Abstract
A strain of Listeria monocytogenes isolated from a drain in a food-processing plant was demonstrated, by determination of D values, to be more resistant to the lethal effect of heat at 56 or 59°C following incubation for 45 min in tryptose phosphate broth (TPB) at pH 12.0 than to that of incubation for the same time in TPB at pH 7.3. Cells survived for at least 6 days when they were suspended in TPB at pHs 9.0, 10.0, and 11.0 and stored at 4 or 21°C. Cells of L. monocytogenes incubated at 37°C for 45 min and then stored for 48 or 144 h in TPB at pH 10.0 were more resistant to heat treatment at 56°C than were cells stored in TPB at pH 7.3. The alkaline-stress response in L. monocytogenes may induce resistance to otherwise lethal thermal-processing conditions. Treatment of cells in 0.05 M potassium phosphate buffer (pH 7.00 ± 0.05) containing 2.0 or 2.4 mg of free chlorine per liter reduced populations by as much as 1.3 log10 CFU/ml, while treatment with 6.0 mg of free chlorine per liter reduced populations by as much as 4.02 log10 CFU/ml. Remaining subpopulations of chlorine-treated cells exhibited some injury, and cells treated with chlorine for 10 min were more sensitive to heating at 56°C than cells treated for 5 min. Contamination of foods by L. monocytogenes cells that have survived exposure to processing environments ineffectively cleaned or sanitized with alkaline detergents or disinfectants may have more severe implications than previously recognized. Alkaline-pH-induced cross-protection of L. monocytogenes against heat has the potential to enhance survival in minimally processed as well as in heat-and-serve foods and in foods on holding tables, in food service facilities, and in the home. Cells surviving exposure to chlorine, in contrast, are more sensitive to heat; thus, the effectiveness of thermal processing in achieving desired log10-unit reductions is not compromised in these cells.
Postprocessing contamination of food with Listeria monocytogenes persists as a serious public health problem, particularly in the production of minimally processed and ready-to-eat foods. Recent outbreaks of listeriosis linked to smoked mussels (5), deli meats and hot dogs (8, 9), pork tongue jelly (16), and corn salad (1) have focused attention on cross-contamination of processed foods from environmental sources. The ubiquity of L. monocytogenes in nature and its acknowledged presence in food-processing environments (14, 26) explain the difficulty in producing minimally processed foods free of the pathogen. Consequently, food product recalls in the United States attributable to detection of L. monocytogenes by random sampling continue to rise (50), even as food processors attempt to comply with federally imposed “zero tolerance” policies.
The ability of microorganisms to adapt to acidic environments and subsequently become resistant to acid or other unrelated stresses has been demonstrated for several food-borne pathogenic bacteria, including Salmonella enterica (21, 32, 33), Escherichia coli O157:H7 (2, 6, 23, 45), and L. monocytogenes (18, 30, 34, 35, 38, 40, 48). Alkaline stress in E. coli has been studied (3, 24, 44, 47), but observations on the ability of L. monocytogenes to survive exposure to highly alkaline environments are limited (11, 31, 35, 49). Information on alkali-induced cross-protection of L. monocytogenes against other environmental stresses, e.g., heat, is lacking. The influence of sanitizer-related stresses on the ability of bacteria to survive thermal treatment has also gained attention as another facet of the cross-protection phenomenon (20, 34, 51), but chlorine-induced cross-protection of L. monocytogenes has not been reported.
Cross-protection against heat as a result of alkaline stress has been documented for both gram-positive and -negative bacteria. Heat resistance (55°C) of Salmonella serovar Enteritidis PT4 in Lemco broth at pH 7.0 ± 0.2 was significantly increased by previous exposure to pH 9.2 ± 0.2 for 5 min or longer (28). Similarly, tolerance to heating at 62°C was induced by treating Enterococcus faecalis cells for 30 min at pH 10.5 (19). Increased resistance of L. monocytogenes to heating at 56°C has been demonstrated following exposure of cells to starvation conditions, ethanol, acid, and H2O2 (34). Since induction of thermotolerance is known to occur in other bacteria exposed to various environmental stresses, including exposure to alkaline environments, the potential for development of thermotolerance in L. monocytogenes concurrent with alkaline shock is likely.
There is a need to determine the effect of sanitizer-induced sublethal injury of L. monocytogenes on subsequent resistance to other stresses. Given the alkaline nature of detergents and some of the chemical sanitizers used to clean and sanitize equipment, floors, pipes, and drains in food- and beverage-processing plants where L. monocytogenes may reside, information on its response to alkaline stress would be useful when designing interventions to prevent postprocessing contamination of foods. Direct application of chlorine is relied upon for reducing microbial populations in foods (especially produce) or on food contact surfaces. However, concentrations of free (available) chlorine reaching microbial cells may not be lethal since the efficacy of chlorine as a disinfectant can be reduced by numerous factors. The objective of this study was to determine the effects of alkaline pH stress and chlorine on the survival and subsequent thermotolerance of L. monocytogenes freshly isolated from a drain in a food-processing facility.
MATERIALS AND METHODS
Strain and culture conditions.
An isolate of L. monocytogenes, serotype 4b, flagellar type ABC, from a drain in a food-processing plant was used in this experiment. Stock cultures were prepared from a subculture of the initial isolate in tryptose phosphate broth (TPB; pH 7.3) (Difco Laboratories, Detroit, Mich.). Cultures were incubated at 37°C for 24 h and then supplemented with 15% glycerol and stored at −20°C until used.
Prior to each experiment, a stock culture was thawed and loop inocula were transferred to Erlenmeyer flasks (250 ml) containing 100 ml of sterile TPB. Flasks were incubated in an incubator shaker (New Brunswick Scientific, New Brunswick, N.J.) set at 37°C and 200 rpm for 13 h, a time at which the culture was at a state of transition between the late logarithmic and early stationary phase of growth, or for 48 h, well into the stationary phase of growth. The culture (25 ml) was dispensed into a 50-ml conical polystyrene centrifuge tube (Becton Dickinson Labware, Franklin Lakes, N.J.) and centrifuged (5,000 × g, 4°C) for 10 min (brake, 3.5 min) in a precooled Marathon 12KBR benchtop refrigerated centrifuge (Fisher Scientific, Pittsburgh, Pa.). Cell pellets were washed three times in sterile, precooled (4°C) 0.05 M potassium phosphate buffer at pH 7.00 ± 0.05 prepared from filtered (pore size, 0.45 μm) laboratory-grade water (PB) and then resuspended in treatment or control (PB or TPB at pH 7.3) solutions.
Preparation of chemical-treatment solutions.
Alkaline-treatment solutions were prepared by adding appropriate volumes of 1 or 2 N filter-sterilized (pore size, 0.22 μm) NaOH to sterile TPB to achieve pHs 9.0, 10.0, 11.0, 12.0, and 13.0 ± 0.1. Samples of alkali-adjusted or unadjusted (control, pH 7.3) TPB were measured with an Accumet pH meter (Denver Instrument Co., Arvada, Colo.) following standardization with pH 4.00, 7.01, and 10.00 buffers. TPB and NaOH solutions were prepared from filtered (pore size, 0.45 μm) laboratory-grade water and used on the same day of pH adjustment.
