Abstract
For decades, basic research on the underlying mechanisms of nociception has held promise to translate into efficacious treatments for patients with pain. Despite great improvement in the understanding of pain physiology and pathophysiology, translation to novel, effective treatments for acute and chronic pain has however been limited, and they remain an unmet medical need. In this opinion paper bringing together pain researchers from very different disciplines, the opportunities and challenges of translational pain research are discussed. The many factors that may prevent the successful translation of bench observations into useful and effective clinical applications are reviewed, including interspecies differences, limited validity of currently available preclinical disease models of pain, and limitations of currently used methods to assess nociception and pain in non‐human and human models of pain. Many paths are explored to address these issues, including the backward translation of observations made in patients and human volunteers into new disease models that are more clinically relevant, improved generalization by taking into account age and sex differences, and the integration of psychobiology into translational pain research. Finally, it is argued that preclinical and clinical stages of developing new treatments for pain can be improved by better preclinical models of pathological pain conditions alongside revised methods to assess treatment‐induced effects on nociception in human and non‐human animals.
Significance:
For decades, basic research of the underlying mechanisms of nociception has held promise to translate into efficacious treatments for patients with pain. Despite great improvement in the understanding of pain physiology and pathophysiology, translation to novel, effective treatments for acute and chronic pain has however been limited, and they remain an unmet medical need.
1. INTRODUCTION
Understanding the neurobiology of pain is of high interest due to the enormous burden of pain for patients and society. There have been very few major breakthroughs leading to effective interventions or treatments for acute and chronic pain in recent times. Indeed, most available interventions have been in use for decades and provide limited long‐term effects. Furthermore, side effects, drug interactions (e.g. analgesics/co‐analgesics) and drug misuse present a major challenge for successful pain management.
Without doubt, accumulated basic knowledge regarding the mechanisms underlying pain perception is today much more refined than what was available two decades ago due to the fascinating opportunities and breakthrough methodologies in neuroscience. The specific understanding of the animal nociceptive system, as well insight into maladaptive processes that underlie pathological conditions in non‐human animals, has advanced significantly. However, as a research community, we must be critical of the lack of translation between instrumental findings about nociceptive processing in non‐human animals and proposed clinical intervention strategies in patients. At present, the clinical relevance of the different pathophysiological processes that have been characterized in preclinical models of pain remains largely unknown. Recently, Yezierski and Hansson outlined a number of concerns that could potentially explain the lack of progress in the field of translational pain research, specifically regarding inflammatory and neuropathic pain explored using behavioural assays (Yezierski & Hansson, 2018). The challenges of translational research on neuropathic pain were also reviewed by Attal and Bouhassira (2019). A lack of relevant pain models and assessment strategies, species differences and repeated failures of clinical trials were just some of the important factors listed.
Prevention of pain in general is a critical clinical issue that has been taken up by the IASP and EFIC as the Global year theme 2020 (https://www.iasp‐pain.org/GlobalYear). Challenges in translational pain research have been addressed in acute postoperative pain and prevention of chronic pain after surgery (Pogatzki‐Zahn et al., 2018). Although multiple neurophysiological mechanisms that could cause persistence of pain have been decoded in preclinical studies, the prevention of chronic pain in patients remains an area of unmet clinical need.
The leadership of the European Pain Federation recognized the need for directions and policies of translational pain research, and installed in 2017 a transdisciplinary working group on translational pain research within its research committee, composed of preclinical and clinical pain researchers with different backgrounds: anaesthesiology, biomedical engineering, neurophysiology, neuropharmacology, experimental psychology and neurology. This paper reflects the outcome of the discussions within this specific working group. The aim of the present paper is to clarify core concepts and definitions, and to discuss the opportunities as well as the challenges of translational pain research exemplified within the 2020 Global Year Against Pain (GYAP) theme. The span across translational concepts from bench to man and the interactions between somatosensory biology and psychobiology is considered (Figure 1). An important strength of translational pain research is collaboration across the many different disciplines involved. Methodological details are provided within experimental human research to illustrate current limitations and potentials for backward translation into mechanistic studies with improved clinical relevance.
FIGURE 1.
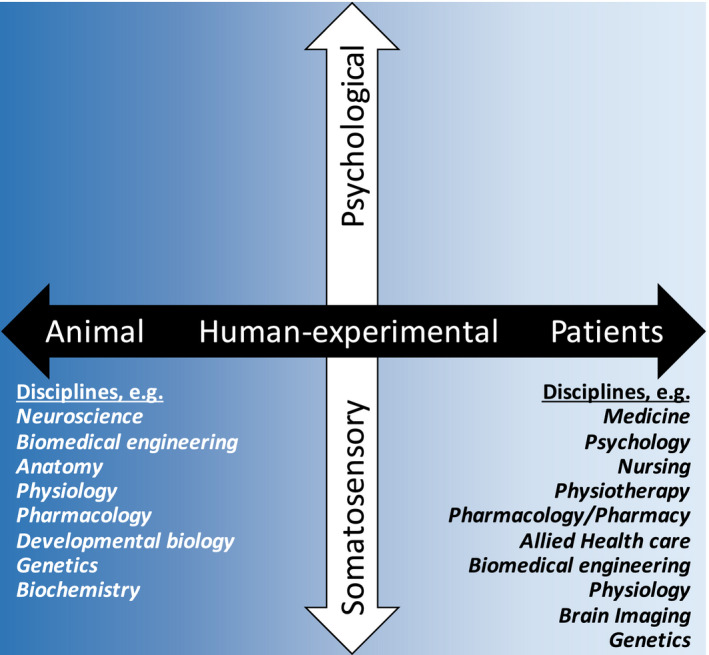
Translational pain research across axes consisting of laboratory animal to human (experimental and patients) and biological systems (somatosensory to psychological). Examples of disciplines involved in translational pain research illustrate the inter‐disciplinary approach
2. TRANSLATIONAL PAIN RESEARCH
The meaning of translational pain research may be understood differently among individuals. Considering the definition of translation as ‘a version in a different language’ or ‘moving something from one place to another’, translational pain researchers are tasked with expressing a particular form of something – for example a pain state or targeted therapeutic strategy – that differs in certain respects from other forms of the same thing. Therefore, the true meaning of translational medicine is open to speculation. When taking into account the mechanistic adaptations that occur in a pain state, basic researchers, pain clinicians (including physicians, psychologists, pain nurses, physiotherapists and others) and even patients might assume and/or hope that a linear movement from bench to bedside, in terms of the molecular and psychosocial processes described or pharmacotherapeutic targets defined, is achievable. However, this is rarely the case. A more realistic goal might be to discover a novel pain pathway or circuit component in the rodent that then provides insight regarding underlying circuitries and potential therapeutic targets or strategies in humans.
2.1. Translating ‘pain’ at the laboratory bench
The translational issues that laboratory scientists face when conducting research to identify mechanisms contributing to a particular pain state are several. The first hurdle relates to terminology since laboratory animals (most often, rats or mice), although sentient, cannot relay directly their pain ‘perception’. As such, basic science researchers must refer to ‘nociceptive behaviour’ or ‘pain related behaviour’. This complexity encompasses translation of an inferred pain level. The process of interpreting, in awake animals, a result that might be symptomatic of pain is a major limitation, and measuring pain thresholds is challenging (Mogil, 2009; Segelcke et al., 2019). However, assessing pain in the clinic is also difficult and should go beyond questions about pain intensity (Pogatzki‐Zahn et al., 2019a). It should focus on mechanisms modulating nociception such as the mechanisms leading to central sensitization or producing endogenous pain modulation. This can be achieved by assessing, for example, hyperalgesia, temporal summation of pain or the effects of heterotopic noxious conditioning stimulation. Psychosocial and functional impacts of pain should also be assessed carefully. Asking patients to score their pain using, for example, a visual analogue scale and distinguishing ‘sensory discriminative’ versus ‘affective motivational’ components of the pain experience is likely an oversimplification of the pain experience. The more, human pain ratings are strongly affected by several social and cognitive factors such as social desirability, context and expectations (Bingel et al., 2011; Deshields et al., 1995). These factors could affect both the subjective experience of pain and the way this pain is reported using ratings.
Following surgery to induce a pain state, some aspects of animal behaviour will change in a manner that relates specifically to the disease model and/or animal setting (e.g. number of animals in the cage, healthy or injured together, both sexes together). Versatile examination of species‐specific behavioural changes and developing ethograms of animals in their home cages might be one future direction for translational research (Mogil, 2009; Yip et al., 2019). Clinically relevant behaviours related to a given aetiology of pain should also be studied. For example, movement‐evoked pain is clinically relevant for patients after surgery (Srikandarajah & Gilron, 2011). In rodents, multidimensional pain assays like gait analysis or locomotor activity monitoring in the home cage or in a novel environment might represent objective correlates for movement‐evoked pain and its functional consequences (Bree et al., 2015; Tappe‐Theodor et al., 2019). The mechanisms underlying these behaviours are different from the mechanisms underlying behaviours referring to spontaneous pain after surgery, and the assessment paradigms differ accordingly (Remeniuk et al., 2015; Segelcke et al., 2019). Another example of complexity is the neuronal response to suprathreshold stimuli measured in anaesthetized animals (Urch & Dickenson, 2003) to provide a quantification of an inferred level of nociception. Interestingly, in a rat model of neuropathy, spinal neuronal responses to peripheral suprathreshold stimulation are comparable between control animals and animals exposed to surgery, and a difference is only revealed when pharmacology is applied (Matthews & Dickenson, 2002). The inhibitory effect of state‐dependent drugs such as gabapentinoids (Bannister, Qu, et al., 2017) provides a focus for the mechanisms driving the affective and sensory pain states in this animal model of pain, and these mechanisms translate to the clinic (Finnerup et al., 2002). Finally, disease‐ and symptom‐specific models need to be studied with the aim of identifying mechanisms related to specific pain states. Identifying mechanisms that drive a pain state opens the potential for treatment targets to be pursued in defined clinical settings. Together, we urgently need advances in preclinical research to translate better our findings to humans, specifically patients, and to provide backward translation of human experimental approaches into animal settings. One aspect that must be considered in future work is whether changes in behaviour or neuronal function that are reported after only a couple of days or weeks in animals models of pain actually relate to clinical pain conditions lasting months or years in patients.
2.2. Animal and human correlates of pain
Experimentally, perceived responses to painful stimuli may be assessed and compared between patients with chronic pain and age‐matched healthy human controls. Self‐reports of pain can be vocalized and combined with objective measurements (for example, heart rate, blood pressure, respiratory rate, pupil diameter and its dynamics, reflexes, cortical evoked responses) although they are not nociceptive specific. Self‐reports and physiological signs do not necessarily correlate and therefore physiological signs cannot be used to validate the severity and complexity of pain reported by humans. The complexity of comparing human perceptual responses to painful stimuli to those inferred in animals (see previous section) is therefore considerable. Nonetheless, this comparison is crucial if pain readouts are to be translated to and from preclinical and clinical realms. Parallel results in rodents and humans validate translational efforts. For example, rat spinal neuronal activity measured using electrophysiology was previously correlated with human thermal pain thresholds (O’Neill et al., 2015). Similarly, Sikandar et al. (2013) observed a strong correlation between dorsal horn activity recorded in rodents and pain perception reported by humans exposed to thermo‐nociceptive laser stimuli, indicating that responses of rat dorsal horn neurones can translate to human nociceptive processing. Restrictions associated with investigating brain function were circumnavigated with the advent of, and advances in, the acquisition and analysis of functional connectivity between spatially distributed but linked brain regions (Kucyi & Davis, 2015; Tracey & Mantyh, 2007). Using pre‐clinical fMRI offers the opportunity to define neuronal activation patterns in rodent models of acute and chronic pain, to validate a potential correlation with clinical data (Amirmohseni et al., 2016; Jia et al., 2017; Just et al., 2019).
2.3. Adequate mechanisms and models for forward translation
Key questions include ‘do basic pain mechanisms, as elucidated in naïve animals, translate to those mechanisms that underlie the normal functioning of pain pathways in man?’ and ‘do data collected from animal models translate to the clinical domain?’ where the animal model used becomes pivotal. At least macroscopically, the anatomy of pain pathways seems to translate relatively well from rodents to humans, but what about peripheral and central mechanisms? Cyclooxygenase inhibition attenuates pain across species (Hagen & Alchin, 2020; Shamsi Meymandi et al., 2019) and the phenomenon of wind up, whereby a persistent barrage of nociceptive stimuli at the periphery results in amplified spinal neuronal responses, corresponds partly to temporal summation in humans (Bosma et al., 2015). Finding adequate animal models for forward translation is complicated by the fact that, in most circumstances, subacute and chronic pain states may be driven by more than one mechanism (e.g. peripheral sensitization due to tissue inflammation, neuroimmune/microglial mechanisms, sensitized central mechanisms, deficient descending control). Furthermore, several of these mechanisms may interact with each other, making it even more complicated to find adequate animal models. Rodent models of joint degeneration have shown reduced intra‐epidermal nerve fibre density and spinal cord microglial activation, suggesting that joint degeneration induces changes in the nociceptive pathways that may contribute to pain (Thakur et al., 2012; Thakur et al., 2014). However, it is noteworthy that whether these observations directly translate to the clinical situation is unknown. Models of spinal cord injury may encompass neuropathic and musculoskeletal pain, but emulating the clinical situation is complex (Nakae et al., 2011). Certainly, identifying specific factors or mechanisms that are lacking in forward translation is more complicated than identifying those present. Regardless of the experimental method of injury and measurement of nociception, laboratory scientists must be able to detect pain‐like responses in a manner that translates to the clinical domain if a true translation is to be achieved.
2.4. Back translation
Why back translate when sophisticated bedside techniques can suitably evoke and/or quantify pain? After all, human psychophysical studies can reveal novel aspects of particular pain mechanisms or phenomena in chronic pain patients; such as expanded referred pain areas (Graven‐Nielsen & Arendt‐Nielsen, 2010), facilitated temporal summation of pain (McPhee & Graven‐Nielsen, 2019; McPhee et al., 2020; Staud et al., 2001), and reduced efficacy of descending inhibitory control system (McPhee & Graven‐Nielsen, 2019; McPhee et al., 2020) or changes in somatosensory sensitivity (Graven‐Nielsen & Arendt‐Nielsen, 2010; Rolke et al., 2006). However, back translation is vital when considering the refinement of animal models used, which itself relates to the pivotal ‘issue’ with forward translation; that is, the appropriateness of the animal model used. By substantiating that a paradigm used in human psychophysical testing can evoke equivalent and/or measurable aspects of a nociceptive response in healthy animals, the field of forward translation would progress. For example, recent evidence that a noxious cuff pressure paradigm activates the descending pain modulatory system in rats and humans has important implications for the forward translation of bench and experimental pain research findings to the clinic (Cummins et al., 2020).
In translational pain research, the species‐difference in key findings is a major issue and widely discussed. Innovative approaches are needed and alternative species for animal experimentations may be an interesting path to follow. As an example, recent initiatives investigate the pain related cortical neuroplastic manifestations in a pig model whose anatomy and physiology is closer to that of humans than rodents (Gigliuto et al., 2014).
3. FROM BENCH TO MAN
At the laboratory bench, numerous approaches may be employed to uncover novel underlying nociceptive mechanisms. For example, the use of in vivo imaging of genetically‐encoded calcium indicators has revolutionized our ability to sample large neuronal populations; in the periphery, dorsal root ganglion primary afferent neurons can be recorded in their thousands, revealing key functional properties at a population level (Kim et al., 2016; Wang et al., 2018). Higher temporal resolution is afforded using electrophysiological techniques that allow analysis of single unit activity in vivo. Through revealing potential sources of nociceptive signals in animal models of, for example, neuropathy, differences between peripherally and centrally driven mechanisms can be identified (Bannister, Qu, et al., 2017; North et al., 2019; Wall & Devor, 1983). Ex vivo preparations can also be applied to obtain data from intact afferent fibres intrinsic to nociception (Guo et al., 2019) and the development of analgesics can be facilitated by in silico techniques (Takasaki et al., 2018). The ultimate aim is to gain a steer on the identity of novel therapeutic targets that translate either to models of experimental pain studies in humans or to pain research and clinical trials in patients (Figure 2). This can appear a large leap when considering bench approaches. However, studying pain circuitry in animals is a vital step towards the identification of such targets.
FIGURE 2.
