Abstract
Background
The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) is a risk‐stratification reporting system that was introduced in 2018. The objective of this multi‐institutional study was to evaluate the utility of the MSRSGC in Japan.
Methods
In total, 1608 fine‐needle aspiration samples with matching histologic diagnoses were retrieved from 12 large institutions in Japan. The diagnostic categories of the MSRSGC were assigned prospectively or retrospectively, and the results were compared with the histologic diagnoses.
Results
The cases were classified as follows: nondiagnostic, 18.1%; non‐neoplastic, 4.1%; atypia of undetermined significance, 11.5%; neoplasm‐benign, 43.7%; salivary gland neoplasm of uncertain malignant potential, 9.6%; suspicious for malignancy, 3.6%; and malignant, 9.4%. The risk of neoplasm and the risk of malignancy in each MSRSGC category were as follows: nondiagnostic, 72.9% and 13.4%, respectively; non‐neoplastic, 15.2% and 9.1%, respectively; atypia of undetermined significance, 77.9% and 24.9%, respectively; neoplasm‐benign, 99% and 1.8%, respectively; salivary gland neoplasm of uncertain malignant potential, 94.8% and 37%, respectively; suspicious for malignancy, 100% and 89.7%, respectively; and malignant, 100% and 99.3%, respectively. The accuracy of the MSRSGC for diagnosing neoplasms was 97.8%, and its accuracy for diagnosing malignancy was 97.3%. Institutions that used Romanowsky‐stained preparations had lower nondiagnostic rates and lower risks of neoplasm and malignancy in the non‐neoplastic category.
Conclusions
The MSRSGC is useful for risk stratification and quality control. Widespread use of the MSRSGC would improve the accuracy of salivary gland cytology and lead to better patient care in Japan.
Keywords: fine‐needle aspiration, Japan, multi‐institutional study, salivary gland cytopathology, the Milan system
Short abstract
This is the first multi‐institutional study of a large‐scale application of the Milan System for Reporting Salivary Gland Cytopathology in Japan. It demonstrates the usefulness of the Milan system in the diagnosis of salivary gland lesions and for the quality control of salivary gland cytology results.
Introduction
Salivary gland fine‐needle aspiration cytology (FNAC) is a useful tool for preoperatively diagnosing salivary gland lesions. It can be used to differentiate neoplastic salivary gland lesions from non‐neoplastic lesions and benign tumors from highly malignant tumors. 1 , 2 , 3 However, because of the inherent complexity of interpreting salivary gland FNAC findings and the lack of a uniform reporting format, salivary gland FNAC performance varies among institutions. 4
The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) was introduced in 2018 and is an evidence‐based classification scheme for addressing these issues with salivary gland cytology. 5 , 6 , 7 It provides the risk of malignancy (ROM) for 7 diagnostic categories, along with recommendations for clinical management. The 7 diagnostic categories are nondiagnostic (ND), non‐neoplastic (NN), atypia of undetermined significance (AUS), neoplasm‐benign (NB), salivary gland neoplasm of uncertain malignant potential (SUMP), suspicious for malignancy (SFM), and malignant (M). 5 , 6 , 7
Use of the MSRSGC is currently spreading throughout the world, and many studies about its application in Asian countries, including Japan, have been published. 8 , 9 , 10 , 11 Salivary gland tumors are as uncommon in Japan, where they represent 6.1% of head and neck cancers and 0.2% of all malignancies, as they are in other countries; eg, they account for 6% of head and neck cancers and 0.3% of all malignancies in the United States. 12 , 13 Consequently, no large‐scale studies of salivary gland FNAC have been conducted in Japan. The study reported herein was 1 of the largest studies of the MSRSGC in the world 7 , 8 , 9 , 10 , 11 , 14 , 15 , 16 , 17 , 18 , 19 and the first multi‐institutional study of the application of the MSRSGC in Japan. With the support of the Japanese Society of Clinical Cytology, we demonstrated the usefulness of the MSRSGC in the diagnosis of salivary gland lesions and for the quality control of salivary gland cytology results.
Materials and Methods
This study was conducted after approval was obtained from each institutional review board. It was conducted prospectively at Kyushu University Hospital and retrospectively at the other institutions. The cytology archives of 12 institutions in Japan were electronically searched for cases of salivary gland lesions that were treated between 2007 and 2019 for which salivary gland FNAC samples and matching histologic diagnoses were available. Each institution was numbered as follows: Tokyo Medical University, 1; Kurume University Hospital, 2; Kyushu University Hospital, 3; Fujita Health University, 4; Naritatomisato Tokushukai Hospital, 5; Kyoto Medical Center, 6; Hiroshima Red Cross Hospital and Atomic‐Bomb Survivors Hospital, 7; Kochi University Hospital, 8; Ehime University Hospital, 9; University of the Ryukyus Hospital, 10; Mita Hospital (International University of Health and Welfare), 11; and Kure Medical Center and Chugoku Cancer Center, 12. The puncture sites were grouped into the parotid gland, the submandibular gland, and others. FNAC was performed with 21‐gauge to 25‐gauge needles and involved 1 to 3 passes. The specimens were processed using the direct smear and/or the liquid‐based cytology method and were stained with Papanicolaou stain or with both Papanicolaou and Romanowsky stains. All FNAC specimens from each institute were processed by the protocol shown in Table 1. Ancillary immunocytochemistry was performed in a total of 15 cases. The MSRSGC was applied to all cases by cytopathologists and/or cytotechnologists at each institution by reviewing glass slides. We also assessed the performance of the MSRSGC by calculating its sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for diagnosing malignancy and neoplasms. In our analysis of the ability of the MSRSGC to diagnose malignancy, we considered the SFM and M categories to be positive and the NN and NB categories to be negative. In our analysis of the ability of the MSRSGC to diagnose neoplasms, we considered the NB, SUMP, SFM, and M categories to be positive and the NN category to be negative. We also characterized AUS, SUMP, false‐positive cases, and false‐negative cases, and nonmucinous cysts. The Student t test was used to compare the average values of continuous variables between 2 groups.
TABLE 1.
