Abstract
Expanded hemodialysis (HDx), using medium cut‐off membrane, is a novel therapy that effectively clears middle molecules (MMs). We aimed to compare HDx to hemodiafiltration (HDF) in an open randomized clinical study. Patients currently on HDF (age 18–80 years; on HDF >3 months) were randomized to switch to HDx (N = 21) or continue HDF (N = 22) with a 24‐week follow‐up. Pre‐ to post‐dialysis reduction ratios (RR) and changes in pre‐dialysis levels over time were evaluated for MMs and clinical biomarkers. Use of erythropoiesis‐stimulating agents (ESAs) was assessed. HDx showed greater RR for YKL‐40 while RR appeared similar between groups for beta2‐microglobulin, FGF‐23, and free light chains. Intradialytic changes in inflammatory biomarkers (IL‐6, CRP, PTX3) did not differ between therapies. Changes from baseline to 12 and 24 weeks did not differ between groups for MMs, inflammatory markers, albumin, fibrinogen, hemoglobin, PTH, and phosphorus. Use of ESAs tended to decrease in HDx arm while remaining stable in HDF arm. HDx appeared safe with similar clinical effectiveness as HDF. With fewer requirements and resource needs, HDx provides an attractive alternative to HDF.
Keywords: erythropoietin resistance index, expanded hemodialysis, hemodiafiltration, large middle molecules, medium cut‐off membrane
1. INTRODUCTION
The efficacy of blood purification by hemodialysis depends on the vascular access condition, type of dialysis membrane, treatment time, and blood flow rate. The choice of membrane becomes particularly important for effective clearance of middle molecular uremic toxins (MMs), for which the membrane's permeability properties are essential [1]. The most well‐studied MM is beta2‐microglobulin (β2m) having a molecular weight of 11.8 kDa; its role in dialysis‐related amyloidosis is well established and its plasma level is associated with mortality risk in dialysis patients [2]. Furthermore, recent research points at a significant role of large MMs, larger than 25 kDa, in the progression of comorbidities and poor outcome in dialysis patients [3, 4]. A chronic state of inflammation appears an important link between large MMs and long‐term dialysis complications [5].
HDF enhances the removal of conventional and large MMs, as increased convection across the membrane helps to overcome limits in membrane permeability [6]. Analysis of pooled data from randomized controlled studies pointed at a survival benefit of HDF vs. HD when HDF was applied in postdilution mode with high convective volume [7]. Although the mechanism of superior outcome has not been firmly established, it is commonly hypothesized that enhanced MM removal by HDF plays a key role.
Recent innovation in membrane design resulted in the MCO membranes with higher retention onset than conventional high‐flux membranes and effective selectivity to limit permeability for albumin [1, 8]. MCO membranes are designed for HDx, a new therapy option to enhance the clearance of large MMs without the need for external replacement fluid as in HDF [9]. The safety of long‐term HDx therapy to prevalent dialysis patients has been established [10, 11] and initial data indicate a positive impact of HDx therapy on hospitalizations, drug utilization, and costs in comparison to conventional high‐flux HD [12]. Short‐term performance studies have shown the HDx therapy to deliver similar clearance of β2m and large middle molecules as HDF [13, 14, 15, 16] with potential to outperform HDF for some large MMs [13]. So far only one observational study presented data on mid‐term clinical effectiveness of HDx therapy in comparison to HDF, indicating noninferiority over a 6‐month period [17]. Here, we report a randomized trial over 24 weeks comparing HDx to HDF under typical treatment conditions in Spain.
2. MATERIALS AND METHODS
2.1. Study design
Patients with chronic kidney disease receiving hemodialysis in the Renal Therapy Services dialysis center, Murcia, Spain, were included in an open‐label, prospective, 1:1 randomized, parallel‐group study with a 24‐weeks follow‐up.
Prevalent dialysis patients aged 18–80 years were eligible if clinically stable, as demonstrated by pertinent medical history, physical examination, and laboratory testing, and on postdilution on‐line HDF three times per week for at least 3 months prior to study enrollment. Exclusion criteria included, but was not limited to, conditions that could interfere with the patient's ability to provide informed consent, unstable vascular access with risk of low and variable extracorporeal blood flow rate, chronic liver disease, bleeding disorder or red blood cell transfusion within 12 weeks prior to enrollment, acute infection within 4 weeks prior to enrollment, or scheduled for interventions requiring hospitalization for more than 1 week.