Sodium hypochlorite (NaOCl) solution (minimum of 4% available chlorine) (Aldrich Chemical Co., Inc., Milwaukee, Wis.) was used to prepare specific concentrations of free available chlorine by dilution with PB. Concentrations were verified using a digital titrator (model 16900; Hach Company, Loveland, Colo.) fitted with a 0.0451 N phenylarsine oxide titration cartridge, an amperometric digital titrator (model 19300), and a TitraStir stir plate by following the forward titration procedure for determining concentrations of free chlorine ranging from 0 to 10 mg/liter. All solutions were prepared using chlorine demand-free glassware and sterile PB made from filtered (pore size, 0.45 μm), laboratory-grade, sterile water. Solutions were protected from light, held at 21 ± 2°C, and used within 1 h of preparation.
Alkaline treatment and heat inactivation.
Washed cell pellets were resuspended in 25 ml of TPB (pH 7.3) or TPB adjusted to pH 9.0 to 13.0 ± 0.1 and incubated with agitation at 37°C for 15 or 45 min. Following incubation, unstressed (pH 7.3, control) and alkali-stressed cells of L. monocytogenes were centrifuged (5,000 × g, 10 min, 4°C) and resuspended in 25 ml of PB (pH 7.00 ± 0.05, 4°C). Cells suspensions (50 μl) were injected into Kimax-51 capillary tubes (inside diameter, 0.8 to 1.0 mm; length, 90 mm; no. 34507-99; Kimble, Vineland, N.J.), and the ends were flame sealed. Capillary tubes were brought to 21 ± 2°C before being subjected to heat treatment in a water bath at 56°C for 0, 1, 2, 5, 10, 20, or 25 min or at 59°C for 0, 0.5, 1, 1.5, 2, 4, 6, 10, and 15 min. Come-up times for tempered fluid-filled capillary tubes measured with a microprocessor thermometer (model HH23; Omega, Stamford, Conn.) connected with a type T thermocouple were 2 and 3 s in the water bath at 56 and 59°C, respectively. Capillary tubes were immediately cooled and sanitized by immersing them in an ice bath and then in 70% ethanol and sterile water before they were aseptically transferred to screw-cap test tubes (inside diameter, 16 mm; length, 125 mm) containing 5 ml of sterile 0.1% peptone water. Within each test tube, the capillary tube containing the heated cell suspension was crushed using a sterile glass rod. The content of each test tube was thoroughly mixed using a vortex mixer, and undiluted and diluted suspensions were surface plated (0.25 ml in quadruplicate or 0.1 ml in duplicate) or serially diluted in 0.1% peptone water and surface plated (0.1 ml in duplicate) on tryptose phosphate agar (TPA; Difco). All plates were incubated for 48 h prior to colonies being counted using manufacturer-recommended modification of the pcount01 file of the Countermat Automated Colony Counter (Cogent Technologies, Cincinnati, Ohio).
Based upon initial observations of heat resistance of alkali-stressed cells, we made modifications to the stressing procedures of cells destined for heating trials. Variations in stressing procedures included incubating cells for 15 or 45 min at 37°C in TPB adjusted with 2.0 N NaOH to pH 12.0; incubating stationary-phase cells (48-h cultures) for 15 or 45 min at 37°C in TPB at pH 7.3, 10.0, or 12.0; and incubating cells for 45 min in TPB containing 20 mg of cycloserine-D per liter, 20 mg of chloramphenicol per liter, or 10 mg of rifampin per liter at pH 7.3 or 12.0.
Cells subjected to alkaline stress for 45 min were also incubated at 4 or 21°C, with agitation, for up to 144 h. Populations of L. monocytogenes were determined after 48 and 144 h of incubation by surface plating undiluted and diluted suspensions on TPA and on TPA supplemented with 4% NaCl (TPAS) as described above. The heat (56°C) tolerance of cells incubated for 45 min at 37°C and then held at 4°C for 48 or 144 h in TPB at pH 7.3, 10.0, or 11.0 was also determined.
NaOCl treatment and heat inactivation.
Flasks (250 ml) containing 50 ml of solutions of 0, 2.0, 2.4, or 6.0 mg of available chlorine per liter were placed on an Innova 2000 platform shaker (New Brunswick Scientific) set at 140 rpm. Cells of L. monocytogenes suspended in PB (10 ml) were added to treatment solutions. After 5 or 10 min, 10 ml was removed from the flask and the chlorine was neutralized by dispensing it into a bottle (120 ml) containing 30 ml of sterile 0.01 N Na2S2O3 (10) and vortexing for 10 s. Samples were surface plated on TPA and TPAS as described above. Chlorine-stressed or unstressed (control) cells were also subjected to heat treatment at 56°C using capillary tubes as described above.
Statistical analyses.
Three replicate experiments were conducted for each trial. Population means, each representing six values (from two duplicate plates from three replicate trials), were analyzed by the general linear-model procedure and means separation analysis of SAS software (SAS Institute, Inc., Cary, N.C.) using Duncan's multiple-range test (17).
The number of viable cells recovered by surface plating heated or unheated cell suspensions on TPA, expressed as log10 CFU per milliliter, was plotted against heating time. Normally, D values are calculated from the absolute value of the reciprocal of the slope of the linear regression line of the plot of survivors versus time (survival curve). However, our data did not fit log-linear inactivation kinetics. Therefore, heat survivor data were analyzed using appropriate forms of the logistic equation (29, 43) applied by the nonlinear-regression procedure of SAS. The log-transformed equations used to analyze data were as follows:
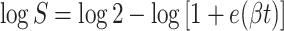 |
1 |
where log S is the log (CFU/CFU0) at any given time (t) and β is the maximum specific death rate.
For survival curves with no initial lag in killing but having two distinct killing phases, (biphasic), data were fitted to the following two-term exponential form of equation 1:
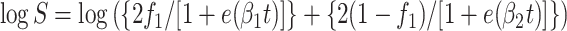 |
2 |
where f1 and (1 − f1) represent two fractions of cells (differing with respect to heat resistance) and β1 and β2 are the specific killing rates for the two fractions. The assumption of this model is that two fractions (subpopulations) are killed exponentially but at different, independent rates.
Curves which included a lag in killing (shoulder) and biphasic inactivation were fitted to the following two-term exponential form of equation 1:
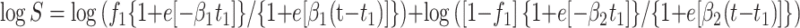 |
3 |
where t1 is the lag period.
For equation 1, logistic D values (43) were calculated as 2.94/β, and for equations 2 and 3, the D value was calculated as ln(19)/β2.
RESULTS
Survival of alkali-stressed L. monocytogenes.