Case Demographics and Fine‐Needle Aspiration Sample Preparation
| Institution | Case No. | Age (Range), y | Sex: Male/Female | Puncture Site: P/SM/O | Pap | Romanowsky | LBC |
|---|---|---|---|---|---|---|---|
| 1 | 331 | 54.8 (0‐96) | 163/168 | 257/70/4 | Yes | Yes | |
| 2 | 121 | 57.1 (15‐90) | 68/53 | 104/17/0 | Yes | Yes | Yes |
| 3 | 44 | 62.0 (22‐92) | 19/25 | 32/8/4 | Yes | Yes | |
| 4 | 72 | 53,9 (11‐91) | 37/35 | 43/12/17 | Yes | Yes | Yes |
| 5 | 165 | NA | NA | NA | Yes | Yes | |
| 6 | 142 | 50.2 (14‐85) | 65/77 | 115/27/0 | Yes | Yes | |
| 7 | 107 | 61.7 (23‐87) | 62/45 | 87/16/4 | Yes | Yes | |
| 8 | 53 | 63.7 (25‐92) | 28/25 | 43/10/0 | Yes | Yes | |
| 9 | 111 | 61.0 (16‐89) | 60/51 | 80/26/5 | Yes | Yes | |
| 10 | 216 | 52.4 (11‐90) | 98/118 | 188/27/1 | Yes | ||
| 11 | 146 | 57.6 (23‐87) | 71/75 | 117/27/2 | Yes | ||
| 12 | 100 | 61.5 (24‐88) | 57/43 | 100/0/0 | Yes | Yes | |
| Total | 1608 | 56.4 (0‐96) | 728/715 | 1166/240/37 | |||
| Ratio, 1.02:1.00 | 80.8%/16.6%/2.6% |
Abbreviations: LBC, liquid‐based cytology; NA, not available; O, others; P, parotid gland; Pap, Papanicolaou staining; SM, submandibular gland.
Results
In total, 1608 cases from 12 institutions were analyzed, with 44 to 331 cases collected from each institution. The mean age of the patients was 56.4 years (range, 0‐96 years), and the male‐to‐female ratio was 728/715 (1.02). The parotid gland accounted for the largest percentage of puncture sites (80.8%), followed by the submandibular gland (16.6%), and other sites (2.6%). Demographic data and information about the FNAC sample preparation methods for each institution are summarized in Table 1.
The 1608 FNAC samples were classified as follows based on the MSRSGC: ND, 291 cases (18.1%); NN, 66 cases (4.1%); AUS, 185 cases (11.5%); NB, 703 cases (43.7%); SUMP, 154 cases (9.6%); SFM, 58 cases (3.6%); and M, 151 cases (9.4%) (Table 2). The matching histologic diagnoses were as follows: 1054 benign neoplasms (65.5%), 363 malignant neoplasms (22.6%), and 191 non‐neoplastic lesions (11.8%). The RON and ROM for each category were as follows: ND, 72.9% and 13.4%, respectively; NN, 15.2% and 9.1%, respectively; AUS, 77.9% and 24.9%, respectively; NB, 99% and 1.8%, respectively; SUMP, 94.8% and 37%, respectively; SFM, 100% and 89.7%, respectively; and M, 100% and 99.3%, respectively (Table 2).
TABLE 2.
Results of the Reclassification of Salivary Gland Lesions Using the Milan System for Reporting Salivary Gland Cytopathology
| Results | Histologic Diagnosis, No. | Total No. of Cases (%) [Range] | RON, % [Range] | ROM, % [Range] | MSRSGC ROM, % | ||
|---|---|---|---|---|---|---|---|
| Non‐Neoplastic | Neoplasm, Benign | Malignant | |||||
| Nondiagnostic | 79 | 173 | 39 | 291 (18.1) [4.7‐34.2] | 72.9 [51.3‐90.9] | 13.4 [0.0‐41.7] | 25.0 |
| Non‐neoplastic | 56 | 4 | 6 | 66 (4.1) [0‐7.7] | 15.2 [0.0‐62.5] | 9.1 [0.0‐50.0] | 10.0 |
| AUS | 41 | 98 | 46 | 185(11.5) [4.1‐25.2] | 77.9 [54.5‐100.0] | 24.9 [3.7‐100.0] | 20.0 |
| Neoplasm, benign | 7 | 683 | 13 | 703 (43.7) [30.2‐52.7] | 99 [95.1‐100.0] | 1.8 [0.0‐6.3] | <5.0 |
| SUMP | 8 | 89 | 57 | 154 (9.6) [3.6‐15.7] | 94.8 [80.0‐100.0] | 37.0 [18.2‐57.1] | 35.0 |
| SFM | 0 | 6 | 52 | 58 (3.6) [0‐5.7] | 100 [100.0] | 89.7 [75.0‐100.0] | 60.0 |
| Malignant | 0 | 1 | 150 | 151 (9.4) [5.6‐14.4] | 100 [100.0] | 99.3 [90.0‐100.0] | 90.0 |
| Total no. (%) | 191 (11.8) | 1054 (65.5) | 363 (22.6) | 1608 | |||
Abbreviations: AUS, atypia of undetermined significance; MSRSGC, Milan System for Reporting Salivary Gland Cytopathology; ROM, risk of malignancy; RON, risk of neoplasm; SFM, suspicious for malignancy; SUMP, salivary gland neoplasm of uncertain malignant potential.
When the AUS and SUMP categories were excluded, the sensitivity, specificity, and accuracy of the MSRSGC for diagnosing malignancy were 91.4%, 99.1%, and 97.3%, respectively (Table 3). The PPV and NPV of the MSRSGC for diagnosing malignancy were 96.7% and 97.5%, respectively (Table 3). When the AUS category was excluded, the sensitivity, specificity, accuracy, PPV, and NPV of the MSRSGC for diagnosing neoplasms were 99.1%, 78.9%, 97.8%, 98.6%, and 84.8%, respectively (Table 4).
TABLE 3.
Performance Characteristics of the Milan System for Reporting Salivary Gland Cytopathology for Detecting Malignancy
| Performance Characteristic | Rate, % |
|---|---|
| Sensitivity | 91.4 |
| Specificity | 99.1 |
| Accuracy | 97.3 |
| PPV | 96.7 |
| NPV | 97.5 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
TABLE 4.