After informed consent was obtained, patients were stratified by residual renal function (anuric [<100 mL/24 h] or nonanuric) and randomized to either transfer to HDx therapy or to stay on previous HDF therapy. HDx treatments were delivered using the Theranova 500 dialyzer (polyarylethersulfone/polyvinylpyrrolidone membrane, 2.0 m2 surface area; Baxter, Hechingen, Germany). HDF treatments were delivered in postdilution mode using the Polyflux 170H dialyzer (polyarylethersulfone/polyamide/polyvinylpyrrolidone membrane, 1.7 m2 surface area; Baxter, Hechingen, Germany) with a target convective volume of at least 23 L. Patients and staff were unblinded to the applied therapy. All treatments were delivered with Artis Physio dialysis systems (Baxter, Medolla, Italy). Treatment duration, blood flow rate (targeted for at least 350 mL/min), dialysis fluid composition, and temperature were to be maintained as before study initiation. Anticoagulation with low molecular weight heparin was provided.
2.2. Ethics
The study protocol was approved by the ethics committee of Reina Sofia General University Hospital, Murcia; the study was conducted in accordance with the Declaration of Helsinki, the ethical and quality standards of good clinical practice, and all applicable regulatory requirements and laws, local, national, and European. Patients were appropriately informed of the study concept and enrolled only after signing an informed consent document. The study was registered as NCT03499691.
2.3. Data collection
Primary outcome assessment included blood sampling pre‐ and post‐dialysis after 12 weeks of treatment and calculation of the reduction ratios (RRs) for a range of MMs: beta2‐microglobulin (β2m, 11.8 kDa), fibroblast growth factor 23 (FGF‐23, 32 kDa), chitinase‐3‐like protein 1 (YKL‐40, 40 kDa), kappa free light chain (FLC, monomers 22.5 kDa), and lambda FLC (dimers 45 kDa).
Secondary outcome assessments were (i) change from pre‐ to post‐dialysis in plasma levels of inflammatory markers—interleukin‐6 (IL‐6), C‐reactive protein (CRP), and pentraxin‐3 (PTX3)—at 12 weeks of treatment, (ii) change from baseline to 12 and 24 weeks of study treatments in mid‐week pre‐dialysis plasma levels of MMs and inflammatory markers, (iii) change from baseline in pre‐dialysis plasma levels of albumin, fibrinogen, hemoglobin, parathyroid hormone (PTH), and phosphorous, (iv) delivered single pool Kt/Vurea, and (v) weekly dose of erythropoiesis‐stimulating agents (ESA) and intravenous iron to manage anemia.
Biomarker analysis was performed by an external laboratory that was blinded to how the samples related to the study subjects. Assay details are listed in Table S1.
2.4. Calculations
For calculations of pre‐ to postdialysis reduction ratios or percent changes, the postdialysis concentrations were corrected for hemoconcentration using the formula by Bergström and Wehle [18].
ESA doses were assessed every 4 weeks and given as the mean weekly dose in the preceding 4 weeks. Darbepoetin alpha doses were converted from mg to IU using a factor of 200. Erythropoietin resistance index (ERI) for a certain week was calculated using the weekly ESA dose divided by the hemoglobin level in that week.
2.5. Statistical analysis
A feasibility analysis indicated that a sample size of approximately 40 patients was possible in the study site, which was deemed to give enough power to evaluate the primary objectives of the study based on previous study results (10). Given the exploratory nature of most other outcomes of the study, this sample size was also seen as enough to establish data for future and more focused studies.
Descriptive data are reported as mean ± SD unless otherwise stated. Differences between study arms in reduction ratio or change during session were analyzed using ANCOVA model. Differences between therapies in change over time were analyzed using mixed‐effect repeated measurement (MMRM) models for most biomarkers. Both models used baseline predialysis concentration and baseline urine output as covariates. The Wilcoxon rank‐sum test was used to analyze change over time for FGF‐23 and CRP due to the non‐normal distribution of data.
3. RESULTS
Forty‐three patients were enrolled in April 2018, fulfilling the inclusion/exclusion criteria and providing informed consent. Twenty‐one patients were assigned to start HDx therapy and 22 to stay on HDF therapy. Baseline demographics were similar between the two study arms (Table 1).
TABLE 1.