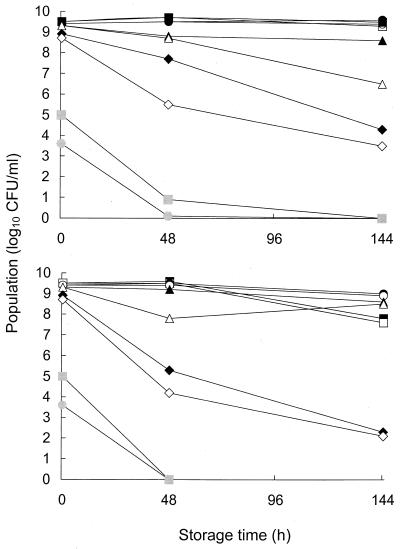
Cells from 13-h cultures of L. monocytogenes treated at 37°C for 45 min in TPB at pH 7.3 or 9.0 followed by holding at 4°C in TPB at pH 7.3 or 9.0, respectively, were essentially unchanged after 144 h (Fig. 1, top). A significant number of cells treated at pH 10.0 were injured as evidenced by their inability to form colonies on TPAS compared to their ability to form colonies on TPA. Incubating cells in TPB at pH 11.0 was more stressful, as the initial population declined by 1 log unit and injury was evident after 48 h; populations further decreased after 144 h. Incubation at 37°C for 45 min in TPB at pH 12.0 reduced the population by almost 5 log units, while subsequent storage at 4°C caused further reductions. Incubation at 37°C for 45 min in TPB at pH 13.0 was lethal to all cells (data not shown).
FIG. 1.
Populations of L. monocytogenes cells held at 4°C (top) or 21°C (bottom) in TPB with the pH adjusted or not adjusted with 1.0 N NaOH. Populations of alkali-injured and uninjured cells were recovered by plating cells onto TPA and TPAS, respectively. Cells were incubated in TPB at various pH values at 37°C for 45 min prior to being held at 4 or 21°C. Samples plated on TPA were taken from TPB at pHs 7.3 (■), 9.0 (●), 10.0 (▴), 11.0 (⧫), and 12.0 (░⃞), and samples plated on TPAS were taken from TPB at pHs 7.3 (□), 9.0 (○), 10.0 (▵), 11.0 (◊), and 12.0 ( ).
Survival of L. monocytogenes in TPB (pH 7.3 to 12.0) at 21°C was similar to survival at 4°C (Fig. 1, bottom). Populations in TPB at pH 7.3, however, declined slightly after 144 h at 21°C. A portion of L. monocytogenes cells incubated for 48 h in TPB at pH 10.0 were injured but resuscitated between 48 and 144 h.
Heat resistance of alkali-stressed L. monocytogenes.
Logistic D56°C values and nonlinear-regression parameter estimates for survivor curves of 13-h cultures of L. monocytogenes cells, which were incubated at 37°C for 15 or 45 min in TPB at pH 7.3 or in TPB adjusted with 1.0 N NaOH to pH 9.0 to 13.0, are listed in Table 1. D56°C values of cells previously incubated in TPB at pH 7.3 for 15 or 45 min were 6.92 and 6.02 min, respectively, and, within the treatment time, were not dissimilar from D56°C values of cells incubated at pH 9.0, 10.0, or 11.0. However, the D56°C value of cells incubated in TPB at pH 12.0 for 15 or 45 min was 8.28 or 14.30 min, respectively, and was higher than that of cells exposed to pH 7.3 for 15 or 45 min. A substantial number of cells were killed during alkali shock in TPB (pH 12.0) incubated at 37°C for 45 min before suspensions were heated at 56°C. The D56°C value of cells subjected to that treatment was derived from the least heat-sensitive fraction of the population (1 − f1), which was 20%. The other fraction of the population (f1, 80%) had a death rate that appeared high but was not accurately estimated by the model as evidenced by the standard deviation.
TABLE 1.
Heat (56°C)-survival-parameter estimates for late-logarithmic-growth-phase (13-h) L. monocytogenes cells incubated at 37°C for 15 or 45 min in TPB at pHs 7.3 to 12.0
| Treatment time (min) | TPB pH (±0.1)a | Eqn.b | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | β (min−1) | Pseudo r2c | Logistic D value (min)d |
|---|---|---|---|---|---|---|---|---|
| 15 | 7.3 | 1 | 0.425 ± 0.021 | 0.973 | 6.92 | |||
| 9.0 | 1 | 0.429 ± 0.014 | 0.987 | 6.85 | ||||
| 10.0 | 1 | 0.539 ± 0.029 | 0.968 | 5.45 | ||||
| 11.0 | 1 | 0.481 ± 0.004 | 0.999 | 6.11 | ||||
| 12.0 | 1 | 0.355 ± 0.020 | 0.958 | 8.28 | ||||
| 12.0e | 1 | 0.350 ± 0.006 | 0.996 | 8.40 | ||||
| 45 | 7.3 | 1 | 0.488 ± 0.013 | 0.991 | 6.02 | |||
| 9.0 | 1 | 0.504 ± 0.014 | 0.991 | 5.83 | ||||
| 10.0 | 1 | 0.520 ± 0.014 | 0.991 | 5.65 | ||||
| 11.0 | 2 | 0.13 | 0.508 ± 0.011 | 0.789 ± 0.081 | 0.997 | 5.79 | ||
| 12.0 | 2 | 0.2 | 0.205 ± 0.010 | 25.7 ± >100 | 0.969 | 14.30 | ||
| 12.0e | 1 | 0.355 ± 0.009 | 0.993 | 8.28 |
The pH of TPB was adjusted using 1.0 N NaOH with noted exceptions.
Parameter estimates were obtained by fitting survival data to the appropriate logistic equation (Eqn.) by nonlinear regression.
Calculated as 1 − (residual sum of squares/corrected total).
The pH was adjusted using 2.0 N NaOH.
Using 2.0 N NaOH rather than 1.0 N NaOH to adjust the pH of the TPB to pH 12.0 had an effect on thermotolerance (Table 1). The D56°C value of cells previously treated in TPB for 15 min at pH 12.0 adjusted with 2.0 N NaOH was higher than the D56°C value of cells previously treated for 15 min at pH 7.3 but similar to the D56°C value of cells treated in TPB adjusted to pH 12.0 with 1.0 N NaOH. The D56°C value of L. monocytogenes cells incubated for 45 min in TPB adjusted to pH 12.0 with 2.0 N NaOH was lower than that of cells subjected to the same treatment in TPB adjusted with 1.0 N NaOH, although it was still higher than the D56°C value of the control.
The enhanced thermotolerance of 13-h cells of L. monocytogenes treated at pH 12.0 compared to that at pH 7.3 warranted further investigation. Table 2 lists D59°C values and nonlinear-regression statistics for survivor curves of L. monocytogenes previously incubated at 37°C for 15 or 45 min in TPB at pH 7.3 or in TPB at pH 12.0. Each survival curve fit equation 2 the best. The D59°C value of cells that were incubated for 45 min in TPB at pH 12.0 was 10.10 min, which was much higher than that of cells incubated for the same time in TPB at pH 7.3.
TABLE 2.
Heat (59°C)-survival-parameter estimates for late-logarithmic-growth-phase (13-h) L. monocytogenes cells incubated at 37°C for 15 or 45 min in TPB at pH 7.3 or 12.0e
| Treatment time (min) | TPB pH (±0.1)a | Eqn.b | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | Pseudo r2c | Logistic D value (min)d |
|---|---|---|---|---|---|---|---|
| 15 | 7.3 | 2 | 0.01 | 1.400 ± 0.124 | 2.510 ± 0.424 | 0.964 | 2.10 |
| 12.0 | 2 | 0.02 | 0.788 ± 0.072 | 1.410 ± 0.245 | 0.951 | 3.73 | |
| 45 | 7.3 | 2 | 0.01 | 1.490 ± 0.177 | 2.920 ± 0.817 | 0.923 | 1.97 |
| 12.0 | 2 | 0.01 | 0.292 ± 0.031 | 1.450 ± 0.172 | 0.967 | 10.10 |
The pH of TPB was adjusted using 1.0 N NaOH.