Performance Characteristics of the Milan System for Reporting Salivary Gland Cytopathology for Detecting Neoplasms
| Performance Characteristic | Rate, % |
|---|---|
| Sensitivity | 99.1 |
| Specificity | 78.9 |
| Accuracy | 97.8 |
| PPV | 98.6 |
| NPV | 84.8 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
The characteristics of the AUS cases are summarized in Table 5. Among the 185 lesions classified as AUS, 41 (22.2%) were non‐neoplastic lesions, 98 (53%) were benign neoplasms, and 46 (24.9%) were malignant neoplasms. Warthin tumors were the most common type of tumor in the AUS category (59 cases; 31.9%), followed by pleomorphic adenoma (PA) (25 cases; 13.5%), and malignant lymphoma (23 cases; 12.4%) (Table 5). Malignant lymphoma was the most common malignant lesion in the AUS category. The malignant lymphomas included 8 mucosa‐associated lymphoid tissue lymphomas, 7 diffuse large B‐cell lymphomas, 6 follicular lymphomas, 1 adult T‐cell leukemia/lymphoma, and 1 Burkitt lymphoma. The most common type of carcinoma among the AUS cases was mucoepidermoid carcinoma (Table 5).
TABLE 5.
Characterization of Atypia of Undetermined Significance Cases
| Histologic Category | Histologic Diagnosis | No. (%) |
|---|---|---|
| Non‐neoplastic | Lymphoepithelial cyst | 14 |
| Lymphoepithelial lesion | 9 | |
| IgG4‐related sialadenitis | 6 | |
| chronic sialadenitis | 3 | |
| Granulation | 2 | |
| Lymphoid hyperplasia | 2 | |
| Fibrosis | 1 | |
| Necrosis | 1 | |
| Nodular oncocytic hyperplasia | 1 | |
| Ranula | 1 | |
| Normal | 1 | |
| Subtotal | 41 (22.2) | |
| Benign neoplasm | Warthin tumor | 59 |
| Pleomorphic adenoma | 25 | |
| Basal cell adenoma | 4 | |
| Lymphadenoma | 4 | |
| Oncocytoma | 1 | |
| Myoepithelioma | 1 | |
| Calcifying epithelioma | 1 | |
| Lipoma | 1 | |
| Schwannoma | 1 | |
| Cystadenoma | 1 | |
| Subtotal | 98 (53.0) | |
| Malignant | Malignant lymphoma | 23 |
| Mucoepidermoid carcinoma | 7 | |
| Acinic cell carcinoma | 3 | |
| Secretory carcinoma | 3 | |
| Carcinoma ex pleomorphic adenoma | 2 | |
| Squamous cell carcinoma | 2 | |
| Small cell carcinoma | 1 | |
| Epithelial‐myoepithelial carcinoma | 1 | |
| Salivary duct carcinoma | 1 | |
| Adenoid cystic carcinoma | 1 | |
| Carcinosarcoma | 1 | |
| Metastatic squamous cell carcinoma | 1 | |
| Subtotal | 46 (24.9) | |
| Total | 185 |
The characteristics of the 154 SUMP cases are summarized in Table 6. There were 8 (5.2%) non‐neoplastic lesions, 89 (57.8%) benign neoplasms, and 57 (37%) malignant neoplasms. The most common neoplasm in the SUMP category was PA (41 cases; 26.6%), followed by basal cell adenoma (31 cases; 20.1%), mucoepidermoid carcinoma (10 cases; 6.5%), adenoid cystic carcinoma (9 cases; 5.8%), and Warthin tumor (9 cases; 5.8%). The most common non‐neoplastic lesion was nodular oncocytic hyperplasia (Table 6).
TABLE 6.
Characterization of Salivary Gland Neoplasm of Uncertain Malignant Potential Cases
| Histologic Category | Histologic Diagnosis | No. (%) |
|---|---|---|
| Non‐neoplastic | Nodular oncocytic hyperplasia | 5 |
| IgG4‐related sialadenitis | 2 | |
| Normal | 1 | |
| Subtotal | 8 (5.2) | |
| Benign neoplasm | Pleomorphic adenoma | 41 |
| Basal cell adenoma | 31 | |
| Warthin tumor | 9 | |
| Myoepithelioma | 8 | |
| Subtotal | 89 (57.8) | |
| Malignant | Mucoepidermoid carcinoma | 10 |
| Adenoid cystic carcinoma | 9 | |
| Epithelial‐myoepithelial carcinoma | 7 | |
| Carcinoma ex pleomorphic adenoma | 7 | |
| Myoepithelial carcinoma | 4 | |
| Basal cell adenocarcinoma | 3 | |
| Secretory carcinoma | 2 | |
| Polymorphous adenocarcinoma | 2 | |
| Adenocarcinoma, NOS | 2 | |
| Acinic cell carcinoma | 2 | |
| Intraductal carcinoma | 2 | |
| MALT lymphoma | 2 | |
| Metastatic carcinoma | 1 | |
| Salivary duct carcinoma | 1 | |
| Squamous cell carcinoma | 1 | |
| Sebaceous adenocarcinoma | 1 | |
| Carcinoma, NOS | 1 | |
| Subtotal | 57 (37.0) | |
| Total | 154 (100.0) |
Abbreviations: MALT, mucosa‐associated lymphoid tissue; NOS, not otherwise specified.
In the ND category, there were 48 nonmucinous cysts (16.5%). Of these, 50% were non‐neoplastic lesions, and the rest were neoplasms. Lymphoepithelial cysts (22 cases; 45.8%) were the most common type of nonmucinous cyst, followed by Warthin tumors (14 cases; 29.2%), PAs (5 cases; 10.4%), and basal cell adenomas (3 cases; 6.3%). The only malignant nonmucinous cyst according to FNAC was a secretory carcinoma (Table 7).
TABLE 7.
