Abstract
What is known and objective
The discussion about health equity in the United States frequently involves concerns over racial and ethnic minority under‐representation in clinical trials and particularly in trials conducted in support of product approvals. The FDA has long worked to encourage diverse participation in clinical trials and through its Drug Trials Snapshots (DTS) program, the U.S. Food and Drug Administration (FDA) has moved to make trial demographic data more accessible and transparent. We conducted a demographic study of U.S. participants in clinical trials for FDA‐approved new drugs (new molecular entities [NMEs], and original Biologics License Applications [BLAs]) from 2015 to 2019, as reported in DTS database with a purpose of understanding the extent to which U.S.‐based trials used to support product approvals represent the racial and ethnic diversity of the U.S. population by therapeutic area.
Methods
Participant‐level trial data were collected by accessing the FDA electronic common technical document (eCTD), for the applications used to publish each Snapshot. The therapeutic area (TA) for each drug was determined by review division assignment. The demographic data were analysed and compared to U.S. census data.
Results and discussion
We examined 102,596 U.S. participants in trials of new drugs that were approved and presented in Drug Trials Snapshots between 2015 and 2019. White participation ranged from 51% in psychiatric trials to 90% in cardiovascular (CV) trials; Black or African American participation ranged from 5% in medical imaging to 45% in psychiatric trials; Asian participation ranged from 0.75% in CV to 4% in dermatologic trials; and Hispanic or Latino participation ranged from 1% in medical imaging to 22% in infectious diseases and gastroenterology trials.
What is new and conclusion
Our data showed variable representation of racial and ethnic minorities across therapeutic areas at the U.S. sites. Blacks or African Americans were represented at or above U.S. census estimates across most therapeutic areas, while Asians and American Indian or Alaska Natives were consistently underrepresented. Hispanic or Latino participation across most therapeutic areas was below U.S. census estimates, however, more variable, and a sizable proportion of data was missing. The next step is a comparison of trial participation based on disease prevalence and epidemiology, which is a more accurate assessment of trial diversity.
Keywords: controlled clinical trial, population analysis, rate
We conducted a demographic study of U.S. participants in clinical trials for FDA‐approved new drugs from 2015 to 2019. U.S. participation within each therapeutic area ranged from 18% in Cardiovascular Diseases to 82% in Ophthalmology and was notable for variable representation of minorities across therapeutic areas.

1. WHAT IS KNOWN AND OBJECTIVE
As clinical research has become more global, public interest in trial demographics, particularly in U.S. trial demographics, has increased and frequently involves concerns over racial and ethnic minority representation in clinical trials. The FDA has long worked to encourage diverse participation in clinical trials during drug development and more recently, to make the trial demographic data more accessible and transparent through the DTS initiative. 1 DTS provide concise demographic information about participation in the clinical trials that supported FDA approval of novel drugs and biologics (new molecular entities and original biologics).
This study is a review of demographic data presented in DTS published between 2015 and 2019, focusing on the participation by therapeutic areas at the U.S. trial sites. Demographic trial data are compared to data obtained from the U.S. Census Bureau 2 to understand the extent to which such trials represent the diversity of the U.S. population.
2. METHODS
Participant‐level clinical trial data were collected by accessing the eCTD of the applications (either NDA or BLA) used to publish each Snapshot.
Race and ethnicity categories were based on definitions outlined in the FDA Guidance. 3 Race was grouped in one of six categories: White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander or other, and ethnicity in one of two categories: Hispanic or Latino or not‐Hispanic or Latino. A ranking order was created to prioritize mentions of race and ethnicity. For example, if a participant reported ‘Black Hispanic’, the participant would have their race assigned to ‘Black or African American’ and their ethnicity would be ‘Hispanic or Latino’. The therapeutic area (TA) for each drug was determined by review division assignment within the Agency at the time of approval. For comparison to census data, census estimates for year 2015 were used because of its mid‐position between census reports from 2010 and 2020.
The data were analysed and visualized using the tableau 2020.3 software program (Tableau).
3. RESULTS
3.1. Overall U.S. participation in clinical trials
In this analysis, we examined demographic data from 517 trials that were the basis for new drugs approvals and published in 231 Drug Trials Snapshots between 2015 and 2019.
The majority of applications used to create the Snapshots contained data both from U.S. and non‐U.S. sites; however, 10.3% had data exclusively from U.S. sites and 5.4% exclusively from non‐U.S. sites, with 0.4% of the submitted applications providing no site location data. Overall, 35% of all participants (102,596) were from U.S. sites. Racial and ethnic breakdowns of the U.S. population are summarized in Tables 1 and 2. The majority of U.S. trial participants were White (78%), and non‐Hispanic or Latino (75%). Census data estimates are presented in Table 3.
