures higher than two‐thirds of the systemic pressures on echocardiography, measured at the first day of life. 19 CLD was defined as oxygen dependency for at least 28 days, determined between day 28 and 56 of life. 21 Need for high frequency oscillation ventilation was not included as outcome parameter since this was not part of routine care in our center from 2013 onwards. 22 Mode of surgery for postnatal repair of the diaphragmatic defect was either thoracoscopy or laparotomy. The initial choice for mode of surgery was based on the patient's stability, the suspected size of the defect and liver position, with “liver up” being an indication for laparotomy. If thoracoscopy had been converted to laparotomy, we classified mode of surgery as laparotomy. 23 Defect size was classified as A, B, C, or D by the pediatric surgeon during surgery or in retrospect from the surgical report, according to Lally et al. 24
Gastrointestinal morbidity was evaluated by two endpoints: (1) age at full enteral feeding, that is, number of days elapsed until partial or complete total parenteral nutrition 6 was stopped; and (2) number of days elapsed until tube feeding was stopped. Physical growth was assessed according to Dutch reference norms, defined as SD scores (SDS) of length and weight at the ages of 6 and 12 months. 25
2.3. Statistical analysis
2.3.1. Baseline characteristics
Patient baseline characteristics are described as absolute numbers (%) for categorical data and median (interquartile range [IQR] for continuous data. Patient parameters were compared between the group of infants who needed treatment for PH and the group of those who did not. We used the Fisher's exact test for nominal or ordinal data and Mann–Whitney tests for continuous data. Consistency between SP at US1, US2, and US3 and the interrater agreement of the graded SP between raters K. Weller and N. C. J. Peters at the three different US time periods was evaluated using the weighted Cohen's kappa coefficient (κ). K. Weller and N. C. J. Peters both graded the SP in 30 randomly selected patients, blinded to each other's result. Correlations between SP and the CDH severity subgroups were analyzed using Spearman's rank correlation coefficient. The association between SP and prenatal liver position was analyzed using a Fisher's exact test.
2.3.2. SP and postnatal outcomes
Differences in dichotomous outcome variables in relation to the different SP grades were analyzed by use of χ 2 or Fisher exact tests. Post hoc pairwise comparisons of the four categories of SP grade were performed using χ 2 or Fisher exact tests with the Bonferroni method to account for the effects of multiple testing, that is, with an adjusted significance level of 0.05/6. A Kruskal–Wallis H test was used to analyze differences in the postnatal defect size amongst the four SP groups. Associations between SP grades and continuous outcomes were analyzed using the Jonckheere–Terpstra test.
2.3.3. SV and postnatal outcomes
To calculate the predictive value of SV (a continuous variable) for dichotomous outcomes, receiver operating characteristics (ROC) curves were made for US1, US2, and US3 separately. Data are presented as area under the curve with the 95% confidence interval (95% CI). The correlation of SV at, respectively, US1, US2, and US3 with continuous postnatal outcomes was evaluated using Spearman's rank correlation coefficient.
All analyses were performed using SPSS version 25.0 for Windows. A two‐sided p value of <0.05 was taken to indicate significance.
3. RESULTS
3.1. Study population
A total of 193 prenatally diagnosed CDH patients were seen in our center, and 101 were included in the study. Reasons for exclusion are detailed in Figure S1. Two patients were excluded because treatment was discontinued shortly after birth (within 3 and 12 h, respectively). Both had a very poor prognosis with bradycardia and respiratory insufficiency directly after birth, nonresponsive to maximal therapeutic interventions. Of the 101 included patients, 19 did not survive until 1 year of age (median days alive: 19, IQR: 11–36 days). Thirteen of them had respiratory complications such PH refractory to medical treatment. Only one fetus of this series, with SP Grade 2, was treated with fetoscopic endoluminal tracheal occlusion without complications. This child died 75 days after birth due to persistent PH, not responding to maximal therapy. One child died at day 11 from untreatable PH, after having received an erroneously high dosage of prostaglandins.