Patient demographics at study baseline
| HDx study arm (N = 21) | HDF study arm (N = 22) | |
|---|---|---|
| Age (years) | 60.7 ± 14.3 | 61.8 ± 9.4 |
| Gender (% males) | 57% | 73% |
| Body weight (kg) | 76.6 ± 13.1 | 75.9 ± 16.0 |
| Dialysis vintage (months; median/range) | 30 / 6–224 | 35 / 5–375 |
| Urine production | ||
| Anuric (<100 mL/24 h) | 48% | 45% |
| Oliguric (100–500 mL/24 h) | 29% | 32% |
| Non‐oliguric (>500 mL/24 h) | 23% | 23% |
| ESRD comorbidity index | 2.5 ± 1.7 | 1.9 ± 1.8 |
| Malnutrition inflammation score | 3.4 ± 2.1 | 3.5 ± 1.4 |
Acceptance to the HDx therapy was high in those patients who switched from HDF to HDx. One patient in the HDF arm died during the study. Two patients were excluded due to severe adverse events not related to dialysis, one in each study arm. In addition, nine patients were discontinued from the study as they left the study unit going on vacation for more than 2 weeks. Most of these left after the week 12 assessment and, by chance, most were in the HDF study arm. The patient flow is summarized in Figure S1.
Treatment characteristics are reported in Table 2. Mean blood flow rate during the study was close to 400 mL/min in both groups over time. Treatment duration was 4 h. HDF treatments showed a substitution volume that averaged 24 L. With 2.4 L of mean UF volume the mean total convective volume was close to 26 L.
TABLE 2.
Study treatment characteristics at week 12
| HDx (N = 21) | HDF (N = 19) | |
|---|---|---|
| Treatment duration (min) | 241 ± 4 | 239 ± 7 |
| Blood flow rate (mL/min) | 400 ± 12 | 396 ± 8 |
| Dialysis fluid flow rate (mL/min) | 500 | 600 |
| Ultrafiltration volume (L) | 2.5 ± 0.8 | 2.1 ± 0.8 |
| Substitution fluid volume (L) | n.a. | 24.4 ± 3.2 |
Abbreviation: n.a., not applicable.
3.1. Intradialytic changes in biomarker levels
Pre‐ to postdialysis RR for MMs was greater in the HDx study arm for YKL‐40 (58.1 ± 9.5 vs. 42.4 ± 12.5%; p < 0.0001) while it appeared similar between HDx and HDF for β2m (76.6 ± 5.6 vs. 77.2 ± 5.6%; p = 0.47) and FGF‐23 (48.1 ± 21.3 vs. 45.1 ± 20.8%; p = 0.63). For FLC, measured by the N Latex assay, the RRs were also similar between therapies for kappa FLC (67.0 ± 5.9 vs. 64.9 ± 6.9%; p = 0.40) and lambda FLC (67.7 ± 6.2 vs. 65.9 ± 8.2%; p = 0.31). These RRs are summarized in Figure 1.
FIGURE 1.
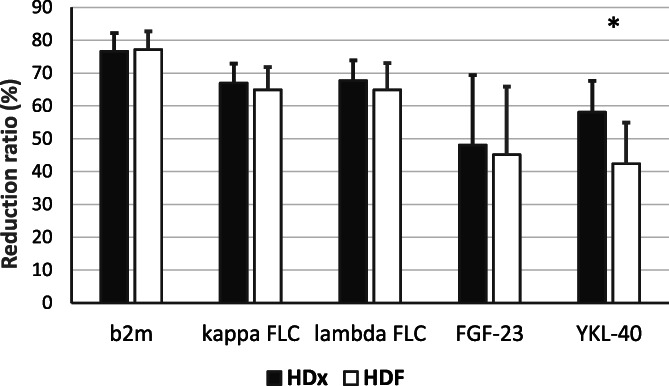
Middle molecule pre‐ to post‐dialysis reduction ratios. Pre‐ to post‐dialysis reduction ratios measured at week 12 (*p < 0.0001). Note that as FLC concentrations were measured by the N Latex assay the lambda FLC reduction ratios likely reflect only the removal of lambda monomers
The HDx and HDF study arms showed similar percent change from pre‐ to postdialysis for inflammatory biomarkers: IL‐6 (−13.7 ± 13.2 vs. −16.9 ± 14.7%; p = 0.29), CRP (−7.2 ± 12.1 vs. −8.8 ± 10.6%; p = 0.62), and PTX3 (+5.2 ± 24.9 vs. +8.6 ± 30.5%; p = 0.45).
Both study arms showed a mean delivered spKt/V of 1.8, ranging between subjects from 1.3 to 2.6.
3.2. Biomarker changes over time
Pre‐dialysis levels of β2m, FGF‐23, FLCs, and YKL‐40 were similar between study arms at baseline and their changes to 12 and 24 weeks did not differ between arms except for borderline differences at week 24 for β2m (p = 0.045) and FGF‐23 (p = 0.039); see Table 3. Inflammatory markers CRP, IL‐6, and PTX3 were also similar between groups at baseline, and changes over the 24‐week study period appeared insignificant and similar between study arms. Likewise, changes over time in plasma levels of albumin, fibrinogen, hemoglobin, PTH, and phosphate did not differ between study arms (see Table 3).
