Abstract
Often thought to be a nutritional issue limited to low‐ and middle‐income countries (LMICs), pediatric thiamine deficiency (PTD) is perceived as being eradicated or anecdotal in high‐income countries (HICs). In HICs, classic beriberi cases in breastfed infants by thiamine‐deficient mothers living in disadvantaged socioeconomic conditions are thought to be rare. This study aims to assess PTD in HICs in the 21st century. Literature searches were conducted to identify case reports of PTD observed in HICs and published between 2000 and 2020. The analyzed variables were age, country, underlying conditions, clinical manifestations of PTD, and response to thiamine supplementation. One hundred and ten articles were identified, totaling 389 PTD cases that were classified into four age groups: neonates, infants, children, and adolescents. Eleven categories of PTD‐predisposing factors were identified, including genetic causes, lifestyle (diabetes, obesity, and excessive consumption of sweetened beverages), eating disorders, cancer, gastrointestinal disorders/surgeries, critical illness, and artificial nutrition. TD‐associated hyperlactatemia and Wernicke encephalopathy were the most frequent clinical manifestations. The circumstances surrounding PTD in HICs differ from classic PTD observed in LMICs and this study delineates its mutiple predisposing factors. Further studies are required to estimate its magnitude. Awareness is of utmost importance in clinical practice.
Keywords: pediatric thiamine deficiency, hyperlactatemia, Wernicke encephalopathy, bariatric surgery, sweetened drinks, anorexia
Pediatric thiamine deficiency (PTD) is perceived as being eradicated or anecdotal in high‐income countries (HICs). This study aims to assess PTD in HICs in the 21st century. Literature searches were conducted to identify case reports of PTD observed in HICs and published between 2000 and 2020. The circumstances surrounding PTD in HICs differ from classic PTD observed in low‐ and middle‐income countries and this study delineates its mutiple predisposing factors.

Introduction
Thiamine, also called vitamin B1 or aneurin, is a water‐soluble vitamin that is an essential micronutrient for human beings, who cannot synthesize it and whose body reserves in tissues can be depleted within a couple of weeks without adequate thiamine intake. 1 Thiamine is crucial factor for both enzymatic and nonenzymatic functions within the cytosolic, mitochondrial, and peroxisomal compartments. Thiamine is involved in many processes, particularly in energy transformation and oxidative and nonoxidative carbohydrate metabolism. As a crucial cofactor in the metabolism of carbohydrates and amino acids, its role is extended to fatty acid metabolism. 1 , 2 The limited body storage in tissues, combined with low intake, contributes to the propensity to thiamine deficiency (TD) during all life stages of human beings.
The daily recommended dietary allowance for thiamine has been revised based on the specific population requirements in some nations, which varies according to age range, gender, life stage (pregnancy and lactation), energy intake (notably carbohydrate intake), and physical activity level (inactive, moderately inactive, moderately active, and active occupational physical activity in children). 3 , 4 These recommended thiamine intakes should cover the metabolic requirements of the main target organs of TD: heart, peripheral and central nervous systems, muscles, and others. 5 , 6 The brain is particularly sensitive to any thiamine deprivation, especially during early childhood and various periods of adolescence. 7
The prevalence of this life‐threatening disease is unevenly distributed around the world. TD is highly prevalent in low‐ and middle‐income countries (LMICs) or in humanitarian emergencies, where various limited resources cause either malnutrition or hunger with severe food insecurity. 8 It can also occur in the context of a monotonous diet or diets that rely on low‐thiamine staple foods (e.g., polished rice), notably in breastfed infants whose mothers are thiamine deficient. 8 , 9 , 10 , 11 , 12
Many factors have likely contributed to the reduction of this public health concern, especially in high‐income countries (HICs), such as significant advancements in large‐scale fortification of widely consumed foods (e.g., wheat flour), although thiamine fortification policies are not mandatory in a number of countries. 13 , 14 , 15 , 16 , 17 , 18 The advances in the diagnosis and treatment of Wernicke encephalopathy (WE) have also contributed to the reduction of its prevalence and burden. 19 As a consequence, there are no studies that have systematically assessed TD in pediatric patients in HICs, and there is only sparse literature in the form of case reports, which contributes to the perception that pediatric thiamine deficiency (PTD) is somewhat anecdotal in HIC. 20 , 21 , 22 , 23 There are no accurate statistics available on the incidence of PTD, which is thought to be associated with a few underlying conditions. 24
Therefore, the aim of this study is to identify cases of PTD reported in HICs during the past 20 years, including a description of the ages, causes of TD, and underlying conditions, as well as clinical manifestations of the affected patients.
Methods
An electronic literature search was conducted on Medline (via Pubmed), Cochrane library, and Google Scholar using the cross‐referenced combination of the following MeSH terms: thiamine deficiency (TD) or disorders, thiamine metabolism, dry beriberi, wet beriberi, WE, heart failure, Shoshin, and lactatemia. Retrieved publications were screened and selected by two authors, B.R. and L.H. (and verified by third author F.G.), following the inclusion criteria: confirmed cases of TD in the pediatric population that were observed in HICs. TD was defined as positive clinical response to thiamine administration or biochemical confirmation of TD (including genetic sequencing) or specific radioimaging findings. The definition of HICs followed the World Bank classification, 25 where low‐ and middle‐ income economies are defined as those with a gross national income per capita of $12,535 or less and high‐income economies are those with a gross national income per capita of $12,536 or more. We followed the American Academy of Pediatrics 3 age groups definition but excluded late adolescents since most of the pediatric units do not report patients above 18 years of age. Neonate (birth to 28 days old), infancy (1 month–2 years old), childhood (2−11 years old), and adolescence (12−18 years old) were considered for inclusion.
This paper focused on the clinical aspects of PTD (as per the study authors’ judgment) and did not cover either laboratory techniques or discuss clinical–laboratory agreement.
The searches were limited to studies published between 2000 and 2020, but there were no language restrictions. The extracted variables for the included studies were: country where the study was conducted, age of the identified cases, causes of TD or underlying conditions (which were classified into categories of predisposing factors), clinical manifestations, and response to thiamine supplementation (as positive or negative, when reported). Variables were reported as numbers or percentages.
Results
A total number of 110 articles were included, covering 389 pediatric patients under 19 years old with confirmed TD in HICs. According to the abovementioned methodology, 74% of all reports, not including genetic sequencing, assessed and had data available on serum thiamine (49 reports), whole blood thiamine diphosphate (5 reports), and erythrocyte transketolase activity coefficient (10 reports).
Country of origin of included studies
The regional distribution of the included publications is displayed in Figure 1. Most of the studies originated from the United States (32.4% of all studies), followed by five countries, namely, Japan, Israel, Italy, Germany, and China. These represent the top six countries among a total of 23 countries.
Figure 1.
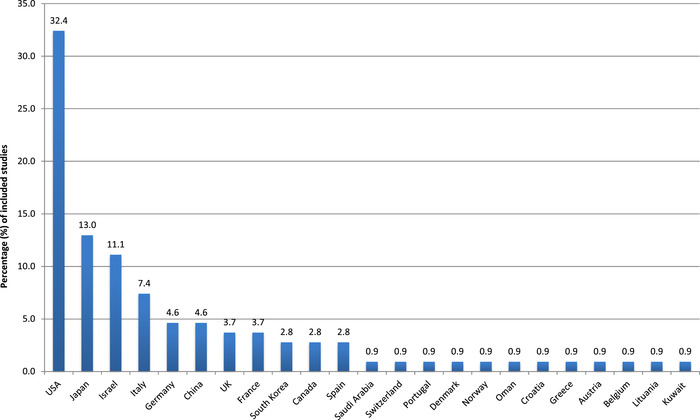
Percentage of included studies per country (case reports/series of pediatric thiamine deficiency published in 110 papers between 2000 and 2020).
Patient age distribution
Figure 2 shows that although infants still represent a large group, the majority of affected PTD patients in HICs are children and adolescents, as opposed to PTD observed in LMICs, where the classic infantile beriberi is well described.
Figure 2.
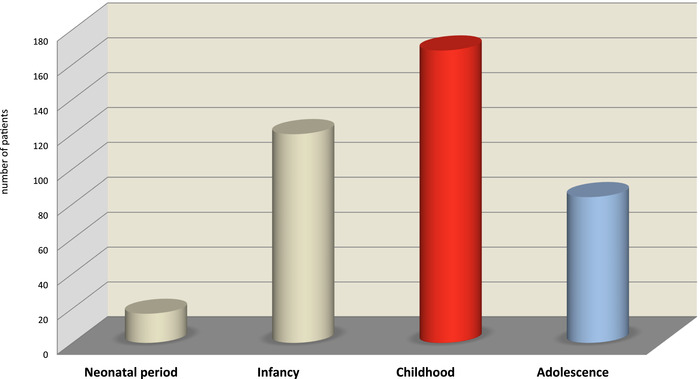
Distribution by four pediatric age groups of the overall 389 patients extracted from the 110 included studies (2000–2020).
Circumstances surrounding PTD in HICs and predisposing risk factors (causes or underlying conditions)
The case reports identified (n = 389) can be divided into 11 main clinical categories (C0–C10) where PTDs have been described. In some conditions, multiple pathophysiological mechanisms might be involved. Table S1 (online only) presents a separate group (C0) of 91 patients described with neurological impairment as a result of earlier TD insult. Table S2 (online only) summarizes the remaining 298 included cases of PTD (C1–C10) divided by categories of predisposing risk factors (causes and underlying conditions), including both genetic thiamine disorders and acquired TD reported in HICs. Figure 3 shows the distributions of included cases into the 10 (C1–C10) categories of predisposing risk factors (causes and underlying conditions) of TD.
Figure 3.
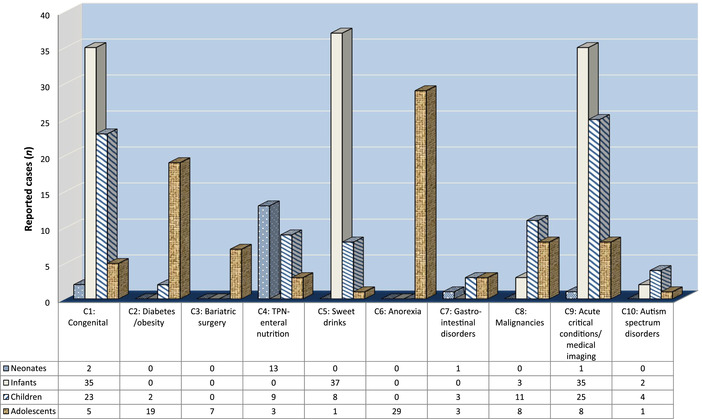
Distribution of the included cases into 10 categories of predisposing risk factors according to the four pediatric age groups.
Below is the description of these 11 categories, classified from C0 to C10:
C0: PTD as a result of early infancy consumption of thiamine‐free formula, followed up in tertiary neurocognitive referral settings. This category is a large group, comprised of mostly young children followed up beyond infancy for neurological sequelae and disability following TD insults during early infancy. They present with neurological and cognitive disorders and are referred to specialized services tied to neuroimaging platforms. 20 , 26 , 27 , 28 , 29 , 30 This category (n = 91) is presented separately in Table S1 (online only).
C1: PTD caused by genetic inborn errors of thiamine metabolism. They account for 22% of all 298 cases and are presented in Table S2 (online only). As expected, neonates and infants represent 57% of the 65 reported cases in this category. Interestingly, children and adolescents represent 35% and 7.7%, respectively.
Using high‐throughput genomic screening, four genetic mutations and their variants have been described in 19 countries, some of them with high incidence of inborn errors of thiamine metabolism disorders that might be increased by consanguinity. 31 , 32 These conditions are characterized by a variable onset of the disorder and a wide range of clinical signs, including recurrent episodic encephalopathy, dystonia, convulsions, ocular abnormalities, microcephaly, anemia, thrombocythemia, and diabetes.