3.2. Prenatal and perinatal characteristics
Baseline characteristics are summarized in Table 1. CDH severity (mild, moderate, or severe) at US3 differed significantly between patients treated for PH and those not treated for PH (p = 0.009). Data on prenatal SP is summarized in Table 2. US data were available for 57 patients at US1, 48 at US2, and 98 at US3. Thirty‐two patients were seen at all three time periods. Missing data were due to referral to our hospital not until the third trimester or to poor quality of the US images. SP grade at US1 was found to be consistent with the SP at US2 (n = 32, κ = 0.97, 95% CI: 0.91–1.03) and US3 (n = 51, κ = 0.87, 95% CI: 0.76–0.97), and SP grade at US2 was consistent with the SP at US3 (n = 48, κ = 0.87, 95% CI: 0.76–0.99). Of the 32 patients seen at US1, US2, and US3, 28 patients (88%) showed a constant SP grade throughout all time periods. Agreement of SP grade between the two raters at US1, US2, and US3 was almost perfect (US1: κ = 0.92, 95% CI: 0.75–1.08; US2: κ = 0.91, 95% CI: 0.73–1.09; US3: κ = 0.88, 95% CI: 0.75–1.02). 3D US data for the assessment of SV was available for 31 (31%) patients (Table S3).
TABLE 1.
Baseline characteristics
| CDH patients (n = 101) | p Value | ||
|---|---|---|---|
| PH‐treated: No (n = 56) | PH‐treated: Yes (n = 45) | ||
| Maternal parameters | |||
| Maternal age (years) | 32 (27–36) | 30 (26–34) | 0.527 |
| Maternal BMI | 23.5 (21.6–26.4) | 23.1 (20.8–28.1) | 0.835 |
| Nulliparous | 32 (57.1) | 20 (44.4) | 0.233 |
| Prenatal parameters | |||
| Polyhydramnios | 14 (25) | 17 (38) | 0.196 |
| US1 (n = 55) | |||
| Prenatal liver position: Up | 13 (23) | 10 (22) | 0.785 |
| CDH severity | 0.771 | ||
| Mild | 19 (34) | 17 (38) | |
| Moderate | 10 (18) | 8 (18) | |
| Severe | 0 (0) | 1 (2) | |
| N.A. | 27 (48) | 19 (42) | |
| US2 (n = 51) | |||
| Prenatal liver position: Up | 11 (20) | 11 (24) | 1.000 |
| CDH severity | 0.202 | ||
| Mild | 17 (30) | 10 (22) | |
| Moderate | 10 (18) | 13 (29) | |
| Severe | 0 (0) | 1 (2) | |
| N.A. | 29 (52) | 21 (47) | |
| US3 (n = 94) | |||
| Prenatal liver position: Up | 16 (29) | 19 (42) | 0.208 |
| CDH severity | 0.009 | ||
| Mild | 42 (75) | 22 (49) | |
| Moderate | 11 (20) | 17 (38) | |
| Severe | 0 (0) | 2 (4) | |
| N.A. | 3 (5) | 4 (9) | |
| Perinatal parameters | |||
| Gender: Male | 37 (66) | 21 (47) | 0.068 |
| GA at delivery (weeks) | 38+3 (37+5–38+6) | 38+1 (37+4–38+6) | 0.570 |
| Caesarean section | 12 (21) | 13 (19) | 0.663 |
| Birth weight (g) | 3000 (2725–3200) | 3000 (2600–3150) | 0.437 |
Note: Data are presented as numbers (% of total inclusion number) or median (IQR), bold type p values indicate statistical significance differences between the groups. US1, ultrasound scan ≤24 weeks gestational age (GA); US2, ultrasound scan 24–30 weeks GA; US3 ultrasound scan ≥30 weeks GA. Prenatal liver position “up”: liver intrathoracic. CDH severity: combination of O/E LHR and liver position according to Jani et al. 16 : mild (O/E LHR > 46% or O/E LHR 36%–45% + liver down), moderate (O/E LHR 36%–45% + liver up or O/E LHR 26%–35%), severe (O/E LHR ≤ 25%).