Parameter estimates were obtained by fitting survival data to the appropriate logistic equation (Eqn.) by nonlinear regression.
Calculated as 1 − (residual sum of squares/corrected total).
Calculated as D = (In19)/β2.
Per minute β values do not apply.
The growth curve of L. monocytogenes in TPB at 37°C was determined following diluting and surface plating of the culture on TPA and TPAS. The presence of 4% NaCl in TPA did not influence the number of cells detected at a given incubation time (data not shown). Nonetheless, alkali-stressed L. monocytogenes cells in a late stationary phase (48 h) of growth responded differently (Table 3) than cells in late logarithmic growth (13 h) used in previous alkaline-stress experiments. Stationary-phase cells treated for 15 min at pH 12.0 had a D56°C value 3.19 times higher than the D56°C value of cells treated at pH 7.3 (control). However, D56°C values of cells treated for 45 min at pH 7.3, 10.0, and 12.0 were not dissimilar. Cells cultured for 48 h may have been entering death phase, making them more sensitive to treatment at pH 12.0. However, injured, i.e., NaCl-sensitive, 48-h cells were not observed.
TABLE 3.
Heat (56°C)-survival-parameter estimates for late-stationary-phase (48-h) L. monocytogenes cells incubated at 37°C for 15 or 45 min in TPB at pH 7.3, 10.0, or 12.0
| Treatment time (min) | TPB pH (±0.1)a | Eqn.b | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | β (min−1) | Pseudo r2c | Logistic D value (min)d |
|---|---|---|---|---|---|---|---|---|
| 15 | 7.3 | 1 | 0.572 ± 0.025 | 0.979 | 5.14 | |||
| 10.0 | 1 | 0.491 ± 0.007 | 0.998 | 5.99 | ||||
| 12.0 | 2 | 0.2 | 0.179 ± 0.007 | 0.542 ± 0.084 | 0.990 | 16.42 | ||
| 45 | 7.3 | 1 | 0.533 ± 0.028 | 0.968 | 5.52 | |||
| 10.0 | 1 | 0.433 ± 0.011 | 0.993 | 6.79 | ||||
| 12.0 | 2 | 0.1 | 0.576 ± 0.021 | 1.850 ± 0.198 | 0.996 | 5.10 |
The pH of TPB was adjusted using 1.0 N NaOH.
Parameter estimates were obtained by fitting survival data to the appropriate logistic equation (Eqn.) by nonlinear regression.
Calculated as 1 − (residual sum of squares/corrected total).
Survival-parameter estimates and logistic D56°C values of late-logarithmio-growth-phase cells (13 h) incubated for 45 min in TPB at pH 7.3, 10.0, or 11.0 at 37°C and then at 4°C for 48 h were determined (Table 4). Long-term exposure of L. monocytogenes to pH 10.0 increased heat tolerance. After 48 h, the D56°C value of cells incubated at 4°C in TPB at pH 10.0 was 2.81 times higher than that of cells incubated at pH 7.3. Similarly, after 144 h, the D56°C value of cells incubated at 4°C in TPB at pH 10.0 was 2.32 times higher than that of cells incubated at pH 7.3. In addition to lower apparent rates of inactivation of alkali-stressed cells than that of control cells, smaller numbers of alkali-stressed cells were inactivated than those of control cells. The least heat-sensitive fraction of cells exposed to pH 11.0 used to derive logistic D56°C values was very small and, therefore, not representative of the original population.
TABLE 4.
Heat (56°C)-survival-parameter estimates for L. monocytogenes cells previously incubated at 37°C for 45 min and then held at 4°C for 48 or 144 h in TPB at pH 7.3, 10.0, or 11.0
| Storage time (h) | TPB pH (±0.1)a | Eqn.b | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | β (min−1) | Pseudo r2c | Logistic D value (min)d |
|---|---|---|---|---|---|---|---|---|
| 48 | 7.3 | 1 | 0.739 ± 0.022 | 0.989 | 3.98 | |||
| 10.0 | 1 | 0.473 ± 0.006 | 0.997 | 6.22 | ||||
| 11.0 | 2 | 0.0001 | 0.263 ± 0.034 | 4.170 ± 0.573 | 0.961 | 11.20 | ||
| 144 | 7.3 | 1 | 0.795 ± 0.040 | 0.968 | 3.70 | |||
| 10.0 | 1 | 0.547 ± 0.021 | 0.981 | 5.37 | ||||
| 11.0 | 2 | 0.005 | 0.343 ± 0.036 | 4.840 ± 0.774 | 0.974 | 8.57 |
The pH of TPB was adjusted using 1.0 N NaOH.
Parameter estimates were obtained by fitting survival data to the appropriate logistic equation (Eqn.) by nonlinear regression.
Calculated as 1 − (residual sum of squares/corrected total).
Addition of antibiotics to the alkaline-stress medium (TPB adjusted to pH 12.0) reduced the resistance of L. monocytogenes to heating at 56°C (Table 5). Survival-parameter estimates of late-logarithmic-growth-phase (13-h) cells previously treated in TPB at pH 7.3 (control) in the presence of antibiotics (Table 5) were similar to those of cells exposed to the same treatment without antibiotics (Table 1). However, 13-h cells treated in solutions of TPB at pH 12.0 containing cycloserine-D, chloramphenicol, or rifampin each had reduced thermal tolerance (Table 5) compared to that of alkali-treated cells not exposed to antibiotics (Table 1) as evidenced by logistic D56°C values. Subjecting cells to alkaline stress in the presence of cycloserine-D had less effect on thermotolerance than other antibiotics.
TABLE 5.
Heat (56°C)-survival-parameter estimates for L. monocytogenes cells previously incubated at 37°C for 45 min in TPB at pH 7.3 or 12.0 in the presence of antibiotics
| Antibiotic (conc [μg/ml]) | TPB pH (±0.1)a | Eqn.b | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | β (min−1) | Pseudo r2c | Logistic D value (min)d |
|---|---|---|---|---|---|---|---|---|
| Cycloserine-D (20) | 7.3 | 2 | 0.01 | 0.587 ± 0.039 | 1.140 ± 0.231 | 0.978 | 5.01 | |
| 12.0 | 2 | 0.2 | 0.302 ± 0.011 | 0.609 ± 0.073 | 0.995 | 9.74 | ||
| Chloramphenicol (20) | 7.3 | 3 | 0.08 | 0.510 ± 0.016 | −0.335 ± 0.092 | 0.993 | 5.76 | |
| 12.0 | 1 | 0.371 ± 0.009 | 0.995 | 7.92 | ||||
| Rifampin (10) | 7.3 | 3 | 0.1 | 0.574 ± 0.027 | −0.192 ± 0.065 | 0.990 | 5.12 | |
| 12.0 | 3 | 0.2 | 0.346 ± 0.046 | −0.037 ± 0.058 | 0.981 | 8.50 |
The pH of TPB was adjusted using 1.0 N NaOH.