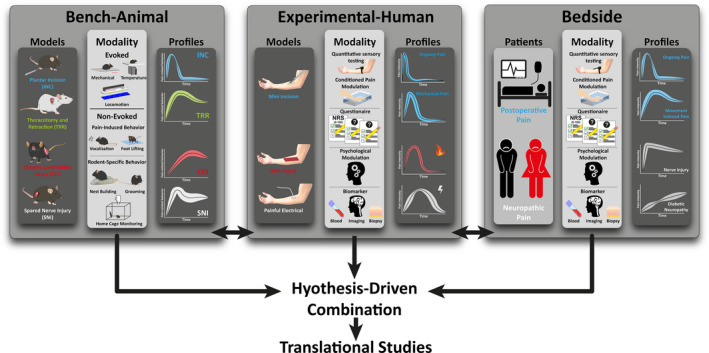
Translational pain research components illustrated for selected conditions of neuropathic and post‐operative pain. Outcome profiles of various assessment modalities is shown for animal (bench) models and human experimental pain models as well as in patients (bedside)
3.1. Relevant animal models and clinical trials
It is possible to produce animal models of nerve damage (Kim & Chung, 1992), inflammation (Guzman et al., 2003), cartilage‐loss (Bellavance & Beitz, 1996), post‐surgical states (Bree et al., 2015; Brennan, 1999; Flatters, 2008; Pogatzki & Raja, 2003; Pogatzki‐Zahn et al., 2018) and cancer states (Kucharczyk et al., 2020). To some extent, it is thus possible to produce models of pathology resembling those of patients with pain using specialized surgical and pharmacological approaches in animals. For example, the clinical observations that oxcarbazepine may be effective only in a specific subgroup of patients with peripheral neuropathic pain displaying the so‐called irritable nociceptor phenotype – characterized by hyperalgesia and allodynia (Demant et al., 2014) – was successfully back‐translated in a rat model of neuropathy in which similar effects are observed following oxcarbazepine treatment (Patel et al., 2019). Likewise, there are several models for musculoskeletal pain that may have clinical relevance (Hoheisel et al., 2013; Sluka et al., 2001). Despite this, animal model outcomes do not always translate directly to man and the failure of an analgesic therapy to relieve symptoms in a clinical trial is often attributed to the ‘poor’ animal model used (Gregory et al., 2013; Hill, 2000). However, failure to translate preclinical results into analgesic action in patients can also be attributable to suboptimal clinical trial designs, inadequate sample numbers, insufficient dosage selection for target engagement, or interspecies differences in pharmacodynamics and/or active/inactive metabolites. Additional study design flaws may relate to the type of pain that is measured (e.g. evoked versus. spontaneous pain) (Sluka et al., 2013) or the subjective manner in which pain is assessed using one‐dimensional ratings in humans (e.g. often relying only on a simple visual analogue scale based measure of the sensory component of pain; Gilron et al., 2019; Pogatzki‐Zahn et al., 2019a)). There is a requirement for preclinical research to more thoroughly consider and model disease specific pain‐related symptoms and the affective‐motivational and cognitive dimensions of the pain experience. These dimensions are highly relevant to the clinical manifestations of pain, often refractory to treatment, and as great or greater burden on patients than the sensory component of pain. This is also a crucial consideration regarding how pain measurements performed in experimental and acute pain settings can be related to assessments performed in more chronic pain settings. There remains a need to develop better measures for the assessment of pain that are specific for experimental, acute and chronic pain. NRS/VAS ratings are still used extensively, despite many known limitations. One important topic not yet elucidated is the definition of the extremities of pain intensity rating scales. Common definitions used across experimental, acute and chronic pain are “worse pain imaginable” or “most intense pain tolerable”. Such descriptions are highly subjective and personal experiences can influence the individual conceptualization of such endpoint anchors and thus ratings of pain (Becker et al., 2020; Dannecker et al., 2007; Yokobe et al., 2014). To circumvent such problems, cross‐model matching procedures have been developed to construct more reliable and valid verbal rating scales of clinical pain (Gracely, 1990; Heft et al., 1980). However, these methods are not commonly used, possibly because their application requires time, which is often a limiting factor in clinical contexts. More research is needed on the definitions of scale endpoints related to pain ratings and how they influence the ratings. Regarding whether healthy volunteers use ratings scales differently to patients with chronic pain, there appears to be some relatively small differences following application of experimental pain stimuli (Becker et al., 2020). For different scopes related to acute versus chronic pain and different chronic pain‐related diseases, there are recent recommendations for outcome domain assessment (“what to measure”) as well as respective patient‐reported and patient‐relevant outcome measures (PROMs and PREMs, “how to measure”) (Pogatzki‐Zahn et al., 2019a). Despite some overlap between the established Core Outcome Sets, many aspects have been considered varyingly, presumably in part due to differences between relevance of outcome for different (chronic) pain states. Placebo studies highlight the complexities surrounding treatment expectations (Petersen et al., 2014), an issue not often contended in animal studies.
The predictive validity of preclinical models, relating to the extent to which drugs work in animals compared to humans, can be improved by the development of animal models based on clinical disease presentation and pathology (i.e. face validity). Ideally, given the complexities of disease states that encompass neuropathic, inflammatory and post‐surgical pain (Pogatzki‐Zahn et al., 2018; Thakur et al., 2014), drugs should be screened in multiple animal models, each predictive of human efficacy. This is naturally difficult because of differing routes of administration, pharmacokinetics, dosing regimens and the varying nature of the human condition (itself a crucial factor; e.g. patients with knee osteoarthritis will often perceive their pain uniquely, and the reasons for this are likely multi‐factorial and not fully understood). It is prudent to note that a laboratory animal cannot tell that it is experiencing pain and therefore we are limited to the interpretation of so‐called pain‐related or nociceptive/nocifensive behaviours. This immediately highlights the limitation of sensory‐evoked measures. Basic science researchers should discuss and draw conclusions with reference to ‘nociception’ (pertaining to transduction, transmission and modulation of a nociceptive stimulus), rather than to ‘pain’. This highlights the issues with translating research outputs gleaned at the bench, to man. Despite these considerations, there are several examples of successful clinical trials based on animal models. TNF‐alpha antibody therapy for rheumatoid arthritis, along with clinical application of ziconotide, high concentration capsaicin (although this could also be considered a refinement of a treatment dating back to the 19th century (Turnbul, 1850)), and tanezumab for chronic pain, and monoclonal antibodies against CGRP for migraine represent some recent examples of effective translation of basic bench research to human therapy (Alonso‐Ruiz et al., 2008; Backonja, 2012). Serendipity plays a role; for neuropathic pain, clinically available front‐line drugs are borne from research of anti‐depressant and anti‐epileptic therapies (Baidya et al., 2011; Sawynok et al., 1999). Of course, promising data collected in animal models does not necessarily translate to a successful clinical trial. Preclinically, tachykinin NK1 receptor antagonism attenuates nociceptive responses, but no analgesic response is observed clinically (Hill, 2000). Similarly, inhibition of low threshold activated T‐type calcium channels that are upregulated in primary sensory neurons in preclinical models of chronic pain, rarely perform better than placebo agents in humans (Snutch & Zamponi, 2018). Such issues with translation highlight the complexity of the physiological processes that must be manipulated to produce analgesia in humans. There are also safety issues, underlining the failure of drugs including nicotine agonists and TRPV1 antagonists, where intolerable side effects render the relevant pharmacotherapies useless.
3.2. Measuring clinically relevant spontaneous/ongoing pain
Modelling spontaneous or ongoing pain in rodents is a significant challenge for preclinical research. Rats and mice rarely develop or exhibit behaviours indicative of spontaneous or ongoing pain, likely because they are prey animals and have evolved to hide signs of weakness or injury. Thus, the vast majority of preclinical pain models and research relies on inducing, through experimental injury, a state of hypersensitivity to non‐noxious and noxious stimuli (evoked pain), or a set of nociceptive behaviours, which manifest over a defined and limited period of time post‐injury (e.g. hind paw licking and flinching in the formalin test). However, in the clinic, spontaneous and ongoing pain often presents more of a problem for patients than evoked pain and, if both occur, the mechanisms behind them are different (Segelcke et al., 2019). Recently, there have been some advances in preclinical modelling of these aspects. Notably, Porreca and colleagues have developed a useful behavioural paradigm for demonstrating and investigating ongoing pain in rodents that is based on conditioned place preference/aversion. Rats/mice that have undergone injury or noxious insult, when placed in a two‐chambered arena, will choose to spend more time in the chamber which has been paired previously with administration of an analgesic drug, likely due to positive affect/reward associated with the relief of ongoing pain (He et al., 2012). In other work, researchers have investigated rodent locomotor activity in the home cage as compared to a novel environment (e.g. an open field arena), or conditioned operant responding, and found that injury or noxious insult typically reduces or suppresses locomotor activity in a novel environment (pain‐depressed behaviour) in a manner which can be reversed by analgesic drugs (Bree et al., 2015; Buvanendran et al., 2008; Martin et al., 2004). However, regarding the translational aspect, it is difficult to ascertain whether animals are less active because they are experiencing ongoing pain, or whether activity itself is evoking pain, with a consequent avoidance‐related reduction in activity. In addition, the analgesic drugs to which the above models have been shown to be sensitive are those often used as standard‐of‐care with varying degrees of success in the clinical setting (e.g. opioids, NSAIDs, local anaesthetics, alpha‐2‐adrenoceptor agonists). The rat and mouse grimace scales represent another attempt to assess ongoing or spontaneous pain in rodents (Langford, Langford, et al., 2010; Sotocinal et al., 2011). They have been used mostly with models of acute or tonic pain, or short‐term post‐operative pain monitoring (in the context of animal welfare) but there is a relative paucity of evidence for their utility in animal models of chronic pain. Non‐evoked resting pain in animals after surgical incision occurs immediately thereafter and might translate to pain at rest in patients after surgery (Segelcke et al., 2019). In vivo electrophysiology may also provide insight into the extent to which dorsal horn neuronal activity occurs in the absence of evoked stimulation of the peripheral receptive field. High levels of spontaneous activity are not evidenced by deep dorsal horn convergent neurons in osteoarthritic or spinal nerve ligated (SNL) male rats (Bee et al., 2011; Rahman et al., 2015). In contrast, convergent neurons in the ventral posterior thalamus do exhibit substantially higher rates of spontaneous firing in SNL male rats compared to their sham‐operated counterparts (Patel & Dickenson, 2016), which may relate to ongoing pain after nerve injury. These results likely represents an increased receptive field and primary afferent input to the dorsal horn that is reflected in increased ascending pathway activity and thus thalamic neuronal activation patterns. Contrasting findings demonstrate spontaneous activity in some dorsal horn neurons after surgical incision, which could relate to non‐evoked post‐surgical resting pain in patients (Pogatzki et al., 2002; Xu et al., 2009). These dorsal horn neurons were different from those related to mechanical hypersensitivity (Pogatzki et al., 2002; Xu et al., 2009), but not fully characterized to allow comparison with prior findings in deep dorsal horn convergent neurons (Bee et al., 2011; Rahman et al., 2015).
3.3. Considering sex and age
The overwhelming majority (approximately 80%) of preclinical studies have been performed in young adult male rats or mice (Zucker & Beery, 2010). However, the clinical reality is that males and females differ with respect to pain tolerance and thresholds, and chronic pain conditions have differing prevalence in females and males, with many being more prevalent in females (Bartley & Fillingim, 2013; Mogil, 2012; Pogatzki‐Zahn et al., 2018). There has been increased recognition of this paradox in recent years, and many grant funding agencies now require the gender issue to be addressed satisfactorily. Furthermore, with an increasingly ageing population worldwide, chronic pain disorders that affect the elderly should be modelled more commonly in aged rats and mice. Aged animals may be sometimes difficult to study because of size and comorbidities, but provide valuable information on age‐related pathophysiology and pharmacology of pain (Jourdan et al., 2002; Pickering et al., 2006). Moreover, further research into pain in children and adolescents is required. But these areas also present their own challenges. Comparing both sexes has the potential to double animal numbers, aged rodents are significantly more expensive than young adult rodents and very young rodents require expensive and labour‐intensive breeding programmes and can be technically challenging.
4. EXPLORING PAIN PERCEPTION AND ITS MODULATION IN HEALTHY HUMAN VOLUNTEERS
Exploring the neural mechanisms underlying the perception of pain and its modulation in healthy human volunteers requires methods to induce pain experimentally in a safe and controlled fashion. Obviously, many of the in vivo techniques that are used in non‐human animal models are not applicable to human volunteers. Despite the inherent limitations to human research, several methods have been developed to activate skin, muscle and visceral nociceptors in humans, to induce peripheral and central sensitization experimentally, and to characterize nociception along the entire human neuraxis. A promising perspective is the use of these methods in phase 1 clinical trials to determine target engagement, in addition to safety, tolerability and pharmacokinetics of a novel compound. The ability to determine at a very early stage whether or not a significant effect on nociception can be observed below the maximum tolerable dose in healthy volunteers could reduce the failure rate of clinical trials.
4.1. Experimental induction of pain in human volunteers
Exploring the neural mechanisms underlying the perception of pain and its modulation in healthy human volunteers requires methods to induce pain experimentally in a safe and controlled fashion. The most‐often used techniques to produce pain aim at activating nociceptors innervating the skin. These include, (1) thermal stimulation of heat‐ or cold‐sensitive skin nociceptors using radiant heat, contact heat or contact cold, (2) mechanical stimulation of mechano‐sensitive skin nociceptors, (3) chemical activation of nociceptors using substances that activate or sensitize nociceptors such as topical capsaicin, and (4) direct electrical stimulation of nociceptive afferents (Arendt‐Nielsen et al., 2007; Plaghki & Mouraux, 2005). These methods can also be used to generate nociceptive input in non‐human animals, and (backward) translation could be improved by better alignment of the methods used. A first important point to consider when comparing the methods used to induce pain is selectivity. All the above‐mentioned stimuli can activate nociceptive afferents and produce pain. However, many of these techniques unavoidably also activate non‐nociceptive somatosensory afferents. Lack of selectivity makes it challenging to determine whether the stimulus‐evoked responses are produced by nociceptive or non‐nociceptive somatic input and as such complicate elucidating which mechanisms are involved, for example, when observing drug‐induced effects on these responses. Mechanical stimuli such as pinprick stimuli can activate nociceptors, but will unavoidably also activate low‐threshold mechanoreceptors (LTM). Similarly, because the electrical activation threshold of peripheral nerve fibres is inversely proportional to their diameter, electrical stimuli having the ability to activate unmyelinated or thinly‐myelinated small‐diameter nociceptive afferents almost unavoidably also activate non‐nociceptive large‐diameter afferents – except when special electrodes are used to selectively activate free nerve endings located in the most superficial layers of the skin (Inui et al., 2002; Mouraux et al., 2010). In contrast, thermal stimuli have the advantage of activating heat‐sensitive free nerve endings without concomitantly activating LTMs (Plaghki & Mouraux, 2005).
Even though most nociceptors are polymodal, different types of nociceptive stimuli can preferentially activate different types of nociceptive afferents. Mechanical stimuli may be expected to preferentially activate mechano‐sensitive nociceptors, heat stimuli to activate heat‐sensitive nociceptors and cold stimuli to activate cold‐sensitive afferents. Moreover, some nociceptors are predominantly sensitive to phasic stimuli whereas other nociceptors respond preferentially to long‐lasting tonic stimuli (Meyer & Campbell, 1981; Treede et al., 1995). Thresholds may also vary (Churyukanov et al., 2012; Treede et al., 1995). As an example, intense short‐lasting phasic heat stimuli such as those generated by a high power infrared laser pulse directed onto the skin have been shown to generate responses almost exclusively related to the activation of one specific type of heat‐sensitive afferent, so‐called A‐fibre Type 2 nociceptors, with very little contribution of other types of heat‐sensitive nociceptors such as Type 1 A‐fibre nociceptors and C‐fibre nociceptors (Bromm & Treede, 1987). Because the respective contribution of these different types of nociceptive afferents to clinical pain remains largely unknown, this raises questions about the translatability to patients of results obtained using transient experimental stimuli in healthy volunteers. Furthermore, when sensitized for example by inflammation, the response properties of nociceptors may change. The afferents activated by a given nociceptive stimulus in physiological conditions could thus differ from those activated in pathological conditions such as inflammatory pain and neuropathic pain. In human skin, so‐called ‘silent nociceptors’ account for almost a quarter of nociceptors (Schmidt et al., 1995). Rodent studies have linked un‐silencing of these nociceptors to hyperalgesia during inflammation and cancer‐induced bone pain (Kucharczyk et al., 2020; Prato et al., 2017).
The above‐mentioned stimulation techniques all activate exteroceptive nociceptors of the skin. Other stimulation techniques have been developed to explore interoceptive nociception, i.e. the neural activity related to noxious stimuli occurring inside the body, such as those involved in musculoskeletal pain and visceral pain. Because pain arising from muscles, ligaments and tendons, bones and the viscera are most common in pathological conditions, understanding interoceptive nociception has a high clinical relevance. However, the techniques to activate interoceptive nociceptors are technically challenging to implement, difficult to control and often intrusive. Such as for skin nociceptors, electrical, mechanical, thermal and chemical stimuli can be used to activate muscle nociceptors and visceral nociceptors (Arendt‐Nielsen et al., 2007; Drewes et al., 2003; Graven‐Nielsen, 2006; Staahl & Drewes, 2004). Intramuscular electrical stimulation using a needle electrode can selectively activate sensory afferents of the stimulated muscle. Similarly, endoscopic probes can be used to activate nociceptors innervating the walls of hollow organs. Such as for electrical stimulation of skin nociceptors, the main drawback of deep tissue electrical stimulation is that the elicited responses can be confounded by concurrent activation of non‐nociceptive afferents and efferents (e.g. motor nerves). Mechanical stimuli can also be used, such as focused muscular pressure using pressure algometry, pressure applied to the entire circumference of a limb using cuff algometry, and distension of hollow organs using balloons. However, mechanical stimulation of muscle nociceptors using external pressure applied against the skin unavoidably also activates skin mechanoreceptors, even though skin nociceptors seem not to play a major role (Graven‐Nielsen et al., 2004; Kosek et al., 1999). Furthermore, the actual pressure applied against the wall of a hollow organ using balloon distension is difficult to control – but new methods have been developed to better estimate stimulus‐evoked tissue strains (Drewes, Gregersen, et al., 2003). One study has used thermal stimulation of muscle nociceptors by injection of warm or cold isotonic saline (Graven‐Nielsen et al., 2002), and a few studies have used thermal stimulation of visceral nociceptors with temperature‐controlled balloons, with the advantage of selectivity for thermosensitive afferents (Villanova et al., 1997). Finally, intramuscular injections of pro‐nociceptive substances or their local delivery in hollow organs using endoscopic probes have been used extensively to induce experimental muscle pain and visceral pain in humans, such as hypertonic saline, acid and capsaicin (Arendt‐Nielsen et al., 2007; Drewes, Gregersen, et al., 2003; Graven‐Nielsen, 2006; Staahl & Drewes, 2004). Endogenous algesic substances can also be used to induce muscle pain, such as the induction of muscular ischemia by contractions during occluded blood flow (Graven‐Nielsen et al., 2003; Issberner et al., 1996), or the induction of muscle soreness by unaccustomed muscular exercise (Newham et al., 1983; Slater et al., 2003). Key characteristics of experimental musculoskeletal pain is their diffuse nature compared to a very localized skin pain and importantly pain referral to other structures not being stimulated.