Abbreviation: BMI, body mass index; IQR, interquartile range; PH, pulmonary hypertension.
TABLE 2.
Stomach position at US1, US2, and US3 correlated to prenatal characteristics
| Stomach position | p Value | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| US1 | n = 8 | n = 13 | n = 29 | n = 4 | |
| Liver position at US1: Up | 1 (13) | 0 (0) | 19 (66) | 3 (75) | <0.001 |
| O/E LHR at US1 | 58 (49–75) | 44 (39–48) | 43 (40–49) | 32 (29–47) | 0.001 |
| CDH severity at US1 | <0.001 | ||||
| Mild | 8 (100) | 10 (77) | 14 (48) | 1 (25) | |
| Moderate | 0 (0) | 1 (8) | 14 (48) | 3 (75) | |
| Severe | 0 (0) | 0 (0) | 1 (3) | 0 (0) | |
| N.A. | 0 (0) | 2 (15) | 0 (0) | 0 (0) | |
| US2 | n = 10 | n = 10 | n = 24 | n = 4 | |
| Liver position at US2: Up | 1 (10) | 0 (0) | 18 (75) | 3 (75) | <0.001 |
| O/E LHR at US2 | 51 (47–63) | 41 (32–54) | 40 (34–47) | 33 (30–40) | 0.014 |
| CDH severity at US2 | 0.001 | ||||
| Mild | 7 (70) | 5 (50) | 8 (33) | 0 (0) | |
| Moderate | 1 (10) | 3 (30) | 15 (63) | 4 (100) | |
| Severe | 0 (0) | 0 (0) | 1 (4) | 0 (0) | |
| N.A. | 2 (20) | 2 (20) | 0 (0) | 0 (0) | |
| US3 | n = 19 | n = 26 | n = 45 | n = 8 | |
| Liver position at US3: Up | 1 (5) | 0 (0) | 26 (58) | 7 (88) | <0.001 |
| O/E LHR at US3 | 58 (49–68) | 46 (39–57) | 43 (40–50) | 29 (25–37) | <0.001 |
| CDH severity at US3 | <0.001 | ||||
| Mild | 17 (90) | 19 (73) | 25 (56) | 1 (13) | |
| Moderate | 1 (5) | 3 (12) | 19 (42) | 5 (63) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | 2 (25) | |
| N.A. | 1 (5) | 4 (15) | 1 (2) | 0 (0) | |
Note: Data are presented as numbers (% of group total), p values represent the association (by use of the Fisher exact test for liver position or Jonckheere–Terpstra test for O/E LHR) or Spearman's rank correlation coefficient (CDH severity) between the four stomach position grades and prenatal characteristics. Bold type p values indicate statistical significance. Liver position “up”: liver intrathoracic. CDH severity: combination of O/E LHR and liver position according to Jani et al. 16 : mild (O/E LHR > 46% or O/E LHR 36%–45% + liver down), moderate (O/E LHR 36%–45% + liver up or O/E LHR 26%–35%), severe (O/E LHR ≤ 25%).
Abbreviation: CDH, congenital diaphragmatic hernia.
At all assessed US time periods, a higher SP grade (Grade 3 and Grade 4) was associated with prenatal intrathoracic liver position (US1: p < 0.001; US2: p < 0.001; US3: p < 0.001) and a lower O/E LHR (US1: p = 0.001; US2: p < 0.014; US3: p < 0.001). In addition, SP was significantly correlated to CDH severity (mild, moderate, or severe) (US1: p ≤ 0.001; US2: p = 0.001; US3: p < 0.001). SV was not associated with liver position, O/E LHR or CDH severity (data not shown).
3.3. Postnatal outcomes
Analyses of the relation between prenatal SP and SV and postnatal outcomes are summarized in Tables 3 and S1–S4.
TABLE 3.