Three of the genetic mutations encode for thiamine transporters, and one encodes for the enzyme thiamine pyrophosphokinase 1 (TPK1), which converts thiamine into its active form:
Mutations in SLC19A2, which cause thiamine‐responsive megaloblastic anemia (TRMA), were described in eight studies (UK, Denmark, China, Portugal, Lithuania, United States, Italy, and Croatia). It is often revealed by a neonatal diabetic ketoacidosis (DKA). 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39
Mutations in SLC19A3, which cause biotin/thiamine‐responsive basal ganglia disease characterized by episodes of severe Leigh‐like encephalopathy, were described in seven studies from six countries (Spain, Saudi Arabia, UK, France, Japan, and Norway). 40 , 41 , 42 , 43 , 44 , 45 , 46
Mutations in SLC25A19 (called Amish microcephaly) were identified in five studies (Canada, United States, Oman, Israel, and Germany). 47 , 48 , 49 , 50 , 51 They are characterized by a very small head at birth, seizures, and episodes of lactic acidosis.
Mutations in TPK1 were found in four studies (China, United States, Austria, and Germany). 32 , 52 , 53 , 54 TPK deficiency can present as episodic encephalopathy or Leigh syndrome–like early‐onset global developmental delay.
C2: PTD related to diabetic patients with ketoacidosis and/or obese patients (7%). In type 1 diabetes, the main concern is DKA with the occurrence of suboptimal thiamine levels even prior to insulin therapy. Then, insulin administration induces PTD. Altered mental status is a common finding of DKA, and therefore TD‐related encephalopathy could be missed. 55 , 56
C3: PTD after bariatric surgery (2%). Clinical signs of TD can occur in adolescents in the first months following surgery. The alerting symptoms are intractable vomiting or minor neurological signs preceding the onset of WE with associated hyperlactatemia. 57 , 58 , 59 , 60 , 61
C4: PTD related to total parenteral nutrition (TPN) or enteral nutrition (EN) (8%) may occur at any age, even in preterm neonates or very low birth‐weight infants and infants suffering from gastrointestinal disorders or postoperative problems. Parenteral/enteral formulations might contain inadequate levels of thiamine for this age group. These patients can also present signs, such as the classic form of TD, refractory lactic acidosis, myocardial injuries, shock with multiorgan failure, and WE. Most of the time the abnormalities can be resolved with the rapid administration of thiamine supplementation. 62 , 63 , 64 , 65 , 66 , 67 , 68
C5: PTD related to high consumption of sugar‐sweetened beverages or isotonic drinks, either in infancy or childhood/adolescence (15%). 22 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 Most of the cases were reported in Japan. A Japanese survey revealed 33 children with regular intake of soft drinks who developed dramatic TD (confirmed by whole blood TDP). 77 Of these, TD was clinically expressed as classical WE in four cases, one resulted in death, and 12 had long‐term neurological sequelae of TD.
C6: PTD related to eating disorders (anorexia) (10%). 79 , 80 , 81 , 82 , 83 , 84 PTD related to eating disorders consistently occurs during adolescence, based on case reports. Two large case series referring to TD‐related anorexia were retrieved. 79 , 83 The largest case series were in pediatric psychiatric units and the majority of cases suffered from Wernicke syndrome. The prevalence of biologically confirmed TD was estimated at 38% of the 37 patients at a specialized pediatric psychiatric unit, 79 while this prevalence only reached 10% in a prospective study of 39 girls admitted to an outpatient day hospital for eating disorders. 83
C7: PTD related to gastrointestinal disorders with reduced thiamine absorption (2%). This encompasses a wide panel of clinical conditions from intestinal resection (short bowel syndrome) to inflammatory bowel disease in children. 85 , 86 , 87 , 88 , 89 , 90 , 91
C8: PTD related to pediatric malignancies (such as leukemia and neuroblastoma) constantly associated with hyperlactatemia and patients undergoing bone marrow transplant (7%). 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 Of note, three cases of drug‐induced TD (namely, methotrexate and metronidazole) have been included in this review. 87 , 93 , 101
C9: PTD related to acute critical illnesses, including pediatric emergency room, surgical setting, pediatric intensive care unit (PICU), pediatric cardiology and nephrology/dialysis units, imaging facilities, and other subspecialty emergency platforms (23%). This category gathers diverse pathologies of various degrees of severity occurring in the very preterm neonatal period until late adolescence. Diverse TD expressions are described, with clinical, biological (associated hyperlactatemia), and radiologically specific or atypical signs of TD. The cases cover a broad panel of clinical manifestations: shock, pulmonary arterial hypertension, Shoshin beriberi, type B lactic acidosis (reversible by thiamine administration), altered mental status that can progress to coma, limb weakness, ocular abnormalities, and signs of truncated or the entire triad of WE. 23 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127
C10: PTD related to a few documented cases of autistic spectrum disorders associated with TD and a case of botulism associated with thiaminase production (2%). 128 , 129 , 130 , 131 , 132
In summary, as opposed to LMICs, many cases of PTD in HICs occur in childhood and adolescence. Both age groups seem to display different underlying medical conditions leading to TD.
Clinical manifestations of PTD
Aside from the case series and reports of disabled patients following TD neurological insults during early infancy, as well as those with entry points of medical imaging or post mortem studies, the main clinical conditions/signs reported are displayed in Figure 4 (expressed in percentage, where each case can present with one or more of these conditions/signs).
Figure 4.
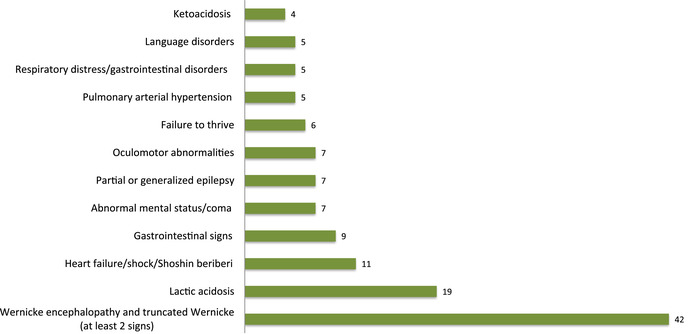
Proportion of the main clinical manifestations of pediatric thiamine deficiency described in the included studies (each case can have one or more signs).
Acute or insidious onset
Contrary to the mainstream medical belief that WE is limited to adults, this study highlights that acute or insidious‐onset WE is the most frequent initial sign. Indeed, 42 out the included 110 studies describe full or truncated WE, which can benefit from a cerebral imaging investigation. The clinical presentation of PTD is broad: severe or refractory underlying TD‐associated hyperlactatemia with acidosis, non‐Wernicke neurocognitive signs (sensorimotor signs or ataxia, altered consciousness, ocular signs, seizures, and dysarthria), and cardiovascular disorders (critical heart failure and shock). Special attention should be given to pulmonary arterial hypertension, remarkably reversed by thiamine supplementation. Failure to thrive is frequently reported and should not be forgotten, as it is a common source of medical referral to pediatricians.
Long‐term sequelae
These mostly include encepalopathy, epilepsy, and language disorders. Small outbreaks of classic beriberi in breastfed infants from thiamine‐deficient mothers in the French islands of Mayotte, 133 and the unfortunate incident of thiamine‐deficient soy‐based infant formula in Israel 30 that jeopardized the neurocognitive outcomes of young patients in the long term, remind the medical community that the classic forms of beriberi in their most detrimental outcomes can still occur in HICs.
It should be also noted that in Israel a number of infants who received the same thiamine‐free, soy‐based formula but who were asymptomatic still presented with later gross‐ and fine‐motor function alterations and learning difficulties. 27 , 134
PTD caused by genetic inborn errors of thiamine metabolism cases (C1) can occur in HICs and especially in migrant and/or consanguineous families. 31 , 32
Response to thiamine supplementation
We chose the following criteria to report whether the patients responded to thiamine supplementation, as it is not always been clearly documented: the authors’ judgment, clinical or radiological signs of improvement, lactatemia dynamics, anemia response, and change in the required dose of insulin or vasopressor drugs in response to thiamine (Tables S1 and S2, online only). A positive clinical response to thiamine administration was identified in 90 of the 110 reports in this review (82%).
Discussion
Despite the heterogeneity in the methodology of reports, they have been grouped into 10 categories (C1–C10) of main underlying conditions potentially leading to PTD. Some case reports could fit in two or more categories, but in order to avoid confusion, they were classified in only one category. These underlying conditions may have one or multiple pathophysiological mechanisms that can lead to TD: (1) low vitamin intake (i.e., an absolute insufficiency in exogenous thiamine intake through foods, drinks, mother's deficient milk, EN or TPN, and/or poor intestinal absorption via impaired intestinal thiamine absorption regardless of the cause); (2) thiamine to caloric ratio imbalance (i.e., an inadequate thiamine intake in comparison with the amount of energy consumed or a mismatch with endogenous hypermetabolic requirements, such in fever, severe illnesses, and infections); (3) cellular machinery dysfunction (i.e., a defect in thiamine transport or an impaired cellular utilization due to a reduced level of thiamine pyrophosphate, the active intracellular form of thiamine); (4) excessive losses (i.e., increased renal or digestive loss, as in renal or peritoneal dialysis, or use of diuretics, or by recurrent diarrhea or vomiting, or postsurgery‐induced hyperemesis); and (5) metabolites or drug interactions with thiamine metabolism (i.e., oxythiamine, thiaminase, or methotrexate and metronidazole).
The fact that one third of the publications are from the United States can be explained by many factors and does not necessarily represent a higher prevalence of PTD in this country. First of all, although there were no language restrictions, most included publications were written in English. The impact of the modern lifestyle on pediatric noncommunicable diseases (NCDs), such as severe obesity and type 2 diabetes, but also diet and drinking habits (of sugar‐sweetened drinks) in young children and adolescents, is particularly noted in the United States. There is likely a reporting bias as the pediatric workforce in the United States is one of the highest in the world; as such, access to pediatric research is common, and therefore the reporting of PTD is likely to be higher.
PTD age‐related specific vulnerability in HICs
In neonates, the category C1 shows a predominant proportion of genetic thiamine disorders with a fixed incidence related to the four most common mutations. They are usually managed by neonatologists and geneticists. The current state of wide globalization is associated with increasing movement of populations; and cases affected by these hereditary disorders, enhanced by consanguinity, might appear in locations where it has not been described previously. 31 , 32 These rare conditions need urgent management as some of them respond to thiamine, such as TRMA. The advances in modern neonatal care have also increased the survival rate of extremely low birth weight preterm babies who are also at high risk of developing TD through a combination of several complex factors, including very limited thiamine stores and organ immaturity with rapid growth. Therefore, they exhibit a significant dependence on the dual‐nutrient “carbohydrate‐thiamine” supply, 135 and inadequate intake or mismatch (not enough thiamine supply compared with carbohydrate supply) with TPN or EN can lead to severe TD. 62 , 67
Children and adolescents represent 65% of all reported cases in this study. In infancy, three categories are predominantly represented (C1, C5, and C9). In childhood, four categories clearly emerged as prominent causes of TD. Category C0, corresponding to the follow‐up of patients who survived TD insults during infancy, is the predominant category, followed by critically ill children in several emergency and subspeciality settings (C9), children on TPN or EN (C4), and then soft drinks habits, as in category C5. The adolescence period is dominated by anorexia (C6) and the combined categories C2 and C3, both related to NCDs.
Thus, the age of the population appears to be the most important determinant that has shaped the current pattern of PTD in HICs, out of the classic TD forms that previously prevailed and still remain in LMICs. The age groups mostly affected by PTD display a shift from early infancy (frequently observed in LMICs) toward childhood and, to a lesser extent, adolescence (in HICs). Another potential explanation for this difference is the higher diagnostic capacity of PTD in HICs, representing a potential bias. It is possible that if those diagnostic capacities were available/accessible in LMICs, more children and adolescents could be diagnosed with PTD.