Characterization of Nonmucinous Cysts
| Histologic Category | Histologic Diagnosis | No. (%) |
|---|---|---|
| Non‐neoplastic | Lymphoepithelial cyst | 22 (45.8) |
| Retention cyst | 2 (4.2) | |
| Subtotal | 24 (50.0) | |
| Neoplasm | ||
| Benign | Warthin tumor | 14 (29.2) |
| Pleomorphic adenoma | 5 (10.4) | |
| Basal cell adenoma | 3 (6.3) | |
| Schwannoma | 1 (2.1) | |
| Malignant | Secretory carcinoma | 1 (2.1) |
| Subtotal | 24 (50.0) | |
| Total | 48 (100.0) |
There were 7 false‐positive cases, including 4 cases of PA and 3 cases of basal cell adenoma (Table 8). The cases of PA in the SFM category involved bizarre myoepithelial cells or oncocytic metaplasia. There were 19 false‐negative cases (Table 9). The most common types were carcinoma ex PA and low‐grade mucoepidermoid carcinoma (5 cases each), followed by adenoid cystic carcinoma and malignant lymphoma (3 cases each). Four of the cases of carcinoma ex PA had small malignant components, and the malignant lymphomas included 2 cases of mucosa‐associated lymphoid tissue lymphoma and 1 case of follicular lymphoma.
TABLE 8.
False‐Positive Cases in the Malignant Category
| Histology | Category of MSRSGC | Total No. | |
|---|---|---|---|
| Suspicious for Malignancy (No.) | Malignancy (No.) | ||
| Pleomorphic adenoma | Acinic cell carcinoma (2) | 4 | |
| Carcinoma ex pleomorphic adenoma (2) | |||
| Basal cell adenoma | Acinic cell carcinoma (1) | Adenoid cystic carcinoma (2) | 3 |
| Total no. | 5 | 2 | 7 |
Abbreviation: MSRSGC, Milan System for Reporting Salivary Gland Cytopathology.
TABLE 9.
False‐Negative Cases in the Benign Category
| Histology | Category of MSRSGC | Total No. | |
|---|---|---|---|
| Non‐Neoplastic (No.) | Neoplasm, Benign (No.) | ||
| Carcinoma ex pleomorphic adenoma | Pleomorphic adenoma (5) | 5 | |
| Mucoepidermoid carcinoma | Inflammation (1) | Pleomorphic adenoma (3) | 5 |
| Warthin tumor (1) | |||
| Adenoid cystic carcinoma | Pleomorphic adenoma (2) | 3 | |
| Basal cell adenoma (1) | |||
| Malignant lymphoma | Lymphoid hyperplasia (3) | 3 | |
| Salivary duct carcinoma | Reactive lesion (1) | 1 | |
| Polymorphous adenocarcinoma | Pleomorphic adenoma (1) | 1 | |
| Metastatic carcinoma, NOS | Inflammation (1) | 1 | |
| Total no. | 6 | 13 | 19 |
Abbreviations: MSRSGC, Milan System for Reporting Salivary Gland Cytopathology; NOS, not otherwise specified.
Next, we analyzed the effects of using Romanowsky staining in addition to Papanicolaou staining for salivary FNAC. Whereas 1082 cases from 8 institutions were diagnosed with Romanowsky staining, 526 cases from 4 institutions were diagnosed without it (Table 1). The RON for each MSRSGC diagnostic category at the institutions that did and did not use Romanowsky staining, respectively, were as follows: ND, 72.3% versus 69.7%; NN, 8.9% versus 28.6%; AUS, 73.1% versus 89.1%; NB, 99% versus 99%; SUMP, 93.3% versus 96.9%; SFM, 100% versus 100%; and M, 100% versus 100% (see Supporting Table 1). The ROM for each MSRSGC category at the institutions that did and did not use Romanowsky staining, respectively, were as follows: ND, 12.8% versus 13.8%; NN, 4.4% versus 19%; AUS, 25.4% versus 23.6%; NB, 2% versus 1.5%; SUMP, 37.8% versus 39.1%; SFM, 87.8% versus 94.2%; and M, 98.9% versus 100% (see Supporting Table 1).
Discussion
Japan's universal health insurance system makes it easy for patients with neck masses to visit medical institutions. When a salivary gland tumor is suspected, ultrasound‐guided FNAC (generally involving 1‐3 passes) is usually performed followed by computed tomography and magnetic reson ance imaging. The Japanese System for Reporting Salivary Gland Cytopathology (JSRSGC) has long been used for salivary FNAC diagnosis in Japan. 20 The JSRSGC consists of 5 categories: nondiagnostic, normal or benign, indeterminate, suspicious for malignancy, and malignant. The JSRSGC can be used to stratify salivary gland lesions according to the ROM, but it is not effective at stratifying them according to the RON (see Supporting Table 2). The MSRSGC was proposed as a tool for stratifying salivary gland lesions according to the RON and the ROM and provides recommendations for clinical management. The National Comprehensive Cancer Network guidelines recommend that all salivary gland tumors, including both benign and malignant tumors, should be resected. 21 Although the JSRSGC demonstrates a good correlation with the MSRSGC (see Supporting Table 3), the MSRSGC is a more practical cytologic reporting system and ensures that appropriate patient care is provided.
The sex and anatomic site distributions seen in the current study were similar to those reported in previous studies (Table 1). 7 , 14 , 15 , 16 , 17 , 19 In contrast to many other studies, the current study included 4 institutions that did not perform Romanowsky staining for FNAC (Table 1). 7 , 8 , 9 , 14 , 15 , 17
In total, 291 cases (18.1%) were categorized as ND (Table 2), which was higher than the percentage (10%) recommended in the MSRSGC 22 and in many other previous studies. 7 , 9 , 14 , 15 , 16 , 18 In the current study, the frequency of ND cases varied among the institutions (range, 4.7%‐34.2%) (Table 2; see Supporting Table 4). The percentage of ND cases is affected by various factors, including the operator's technique, the appearance of the tumor, and the specimen‐processing methods used, eg, the staining method(s) used for FNAC specimens. Romanowsky staining of air‐dried materials results in better specimen preservation and is useful for detecting metachromatic stromal mucin in myoepithelial salivary gland tumors. 6 , 23 In this study, the mean percentage of ND cases tended to be lower at the facilities that used Romanowsky‐stained preparations compared with those that only used Papanicolaou staining (14.1% vs 21.9%) (see Supporting Table 4), but the difference was not statistically significant (P = .19). Rapid on‐site evaluation is another well known method for reducing the percentage of ND cases on FNAC. 6 , 18 However, in Japan, rapid on‐site evaluation is only performed at a limited number of facilities because of manpower shortages. It was only performed at 1 facility in the current study (institution 5), where the frequency of ND lesions was as low as 7.3%. Among the ND cases, 48 lesions (16.5%) were classified as nonmucinous cysts (Table 7). The MSRSGC classifies nonmucinous cysts as ND rather than as non‐neoplastic lesions 22 because some of these cysts may be neoplastic cystic lesions. In our study, 50% of the nonmucinous cysts were of neoplastic origin, confirming the validity of the MSRSGC. A recent report revealed that 29.2% of cystic FNAC specimens were reclassified as ND when the MSRSGC was applied. 24 Pantanowitz et al 25 reported the utility of classifying the FNAC findings of cystic lesions based on the presence or absence of mucin and admixed lymphocytes. Our results support their proposal because approximately one‐half of the nonmucinous cysts were derived from lymphoepithelial cysts.