Stomach position at US3 and postnatal outcomes
| Stomach position | p Value | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| US3 | n = 19* | n = 26 | n = 45 a | n = 8 a | |
| PH‐treated | 4 (21) | 16 (62) | 16 (36) | 8 (100) | <0.001 |
| PH‐echocardiogram | 11 (56) | 20 (77) | 34 (76) | 7 (88) | 0.116 |
| CLD b | 3 (16) | 20 (77) | 23 (51) | 5 (63) | <0.001 |
| ECMO | 0 (0) | 9 (35) | 7 (16) | 7 (88) | <0.001 |
| Postnatal liver position: Up | 1 (5) | 6 (23) | 24 (53) | 5 (63) | <0.001 |
| Mode of surgery: Laparotomy | 3 (16) | 16 (62) | 29 (64) | 6 (75) | <0.001 |
| Defect size | <0.001 | ||||
| A | 6 (32) | 0 (0) | 3 (7) | 0 (0) | |
| B | 7 (37) | 8 (31) | 11 (24) | 0 (0) | |
| C | 5 (26) | 16 (62) | 27 (60) | 4 (50) | |
| D | 0 (0) | 2 (8) | 3 (7) | 2 (25) | |
| Not operated | 1 (5) | 0 (0) | 1 (2) | 2 (25) | |
| Use of patch | 8 (42) | 24 (92) | 37 (82) | 6 (75) | 0.001 |
| Duration TPN (days) | 9 (7–12) | 20 (14–35) | 14 (11–22) | 16 (10–42) | 0.138 |
| Duration TF (days) | 25 (12–48) | 116 (38–842) | 47 (17–188) | 33 (11–393) | 0.984 |
| ICU stay (days) | 9 (6–25) | 30 (19–58) | 19 (10–37) | 33 (11–97) | 0.117 |
| Total hospital stay (days) | 20 (13–38) | 47 (34–71) | 34 (21–53) | 33 (11–100) | 0.509 |
| Survival >28 days | 18 (95) | 24 (92) | 42 (93) | 5 (63) | 0.083 |
| Survival >1 year | 18 (95) | 21 (81) | 39 (87) | 2 (25) | 0.001 |
Note: Data are presented as numbers (% of group total) or median (IQR), p values represent the association (by use of the χ 2 or Fisher exact tests for nominal, Kruskal–Wallis H test for ordinal or Jonckheere–Terpstra test for continuous variables), bold type indicates statistical significance. Defect size was classified according to Lally et al. 21 with “A” being defects entirely surrounded by muscle, “B” defects having a small (<50%), and “C” defects having a large (>50%) portion of the chest wall devoid of diaphragm tissue, and “D” patients having complete or near complete absence of the left diaphragm.
Abbreviations: CLD, chronic lung disease; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; PH, pulmonary hypertension; TF, tube feeding; TPN, total parenteral nutrition.
Missing cases due to death before operation.
CLD in surviving infants (≥28 days).
3.4. Respiratory outcomes
SP grade at any US time period was significantly associated with an increased need for treatment of PH (US1: p < 0.001; US2: p = 0.015; US3: p < 0.001) and ECMO (US1: p = 0.014; US2: p = 0.017; US3: p < 0.001). In addition, SP grade at US2 or US3 was significantly associated with the development of CLD in surviving infants (p = 0.023 and p < 0.001, respectively). No associations were found between SP grade and PH on echocardiogram (US1: p = 0.116; US2: p = 0.419; US3: p = 0.671). In post hoc pairwise comparisons between SP grades, infants with a prenatal SP Grade 2 at US3 showed higher incidences of treatment of PH (62% vs. 36%, p = 0.034) and development of CLD (77% vs. 51%, p = 0.033) than infants with a prenatal SP Grade 3 at US3, but these differences were not statistically significant after adjustment for multiple testing. Results for other pairwise comparisons are not shown. The patients who were excluded from analysis because of death within 24 h had been diagnosed with isolated left‐sided CDH (n = 2) and had a prenatal SP Grade 3 (n = 1) or SP Grade 4 (n = 1). ROC analyses of SV for respiratory outcomes did not show a significant association (Table S3).