Impact of the modern lifestyle in HICs and the growing burden of NCDs on thiamine metabolism in pediatrics
Excessive consumption of soft drinks in the pediatric population
This literature review raises two important points about two periods of the pediatric lifespan.
On the one hand, there is the description of a rapid onset of acute TD in infants who have an excessive consumption of soft drinks, as shown in category C5. Okumura et al. report a series of concerning and ongoing PTD cases in Japanese infants and young children (7–30 months old) as a result of regular consumption of an average of 1000 mL/day of iso‐/hypotonic soft drinks in nonnurturing environments. 77 Reports include cases of heart failure, pulmonary arterial hypertension, and WE consistently reversed with additional thiamine, and neurological sequelae and death in some.
On the other hand, there is the slow build‐up, insidious TD resulting from the consumption of sweetened sugar drinks in childhood and adolescence. Continuing from childhood habits, the obesogenic effect of sugar‐sweetened beverages is extended into adolescent dietary patterns associated with the consumption of carbonated soft drinks and calorie‐dense snack foods. 136 , 137 , 138 , 139 , 140 , 141 Without thiamine supplementation, this can lead to PTD. The availability of varied and balanced diets, such as those provided by school meal programs, may mitigate the expression of TD, rarely overtly observed in this population. Adolescent binge drinking, often associated with excessive carbohydrate consumption, puts this group at risk of TD and particularly of WE. 78 , 142
Diabetes
During the last two decades, the burden of type 2 diabetes and “diabesity” (the association of obesity with type 2 diabetes) has dramatically increased. 143 , 144 , 145 , 146 Pediatric type 1 diabetes incidence is also increasing, and almost half of them are in European or North American countries. 147
DKA is the most common life‐threatening complication of pediatric type 1 diabetes and is occasionally associated with cerebral damage, 148 , 149 and it has been described in all age ranges, including in neonatal diabetes associated with TRMA syndrome. 31 , 34 , 148 Ketoacidosis is a common reason for the discovery of diabetes in children and results in a subclinical cerebral edema that progresses (even in the first hours of insulin therapy) to encephalopathy. 55 , 56 , 149 The DKA‐related cerebral edema associated with hyperlactatemia is still not completely understood in some respects. 150 , 151 In addition to osmotherapy, 148 , 152 recent data suggest that thiamine might have a potential adjuvant role in the global management of DKA 55 , 148 , 150 , 153 , 154 A positive correlation is also observed between diastolic myocardial dysfunction and serum thiamine levels in children with DKA. 155 Thus, converging evidence suggests that thiamine may exert a beneficial effect in the management of DKA in children and even those with TRMA. 34 , 56 , 155 Unlike in adults, no studies have addressed the potential effect of thiamine to prevent specific complications in the course of chronic pediatric diabetes. 156 , 157 , 158
Severe obesity and bariatric surgery
The prevalence of overweight and obesity in children is plateauing after many years of sharp increase in HICs. 159 , 160 However, morbid obesity still continues to grow silently in an obesogenic environment, most notably in 12‐ to 19‐year‐olds. 159 , 161 , 162 Despite multidisciplinary programs to manage pediatric obesity, bariatric surgery is one of the primary management methods. Operations are costly and constraining, using either restrictive or gastrointestinal bypass procedures now extensively performed by well‐trained surgeons in HICs, even in pediatric populations. 163
It is a common belief that TD after bariatric surgery is a frequent complication that occurs insidiously within 6 months after surgery in pediatrics, as in adults. No recent large studies or cohorts have yet established the true prevalence of TD in pediatric bariatric surgery patients. 164 Conversely, the literature abounds with scattered and somewhat informative case reports on TD in obese children after pediatric surgery, 58 , 164 requiring mulidisciplinary teams. Postbariatric TD usually presents as episodes of repetitive, uncontrolled diarrhea or vomiting, associated with minor neuropathic symptoms or atypical ocular signs, or full blown WE. 165 Our review suggests that most cases of WE occur in adolescents, indicating a profound depletion of the body's thiamine reserves over time.
Deficiencies of micronutrients, including vitamins D and B12, folate, iron, and thiamine, are also reported prior to bariatric surgery, likely due to attempts to lose weight tied to self‐induced vomiting or an unbalanced diet. 166
Eating disorders and anorexia
Anorexia in adolescence (C6) occupies a predominant proportion of cases in this review. In most HICs, these patients are managed by specialized multidisciplinary eating disorder units. Eating disorders, including anorexia, have also become a significant public health issue among adolescents in the industrialized world 167 , 168 , 169 and middle‐income countries (MICs) 170 They affect mostly girls, with an initial onset between 10 and 20 years of age and a peak age of onset in early to mid‐adolescence, according to studies in six European countries. 171 Two studies have described a variable frequency of anorexia‐related TD of between 10% and 32%. 79 , 83 Anorexia is a complex neuropsychiatric disorder with multifactorial components (sociocultural, epigenetic, and genetic). 172 Our review shows that anorexia is associated with various phenotypes of TD, WE being the culminating neurological presentation associated with other reported minor neuropsychiatric symptoms. 79 , 80 , 81 , 82 , 84 The multiple mechanisms leading to TD in anorexia include malnutrition associated with poor intake, as well as laxative use and self‐induced vomiting in patients with purging behaviors. A hypothesis for the occurrence of WE could be that pronounced depletion of thiamine stores and inadequate intake of thiamine affects the brain that is still undergoing maturation in adolescence 7 , 10 An underlying genetic error of thiamine metabolism has been described in some patients, making them more susceptible to any thiamine‐deficient diet. 19 , 172 , 173 Refeeding requires special attention in these cases. 174
To what extent can higher diagnostic capacities in HICs influence the detection of clinical expressions of PTD compared with resource‐limited settings?
The classic TD clinical spectrum is summarized in Figure 5. These clinical presentations are discussed in recent publications. 9 , 175 , 176
Figure 5.
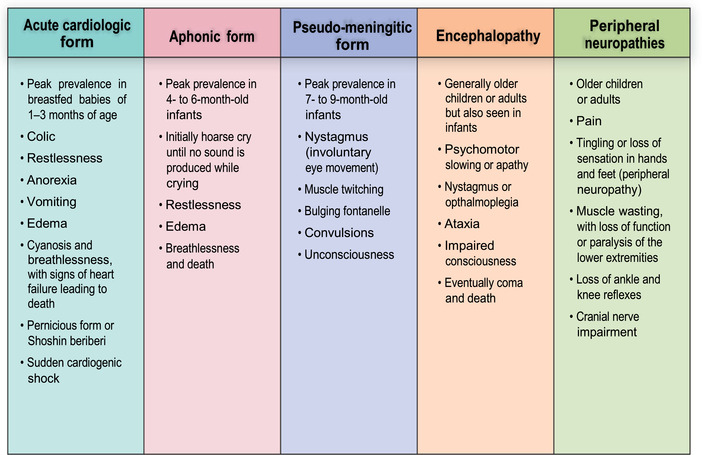
Clinical spectrum of classic thiamine deficiency. Reproduced from Ref. 9.
Classic infantile beriberi
Classic infantile beriberi in early months of infancy caused by feeding by thiamine‐deficient mothers is very rarely observed in HICs. Only two sets of cases are reported in this review. The first occurred on an epidemic scale in families living in disadvantaged socioeconomic conditions on the French island of Mayotte. Some sporadic cases have also been observed in the island of Reunion. 109 , 133 The second case series is related to the unfortunate case of infants fed thiamine‐deficient soy milk formula, which occurred on a large scale in Israel in 2003 30 , 122 and had a major long‐term impact, as discussed below.
In this study, there was no report of the nonspecific signs associated with infant TD, such as loud piercing cries, colic, refusal to breastfeed, vomiting, and agitation. 177 , 178 Nevertheless, our current review may suffer from underreporting of this early form of TD because most studies originate from specialized settings and not from primary care pediatrics. Few available studies have addressed the issue of the micropockets of poverty that are consistently present in the large megacities of rich countries. This context specifically relates to the hidden and high‐risk young population susceptible to overlooked multiple nutritional deficiencies, in addition to infectious and/or respiratory diseases within the general framework of pathology affecting minority families, poor migrants, and even homeless breastfeeding women, who can be marginalized from access to primary and specialist care. 179 , 180 , 181 , 182
Cardiovascular beriberi
Cardiovascular beriberi consists of the onset of heart failure, which can occur suddenly. It is described as hyperlactatemia associated with a high‐output cardiac failure, predominantly in the right or both cardiac chambers, and in its edematous form with a pitting edema in the lower limbs. The outcome can be exacerbated by loop diuretics without prompt thiamine administration. 66 , 113 , 114 , 125
One of the major findings was the occurrence of acute respiratory distress in tachycardic infants, expressed as a pulmonary arterial hypertension with hyperlactatemia, which is remarkably reversed by thiamine treatment, as noted earlier. 72 , 74 , 111 , 125 , 127 , 131
The popularization of pediatric bedside cardiac Doppler ultrasound in infants and the readily available assay of lactate point‐of‐care testing have allowed such a refined diagnostic and therapeutic achievement, which is becoming routine practice in HICs. 183 However, case reports of TD related to pulmonary arterial hypertension were emerging not long ago in MICs, where ultrasound cardiac Doppler and trained local staff are becoming more available. 184 , 185 , 186 This highlights that LMICs and HICs differ more from diagnostic capacities than clinical presentations.
Neurological signs of PTD
Early minor manifestations of TD neuropathy are characterized in this review by symmetrical sensory‐motor impairments (such as muscle pain and weakness), 84 dysarthria, dysfunction or paralysis of the lower extremities, progressive (gait) ataxia with or without inaugural signs of encephalopathy, and decreased deep tendon reflex function of the extremities, affecting mainly the lower limbs. This can progressively lead to altered consciousness. 80 , 82 , 84 , 108 , 122
The diagnosis of unexplained severe neurological signs of encephalopathy can benefit from modern neuroimaging and/or response to thiamine administration or both. In this review, there is no report of pseudo‐meningitic forms that usually occur around 6−12 months of age. 8 , 187 WE was the most frequent manifestation of TD in our review (Fig. 4), which can occur at any age, 57 , 89 but a significant subset of the reports in this review are during adolescence, as seen in categories C7–C9, as opposed to infancy in LMICs. 188 , 189 Its onset can have a rapid progression, with or without ataxia, and can be associated with other minor clinical manifestations, including muscular fasciculation or weakness, atypical ocular symptoms, nystagmus, ophthalmoplegia, seizures, and altered mental status until coma. 59 , 61 , 65 , 122 , 173 The main causative pathological conditions in our review were from anorexia, morbid obesity, and bariatric surgery; all of them are caused by an insufficient supply of thiamine tied to the lack of risk awareness. 62 , 90 , 105
In pediatric medical imaging platforms, as found in tertiary hospitals in HICs, the imaging modalities are useful tools for assessment and making a rapid as well as decisive diagnosis of PTD. Magnetic resonance imaging (MRI) is more available and feasible in HICs as a screening modality because of its greater diagnostic potential over CT. In adult studies, the MRI sensitivity is 53%, but its specificity is 93%, with a positive predictive value of 89%. 190 However, it is now widely used in pediatrics in HICs. 191 Unlike in adults, the pediatric brain MRI of Wernicke‐like syndrome can display peculiar lesions in the frontal lobe and basal ganglia, notably the striatum and putamen, all of these regions being sensitive to thiamine deprivation. Atypical lesions can also be located in the cerebellum. An evaluation of the cortex serves as an indicator of prognosis. 191 , 192 , 193 However, adults and children affected by TD show the same symmetrical high‐intensity signal on T2 weighting in mammillary bodies and periaqueductal and thalamic areas, sites of neuronal damage, and disruption to myelination confirmed by cerebral spectrum MRI.