In the NN category, the RON and ROM for NN lesions tended to be lower at the facilities that used Romanowsky‐stained preparations compared with those that only used Papanicolaou staining (8.9% vs 28.6% and 4.4% vs 19%, respectively) (see Supporting Table 1). However, the differences were not statistically significant (P = .31 and P = .57 for RON and ROM, respectively). Romanowsky staining might be beneficial to facilitate the correct classification of neoplasms partly because it prevents the underdiagnosis of neoplastic lesions, including malignant tumors. It is important to highlight the advantages of using Romanowsky staining in combination with Papanicolaou staining for salivary FNAC in Japan.
In total, 185 cases were categorized as AUS (Table 5). Warthin tumors were the most common type of tumor (59 cases) in the AUS category, accounting for 31.9% of all AUS cases. Japanese studies tend to include more Warthin tumors in the AUS category 11 , 26 (range, 41.9%‐50%) than those from other countries (range, 5.6%‐16.7%). 15 , 16 , 17 Cytologic specimens of typical Warthin tumors show oncocytic cell clusters with lymphocytes on a necrotic background, and such cases can be diagnosed by cytology with a high degree of certainty. 27 However, aspiration cytology often yields necrotic material containing only degenerated columnar cells (Fig. 1) or a few oncocytic cells. 28 Such FNAC specimens tend to be classified as AUS in Japan, although these features are characteristic of Warthin tumors. 28 For these reasons, the RON in the AUS category tended to be higher than that reported in previous studies (Table 2). 15 , 16 , 17 Warthin tumors have characteristic clinical features, eg, they are soft, cystic tumors arising in the lower pole of the parotid gland in middle‐aged to older men. On the basis of these characteristic features, a clinical diagnosis can be made in many cases. 29 It might be necessary to reconsider the clinicocytologic diagnostic criteria for Warthin tumors used in Japan to reduce the RON value of the AUS category.
Figure 1.
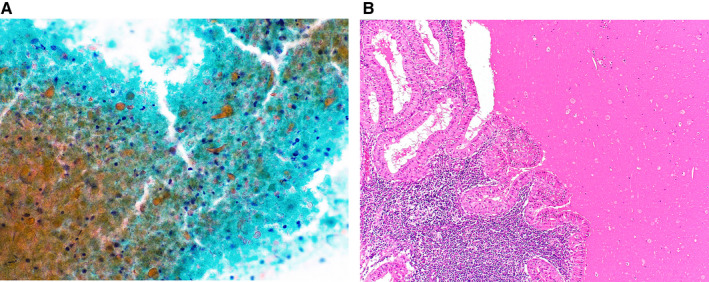
(A) Cytological features of a Warthin tumor that was categorized as atypia of undetermined significance. Only a few degenerated columnar cells and degenerated lymphocytes can be seen on a dirty background (Papanicolaou stain, original magnification ×400). (B) The histologic specimen shows a Warthin tumor (H&E stain, original magnification ×100).
Among the malignant tumors classified as AUS, malignant lymphoma was the most common type (23 cases) (Fig. 2), accounting for 12.4% of all cases in the AUS category (Table 5). Lymphoid‐rich entities, including IgG4‐related sialadenitis, chronic sialadenitis, lymphoid hyperplasia, and malignant lymphoma, accounted for 23.2% of the AUS lesions in this study. Alruwaii et al 30 reported a higher percentage (50%) of lymphoid‐rich lesions in the AUS category, which pushed up the ROM in the AUS category to 37.5%. In general, making a definitive diagnosis of malignant lymphoma based solely on cytology is difficult. 31 Ancillary immunophenotyping examinations, especially flow cytometry, are useful for differentiating malignant lymphoma from other lymphoid lesions. 32 , 33 In the current study, the ROM for AUS lesions was 24.9%, which was slightly higher than the percentage recommended in the MSRSGC (20%). Ancillary examinations were only performed in 15 cases in this study, and flow cytometry was not applied to any lymphoid lesions. It will be necessary to further promote the use of flow cytometry for cases in which lymphoid lesions are suspected to reduce the ROM value for the AUS category in Japan.
Figure 2.
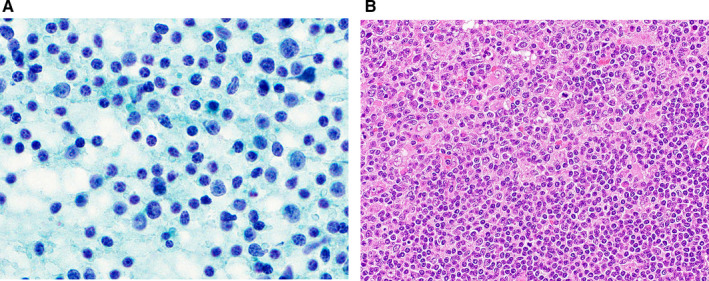
(A) Cytological features of a case of T‐cell lymphoma that was categorized as atypia of undetermined significance. The aspirate shows a mixed lymphoid pattern with small, intermediate‐sized, and large lymphocytes without distinct atypia (Papanicolaou stain, original magnification ×1000). (B) The histologic specimen shows a transition from an area of non‐neoplastic, small lymphocytes (lower right) to an area of atypical neoplastic lymphocytes (upper left; H&E stain, original magnification ×400).