Parameter estimates were obtained by fitting survival data to the appropriate logistic equation (Eqn.) by nonlinear regression.
Calculated as 1 − (residual sum of squares/corrected total).
Chlorine stress and effect on thermotolerance.
Treatment of late-logarithmic-growth-phase (13 h) L. monocytogenes cells for 5 or 10 min with 2.0, 2.4, and 6.0 mg of chlorine per liter resulted in injury of cells as evidenced by a significant difference (P ≤ 0.05) in the ability to form colonies on TPAS compared to that on TPA (Table 6). Populations of cells treated with 2.0, 2.4, or 6.0 mg/liter for 10 min were reduced by 0.62, 1.30, or 4.02 log units, respectively, compared to that of the control. At each chlorine concentration tested, treatment time did not have a significant effect on the number of cells recovered on TPA or TPAS.
TABLE 6.
Populations of late-logarithmic-growth-phase (13-h) L. monocytogenes cells recovered on TPA and TPAS following treatment with chlorine
| Chlorine concn (mg/liter) | Treatment time (min) | Mean population ± SE (log10
CFU/ml)a on:
|
|
|---|---|---|---|
| TPA | TPAS | ||
| 0 | 5 | a 8.73 ± 0.0219 | a 8.73 ± 0.1790 |
| 10 | a 8.77 ± 0.0133 | a 8.70 ± 0.2190 | |
| 2.0 | 5 | a 8.21 ± 0.0556 | b 7.56 ± 0.0760 |
| 10 | a 8.15 ± 0.0514 | b 7.61 ± 0.0982 | |
| 2.4 | 5 | a 7.54 ± 0.1880 | b 7.09 ± 0.0999 |
| 10 | a 7.47 ± 0.1270 | b 6.78 ± 0.1540 | |
| 6.0 | 5 | a 4.90 ± 0.1610 | b 4.03 ± 0.1590 |
| 10 | a 4.75 ± 0.1690 | b 3.30 ± 0.2960 | |
Values in the same row that are not preceded by the same letter are significantly different (P ≤ 0.05).
Heat-survival-parameter estimates for chlorine-treated cells were varied (Table 7). Survivor curves of populations not exposed to chlorine (controls) were analyzed with equation 3 due to an initial lag in death (shoulder) and biphasic inactivation. In these cases, the least heat-sensitive fraction was rather large and estimated as 50%. Generally, logistic D56°C values of cells treated for 5 min with chlorine were higher than those of cells treated for 10 min. Logistic D56°C values of cells treated with 6.0 mg of available chlorine per liter for 5 or 10 min were 7.12 and 6.93, respectively, and were larger than those of controls.
TABLE 7.
Heat (56°C)-survival-parameter estimates for late-logarithmic-growth-phase (13-h) L. monocytogenes cells previously treated with chlorine
| Chlorine concn (mg/liter) | Treatment time (min) | Eqn.a | (1 − f) least heat-sensitive fraction | β2 − least heat-sensitive fraction (min−1) | β1 − most heat-sensitive fraction (min−1) | β (min−1) | Pseudo r2b | Logistic D value (min)c |
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 3 | 0.5 | 0.709 ± 0.056 | −0.604 ± 0.144 | 0.973 | 4.15 | |
| 0 | 10 | 3 | 0.5 | 0.738 ± 0.047 | −0.555 ± 0.121 | 0.983 | 3.98 | |
| 2.0 | 5 | 1 | 0.593 ± 0.014 | 0.994 | 4.96 | |||
| 2.0 | 10 | 1 | 0.678 ± 0.011 | 0.997 | 4.34 | |||
| 2.4 | 5 | 2 | 0.2 | 0.512 ± 0.024 | 0.809 ± 0.160 | 0.990 | 5.74 | |
| 2.4 | 10 | 1 | 0.962 ± 0.040 | 0.985 | 3.06 | |||
| 6.0 | 5 | 2 | 0.1 | 0.413 ± 0.053 | 2.090 ± 0.651 | 0.950 | 7.12 | |
| 6.0 | 10 | 2 | 0.1 | 0.424 ± 0.008 | 3.950 ± 0.314 | 0.999 | 6.93 |
DISCUSSION
Following incubation at 37°C for 45 min, L. monocytogenes survived well at 4°C in TPB at pHs 9.0, 10.0, and 11.0. These high-nutrient, high-pH conditions represent conditions on floors, in drains, within conveyor belt rollers, and in other areas within food-processing facilities that may harbor food debris and alkaline detergent or sanitizer residues. Demonstration of injury to L. monocytogenes cells held for 144 h in TPB at pH 10.0, and after 48 h in TPB at pH 11.0, suggests that some cells were better at coping with alkaline environments than others. At 21°C, the slight decline in the number of viable cells held in TPB at pH 7.3 may be due to a mild acidification of TPB caused by metabolism of sugars by L. monocytogenes that killed weaker cells. Populations of cells stored at 21°C in TPB at pH 9.0, which is below the upper pH limit for growth of L. monocytogenes (13, 27), were constant up to 144 h, perhaps because acid produced by the pathogen was neutralized by the alkaline environment to prevent the pH from decreasing to a stressful range. The pH of the stressing medium generally decreased by ca. 1 pH unit during incubation.
Alkaline resistance of L. monocytogenes at higher temperatures has been documented by other researchers. The pathogen has been shown to be resistant to storage in NaHCO3-NaOH buffer at pHs 9.0, 10.0, 11.0, and 12.0 at 37 or 45°C (35). Laird et al. (31) observed less than a 1-log-unit decrease in the viability of L. monocytogenes after a 4-h incubation at 33°C in synthetic egg washwater adjusted to pH values of 8.0 to 10.5 by titration with an alkaline-detergent product and up to a 3-log-unit decrease in the number of viable cells in a neutral-pH control.
Other researchers (11, 49) have reported the ability of food factory isolates of L. monocytogenes to grow in alkali-adjusted media. It is likely that growth would have been observed in our studies if smaller inocula had been used. Nonetheless, our data confirm that L. moncytogenes has the ability to survive in alkaline media at refrigeration and ambient temperature and give insight into the extent of injury and death that occurs over time. Implications are that standing pools of detergent and possibly sanitizer residue in food-processing environments may permit survival of L. monocytogenes for extended periods, with potential development of cross-protection against other stress conditions.
The D56°C value of cells previously exposed to pH 12.0 was larger than the D56°C value of cells exposed to lower-pH environments (Table 1). The induction of cross-protection of bacteria against heat may, theoretically, increase gradually in proportion to increased levels of stress. A gradual increase in the heat resistance of L. monocytogenes cells proportional to increasing alkalinity was not observed, although a gradual increase of the thermotolerance of cells treated at pH 12.0 was observed with increasing length of exposure time. Results may indicate that, after incubation at 37°C for 45 min at pH 12.0, the weaker, less resistant cells died, leaving only the more stable cells, perhaps those approaching stationary phase, to survive thermal treatment. Gilbert et al. (22) stated that populations of microorganisms will respond as collections of individuals from related backgrounds, rather than as heterogeneous mixtures of all possible biotypes. However, thermal-inactivation experiments using cells stressed for prolonged periods in alkaline TPB at 4°C (Table 4) verified enhanced thermotolerance due to alkaline stress. After prolonged storage at 4°C in TPB at pH 7.3 or 10.0 before heating, populations of alkali-stressed cells were similar to populations of unstressed cells (pH 7.3, control), so D56°C values were calculated using survival curves beginning with roughly the same number of cells. In this case, heating was still more lethal to control cells than to alkali-stressed cells treated and stored at pH 10.0, as evidenced by lower rates of death and less total inactivation of alkali-stressed cells.