3.5. Gastrointestinal outcomes
The duration of total parenteral feeding was reported for all patients, but the duration of tube feeding was not available for 10 patients due to incomplete registration of time of tube removal. No significant associations were found between prenatal SP or SV and the duration of total parenteral feeding, tube feeding or SDS of growth parameters (Tables 3, S1, S2, and S4, respectively).
3.6. Surgery
Prenatal SP grade was significantly associated with postnatal defect size, classified A–D (US1: p = 0.003; US2: p = 0.007; US3: p < 0.001). In patients with a higher SP grade at US3, a laparotomy was performed significantly (p < 0.001) more often than in patients with a lower SP grade. This significant association was not seen between SP grades at US1 (p = 0.11) and US2 (p = 0.10) and mode of surgery. At US3, SP grade was associated with the need for a patch closure (p = 0.002). ROC analyses of SV for mode of surgery and use of patch did not show a significant association (Table S3). No significant association was found between SV and postnatal defect size (Table S4).
3.7. Hospital stay
A higher prenatal SP at US2, but not at US1 or US3, was significantly associated with a longer stay at the ICU (p = 0.03). No other significant associations existed between SP or SV and the duration of ICU and/or hospital stay.
4. DISCUSSION
In this cohort of infants with isolated left‐sided CDH, prenatal SP grade was significantly associated with respiratory morbidity and defect size. Prenatal SP in fetuses with left‐sided CDH, graded according to Cordier et al. 13 can be reliably assessed by prenatal US, as the interrater agreement is almost perfect. While other studies assessed SP only at one time point in pregnancy, 12 , 13 , 14 , 26 we considered more time points and found that SP did not vary significantly throughout pregnancy.
This is the first study that shows that a higher SP grade, on a scale from Grade 1 up to Grade 4, is positively correlated to an increasing postnatal defect size classified A–D, and therefore might serve as a proxy for postnatal defect size. 24 This finding is in line with previous observations reporting a higher need of a patch closure. 12 , 14 Previous studies have shown that defect size is a major predictor of mortality and is a reliable predictor for long‐term gastrointestinal, neurological and pulmonary morbidity in these children. 27 , 28 Prenatal determination of defect size has only been possible with the use of fetal magnetic resonance imaging. 29 Measurement of the size of the defect by prenatal US is still not possible, hence the degree of lung hypoplasia can be used as a proxy as we have previously reported. 7 However, contrary to SP grading the learning curve to accurately assess the O/E LHR is extensive. 30 Therefore SP grading might be a valuable addition to determination of the lung size in fetuses with left‐sided CDH, as earlier suggested in previous papers. 5 , 15 Due to small subgroup sizes in our cohort, specifically fetuses with SP Grade 4 (n = 8 at US3), multivariable regression analysis to evaluate the individual predictive value of SP grade for postnatal outcomes was not feasible. This limitation should be taken into account in interpreting the results.
The prenatal prediction of respiratory morbidity in infants born with a CDH has proven to be challenging, in part because the lungs will only be fully functional after birth. Both SP grading and O/E LHR are considered as a proxy for the degree of pulmonary hypoplasia, reflecting the chances of survival and the severity of respiratory morbidity. In this study we have confirmed that the position of the stomach is indeed associated with clinically relevant PH and CLD. A previous study, too, showed a correlation between prenatal SP and a longer length of time until resolution of PH on echocardiogram. 31 In contrast, the incidence of need for treatment of PH in our study (45%) is lower than previously reported incidence rates (>60%). These incidences are based, however, on diagnosis of PH by echocardiography, not purely on need for treatment. 32 , 33 , 34 In general, PH is defined as pulmonary pressures higher than two‐thirds of the systemic pressures on echocardiography, measured at the first day of life. In our opinion this definition for PH overestimates the number of children with a clinical need for treatment of PH, as was shown by our data. Thus we propose that the number of treated infants is a more relevant clinical outcome measure. Although we found a significant association between a higher SP grade and respiratory morbidity, caution remains warranted for individual cases. With the current prenatal imaging techniques, prenatal prediction of postnatal lung function is still highly challenging.