In total, 154 cases were classified as SUMP (Table 6). Of the 57 malignant tumors, so‐called basaloid neoplasms, including adenoid cystic carcinoma, myoepithelial carcinoma, and basal cell adenocarcinoma, accounted for 16 of the malignant SUMP cases (28.1%). Other low‐grade carcinomas that exhibited slight atypia, including mucoepidermoid carcinoma, epithelial‐myoepithelial carcinoma, secretory carcinoma, polymorphous adenocarcinoma, acinic cell carcinoma, and intraductal carcinoma, were also included in the SUMP category. When combined with basaloid cell tumors, they accounted for 41 cases (71.9%). To differentiate among them, we should use ancillary techniques, for example, immunostaining of PLAG1 or MYB or ETV6 fluorescence in situ hybridization using cell blocks or liquid‐based cytology samples. 34 Of the benign tumors, 41 (46.1%) were PAs, and 31 (34.8%) were basal cell adenomas. The PAs categorized under SUMP included cases of the spindle cell type; the basaloid cell type; PA with bizarre giant myoepithelial cells (Fig. 3); and PA with many hyaline globules, which is sometimes difficult to differentiate from adenoid cystic carcinoma. 28 The most common non‐neoplastic lesion was nodular oncocytic hyperplasia (5 cases) (Fig. 4). Liu et al also reported 59 cases of SUMP, including 7 cases of nodular oncocytosis, 35 and Rooper et al reported that nodular oncocytosis was difficult to differentiate from oncocytoma. 36
Figure 3.
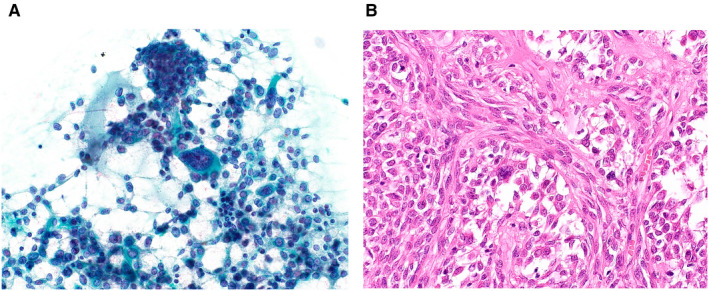
(A) Cytological features of a pleomorphic adenoma that was categorized as salivary gland neoplasm of uncertain malignant potential. Many myoepithelial cells and mucin can be seen. Among these, a few large, atypical cells are present (Papanicolaou stain, original magnification ×400). (B) Histology of the resected specimen reveals cellular pleomorphic adenoma in which interspersed, bizarre myoepithelial cells can be seen (H&E stain, original magnification ×400).
Figure 4.
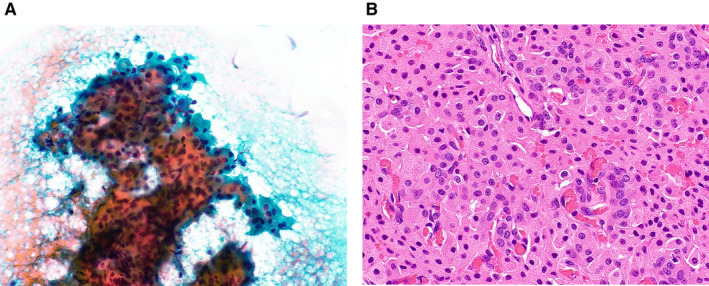
(A) Cytological features of a case of nodular oncocytic hyperplasia that was categorized as salivary gland neoplasm of uncertain malignant potential. Large oncocytic cell clusters with polygonal eosinophilic cytoplasm can be seen (Papanicolaou stain, original magnification ×400). (B) The histologic specimen shows hyperplasia with ductal components (H&E stain, original magnification ×200).
There were significant differences in the RON and ROM values for each MSRSGC category among the institutions (Table 2). The adoption of the MSRSGC will require education and quality control to ensure appropriate performance. 37
Although overall ROM values for each category were not calculated in this study, the mean percentage of malignant lesions among all of the aspiration samples from 7 institutions (institutions 1, 2, 4, 5, 8, 10, and 11) was 11.8% (range, 5.9%‐20%), which is similar to that reported in another study. 17
The sensitivity, specificity, accuracy, PPV, and NPV of the MSRSGC for diagnosing malignant lesions were as good as those described in previous studies. 11 , 14 , 16 , 17 , 18 , 19 In the current study, there were 7 false‐positive cases (see Supporting Table 2). One PA that was misdiagnosed as an acinic cell carcinoma showed cellular atypia with granular cytoplasm, enlarged nuclei, and conspicuous nucleoli. A retrospective examination indicated that these features were caused by oncocytic metaplasia (Fig. 5). 38 There were 19 false‐negative cases (see Supporting Table 3). The most common misdiagnosis was carcinoma ex PA being misdiagnosed as PA (5 cases; 26.3%). The cytologic diagnosis of carcinoma ex PA might be difficult when a carcinoma component is poorly sampled. 39 When relevant clinical findings, such as a sudden increase in the size of a long‐standing mass in an elderly patient, are present, the possibility of carcinoma ex PA should be considered, even if PA is suspected.
Figure 5.
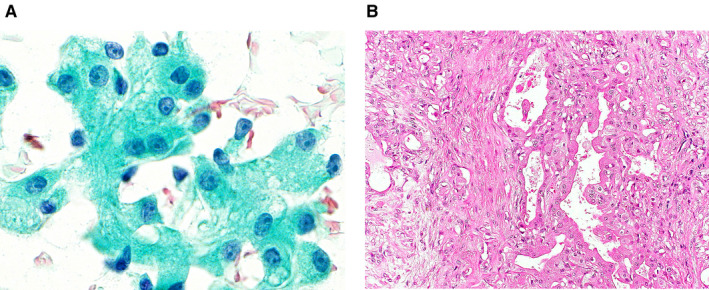
(A) Cytological features of a pleomorphic adenoma that was misdiagnosed as acinic cell carcinoma. Acinar‐like clusters of epithelial cells with large amounts of lacy and granular cytoplasm and distinct nucleoli can be seen (Papanicolaou stain, original magnification ×1000). (B) On histology of the resected specimen, oncocytic changes in the ductal epithelium (right) can be seen in this typical pleomorphic adenoma (left) (H&E, original magnification ×200).