TABLE 3.
Baseline pre‐dialysis levels and changes from baseline for the studied biomarkers
| Baseline | Change to week 12 | Change to week 24 | ||
|---|---|---|---|---|
| B2m (mg/L) | HDx | 25.4 ± 7.6 | −0.6 ± 3.6 | −0.6 ± 3.9 |
| HDF | 24.3 ± 7.5 | −1.0 ± 4.5 | +3.3 ± 6.1 | |
| p‐value | 0.62 | 0.55 | 0.046 | |
| FGF‐23 (pg/mL) | HDx | 1153 (402, 1979) | −20 (−597, +512) | −24 (−623, +202) |
| HDF | 825 (277, 1438) | +208 (−537, +494) | +343 (+44, +1152) | |
| p‐value | 0.28 | 0.45 | 0.039 | |
| YKL‐40 (ng/mL) | HDx | 432 ± 325 | +2 ± 114 | 0 ± 132 |
| HDF | 507 ± 491 | −3 ± 234 | +103 ± 432 | |
| p‐value | 0.56 | 0.94 | 0.16 | |
| Kappa FLC (mg/L) | HDx | 143 ± 41 | −5 ± 17 | +7 ± 35 |
| HDF | 151 ± 45 | −4 ± 30 | +20 ± 46 | |
| p‐value | 0.52 | 0.75 | 0.56 | |
| Lambda FLC (mg/L) | HDx | 129 ± 37 | −3 ± 28 | +19 ± 37 |
| HDF | 173 ± 141 | 8 ± 43 | +44 ± 58 | |
| p‐value | 0.17 | 0.65 | 0.28 | |
| IL‐6 (pg/mL) | HDx | 8.6 ± 5.3 | −0.3 ± 2.6 | −0.5 ± 2.2 |
| HDF | 7.2 ± 3.2 | −0.1 ± 3.1 | −0.1 ± 2.3 | |
| p‐value | 0.32 | 0.83 | 0.49 | |
| CRP (mg/L) | HDx | 2.4 (1.3, 4.7) | −0.3 (−1.0, +0.3) | 0.0 (−0.7, +1.1) |
| HDF | 5.6 (2.6, 8.4) | −0.7 (−4.0, +0.1) | −0.4 (−1.6, +4.2) | |
| p‐value | 0.18 | 0.34 | 0.68 | |
| PTX‐3 (pg/mL) | HDx | 4.8 ± 2.7 | +0.6 ± 2.1 | 0.0 ± 1.8 |
| HDF | 6.8 ± 4.8 | −0.9 ± 2.4 | −1.0 ± 3.9 | |
| p‐value | 0.10 | 0.39 | 0.75 | |
| Albumin (g/dL) | HDx | 3.67 ± 0.38 | +0.06 ± 0.46 | −0.02 ± 0.25 |
| HDF | 3.78 ± 0.32 | −0.01 ± 0.26 | −0.02 ± 0.34 | |
| p‐value | 0.33 | 0.89 | 0.59 | |
| Fibrinogen (mg/dL) | HDx | 352 ± 95 | −45 ± 75 | −22 ± 74 |
| HDF | 376 ± 126 | −72 ± 117 | −94 ± 103 | |
| p‐value | 0.49 | 0.78 | 0.13 | |
| Hemoglobin (g/dL) | HDx | 11.3 ± 0.8 | +0.3 ± 1.2 | −0.2 ± 1.0 |
| HDF | 11.4 ± 0.8 | −0.3 ± 1.1 | +0.1 ± 1.0 | |
| p‐value | 0.69 | 0.19 | 0.16 | |
| PTH (pg/mL) | HDx | 413 ± 214 | +36 ± 328 | +51 ± 330 |
| HDF | 309 ± 181 | +34 ± 191 | +210 ± 293 | |
| p‐value | 0.09 | 0.66 | 0.30 | |
| Phosphorous (mg/dL) | HDx | 4.25 ± 1.11 | +0.04 ± 1.54 | +0.17 ± 1.16 |
| HDF | 3.79 ± 0.68 | +0.36 ± 0.96 | +0.67 ± 0.76 | |
| p‐value | 0.11 | 0.87 | 0.67 |
Note: Data presented as mean ± SD or as median (25th, 75th percentile).