Achieving a pH of 12.0 in TPB by adding 1.0 N NaOH diluted the TPB by approximately 20%, thereby diluting the nutrient concentration. The procedure for adjusting pH was therefore modified by using 2.0 N NaOH to adjust TPB to pH 12.0. With this modification, cells stressed at pH 12.0 for 15 min appeared more thermotolerant than those stressed at pH 12.0 for 45 min. The increased nutrient availability in TPB adjusted to pH 12.0 with 2.0 N NaOH, compared to that of the original procedure, may have caused cells to use alternative mechanisms to cope with the high alkalinity. Cells may also have undergone cytoplasmic buffering due to synthesis of intracellular metabolites in an attempt to stabilize pH. Dilworth and Glenn (15) noted that the use of cytoplasmic buffering by bacteria to cope with continued ingress to OH− might avert such stress temporarily but that it would not be successful long term. This supposition is consistent with our findings, since treatment for 45 min in TPB adjusted to pH 12.0 with 2.0 N NaOH tended to sensitize cells to heating compared to treatment for 15 min. This phenomenon did not occur in TPB adjusted to pH 12.0 with 1.0 N NaOH.
Another possible explanation for the differences in the heat sensitivities of cells based on increased normality of NaOH used to adjust the pH of TPB is that differences in the concentrations of Na+ may have influenced heat resistance. Since cells treated for 15 min were significantly more thermotolerant than populations treated for 45 min, Na+ may take longer than 15 min to injure the cell. Small et al. (46) showed that the use of KOH rather than NaOH in broth used to stress E. coli resulted in increased survival from 0.06 to 50% at pH 10.2. Higher rates of entry of Na+ occur at alkaline pH (4), and Na+ may have damaged L. monocytogenes cells and sensitized them to heating.
Resistance of bacteria to stress is generally believed to increase in stationary-phase cells. Our observations on the alkaline-stress-induced thermotolerance of stationary-phase cells of L. monocytogenes were inconsistent with that generalization. Stationary-phase cells (48 h) stressed at pH 12.0 for 15 min were considerably more thermotolerant than cells exposed to other treatments, but exposure to the stress for 45 min was lethal and the majority of cells in the remaining population had decreased tolerance to heat. Being relatively inactive metabolically after treatment in alkali-adjusted TPB, stationary-phase cells may have had limited or inoperable mechanisms to recover from alkaline stress and resorted to a short-term intracellular-pH compensation mechanism to remain viable.
Information on the effects of alkalinity on thermotolerance of L. monocytogenes is limited. Palumbo et al. (39) used a submerged vial heating technique to determine D56.6°C values for L. moncytogenes in egg whites as affected by pH. In that study, D56.6°C values were calculated to be 2.6 times greater at pH 9.3 than at pH 7.8. Conversely, with Salmonella, the D values were reduced with increasing pH. These researchers attributed the effect of high pH on increased heat resistance of L. monocytogenes to a lack of heat stability of an antilisterial enzyme, lysozyme, which is more heat stable at pH 7 than at pH 9. While heat destruction of lysozyme in egg whites may have played a role in thermotolerance, our study indicates that alkaline pH itself enhances thermotolerance of L. monocytogenes.
Mendonca et al. (36) examined the morphology of alkali-stressed L. monocytogenes cells using scanning electron microscopy and transmission electron microscopy. Their study revealed that, unlike with the gram-negative bacteria tested, L. monocytogenes cells did not leak cell constituents following exposure to pH 9.0, 10.0, 11.0, or 12.0 and DNA was not detected in any filtrates from suspensions of the organism. While gram-negative cells appeared collapsed and wrinkled, L. monocytogenes cells retained their shape. Interestingly, exposure of L. monocytogenes cells to alkaline pH caused the cytoplasmic membrane to bulge against the cell wall. These researchers concluded that the presence of a thick rigid peptidoglycan layer in gram-positive bacteria probably prevents the cytoplasmic membrane from expanding and bursting.
Addition of antibiotics to alkali-stressing media resulted in reduction of thermotolerance of L. monocytogenes cells exposed to pH 12.0, implying possible mechanisms of injury repair. Phan-Thanh and Gormon (42) reported that alkaline stress (pH 10.0, 5 min) of L. monocytogenes repressed 67% of protein spots otherwise detected in control cells on two-dimensional sodium dodecyl sulfate-polyacrylamide gels. However, among 254 protein spots that were resolved from alkali-stressed cells, 11 novel proteins were revealed and 16 others were up-regulated from 2- to 14-fold. Cycloserine inhibits peptidoglycan synthesis by inhibiting the enzymes involved in synthesis of the pentapeptide side chains, whereas chloramphenicol inhibits protein synthesis by combining with the 50S-subunit ribosome and blocking the associated transpeptidation and translocation functions (41). Rifampin blocks initiation, but not ongoing mRNA synthesis by binding to the β-subunit of DNA-dependent RNA polymerase (37). The presence of cycloserine-D or rifampin in TPB during stress of L. monocytogenes at pH 12.0 resulted in less thermotolerant cells, but chloramphenicol reduced the thermotolerance even more noticeably. As with the acid tolerance response of L. monocytogenes, the synthesis of certain proteins probably associated with the cell wall or plasma membrane as a result of alkaline stress is necessary for survival and may be similar to what occurs with heat shock proteins when they confer thermotolerance to the cell.
Exposure of L. monocytogenes to alkaline stress may occur in a variety of situations that have implications to food safety. Goodson and Rowbury (25) stated that alkalinization of natural waters can be caused by runoff from naturally alkaline soils but that it more likely occurs from the discharge of alkaline chemical wastes or highly ammoniacal agricultural slurries. Environmental exposure of L. monocytogenes to the pH levels shown to induce heat resistance in our study are more likely to occur as a result of the latter of the two mechanisms. Alkali-stressed L. monocytogenes may contaminate food by these vectors prior to even reaching processing, production, and distribution. Exposure of L. monocytogenes cells to alkaline stress in food-processing facilities may occur repeatedly through the use of alkaline detergents and disinfectants routinely used to remove food residues from equipment and floors and for cleaning food contact surfaces. One of the most commonly used inorganic alkalies in the food industry is NaOH or caustic soda (12). Alkaline chemicals are also used to assist in lye peeling of fruits and vegetables and to clean and disinfect produce surfaces. Accumulation of these chemicals may occur in areas of food-processing environments that also happen to favor survival and growth of L. monocytogenes. Routine sublethal exposure to alkaline chemicals may in fact induce stress responses that cross-protect the organism against heat and possibly other nonrelated stresses.