Additional diagnostic tools can also be useful in identifying and characterizing TD. Cranial transfontanelar ultrasound in infants could be an alternative to MRI, as it can be performed at the bedside (point of care), revealing hyperechoic lesions of the basal ganglia. 188 Other techniques, such as the use of contrast enhancement, fluid‐attenuated inversion recovery (FLAIR) images, and diffusion‐weighted imaging, 194 as well as MR spectroscopy (displaying high lactate peaks), 195 have additional diagnostic value. 195 In addition, MRI can be used to monitor in situ recovery of neurological damage during thiamine treatment. 191 , 196
The MRI findings in HICs and LMICs are similar, with basal ganglia involvement (especially putamen and caudate nucleus 20 , 77 , 189 , 191 ), and the two differ largely by the accessibility of such neuroimaging tools. Some differences found between LMIC and HIC cohorts 189 regarding less involvement of other cerebral regions (e.g., mammillary bodies and periaqueductal gray matter) might be explained by multiple factors. Mammillary bodies and periaqueductal area abnormalities seem to be more frequent in children and adolescents than in young infants. 23 , 60 , 61 , 68 , 82 Similarly, nutrition, environment, and genetic diseases (such as Leigh syndrome) potentially overlapping with the TD spectrum might also contribute to these differences. 188 , 189 , 197 MRI protocols, such as contrast enhancement, might increase the sensitivity for mammillary bodies detection and has not been used systematically in some studies. 20 , 23 , 198 , 199 , 200 , 201
Neurological presentations, such as WE in adolescents, seem more frequent in HICs than in LMICs, but this may be biased as neuroimaging is the standard of care in HICs and has contributed significantly to its diagnosis.
Long‐term neurological sequelae (C1) was the most commonly reported of the categories in children. It includes outpatient care in pediatric neurology departments, or follow‐up in pediatric neurological services or neuroimaging department. 26 , 27 They are investigated in the neurolinguistic and psychological settings for their alteration in the syntactic and lexical modalities of language acquisition. 28 Most overt symptomatic cases who survived later developed complications in the form of epilepsy or lack of acquisition of developmental milestones. 29 Some children also presented permanent auditory dysfunction. 134
A number of infants who received this thiamine‐deficient soy‐based formula in Israel, but who were initially asymptomatic still presented years later with gross‐ and fine‐motor function alterations and learning difficulties. 9 , 28 This reflects how important thiamine is for children's overall neurodevelopment, and it highlights the importance of early diagnosis of TD and subclinical TD during this critical period in infancy of neurodevelopment that involves the acquisition of cognitive skills. In utero exposure to TD may also lead to fetal brain dysfunction, as observed in fetal alcohol syndrome. 202
This nutritional incident in Israel has probably contributed to reducing the extent of TD in infants in HICs because many national regulatory agencies in every industrialized country, the major international pediatric societies, and the infant formula industry have complied with the current minimum standards for infant formula, 203 in addition to the WHO promotion of breastfeeding in the first 6 months of life. However, such poor outcomes may be the fate of children from very underprivileged or homeless families who gradually develop TD, which can be expressed months or years later as neurocognitive disabilities. 182
High‐level medical management of pediatric complex illnesses can be associated with PTD in HICs
Most of the cases from this review occurred in high‐level tertiary centers, as opposed to classic beriberi in LMICs that tends to occur in primary settings. This is in line with the Lallas and Desai review 173 that describes WE in children and that points out three main scenarios, such as bariatric surgery, malignancies, and intensive care management. Our study reveals similar findings with more recent and detailed categories.
Owing to the increasing prevalence of morbid obesity and the recent advances in bariatric surgery procedures, 204 more and more adolescents follow this option, as represented by the C3 category. Bariatric surgery is a highly specialized, requiring multidisciplinary teams, including nutritionists, among other specialists. The reduction of both thiamine absorption following surgery and thiamine intake in the first months can lead to severe TD, including WE. 57 , 58 , 59 , 60 , 61
Categories C8 and C9 include diverse critical conditions that increase the risk of developing TD. Many highly specialized pediatric services were involved in these categories: oncology units, pediatric emergency rooms, PICUs or neonatal intensive care units, specialized surgical services (abdominal, cardiac, and transplantation), gastroenterology, and imaging and dialysis units. This is also reflected by the large number of different critical conditions, including cases of coma, shock with hyperlactatemia, heart failure, pulmonary arterial hypertension detected by color Doppler sonography, postsurgical nutritional complications, nephrological cases, and neurological cases with minor sensorimotor signs or atypical or full WE. 23 , 91 , 110 , 111 , 116 , 119 , 123 , 125 , 126 , 131
In these categories, multiple pathogenic mechanisms leading to TD are often involved. This can be observed in postsurgical cases of heart operation or organ transplantation. 119 The predominant cause is a mismatch between an increased metabolic demand and an inadequate thiamine availability. This can be caused by the coexistence of underlying malabsorption or excessive digestive or renal loss of thiamine, and/or insufficient removal of oxythiamine (a uremic toxin inhibiting transketolase), as in end‐stage renal disease. 205 , 206 , 207 As such, an unusual cluster of TD cases has been reported to be mostly tied to advanced techniques in dialysis or hemofiltration. 114 , 121 , 126 Such a subcategory of PTD risk linked with end‐stage renal diseases has not been fully delineated by Lallas and Desai, 173 while there has been significant and incremental advances in renal replacement‐dialysis modalities (peritoneal dialysis, intermittent hemodialysis, and continuous and hybrid therapies). 208
C4 is represented by a set of reports of neonates and infants/children on TPN or EN with poor thiamine intake or increased intestinal losses in which TD appears after insufficient thiamine supply in therapeutic feeding. 62 , 67
Critically ill patients, often malnourished and/or on prolonged TPN, have complex metabolisms that can be further compromised by receiving polychemotherapy that can interact with thiamine metabolism: diuretics, proton pump inhibitor drugs, metronidazole and some other antibiotics, tacrolimus, cyclosporin, 209 , 210 5‐fluorouracil, and methotrexate, 93 , 101 among others. This is represented to some extent in our C8 category, especially in perioperative bone marrow transplantations; however, drugs that potentially interact with thiamine metabolism are not uncommon in other categories. Transient reduction of micronutrients has been described in systemic inflammation, 211 , 212 , 213 and Peixoto de Lima et al. described an independent correlation with C‐reactive protein (CRP) levels and TD. 214 In this review, very few reports mention CRP monitoring, 70 , 85 , 86 , 87 , 117 , 127 and none monitored CRP and thiamine trends simultaneously, apart from Peixoto de Lima et al. The not insignificant consequences of transient TD on children's developing brains are difficult to document in daily clinical practice as no clinician would take the risk of delaying such treatment (due to the potential long‐term sequelae that have been described even in initially asymptomatic thiamine‐deficient infants, as documented in the C0 category of this study).
While the major cause of classic diet thiamine deprivation is no longer relevant in 2020 in HICs, this study has revealed a small but noticeable number of PTD cases arising from critical illnesses or chronic conditions that are managed in highly specialized pediatric services. All of these patients suffer various risks of PTD.
Strengths and limitations
Strengths
This up‐to‐date review is a condensed and unique study that comprehensively addresses complex PTD issues, including age, clinical presentation, and predisposing risk factors, observed in HICs.
Limitations
First, as this is not a systematic review, the number of retrieved articles cannot reflect the magnitude or prevalence of overall PTD in HICs. The number of countries included in this review accounts for only 28% of the total HICs, according to the World Bank. 25 We also excluded opinion pieces and commentaries, which do not constitute original research.
Second, the included studies are only case studies and series, which are not evidence of the best quality (i.e., high‐quality, randomized clinical trials or systematic reviews). Nevertheless, they should not be excluded from scientific consideration. These instructive case series or reports deal with facts and are based on observational data, so they remain illustrative of the real world of medical practice in PTD issues.
Third, the methodology of using a compilation of several scattered cases led to a high level of clinical heterogeneity. Nevertheless, in light of the likely low prevalence of the phenomenon, this method proves to be the only pragmatic approach to account for it. They result in concordant PTD observations encountered all over the world within the limited circle of HICs.
Conclusion
This study provides an updated and holistic overview of PTD in HICs over the last two decades and highlights two paradigm changes. First, there is a clear shift toward older children as opposed to young infants, as seen in LMICs. Second, multiple underlying conditions, either individually or concomitantly, can lead to PTD in HICs, such as anorexia, critical illnesses, malignancies, and renal replacement therapies. The growing burden of NCDs, such as morbid obesity, is beginning to have an impact on PTD. In addition, this study supports the idea that the nervous system, notably the growing brain, is a crucial target of thiamine deprivation among the pediatric population. WE is the predominant manifestation of PTD, and its early signs need to be known in order to avoid long‐term sequelae. Neuroimaging with MRI is a common diagnostic tool, with characteristic TD findings that allow clinicians to monitor treatment response as well. PTD is mostly described in high‐level tertiary centers, where medical awareness needs to be high as PTD can also be associated with complex medical management. The number of children with neurocognitive disabilities, followed up after an overt clinical or subclinical TD insult that occurred during early infancy, should call for special attention toward the most vulnerable populations in HICs, including pregnant migrant women and young infants living in precarious conditions in HICs.
Author contributions
B.R. and L.H. were involved in the conception and drafting of the manuscript, performed the literature searches, and extracted the data. All authors were involved in the design (including table and figures) and revision of the manuscript, and the approval of its final version.
Competing interests
The authors declare no competing interests.
Supporting information
Table S1. TD in early infancy followed up in tertiary neurocognitive referral centers.
Table S2. Overall description of cases of pediatric thiamine deficiency divided by categories of predisposing risk factors.
Acknowledgments
This research was supported by funding from the Bill & Melinda Gates Foundation to the New York Academy of Sciences (Grant number OPP1176128). We thank Megan Bourassa for her valuable comments to the manuscript and Rado Randriambovonjy, as well as Rija Rakotondralambo for their contribution (partial literature sorting).
References
- 1. Manzetti, S. , Zhang J. & Van Der Spoel D.. 2014. Thiamin function, metabolism, uptake, and transport. Biochemistry 53: 821–835. [DOI] [PubMed] [Google Scholar]
- 2. Singleton, C.K. & Martin P.R.. 2001. Molecular mechanisms of thiamine utilization. Curr. Mol. Med. 1: 197–207. [DOI] [PubMed] [Google Scholar]
- 3. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) . 2013. Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 11: 3408. [Google Scholar]
- 4. Strohm , D., Bechthold A., Isik N., et al. 2016. Revised reference values for the intake of thiamin (vitamin B 1), riboflavin (vitamin B 2), and niacin. NFS J. 3: 20–24. [Google Scholar]
- 5. Bettendorff, L. , Mastrogiacomo F., Kish S.J., et al. 1996. Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain. J. Neurochem. 66: 250–258. [DOI] [PubMed] [Google Scholar]
- 6. DiNicolantonio, J.J. , Liu J. & O'Keefe J.H.. 2018. Thiamine and cardiovascular disease: a literature review. Prog. Cardiovasc. Dis. 61: 27–32. [DOI] [PubMed] [Google Scholar]
- 7. Giedd, J.N. , Blumenthal J., Jeffries N.O., et al. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2: 861–863. [DOI] [PubMed] [Google Scholar]
- 8. Hiffler, L. , Rakotoambinina B., Lafferty N., et al. 2016. Thiamine deficiency in tropical pediatrics: new insights into a neglected but vital metabolic challenge. Front. Nutr. 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitfield, K. , Bourassa M.W., Adamolekun B., et al. 2018. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann. N.Y. Acad. Sci. 1430: 3–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhir, S. , Tarasenko M., Napoli E., et al. 2019. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front. Psychiatry 10: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rakotoambinina, B. & Hiffler L.. 2020. Aspectos atuais da deficiência de tiamina em pediatria. In Micronutrientes em pediatria. Leite H.P. & Konstantyner T., Eds.: 56–103. Editora Manole Ltda. [Google Scholar]
- 12. Nazir, M. , Lone R. & Charoo B.A.. 2019. Infantile thiamine deficiency: new insights into an old disease. Indian Pediatr. 56: 673–681. [PubMed] [Google Scholar]
- 13. Tiong, S.A. , Chandra‐Hioe M.V. & Arcot J.. 2015. Thiamin fortification of bread‐making flour: retention in bread and levels in Australian commercial fortified bread varieties. J. Food Compos. Anal. 38: 27–31. [Google Scholar]
- 14. Brown, J.V. , Lin L., Embleton N.D., et al. 2020. Multi‐nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 8: CD000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harper, C.G. , Sheedy D.L., Lara A.I., et al. 1998. Prevalence of Wernicke–Korsakoff syndrome in Australia: has thiamine fortification made a difference? Med. J. Aust. 168: 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacco, J. & Tarasuk V.. 2013. Limitations of food composition databases and nutrition surveys for evaluating food fortification in the United States and Canada. Procedia Food Sci. 2: 203–210. [Google Scholar]
- 17. Allen, L.H. , de Benoist B., Dary O., et al. 2006. Guidelines on Food Fortification with Micronutrients. WHO. [Google Scholar]
- 18. Food Standards Australia New Zealand . 2016. Thiamin levels in Australian breads: results from the 2010 and 2012 National Bread Surveys. Report by Food Standards Australia New Zealand (FSANZ).