In conclusion, the MSRSGC can be implemented as an alternative reporting format for salivary gland cytology to the JSRSGC. The MSRSGC is an excellent tool for ensuring quality control of salivary gland cytology, and the widespread use of the MSRSGC would improve the accuracy of salivary gland cytology and lead to better patient care in Japan.
Funding Support
This study was supported by the research fund of the Japanese Society of Clinical Cytology.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Kayoko Higuchi: Conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, writing–original draft, and writing–review and editing. Makoto Urano: Conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Jun Akiba: Conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Miwako Nogami: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Yukiya Hirata: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Yoko Zukeran: Data curation, formal analysis, investigation, resources, and validation. Koki Moriyoshi: Data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Yuichiro Tada: Data curation, resources, validation, and writing–review and editing. Mana Fukushima: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Mariko Obayashi: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Shinnichi Sakamoto: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Kazuya Kuraoka: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Kana Kira: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Akihiko Kawahara: Data curation, formal analysis, investigation, resources, validation, and writing–review and editing. Taku Kato: Data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Maki Tanigawa: Data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Masato Nakaguro: Conceptualization, validation, and writing–review and editing. Hidetaka Yamamoto: Data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing. Toshitaka Nagao: Conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, and writing–review and editing.
Supporting information
Table S1
Table S2
Table S3
Table S4
Higuchi K, Urano M, Akiba J, Nogami M, Hirata Y, Zukeran Y, Moriyoshi K, Tada Y, Fukushima M, Obayashi M, Sakamoto S, Kuraoka K, Kira K, Kawahara A, Kato T, Tanigawa M, Nakaguro M, Yamamoto H, Nagao T. A multi‐institutional study of salivary gland cytopathology: Application of the Milan System for Reporting Salivary Gland Cytopathology in Japan. Cancer Cytopathol.2022. 10.1002/cncy.22505
See related editorial on pages 16‐17, this issue.
References
- 1. Layfield LJ, Gopez E, Hirschowits S. Cost efficiency analysis for fine‐needle aspiration in the workup of parotid and submandibular gland nodules. Diagn Cytopathol. 2006;34:734‐738. [DOI] [PubMed] [Google Scholar]
- 2. Ali NS, Akhtar S, Junaid M, Awan S, Aftab K. Diagnostic accuracy of fine needle aspiration cytology in parotid lesions. ISRN Surg. 2011;2011:721525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eytan DF, Yin LX, Maleki Z, et al. Utility of preoperative fine needle aspiration in parotid lesions. Laryngoscope. 2018;128:398‐402. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systemic review and meta‐analysis of the diagnostic accuracy of fine‐needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011;136:45‐59. [DOI] [PubMed] [Google Scholar]
- 5. Rossi ED, Faquin WC, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology: analysis and suggestions of initial survey. Cancer Cytopathol. 2017;125:757‐766. [DOI] [PubMed] [Google Scholar]
- 6. Baloch Z, Field AS, Katabi N, Wenig BM. Chapter 1: The Milan System for Reporting Salivary Gland Cytopathology. In: Faquin WC, Rossi ED, Baloch Z, et al, eds. The Milan System for Reporting Salivary Gland Cytopathology. Springer; 2018:1‐9. [Google Scholar]
- 7. Song SJ, Shafique K, Wong LQ, LiVolsi VA, Montone KT, Baloch Z. The utility of the Milan system as a risk stratification tool for salivary gland fine needle aspiration cytology specimens. Cytopathology. 2019;30:91‐98. [DOI] [PubMed] [Google Scholar]
- 8. Hang JF, Alruwaii F, Zeng BR, Lai CR, Wu HH. Subtyping salivary gland neoplasm of uncertain malignant potential based on cell type demonstrates differential risk of malignancy. Cancer Cytopathol. 2018;126:924‐933. [DOI] [PubMed] [Google Scholar]
- 9. Park W, Bae H, Park MH, et al. Risk of high‐grade malignancy in parotid gland tumors as classified by the Milan System for Reporting Salivary Gland Cytopathology. J Oral Pathol Med. 2019;48:222‐231. [DOI] [PubMed] [Google Scholar]
- 10. Lee JJL, Tan HM, Chua DYS, Chung JGK, Nga ME. The Milan System for Reporting Salivary Gland Cytology: a retrospective analysis of 1384 cases in a tertiary Southeast Asian institution. Cancer Cytopathol. 2020;128:348‐358. [DOI] [PubMed] [Google Scholar]
- 11. Hirata Y, Higuchi K, Tamashiro K, et al. Application of the Milan System for Reporting Salivary Gland Cytopathology: a 10‐year experience in a single Japanese Institution. Acta Cytol. 2021;65:123‐131. [DOI] [PubMed] [Google Scholar]
- 12. National Cancer Center of Japan . Cancer Registry and Statistics [homepage on the internet]. Cancer Information Service, National Cancer Center of Japan (Vital Statistics of Japan); 2016. Accessed June 4, 2020; https://ganjoho.jp/reg_stat/statistics/dl/index.html
- 13. Ries LAG, Harkey BF, Hartman AM, Edwards BK. Cancer Statics Review, 1973‐1988. NIH Publication 91‐2789. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 1991. [Google Scholar]
- 14. Rohilla M, Singh P, Rajwanshi A, et al. Three‐year cytohistological correlation of salivary gland FNA cytology at a tertiary center with the application of the Milan system for risk stratification. Cancer Cytopathol. 2017;125:767‐775. [DOI] [PubMed] [Google Scholar]