4.2. Experimental induction of peripheral sensitization and facilitation of central mechanisms
In patients, pain is most often a consequence of tissue lesion and inflammation, which induce peripheral sensitization, i.e. increased sensitivity of peripheral nociceptors. It is increasingly acknowledged that sustained peripheral nociceptive input even without peripheral sensitization tends to enhance the responsiveness of the central nervous system to that peripheral nociceptive input, such as the phenomenon of central sensitization at the level of the spinal cord that has been studied extensively in rodent models of pain. However, central sensitization can occur in the absence of a peripheral insult. These changes can dramatically modify the function of the nociceptive system and could explain pain symptoms in many patients. To induce peripheral and central sensitization in human volunteers, several approaches have been developed (Binder, 2016). These include experimental lesions of the skin such as burn and freeze lesions (Martin et al., 2019), UVB‐induced tissue damage and inflammation, topical application or intradermal injection of nociceptive or inflammatory substances such as capsaicin, menthol, cinnamaldehyde and nerve growth factor (NGF), high‐frequency electrical stimulation of the skin (Klein et al., 2005; Vollert et al., 2018) and an experimental incision in the skin of human volunteers (Fißmer et al., 2011; Pogatzki‐Zahn et al., 2010, 2019b). Some of these approaches have also been used to induce sensitization in muscle tissues or hollow viscera (e.g. repeated intramuscular infusions of nociceptive substances (Hayashi et al., 2013), high‐frequency electrical stimulation of muscular and fascia afferents (Schilder et al., 2016), capsaicin infusions in the gastro‐intestinal tract (Drewes, Schipper, et al., 2003).
The commonality of all these experimental techniques is that they all induce some amount of hyperalgesia. All but high‐frequency electrical stimulation of nociceptive afferents markedly sensitize transduction by peripheral nociceptors, either via a direct action on the molecular receptors responsible for this transduction (e.g. agonists of TRP channels such as capsaicin, menthol and cinnamaldehyde), or by producing local inflammation which in turn sensitizes the nociceptors (e.g. tissue lesions producing tissue inflammation). Notably, any sustained experimental activation of peripheral nociceptors will also produce some amount of tissue inflammation, because of the local release of neuropeptides by peptidergic nociceptors, and this explains why topical capsaicin or high‐frequency electrical stimulation of the skin can produce a cutaneous flare. Furthermore, any sustained activation of peripheral nociceptors is likely to induce some amount of central sensitization, hallmarked by the development of secondary mechanical hyperalgesia, i.e. an increased sensitivity to noxious mechanical stimuli that extends beyond the zone of injury or inflammation. This is most evident with high‐frequency electrical stimulation of the skin although leaving the stimulation site less affected. In healthy volunteers, five seconds of high‐frequency electrical stimulation using an electrode designed to preferentially activate nociceptive free nerve endings induces an increase in sensitivity to mechanical pinprick stimuli that lasts several hours and extends well beyond the stimulated skin (Klein et al., 2004). A similar time course of pinprick hyperalgesia can be produced by an experimental surgical incision that is, however, accompanied by transient spontaneous pain and hyperalgesia to heat (Fißmer et al., 2011; Pfau et al., 2011). Following prolonged stimulation of muscle nociceptors, deep‐tissue hyperalgesia is sometimes accompanied with widespread hyperalgesia and facilitation of central pain mechanisms (e.g. temporal summation of pain and expanded pain referral) (Andersen et al., 2008; Doménech‐García et al., 2016; Hayashi et al., 2013).
Interestingly, these experimental pain models in human volunteers have also often been used in animal models of pain, thereby making it possible to establish links between pathophysiological observations that are possible only in non‐human animals – such as the recording of spinal neurons or the immunohistochemical assessment of glial activation in the dorsal horn – and behavioural observations that are possible only in humans such as assessing self‐reported changes in pain perception (Pogatzki‐Zahn et al., 2017).
Several authors have referred to some of the above‐mentioned methods as “surrogate models of neuropathic pain” (Klein et al., 2005). Although peripheral sensitization and facilitated central mechanisms may contribute to the positive symptoms of neuropathic pain, there are arguments against this because neuropathic pain obligatorily involves a lesion or disease of the somatosensory system, and none of these models reproduce any such lesion or disease. Furthermore, all these methods consistently produce some amount of hyperalgesia, but generate little or no static or dynamic tactile allodynia and cold allodynia, although these symptoms are highly prevalent and most disabling in many neuropathic pain conditions. For obvious ethical reasons, there are thus currently no experimental models of neuropathic pain in human volunteers. One exception could have been the prolonged and/or high‐concentration treatment of the skin with capsaicin, as it induces a selective nociceptor denervation of the epidermis (Nolano et al., 1999). However, although this intervention reproduces the negative symptoms of neuropathic pain (i.e. a reduced sensitivity to nociceptive stimuli lasting several days), the capsaicin‐induced denervation does not reproduce the positive symptoms such as spontaneous pain, allodynia and hyperalgesia. Another exception could be the thermal grill illusion, in which interlaced warm and cool temperatures applied against the skin may produce a paradoxical unpleasant sensation mimicking some of the positive symptoms of neuropathic pain (Craig & Bushnell, 1994).
4.3. Experimental manipulation of descending modulatory pathways
The discovery in rats that spinal neurons relaying nociceptive input can be inhibited by nociceptive input applied outside their own segmental receptive field – a phenomenon referred to as diffuse noxious inhibitory controls (DNIC) and involving a spinal‐bulbar‐spinal loop (Le Bars et al., 1979a, 1979b) – prompted extensive research in humans on the involvement of DNIC in the modulation of pain. In human volunteers, the phenomenon is typically studied by characterizing the effect of a remote or heterotopic noxious conditioning stimulus (HNCS) on the responses to a second painful test stimulus – so‐called conditioned pain modulation (CPM) (Pud et al., 2009). Most often, HNCS is achieved by immersing an upper limb extremity in noxious cold or hot water, and the outcome measure is the reduction in pain ratings to brief noxious test stimuli applied to another region of the body, such as the contralateral lower limb, during the application of HNCS. Other modalities for HNCS have been extensively used in experimental and clinical studies, such as cuff pressure stimulation (Cummins et al., 2020; Graven‐Nielsen et al., 2017). Animal studies have also shown that the inhibitory effect of HNCS on spinal neurons persists some minutes after the termination of HNCS. For this reason, other studies have assessed in humans the changes in sensitivity to the test stimulus not only during but also after HNCS (Willer et al., 1989). Although unequivocal evidence that malfunctioning descending controls may be a primary driver of any chronic pain condition is lacking, it is noteworthy that dysregulation of DNIC is observed in several rodent models of chronic pain (Bannister et al., 2015, 2017; Lockwood et al., 2019), and that the effects of CPM can be lower in patients with chronic pain as compared to controls (Jennings et al., 2014; Lewis et al., 2012; McPhee et al., 2020; Petersen et al., 2019; Potvin & Marchand, 2016; Yarnitsky, 2010). One hint that descending modulation is involved in the severity of pain conditions comes from studies showing that CPM is predictive of the development of post‐surgical acute and chronic pain (Ruscheweyh et al., 2017; Yarnitsky et al., 2008). Underlying noradrenergic mechanisms could explain, in patients with chronic pain, the relationship between reduced CPM and the beneficial effects of tapentadol (μ‐opioid receptor agonist and noradrenaline reuptake inhibitor, NRI) and duloxetine (serotonin norepinephrine reuptake inhibitor, SNRI) (Niesters et al., 2014; Yarnitsky et al., 2012). This back‐translates since NRIs reinstate the functional expression of DNIC in rat models of neuropathic pain (Bannister et al., 2015). Even so, whether the pain reduction induced during and/or after HNCS in humans can be attributed in full or in part to a modulation of nociception at spinal level, and whether this modulation results from an engagement of DNIC remain open questions. When one relies on pain perception, higher‐order cognitive processes such as HNCS‐induced distraction from the test stimulus are likely to contribute to the observed effects. The finding that the spinal nociceptive withdrawal reflex (NWR) can be depressed by HNCS suggests that HNCS can modulate nociception at spinal level in humans (Jure et al., 2019; Willer et al., 1989). Furthermore, studies in rodents have shown that the functional expression of DNIC is influenced by subcortical brain regions associated with emotional processing (Phelps et al., 2019), indicating that cognitive and affective factors may modulate the experience of pain by modulating this mechanism.
Beyond DNIC, the NWR can also be modulated directly by cognition and affect. An increasing number of studies explore how these factors may modulate pain through an effect on the descending pathways that can inhibit or facilitate spinal nociception (Tracey & Mantyh, 2007). This is exemplified by recent studies exploring how induction of placebo or nocebo effects may modulate nociception – and, thereby, pain perception – using functional neuroimaging techniques to identify placebo/nocebo‐related changes in activity at brain, brainstem and spinal levels (Geuter & Büchel, 2013), as well as pharmacological manipulations to understand the role of different neurotransmitter systems such as descending opioidergic pathways (Eippert, Bingel, et al., 2009).
Part of the effects of psychological factors on pain perception may thus involve descending mechanisms controlling nociception at spinal level. However, supraspinal effects are probably equally important, and changes in the function of peripheral nociceptors might also occur. For example, the phenomena of stress‐induced analgesia and stress‐induced hyperalgesia have been shown to involve multiple mechanisms that modulate both spinal (via descending pain pathways) and supraspinal components of the pain pathways, and stress‐induced responses may also impact nociception in the periphery (Burke et al., 2017; Butler & Finn, 2009; Jennings et al., 2014).
4.4. What can be recorded in humans?
As compared to studies conducted in animals, studies in human volunteers offer the advantage that the results may be more closely related to what is observed in patients because there will be no inter‐species difference, and because it is possible to obtain self‐reports of pain intensity or unpleasantness. Furthermore, in patients, questionnaires can be used to assess how pain impacts mood, daily life activities and quality of life. On the other hand, human research is limited by the techniques that are available to sample neural activity (and proxies of this) and, even more so, the techniques that can be used to induce and characterize pain‐related changes in function or structure of the nervous system that may occur in patients exposed to various pathologies such as tissue lesions and inflammation, and lesions of the nervous system. Nevertheless, several techniques allow sampling nociception‐related activity at all levels of the human neuraxis and examples are given below.
At the peripheral level, microneurography is a minimally invasive technique to measure action potentials of single (C‐fibre) nociceptors or sets of nociceptors (Ackerley & Watkins, 2018; Serra, 2009). Also at the peripheral level, electrophysiological and psychophysical sensory “threshold tracking techniques” have been developed to assess the excitability of large‐diameter sensory axons and gain insight into ion channel activity using a combination of transcutaneous conditioning and test electrical pulses, and attempts have been made to extend these techniques to the assessment of small‐diameter nociceptive afferents (Bostock et al., 1998; Hugosdottir et al., 2019; Kiernan et al., 2020).
To assess nociception at spinal level, the NWR has been used extensively (Neziri et al., 2010; Sandrini et al., 2005). Most‐often, it is elicited by noxious stimulation of the lower limb (e.g. transcutaneous electrical stimulation of the sural nerve at the lateral malleolus or electrical stimulation of the foot sole) and recorded from a flexor muscle of that same lower limb. Although this reflex is not a direct measure of the activity of spinal neurons relaying nociceptive input to the brain, and while isolating this nociceptive reflex from reflex activity triggered by non‐nociceptive large‐diameter afferents can be tricky, the threshold and amplitude of the NWR can be expected to relate to the responsiveness of spinal neurons responding to nociceptive input. The reflex receptive fields from where the NWR can be elicited (e.g. on the foot) is an additional measure (Andersen et al., 2001) where objective quantification of the area have demonstrated enlargement in clinical conditions (Biurrun Manresa et al., 2013). Complementary to the NWR, and since the technique remains very challenging, several research centres are now able to sample nociception‐related activity at spinal level using BOLD‐fMRI (Powers et al., 2018).
At brainstem level, the blink reflex is a trigemino‐facial reflex aimed at triggering eyelid closure in response to a threatening stimulus (Cruccu et al., 2005). It can be easily measured by stimulating skin innervated by the supraorbital nerve and sampling the elicited response from the orbicularis oculi muscle. The R2 component of this reflex is mediated by A‐beta and A‐delta fibres, which synapse with wide dynamic range neurons in the medulla. Autonomic and arousal responses such as variations in skin conductance, pupil diameter and heart rate can also be used to assess involvement of the brainstem and hypothalamus. Using high‐resolution fMRI, it is now also possible to measure BOLD activity related to nociception and its modulation in subcortical and brainstem structures, including the periaqueductal grey (PAG) and rostral ventromedial medulla (RVM) which are thought to play a critical role in descending pain modulation (Bingel & Tracey, 2008).
Finally, at the level of the cortex, nociception‐evoked neural activity can be readily assessed using non‐invasive scalp electroencephalography (EEG) and magnetoencephalography (MEG) (Mouraux, 2019). The recorded signals constitute a direct but macroscopic or population‐level measure of synchronized electrocortical activity. This includes the recording of evoked brain potentials elicited by brief nociceptive stimuli such as laser‐evoked brain potentials, pinprick‐evoked brain potentials (Iannetti et al., 2013) and some more recent approaches attempting to isolate cortical activity related to sustained noxious input such as time‐frequency analyses to measure tonic variations in ongoing oscillatory activity (reviewed by Ploner et al. (2017)) and the recording of nociceptive steady‐state responses (Colon et al., 2017). Using depth electrodes or subdural electrode grids implanted for the presurgical evaluation of epilepsy (intracerebral EEG or electrocorticography), several centres have also measured directly nociception‐evoked neural activity from various brain structures such as the operculo‐insular cortex (Frot et al., 2007). Interestingly, studies in animals have suggested that experimental modulation of some of these responses may modulate pain behaviour. For example, Tan et al. (2019) showed that stimulus‐evoked gamma‐band oscillations in the somatosensory cortex which have also been observed in humans (Gross et al., 2007) are enhanced in mice with inflammatory pain, and that optogenic induction of similar oscillations induces behaviours suggestive of pain, allodynia or paraesthesia.
Functional neuroimaging techniques that measure local variations in cerebral blood flow or oxygenation due to neurovascular coupling and the hemodynamic response have also been used extensively (Tracey & Mantyh, 2007): BOLD fMRI, but also magnetic resonance arterial spin labelling (ASL), positron emission tomography (PET), and functional near‐infrared spectroscopy (fNIRS). The high spatial resolution provided by these techniques to sample brain activity, as well as the ability to of these techniques to characterize activity from deep brain structures, has provided an unprecedented view of how the brain responds to painful stimuli, but also how the brain may modulate pain perception including through the activation of descending modulatory pathways although it is not yet possible to clearly study brainstem to dorsal horn pathways using fMRI.
4.5. From healthy volunteers to patients
Observations made in healthy volunteers – including several observations that tend to replicate observations made in animal models of pain such as the induction of secondary hyperalgesia by high‐frequency electrical stimulation of nociceptive afferents, cold injury, an experimental incision or capsaicin (Fißmer et al., 2011; Klein et al., 2005; Madden et al., 2019), and the inhibitory effects of CPM have contributed to a better understanding of the mechanisms underlying pain perception and its modulation. A driving motivation for this research is that it could also lead to a better understanding of the mechanisms underlying the pain experienced by patients and, thereby, to new diagnostic and therapeutic approaches. However, translating findings in healthy volunteers to what occurs in patients is an ongoing challenge.
The mechanisms underlying short‐lasting experimental pain in healthy human volunteers could be quite different from the mechanisms underlying the pain experienced by patients during days, weeks, months or even years (Figure 2). Similarly, the mechanisms at play in experimental models of inflammation, sensitization of peripheral and central mechanisms induced by experimental procedures that induce spatially restricted changes in pain sensitivity lasting minutes to hours could be very different from the mechanisms that are at play in patients suffering from chronic nociceptive, inflammatory or neuropathic pain. In other words, generalization may be limited to the process from acute to prolonged pain but less appropriate to generalize from healthy volunteers to patients. Several studies have suggested a mismatch between the brain regions that are typically activated by acute experimental pain and those that are most active in patients suffering from clinical pain such as low back pain (Baliki et al., 2006; Wasan et al., 2011) or pain following a surgical tooth extraction (Hodkinson et al., 2013; Howard et al., 2011). On a positive note, characterizing and understanding these differences could be a way to understand the changes in structure and function of the nervous system that underlie the chronification of pain. Interestingly, there are also examples of manifestations that are comparable to what is observed in patients. Recently several attempts have successfully studied prolonged experimental pain models in otherwise healthy subjects by following the cortical neuroplastic manifestations in the course of pain for several days (De Martino et al., 2018; De Martino et al., 2018, De Martino 2019; Schabrun et al., 2016). Another very important issue is the discussion of causality when interpreting differences observed in patients with pain as compared to controls. Except in the case of post‐surgical pain, the lack of pre‐pain measurements makes it often impossible to determine whether a particular finding is causal or consequential to the presence of ongoing pain for a long time, or whether it constitutes a pre‐existing trait present in these patients. Furthermore, it is often difficult to disentangle whether observed differences reflect an unspecific change in state associated to the condition. Here, large cohort studies or the relative rare patient conditions where baseline recordings can be obtained may be useful, as well as prolonged experimental pain studies where baseline recordings are included. Recurrent pain conditions may also be useful to understand the pain systems with and without ongoing pain, e.g. in recurrent low back pain patients where measures related to pro‐nociceptive mechanisms (temporal summation of pain) were enhanced only during ongoing clinical pain, while measures related to anti‐nociceptive mechanisms (CPM) seem depressed both in periods with pain and periods without pain (McPhee & Graven‐Nielsen, 2019).
4.6. Considering sex and age
Epidemiological studies as well as clinical studies have shown that women are at greater risk for many pain conditions (Mogil, 2012). Except for studies involving administration of a drug, the majority of studies conducted in healthy human volunteers recruit both men and women, and several studies have explored sex‐related differences specifically. Differences in the responses to experimental pain and its modulation in male and female have been reported, but these appear to be somewhat marginal as compared to the differences observed in patients. This is in line with the notion that chronic pain is not necessarily related to changes in the responses to experimental pain, and indicates that further research is needed.
Although the prevalence of chronic pain greatly increases with age, and is a main determinant of disability in the elderly (Gibson, 2007), the majority of studies on pain perception and its modulation conducted in human volunteers are biased towards younger individuals. Studies conducted in aged healthy volunteers indicate reduced sensitivity to experimental nociceptive stimuli (Chakour et al., 1996; Pickering et al., 2002, 2014), which could be explained, at least in part, to an age‐related reduction of nociceptor innervation density (Besné et al., 2002), but also tendency for reduced pain tolerance which might result from age‐related differences in the mechanisms modulating pain perception, as well as changes in cognitive function (Defrin et al., 2015).