- 19. Sechi, G. & Serra A.. 2007. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 6: 442–455. [DOI] [PubMed] [Google Scholar]
- 20. Kornreich, L. , Bron‐Harlev E., Hoffmann C., et al. 2005. Thiamine deficiency in infants: MR findings in the brain. Am. J. Neuroradiol. 26: 1668–1674. [PMC free article] [PubMed] [Google Scholar]
- 21. Salvatori, G. , Mondì V., Piersigilli F., et al. 2016. Thiamine deficiency in a developed country. J. Parenter. Enter. Nutr. 40: 886–889. [DOI] [PubMed] [Google Scholar]
- 22. Saeki, K. , Saito Y., Komaki H., et al. 2010. Thiamine‐deficient encephalopathy due to excessive intake of isotonic drink or overstrict diet therapy in Japanese children. Brain Dev. 32: 556–563. [DOI] [PubMed] [Google Scholar]
- 23. Lamdhade, S. , Almulla A. & Alroughani R.. 2014. Recurrent Wernicke's encephalopathy in a 16‐year‐old girl with atypical clinical and radiological features. Case Rep. Neurol. Med. 2014: 582482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen‐Khoa, D.‐T. 2020. Accessed May 12, 2020. https://emedicine.medscape.com/article/116930‐overview.
- 25. The World Bank . Accessed June 12, 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 26. Mimouni‐Bloch, A. , Goldberg‐Stern H., Strausberg R., et al. 2014. Thiamine deficiency in infancy: long‐term follow‐up. Pediatr. Neurol. 51: 311–316. [DOI] [PubMed] [Google Scholar]
- 27. Harel, Y. , Zuk L., Guindy M., et al. 2017. The effect of subclinical infantile thiamine deficiency on motor function in preschool children. Matern. Child Nutr. 13: e12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fattal‐Valevski, A. , Azouri‐Fattal I., Greenstein Y.J., et al. 2009. Delayed language development due to infantile thiamine deficiency. Dev. Med. Child Neurol. 51: 629–634. [DOI] [PubMed] [Google Scholar]
- 29. Fattal‐Valevski, A. , Bloch‐Mimouni A., Kivity S., et al. 2009. Epilepsy in children with infantile thiamine deficiency. Neurology 73: 828–833. [DOI] [PubMed] [Google Scholar]
- 30. Fattal‐Valevski, A. , Kesler A., Sela B.‐A., et al. 2005. Outbreak of life‐threatening thiamine deficiency in infants in Israel caused by a defective soy‐based formula. Pediatrics 115: e233–e238. [DOI] [PubMed] [Google Scholar]
- 31. Olsen, B.S. , Hahnemann J.M.D., Schwartz M., et al. 2007. Thiamine‐responsive megaloblastic anaemia: a cause of syndromic diabetes in childhood. Pediatr. Diabetes 8: 239–241. [DOI] [PubMed] [Google Scholar]
- 32. Mayr, J.A. , Freisinger P., Schlachter K., et al. 2011. Thiamine pyrophosphokinase deficiency in encephalopathic children with defects in the pyruvate oxidation pathway. Am. J. Hum. Genet. 89: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lagarde, W.H. , Underwood L.E., Moats‐Staats B.M., et al. 2004. Novel mutation in the SLC19A2 gene in an African‐American female with thiamine‐responsive megaloblastic anemia syndrome. Am. J. Med. Genet. Part A 125A: 299–305. [DOI] [PubMed] [Google Scholar]
- 34. Shaw‐Smith, C. , Flanagan S.E., Patch A.‐M., et al. 2012. Recessive SLC19A2 mutations are a cause of neonatal diabetes mellitus in thiamine‐responsive megaloblastic anaemia. Pediatr. Diabetes 13: 314–321. [DOI] [PubMed] [Google Scholar]
- 35. Mozzillo, E. , Melis D., Falco M., et al. 2013. Thiamine responsive megaloblastic anemia: a novel SLC19A2 compound heterozygous mutation in two siblings. Pediatr. Diabetes 14: 384–387. [DOI] [PubMed] [Google Scholar]
- 36. Tahir, S. , Leijssen L.G.J., Sherif M., et al. 2015. A novel homozygous SLC19A2 mutation in a Portuguese patient with diabetes mellitus and thiamine‐responsive megaloblastic anaemia. Int. J. Pediatr. Endocrinol. 2015: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikstiene, V. , Songailiene J., Byckova J., et al. 2015. Thiamine responsive megaloblastic anemia syndrome: a novel homozygous SLC19A2 gene mutation identified. Am. J. Med. Genet. Part A 167: 1605–1609. [DOI] [PubMed] [Google Scholar]
- 38. Li, X. , Cheng Q., Ding Y., et al. 2019. TRMA syndrome with a severe phenotype, cerebral infarction, and novel compound heterozygous SLC19A2 mutation: a case report. BMC Pediatr. 19: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uroic, A. , Milenkovic D., Franco E., et al. 2020. Importance of immediate thiamine therapy in children with suspected thiamine‐responsive megaloblastic anemia—report on two patients carrying a novel SLC19A2 gene mutation. J. Pediatr. Genet. 10.1055/s-0040-1717136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Debs, R. , Depienne C., Rastetter A., et al. 2010. Biotin‐responsive basal ganglia disease in ethnic Europeans with novel SLC19A3 mutations. Arch. Neurol. 67: 126–130. [DOI] [PubMed] [Google Scholar]
- 41. Yamada, K. , Miura K., Hara K., et al. 2010. A wide spectrum of clinical and brain MRI findings in patients with SLC19A3mutations. BMC Med. Genet. 11: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pérez‐Dueñas, B. , Serrano M., Rebollo M., et al. 2013. Reversible lactic acidosis in a newborn with thiamine transporter‐2 deficiency. Pediatrics 131: e1670 LP–e1675. [DOI] [PubMed] [Google Scholar]
- 43. Fassone, E. , Wedatilake Y., DeVile C.J., et al. 2013. Treatable Leigh‐like encephalopathy presenting in adolescence. BMJ Case Rep. 2013: bcr2013200838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aljabri, M.F. , Kamal N.M., Arif M., et al. 2016. A case report of biotin–thiamine‐responsive basal ganglia disease in a Saudi child: is extended genetic family study recommended? Medicine (Baltimore) 95: e4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flønes, I. , Sztromwasser P., Haugarvoll K., et al. 2016. Novel SLC19A3 promoter deletion and allelic silencing in biotin‐thiamine‐responsive basal ganglia encephalopathy. PLoS One 11: e0149055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alfadhel, M. , Almuntashri M., Jadah R.H., et al. 2013. Biotin‐responsive basal ganglia disease should be renamed biotin‐thiamine‐responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J. Rare Dis. 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kelley, R.I. , Robinson D., Puffenberger E.G., et al. 2002. Amish lethal microcephaly: a new metabolic disorder with severe congenital microcephaly and 2‐ketoglutaric aciduria. Am. J. Med. Genet. 112: 318–326. [DOI] [PubMed] [Google Scholar]
- 48. Rajab, A. , Manzini M.C., Mochida G.H., et al. 2007. A novel form of lethal microcephaly with simplified gyral pattern and brain stem hypoplasia. Am. J. Med. Genet. Part A 143A: 2761–2767. [DOI] [PubMed] [Google Scholar]
- 49. Spiegel, R. , Shaag A., Edvardson S., et al. 2009. SLC25A19 mutation as a cause of neuropathy and bilateral striatal necrosis. Ann. Neurol. 66: 419–424. [DOI] [PubMed] [Google Scholar]
- 50. Siu, V.M. , Ratko S., Prasad A.N., et al. 2010. Amish microcephaly: long‐term survival and biochemical characterization. Am. J. Med. Genet. Part A 152A: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 51. Dunckelmann, R.J. , Ebinger F., Schulze A., et al. 2000. 2‐Ketoglutarate dehydrogenase deficiency with intermittent 2‐ketoglutaric aciduria. Neuropediatrics 31: 35–38. [DOI] [PubMed] [Google Scholar]
- 52. Huang, W. , Qin J., Liu D., et al. 2019. Reduced thiamine binding is a novel mechanism for TPK deficiency disorder. Mol. Genet. Genomics 294: 409–416. [DOI] [PubMed] [Google Scholar]
- 53. Nyhan, W.L. , McGowan K. & Barshop B.A.. 2019. Thiamine phosphokinase deficiency and mutation in TPK1 presenting as biotin responsive basal ganglia disease. Clin. Chim. Acta 499: 13–15. [DOI] [PubMed] [Google Scholar]
- 54. Eckenweiler, M. , Mayr J., Grünert S., et al. 2020. Thiamine treatment and favorable outcome in an infant with biallelic TPK1 variants. Neuropediatrics 52: 123–125. [DOI] [PubMed] [Google Scholar]
- 55. Clark, J.A. , Burny I., Sarnaik A.P., et al. 2006. Acute thiamine deficiency in diabetic ketoacidosis: diagnosis and management. Pediatr. Crit. Care Med. 7: 595–599. [DOI] [PubMed] [Google Scholar]
- 56. Rosner, E.A. , Strezlecki K.D., Clark J.A., et al. 2015. Low thiamine levels in children with type 1 diabetes and diabetic ketoacidosis: a pilot study. Pediatr. Crit. Care Med. 16: 114–118. [DOI] [PubMed] [Google Scholar]
- 57. Towbin, A. , Inge T.H., Garcia V.F., et al. 2004. Beriberi after gastric bypass surgery in adolescence. J. Pediatr. 145: 263–267. [DOI] [PubMed] [Google Scholar]
- 58. Stenerson, M. , Renaud D., Dufendach K., et al. 2013. Recurrent Wernicke encephalopathy in an adolescent female following laparoscopic gastric bypass surgery. Clin. Pediatr. (Phila.) 52: 1067–1069. [DOI] [PubMed] [Google Scholar]
- 59. Armstrong‐Javors, A. , Pratt J. & Kharasch S.. 2016. Wernicke encephalopathy in adolescents after bariatric surgery: case report and review. Pediatrics 138: e20161039. [DOI] [PubMed] [Google Scholar]
- 60. Samanta, D. 2015. Dry beriberi preceded Wernicke's encephalopathy: thiamine deficiency after laparoscopic sleeve gastrectomy. J. Pediatr. Neurosci. 10: 297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Takacs, D. & Lyons‐Warren A.. 2018. Isolated ophthalmoplegia as presenting sign of pediatric Wernicke encephalopathy (P3.333). Neurology 90: P3.333. [Google Scholar]
- 62. Thauvin‐Robinet, C. , Faivre L., Barbier M.L., et al. 2004. Severe lactic acidosis and acute thiamin deficiency: a report of 11 neonates with unsupplemented total parenteral nutrition. J. Inherit. Metab. Dis. 27: 700–704. [DOI] [PubMed] [Google Scholar]