TABLE 1.
Clinical trials participation at the U.S. Sites by Race (N = 102,596)
| White | Black or African American | Asian | American Indian or Alaska Native | Other* |
|---|---|---|---|---|
| 80,310 (78.27%) | 16,733 (16.31%) | 2,139 (2.08%) | 531 (0.52%) | 2,883 (2.81%) |
combined categories ‘Native Hawaiian or Other Pacific Islander’, ‘Other race’, ‘Mixed race’ and ‘Unknown/Unreported/Missing’.
TABLE 2.
Clinical Trials Participation at the U.S. Sites by Ethnicity (N = 102,596)
| Hispanic or Latino | Not Hispanic or Latino | Missing |
|---|---|---|
| 15,691 (15.29%) | 77,353 (75.39%) | 9,552 (9.31%) |
TABLE 3.
US Census Bureau Estimate* of the Resident Population in 2015 (N = 320,635,163)
| Demographic Category | U.S. Population |
|---|---|
| Race | |
| White | 247,382,690 (77.15%) |
| Black or African American | 42,532,491 (13.26) |
| Asian | 17,752,744 (5.53%) |
| American Indian or Alaska Native | 4,004,358 (1.24%) |
| Ethnicity | |
| Hispanic or Latino | 56,254,742 (17.54%) |
| Not Hispanic or Latino | 129,427,521 (40.36%) |
Adapted from US Census Bureau Population Division, Annual Estimates of the Resident Population.
Geographic distribution of participants by 13 therapeutic areas is presented in Figure 1.
FIGURE 1.
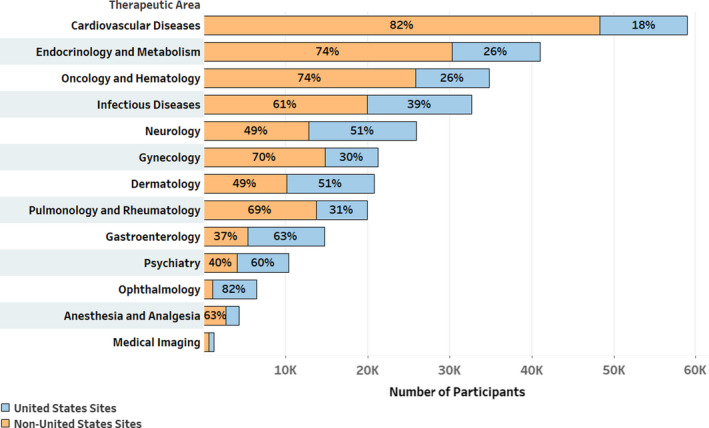
Geographic breakdown across therapeutic areas‐overall trials population
U.S. participation within each therapeutic area varied from 18% in cardiovascular diseases to 82% in Ophthalmology. At the midpoint are Neurology and Dermatology, with 51% U.S. participants.
3.2. U.S. participation per therapeutic area
The two therapeutic areas with the highest numbers of participants from the U.S. sites were Neurology, with 13,089, and infectious diseases, with 12,758. Anaesthesiology and medical imaging had the lowest numbers of participants (1,586 and 624, respectively). Gastroenterology represented the group median (9,265 participants). A comparative presentation of total U.S. participants across therapeutic areas is shown in Figure 2.
FIGURE 2.
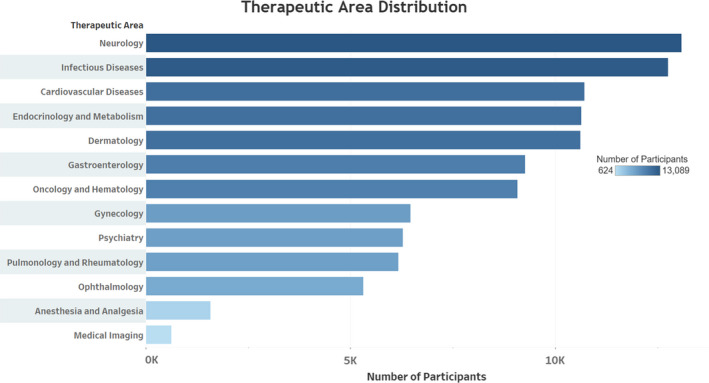
Therapeutic area distribution of U.S. trial participants
3.2.1. Racial composition across therapeutic areas at U.S. sites
In every TA, more than half of all participants were White. (Table 4). The highest percentage of White participants was in cardiovascular diseases (90%), followed by pulmonology and rheumatology (84%) and neurology (81%).