5. TRANSLATING EXPERIMENTAL TECHNIQUES TO STUDY PAIN INTO CLINICAL TOOLS
5.1. Tools for measuring pain in patients
Some researchers have put forward the idea that pain‐related brain activity sampled using functional neuroimaging could be used as objective measures of pain perception. A crucial point to consider here is that pain, like any other percept, is an intrinsically subjective experience. Therefore, even if the measure itself is objective – like an objective change in regional cerebral blood flow – measures of pain derived from functional neuroimaging will be relevant if and only if they account for the subjectivity of the pain experience. Another point to consider is generalizability. For example, a marker for pain derived from brain activity sampled in healthy volunteers exposed to acute experimental pain might be efficient at identifying and quantifying this acute experimental pain, but have limited abilities to identify and quantify pain in clinical conditions. Pain assessment in persons who cannot communicate is done with observational scales providing only some degree of detection and possible presence of pain (Pickering et al., 2018). Recently, brain imaging in pain conditions was thoroughly reviewed and it was concluded that it is not possible to use it for supporting or disputing a claim of chronic pain but rather imaging is justified for studies of pain mechanisms (Davis et al., 2017).
5.2. Tools for mechanism‐based diagnosis of pain in clinical conditions
Researchers have also suggested that the different neuroimaging techniques used to explore pain perception in humans could be used to characterize the engagement of the different mechanisms or systems that can modulate the pain experience, such as the top‐down influence of nociceptive transmission at the level of the spinal cord, the changes in responsiveness due to central sensitization, and the modulation of pain by placebo, nocebo, cognitive control, emotions and attention (Bingel & Tracey, 2008). Similarly, neuropathic pain – i.e. pain caused by a lesion or disease of the somatosensory nervous system – is thought to involve specific mechanisms leading to specific changes in nociceptive function. The above‐mentioned techniques can be used to demonstrate the existence of a lesion affecting the nociceptive system. Furthermore, techniques such as microneurography can be used to characterize abnormal excitability of peripheral afferents in patients with peripheral neuropathic pain.
Altogether, this could open the prospect of stratifying patients in mechanistically distinct groups, of predicting response to treatment, and of proposing tailored treatments most likely to provide pain relief (Baron et al., 2017). A few studies have followed this line in predicting treatment response to drugs and generated encouraging results (Harris et al., 2013; Yarnitsky et al., 2012), including the ability to predict placebo responses (Vachon‐Presseau et al., 2018). These promising results have wide clinical implications in terms of patient stratification and design of future clinical trials: failures of a treatment may be due to the fact that it is given to the wrong patients, or at a wrong moment in time relative to disease progression.
5.3. Tools for improving future clinical trials
Several studies have shown that the methods described above can be used to characterize drug‐induced modulation of nociception in healthy human volunteers. Among many examples, in patients with neuropathy, peripheral nerve excitability testing is sensitive to the suppression of abnormal sodium currents induced by mexiletine (Isose et al., 2010). Similarly, at the level of the spinal cord, several studies have demonstrated drug‐induced effects on the NWR (Sandrini et al., 2005). Also in healthy volunteers, Truini et al. (2010) showed that laser‐evoked brain potentials are significantly affected by a single administration of tramadol compared to placebo, and it has been shown that the BOLD‐fMRI responses to mechanical pinprick stimuli delivered to skin sensitized by capsaicin can be suppressed by known analgesics including opioids and gabapentinoids (Wanigasekera et al., 2016, 2018; Wise et al., 2002).
A critical step in clinical drug studies is to determine whether the candidate drug achieves target engagement and at which dose. If feasible, this should be done already during Phase 1 studies in healthy subjects, i.e. before full clinical development. The fact that several measures of nociception can readily be modulated by the administration of known analgesics in healthy volunteers suggests that this might be possible, and measures of drug‐induced effects on nociception and its modulation in healthy volunteers could be used as a means to improve the odds of drug development success (Davis et al., 2020). Stratification of patients with peripheral neuropathies in clinical trials using quantitative sensory testing (QST, based on studies like Baron et al. (2017) and Vollert et al. (2018)) or specific screening questionnaires aiming, for example, to identify patients with symptoms of neuropathic pain (Attal et al., 2018) has been encouraged already by the European Medicines Agency in its new guideline as a step towards increasing response rates in clinical trials by a mechanism‐based treatment approach (European Medicines Agency, 2016). Furthermore, the markers of nociception that are sensitive to drug‐induced effects in human volunteers could be back‐translated into relevant preclinical models.
5.4. Non‐pharmacological neuromodulation of the nociceptive system
The observations in animals that stimulation of a given neuronal structure may inhibit selectively transmission of nociceptive input in the central nervous system and suppress pain behaviour has also translated into several non‐pharmacological neuromodulation techniques aiming at reducing pain in patients by acting directly on the mechanisms involved in its modulation. For example, thousands of patients are currently implanted each year with spinal cord stimulators, a technique that was initially justified by the concept of the “gate control” theory of pain and the observation in animals that electrical stimulation of the dorsal columns inhibits nociceptive transmission at the level of the spinal cord. Yet, the actual mechanism of action of spinal cord stimulation and other neuromodulation techniques such as motor cortex stimulation remains largely unknown. A better understanding of the effects of neuromodulation therapies could benefit from further back‐translation to preclinical models.
6. INTERACTION BETWEEN SOMATOSENSORY AND PSYCHOLOGICAL SYSTEMS
Translational pain research is not only challenged by the forward and backward translation between different species and from health and disease states, but also by bi‐directional, multifaceted influences between somatosensory and psychological systems. Results from psychobiological research, targeting such bi‐directional influences, have highlighted the impact of psychobiological mechanisms in the development, maintenance, and therapy of chronic pain (Edwards et al., 2016; Meints & Edwards, 2018).
The effect of psychological processes on somatosensory processing, in particular pain processing, is well‐described in human research. For example, expectations (including expectations induced by placebo or nocebo), perceived threat, catastrophizing, stress, self‐efficacy, to name just a few examples, have been shown to strongly affect pain perception in acute as well as chronic pain states (Atlas & Wager, 2012; Butler & Finn, 2009; Elman & Borsook, 2018; Jennings et al., 2014; Meints & Edwards, 2018). All these factors can inhibit and facilitate nociceptive signalling at all levels of the neuraxis (Bräscher et al., 2016; Bushnell et al., 2013; Eippert et al., 2009; Galambos et al., 2019; Terry et al., 2016). In the context of placebo/nocebo research, it has been shown that effects of expectations can even outperform and override the effects of short‐acting, highly potent opioidergic drugs (Bingel et al., 2011). In addition, the effects of psychological processes on somatosensory processing have also been highlighted with respect to clinical pain. For example, such effects have been well‐described in the context of visceral pain in functional gastrointestinal disorders (e.g. Elsenbruch et al., 2010; Icenhour et al., 2019), tellingly also termed disorders of brain‐gut interactions (Mayer et al., 2019).
However, while there is extensive work on psychobiological mechanisms in humans, animal work is limited. This is not surprising, as many psychological processes are hard to assess in animals. Nevertheless, studies have demonstrated impressively similar effects in laboratory animal research compared to human work. For example, positive social interactions decrease pain behaviour and nociceptive signalling in mice (Langford, Tuttle, et al., 2010), mild threat induced by the presence of stranger male mice induces hyperalgesia in male mice, while severe threat induces analgesia (Butler & Finn, 2009; Jennings et al., 2014; Langford et al., 2011), and placebo analgesia has also been demonstrated in rodents (Nolan et al., 2012; Xu et al., 2018). Converging insights of human and animal research have been found recently on the interaction of cognition and chronic pain. Chronic pain has often been shown to be accompanied by impaired cognition (e.g. Rouch et al., 2020; Samartin‐Veiga et al., 2019), for review: Moriarty et al. (2011). Importantly, based on the results from basic science, a causal role of pain on impaired cognition can be assumed and possibly underlying mechanisms delineated (Shiers et al., 2018, 2020; Xiong et al., 2020), indicating a high potential for successful translational science in the context of psychobiological mechanisms. Nevertheless, one limiting factor in animal research is that the effect of psychological factors has always to be inferred from behaviour, necessitating several assumptions on the psychological effects of experimental manipulations and for interpretation of the displayed behaviour.
Not only do psychological systems affect somatosensory signalling, but the somatosensory system can also modulate psychological processes. One well‐known example is the effect of disturbed sleep on the perception on pain. While disrupted sleep leads to an increased sensitivity to experimental pain (Seminowicz et al., 2019; Staffe et al., 2019), disturbed pain can also predict the development and maintenance of persistent pain (Finan et al., 2013; Nitter et al., 2012, for review). In general, the embodiment framework assumes influences of our body on cognition, emotion, and behaviour as well as sensory and motor systems (Glenberg, 2010). Pain has been described as “inescapably embodied and embedded” (Tabor et al., 2017), shifting the concept of pain from a passive, sensory experience to an active, motor experience. In a broader context, situational feelings have been described as being engrained by “somatic markers” in our bodies, thereby forming future actions (Bechara et al., 2000). Furthermore, the high comorbidity of chronic pain with affective disorders, with some evidence pointing at a possible causal role of pain in the development of these affective disorders (Humo et al., 2019), suggests that the experience of pain can modulate mental well‐being probably through shared underlying mechanisms (Becker et al., 2018; Borsook et al., 2016; Burke et al., 2015; Fitzgibbon et al., 2016).
In translational research, interactions of psychological and somatosensory systems are often neglected (Mao, 2012). However, considering the impact of psychological factors in the development, maintenance and therapy of chronic pain in humans, it appears essential to take such interactions into account in translational research, in particular to foster a better prediction from preclinical trials. In line with this reasoning, it has been shown that factors such as environmental enrichment reduce pain sensitivity and pain behaviour in rodents (e.g. Gabriel et al., 2010; Wang et al., 2019), but a direct translation of these results to societal aspects on pain in humans is lacking so far (Bushnell et al., 2015). Nevertheless, an important step towards integrating psychological aspects in animal research has been taken by basic science focusing more and more on pain aversiveness and emotional‐motivational pain responses, utilizing, for example, grimacing, operant behaviour, and conditioned place preference in rodents (Gomtsian et al., 2018; Leitl & Negus, 2016; Tappe‐Theodor et al., 2019; Tuttle et al., 2018). However, it remains open how such proxies relate to emotional‐motivational pain experiences in humans. It appears an insolvable task to incorporate all known psychological factors from human research in animal research. Identifying central psychological factors and finding appropriate proxies in animals is one challenge in backward translation, which has received surprisingly little attention so far.
Focusing on psychological systems and backward translation, one could carry the question to extremes, asking whether we need backward translation to basic science for psychological pain treatments. While this would allow, as outlined above, urgently needed investigations into fine‐grained neurobiological mechanisms, impossible to investigate in humans, a proper implementation in animal research appears inconceivable. In contrast, human pain research, in particular on primary pain, has strongly focused on psychological mechanisms, in part almost neglecting the knowledge on nociceptive mechanisms gained from basic research, and devaluating the role of nociception in chronic pain (Schweinhardt, 2019). Despite the fact that psychological factors can modulate pain perception, ascending nociceptive input (evoked or spontaneous) can be assumed to be present in almost all pain patients. A large proportion of patients with chronic pain do not report restrictions in activity or participation (Pitcher et al., 2019), suggesting that psychological factors do not play a predominant role in these patients, while nociceptive processing might have been underestimated (Schweinhardt, 2019). In this context, human research could be fostered by forward translation utilizing the knowledge from animal research to understand pain mechanisms in humans, particularly in incorporating and balancing the interaction between somatosensory and psychological mechanisms. Furthermore, backward translation could enrich animal research, for example, by mechanistic investigations using methods such as optogenetics or intracerebral drug injections in specific brain regions identified as important in human pain processing using brain imaging and incorporating somatosensory and psychological interactions. To solve the raised issues on interactions of somatosensory and psychological systems in the context of translational research, interdisciplinary work is essential.
7. MAIN CHALLENGES IN PREVENTION OF PAIN
Translational approaches in pain research are important not only to treat but also to prevent pain from becoming chronic or getting worse by causing secondary effects beyond pain, termed tertiary prevention. Currently, primary prevention of pain focuses on exercise and workouts in general and in those experiencing an acute pain episode (secondary prevention) like low back pain (Geneen et al., 2017). Clinical risk factors have been established in patients for early identification of patients at major risk for musculoskeletal chronic pain which are summarized for easy use by red (biological) and yellow (psychosocial) “flags” as a most common construct, and added by blue (occupational), black (compensation) and white (socio‐cultural) flags (Meyer et al., 2018) (see also https://www.iasp‐pain.org/GlobalYear). Most of the evidence behind these clinical risk factors and approaches to prevent pain from becoming chronic come from clinical investigations.
Screening for subgroups of patients regarding their risk for chronification is possible and might help to identify those patients with specific need for prevention (Hill et al., 2010). A similar approach has been taken for postoperative pain that, under certain circumstances, becomes persistent (Lavand’homme et al., 2018). Here, risk factors are indicated but not yet finally determined (Yang et al., 2019). There is however one important missing link for preventive approaches that in fact relates to translational aspects. To investigate preventive approaches related to the underlying biological mechanisms, animal studies are still required in order to investigate new and specific targets. However, the back translation to animals and healthy human volunteers is hindered here by the fact that most available preclinical models lack translatability. For example, approximately 10% of patients after surgery develop chronic pain and up to 40% after some specific surgical procedures like thoracotomy and breast surgery (Fletcher et al., 2015; Haroutiunian et al., 2013). The percentage of patients presenting signs suggestive of neuropathic pain is even less (Haroutiunian et al., 2013). In most preclinical animal models of neuropathic pain, almost all animals exhibit behaviours suggesting of hyperalgesia and allodynia. The same is true for animal models of musculoskeletal (arthritis) pain. Therefore, the findings from these animal models might not translate well to the mechanisms of chronic pain in patients and prevention strategies based on these studies might therefore fail. Only very few preclinical models were able to address this issue. One example for postsurgical pain is a model for evaluation of persistent post‐thoracotomy pain (Buvanendran et al., 2004). Here, approximately half of the animals developed long‐lasting signs suggestive of hyperalgesia after surgery, and reminiscent of clinical observations (Haroutiunian et al., 2013). Similar, signs of persistent increased sensitivity to mechanical stimulation at the joint after antigen‐induced arthritis (AIA) was only found in a subgroup of rats (Leuchtweis et al., 2020). These models are disease‐related as well as mechanisms‐based and should therefore be preferred for translational research in order to identify preventive targets relevant for disease specific chronification of pain. However, using these models in preclinical studies to investigate mechanisms related to the development of persistent pain and preventive strategies are rare. In addition, translation is still required to prove that treatments targeting these mechanisms potentially prevent chronification of pain in humans (Martinez et al., 2017; Weinstein et al., 2018). Preventive translational approaches with drugs are not very numerous, but these bring a very valuable guide for chronic pain treatment, as has been developed with NMDA antagonists in animals, healthy volunteers and patients (Martin et al., 2019; Morel et al., 2013, 2016). In addition, an interdisciplinary approach is needed because molecular, psychological, social and therapeutic aspects influence the development of chronic pain. There is still a long way to go before we know what combination of factors is beneficial for the prevention of pain, and the duration and types of treatments required. Understanding what patients respond to which kind of preventive strategy, and coupling this with translational approaches, may add valuable knowledge.
8. CONCLUSION
This opinion paper brings together pain researchers from very different disciplines to discuss the opportunities within, and challenges of, translational pain research. Examples are provided in Figure 2 illustrating some components of translational pain research. The many reasons that can prevent the successful translation of bench observations into useful and effective clinical applications are reviewed. Different exploratory paths can address these issues and include (1) the backward translation of observations made in patients and human volunteers into new disease models with improved clinical relevance, (2) improved generalizability by taking into account age and sex differences, and (3) the integration of psychobiology into translational pain research. Moreover, preclinical and clinical stages of developing new treatments for pain can be improved by better preclinical models of pathological pain conditions, and improved methods to assess treatment‐induced effects on pain perception and nociceptive processing in humans and non‐human animals, respectively. Finally, persistence of pain involves plasticity in the peripheral and central nervous system. It is therefore likely that successful outcome of a treatment given at a particular time in preclinical research will not be efficacious at all stages of disease initiation, progression and maintenance in patients. Future research should thus probably take into consideration the dynamics of the mechanisms as they evolve over time.
DISCLOSURES
TGN is a part of Center for Neuroplasticity and Pain (CNAP) supported by the Danish National Research Foundation (DNRF121). EPZ received in the past 5 years financial support from Mundipharma GmbH and Grüenenthal for research activities (to her institution) and advisory and lecture fees from Grüenenthal, MSD Sharp & DOHME GmbH, Mundipharma GmbH, Mundipharma International, Janssen‐Cilag GmbH; TAD pharma, Fresenius Kabi and AcelRx. Currently, EPZ receives scientific support from the DFG (PO1319/3‐1, PO1319/4‐1 and PO1319/5‐1), the BMBF and the EU/Innovative Medicines Initiative 2 (777,500, includes EFPIA).
ACKNOWLEDGEMENT
Dr. Daniel Segelcke, (postdoc, Translational Research Group, Muenster, Germany) is acknowledged for intellectual and practical contributions to Figure 2.