A recent study showed significant associations between SP grades and gastrointestinal outcomes, defined as the duration of parenteral nutrition and the persistence of oral aversion at the age of 2. 35 In our study population we did not find such a relationship between SP grade and gastrointestinal outcomes (duration of total parenteral feeding and duration of tube feeding). Of note, the retrospective design of this study is a potential bias and may have contributed to many missing data and outliers for duration of tube feeding, consequently considered this an unreliable outcome parameter for this population. The relatively low proportion of both severe CDH cases and fetuses with Grade 4 SP (n = 8, 8%) at 30 weeks GA in our study compared to earlier reported ranges of Grade 4 SP (17%–26%), might explain the differences found between our and previous studies, regarding gastrointestinal outcomes. 12 , 13 , 14 , 35 We recommend future prospective research to focus on gastrointestinal morbidity at the age of 2 years, and study other gastrointestinal outcome parameters, that is, oral aversion or gastrointestinal reflux, in relation to prenatal SP. Since assessment of gastrointestinal reflux through esophageal 24‐h pH/impedance monitoring was not part of the standard follow‐up protocol in our center during the entire study period, we were not able to include this in the present study.
In our cohort, fetuses with a prenatal SP Grade 2 appeared to have higher incidences of postnatal respiratory morbidity (need for treatment of PH and development of CLD) than those with a Grade 3, however the difference was not significant after correction for multiple testing. More severe respiratory outcomes in Grade 2 SP compared to Grade 3 are in contrast to Basta et al. 12 who found a linear association between SP grade and days of ventilation. Possibly this could be explained by the different definitions of postnatal outcome parameters (number of days of ventilation vs. presence of CLD) in their study compared to our study and previous studies. 33 In addition, the same effect was seen for Grades 2 and 3 and gastrointestinal outcomes. These results seem in concordance with Cordier et al. 35 who also found that the median duration of total parenteral feeding in fetuses with a prenatal Grade 2 SP is higher, although not significantly, than that in fetuses with a prenatal Grade 3 SP. One could hypothesize that an anterior position of the stomach (Grade 2 SP) indicates a large shift of bowel and stomach, herniating from a posterior defect, to an anterior position within the thorax. Possibly this leads to a larger intrathoracic pressure on the fetal heart and lungs, whereas in fetuses with a Grade 3 SP the stomach remains closer to the posterior defect. Despite the fact that we have shown a significant consistency of SP grade between different time periods of gestation, we are limited by the number of patients with US data at all three time periods (n = 32) and potential changes in SP grade might have been biased. Larger patient numbers are needed to explore this hypothesis.
Hata et al. 36 measured 3D (abdominal) SV in healthy fetuses (n = 35) in 2010, showing feasibility of SV measurement and changing SV within a one hour examination. To our knowledge, the additional value of thoracic SV measurements has not yet been investigated in left‐sided CDH. In our study, mean SV was assessed in a relatively small number of patients and was not associated with treatment for PH or other postnatal outcome parameters.
5. CONCLUSION
SP grade, assessed by prenatal US, is associated with respiratory morbidity and the postnatal defect size in left‐sided CDH. Since SP is easy to assess, with hardly any interrater variability, and is constant throughout pregnancy, we consider SP grading an easily applicable tool in the prenatal assessment of fetuses with left‐sided CDH and a valuable contribution to counseling of future parents.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Supporting information
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
ACKNOWLEDGMENT
Ko Hagoort provided editorial advice.
Weller K, Peters NCJ, van Rosmalen J, et al. Prenatal stomach position and volume in relation to postnatal outcomes in left‐sided congenital diaphragmatic hernia. Prenat Diagn. 2022;42(3):338‐347. 10.1002/pd.6019
DATA AVAILABILITY STATEMENT
Data available on request from the corresponding author.