TABLE 4.
Racial distribution per therapeutic areas at U.S. Sites
| Therapeutic area | Race | ||||
|---|---|---|---|---|---|
| White n (%) | Black or African American n (%) | Asian n (%) | American Indian or Alaska Native n (%) | Other* n (%) | |
| Neurology (N = 13,089) | 10,643 (81.31) | 1,848 (14.12) | 162 (1.24) | 72 (0.55) | 364 (2.78) |
| Infectious diseases (N = 12,758) | 9,266 (72.63) | 2,789 (21.86) | 277 (2.17) | 111 (0.87) | 315 (2.47) |
| Cardiovascular diseases (N = 10,718) | 9,602 (89.59) | 885 (8.26) | 80 (0.75) | 27 (0.25) | 124 (1.16) |
| Endocrinology and metabolism (N = 10,639) | 8,453 (79.45) | 1,689 (15.88) | 272 (2.56) | 56 (0.53) | 169 (1.59) |
| Dermatology (N = 10,618) | 8,383 (78.95) | 1359 (12.80) | 418 (3.94) | 84 (0.79) | 374 (3.52) |
| Gastroenterology (N = 9,265) | 7,281 (78.59) | 1,598 (17.25) | 153 (1.65) | 38 (0.41) | 195 (2.10) |
| Oncology and haematology (N = 9,079) | 7,193 (79.23) | 987 (10.87) | 316 (3.48) | 32 (0.35) | 551 (6.07) |
| Gynaecology (N = 6,461) | 5,238 (81.07) | 827 (12.80) | 139 (2.15) | 24 (0.37) | 233 (3.61) |
| Psychiatry (N = 6,282) | 3,211 (51.11) | 2,828 (45.02) | 78 (1.24) | 28 (0.45) | 137 (2.18) |
| Pulmonology and rheumatology (N = 6,169) | 5,211 (84.47) | 746 (12.09) | 86 (1.39) | 38 (0.62) | 88 (1.43) |
| Ophthalmology (N = 5,308) | 4,230 (79.69) | 868 (16.35) | 134 (2.52) | 16 (0.30) | 60 (1.13) |
| Anaesthesia and analgesia (N = 1,586) | 1,187 (74.84) | 278 (17.53) | 19 (1.20) | 4 (0.25) | 98 (6.18) |
| Medical imaging (N = 624) | 412 (66.03) | 31 (4.97) | 5 (0.80) | 1 (0.16) | 175 (28.04) |
Due to small percentages, the ‘Other’ category combines ‘Native Hawaiian or Other Pacific Islander’, ‘Unknown/Unreported/Missing’, ‘Other race’, and ‘Mixed race’.
Therapeutic areas with the highest participation of Black or African Americans were psychiatry (45%) and infectious diseases (22%). Representation of Asians was highest in dermatology (4%) and oncology and haematology (3%), and for American Indian or Alaska Natives, participation was highest in infectious diseases (0.9%) and dermatology (0.8%).
The lowest participation percentages in each racial group were 51% in psychiatric trials for Whites, 5% in medical imaging trials for Black or African Americans, 0.8% in cardiovascular and medical imaging trials for Asians, and 0.2% in medical imaging trials for American Indian or Alaska Natives.
3.2.2. Ethnic composition across therapeutic areas
Hispanics or Latinos comprised 15% of all U.S. participants. The highest participation of Hispanics or Latinos was in infectious diseases and gastroenterology (22%), followed by endocrinology and metabolism and dermatology (20%); and the lowest was in medical imaging (2%), followed by oncology and haematology and cardiovascular diseases (7%). (Table 5) It should be noted that approximately 9% of U.S. participants had missing ethnicity data (range <1% to 31% per TA).
TABLE 5.