Mouraux A, Bannister K, Becker S, et al. Challenges and opportunities in translational pain research – An opinion paper of the working group on translational pain research of the European pain federation (EFIC). Eur J Pain. 2021;25:731–756. 10.1002/ejp.1730
REFERENCES
- Ackerley, R. , & Watkins, R. H. (2018). Microneurography as a tool to study the function of individual C‐fiber afferents in humans: Responses from nociceptors, thermoreceptors, and mechanoreceptors. Journal of Neurophysiology, 120, 2834–2846. 10.1152/jn.00109.2018 [DOI] [PubMed] [Google Scholar]
- Alonso‐Ruiz, A. , Pijoan, J. I. , Ansuategui, E. , Urkaregi, A. , Calabozo, M. , & Quintana, A. (2008). Tumor necrosis factor alpha drugs in rheumatoid arthritis: Systematic review and metaanalysis of efficacy and safety. BMC Musculoskeletal Disorders, 9, 52. 10.1186/1471-2474-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirmohseni, S. , Segelcke, D. , Reichl, S. , Wachsmuth, L. , Görlich, D. , Faber, C. , & Pogatzki‐Zahn, E. (2016). Characterization of incisional and inflammatory pain in rats using functional tools of MRI. NeuroImage, 127, 110–122. 10.1016/j.neuroimage.2015.11.052 [DOI] [PubMed] [Google Scholar]
- Andersen, H. , Arendt‐Nielsen, L. , Svensson, P. , Danneskiold‐Samsøe, B. , & Graven‐Nielsen, T. (2008). Spatial and temporal aspects of muscle hyperalgesia induced by nerve growth factor in humans. Experimental Brain Research, 191, 371–382. 10.1007/s00221-008-1531-5 [DOI] [PubMed] [Google Scholar]
- Andersen, O. K. , Sonnenborg, F. A. , & Arendt‐Nielsen, L. (2001). Reflex receptive fields for human withdrawal reflexes elicited by non‐painful and painful electrical stimulation of the foot sole. Clinical Neurophysiology, 112, 641–649. 10.1016/S1388-2457(01)00485-0 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Curatolo, M. , & Drewes, A. (2007). Human experimental pain models in drug development: Translational pain research. Current Opinion in Investigational Drugs, 8, 41–53. [PubMed] [Google Scholar]
- Atlas, L. Y. , & Wager, T. D. (2012). How expectations shape pain. Neuroscience Letters, 520, 140–148. 10.1016/j.neulet.2012.03.039 [DOI] [PubMed] [Google Scholar]
- Attal, N. , & Bouhassira, D. (2019). Translational neuropathic pain research. Pain, 160, S23–S28. 10.1097/j.pain.0000000000001522 [DOI] [PubMed] [Google Scholar]
- Attal, N. , Bouhassira, D. , & Baron, R. (2018). Diagnosis and assessment of neuropathic pain through questionnaires. The Lancet Neurology, 17, 456–466. 10.1016/S1474-4422(18)30071-1 [DOI] [PubMed] [Google Scholar]
- Backonja, M. M. (2012). Neuropathic pain therapy: From bench to bedside. Seminars in Neurology, 32, 264–268. 10.1055/s-0032-1329204 [DOI] [PubMed] [Google Scholar]
- Baidya, D. K. , Agarwal, A. , Khanna, P. , & Arora, M. K. (2011). Pregabalin in acute and chronic pain. J Anaesthesiol Clin Pharmacol, 27, 307–314. 10.4103/0970-9185.83672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki, M. N. , Chialvo, D. R. , Geha, P. Y. , Levy, R. M. , Harden, R. N. , Parrish, T. B. , & Apkarian, A. V. (2006). Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. Journal of Neuroscience, 26, 12165–12173. 10.1523/JNEUROSCI.3576-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister, K. , Lockwood, S. , Goncalves, L. , Patel, R. , & Dickenson, A. H. (2017). An investigation into the inhibitory function of serotonin in diffuse noxious inhibitory controls in the neuropathic rat. Eur J Pain (United Kingdom), 21, 750–760. 10.1002/ejp.979 [DOI] [PubMed] [Google Scholar]
- Bannister, K. , Patel, R. , Goncalves, L. , Townson, L. , & Dickenson, A. H. (2015). Diffuse noxious inhibitory controls and nerve injury: Restoring an imbalance between descending monoamine inhibitions and facilitations. Pain, 156, 1803–1811. 10.1097/j.pain.0000000000000240 [DOI] [PubMed] [Google Scholar]
- Bannister, K. , Qu, C. , Navratilova, E. , Oyarzo, J. , Xie, J. Y. , King, T. , Dickenson, A. H. , & Porreca, F. (2017). Multiple sites and actions of gabapentin‐induced relief of ongoing experimental neuropathic pain. Pain, 158, 2386–2395. 10.1097/j.pain.0000000000001040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, R. , Maier, C. , Attal, N. , Binder, A. , Bouhassira, D. I. , Cruccu, G. , Finnerup, N. B. , Haanpaä, M. , Hansson, P. , Hüllemann, P. , Jensen, T. S. , Freynhagen, R. , Kennedy, J. D. , Magerl, W. , Mainka, T. , Reimer, M. , Rice, A. S. C. , Segerdahl, M. , Serra, J. , … Treede, R. D. (2017). Peripheral neuropathic pain: A mechanism‐related organizing principle based on sensory profiles. Pain, 158, 261–272. 10.1097/j.pain.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley, E. , & Fillingim, R. (2013). Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia, 111, 52–58. 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, A. , Damasio, H. , & Damasio, A. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10, 295–307. 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- Becker, S. , Fuchs, X. , Schakib‐Ekbatan, K. , & Schweiker, M. (2020). What does “moderate pain” mean? Subgroups holding different conceptions of rating scales evaluate experimental pain differently. European Journal of Pain (United Kingdom), 24, 625–638. 10.1002/ejp.1514 [DOI] [PubMed] [Google Scholar]
- Becker, S. , Navratilova, E. , Nees, F. , & Van Damme, S. (2018). Emotional and motivational pain processing: Current state of knowledge and perspectives in translational research. Pain Research and Management, 2018, 5457870. 10.1155/2018/5457870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee, L. A. , Bannister, K. , Rahman, W. , & Dickenson, A. H. (2011). Mu‐opioid and noradrenergic α2‐adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve‐injured rats. Pain, 152, 131–139. 10.1016/j.pain.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Bellavance, L. , & Beitz, A. (1996). Altered C‐fos expression in the Parabrachial nucleus in a rodent model of CFA‐induced peripheral inflammation. The Journal of Comparative Neurology, 366, 431–447. [DOI] [PubMed] [Google Scholar]
- Besné, I. , Descombes, C. , & Breton, L. (2002). Effect of age and anatomical site on density of sensory innervation in human epidermis. Archives of Dermatology, 138, 1445–1450. 10.1001/archderm.138.11.1445 [DOI] [PubMed] [Google Scholar]
- Binder, A. (2016). Human surrogate models of neuropathic pain. Pain, 157(Supplement 1), S48–S52. [DOI] [PubMed] [Google Scholar]
- Bingel, U. , & Tracey, I. (2008). Imaging CNS modulation of pain in humans. Physiology, 23, 371–380. 10.1152/physiol.00024.2008 [DOI] [PubMed] [Google Scholar]
- Bingel, U. , Wanigasekera, V. , Wiech, K. , Mhuircheartaigh, R. N. , Lee, M. C. , Ploner, M. , & Tracey, I. (2011). The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Science Translational Medicine, 3, 70ra14. 10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- Biurrun Manresa, J. A. , Neziri, A. Y. , Curatolo, M. , Arendt‐Nielsen, L. , & Andersen, O. K. (2013). Reflex receptive fields are enlarged in patients with musculoskeletal low back and neck pain. Pain, 154, 1318–1324. 10.1016/j.pain.2013.04.013 [DOI] [PubMed] [Google Scholar]
- Borsook, D. , Linnman, C. , Faria, V. , Strassman, A. M. , Becerra, L. , & Elman, I. (2016). Reward deficiency and anti‐reward in pain chronification. Neuroscience and Biobehavioral Reviews, 68, 282–297. 10.1016/j.neubiorev.2016.05.033 [DOI] [PubMed] [Google Scholar]
- Bosma, R. L. , Ameli Mojarad, E. , Leung, L. , Pukall, C. , Staud, R. , & Stroman, P. W. (2015). Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Human Brain Mapping, 36, 5038–5050. 10.1002/hbm.22993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock, H. , Cikurel, K. , & Burke, D. (1998). Threshold tracking techniques in the study of human peripheral nerve. Muscle and Nerve, 21, 137–158. [DOI] [PubMed] [Google Scholar]
- Bräscher, A. K. , Becker, S. , Hoeppli, M. E. , & Schweinhardt, P. (2016). Different brain circuitries mediating controllable and uncontrollable pain. Journal of Neuroscience, 36, 5013–5025. 10.1523/JNEUROSCI.1954-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bree, D. , Moriarty, O. , O’Mahony, C. M. , Morris, B. , Bannerton, K. , Broom, D. C. , Kelly, J. P. , Roche, M. , & Finn, D. P. (2015). Development and characterization of a novel, anatomically relevant rat model of acute postoperative pain. The Journal of Pain, 16, 421–435. 10.1016/j.jpain.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Brennan, T. J. (1999). Postoperative models of nociception. ILAR Journal, 40, 129–136. 10.1093/ilar.40.3.129 [DOI] [PubMed] [Google Scholar]
- Bromm, B. , & Treede, R. D. (1987). Human cerebral potentials evoked by CO2 laser stimuli causing pain. Experimental Brain Research, 67, 153–162. 10.1007/BF00269463 [DOI] [PubMed] [Google Scholar]
- Burke, N. N. , Finn, D. P. , McGuire, B. E. , & Roche, M. (2017). Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. Journal of Neuroscience Research, 95, 1257–1270. [DOI] [PubMed] [Google Scholar]
- Burke, N. N. , Finn, D. P. , & Roche, M. (2015). Neuroinflammatory mechanisms linking pain and depression. Modern Trends in Pharmacopsychiatry, 30, 36–50. [DOI] [PubMed] [Google Scholar]
- Bushnell, M. C. , Case, L. K. , Ceko, M. , Cotton, V. A. , Gracely, J. L. , Low, L. A. , Pitcher, M. H. , & Villemure, C. (2015). Effect of environment on the long‐term consequences of chronic pain. Pain, 156, S42–S49. 10.1097/01.j.pain.0000460347.77341.bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, M. C. , Čeko, M. , & Low, L. A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience, 14, 502–511. 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, R. K. , & Finn, D. P. (2009). Stress‐induced analgesia. Progress in Neurobiology, 88, 184–202. 10.1016/j.pneurobio.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Buvanendran, A. , Kroin, J. S. , Kari, M. R. , & Tuman, K. J. (2008). A new knee surgery model in rats to evaluate functional measures of postoperative pain. Anesthesia and Analgesia, 107, 300–308. 10.1213/ane.0b013e3181732f21 [DOI] [PubMed] [Google Scholar]
- Buvanendran, A. , Kroin, J. S. , Kerns, J. M. , Nagalla, S. N. K. , & Tuman, K. J. (2004). Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesthesia and Analgesia, 99, 1453–1460. 10.1213/01.ANE.0000134806.61887.0D [DOI] [PubMed] [Google Scholar]
- Chakour, M. C. , Gibson, S. J. , Bradbeer, M. , & Helme, R. D. (1996). The effect of age on Aδ‐ and C‐fibre thermal pain perception. Pain, 64, 143–152. 10.1016/0304-3959(95)00102-6 [DOI] [PubMed] [Google Scholar]
- Churyukanov, M. , Plaghki, L. , Legrain, V. , Mouraux, A. , & El‐Deredy, W. (2012). Thermal detection thresholds of Aδ‐ and C‐fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PLoS One, 7, e35817. 10.1371/journal.pone.0035817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon, E. , Liberati, G. , & Mouraux, A. (2017). EEG frequency tagging using ultra‐slow periodic heat stimulation of the skin reveals cortical activity specifically related to C fiber thermonociceptors. NeuroImage, 146, 266–274. 10.1016/j.neuroimage.2016.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. , & Bushnell, M. C. (1994). The thermal grill illusion: Unmasking the burn of cold pain. Science (80‐, ), 265, 252–255. 10.1126/science.8023144 [DOI] [PubMed] [Google Scholar]
- Cruccu, G. , Iannetti, G. , Marx, J. , Thoemke, F. , Truini, A. , Fitzek, S. , Galeotti, F. , Urban, P. , Romaniello, A. , Stoeter, P. , Manfredi, M. , & Hopf, H. (2005). Brainstem reflex circuits revisited. Brain, 128, 386–394. 10.1093/brain/awh366 [DOI] [PubMed] [Google Scholar]
- Cummins, T. M. , Kucharczyk, M. M. , Graven‐Nielsen, T. , & Bannister, K. (2020). Activation of the descending pain modulatory system using cuff pressure algometry: Back translation from man to rat. European Journal of Pain, 24, 1330–1338. 10.1002/ejp.1580 [DOI] [PubMed] [Google Scholar]
- Dannecker, E. A. , George, S. Z. , & Robinson, M. E. (2007). Influence and stability of pain scale anchors for an investigation of cold pressor pain tolerance. The Journal of Pain, 8, 476–482. 10.1016/j.jpain.2007.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, K. D. , Aghaeepour, N. , Ahn, A. H. , Angst, M. S. , Borsook, D. , Brenton, A. , Burczynski, M. E. , Crean, C. , Edwards, R. , Gaudilliere, B. , Hergenroeder, G. W. , Iadarola, M. J. , Iyengar, S. , Jiang, Y. , Kong, J. T. , Mackey, S. , Saab, C. Y. , Sang, C. N. , Scholz, J. , … Pelleymounter, M. A. (2020). Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nature Reviews. Neurology, 16, 381–400. 10.1038/s41582-020-0362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, K. D. , Flor, H. , Greely, H. T. , Iannetti, G. D. , MacKey, S. , Ploner, M. , Pustilnik, A. , Tracey, I. , Treede, R. D. , & Wager, T. D. (2017). Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nature Reviews Neurology, 13, 624–638. 10.1038/nrneurol.2017.122 [DOI] [PubMed] [Google Scholar]
- De Martino, E. , Petrini, L. , Schabrun, S. , & Graven‐Nielsen, T. (2018). Cortical somatosensory excitability is modulated in response to several days of muscle soreness. The Journal of Pain, 19, 1296–1307. 10.1016/j.jpain.2018.05.004 [DOI] [PubMed] [Google Scholar]
- De Martino, E. , Seminowicz, D. A. , Schabrun, S. M. , Petrini, L. , & Graven‐Nielsen, T. (2019). High frequency repetitive transcranial magnetic stimulation to the left dorsolateral prefrontal cortex modulates sensorimotor cortex function in the transition to sustained muscle pain. NeuroImage, 186, 93–102. 10.1016/j.neuroimage.2018.10.076 [DOI] [PubMed] [Google Scholar]
- De Martino, E. , Zandalasini, M. , Schabrun, S. , Petrini, L. , & Graven‐Nielsen, T. (2018). Experimental muscle hyperalgesia modulates sensorimotor cortical excitability, which is partially altered by unaccustomed exercise. Pain, 159, 2493–2502. 10.1097/j.pain.0000000000001351 [DOI] [PubMed] [Google Scholar]
- Defrin, R. , Amanzio, M. , De Tommaso, M. , Dimova, V. , Filipovic, S. , Finn, D. P. , Gimenez‐Llort, L. , Invitto, S. , Jensen‐Dahm, C. , Lautenbacher, S. , Oosterman, J. M. , Petrini, L. , Pick, C. G. , Pickering, G. , Vase, L. , & Kunz, M. (2015). Experimental pain processing in individuals with cognitive impairment: Current state of the science. Pain, 156, 1396–1408. 10.1097/j.pain.0000000000000195 [DOI] [PubMed] [Google Scholar]
- Demant, D. T. , Lund, K. , Vollert, J. , Maier, C. , Segerdahl, M. , Finnerup, N. B. , Jensen, T. S. , & Sindrup, S. H. (2014). The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double‐blind, placebo‐controlled phenotype‐stratified study. Pain, 155, 2263–2273. 10.1016/j.pain.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Deshields, T. L. , Tait, R. C. , Gfeller, J. D. , & Chibnall, J. T. (1995). Relationship between social desirability and self‐report in chronic pain patients. Clinical Journal of Pain, 11, 189–193. 10.1097/00002508-199509000-00005 [DOI] [PubMed] [Google Scholar]
- Doménech‐García, V. , Palsson, T. S. , Herrero, P. , & Graven‐Nielsen, T. (2016). Pressure‐induced referred pain is expanded by persistent soreness. Pain, 157, 1164–1172. 10.1097/j.pain.0000000000000497 [DOI] [PubMed] [Google Scholar]
- Drewes, A. M. , Gregersen, H. , & Arendt‐Nielsen, L. (2003). Experimental pain in gastroenterology: A reappraisal of human studies. Scandinavian Journal of Gastroenterology, 38, 1115–1130. 10.1080/00365520310004399 [DOI] [PubMed] [Google Scholar]
- Drewes, A. M. , Schipper, K. P. , Dimcevski, G. , Petersen, P. , Gregersen, H. , Funch‐Jensen, P. , & Arendt‐Nielsen, L. (2003). Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain, 104, 333–341. 10.1016/S0304-3959(03)00039-3 [DOI] [PubMed] [Google Scholar]
- Edwards, R. R. , Dworkin, R. H. , Sullivan, M. D. , Turk, D. C. , & Wasan, A. D. (2016). The role of psychosocial processes in the development and maintenance of chronic pain. The Journal of Pain, 17, T70–T92. 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert, F. , Bingel, U. , Schoell, E. D. , Yacubian, J. , Klinger, R. , Lorenz, J. , & Büchel, C. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron, 63, 533–543. 10.1016/j.neuron.2009.07.014 [DOI] [PubMed] [Google Scholar]
- Eippert, F. , Finsterbusch, J. , Bingel, U. , & Büchel, C. (2009). Direct evidence for spinal cord involvement in Placebo Analgesia. Science (80‐, ) 326, 404. 10.1126/science.1180142 [DOI] [PubMed] [Google Scholar]
- Elman, I. , & Borsook, D. (2018). Threat response system: Parallel brain processes in pain vis‐à‐vis fear and anxiety. Frontiers in Psychiatry, 9, 29. 10.3389/fpsyt.2018.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch, S. , Rosenberger, C. , Bingel, U. , Forsting, M. , Schedlowski, M. , & Gizewski, E. R. (2010). Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology, 139, 1310–1319. 10.1053/j.gastro.2010.06.054 [DOI] [PubMed] [Google Scholar]
- Finan, P. H. , Goodin, B. R. , & Smith, M. T. (2013). The association of sleep and pain: An update and a path forward. The Journal of Pain, 14, 1539–1552. 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2016). Guideline for the Clinical development of medicinal products intended for the treatment of pain. EMA/CHMP/970057/2011. Available at https://www.ema.europa.eu/en/clinical‐development‐medicinal‐products‐intended‐treatment‐pain