REFERENCES
- 1. Lally KP. Congenital diaphragmatic hernia—the past 25 (or so) years. J Pediatr Surg. 2016;51(5):695‐698. [DOI] [PubMed] [Google Scholar]
- 2. Dao DT, Hayden LP, Buchmiller TL, et al. Longitudinal analysis of pulmonary function in survivors of congenital diaphragmatic hernia. J Pediatr. 2020;216:158‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benachi A, Cordier AG, Cannie M, Jani J. Advances in prenatal diagnosis of congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19(6):331‐337. [DOI] [PubMed] [Google Scholar]
- 4. Russo FM, Eastwood MP, Keijzer R, et al. Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;49(6):704‐713. [DOI] [PubMed] [Google Scholar]
- 5. Russo FM, Cordier AG, De Catte L, et al. Proposal for standardized prenatal ultrasound assessment of the fetus with congenital diaphragmatic hernia by the European reference network on rare inherited and congenital anomalies (ERNICA). Prenat Diagn. 2018;38(9):629‐637. [DOI] [PubMed] [Google Scholar]
- 6. Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33(1):64‐69. [DOI] [PubMed] [Google Scholar]
- 7. Snoek KG, Peters NCJ, van Rosmalen J, et al. The validity of the observed‐to‐expected lung‐to‐head ratio in congenital diaphragmatic hernia in an era of standardized neonatal treatment; a multicenter study. Prenat Diagn. 2017;37(7):658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Congenital Diaphragmatic Hernia Study Group , Lally KP, Lally PA, et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120(3):e651‐e657. [DOI] [PubMed] [Google Scholar]
- 9. Rudra S, Adibe OO, Malcolm WF, Smith PB, Cotten CM, Greenberg RG. Gastrostomy tube placement in infants with congenital diaphragmatic hernia: frequency, predictors, and growth outcomes. Early Hum Dev. 2016;103:97‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Power B, Shibuya S, Lane B, Eaton S, De Coppi P. Long‐term feeding issue and its impact on the daily life of congenital diaphragmatic hernia survivors: results of the first patient‐led survey. Pediatr Surg Int. 2020;36(1):63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haliburton B, Mouzaki M, Chiang M, et al. Long‐term nutritional morbidity for congenital diaphragmatic hernia survivors: failure to thrive extends well into childhood and adolescence. J Pediatr Surg. 2015;50(5):734‐738. [DOI] [PubMed] [Google Scholar]
- 12. Basta AM, Lusk LA, Keller RL, Filly RA. Fetal stomach position predicts neonatal outcomes in isolated left‐sided congenital diaphragmatic hernia. Fetal Diagn Ther. 2016;39(4):248‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cordier AG, Cannie MM, Guilbaud L, et al. Stomach position versus liver‐to‐thoracic volume ratio in left‐sided congenital diaphragmatic hernia. J Matern Fetal Neonatal Med. 2015;28(2):190‐195. [DOI] [PubMed] [Google Scholar]
- 14. Cordier AG, Jani JC, Cannie MM, et al. Stomach position in prediction of survival in left‐sided congenital diaphragmatic hernia with or without fetoscopic endoluminal tracheal occlusion. Ultrasound Obstet Gynecol. 2015;46(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 15. Sananes N, Britto I, Akinkuotu AC, et al. Improving the prediction of neonatal outcomes in isolated left‐sided congenital diaphragmatic hernia by direct and indirect sonographic assessment of liver herniation. J Ultrasound Med. 2016;35(7):1437‐1443. [DOI] [PubMed] [Google Scholar]
- 16. Ibirogba ER, Novoa YNVA, Sutton LF, et al. Standardization and reproducibility of sonographic stomach position grades in fetuses with congenital diaphragmatic hernia. J Clin Ultrasound. 2019;47(9):513‐517. [DOI] [PubMed] [Google Scholar]
- 17. Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. 2007;30(1):67‐71. [DOI] [PubMed] [Google Scholar]
- 18. Carlson DE, Platt LD, Medearis AL, Horenstein J. Quantifiable polyhydramnios: diagnosis and management. Obstet Gynecol. 1990;75(6):989‐993. [PubMed] [Google Scholar]
