ABSTRACT
Sloths are unusual mobile ecosystems, containing a high diversity of epibionts living and growing in their fur as they climb slowly through the canopies of tropical forests. These epibionts include poorly studied algae, arthropods, fungi, and bacteria, making sloths likely reservoirs of unexplored biodiversity. This review aims to identify gaps and eliminate misconceptions in our knowledge of sloths and their epibionts, and to identify key questions to stimulate future research into the functions and roles of sloths within a broader ecological and evolutionary context. This review also seeks to position the sloth fur ecosystem as a model for addressing fundamental questions in metacommunity and movement ecology. The conceptual and evidence‐based foundation of this review aims to serve as a guide for future hypothesis‐driven research into sloths, their microbiota, sloth health and conservation, and the coevolution of symbioses in general.
Keywords: symbiosis, mutualism, algae, fungi, arthropods, hair, fur, microbiome, epibiont, movement ecology
I. INTRODUCTION
Sloths spend much of their lives hanging from trees in Central and South America and are unique in that they have some of the slowest metabolisms of all mammals (Pauli et al., 2016). There are six extant species of sloths in two genera: two‐fingered (Choloepus spp., family Choloepodidae) and three‐fingered (Bradypus spp., family Bradypodidae) (Slater et al., 2016). Historically, the names ‘two‐toed’ and ‘three‐toed’ have been used, although this is a misnomer; we use ‘two‐fingered’ and ‘three‐fingered’ herein because all sloths have three toes but differ in the number of ‘fingers’ they have on their upper limbs. Despite both genera being slow‐moving arboreal folivores, two‐ and three‐fingered sloths are very different as revealed by molecular, morphological, and behavioural data (Table 1). Mitogenome and ancient collagen DNA phylogenetic analyses have revealed that these two sloth genera diverged ~31 million years ago (Fig. 1; Delsuc et al., 2019; Presslee et al., 2019). Both sloth genera host an array of largely unexplored symbioses (i.e. persistent, physical associations; Bronstein, 2015) involving taxonomically diverse microorganisms and arthropods in a multi‐trophic assemblage within their fur or pelage (Aiello, 1985; Gilmore, da Costa & Duarte, 2001; Suutari et al., 2010; Higginbotham et al., 2014). The structure of sloth hair is also unusual, being characterized by cracks or grooves that are hypothesized to facilitate algal growth (Aiello, 1985; Suutari et al., 2010), giving them a distinct green coloration in the wild.
Table 1.
Comparison of two‐ and three‐fingered sloth characteristics. Synthesized from Aiello (1985), Anderson & Handley (2001), Britton (1941), Chiarello (2008), Falconi et al. (2015), Feldhamer et al. (2015), Goodwin & Ayres (2014), Higginbotham et al. (2014), Mendoza et al. (2015), Montgomery & Sunquist (1978), Nie et al. (2015), Nyakatura (2012), Pauli & Peery (2012), Pauli et al. (2014, 2016), Peery & Pauli (2012), Ramirez et al. (2011), Sunquist & Montgomery (1973), Taube et al. (2001), Urbani & Bosque (2007), Vaughan et al. (2007), and Wetzel (1985). It should be noted that Choloepus hoffmanni and Bradypus variegatus home range sizes were based largely on observations in mixed‐cacao plantation agroecosystems and thus may not truly represent native home ranges for these species (Montgomery & Sunquist, 1978; Vaughan et al., 2007; Ramirez et al., 2011). Predicted home range values (H pred ) are based on Jetz et al.'s (2004) scaling relation for mammalian herbivores: (1.02 ± 0.9 ha/kg) × M + (2.05 ± 0.5 ha), where M is sloth body weight (kg); upper and lower values were calculated using extreme upper and lower bounds of input values