- Finnerup, N. B. , Gottrup, H. , & Jensen, T. S. (2002). Anticonvulsants in central pain. Expert Opinion on Pharmacotherapy, 3, 1411–1420. 10.1517/14656566.3.10.1411 [DOI] [PubMed] [Google Scholar]
- Fißmer, I. , Klein, T. , Magerl, W. , Treede, R. D. , Zahn, P. K. , & Pogatzki‐Zahn, E. M. (2011). Modality‐specific somatosensory changes in a human surrogate model of postoperative pain. Anesthesiology, 115, 387–397. 10.1097/ALN.0b013e318219509e [DOI] [PubMed] [Google Scholar]
- Fitzgibbon, M. , Finn, D. P. , & Roche, M. (2016). High times for painful blues: The endocannabinoid system in pain‐depression comorbidity. International Journal of Neuropsychopharmacology, 19, pyv095. 10.1093/ijnp/pyv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters, S. J. L. (2008). Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain, 135, 119–130. 10.1016/j.pain.2007.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, D. , Stamer, U. M. , Pogatzki‐Zahn, E. , Zaslansky, R. , Tanase, N. V. , Perruchoud, C. , Kranke, P. , Komann, M. , Lehman, T. , Lavand homme, P. , Vercauteren, M. , Meissner, W. , Iohom, G. , Cinnella, G. , Aurilio, C. , Belii, A. , Filipescu, D. , Rehberg‐Klug, B. , Decostered, I. , Suter, M. R. , Blumenthal, S. , Puig, M. , Garcia‐Filoso, A. , Brandner, B. , Varvinskiy, A. M. , Lisnyy, I. , & Kuchyn, I. (2015). Chronic postsurgical pain in Europe: An observational study. European Journal of Anaesthesiology, 32, 725–734. 10.1097/EJA.0000000000000319 [DOI] [PubMed] [Google Scholar]
- Frot, M. , Magnin, M. , Mauguière, F. , & Garcia‐Larrea, L. (2007). Human SII and posterior insula differently encode thermal laser stimuli. Cerebral Cortex, 17, 610–620. 10.1093/cercor/bhk007 [DOI] [PubMed] [Google Scholar]
- Gabriel, A. F. , Paoletti, G. , Seta, D. D. , Panelli, R. , Marcus, M. A. E. , Farabollini, F. , Carli, G. , & Joosten, E. A. J. (2010). Enriched environment and the recovery from inflammatory pain: Social versus physical aspects and their interaction. Behavioral Brain Research, 208, 90–95. 10.1016/j.bbr.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Galambos, A. , Szabó, E. , Nagy, Z. , Édes, A. E. , Kocsel, N. , Juhász, G. , & Kökönyei, G. (2019). A systematic review of structural and functional MRI studies on pain catastrophizing. Journal of Pain Research, 12, 1155–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneen, L. J. , Moore, R. A. , Clarke, C. , Martin, D. , Colvin, L. A. , & Smith, B. H. (2017). Physical activity and exercise for chronic pain in adults: An overview of Cochrane reviews. Cochrane Database Systematic Review, 1, CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter, S. , & Büchel, C. (2013). Facilitation of pain in the human spinal cord by nocebo treatment. Journal of Neuroscience, 33, 13784–13790. 10.1523/JNEUROSCI.2191-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, S. J. (2007). IASP global year against pain in older persons: Highlighting the current status and future perspectives in geriatric pain. Expert Review of Neurotherapeutics, 7, 627–635. 10.1586/14737175.7.6.627 [DOI] [PubMed] [Google Scholar]
- Gigliuto, C. , De Gregori, M. , Malafoglia, V. , Raffaeli, W. , Compagnone, C. , Visai, L. , Petrini, P. , Avanzini, M. A. , Muscoli, C. , Viganò, J. , Calabrese, F. , Dominioni, T. , Allegri, M. , & Cobianchi, L. (2014). Pain assessment in animal models: Do we need further studies? J Pain Res, 7, 227–236. 10.2147/JPR.S59161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron, I. , Kehlet, H. , & Pogatzki‐Zahn, E. (2019). Current status and future directions of pain‐related outcome measures for post‐surgical pain trials. Canadian Journal of Pain, 3, 36–43. 10.1080/24740527.2019.1583044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenberg, A. M. (2010). Embodiment as a unifying perspective for psychology. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 586–596. 10.1002/wcs.55 [DOI] [PubMed] [Google Scholar]
- Gomtsian, L. , Bannister, K. , Eyde, N. , Robles, D. , Dickenson, A. H. , Porreca, F. , & Navratilova, E. (2018). Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain, 159, 2512–2521. 10.1097/j.pain.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely, R. H. (1990). Measuring pain in the clinic. Anesthesia Progress, 37, 88–92. [PMC free article] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. (2006). Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scandinavian Journal of Rheumatology, 35, 1–43. 10.1080/03009740600865980 [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. , & Arendt‐Nielsen, L. (2010). Assessment of mechanisms in localized and widespread musculoskeletal pain. Nature Reviews Rheumatology, 6, 599–606. 10.1038/nrrheum.2010.107 [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. , Arendt‐Nielsen, L. , & Mense, S. (2002). Thermosensitivity of muscle: High‐intensity thermal stimulation of muscle tissue induces muscle pain in humans. Journal of Physiology, 540, 647–656. 10.1113/jphysiol.2001.013336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. , Izumi, M. , Petersen, K. K. , & Arendt‐Nielsen, L. (2017). User‐independent assessment of conditioning pain modulation by cuff pressure algometry. European Journal of Pain, 21, 552–561. 10.1002/ejp.958 [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. , Jansson, Y. , Segerdahl, M. , Kristensen, J. D. , Mense, S. , Arendt‐Nielsen, L. , & Sollevi, A. (2003). Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. European Journal of Pain, 7, 93–102. 10.1016/S1090-3801(02)00069-1 [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen, T. , Mense, S. , & Arendt‐Nielsen, L. (2004). Painful and non‐painful pressure sensations from human skeletal muscle. Experimental Brain Research, 159, 273–283. 10.1007/s00221-004-1937-7 [DOI] [PubMed] [Google Scholar]
- Gregory, N. S. , Harris, A. L. , Robinson, C. R. , Dougherty, P. M. , Fuchs, P. N. , & Sluka, K. A. (2013). An overview of animal models of pain: Disease models and outcome measures. The Journal of Pain, 14, 1255–1269. 10.1016/j.jpain.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Schnitzler, A. , Timmermann, L. , & Ploner, M. (2007). Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biology, 5, 1168–1173. 10.1371/journal.pbio.0050133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, T. , Bian, Z. , Trocki, K. , Chen, L. , Zheng, G. , & Feng, B. (2019). Optical recording reveals topological distribution of functionally classified colorectal afferent neurons in intact lumbosacral DRG. Physiological Reports, 7, e14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman, R. E. , Evans, M. G. , Bove, S. , Morenko, B. , & Kilgore, K. (2003). Mono‐iodoacetate‐induced histologic changes in subchondral bone and articular cartilage of rat Femorotibial joints: An animal model of osteoarthritis. Toxicologic Pathology, 31, 619–624. 10.1080/01926230390241800 [DOI] [PubMed] [Google Scholar]
- Hagen, M. , & Alchin, J. (2020). Nonprescription drugs recommended in guidelines for common pain conditions. Pain Management, 10, 117–129. 10.2217/pmt-2019-0057 [DOI] [PubMed] [Google Scholar]
- Haroutiunian, S. , Nikolajsen, L. , Finnerup, N. B. , & Jensen, T. S. (2013). The neuropathic component in persistent postsurgical pain: A systematic literature review. Pain, 154, 95–102. 10.1016/j.pain.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Harris, R. E. , Napadow, V. , Huggins, J. P. , Pauer, L. , Kim, J. , Hampson, J. , Sundgren, P. C. , Foerster, B. , Petrou, M. , Schmidt‐Wilcke, T. , & Clauw, D. J. (2013). Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology, 119, 1453–1464. 10.1097/ALN.0000000000000017 [DOI] [PubMed] [Google Scholar]
- Hayashi, K. , Shiozawa, S. , Ozaki, N. , Mizumura, K. , & Graven‐Nielsen, T. (2013). Repeated intramuscular injections of nerve growth factor induced progressive muscle hyperalgesia, facilitated temporal summation, and expanded pain areas. Pain, 154, 2344–2352. 10.1016/j.pain.2013.07.007 [DOI] [PubMed] [Google Scholar]
- He, Y. , Tian, X. , Hu, X. , Porreca, F. , & Wang, Z. J. (2012). Negative reinforcement reveals non‐evoked ongoing pain in mice with tissue or nerve injury. The Journal of Pain, 13, 598–607. 10.1016/j.jpain.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heft, M. W. , Gracely, R. H. , Dubner, R. , & McGrath, P. A. (1980). A validation model for verbal descriptor scaling of human clinical pain. Pain, 9, 363–373. 10.1016/0304-3959(80)90050-0 [DOI] [PubMed] [Google Scholar]
- Hill, J. C. , Dunn, K. M. , Main, C. J. , & Hay, E. M. (2010). Subgrouping low back pain: A comparison of the STarT back Tool with the Örebro musculoskeletal Pain screening questionnaire. European Journal of Pain, 14, 83–89. 10.1016/j.ejpain.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, R. (2000). NK1 (substance P) receptor antagonists ‐ Why are they not analgesic in humans? Trends in Pharmacological Sciences, 21, 244–246. 10.1016/S0165-6147(00)01502-9 [DOI] [PubMed] [Google Scholar]
- Hodkinson, D. J. , Krause, K. , Khawaja, N. , Renton, T. F. , Huggins, J. P. , Vennart, W. , Thacker, M. A. , Mehta, M. A. , Zelaya, F. O. , Williams, S. C. R. , & Howard, M. A. (2013). Quantifying the test‐retest reliability of cerebral blood flow measurements in a clinical model of on‐going post‐surgical pain: A study using pseudo‐continuous arterial spin labelling. NeuroImage Clinical, 3, 301–310. 10.1016/j.nicl.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel, U. , Reuter, R. , De Freitas, M. F. , Treede, R. D. , & Mense, S. (2013). Injection of nerve growth factor into a low back muscle induces long‐lasting latent hypersensitivity in rat dorsal horn neurons. Pain, 154, 1953–1960. 10.1016/j.pain.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Howard, M. A. , Krause, K. , Khawaja, N. , Massat, N. , Zelaya, F. , Schumann, G. , Huggins, J. P. , Vennart, W. , Williams, S. C. R. , & Renton, T. F. (2011). Beyond patient reported pain: Perfusion magnetic resonance imaging demonstrates reproducible cerebral representation of ongoing post‐surgical pain. PLoS One, 6, e17096. 10.1371/journal.pone.0017096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugosdottir, R. , Mørch, C. D. , Jørgensen, C. K. , Nielsen, C. W. , Olsen, M. V. , Pedersen, M. J. , & Tigerholm, J. (2019). Altered excitability of small cutaneous nerve fibers during cooling assessed with the perception threshold tracking technique. BMC Neuroscience, 20, 47. 10.1186/s12868-019-0527-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humo, M. , Lu, H. , & Yalcin, I. (2019). The molecular neurobiology of chronic pain–induced depression. Cell and Tissue Research, 377, 21–43. 10.1007/s00441-019-03003-z [DOI] [PubMed] [Google Scholar]
- Iannetti, G. D. , Baumgärtner, U. , Tracey, I. , Treede, R. D. , & Magerl, W. (2013). Pinprick‐evoked brain potentials: A novel tool to assess central sensitization of nociceptive pathways in humans. Journal of Neurophysiology, 110, 1107–1116. 10.1152/jn.00774.2012 [DOI] [PubMed] [Google Scholar]
- Icenhour, A. , Tapper, S. , Bednarska, O. , Witt, S. T. , Tisell, A. , Lundberg, P. , Elsenbruch, S. , & Walter, S. (2019). Elucidating the putative link between prefrontal neurotransmission, functional connectivity, and affective symptoms in irritable bowel syndrome. Scientific Reports, 9, 13590. 10.1038/s41598-019-50024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui, K. , Tran, T. D. , Hoshiyama, M. , & Kakigi, R. (2002). Preferential stimulation of Aδ fibers by intra‐epidermal needle electrode in humans. Pain, 96, 247–252. 10.1016/S0304-3959(01)00453-5 [DOI] [PubMed] [Google Scholar]
- Isose, S. , Misawa, S. , Sakurai, K. , Kanai, K. , Shibuya, K. , Sekiguchi, Y. , Nasu, S. , Noto, Y. , Fujimaki, Y. , Yokote, K. , & Kuwabara, S. (2010). Mexiletine suppresses nodal persistent sodium currents in sensory axons of patients with neuropathic pain. Clinical Neurophysiology, 121, 719–724. 10.1016/j.clinph.2009.12.034 [DOI] [PubMed] [Google Scholar]
- Issberner, U. , Reeh, P. W. , & Steen, K. H. (1996). Pain due to tissue acidosis: A mechanism for inflammatory and ischemic myalgia? Neuroscience Letters, 208, 191–194. 10.1016/0304-3940(96)12576-3 [DOI] [PubMed] [Google Scholar]
- Jennings, E. M. , Okine, B. N. , Roche, M. , & Finn, D. P. (2014). Stress‐induced hyperalgesia. Progress in Neurobiology, 121, 1–18. 10.1016/j.pneurobio.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Jia, Z. , Tang, W. , Zhao, D. , & Yu, S. (2017). Disrupted functional connectivity between the periaqueductal gray and other brain regions in a rat model of recurrent headache. Scientific Reports, 7, 3960. 10.1038/s41598-017-04060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, D. , Pickering, G. , Marchand, F. , Gaulier, J. M. , Alliot, J. , & Eschalier, A. (2002). Impact of ageing on the antinociceptive effect of reference analgesics in the Lou/c rat. British Journal of Pharmacology, 137, 813–820. 10.1038/sj.bjp.0704944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jure, F. A. , Arguissain, F. G. , Biurrun Manresa, J. A. , & Andersen, O. K. (2019). Conditioned pain modulation affects the withdrawal reflex pattern to nociceptive stimulation in humans. Neuroscience, 408, 259–271. 10.1016/j.neuroscience.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Just, N. , Segelcke, D. , Sandbrink, M. , Pogatzki‐Zahn, E. , & Faber, C. (2019). Development of a stimulator for the characterization of mechanical‐evoked pain‐related supra‐spinal processing using BOLD‐fMRI in rodents. IEEE Transactions on Biomedical Engineering, 67, 1349–1356. 10.1109/TBME.2019.2936571 [DOI] [PubMed] [Google Scholar]
- Kiernan, M. C. , Bostock, H. , Park, S. B. , Kaji, R. , Krarup, C. , Krishnan, A. V. , Kuwabara, S. , Lin, C. S. Y. , Misawa, S. , Moldovan, M. , Sung, J. , Vucic, S. , Wainger, B. J. , Waxman, S. , & Burke, D. (2020). Measurement of axonal excitability: Consensus guidelines. Clinical Neurophysiology, 131, 308–323. 10.1016/j.clinph.2019.07.023 [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Chung, J. M. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain, 50, 355–363. 10.1016/0304-3959(92)90041-9 [DOI] [PubMed] [Google Scholar]
- Kim, Y. S. , Anderson, M. , Park, K. , Zheng, Q. , Agarwal, A. , Gong, C. , Saijilafu, Y. L. A. , He, S. , LaVinka, P. C. , Zhou, F. , Bergles, D. , Hanani, M. , Guan, Y. , Spray, D. C. , & Dong, X. (2016). Coupled activation of primary sensory neurons contributes to chronic pain. Neuron, 91, 1085–1096. 10.1016/j.neuron.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T. , Magerl, W. , Hopf, H. C. , Sandkühler, J. , & Treede, R. D. (2004). Perceptual correlates of nociceptive long‐term potentiation and long‐term depression in humans. Journal of Neuroscience, 24, 964–971. 10.1523/JNEUROSCI.1222-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T. , Magerl, W. , Rolke, R. , & Treede, R. D. (2005). Human surrogate models of neuropathic pain. Pain, 115, 227–233. 10.1016/j.pain.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Kosek, E. , Ekholm, J. , & Hansson, P. (1999). Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scandinavian Journal of Rehabilitation Medicine, 31, 89–93. 10.1080/003655099444597 [DOI] [PubMed] [Google Scholar]
- Kucharczyk, M. W. , Chisholm, K. I. , Denk, F. , Dickenson, A. H. , Bannister, K. , & McMahon, S. B. (2020). The impact of bone cancer on the peripheral encoding of mechanical pressure stimuli. Pain, 161, 1894–1905. 10.1097/j.pain.0000000000001880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2015). The dynamic pain connectome. Trends in Neurosciences, 38, 86–95. 10.1016/j.tins.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Langford, D. J. , Langford, D. J. , Bailey, A. L. , Chanda, M. L. , Chanda, M. L. , Clarke, S. E. , Clarke, S. E. , Drummond, T. E. , Drummond, T. E. , Echols, S. , Echols, S. , Glick, S. , Glick, S. , Ingrao, J. C. , Klassen‐Ross, T. , Lacroix‐Fralish, M. L. , Matsumiya, L. , Matsumiya, L. , Sorge, R. E. , … Mogil, J. S. (2010). Coding of facial expressions of pain in the laboratory mouse. Nature Methods, 7, 447–449. 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- Langford, D. J. , Tuttle, A. H. , Briscoe, C. , Harvey‐Lewis, C. , Baran, I. , Gleeson, P. , Fischer, D. B. , Buonora, M. , Sternberg, W. F. , & Mogil, J. S. (2011). Varying perceived social threat modulates pain behavior in male mice. J Pain, 12, 125–132. 10.1016/j.jpain.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Langford, D. J. , Tuttle, A. H. , Brown, K. , Deschenes, S. , Fischer, D. B. , Mutso, A. , Root, K. C. , Sotocinal, S. G. , Stern, M. A. , Mogil, J. S. , & Sternberg, W. F. (2010). Social approach to pain in laboratory mice. Social Neuroscience, 5, 163–170. 10.1080/17470910903216609 [DOI] [PubMed] [Google Scholar]
- Lavand’homme, P. , Wu, C. , Katz, J. (2018). From acute to chronic postoperative pain. In Pain 2018. Refresher Courses, 17th World Congress on Pain, M. Gold, E. Pogatzki‐Zahn, & M. Wallaces (Ed.). (Washington (DC): IASP Press), pp. 147–157.