Cross-contamination of foods with L. monocytogenes from environmental sources remains a critical issue in food product manufacture. It is reasonable to propose that alkali-stressed cells may survive otherwise lethal heat treatments and proliferate on foods during subsequent storage. Postprocessing contamination of food with alkali-stressed L. monocytogenes cells may permit survival of the pathogen during reheating just prior to consumption. Similar scenarios may compromise the safety of minimally processed, ready-to-eat, or heat-and-serve foods in retail, food service, and home settings, where alkaline detergents and sanitizers are being used with increased frequency.
It is likely that the alkaline-stress response of L. monocytogenes is transient, as has been shown for E. coli (47). When stressed cells are placed in a nonalkaline environment, they may revert to an original state of tolerance to a secondary chemical or physical assault. Fortunately, no food is alkaline enough to induce the stress response and cross-protection to heat observed in our study. However, the duration of the alkaline-stress response in L. monocytogenes upon exposure to near-neutral pH in situ and in food matrices needs to be determined.
The degree of chlorine injury of L. monocytogenes was assessed by plating cells onto nonselective (TPA) and selective (TPAS) media. Demonstration of injury ensured that subsequent heating studies will be performed on a range of stressed and unstressed cells. Generally, within chlorine concentration, higher logistic D56°C values were calculated from populations of cells exposed to chlorine for 5 min as opposed to 10 min, indicating that longer exposure sensitizes cells to heat. However, chlorine treatment alone was also lethal to portions of the population, as shown in Table 6. Therefore, higher logistic D56°C values reported for cells previously exposed to 6.0 mg of chlorine per liter (Table 7) are probably not representative of the entire population. Other researchers' observations on E. coli O157:H7 subjected to chlorine stress revealed a decrease in heat tolerance (20). Our data suggest that oxidative stress from chlorine creates two subpopulations of L. monocytogenes differing in their capacities to survive heating at 56°C. Bunduki et al. (7) proposed that in order for L. monocytogenes to repair from heat- or sanitizer-induced injury, cells require mRNA, protein synthesis, and oxidative phosphorylation. They further reported that the cell wall was not damaged by heat (56°C for 20 min) or by a chlorine-based sanitizer (100 mg/liter for 2 min). Whatever the site of injury, our data show that treating cells with chlorine for 10 min causes more rapid death during subsequent heating than does treating cells with chlorine for 5 min.
It should be noted that this study used planktonic cells of L. monocytogenes and that biofilm-associated cells may have more closely resembled conditions in food-processing environments. Also, some of the pretreatments applied to cells, such as pH 12.0 for 45 min at 37°C and 6.0 mg of chlorine per liter for 5 or 10 min, were in themselves lethal, and therefore populations subsequently subjected to heating were not identical to control populations. However, based upon heat-survival-parameter estimates of sublethal treatments, these data indicate that alkaline stress causes cells to become more tolerant to mild heating and that chlorine sensitizes cells to heat.
Observations that chlorine stress creates two subpopulations of L. monocytogenes cells and that longer exposure time to chlorine sensitizes cells to subsequent heating warrant further investigations into chlorine-induced heat sensitivity and application to safe food production. Alkaline-pH-induced cross-protection of L. monocytogenes against heat has the potential to enhance survival in foods. Storage of food containing alkali-adapted L. monocytogenes at temperatures as low as 4°C may permit growth and increased risk of illness. Further studies should be conducted to define the behavior of alkali- and chlorine-stressed L. monocytogenes on foods and in food-processing environments.
ACKNOWLEDGMENT
We thank Jerry Davis for his advice and assistance with statistical analyses.
REFERENCES
- 1.Aureli P, Fiorucci G C, Caroli D, Marchiaro G, Novara O, Leonello L, Salmaso S. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N Engl J Med. 2000;342:1236–1241. doi: 10.1056/NEJM200004273421702. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin M M, Datta A R. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61:1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingham R J, Hall K S, Slonczewski J L. Alkaline induction of a novel gene locus, alx, in Escherichia coli. J Bacteriol. 1990;172:2184–2186. doi: 10.1128/jb.172.4.2184-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boothe I R. The regulation of intracellular pH in bacteria. Novartis Found Symp. 1999;221:19–37. doi: 10.1002/9780470515631.ch3. [DOI] [PubMed] [Google Scholar]
- 5.Brett M S Y, Short P, McLauchlin J. A small outbreak of listeriosis associated with smoked mussels. Int J Food Microbiol. 1998;43:223–229. doi: 10.1016/s0168-1605(98)00116-0. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan R L, Edelson S G. Effect of pH-dependent, stationary phase acid resistance on the thermal tolerance of Escherichia coliO157:H7. Food Microbiol. 1999;16:447–458. doi: 10.4315/0362-028x-62.3.211. [DOI] [PubMed] [Google Scholar]
- 7.Bunduki M M-C, Flanders K J, Donnelly C W. Metabolic and structural sites of damage in heat- and sanitizer-injured populations of Listeria monocytogenes. J Food Prot. 1995;58:410–415. doi: 10.4315/0362-028X-58.4.410. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Multistate outbreak of listeriosis—United States, 1998. Morb Mortal Wkly Rep. 1998;47:1085–1086. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Update: multistate outbreak of listeriosis—United States, 1998–1999. Morb Mortal Wkly Rep. 1999;47:1117–1118. [PubMed] [Google Scholar]
- 10.Chambers C W. A procedure for evaluating the efficiency of bactericidal agents. J Milk Food Technol. 1956;19:183–187. [Google Scholar]
- 11.Cheroutre-Vialette M, Lebert I, Hebraud M, Labadie J C, Lebert A. Effects of pH or aw stress on growth of Listeria monocytogenes. Int J Food Microbiol. 1998;42:71–77. doi: 10.1016/s0168-1605(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 12.Cliver D O, Marth E H. Preservation, sanitation, and microbiological specifications for food. In: Cliver D O, editor. Foodborne diseases. San Diego, Calif: Academic Press, Inc.; 1990. pp. 45–63. [Google Scholar]
- 13.Conner D E, Brackett R E, Beuchat L R. Effect of temperature, sodium chloride, and pH on growth of Listeria monocytogenesin cabbage juice. Appl Environ Microbiol. 1986;52:59–63. doi: 10.1128/aem.52.1.59-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox L J, Kleiss T, Cordier J L, Cordellana C, Konkel P, Pedrazzini C, Beumer R, Siebenga A. Listeriaspp. in food processing, non-food and domestic environments. Food Microbiol. 1989;6:49–61. [Google Scholar]
- 15.Dilworth M J, Glenn A R. Problems of adverse pH and bacterial strategies to combat it. Novartis Found Symp. 1999;221:4–18. doi: 10.1002/9780470515631.ch2. [DOI] [PubMed] [Google Scholar]