Ethnicity distribution for therapeutic areas at U.S. Sites
| Therapeutic Area | Ethnicity | ||
|---|---|---|---|
| Hispanic or Latino n (%) | Not Hispanic or Latino n (%) | Missing n (%) | |
| Neurology (N = 13,089) | 1,841 (14.06) | 10,041 (76.7) | 1,207 (9.22) |
| Infectious diseases (N = 12,758) | 2,851 (22.35) | 9,845 (77.17) | 62 (0.48) |
| Cardiovascular diseases (N = 10,718) | 767 (7.16) | 9,279 (86.57) | 672 (6.27) |
| Endocrinology and metabolism (N = 10,639) | 2,123 (19.95) | 8,491(79.81) | 25 (0.24) |
| Dermatology (N = 10,618) | 2,152 (20.26) | 7,343 (69.16) | 1,123 (10.58) |
| Gastroenterology (N = 9,256) | 2,044 (22.06) | 6,179 (66.69) | 1,042 (11.25) |
| Oncology and haematology (N = 9,079) | 636 (7.01) | 5,124 (56.43) | 3,319 (36.56) |
| Gynaecology (N = 6,461) | 878 (13.59) | 4526 (70.05) | 1,057 (16.36) |
| Psychiatry (N = 6,282) | 710 (11.30) | 5,570 (88.67) | 2 (0.03) |
| Pulmonology and rheumatology (N = 6,169) | 712 (11.54) | 4,667 (75.65) | 790 (12.81) |
| Ophthalmology (N = 5,308) | 777 (14.64) | 4,520 (85.15) | 11 (0.21) |
| Anaesthesia and analgesia (N = 1,586) | 192 (12.10) | 1,379 (86.95) | 15 (0.95) |
| Medical imaging (N = 624) | 8 (1.28) | 389 (62.34) | 227 (36.38) |
4. DISCUSSION
Multiple authors have written about low participation of racial and ethnic minorities in global clinical trials in comparison with U.S. census data 4 , 5 but very few have actually compared participation in U.S. trials to U.S. census data. 6 Further, to better support the efforts of clinical trial communities to diversify the trial population within the United States, understanding the current rates of participation of racial and ethnic groups within the United States is critical. We have previously published global participation data as a part of the Drug Trials Snapshots initiative; our focus here is therefore on the representation in the U.S. trials. In addition to examining overall U.S. participation, we have provided data for each of 13 major therapeutic areas.
Trials for cardiovascular diseases frequently are cited as an area with low racial and ethnic minority participation. 7 Our data showed that for the U.S.‐based trials, participation rates were lower than census rates for all racial and ethnic minority groups, and above the census rates (90% v. 77%) only for White participants.
Oncology and haematology is another TA of interest with respect to trial diversity, and those populations showed a similar lack of diversity. All racial and ethnic minorities were underrepresented, although, not to the extent seen in cardiovascular disease trials, and closely aligned with previously published data. 8
Neurology, with the largest trials' population at U.S. sites, had White and Black or African American representation above the census rate (81% and 14%, respectively), while Asians, American Indian or Alaska Natives, and Hispanics or Latinos were all below census rates.
Of interest, participation of Blacks or African Americans in infectious disease trials was well above census, at 22%. A similar, but more striking pattern was evident for psychiatric diseases, for which overall Black or African American participation was 45%.
In nine of 13 TAs, Black or African American participation within the United States was 13% or higher, highlighting that participation was at or above the U.S. census for this population.
We found the opposite to be true for Asians, who were regularly underrepresented, from 1% in cardiovascular trials to 4% in dermatology trials. The low participation of Asian Americans is frequently overlooked in recruitment efforts within the United States. One reason may be that, in global trials, Asians are relatively well represented because of high numbers of research sites in Asia, offsetting the low participation within the United States. Low Asian participation within the United States is multifactorial, 9 but a study by Lan et al. 10 showed an increase from 2.5% in 2011 to 12.1% in 2016 in U.S. National Institutes of Health‐funded trials, which highlights the possibility for improvement in this area.
A similar, although less consistent finding among racial groups, was in American Indian or Alaska Natives, whose participation across therapeutic areas was generally below census rates and approaching the census rate in only two out of 13 TAs. The major obstacle to higher participation of this group has been frequently attributed to its population dispersion across the rural United States; however, about 60% of American Indians and Alaska Natives reside in metropolitan areas. 11
The least consistent participation level across TAs was for the Hispanic or Latino population, ranging from 1% in Medical Imaging to 22% in gastroenterology and infectious diseases. Oncology and haematology and cardiovascular diseases had approximately 7% Hispanic or Latino participants, an increase in comparison with the previously reported 3%, 12 despite a higher proportion of unreported ethnicity. Per some authors, 13 fewer interventions have targeted Hispanics or Latinos, whose participation barriers may be different from those of other demographic groups.
5. WHAT IS NEW AND CONCLUSION
The complexities of trial participation by demographic groups have long been recognized. Our data showed that at U.S. sites, Blacks or African Americans were well represented across most therapeutic areas, while Asians were consistently underrepresented in comparison with U.S. census data. Hispanic or Latino participation was more variable, with a sizable proportion of missing data. Some racial and ethnic minorities are still not well represented in most drug development programmes, and this remains an area where improvement is needed.