- Le Bars, D. , Dickenson, A. H. , & Besson, J. M. (1979b). Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain, 6, 283–304. 10.1016/0304-3959(79)90049-6 [DOI] [PubMed] [Google Scholar]
- Le Bars, D. , Dickenson, A. H. , Besson, J. , & marie,, (1979a). Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non‐convergent neurones, supraspinal involvement and theoretical implications. Pain, 6, 305–327. 10.1016/0304-3959(79)90050-2 [DOI] [PubMed] [Google Scholar]
- Leitl, M. D. , & Negus, S. S. (2016). Pharmacological modulation of Neuropathic pain‐related depression of behavior: Effects of morphine, ketoprofen, bupropion and Δ9‐tetrahydrocannabinol on formalin‐induced depression of intracranial self‐stimulation in rats. Behavioural Pharmacology, 27, 364–376. 10.1097/FBP.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchtweis, J. , Segond von Banchet, G. , Eitner, A. , Ebbinghaus, M. , & Schaible, H. G. (2020). Pain‐related behaviors associated with persistence of mechanical hyperalgesia after antigen‐induced arthritis in rats. Pain, 161, 1571–1583. 10.1097/j.pain.0000000000001852 [DOI] [PubMed] [Google Scholar]
- Lewis, G. N. , Rice, D. A. , & McNair, P. J. (2012). Conditioned pain modulation in populations with chronic pain: A systematic review and meta‐analysis. The Journal of Pain, 13, 936–944. 10.1016/j.jpain.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Lockwood, S. M. , Bannister, K. , & Dickenson, A. H. (2019). An investigation into the noradrenergic and serotonergic contributions of diffuse noxious inhibitory controls in a monoiodoacetate model of osteoarthritis. Journal of Neurophysiology, 121, 96–104. 10.1152/jn.00613.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden, V. J. , Bedwell, G. J. , Chikezie, P. C. , Rice, A. S. C. , & Kamerman, P. R. (2019). A systematic review of experimental methods to manipulate secondary hyperalgesia in humans: Protocol. Systematic Reviewss, 8, 208. 10.1186/s13643-019-1120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J. (2012). Current challenges in translational pain research. Trends in Pharmacological Sciences, 33, 568–573. 10.1016/j.tips.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, E. , Narjoz, C. , Decleves, X. , Labat, L. , Lambert, C. , Loriot, M. A. , Ducheix, G. , Dualé, C. , Pereira, B. , & Pickering, G. (2019). Dextromethorphan analgesia in a human experimental model of hyperalgesia. Anesthesiology, 131, 356–368. 10.1097/ALN.0000000000002736 [DOI] [PubMed] [Google Scholar]
- Martin, T. J. , Buechler, N. L. , Kahn, W. , Crews, J. C. , & Eisenach, J. C. (2004). Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: A model for postoperative pain. Anesthesiology, 101, 191–203. 10.1097/00000542-200407000-00030 [DOI] [PubMed] [Google Scholar]
- Martinez, V. , Pichard, X. , & Fletcher, D. (2017). Perioperative pregabalin administration does not prevent chronic postoperative pain: Systematic review with a meta‐analysis of randomized trials. Pain, 158, 775–783. 10.1097/j.pain.0000000000000838 [DOI] [PubMed] [Google Scholar]
- Matthews, E. A. , & Dickenson, A. H. (2002). A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology, 96, 633–640. 10.1097/00000542-200203000-00020 [DOI] [PubMed] [Google Scholar]
- Mayer, E. A. , Labus, J. , Aziz, Q. , Tracey, I. , Kilpatrick, L. , Elsenbruch, S. , Schweinhardt, P. , Van Oudenhove, L. , & Borsook, D. (2019). Role of brain imaging in disorders of brain‐gut interaction: A Rome Working Team Report. Gut, 68, 1701–1715. 10.1136/gutjnl-2019-318308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee, M. E. , & Graven‐Nielsen, T. (2019). Recurrent low back pain patients demonstrate facilitated pronociceptive mechanisms when in pain, and impaired antinociceptive mechanisms with and without pain. Pain, 160, 2866–2876. 10.1097/j.pain.0000000000001679 [DOI] [PubMed] [Google Scholar]
- McPhee, M. E. , Vaegter, H. B. , & Graven‐Nielsen, T. (2020). Alterations in pronociceptive and antinociceptive mechanisms in patients with low back pain: A systematic review with meta‐analysis. Pain, 161, 464–475. 10.1097/j.pain.0000000000001737 [DOI] [PubMed] [Google Scholar]
- Meints, S. M. , & Edwards, R. R. (2018). Evaluating psychosocial contributions to chronic pain outcomes. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 87, 168–182. 10.1016/j.pnpbp.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, C. , Denis, C. M. , & Berquin, A. D. (2018). Secondary prevention of chronic musculoskeletal pain: A systematic review of clinical trials. Annals of Physical and Rehabilitation Medicine, 61, 323–338. 10.1016/j.rehab.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Meyer, R. A. , & Campbell, J. N. (1981). Evidence for two distinct classes of unmyelinated nociceptive afferents in monkey. Brain Research, 224, 149–152. 10.1016/0006-8993(81)91124-0 [DOI] [PubMed] [Google Scholar]
- Mogil, J. S. (2009). Animal models of pain: Progress and challenges. Nature Reviews Neuroscience, 10, 283–294. 10.1038/nrn2606 [DOI] [PubMed] [Google Scholar]
- Mogil, J. S. (2012). Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience, 13, 859–866. 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- Morel, V. , Etienne, M. , Wattiez, A. S. , Dupuis, A. , Privat, A. M. , Chalus, M. , Eschalier, A. , Daulhac, L. , & Pickering, G. (2013). Memantine, a promising drug for the prevention of neuropathic pain in rat. European Journal of Pharmacology, 721, 382–390. 10.1016/j.ejphar.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Morel, V. , Joly, D. , Villatte, C. , Dubray, C. , Durando, X. , Daulhac, L. , Coudert, C. , Roux, D. , Pereira, B. , & Pickering, G. (2016). Memantine before mastectomy prevents post‐surgery pain: A randomized, blinded clinical trial in surgical patients. PLoS One, 11, e0152741. 10.1371/journal.pone.0152741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty, O. , McGuire, B. E. , & Finn, D. P. (2011). The effect of pain on cognitive function: A review of clinical and preclinical research. Progress in Neurobiology, 93, 385–404. 10.1016/j.pneurobio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Mouraux, A. (2019). Electrophysiologic techniques to study the supraspinal responses to nociceptive input in humans. In Pain after Surgery, D.B. Carr, L. Arendt‐Nielsen, and K. Vissers, eds. (IASP Press), pp. 133–151.
- Mouraux, A. , Iannetti, G. D. , & Plaghki, L. (2010). Low intensity intra‐epidermal electrical stimulation can activate Aδ‐nociceptors selectively. Pain, 150, 199–207. 10.1016/j.pain.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Nakae, A. , Nakai, K. , Yano, K. , Hosokawa, K. , Shibata, M. , & Mashimo, T. (2011). The animal model of spinal cord injury as an experimental pain model. Journal of Biomedicine & Biotechnology, 2011, 939023. 10.1155/2011/939023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham, D. J. , Mills, K. R. , Quigley, B. M. , & Edwards, R. H. T. (1983). Pain and fatigue after concentric and eccentric muscle contractions. Clinical Science, 64, 55–62. 10.1042/cs0640055 [DOI] [PubMed] [Google Scholar]
- Neziri, A. Y. , Andersen, O. K. , Petersen‐Felix, S. , Radanov, B. , Dickenson, A. H. , Scaramozzino, P. , Arendt‐Nielsen, L. , & Curatolo, M. (2010). The nociceptive withdrawal reflex: Normative values of thresholds and reflex receptive fields. European Journal of Pain, 14, 134–141. 10.1016/j.ejpain.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Niesters, M. , Proto, P. L. , Aarts, L. , Sarton, E. Y. , Drewes, A. M. , & Dahan, A. (2014). Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. British Journal of Anaesthesia, 113, 148–156. 10.1093/bja/aeu056 [DOI] [PubMed] [Google Scholar]
- Nitter, A. K. , Pripp, A. H. , & Forseth, K. (2012). Are sleep problems and non‐specific health complaints risk factors for chronic pain? A prospective population‐based study with 17 year follow‐up. Scandinavian Journal of Pain, 3, 210–217. 10.1016/j.sjpain.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Nolan, T. A. , Price, D. D. , Caudle, R. M. , Murphy, N. P. , & Neubert, J. K. (2012). Placebo‐induced analgesia in an operant pain model in rats. Pain, 153, 2009–2016. 10.1016/j.pain.2012.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolano, M. , Simone, D. A. , Wendelschafer‐Crabb, G. , Johnson, T. , Hazen, E. , & Kennedy, W. R. (1999). Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain, 81, 135–145. 10.1016/S0304-3959(99)00007-X [DOI] [PubMed] [Google Scholar]
- North, R. , Li, Y. , Ray, P. , Rhines, L. , Tatsui, C. , Rao, G. , Johansson, C. , Zhang, H. , Kim, Y. , Zhang, B. , Dussor, G. , Kim, T. , Price, T. , & Dougherty, P. (2019). Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain, 142, 1215–1226. 10.1093/brain/awz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill, J. , Sikandar, S. , Mcmahon, S. B. , & Dickenson, A. H. (2015). Human psychophysics and rodent spinal neurones exhibit peripheral and central mechanisms of inflammatory pain in the UVB and UVB heat rekindling models. Journal of Physiology, 593, 4029–4042. 10.1113/JP270294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , & Dickenson, A. H. (2016). Neuronal hyperexcitability in the ventral posterior thalamus of neuropathic rats: Modality selective effects of pregabalin. Journal of Neurophysiology, 116, 159–170. 10.1152/jn.00237.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , Kucharczyk, M. , Montagut‐Bordas, C. , Lockwood, S. , & Dickenson, A. H. (2019). Neuropathy following spinal nerve injury shares features with the irritable nociceptor phenotype: A back‐translational study of oxcarbazepine. European Journal of Pain (United Kingdom), 23, 183–197. 10.1002/ejp.1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, G. L. , Finnerup, N. B. , Grosen, K. , Pilegaard, H. K. , Tracey, I. , Benedetti, F. , Price, D. D. , Jensen, T. S. , & Vase, L. (2014). Expectations and positive emotional feelings accompany reductions in ongoing and evoked neuropathic pain following placebo interventions. Pain, 155, 2687–2698. 10.1016/j.pain.2014.09.036 [DOI] [PubMed] [Google Scholar]
- Petersen, K. K. , McPhee, M. E. , Hoegh, M. S. , & Graven‐Nielsen, T. (2019). Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: Manifestations and implications for pain progression. Current Opinion in Supportive & Palliative Care, 13, 99–106. 10.1097/SPC.0000000000000419 [DOI] [PubMed] [Google Scholar]
- Pfau, D. B. , Klein, T. , Putzer, D. , Pogatzki‐Zahn, E. M. , Treede, R. D. , & Magerl, W. (2011). Analysis of hyperalgesia time courses in humans after painful electrical high‐frequency stimulation identifies a possible transition from early to late LTP‐like pain plasticity. Pain, 152, 1532–1539. 10.1016/j.pain.2011.02.037 [DOI] [PubMed] [Google Scholar]
- Phelps, C. E. , Navratilova, E. , Dickenson, A. H. , Porreca, F. , & Bannister, K. (2019). Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain, 160, 1614–1621. 10.1097/j.pain.0000000000001553 [DOI] [PubMed] [Google Scholar]
- Pickering, G. , Jourdan, D. , Eschalier, A. , & Dubray, C. (2002). Impact of age, gender and cognitive functioning on pain perception. Gerontology, 48, 112–118. 10.1159/000048937 [DOI] [PubMed] [Google Scholar]
- Pickering, G. , Jourdan, D. , Millecamps, M. , Chapuy, E. , Alliot, J. , & Eschalier, A. (2006). Age‐related impact of neuropathic pain on animal behaviour. European Journal of Pain, 10, 749. 10.1016/j.ejpain.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Pickering, G. , Pereira, B. , Clère, F. , Sorel, M. , de Montgazon, G. , Navez, M. , Picard, P. , Roux, D. , Morel, V. , Salimani, R. , Adda, M. , Legout, V. , & Dubray, C. (2014). Cognitive function in older patients with postherpetic neuralgia. Pain Pract, 14, E1–7. 10.1111/papr.12079 [DOI] [PubMed] [Google Scholar]
- Pickering, G. , Monacelli, F. , Garrote, J. M. P. C. , Guarda, H. , Batalha, L. , Gibson, S. , Savas, S. , Odetti, P. , Gandolfo, F. , Pastorino, E. , Mugeiro, M. J. C. , Dias, I. P. , Kilavuz, A. , & Macian, N. , The Doloplus Team, Pereira, B (2018). Reliability study in five languages of the translation of the pain observational scale Algoplus. Pain Med (United States), 19, 252–261. 10.1093/pm/pnw356 [DOI] [PubMed] [Google Scholar]
- Pitcher, M. H. , Von Korff, M. , Bushnell, M. C. , & Porter, L. (2019). Prevalence and profile of high‐impact chronic pain in the United States. The Journal of Pain, 20, 146–160. 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaghki, L. , & Mouraux, A. (2005). EEG and laser stimulation as tools for pain research. Current Opinion in Investigational Drugs, 6, 58–64. [PubMed] [Google Scholar]
- Ploner, M. , Sorg, C. , & Gross, J. (2017). Brain rhythms of Pain. Trends in Cognitive Sciences, 21, 100–110. 10.1016/j.tics.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki, E. M. , & Raja, S. N. (2003). A mouse model of incisional pain materials and methods. Anesthesiology, 99, 1023–1027. 10.1097/00000542-200310000-00041 [DOI] [PubMed] [Google Scholar]
- Pogatzki, E. M. , Vandermeulen, E. P. , & Brennan, T. J. (2002). Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain, 97, 151–161. 10.1016/S0304-3959(02)00014-3 [DOI] [PubMed] [Google Scholar]
- Pogatzki‐Zahn, E. M. , Drescher, C. , Englbrecht, J. S. , Klein, T. , Magerl, W. , & Zahn, P. K. (2019b). Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain, 160, 1781–1793. 10.1097/j.pain.0000000000001561 [DOI] [PubMed] [Google Scholar]
- Pogatzki‐Zahn, E. , Schnabel, K. , & Kaiser, U. (2019a). Patient‐reported outcome measures for acute and chronic pain: Current knowledge and future directions. Current Opinion in Anaesthesiology, 32, 616–622. 10.1097/ACO.0000000000000780 [DOI] [PubMed] [Google Scholar]
- Pogatzki‐Zahn, E. M. , Segelcke, D. , & Schug, S. A. (2017). Postoperative pain ‐ from mechanisms to treatment. Pain Reports, 2, e588. 10.1097/PR9.0000000000000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki‐Zahn, E. , Segelcke, D. , & Zahn, P. (2018). Mechanisms of acute and chronic pain after surgery: Update from findings in experimental animal models. Current Opinion in Anaesthesiology, 31, 575–585. 10.1097/ACO.0000000000000646 [DOI] [PubMed] [Google Scholar]
- Pogatzki‐Zahn, E. M. , Wagner, C. , Meinhardt‐Renner, A. , Burgmer, M. , Beste, C. , Zahn, P. K. , & Pfleiderer, B. (2010). Coding of incisional pain in the brain: A functional magnetic resonance imaging study in human volunteers. Anesthesiology, 112, 406–417. 10.1097/ALN.0b013e3181ca4c82 [DOI] [PubMed] [Google Scholar]
- Potvin, S. , & Marchand, S. (2016). Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain, 157, 1704–1710. 10.1097/j.pain.0000000000000573 [DOI] [PubMed] [Google Scholar]
- Powers, J. M. , Ioachim, G. , & Stroman, P. W. (2018). Ten key insights into the use of spinal cord fMRI. Brain Sciences, 8, 173. 10.3390/brainsci8090173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato, V. , Taberner, F. J. , Hockley, J. R. F. , Callejo, G. , Arcourt, A. , Tazir, B. , Hammer, L. , Schad, P. , Heppenstall, P. A. , Smith, E. S. , & Lechner, S. G. (2017). Functional and molecular characterization of mechanoinsensitive “silent” nociceptors. Cell Reports, 21, 3102–3115. 10.1016/j.celrep.2017.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pud, D. , Granovsky, Y. , & Yarnitsky, D. (2009). The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)‐like effect in humans. Pain, 144, 16–19. 10.1016/j.pain.2009.02.015 [DOI] [PubMed] [Google Scholar]
- Rahman, W. , Patel, R. , & Dickenson, A. H. (2015). Electrophysiological evidence for voltage‐gated calcium channel 2 (Cav2) modulation of mechano‐ and thermosensitive spinal neuronal responses in a rat model of osteoarthritis. Neuroscience, 305, 76–85. 10.1016/j.neuroscience.2015.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remeniuk, B. , Sukhtankar, D. , Okun, A. , Navratilova, E. , Xie, J. Y. , King, T. , & Porreca, F. (2015). Behavioral and neurochemical analysis of ongoing bone cancer pain in rats. Pain, 156, 1864–1873. 10.1097/j.pain.0000000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke, R. , Magerl, W. , Campbell, K. A. , Schalber, C. , Caspari, S. , Birklein, F. , & Treede, R. D. (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. European Journal of Pain, 10, 77–88. 10.1016/j.ejpain.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Rouch I., Edjolo A., Laurent B., Pongan E., Dartigues J.F., Amieva H. (2021). Association between chronic pain and long‐term cognitive decline in a population‐based cohort of elderly participants. Pain, 162, (2), 552–560. 10.1097/j.pain.0000000000002047 [DOI] [PubMed] [Google Scholar]