3.3. Exploratory—Anemia management
All subjects in the HDF study arm and 20 out of 21 subjects in the HDx study arm received ESA during the study. Thirty‐one subjects received erythropoietin (HDF: 17; HDx:14) while 9 received darbepoetin alpha (HDF: 3; HDx:6); in addition, 2 patients in the HDF arm were on darbepoetin alpha at the start of the study but shifted to erythropoietin during the study. When evaluating subjects who completed the 24‐week study period, those in the HDF study arm (N = 12) showed stable mean weekly dose of ESA over time (baseline: 99 ± 74 IU/kg, week 12: 113 ± 75 IU/kg, week 24: 102 ± 74 IU/kg) while the HDx study arm subjects (N = 19) showed a trend toward a decrease in weekly ESA dose from week 8 (baseline: 106 ± 87 IU/kg, week 12: 98 ± 102 IU/kg, week 24: 77 ± 103 IU/kg) (Figure 2a). ERI in ESA‐treated patients showed a similar trend without significant difference between groups (Figure 2b) while hemoglobin level appeared stable over time in both groups (Figure 2c). The use of intravenous iron did not differ significantly between treatment arms, although with a trend of reduced need over time in the HDx arm. Transferrin saturation and ferritin levels were comparable at baseline (23.1 ± 7.8% and 297 ± 275 ng/mL for HDx arm vs. 20.8 ± 7.4% and 231 ± 201 ng/mL for HDF arm) and changes during the study period were similar between groups.
FIGURE 2.
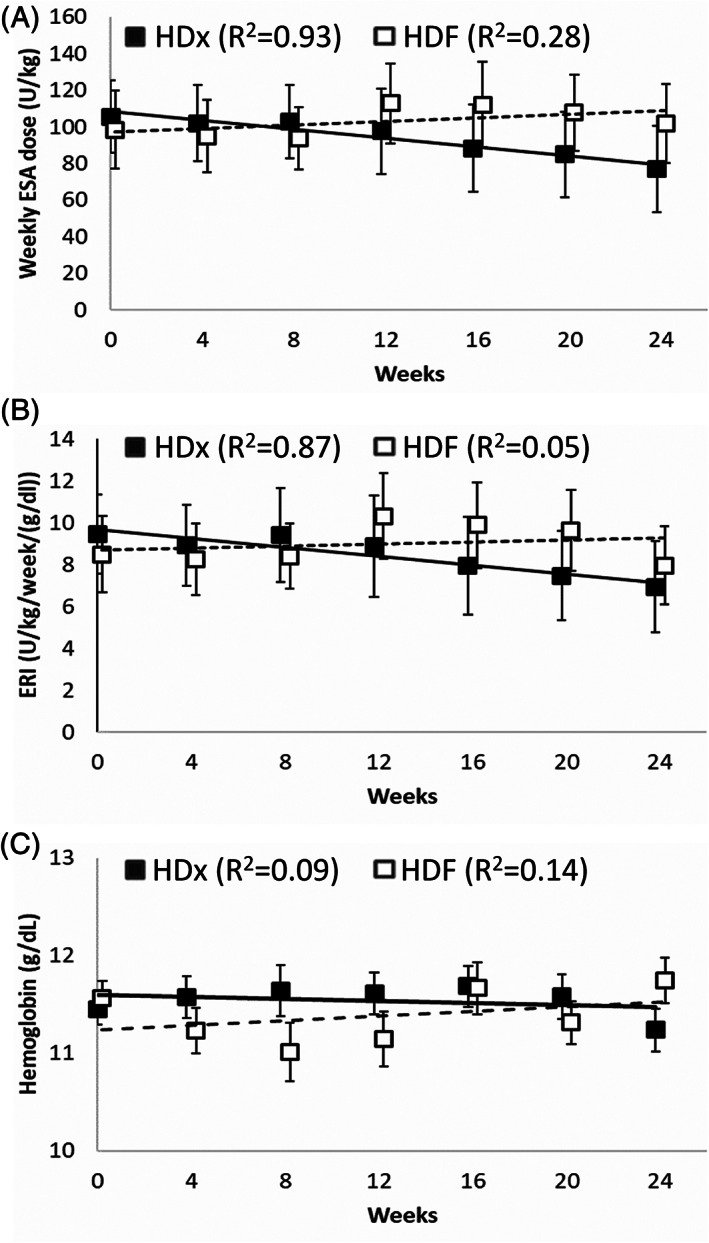
Anemia parameters over time in subjects who completed the 24‐week study period. (a) Weekly ESA dose per kg body weight. (b) Erythropoietin resistance index (ERI). (c) Blood hemoglobin level. Filled squares show means for patients switching to HDx, open squares show means for patients maintained on HDF. Error bars indicate standard errors of the means. Lines and R 2 values refer to linear regressions
3.4. Adverse events
Overall, a total of 37 subjects (86%) were reported to experience 134 adverse events (AEs, serious or nonserious) during the study; 18 subjects with 79 AEs in the HDx group and 19 subjects with 55 AEs in the HDF group. Hypotension, muscle cramps, and hypertension were the most reported AEs. Intradialytic hypotension episodes were not seen in any patient during the study period. Eight AEs were rated as serious (3 in HDx, 5 in HDF); none of these were judged to be related to the dialysis procedure.