5.1. Limitations
Our analysis had some limitations. Collected data on racial and ethnic minority representation was not always complete. It should also be noted that data were generated from pivotal trials conducted for new molecular entities, which frequently, particularly in oncology, are approved via an accelerated approval process, or are tested on limited populations, such as participants with rare diseases. And finally, our study does not consider disease prevalence; therefore, the next step is a comparison of trial participation based on disease prevalence and epidemiology, which is a more accurate assessment of trial diversity.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. The contents of this publication reflect the thoughts of the authors and do not represent the official views of, nor an endorsement by, the FDA, Department of Health and Human Services, or US government.
ACKNOWLEDGEMENTS
The authors thank Joanne Berger, MS, FDA Library, for manuscript editing.
Lolic M, Araojo R, Okeke M, Temple R. U.S. racial and ethnic participation in global clinical trials by therapeutic areas. J Clin Pharm Ther. 2021;46:1576–1581. 10.1111/jcpt.13532
Funding information
This study was supported in part by an appointment to the Oak Ridge Institute for Science and Education (ORISE) Research Participation Program at the Center for Drug Evaluation and Research administered by the ORISE through an agreement between the US Department of Energy and CDER (M.O)
DATA AVAILABILITY STATEMENT
Based on several federal statutes and regulations governing the disclosure of information, including patient‐level data that support the findings of this study, FDA cannot share this data.
REFERENCES
- 1. U.S. Food and Drug Administration . Drug trials snapshots. Available at: https://www.fda.gov/drugs/drug‐approvals‐and‐databases/drug‐trials‐snapshots. Accessed March 4, 2021.
- 2. US Census Bureau Population Division Annual estimates of the resident population by sex, race, and hispanic origin for the United States: April 1, July 1, 2019 (NC‐EST2019‐SR11H), Release Date: June 2020. https://www.census.gov/data/datasets/time‐series/demo/popest/2010s‐national‐detail.html. Accessed July 2,2021.
- 3. US Food and Drug Administration . Collection of race and ethnicity data in clinical trials, guidance for industry and food and drug administration staff. Available at: https://www.fda.gov/media/75453/download. Accessed July 2, 2021.
- 4. The Editors . Clinical trials have far too little racial and ethnic diversity. Scientific American. https://www.scientificamerican.com/article/clinical‐trials‐have‐far‐too‐little‐racial‐and‐ethnic‐diversity. Accessed March 9, 2021.
- 5. Geller SE, Koch A, Pellettieri B, Carnes M, . Inclusion, analysis and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Women's Health. 2011;20(3):315‐320. 10.1089/jwh.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rottas M, Thadeio P, Simons R, et al. Demographic diversity of participants in Pfizer sponsored clinical trials in the United States. Contemp Clin Trials. 2021;106:106421. 10.1016/j.cct.2021.106421 [DOI] [PubMed] [Google Scholar]
- 7. Ortega RF, Yancy CW, Mehran R, Batchelor W. Overcoming lack of diversity in cardiovascular clinical trials. Circulation. 2019;140(21):1690‐1692. [DOI] [PubMed] [Google Scholar]
- 8. Tharakan S, Zhong X, Galsky MD. The impact of the globalization of cancer clinical trials on the enrollment of black patients. Cancer. 2021;127(13):2294‐2301. 10.1002/cncr.33463 [DOI] [PubMed] [Google Scholar]
- 9. Paterniti DA, Chen MS, Chiech C, et al. Asian Americans and clinical trials: a mixed‐methods approach to understanding awareness and experience. Cancer. 2005;104(12 Suppl):3015‐3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ðoàn LN, Takata Y, Sakuma K‐LK, Irvin VL. Trends in Clinical Research Including Asian American, Native Hawaiian, and Pacific Islander Participants Funded by the US National Institutes of Health, 1992 to 2018. JAMA Network Open. 2019;2:(7):e197432. 10.1001/jamanetworkopen.2019.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Department of Health and Human Services Office of Minority Health. Policy and Data. Minority Population Profiles. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=62. Accessed August 4, 2021.
- 12. Fashoyin‐Aje LA, Fernandes LL, Keegan P, Sridhara R, Pazdur R. Enrollment of hispanics in cancer clinical trials: an FDA analysis. J Clin Oncol. 2018;36(15_suppl):e18670. 10.1200/JCO.2018.36.15_suppl.e18670 [DOI] [Google Scholar]
- 13. Rangel ML, Heredia NI, Reininger B, et al. Educating hispanics about clinical trials and biobanking. J Cancer Educ. 2019;34(6):1112‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Based on several federal statutes and regulations governing the disclosure of information, including patient‐level data that support the findings of this study, FDA cannot share this data.