- Ruscheweyh, R. , Viehoff, A. , Tio, J. , & Pogatzki‐Zahn, E. M. (2017). Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre‐existing chronic pain. Pain, 158, 1030–1038. 10.1097/j.pain.0000000000000873 [DOI] [PubMed] [Google Scholar]
- Samartin‐Veiga, N. , González‐Villar, A. J. , & Carrillo‐de‐la‐Peña, M. T. (2019). Neural correlates of cognitive dysfunction in fibromyalgia patients: Reduced brain electrical activity during the execution of a cognitive control task. NeuroImage Clinical, 23, 101817. 10.1016/j.nicl.2019.101817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini, G. , Serrao, M. , Rossi, P. , Romaniello, A. , Cruccu, G. , & Willer, J. C. (2005). The lower limb flexion reflex in humans. Progress in Neurobiology, 77, 353–395. 10.1016/j.pneurobio.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Sawynok, J. , Esser, M. J. , & Reid, A. R. (1999). Peripheral antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain, 82, 149–158. 10.1016/S0304-3959(99)00043-3 [DOI] [PubMed] [Google Scholar]
- Schabrun, S. M. , Christensen, S. W. , Mrachacz‐Kersting, N. , & Graven‐Nielsen, T. (2016). Motor cortex reorganization and impaired function in the transition to sustained muscle pain. Cerebral Cortex, 26, 1878–1890. 10.1093/cercor/bhu319 [DOI] [PubMed] [Google Scholar]
- Schilder, A. , Magerl, W. , Hoheisel, U. , Klein, T. , & Treede, R. D. (2016). Electrical high‐frequency stimulation of the human thoracolumbar fascia evokes long‐term potentiation‐like pain amplification. Pain, 157, 2309–2317. 10.1097/j.pain.0000000000000649 [DOI] [PubMed] [Google Scholar]
- Schmidt, R. , Schmelz, M. , Forster, C. , Ringkamp, M. , Torebjork, E. , & Handwerker, H. (1995). Novel classes of responsive and unresponsive C nociceptors in human skin. Journal of Neuroscience, 15, 333–341. 10.1523/JNEUROSCI.15-01-00333.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt, P. (2019). Where has the “bio” in bio‐psycho‐social gone? Current Opinion in Supportive & Palliative Care, 13, 94–98. 10.1097/SPC.0000000000000420 [DOI] [PubMed] [Google Scholar]
- Segelcke, D. , Pradier, B. , & Pogatzki‐Zahn, E. (2019). Advances in assessment of pain behaviors and mechanisms of post‐operative pain models. Current Opinion in Physiology, 11, 85–92. 10.1016/j.cophys.2019.07.002 [DOI] [Google Scholar]
- Seminowicz, D. A. , Remeniuk, B. , Krimmel, S. R. , Smith, M. T. , Barrett, F. S. , Wulff, A. B. , Furman, A. J. , Geuter, S. , Lindquist, M. A. , Irwin, M. R. , & Finan, P. H. (2019). Pain‐related nucleus accumbens function: Modulation by reward and sleep disruption. Pain, 160, 1196–1207. 10.1097/j.pain.0000000000001498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, J. (2009). Re‐emerging microneurography. Journal of Physiology, 587, 295–296. 10.1113/jphysiol.2008.167858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi Meymandi, M. , Sepehri, G. , Izadi, G. , & Zamiri, Z. (2019). Evidence for antinociceptive effects of combined administration of vitamin E and celecoxib in tail‐flick and formalin test in male rats. Pharmacol Reports, 71, 457–464. 10.1016/j.pharep.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Shiers, S. , Mwirigi, J. , Pradhan, G. , Kume, M. , Black, B. , Barragan‐Iglesias, P. , Moy, J. K. , Dussor, G. , Pancrazio, J. J. , Kroener, S. , & Price, T. J. (2020). Reversal of peripheral nerve injury‐induced neuropathic pain and cognitive dysfunction via genetic and tomivosertib targeting of MNK. Neuropsychopharmacology, 45, 524–533. 10.1038/s41386-019-0537-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiers, S. , Pradhan, G. , Mwirigi, J. , Mejia, G. , Ahmad, A. , Kroener, S. , & Price, T. (2018). Neuropathic pain creates an enduring prefrontal cortex dysfunction corrected by the type II diabetic drug metformin but not by gabapentin. Journal of Neuroscience, 38, 7337–7350. 10.1523/JNEUROSCI.0713-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar, S. , Ronga, I. , Iannetti, G. D. , & Dickenson, A. H. (2013). Neural coding of nociceptive stimuli ‐ From rat spinal neurones to human perception. Pain, 154, 1263–1273. 10.1016/j.pain.2013.03.041 [DOI] [PubMed] [Google Scholar]
- Slater, H. , Arendt‐Nielsen, L. , Wright, A. , & Graven‐Nielsen, T. (2003). Experimental deep tissue pain in wrist extensors ‐ A model of lateral epicondylalgia. European Journal of Pain, 7, 277–288. 10.1016/S1090-3801(02)00141-6 [DOI] [PubMed] [Google Scholar]
- Sluka, K. A. , Bjordal, J. M. , Marchand, S. , & Rakel, B. A. (2013). What makes transcutaneous electrical nerve stimulation work? making sense of the mixed results in the clinical literature. Physical Therapy, 93, 1397–1402. 10.2522/ptj.20120281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka, K. A. , Kalra, A. , & Moore, S. A. (2001). Unilateral intramuscular injections of acidic saline produce a bilateral, long‐lasting hyperalgesia. Muscle and Nerve, 24, 37–46. [DOI] [PubMed] [Google Scholar]
- Snutch, T. P. , & Zamponi, G. W. (2018). Recent advances in the development of T‐type calcium channel blockers for pain intervention. British Journal of Pharmacology, 175, 2375–2383. 10.1111/bph.13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotocinal, S. G. , Sorge, R. E. , Zaloum, A. , Tuttle, A. H. , Martin, L. J. , Wieskopf, J. S. , Mapplebeck, J. C. S. , Wei, P. , Zhan, S. , Zhang, S. , McDougall, J. J. , King, O. D. , & Mogil, J. S. (2011). The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Molecular Pain, 7, 55. 10.1186/1744-8069-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikandarajah, S. , & Gilron, I. (2011). Systematic review of movement‐evoked pain versus pain at rest in postsurgical clinical trials and meta‐analyses: A fundamental distinction requiring standardized measurement. Pain, 152, 1734–1739. 10.1016/j.pain.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Staahl, C. , & Drewes, A. M. (2004). Experimental human pain models: A review of standardised methods for preclinical testing of analgesics. Basic & Clinical Pharmacology & Toxicology, 95, 97–111. 10.1111/j.1742-7843.2004.950301.x [DOI] [PubMed] [Google Scholar]
- Staffe, A. T. , Bech, M. W. , Clemmensen, S. L. K. , Nielsen, H. T. , Larsen, D. B. , & Petersen, K. K. (2019). Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One, 14, e0225849. 10.1371/journal.pone.0225849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud, R. , Vierck, C. J. , Cannon, R. L. , Mauderli, A. P. , & Price, D. D. (2001). Abnormal sensitization and temporal summation of second pain (wind‐up) in patients with fibromyalgia syndrome. Pain, 91, 165–175. 10.1016/S0304-3959(00)00432-2 [DOI] [PubMed] [Google Scholar]
- Tabor, A. , Keogh, E. , & Eccleston, C. (2017). Embodied pain ‐ Negotiating the boundaries of possible action. Pain, 158, 1007–1011. 10.1097/j.pain.0000000000000875 [DOI] [PubMed] [Google Scholar]
- Takasaki, I. , Watanabe, A. , Yokai, M. , Watanabe, Y. , Hayakawa, D. , Nagashima, R. , Fukuchi, M. , Okada, T. , Toyooka, N. , Miyata, A. , Gouda, H. , & Kurihara, T. (2018). In silico screening identified novel small‐molecule antagonists of PAC1 receptors. Journal of Pharmacology and Experimental Therapeutics, 365, 1–8. 10.1124/jpet.117.245415 [DOI] [PubMed] [Google Scholar]
- Tan, L. L. , Oswald, M. J. , Heinl, C. , Retana Romero, O. A. , Kaushalya, S. K. , Monyer, H. , & Kuner, R. (2019). Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nature Communications, 10, 983. 10.1038/s41467-019-08873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe‐Theodor, A. , King, T. , & Morgan, M. M. (2019). Pros and cons of clinically relevant methods to assess pain in rodents. Neuroscience and Biobehavioral Reviews, 100, 335–343. 10.1016/j.neubiorev.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, E. L. , Thompson, K. A. , & Rhudy, J. L. (2016). Does pain catastrophizing contribute to threat‐evoked amplification of pain and spinal nociception? Pain, 157, 456–465. 10.1097/j.pain.0000000000000392 [DOI] [PubMed] [Google Scholar]
- Thakur, M. , Dickenson, A. H. , & Baron, R. (2014). Osteoarthritis pain: Nociceptive or neuropathic? Nature Reviews Rheumatology, 10, 374–380. 10.1038/nrrheum.2014.47 [DOI] [PubMed] [Google Scholar]
- Thakur, M. , Rahman, W. , Hobbs, C. , Dickenson, A. H. , & Bennett, D. L. H. (2012). Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One, 7, e33730. 10.1371/journal.pone.0033730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey, I. , & Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron, 55, 377–391. 10.1016/j.neuron.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Treede, R. D. , Meyer, R. A. , Raja, S. N. , & Campbell, J. N. (1995). Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. Journal of Physiology, 483, 747–758. 10.1113/jphysiol.1995.sp020619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini, A. , Panuccio, G. , Galeotti, F. , Maluccio, M. R. , Sartucci, F. , Avoli, M. , & Cruccu, G. (2010). Laser‐evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. European Journal of Pain, 14, 222–225. 10.1016/j.ejpain.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbul, A. (1850). Tincture of capsaicin as a remedy for chilblains and toothache. (Dublin Free Press). [Google Scholar]
- Tuttle, A. H. , Molinaro, M. J. , Jethwa, J. F. , Sotocinal, S. G. , Prieto, J. C. , Styner, M. A. , Mogil, J. S. , & Zylka, M. J. (2018). A deep neural network to assess spontaneous pain from mouse facial expressions. Molecular Pain, 14, 1744806918763658. 10.1177/1744806918763658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch, C. E. , & Dickenson, A. H. (2003). In vivo single unit extracellular recordings from spinal cord neurones of rats. Brain Research Protocols, 12, 26–34. 10.1016/S1385-299X(03)00068-0 [DOI] [PubMed] [Google Scholar]
- Vachon‐Presseau, E. , Berger, S. E. , Abdullah, T. B. , Huang, L. , Cecchi, G. A. , Griffith, J. W. , Schnitzer, T. J. , & Apkarian, A. V. (2018). Brain and psychological determinants of placebo pill response in chronic pain patients. Nature Communications, 9, 3397. 10.1038/s41467-018-05859-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanova, N. , Azpiroz, F. , & Malagelada, J. R. (1997). Perception and gut reflexes induced by stimulation of gastrointestinal thermoreceptors in humans. Journal of Physiology, 502, 215–222. 10.1111/j.1469-7793.1997.215bl.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollert, J. , Magerl, W. , Baron, R. , Binder, A. , Enax‐Krumova, E. K. , Geisslingere, G. , Gierthmuhlen, J. , Henrich, F. , Hullemann, P. , Klein, T. , Lotsch, J. , Maier, C. , Oertel, B. , Schuh‐Hofer, S. , Tolle, T. R. , & Treede, R. D. (2018). Pathophysiological mechanisms of neuropathic pain: Comparison of sensory phenotypes in patients and human surrogate pain models. Pain, 159, 1090–1102. 10.1097/j.pain.0000000000001190 [DOI] [PubMed] [Google Scholar]
- Wall, P. D. , & Devor, M. (1983). Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain, 17, 321–339. 10.1016/0304-3959(83)90164-1 [DOI] [PubMed] [Google Scholar]
- Wang, F. , Bélanger, E. , Côté, S. L. , Desrosiers, P. , Prescott, S. A. , Côté, D. C. , & De Koninck, Y. (2018). Sensory afferents use different coding strategies for heat and cold. Cell Reports, 23, 2001–2013. 10.1016/j.celrep.2018.04.065 [DOI] [PubMed] [Google Scholar]
- Wang, X.‐M. , Zhang, G.‐F. , Jia, M. , Xie, Z.‐M. , Yang, J.‐J. , Shen, J.‐C. , & Zhou, Z.‐Q. (2019). Environmental enrichment improves pain sensitivity, depression‐like phenotype, and memory deficit in mice with neuropathic pain: Role of NPAS4. Psychopharmacology (Berl), 236, 1999–2014. 10.1007/s00213-019-5187-6 [DOI] [PubMed] [Google Scholar]
- Wanigasekera, V. , Mezue, M. , Andersson, J. , Kong, Y. , & Tracey, I. (2016). Disambiguating pharmacodynamic efficacy from behavior with neuroimaging. Anesthesiology, 124(1), 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera, V. , Wartolowska, K. , Huggins, J. P. , Duff, E. P. , Vennart, W. , Whitlock, M. , Massat, N. , Pauer, L. , Rogers, P. , Hoggart, B. , & Tracey, I. (2018). Disambiguating pharmacological mechanisms from placebo in neuropathic pain using functional neuroimaging. British Journal of Anaesthesia, 120, 299–307. 10.1016/j.bja.2017.11.064 [DOI] [PubMed] [Google Scholar]
- Wasan, A. D. , Loggia, M. L. , Chen, L. Q. , Napadow, V. , Kong, J. , & Gollub, R. L. (2011). Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology, 115, 364–374. 10.1097/ALN.0b013e318220e880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, E. J. , Levene, J. L. , Cohen, M. S. , Andreae, D. A. , Chao, J. Y. , Johnson, M. , Hall, C. B. , & Andreae, M. H. (2018). Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Systematic Review, 4, CD007105. 10.1002/14651858.CD007105.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer, J. C. , De Broucker, T. , & Le Bars, D. (1989). Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. Journal of Neurophysiology, 62, 1028–1038. 10.1152/jn.1989.62.5.1028 [DOI] [PubMed] [Google Scholar]
- Wise, R. G. , Rogers, R. , Painter, D. , Bantick, S. , Ploghaus, A. , Williams, P. , Rapeport, G. , & Tracey, I. (2002). Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. NeuroImage, 16, 999–1014. 10.1006/nimg.2002.1146 [DOI] [PubMed] [Google Scholar]
- Xiong, B. , Zhang, W. , Zhang, L. , Huang, X. , Zhou, W. , Zou, Q. , Manyande, A. , Wang, J. , Tian, Y. , & Tian, X. (2020). Hippocampal glutamatergic synapses impairment mediated novel‐object recognition dysfunction in rats with neuropathic pain. Pain, 161, 1824–1836. 10.1097/j.pain.0000000000001878 [DOI] [PubMed] [Google Scholar]
- Xu, J. , Richebe, P. , & Brennan, T. J. (2009). Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. European Journal of Pain, 13, 820–828. 10.1016/j.ejpain.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Xu, L. , Wan, Y. , Ma, L. , Zheng, J. , Han, B. , Liu, F. Y. , Yi, M. , & Wan, Y. (2018). A context‐based analgesia model in rats: Involvement of prefrontal cortex. Neurosci Bull, 34, 1047–1057. 10.1007/s12264-018-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. M. H. , Hartley, R. L. , Leung, A. A. , Ronksley, P. E. , Jetté, N. , Casha, S. , & Riva‐Cambrin, J. (2019). Preoperative predictors of poor acute postoperative pain control: A systematic review and meta‐analysis. British Medical Journal Open, 9, e025091. 10.1136/bmjopen-2018-025091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky, D. (2010). Conditioned pain modulation (the diffuse noxious inhibitory control‐like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol, 23, 611–615. 10.1097/ACO.0b013e32833c348b [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. , Crispel, Y. , Eisenberg, E. , Granovsky, Y. , Ben‐Nun, A. , Sprecher, E. , Best, L. A. , & Granot, M. (2008). Prediction of chronic post‐operative pain: Pre‐operative DNIC testing identifies patients at risk. Pain, 138, 22–28. 10.1016/j.pain.2007.10.033 [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. , Granot, M. , Nahman‐Averbuch, H. , Khamaisi, M. , & Granovsky, Y. (2012). Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain, 153, 1193–1198. 10.1016/j.pain.2012.02.021 [DOI] [PubMed] [Google Scholar]
- Yezierski, R. P. , & Hansson, P. (2018). Inflammatory and neuropathic pain from bench to bedside: What went wrong? The Journal of Pain, 19, 571–588. 10.1016/j.jpain.2017.12.261 [DOI] [PubMed] [Google Scholar]
- Yip, P. K. , Chapman, G. E. , Sillito, R. R. , Ip, T. H. R. , Akhigbe, G. , Becker, S. C. , Price, A. W. , Michael‐Titus, A. T. , Armstrong, J. D. , & Tremoleda, J. L. (2019). Studies on long term behavioural changes in group‐housed rat models of brain and spinal cord injury using an automated home cage recording system. Journal of Neuroscience Methods, 321, 49–63. 10.1016/j.jneumeth.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Yokobe, J. , Kitahara, M. , Matsushima, M. , & Uezono, S. (2014). Preference for different anchor descriptors on visual analogue scales among Japanese patients with chronic pain. PLoS One, 9, e99891. 10.1371/journal.pone.0099891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker, I. , & Beery, A. K. (2010). Males still dominate animal studies. Nature, 465, 690. 10.1038/465690a [DOI] [PubMed] [Google Scholar]