- 63. Masumoto, K. , Esumi G., Teshiba R., et al. 2009. Need for thiamine in peripheral parenteral nutrition after abdominal surgery in children. J. Parenter. Enter. Nutr. 33: 417–422. [DOI] [PubMed] [Google Scholar]
- 64. Ramsi, M. , Mowbray C., Hartman G., et al. 2014. Severe lactic acidosis and multiorgan failure due to thiamine deficiency during total parenteral nutrition. BMJ Case Rep. 2014: bcr2014205264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Long, L. , Cai X.‐D., Bao J., et al. 2014. Total parenteral nutrition caused Wernicke's encephalopathy accompanied by wet beriberi. Am. J. Case Rep. 15: 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lefevre, C. , Mallett L.H. & Wick L.. 2018. Myocardial ischemia in an adolescent secondary to nutritional thiamine deficiency. Baylor Univ. Med. Cent. Proc. 31: 51–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Salvatori, G. , Mondì V., Piersigilli F., et al. 2016. Thiamine deficiency in a developed country: acute lactic acidosis in two neonates due to unsupplemented parenteral nutrition. J. Parenter. Enter. Nutr. 40: 886–889. [DOI] [PubMed] [Google Scholar]
- 68. Benidir, A.N. , Laughlin S. & Ng V.L.. 2014. Visual disturbances in total parenteral nutrition dependent liver transplant pediatric patient. Gastroenterology 146: e10–1. [DOI] [PubMed] [Google Scholar]
- 69. Nozue, H. , Kamoda T. & Matsui A.. 2001. Water intoxication and lactic acidosis caused by excessive intake of commercial sports drink. Acta Paediatr. 90: 1357–1358. [PubMed] [Google Scholar]
- 70. Majima, N. , Umegaki O. & Soen M.. 2013. Use of central venous saturation monitoring in a patient with pediatric cardiac beriberi. World J. Clin. Cases 1: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takemoto, T. , Toshio H., Aya K., et al. 2008. A 20‐month‐old infant with beriberi caused by excessive intake of isotonic drink. J. Jpn. Pediatr. Soc. 112: 1710–1171. [Google Scholar]
- 72. Sakurai, K. , Fujiwara N., Takahashi K., et al. 2019. Excessive soft drink may induce pulmonary hypertension via thiamine deficiency. Pediatr. Int. 61: 823–824. [DOI] [PubMed] [Google Scholar]
- 73. Fujii, T. , Hojo A., Nakamura T., et al. 2012. Rapid improvement of echocardiographic and electrocardiographic findings in a 1‐year‐old patient with beriberi heart. J. Jpn. Pediatr. Soc. 116: 84–91. [Google Scholar]
- 74. Kataoka, H. , Arakaki Y., Waki K., et al. 2012. Beriberi presenting reversible severe pulmonary hypertension by excessive intake of isotonic drink. J. Jpn. Pediatr. Soc. 116: 1228–1232. [Google Scholar]
- 75. Shioda, T. , Watanabe S., Kyogoku T., et al. 2014. A case of pediatric Wernicke's encephalopathy due to excessive intake of isotonic drinks. J. Jpn. Pediatr. Soc. 111: 930–936. [Google Scholar]
- 76. Hiraki, A. & Kikuchi M.. 2014. Wernicke's encephalopathy due to excessive intake of isotonic drink — report of 2 cases. No to Hattatsu 46: 34–38. [PubMed] [Google Scholar]
- 77. Okumura, A. , Ida S., Mori M., et al. 2018. Vitamin B1 deficiency related to excessive soft drink consumption in Japan. J. Pediatr. Gastroenterol. Nutr. 66: 838–842. [DOI] [PubMed] [Google Scholar]
- 78. Ko, C. , Lau W. & Cheng W.. 2011. Wernicke's encephalopathy in an adolescent who leads a high risk lifestyle. HK J. Paediatr. 16: 285–288. [Google Scholar]
- 79. Winston, A.P. , Jamieson C.P., Madira W., et al. 2000. Prevalence of thiamin deficiency in anorexia nervosa. Int. J. Eat. Disord. 28: 451–454. [DOI] [PubMed] [Google Scholar]
- 80. Heiser, P. , Teepker M., Möller J.C., et al. 2004. Neuropathy due to hypovitaminosis following excessive weight loss. J. Am. Acad. Child Adolesc. Psychiatry 43: 928–929. [DOI] [PubMed] [Google Scholar]
- 81. Peters, T.E. , Parvin M., Petersen C., et al. 2007. A case report of Wernicke's encephalopathy in a pediatric patient with anorexia nervosa–restricting type. J. Adolesc. Health 40: 376–383. [DOI] [PubMed] [Google Scholar]
- 82. Renthal, W. , Marin‐Valencia I. & Evans P.A.. 2014. Thiamine deficiency secondary to anorexia nervosa: an uncommon cause of peripheral neuropathy and Wernicke encephalopathy in adolescence. Pediatr. Neurol. 51: 100–103. [DOI] [PubMed] [Google Scholar]
- 83. Bahat, H. , Reisler G., Brandriss N., et al. 2020. Thiamine deficiency in adolescents with eating disorders: a prospective cohort study. Nutrition 12: 1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. French, K.F. , Candee M.S., Stahl J.L., et al. 2013. Clinical reasoning: a 12‐year‐old boy with ascending weakness. Neurology 80: e110 LP–e114. [DOI] [PubMed] [Google Scholar]
- 85. Greenspon, J. , Perrone E.E. & Alaish S.M.. 2010. Shoshin beriberi mimicking central line sepsis in a child with short bowel syndrome. World J. Pediatr. 6: 366–368. [DOI] [PubMed] [Google Scholar]
- 86. Barnes, D. & Kerner J.. 2016. Severe lactic acidosis in a parenteral nutrition‐dependent teenager with ulcerative colitis. Dig. Dis. Sci. 61: 2804–2806. [DOI] [PubMed] [Google Scholar]
- 87. Salloum, S. , Goenka A. & Mezoff A.. 2018. Beriberi disease in an 11‐year‐old girl with total colectomy. Oxford Med. Case Rep. 2018(11): omy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roilides, I. , Vasilaki K., Xinias I., et al. 2019. Thiamine deficiency in a child with short bowel syndrome and review. Pediatr. Gastroenterol. Hepatol. Nutr. 22: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang, Y. , Zhou B., Wu L., et al. 2020. Short bowel syndrome in an extremely low birth weight premature infant with Wernicke encephalopathy: a case report. Am. J. Case Rep. 21: e924830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Anderson, J. , Kandeepan A., Wratney A., et al. 2020. Type B lactic acidosis patient with Crohn disease and thiamine deficiency. Crit. Care Med. 48: 212. [Google Scholar]
- 91. Rodan, L.H. , Mishra N. & Tein I.. 2013. MR spectroscopy in pediatric Wernicke encephalopathy. Neurology 80: 969 LP–969. [DOI] [PubMed] [Google Scholar]
- 92. D'Aprile, P. , Tarantino A., Santoro N., et al. 2000. Wernicke's encephalopathy induced by total parenteral nutrition in patient with acute leukaemia: unusual involvement of caudate nuclei and cerebral cortex on MRI. Neuroradiology 42: 781–783. [DOI] [PubMed] [Google Scholar]
- 93. Svahn, J. , Schiaffino M.C., Caruso U., et al. 2003. Severe lactic acidosis due to thiamine deficiency in a patient with B cell leukemia/lymphoma on total parenteral nutrition during high‐dose methotrexate therapy. J. Pediatr. Hematol. Oncol. 25: 965–968. [DOI] [PubMed] [Google Scholar]
- 94. Cefalo, M.G. , De Ioris M.A., Cacchione A., et al. 2014. Wernicke encephalopathy in pediatric neuro‐oncology: presentation of 2 cases and review of literature. J. Child Neurol. 29: NP181–NP185. [DOI] [PubMed] [Google Scholar]
- 95. La Spina, M. , Catania R., Lo Nigro L., et al. 2010. Neurological complications during treatment of childhood cancer: mind Wernicke encephalopathy. Pediatr. Hematol. Oncol. 27: 320–323. [DOI] [PubMed] [Google Scholar]
- 96. Zensho, K. , Ishida H., Nagai H., et al. 2018. Wernicke's encephalopathy in a child with autism during chemotherapy for T cell acute leukemia. Pediatr. Int. 60: 757–758. [DOI] [PubMed] [Google Scholar]
- 97. Didisheim, C. , Ballhausen D., Choucair M., et al. 2020. Severe lactic acidosis in a critically ill child: think about thiamine! A case report. J. Pediatr. Intensive Care 10.1055/s-0040-1713382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Derespina, K. , Greenberg M., Mahadeo K., et al. 2018. 416: Lactic acidosis secondary to thiamine deficiency following autologous stem cell transplantation. Crit. Care Med. 46: 191. [DOI] [PubMed] [Google Scholar]
- 99. Murali, C.N. & Ganetzky R.. 2020. Persistent lactic acidosis in an 18‐month‐old girl status post bone marrow transplant. Pediatr. Rev. 41: S20 LP–S22. [DOI] [PubMed] [Google Scholar]
- 100. Byun, S.W. , Choi S.‐H., Park H.‐G., et al. 2007. A case of lactic acidosis caused by thiamine deficiency. Korean J. Med. 73: 443–447. [Google Scholar]
- 101. Han, J.W. , Lim S., Shin H.S., et al. 2012. Two cases of Wernicke's encephalopathy in young age patients receiving allogeneic hematopoietic stem cell transplantation. Yonsei Med. J. 53: 1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Takahashi, H. , Koh K., Yasui N., et al. 2013. Wernicke's encephalopathy in a 21‐trisomy girl with acute lymphoblastic leukemia. J. Jpn. Pediatr. Soc. 117: 113–117. [Google Scholar]
- 103. Lerner, R.K. , Pessach I., Rubinstein M., et al. 2017. Lactic acidosis as presenting symptom of thiamine deficiency in children with hematologic malignancy. J. Pediatr. Intensive Care 6: 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bae, S.‐N. , Kim B.‐J., Park S.‐S., et al. 2001. A case of Wernicke's encephalopathy which occurred during chemotherapy in a child with acute myeloblastic leukemia. J. Korean Pediatr. Soc. 44: 1320–1325. [Google Scholar]
- 105. Perko, R. , Harreld J.H., Helton K.J., et al. 2012. What goes around comes around? Wernicke encephalopathy and the nationwide shortage of intravenous multivitamins revisited. J. Clin. Oncol. 30: e318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Park, S.W. , Yi Y.Y., Han J.W., et al. 2014. Wernicke's encephalopathy in a child with high dose thiamine therapy. Korean J. Pediatr. 57: 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Darlington, W.S. , Pinto N., Hecktman H.M., et al. 2015. Stem cell transplant‐associated Wernicke encephalopathy in a patient with high‐risk neuroblastoma. Pediatr. Blood Cancer 62: 2232–2234. [DOI] [PubMed] [Google Scholar]