- 19. Reiss I, Schaible T, van den Hout L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98(4):354‐364. [DOI] [PubMed] [Google Scholar]
- 20. Snoek KG, Reiss IK, Greenough A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus—2015 update. Neonatology. 2016;110(1):66‐74. [DOI] [PubMed] [Google Scholar]
- 21. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723‐1729. [DOI] [PubMed] [Google Scholar]
- 22. Snoek KG, Capolupo I, van Rosmalen J, et al. Conventional mechanical ventilation versus high‐frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (the VICI‐trial). Ann Surg. 2016;263(5):867‐874. [DOI] [PubMed] [Google Scholar]
- 23. Vijfhuize S, Schaible T, Kraemer U, Cohen‐Overbeek TE, Tibboel D, Reiss I. Management of pulmonary hypertension in neonates with congenital diaphragmatic hernia. Eur J Pediatr Surg. 2012;22(5):374‐383. [DOI] [PubMed] [Google Scholar]
- 24. Lally KP, Lasky RE, Lally PA, et al. Standardized reporting for congenital diaphragmatic hernia—an international consensus. J Pediatr Surg. 2013;48(12):2408‐2415. [DOI] [PubMed] [Google Scholar]
- 25. Talma H, Schonbeck Y, Bakker B, Hirasing R, van Buuren S. Groeidiagrammen 2010: Handleiding bij het meten en wegen van kinderen en het invullen van groeidiagrammen. Leiden: TNO; 2010. [Google Scholar]
- 26. Kitano Y, Okuyama H, Saito M, et al. Re‐evaluation of stomach position as a simple prognostic factor in fetal left congenital diaphragmatic hernia: a multicenter survey in Japan. Ultrasound Obstet Gynecol. 2011;37(3):277‐282. [DOI] [PubMed] [Google Scholar]
- 27. Putnam LR, Harting MT, Tsao K, et al. Congenital diaphragmatic hernia defect size and infant morbidity at discharge. Pediatrics. 2016;138(5):e20162043. [DOI] [PubMed] [Google Scholar]
- 28. Congenital Diaphragmatic Hernia Study Group , Morini F, Valfre L, et al. Congenital diaphragmatic hernia: defect size correlates with developmental defect. J Pediatr Surg. 2013;48(6):1177‐1182. [DOI] [PubMed] [Google Scholar]
- 29. Prayer F, Metzelder M, Krois W, et al. Three‐dimensional reconstruction of defects in congenital diaphragmatic hernia: a fetal MRI study. Ultrasound Obstet Gynecol. 2019;53(6):816‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cruz‐Martinez R, Figueras F, Moreno‐Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2010;36(1):32‐36. [DOI] [PubMed] [Google Scholar]
- 31. Lusk LA, Wai KC, Moon‐Grady AJ, Basta AM, Filly R, Keller RL. Fetal ultrasound markers of severity predict resolution of pulmonary hypertension in congenital diaphragmatic hernia. Am J Obstet Gynecol. 2015;213(2):216.e1‐216.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brindle ME, Cook EF, Tibboel D, Lally PA, Lally KP, Congenital Diaphragmatic Hernia Study Group . A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics. 2014;134(2):e413‐e419. [DOI] [PubMed] [Google Scholar]
- 33. Cochius‐den Otter SCM, Erdem O, van Rosmalen J, et al. Validation of a prediction rule for mortality in congenital diaphragmatic hernia. Pediatrics. 2020;145(4):e20192379. [DOI] [PubMed] [Google Scholar]
- 34. Bent DP, Nelson J, Kent DM, Jen HC. Population‐based validation of a clinical prediction model for congenital diaphragmatic hernias. J Pediatr. 2018;201:160‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cordier AG, Laup L, Letourneau A, et al. Prenatal stomach position predicts gastrointestinal morbidity at 2 years in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2021;57(6):959‐967. [DOI] [PubMed] [Google Scholar]
- 36. Hata T, Tanaka H, Noguchi J, Inubashiri E, Yanagihara T, Kondoh S. Three‐dimensional sonographic volume measurement of the fetal stomach. Ultrasound Med Biol. 2010;36(11):1808‐1812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Data Availability Statement
Data available on request from the corresponding author.