4. DISCUSSION
The clinical effectiveness of expanded hemodialysis (HDx) using the recently introduced MCO membrane is not yet fully characterized in comparison to on‐line HDF. We here report what we believe to be the first randomized controlled study comparing HDx to HDF with a 24‐week follow‐up. The key finding is that the HDx therapy provided similar reduction ratios as high‐efficiency HDF for a wide range of MMs, with greater reduction ratio for YKL‐40. Compared to patients who were continued on HDF, patients assigned to HDx exhibited no change over time in biomarker levels, indicating that HDx is non‐inferior to HDF in clinical effectiveness.
It should be recognized that large MM clearance in HDF depends on membrane properties, dilution mode, convective volume, and convective flow rate in relation to blood flow rate. The current study applied high‐efficiency HDF therapy according to the normal HDF prescription in the study site, which included a high‐flux membrane commonly used in convective therapies, post‐dilution mode, an average HDF convective volume close to 26 L/treatment, and a convective flow rate close to 28% of blood flow rate on average. Using a more protein permeable membrane in HDF would likely increase MM clearance but probably also result in greater loss of albumin that may be unwanted. Greater convective flow in relation to blood flow, like the 35% reached in a recent study by Maduell et al., may also increase MM removal in HDF but may also result in greater albumin loss and frequent coagulation problems [19]. We therefore consider HDF therapy as applied in our study as clinically relevant for most HDF users.
Effective removal of a wide spectrum of uremic toxins is a key objective of the dialysis treatment. While β2m has long been the marker of conventional MMs, there is currently no consensus of what is appropriate as marker for the large middle molecule class of uremic toxins. A global removal score was recently proposed [16], calculated from reduction ratios of urea, several middle molecules, and albumin; α1‐microglobulin and α1‐acid glycoprotein were included as large middle molecules. In our study, we measured the removal of four other large middle molecules—FGF‐23, kappa FLC, lambda FLC, and YKL‐40—for which a high plasma level has been found to be predictive of poor outcome in CKD patients [20, 21, 22, 23]. We suggest these are appropriate markers for a global removal score when assessing therapy performance.
In terms of blood purification performance for middle molecules, our results are comparable to the single‐treatment data reported by Kirsch et al. for the Theranova MCO membrane (initially reported as MCO AA dialyzer) [13], except for lambda FLC. While Kirsch et al. found increased removal of lambda FLC with the MCO dialyzer in comparison to HDF, we did not. Importantly, Kirsch et al. found for both therapies a lower RR for lambda FLC than for kappa FLC, consistent with the fact that lambda circulates mostly as dimers while kappa circulates as monomers. In our analysis the RRs for kappa and lambda were high and of a similar magnitude, which indicates that the dialytic removal of lambda dimers was not accurately captured. We believe these differences are explained by the assay used, being a Siemens N Latex assay in our study and the Binding Site Freelite assay in the Kirsch study. Recent comparisons of these two immunochemical methods have revealed significant differences in their ability to discriminate between monomers and dimers of lambda FLC [24, 25]. Based on these data, we would recommend the use of the Freelite assay in future assessments of the removal of lambda FLC by dialysis.
We did not find a change over time in pre‐dialysis plasma levels of MMs in study patients transferring from HDF to HDx but observed a trend for increased levels in those patients who stayed on HDF. The apparent difference between groups in these changes for β2m and FGF‐23 at week 24 (p‐values 0.048 and 0.039, respectively) should be interpreted with caution, considering the multiple testing and the risk of attrition bias in the week 24 data.
Interestingly, we saw a trend for reduced ESA dose over time in the HDx study arm without a concomitant reduction in hemoglobin level, indicating an improved response to the ESA administered in comparison to HDF. HDF has been found superior to conventional HD in reducing ESA resistance in some [26] but not all studies [27]. A positive effect on anemia management is possibly limited to patients receiving intravenous erythropoietin [28]. ESA hyporesponsiveness in iron‐replete dialysis patients is considered linked to a state of inflammation. We did not find evidence of a difference between HDx and HDF in inflammatory biomarkers; it should be noted, though, that the variability in the data and the small sample size gave us low power to detect such a difference. When Lim et al. in a randomized controlled trial found reduced ESA dose and ERI with HDx in comparison to high‐flux HD they also found with HDx a significantly lower serum TNF‐α level at 12 weeks [29]. Whether ESA sparing and/or cytokine lowering effects are due to increased MM removal or improved biocompatibility of the newer generation membrane is currently unclear.