- 108. Decker, M.J. & Isaacman D.J.. 2000. A common cause of altered mental status occurring at an uncommon age. Pediatr. Emerg. Care 16: 94–96. [DOI] [PubMed] [Google Scholar]
- 109. Moulin, P. , Cinq‐Frais C., Gangloff C., et al. 2014. [Thiamine deficiency in infants: a case report]. Arch. Pediatr. 21: 392–395. [DOI] [PubMed] [Google Scholar]
- 110. Cottini, M. , Ranucci M., Facciolo C., et al. 2016. An unusual case of cardiogenic shock in which thiamine administration led to reversal of lactic acidosis and heart function recovery: Shoshin beriberi in an adolescent. Int. J. Cardiol. 222: 401–403. [DOI] [PubMed] [Google Scholar]
- 111. Teagarden, A.M. , Leland B.D., Rowan C.M., et al. 2017. Thiamine deficiency leading to refractory lactic acidosis in a pediatric patient. Case Rep. Crit. Care 2017: 5121032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Harshman, L.A. , Lee‐Son K. & Jetton J.G.. 2018. Vitamin and trace element deficiencies in the pediatric dialysis patient. Pediatr. Nephrol. 33: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vicinanza, A. , De Laet C., Rooze S., et al. 2019. Shoshin beriberi and severe accidental hypothermia as causes of heart failure in a 6‐year‐old child: a case report and brief review of literature. Front. Pediatr. 7: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Elias, I.M. , Sinclair G. & Blydt‐Hansen T.D.. 2019. Acute Shoshin beriberi syndrome immediately post–kidney transplant with rapid recovery after thiamine administration. Pediatr. Transplant. 23: e13493. [DOI] [PubMed] [Google Scholar]
- 115. Weiss, S.L. , Blowey B., Keele L., et al. 2019. Matched retrospective cohort study of thiamine to treat persistent hyperlactatemia in pediatric septic shock. Pediatr. Crit. Care Med. 20: e452–e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tiwana, H.K. , Raymond G., Littleton K., et al. 2020. Thiamine deficiency with mashed potatoes: a novel case of Wernicke encephalopathy manifesting with retinal hemorrhages in a pediatric patient. Cureus 12: e10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kubota, S. , Fuchigami T., Ishii W., et al. 2015. A 3‐year‐old girl with Wernicke's encephalopathy due to a severely unbalanced diet. Neurol. Asia 20: 95–99. [Google Scholar]
- 118. Coe, M. , Carfagnini F., Tani G., et al. 2001. Wernicke's encephalopathy in a child: case report and MR findings. Pediatr. Radiol. 31: 167–168. [DOI] [PubMed] [Google Scholar]
- 119. Shamir, R. , Dagan O., Abramovitch D., et al. 2000. Thiamine deficiency in children with congenital heart disease before and after corrective surgery. J. Parenter. Enter. Nutr. 24: 154–158. [DOI] [PubMed] [Google Scholar]
- 120. Ahmed, R. 2012. Wernicke like encephalopathy in a child: a reversible cause. J. Pediatr. Neurosci. 7: 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pela, I. , Seracini D., Lavoratti G.C., et al. 2000. Efficacy of hemodiafiltration in a child with severe lactic acidosis due to thiamine deficiency. Clin. Nephrol. 53: 400–403. [PubMed] [Google Scholar]
- 122. Kesler, A. , Stolovitch C., Hoffmann C., et al. 2005. Acute ophthalmoplegia and nystagmus in infants fed a thiamine‐deficient formula: an epidemic of Wernicke encephalopathy. J. Neuro‐ophthalmol. 25: 169–172. [DOI] [PubMed] [Google Scholar]
- 123. Cooke, C.A. , Hicks E., Page A.B., et al. 2006. An atypical presentation of Wernicke's encephalopathy in an 11‐year‐old child. Eye 20: 1418–1420. [DOI] [PubMed] [Google Scholar]
- 124. Abu‐Kishk, I. , Rachmiel M., Hoffmann C., et al. 2009. Infantile encephalopathy due to vitamin deficiency in industrial countries. Child's Nerv. Syst. 25: 1477–1480. [DOI] [PubMed] [Google Scholar]
- 125. Sakuma, H. , Hye Kim J., Ichikawa Y., et al. 2013. Thiamine deficiency induced remarkable pulmonary hypertension in an infant. Pediatr. Cardiol. Card. Surg. 29: 352–356. [Google Scholar]
- 126. Baracco, R. , Ku L., Adabala M., et al. 2013. An adolescent on peritoneal dialysis with acute encephalopathy. Pediatr. Nephrol. 28: 1221–1225. [DOI] [PubMed] [Google Scholar]
- 127. Quatresous, I. , Tajahmady A. & Sissoko D.. 2004. Epidémie de Béribéri infantile à Mayotte ‐ Rapport d'investigation .
- 128. Ringe, H. , Schuelke M., Weber S., et al. 2014. Infant botulism: is there an association with thiamine deficiency? Pediatrics 134: e1436–e1440. [DOI] [PubMed] [Google Scholar]
- 129. Baird, J.S. & Ravindranath T.M.. 2015. Vitamin B deficiencies in a critically ill autistic child with a restricted diet. Nutr. Clin. 30: 100–103. [DOI] [PubMed] [Google Scholar]
- 130. Watanabe, S. , Yamakura S., Hirano K., et al. 2009. [Case of infantile autism with pediatric Wernicke's encephalopathy due to severe eating disorder]. No to hattatsu = Brain Dev. 41: 43–46. [PubMed] [Google Scholar]
- 131. Duvall, M.G. , Pikman Y., Kantor D.B., et al. 2013. Pulmonary hypertension associated with scurvy and vitamin deficiencies in an autistic child. Pediatrics 132: e1699–703. [DOI] [PubMed] [Google Scholar]
- 132. Lonsdale, D. , Shamberger R.J. & Audhya T.. 2002. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: a pilot study. Neuro Endocrinol. Lett. 23: 303–308. [PubMed] [Google Scholar]
- 133. Tajahmady, A. , Quatresous I., Sissoko D., et al. 2004. Une épidémie de béribéri infantile à Mayotte. 213–216.
- 134. Attias, J. , Raveh E., Aizer‐Dannon A., et al. 2012. Auditory system dysfunction due to infantile thiamine deficiency: long‐term auditory sequelae. Audiol. Neurootol. 17: 309–320. [DOI] [PubMed] [Google Scholar]
- 135. Agostoni, C. , Buonocore G., Carnielli V.P., et al. 2010. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 50: 85–91. [DOI] [PubMed] [Google Scholar]
- 136. Libuda, L. & Kersting M.. 2009. Soft drinks and body weight development in childhood: is there a relationship? Curr. Opin. Clin. Nutr. Metab. Care 12: 596–600. [DOI] [PubMed] [Google Scholar]
- 137. Berkey, C.S. , Rockett H.R.H., Field A.E., et al. 2004. Sugar‐added beverages and adolescent weight change. Obes. Res. 12: 778–788. [DOI] [PubMed] [Google Scholar]
- 138. Vartanian, L.R. , Schwartz M.B. & Brownell K.D.. 2007. Effects of soft drink consumption on nutrition and health: a systematic review and meta‐analysis. Am. J. Public Health 97: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Phillips, S.M. , Bandini L.G., Naumova E.N., et al. 2004. Energy‐dense snack food intake in adolescence: longitudinal relationship to weight and fatness. Obes. Res. 12: 461–472. [DOI] [PubMed] [Google Scholar]
- 140. Ludwig, D.S. , Peterson K.E. & Gortmaker S.L.. 2001. Relation between consumption of sugar‐sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet (London, England) 357: 505–508. [DOI] [PubMed] [Google Scholar]
- 141. Hardy, L.L. , Grunseit A., Khambalia A., et al. 2012. Co‐occurrence of obesogenic risk factors among adolescents. J. Adolesc. Health 51: 265–271. [DOI] [PubMed] [Google Scholar]
- 142. Hermens, D.F. , Lagopoulos J., Tobias‐Webb J., et al. 2013. Pathways to alcohol‐induced brain impairment in young people: a review. Cortex 49: 3–17. [DOI] [PubMed] [Google Scholar]
- 143. SEARCH for Diabetes in Youth Study Group . 2006. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 118: 1510 LP–1518. [DOI] [PubMed] [Google Scholar]
- 144. Wherrett, D.K. , Ho J., Huot C., et al. 2018. Type 1 diabetes in children and adolescents. Can. J. Diabetes 42(Suppl. 1): S234–S246. [DOI] [PubMed] [Google Scholar]
- 145. Cali, A.M.G. & Caprio S.. 2008. Prediabetes and type 2 diabetes in youth: an emerging epidemic disease? Curr. Opin. Endocrinol. Diabetes Obes. 15: 123–127. [DOI] [PubMed] [Google Scholar]
- 146. Harding, J.L. , Pavkov M.E., Magliano D.J., et al. 2019. Global trends in diabetes complications: a review of current evidence. Diabetologia 62: 3–16. [DOI] [PubMed] [Google Scholar]
- 147. Patterson, C.C. , Karuranga S., Salpea P., et al. 2019. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157: 107842. [DOI] [PubMed] [Google Scholar]
- 148. Wolfsdorf, J. , Glaser N. & Sperling M.A.. 2006. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 29: 1150–1159. [DOI] [PubMed] [Google Scholar]
- 149. Glaser, N.S. , Wootton‐Gorges S.L., Buonocore M.H., et al. 2006. Frequency of sub‐clinical cerebral edema in children with diabetic ketoacidosis. Pediatr. Diabetes 7: 75–80. [DOI] [PubMed] [Google Scholar]
- 150. Moskowitz, A. , Graver A., Giberson T., et al. 2014. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J. Crit. Care 29: 182.e5–182.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Glaser, N. , Barnett P., McCaslin I., et al. 2001. Risk factors for cerebral edema in children with diabetic ketoacidosis. N. Engl. J. Med. 344: 264–269. [DOI] [PubMed] [Google Scholar]
- 152. Wolfsdorf, J.I. , Glaser N., Agus M., et al. 2018. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes 19(Suppl. 2): 155–177. [DOI] [PubMed] [Google Scholar]
- 153. Gupta, R. & McNally J.D.. 2016. 1685: Type B lactic acidosis in diabetic ketoacidosis responding to thiamine therapy. Crit. Care Med. 44: 496.26540401 [Google Scholar]
- 154. Liu, X. , Uber A., Holmberg M., et al. 2017. P452 — In vitro administration of ubiquinol and thiamine improve cellular oxygen consumption in patients with diabetic ketoacidosis. In 37th International Symposium on Intensive Care and Emergency Medicine (part 3 of 3) .