- 15. Savant D, Jin C, Chau K, et al. Risk stratification of salivary gland cytology using the Milan system of classification. Diagn Cytopathol. 2019;47:172‐180. [DOI] [PubMed] [Google Scholar]
- 16. Viswanathan K, Sung S, Scognamiglio T, Yang GCH, Siddiqui MT, Rao RA. The role of the Milan System for Reporting Salivary Gland Cytopathology: a 5‐year institutional experience. Cancer Cytopathol. 2018;126:541‐551. [DOI] [PubMed] [Google Scholar]
- 17. Wu HH, Alruwaii F, Zeng BR, Cramer HM, Lai CR, Hang JF. Application of the Milan System for Reporting Salivary Gland Cytopathology: a retrospective 12‐year bi‐institutional study. Am J Clin Pathol. 2019;151:613‐621. [DOI] [PubMed] [Google Scholar]
- 18. Lubin D, Buonocore D, Wei XJ, Cohen JM, Lin O. The Milan System at Memorial Sloan Kettering: utility of the categorization system for in‐house salivary gland fine‐needle aspiration cytology at a comprehensive cancer center. Diagn Cytopathol. 2020;48:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolon MR, Schnadig VJ, Faiz S, Nawgiri R, Clement CG. Salivary gland fine‐needle aspiration cytology with the application of the Milan system for risk stratification and histological correlation: a retrospective 6‐year study. Diagn Cytopathol. 2020;48:1067‐1074. [DOI] [PubMed] [Google Scholar]
- 20. Hirokawa M, Koshikawa S, Higuchi K, Minato H, Tsuzuku M, Mukai K. Reporting system of salivary gland aspiration cytology. J Jpn Soc Clin Cytol. 2007;46:160‐163. [Google Scholar]
- 21. National Comprehensive Cancer Network (NCCN) . NCCN Guidelines® for Head and Neck Cancers. Accessed June 15, 2021. https://www.nccn.org/guidelines/guidelines‐detail?category=1&id=1437
- 22. Foschini MP, Rossi ED, Higuchi K, et al. Chapter 2: Non‐diagnostic. In: Faquin WC, Rossi ED, Baloch Z, et al, eds. The Milan System for Reporting Salivary Gland Cytopathology. Springer; 2018:11‐20. [Google Scholar]
- 23. Faquin WC, Powers CN. Salivary gland cytopathology. In: Rosenthal DL, series ed. Essentials in Cytopathology Series. Vol 5. Springer; 2008. [Google Scholar]
- 24. Maleki Z, Allison DB, Butcher M, Kawamoto S, Eisele DW, Pantanowitz L. Application of the Milan System for Reporting Salivary Gland Cytopathology to cystic salivary gland lesions. Cancer Cytopathol. 2021;129:214‐225. [DOI] [PubMed] [Google Scholar]
- 25. Pantanowitz L, Thompson LDR, Rossi ED. Diagnostic approach to fine needle aspirations of cystic lesions of the salivary gland. Head Neck Pathol. 2018;12:548‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akiba J, Kawahara A, Abe H, Yoshida T, Takase Y, Fukumitsu C. A retrospective analysis of fine‐needle aspiration findings in the salivary gland region using the Milan System for Reporting Salivary Gland Cytopathology. J Jpn Soc Clin Cytol. 2020;59:24‐29. [Google Scholar]
- 27. Veder LL, Kerrebijin JDF, Smedts FM, den Bakker MA. Diagnostic accuracy of fine‐needle aspiration cytology in Warthin tumors. Head Neck. 2010;32:1635‐1640. [DOI] [PubMed] [Google Scholar]
- 28. Baloch Z, Fadda G, Firat P, et al. Chapter 5: Neoplasm. In: Faquin WC, Rossi ED, Baloch Z, et al, eds. The Milan System for Reporting Salivary Gland Cytopathology. Springer; 2018:85‐95. [Google Scholar]
- 29. Lee DH, Yoon TM, Lee JK, Lim SC. Surgical treatment strategy in Warthin tumor of the parotid gland. Braz J Otorhinolaryngol. 2019;85:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alruwaii F, Hang JF, Zeng BR, et al. Risk of malignancy in “atypia of undetermined significance” category of salivary gland fine‐needle aspiration: a bi‐institutional experience. Diagn Cytopathol. 2020;48:138‐143. [DOI] [PubMed] [Google Scholar]
- 31. Chhieng DC, Cangiarella JF, Cohen JM. Fine‐needle aspiration cytology of lymphoproliferative lesions involving the major salivary gland. Am J Clin Pathol. 2000;113:563‐571. [DOI] [PubMed] [Google Scholar]
- 32. Stacchini A, Aliberti S, Pacchioni D, et al. Flow cytometry significantly improves the diagnostic value of fine‐needle aspiration cytology of lymphoproliferative lesions of salivary glands. Cytopathology. 2014;25:231‐240. [DOI] [PubMed] [Google Scholar]
- 33. Pusztaszeri M, Baloch Z, Faquin WC, Rossi ED, Tabatabai LZ. Chapter 4: Atypia of undetermined significance. In: Faquin WC, Rossi ED, Baloch Z, et al, eds. The Milan System for Reporting Salivary Gland Cytopathology. Springer; 2018:43‐54. [Google Scholar]
- 34. Pusztaszeri M, Garcia JJ, Faquin WC. Salivary gland FNA: new markers and new opportunities for improved diagnosis. Cancer Cytopathol. 2015;32:264‐274. [DOI] [PubMed] [Google Scholar]
- 35. Liu H, Ljungren C, Lin F, Zarka MA, Chen L. Analysis of histologic follow‐up and risk of malignancy for salivary gland neoplasm of uncertain malignant potential proposed by the Milan System for Reporting Salivary Gland Cytopathology. Cancer Cytopathol. 2018;126:490‐497. [DOI] [PubMed] [Google Scholar]
- 36. Rooper LM, Onenerk M, Siddiqui MT, Faquin WC, Bishop JA, Ali SZ. Multinodular oncocytic hyperplasia: can cytomorphology allow preoperative diagnosis of a non‐neoplastic salivary disease? Cancer Cytopathol. 2017;125:627‐634. [DOI] [PubMed] [Google Scholar]
- 37. Maleki Z. The Milan System for Reporting Salivary Gland Cytopathology: a universal language to improve patient care. J Am Soc Cytopathol. 2020;9:113‐115. [DOI] [PubMed] [Google Scholar]
- 38. Ito H, Ishida M, Miyasaka C, et al. Prominent oncocytic metaplasia in pleomorphic adenoma: a potential diagnostic pitfall. Diagn Cytopathol. 2020;48:765‐768. [DOI] [PubMed] [Google Scholar]
- 39. Allison DB, Smith AP, An D, et al. Assessing the diagnostic accuracy for pleomorphic adenoma and Warthin tumor by using the Milan System for Reporting Salivary Gland Cytopathology: an international, multi‐institutional study. Cancer Cytopathol. 2021;129:43‐52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