- 16.Dorozynski A. Seven die in French Listeriaoutbreak. Brit Med J. 2000;320:601. [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan D B. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 18.Farber J M, Pagotto F. The effect of acid shock on the heat resistance of Listeria monocytogenes. Lett Appl Microbiol. 1992;15:197–201. [Google Scholar]
- 19.Flahaut S, Hartke A, Giard J-C, Auffray Y. Alkaline stress response in Enterococcus faecalis: adaptation, cross-protection, and changes in protein synthesis. Appl Environ Microbiol. 1997;63:812–814. doi: 10.1128/aem.63.2.812-814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folsom J P, Frank J F. Heat inactivation of Escherichia coliO157:H7 in apple juice exposed to chlorine. J Food Prot. 2000;63:1021–1025. doi: 10.4315/0362-028x-63.8.1021. [DOI] [PubMed] [Google Scholar]
- 21.Foster J W, Hall H K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert P, Brown M R W, Costerton J W. Inocula for antimicrobial sensitivity testing: a critical review. J Antimicrob Chemother. 1987;20:147–154. doi: 10.1093/jac/20.2.147. [DOI] [PubMed] [Google Scholar]
- 23.Goodson M, Rowbury R J. Habituation to normally lethal acidity by prior growth of Escherichia coliat a sub-lethal acid pH value. Lett Appl Microbiol. 1989;8:77–79. [Google Scholar]
- 24.Goodson M, Rowbury R J. Habituation to alkali in Escherichia coli. Lett Appl Microbiol. 1989;9:71–73. [Google Scholar]
- 25.Goodson M, Rowbury R J. Habituation to alkali and increased u.v.-resistance in DNA repair-proficient and -deficient strains of Escherichia coligrown at pH 9.0. Lett Appl Microbiol. 1990;11:123–125. [Google Scholar]
- 26.Gravani R. Incidence and control of Listeria in food-processing facilities. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food Safety. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1999. pp. 657–709. [Google Scholar]
- 27.Gray M L, Killinger A H. Listeria monocytogenesand listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey T J, Richardson N P, Gawler A H L, Allen M J. Heat resistance of Salmonella enteritidisPT4: the influence of prior exposure to alkaline conditions. Lett Appl Microbiol. 1991;12:258–260. [Google Scholar]
- 29.Kamau D N, Doores S, Pruitt K M. Enhanced thermal destruction of Listeria monocytogenes and Staphylococcus aureusby the lactoperoxidase system. Appl Environ Microbiol. 1990;56:2711–2716. doi: 10.1128/aem.56.9.2711-2716.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroll R G, Patchett R A. Induced acid tolerance in Listeria monocytogenes. Lett Appl Microbiol. 1992;14:224–227. [Google Scholar]
- 31.Laird J M, Bartlett F M, McKellar R C. Survival of Listeria monocytogenesin egg washwater. Int J Food Microbiol. 1991;12:115–122. doi: 10.1016/0168-1605(91)90060-3. [DOI] [PubMed] [Google Scholar]
- 32.Leyer G J, Johnson E A. Acid adaptation promotes survival of Salmonellaspp. in cheese. Appl Environ Microbiol. 1992;58:2075–2080. doi: 10.1128/aem.58.6.2075-2080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyer G J, Johnson E A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl Environ Microbiol. 1993;59:1842–1847. doi: 10.1128/aem.59.6.1842-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lou A, Yousef A E. Resistance of Listeria monocytogenesto heat after adaptation to environmental stresses. J Food Prot. 1996;59:465–471. doi: 10.4315/0362-028X-59.5.465. [DOI] [PubMed] [Google Scholar]
- 35.Lou A, Yousef A E. Adaptation to sublethal environmental stresses protects Listeria monocytogenesagainst lethal preservation factors. Appl Environ Microbiol. 1997;63:1252–1255. doi: 10.1128/aem.63.4.1252-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendonca A F, Amoroso T L, Knabel S J. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl Environ Microbiol. 1994;60:4009–4014. doi: 10.1128/aem.60.11.4009-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray P R, Kobayashi G S, Pfaller M A, Rosenthal K S. Medical microbiology. 2nd ed. St. Louis, Mo: Mosby; 1994. [Google Scholar]
- 38.Okereke A, Thompson S S. Induced acid-tolerance response confers limited nisin resistance on Listeria monocytogenes. J Food Prot. 1996;59:1003–1006. doi: 10.4315/0362-028X-59.9.1003. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo M S, Beers S M, Bhaduri S, Palumbo S A. Thermal resistance of Listeria monocytogenes and Salmonellaspp. in liquid egg white. J Food Prot. 1996;59:1182–1186. doi: 10.4315/0362-028X-59.11.1182. [DOI] [PubMed] [Google Scholar]
- 40.Parish M E, Higgins D P. Survival of Listeria monocytogenesin low pH model broth systems. J Food Prot. 1989;52:144–147. doi: 10.4315/0362-028X-52.3.144. [DOI] [PubMed] [Google Scholar]
- 41.Pelczar M J, Chan E C S, Krieg N R. Microbiology. 5th ed. New York, N.Y: McGraw-Hill; 1986. [Google Scholar]
- 42.Phan-Thanh L, Gormon T. Stress proteins in Listeria monocytogenes. Electrophoresis. 1997;18:1464–1471. doi: 10.1002/elps.1150180821. [DOI] [PubMed] [Google Scholar]
- 43.Pruitt K M, Kamau D N. Mathematical models of bacterial growth, inhibition and death under combined stress conditions. J Ind Microbiol. 1993;12:221–231. [Google Scholar]
- 44.Rowbury R J, Lazim Z, Goodson M. Regulatory aspects of alkali tolerance induction in Escherichia coli. Lett Appl Microbiol. 1996;22:429–432. doi: 10.1111/j.1472-765x.1996.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 45.Ryu J-H, Deng Y, Beuchat L R. Behavior of acid-adapted and unadapted Escherichia coliO157:H7 when exposed to reduced pH achieved with various organic acids. J Food Prot. 1999;62:451–455. doi: 10.4315/0362-028x-62.5.451. [DOI] [PubMed] [Google Scholar]
- 46.Small P, Blankenhorn D, Welty D, Zinser E, Slonczewski J L. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoSand growth pH. J Bacteriol. 1994;176:1729–1737. doi: 10.1128/jb.176.6.1729-1737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taglicht D, Padan E, Oppenheim A B, Schuldiner S. An alkaline shift induces the heat shock response in Escherichia coli. J Bacteriol. 1987;169:885–887. doi: 10.1128/jb.169.2.885-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schaik W, Gahan C G M, Hill C. Acid-adapted Listeria monocytogenesdisplays enhanced tolerance against lantibiotics nisin and lacticin 3147. J Food Prot. 1999;62:536–539. doi: 10.4315/0362-028x-62.5.536. [DOI] [PubMed] [Google Scholar]
- 49.Vasseur C, Baverel L, Hébraud M, Labadie J. Effect of osmotic, alkaline, acid or thermal stresses on the growth and inhibition of Listeria monocytogenes. J Appl Microbiol. 1999;86:469–476. doi: 10.1046/j.1365-2672.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 50.Wong S, Street D, Delgado S I, Klontz K C. Recalls of foods and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J Food Prot. 2000;63:1113–1116. doi: 10.4315/0362-028x-63.8.1113. [DOI] [PubMed] [Google Scholar]
- 51.Zook C D, Brady L J, Busta F F. Sanitizer-induced adaptive stress response of Escherichia coli O157:H7. In: Tuijtelaars A C J, Samson R A, Rombouts F M, Notermans S, editors. Food microbiology and food safety into the next millennium. Proceedings of the 17th International Conference of the International Committee on Food Microbiology and Hygiene. 1999. p. 821. [Google Scholar]