This study has several limitations. It was not designed for a formal noninferiority analysis of HDx therapy in comparison to HDF but was merely exploratory. Although our sample size appears appropriate to evaluate differences in reduction ratios for middle molecules, it was too low to draw firm conclusions on the impact of therapy over time. The fact that we lost several patients in the HDF arm after the week 12 visit translates into an increased risk of attrition bias for the week 24 comparison. All study patients were on HDF treatments at study enrollment; our results may not reflect a situation where patients are on HD for transfer to HDF or HDx.
5. CONCLUSION
In a randomized controlled study, we found that HDx therapy using MCO membrane delivers large middle molecule removal at least at level with high‐efficiency HDF. We found for HDx therapy a trend for reduced need of ESAs without indication that HDx is inferior to HDF in other parameters of clinical effectiveness, results that should be viewed as preliminary while awaiting additional and larger studies in this field. Being easy to implement and not dependent on on‐line HDF equipment, the HDx therapy appears an attractive option to manage long‐term dialysis.
CONFLICT OF INTEREST
Fernando Hadad‐Arrascue was an employee of Renal Therapy Services (RTS), an independent entity owned by Baxter International, Inc, at the time the study was conducted. Lars‐Göran Nilsson, Angela S. Rivera, and Angelito A. Bernardo are employees of Baxter Healthcare.
Supporting information
Table S1 Analytical assays used to measure biomarker plasma concentrations
Figure S1: CONSORT patient flow diagram
ACKNOWLEDGMENTS
Baxter Healthcare Corporation acted as sponsor of the study. We thank the clinical staff at the Renal Therapy Services Hemodialysis unit in Murcia for their work in collecting the study data, and the Baxter clinical development team for setting up the study framework. We also thank Rhea Parreno, Baxter, for conducting statistical analyses.
Hadad‐Arrascue F, Nilsson L‐G, Rivera AS, Bernardo AA, Cabezuelo Romero JB. Expanded hemodialysis as effective alternative to on‐line hemodiafiltration: A randomized mid‐term clinical trial. Ther Apher Dial. 2022;26:37–44. 10.1111/1744-9987.13700
Funding information Baxter Healthcare Corporation
REFERENCES
- 1. Ronco C, Clark WR. Haemodialysis membranes. Nat Rev Nephrol. 2018;14:394–410. [DOI] [PubMed] [Google Scholar]
- 2. Okuno S, Ishimura E, Kohno K, et al. Serum β2‐microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24:571–7. [DOI] [PubMed] [Google Scholar]
- 3. Chmielewski M, Cohen G, Wiecek A, Jesús CJ. The peptidic middle molecules: Is molecular weight doing the trick? Semin Nephrol. 2014;34:118–34. [DOI] [PubMed] [Google Scholar]
- 4. Wolley M, Jardine M, Hutchison CA. Exploring the clinical relevance of providing increased removal of large middle molecules. Clin J Am Soc Nephrol. 2018;13:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end‐stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl_3):iii35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blankestijn P, Ledebo I, Canaud B. Hemodiafiltration: Clinical evidence and remaining questions. Kidney Int. 2010;77:581–7. [DOI] [PubMed] [Google Scholar]
- 7. Peters SA, Bots ML, Canaud B, et al. Haemodiafiltration and mortality in end‐stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31:978–84. [DOI] [PubMed] [Google Scholar]
- 8. Boschetti‐de‐Fierro A, Voigt M, Storr M, Krause B. MCO membranes: Enhanced selectivity in high‐flux class. Sci Rep. 2015;5:18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ronco C. The rise of expanded hemodialysis. Blood Purif. 2017;44:I–VIII. [DOI] [PubMed] [Google Scholar]
- 10. Krishnasamy R, Hawley CM, Jardine MJ, et al. A trial evaluating mid cut‐off value membrane clearance of albumin and light chains in hemodialysis patients: A safety device study. Blood Purif. 2020;49:468–78. [DOI] [PubMed] [Google Scholar]