- 155. Mohamed, R.A.‐E. , Abu Farag I.M., Elhady M., et al. 2019. Myocardial dysfunction in relation to serum thiamine levels in children with diabetic ketoacidosis. J. Pediatr. Endocrinol. Metab. 32: 335–340. [DOI] [PubMed] [Google Scholar]
- 156. Thornalley, P.J. , Babaei‐Jadidi R., Al Ali H., et al. 2007. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 50: 2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Jermendy, G. 2006. Evaluating thiamine deficiency in patients with diabetes. Diabetes Vasc. Dis. Res. 3: 120–121. [DOI] [PubMed] [Google Scholar]
- 158. Rabbani, N. & Thornalley P.J.. 2011. Emerging role of thiamine therapy for prevention and treatment of early‐stage diabetic nephropathy. Diabetes Obes. Metab. 13: 577–583. [DOI] [PubMed] [Google Scholar]
- 159. Farpour‐Lambert, N.J. , Baker J.L., Hassapidou M., et al. 2015. Childhood obesity is a chronic disease demanding specific health care—a position statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO). Obes. Facts 8: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. ALNohair, S. 2014. Obesity in gulf countries. Int. J. Health Sci. (Qassim) 8: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Skinner, A.C. , Ravanbakht S.N., Skelton J.A., et al. 2018. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 141: e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Skelton, J.A. , Cook S.R., Auinger P., et al. 2009. Prevalence and trends of severe obesity among US children and adolescents. Acad. Pediatr. 9: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Angrisani, L. , Santonicola A., Iovino P., et al. 2015. Bariatric surgery worldwide 2013. Obes. Surg. 25: 1822–1832. [DOI] [PubMed] [Google Scholar]
- 164. Stanford, A. , Glascock J.M., Eid G.M., et al. 2003. Laparoscopic Roux‐en‐Y gastric bypass in morbidly obese adolescents. J. Pediatr. Surg. 38: 430–433. [DOI] [PubMed] [Google Scholar]
- 165. Juhasz‐Pocsine, K. , Rudnicki S.A., Archer R.L., et al. 2007. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology 68: 1843 LP–1850. [DOI] [PubMed] [Google Scholar]
- 166. Carrodeguas, L. , Kaidar‐Person O., Szomstein S., et al. 2005. Preoperative thiamine deficiency in obese population undergoing laparoscopic bariatric surgery. Surg. Obes. Relat. Dis. 1: 517–522. [DOI] [PubMed] [Google Scholar]
- 167. Smink, F.R.E. , van Hoeken D., Donker G.A., et al. 2016. Three decades of eating disorders in Dutch primary care: decreasing incidence of bulimia nervosa but not of anorexia nervosa. Psychol. Med. 46: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 168. Steinhausen, H.‐C. & Jensen C.M.. 2015. Time trends in lifetime incidence rates of first‐time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a Danish nationwide psychiatric registry study. Int. J. Eat. Disord. 48: 845–850. [DOI] [PubMed] [Google Scholar]
- 169. Pike, K.M. & Borovoy A.. 2004. The rise of eating disorders in Japan: issues of culture and limitations of the model of “westernization”. Cult. Med. Psychiatry 28: 493–531. [DOI] [PubMed] [Google Scholar]
- 170. Kolar, D.R. , Rodriguez D.L.M., Chams M.M., et al. 2016. Epidemiology of eating disorders in Latin America: a systematic review and meta‐analysis. Curr. Opin. Psychiatry 29: 363–371. [DOI] [PubMed] [Google Scholar]
- 171. Preti, A. , de Girolamo G., Vilagut G., et al. 2009. The epidemiology of eating disorders in six European countries: results of the ESEMeD‐WMH project. J. Psychiatr. Res. 43: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 172. Yilmaz, Z. , Hardaway J.A. & Bulik C.M.. 2015. Genetics and epigenetics of eating disorders. Adv. Genomics Genet. 5: 131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lallas, M. & Desai J.. 2014. Wernicke encephalopathy in children and adolescents. World J. Pediatr. 10: 293–298. [DOI] [PubMed] [Google Scholar]
- 174. Pulcini, C.D. , Zettle S. & Srinath A.. 2016. Refeeding syndrome. Pediatr. Rev. 37: 516–523. [DOI] [PubMed] [Google Scholar]
- 175. Smith, T.J. & Hess S.Y.. 2021. Infantile thiamine deficiency in South and Southeast Asia: an age‐old problem needing new solutions. Nutr. Bull. 46: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Qureshi, U.A. , Bhat A.S., Qureshi U., et al. 2021. Infantile thiamine deficiency: redefining the clinical patterns. Nutrition 84: 111097. [DOI] [PubMed] [Google Scholar]
- 177. Heird, W.C. 2004. Nutrition in Pediatrics: Basic Science and Clinical Applications. Hamilton, ON: BC Decker Inc. [Google Scholar]
- 178. Rabinowitz, S. 2018. Accessed August 12, 2020. emedicine.medscape.com/article/984721‐overview.
- 179. Beharry, M.S. & Christensen R.. 2020. Homelessness in pediatric populations: strategies for prevention, assistance, and advocacy. Pediatr. Clin. North Am. 67: 357–372. [DOI] [PubMed] [Google Scholar]
- 180. Grant, R. , Shapiro A., Joseph S., et al. 2007. The health of homeless children revisited. Adv. Pediatr. 54: 173–187. [DOI] [PubMed] [Google Scholar]
- 181. Hacker, K. , Anies M., Folb B.L., et al. 2015. Barriers to health care for undocumented immigrants: a literature review. Risk Manag. Healthc. Policy 8: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dias, F.M.V. , de Silva D.M.F., de Doyle F.C.P., et al. 2013. The connection between maternal thiamine shortcoming and offspring cognitive damage and poverty perpetuation in underprivileged communities across the world. Med. Hypotheses 80: 13–16. [DOI] [PubMed] [Google Scholar]
- 183. Market . 2021. Global biochemistry glucose lactate analyzer market research report .
- 184. Panigrahy, N. , Chirla D.K., Shetty R., et al. 2020. Thiamine‐responsive acute pulmonary hypertension of early infancy (TRAPHEI)—a case series and clinical review. Child (Basel, Switzerland) 7: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sastry, U.M.K., Jayranganath M., Kumar R.K., et al. 2021. Thiamine‐responsive acute severe pulmonary hypertension in exclusively breastfeeding infants: a prospective observational study. Arch. Dis. Child. 106: 241 LP–246. [DOI] [PubMed] [Google Scholar]
- 186. Bhat, J.I. , Rather H.A., Ahangar A.A., et al. 2017. Shoshin beriberi‐thiamine responsive pulmonary hypertension in exclusively breastfed infants: a study from northern India. Indian Heart J. 69: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Newland, J.G. , Shah S.S. & Zaoutis T.E.. 2003. The child with aseptic meningitis. Pediatr. Case Rev. 3: 218–221. [DOI] [PubMed] [Google Scholar]
- 188. Wani, N.A. , Qureshi U.A., Ahmad K., et al. 2016. Cranial ultrasonography in infantile encephalitic beriberi: a useful first‐line imaging tool for screening and diagnosis in suspected cases. Am. J. Neuroradiol. 37: 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wani, N.A. , Qureshi U.A., Jehangir M., et al. 2016. Infantile encephalitic beriberi: magnetic resonance imaging findings. Pediatr. Radiol. 46: 96–103. [DOI] [PubMed] [Google Scholar]
- 190. Antunez, E. , Estruch R., Cardenal C., et al. 1998. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. Am. J. Roentgenol. 171: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 191. Zuccoli, G. , Siddiqui N., Bailey A., et al. 2010. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology 52: 523–529. [DOI] [PubMed] [Google Scholar]
- 192. Fei, G‐Q. , Zhong C., Jin L., et al. 2008. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. Am. J. Neuroradiol. 29: 164 LP–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Xin, Y. , Wan D.H., Chu Q., et al. 2011. Severe sepsis as an initial presentation in children with Wernicke's encephalopathy: report of a case and literature review. Chinese J. Pediatr. 49: 612–616. [PubMed] [Google Scholar]
- 194. Chung, T.‐I. , Kim J.‐S., Park S.‐K., et al. 2003. Diffusion weighted MR imaging of acute Wernicke's encephalopathy. Eur. J. Radiol. 45: 256–258. [DOI] [PubMed] [Google Scholar]
- 195. Rugilo, C.A. , Uribe Roca M.C., Zurru M.C., et al. 2003. Proton MR spectroscopy in Wernicke encephalopathy. Am. J. Neuroradiol. 24: 952–955. [PMC free article] [PubMed] [Google Scholar]
- 196. Murata, T. , Fujito T., Kimura H., et al. 2001. Serial MRI and (1)H‐MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res. 108: 49–55. [DOI] [PubMed] [Google Scholar]
- 197. Mani, S. , Rao S.N. & Kranthi Kumar M.V.. 2019. Genetic heterogeneity of mitochondrial genome in thiamine deficient Leigh syndrome patients. J. Neurol. Sci. 404: 91–100. [DOI] [PubMed] [Google Scholar]
- 198. D'Aprile, P. , Gentile M.A. & Carella A.. 1994. Enhanced MR in the acute phase of Wernicke encephalopathy. Am. J. Neuroradiol. 15: 591–593. [PMC free article] [PubMed] [Google Scholar]
- 199. Mascalchi, M. , Simonelli P., Tessa C., et al. 1999. Do acute lesions of Wernicke's encephalopathy show contrast enhancement? Report of three cases and review of the literature. Neuroradiology 41: 249–254. [DOI] [PubMed] [Google Scholar]
- 200. Kavuk, I. , Agelink M.W., Gaertner T., et al. 2003. Wernicke's encephalopathy: unusual contrast enhancement revealed by magnetic resonance imaging. Eur. J. Med. Res. 8: 492–494. [PubMed] [Google Scholar]
- 201. Konno, Y. , Kanoto M., Hosoya T., et al. 2014. Clinical significance of mammillary body enhancement in Wernicke encephalopathy: report of 2 cases and review of the literature. Magn. Reson. Med. Sci. 13: 123–126. [DOI] [PubMed] [Google Scholar]
- 202. Kloss, O. , Eskin N.A.M. & Suh M.. 2018. Thiamin deficiency on fetal brain development with and without prenatal alcohol exposure. Biochem. Cell Biol. 96: 169–177. [DOI] [PubMed] [Google Scholar]
- 203. Codex Alimentarius . 2007. Standard for infant formula and formulas for special medical puporses intended for infants .
- 204. Thakkar, R.K. & Michalsky M.P.. 2015. Update on bariatric surgery in adolescence. Curr. Opin. Pediatr. 27: 370–376. [DOI] [PubMed] [Google Scholar]
- 205. Zhang, F. , Masania J., Anwar A., et al. 2016. The uremic toxin oxythiamine causes functional thiamine deficiency in end‐stage renal disease by inhibiting transketolase activity. Kidney Int. 90: 396–403. [DOI] [PubMed] [Google Scholar]
- 206. Hung, S.C. , Hung S.H., Tarng D.C., et al. 2001. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am. J. Kidney Dis. 38: 941–947. [DOI] [PubMed] [Google Scholar]
- 207. Golan, O. , Dyer R., Sinclair G., et al. 2021. Investigating oxythiamine levels in children undergoing kidney transplantation and the risk of immediate post‐operative metabolic and hemodynamic decompensation. Pediatr. Nephrol. 36: 987–993. [DOI] [PubMed] [Google Scholar]
- 208. de Galasso, L. , Picca S. & Guzzo I.. 2020. Dialysis modalities for the management of pediatric acute kidney injury. Pediatr. Nephrol. 35: 753–765. [DOI] [PubMed] [Google Scholar]
- 209. Brown, R.D. 1990. The proton channel blocking agent omeprazole is an inhibitor of the thiamin shuttle. J. Theor. Biol. 143: 565–573. [DOI] [PubMed] [Google Scholar]
- 210. Wilson, R.B. 2020. Pathophysiology, prevention, and treatment of beriberi after gastric surgery. Nutr. Rev. 78: 1015–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Marino, L.V. , Valla F.V., Beattie R.M., et al. 2020. Micronutrient status during paediatric critical illness: a scoping review. Clin. Nutr. 39: 3571–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Dao, D.T. , Anez‐Bustillos L., Cho B.S., et al. 2017. Assessment of micronutrient status in critically ill children: challenges and opportunities. Nutrients 9: 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Gerasimidis, K. , Bronsky J., Catchpole A., et al. 2020. Assessment and interpretation of vitamin and trace element status in sick children: a position paper from the European Society for Paediatric Gastroenterology Hepatology, and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 70: 873–881. [DOI] [PubMed] [Google Scholar]
- 214. Peixoto de Lima, L.F. , Leite H.P. & de AC Taddei J.A.. 2011. Low blood thiamine concentrations in children upon admission to the intensive care unit: risk factors and prognostic significance. Am. J. Clin. Nutr. 93: 57–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. TD in early infancy followed up in tertiary neurocognitive referral centers.
Table S2. Overall description of cases of pediatric thiamine deficiency divided by categories of predisposing risk factors.