- 11. Bunch A, Sanchez R, Nilsson LG, et al. Medium cut‐off dialyzers in a large population of hemodialysis patients in Colombia: COREXH registry. Ther Apher Dial. 2021;25:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ariza JG, Walton SM, Suarez AM, Sanabria M, Vesga JI. An initial evaluation of expanded hemodialysis on hospitalizations, drug utilization, costs, and patient utility in Colombia. Ther Apher Dial. 2021. 10.1111/1744-9987.13620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirsch AH, Lyko R, Nilsson LG, et al. Performance of hemodialysis with novel medium cut‐off dialyzers. Nephrol Dial Transplant. 2017;32:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reque J, Pérez Alba A, Panizo N, Sánchez‐Canel JJ, Pascual MJ, Pons PR. Is expanded hemodialysis an option to online Hemodiafiltration for small‐ and middle‐sized molecules clearance? Blood Purif. 2019;47:126–31. [DOI] [PubMed] [Google Scholar]
- 15. García‐Prieto A, Vega A, Linares T, et al. Evaluation of the efficacy of a medium cut‐off dialyser and comparison with other high‐flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin Kidney J. 2018;11:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maduell F, Rodas L, Broseta JJ, et al. Medium cut‐off dialyzer versus eight hemodiafiltration dialyzers: Comparison using a global removal score. Blood Purif. 2019;48:167–74. [DOI] [PubMed] [Google Scholar]
- 17. Belmouaz M, Diolez J, Bauwens M, et al. Comparison of hemodialysis with medium cut‐off dialyzer and on‐line hemodiafiltration on the removal of small and middle‐sized molecules. Clin Nephrol. 2018;89:50–6. [PubMed] [Google Scholar]
- 18. Bergström J, Wehle B. No change in corrected beta 2‐microglobulin concentration after cuprophane haemodialysis. Lancet. 1987;1:628–9. [DOI] [PubMed] [Google Scholar]
- 19. Maduell F, Broseta JJ, Rodas L, et al. Comparison of solute removal properties between high‐efficient dialysis modalities in low blood flow rate. Ther Apher Dial. 2020;24:387–92. [DOI] [PubMed] [Google Scholar]
- 20. Marthi A, Donovan K, Haynes R, et al. Fibroblast growth factor‐23 and risks of cardiovascular and noncardiovascular diseases: A meta‐analysis. J Am Soc Nephrol. 2018;29:2015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lorenz G, Schmalenberg M, Kemmner S, et al. Mortality prediction in stable hemodialysis patients is refined by YKL‐40, a 40‐kDa glycoprotein associated with inflammation. Kidney Int. 2018;93:221–30. [DOI] [PubMed] [Google Scholar]
- 22. Vega A, Sanchez‐Niño MD, Ortiz A, et al. The new marker YKL‐40, a molecule related to inflammation, is associated with cardiovascular events in stable haemodialysis patients. Clin Kidney J. 2019;13:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchison CA, Burmeister A, Harding SJ, Basnayake K, Church H, Jesky MD, et al. Serum polyclonal immunoglobulin free light chain levels predict mortality in people with chronic kidney disease. Mayo Clin Proc. 2014;89:615–22. [DOI] [PubMed] [Google Scholar]
- 24. Kennard A, Hawley C, Tate J, Klingberg S, Pretorius C, Hutchison C, et al. Comparison of Freelite™ and N Latex serum free light chain assays in subjects with end stage kidney disease on haemodialysis. Clin Chem Lab Med. 2016;54:1045–52. [DOI] [PubMed] [Google Scholar]
- 25. Caponi L, Koni E, Romiti N, Paolicchi A, Franzini M. Free light chain UV quantification compared with immunochemical measurement: How dimers and monomers may influence the results. Clin Chim Acta. 2020;510:278–84. [DOI] [PubMed] [Google Scholar]
- 26. Panichi V, Scatena A, Rosati A, et al. High‐volume online haemodiafiltration improves erythropoiesis‐stimulating agent (ESA) resistance in comparison with low‐flux bicarbonate dialysis: Results of the REDERT study. Nephrol Dial Transplant. 2015;30:682–9. [DOI] [PubMed] [Google Scholar]
- 27. Locatelli F, Altieri P, Andrulli S, et al. Predictors of haemoglobin levels and resistance to erythropoiesis‐stimulating agents in patients treated with low‐flux haemodialysis, haemofiltration and haemodiafiltration: Results of a multicentre randomized and controlled trial. Nephrol Dial Transplant. 2012;27:3594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marcelli D, Bayh I, Merello JI, et al. Dynamics of the erythropoiesis stimulating agent resistance index in incident hemodiafiltration and high‐flux hemodialysis patients. Kidney Int. 2016;90:192–202. [DOI] [PubMed] [Google Scholar]
- 29. Lim JH, Jeon Y, Yook JM, et al. Medium cut‐off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin‐independent manner in maintenance hemodialysis patients: Results from a randomized controlled trial. Sci Rep. 2020;10:16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Analytical assays used to measure biomarker plasma concentrations
Figure S1: CONSORT patient flow diagram