| Two‐fingered sloths | Three‐fingered sloths |
|---|---|
| Gross anatomy/morphology | |
| Modified, hook‐like arms and feet | Modified, hook‐like arms and feet |
| Rounded thorax with a small diameter | Rounded thorax with a small diameter |
| Relatively long arms with a relatively short scapula | Relatively long arms with a relatively short scapula |
| High mobility of all joints proximal to the midcarpal and transverse tarsal joints | High mobility of all joints proximal to the midcarpal and transverse tarsal joints |
| Highly mobile sterno‐clavicular articulation | Highly mobile sterno‐clavicular articulation |
| Powerful flexion in the proximal limb joints via advantageous lever arms | Powerful flexion in the proximal limb joints via advantageous lever arms |
| Two forelimb fingers | Three forelimb fingers |
| 5–8 neck vertebrae | 8–9 neck vertebrae |
| Body mass: up to 8.5 kg | Body mass: up to 6 kg |
| C. hoffmanni: mean (sd) = 5.7 ± 0.7 kg | B. variegatus: mean = 4.3 ± 0.9 kg |
| C. didactylus: mean (sd) = 6 ± 1 kg | B. torquatus: range = 4.1–6.0 kg |
| B. tridactylus: mean = 4.0 ± 0.3 kg | |
| B. pygmaeus: range = 2.5–3.5 kg | |
| Similar limb length | Forelimbs longer than hindlimbs |
| No tail | Small tail |
| Caniniform premolars | Only cylindrical teeth |
| Physiology and diet | |
| Diet is mostly leaves, but also fruits, eggs, and insects | Diet is almost exclusively leaves |
| C. hoffmanni: third slowest metabolism of all mammals (energy expenditure = 234 kJ/d/kg) | B. variegatus: slowest metabolism of all mammals (energy expenditure = 162 kJ/d/kg) |
| 10‐month gestation | 5–6‐month gestation |
| Behaviour and range | |
| Suspensory, arboreal locomotion | Suspensory, arboreal locomotion |
| No basking behaviour | Basking behaviour |
| Vigorous self‐defence | Minimal self‐defence |
| Nocturnal | Cathemeral (sporadic activity over 24 h), although B. torquatus observed to be nocturnal in warmer temperatures |
| Promiscuous | Polygynous |
| Movements (C. hoffmanni): | Movements (B. variegatus): |
| >50% travel ≥38 m per day | ~90% travel <38 m per day |
| <10% on same tree in successive days | <40% on same tree in successive days |
| Total activity per day: 7.6 ± 1.5 h | Total activity per day: 10.1 ± 2.2 h |
| Continuous bouts of activity: | Continuous bouts of activity: |
| <1 h, 45% | <1 h, 66% |
| 1–2 h, 29% | 1–2 h, 19.5% |
| *2–6 h, 23% | *2–6 h, 13% |
| 6–10 h, 3% | 6–10 h, 1.5% |
| *accounts for majority (52%) of total time active | *accounts for majority (43%) of total time active |
| Home range: | Home range: |
| C. hoffmanni: | B. variegatus: |
| median = 4.4–7.5 ha | median = 5.2 ha |
| male mean (sd) = 9 ± 53 ha | male mean (sd) = 22 ± 57 ha |
| female mean (sd) = 6 ± 9 ha | female mean (sd) = 2 ± 25 ha |
| H pred = 6.7–9.2 ha | H pred = 5.2–7.8 ha |
| C. didactylus: unknown; H pred = 6.7–9.9 ha | B. torquatus: median = 6.6 ha; range = 0.4–22 ha |
| H pred = 5.9–8.7 ha | |
| B. tridactylus: unknown; H pred = 5.5–6.9 ha | |
| B. pygmaeus: unknown; H pred = 4.3–6.0 ha | |
| Fur‐related | |
| Visible algal growth on hair, four known genera | Visible algal growth on hair, six known genera |
| Fungal genera unclear | 16 fungal genera identified |
| Longitudinal hair grooves | Transverse hair cracks |
Fig 1.
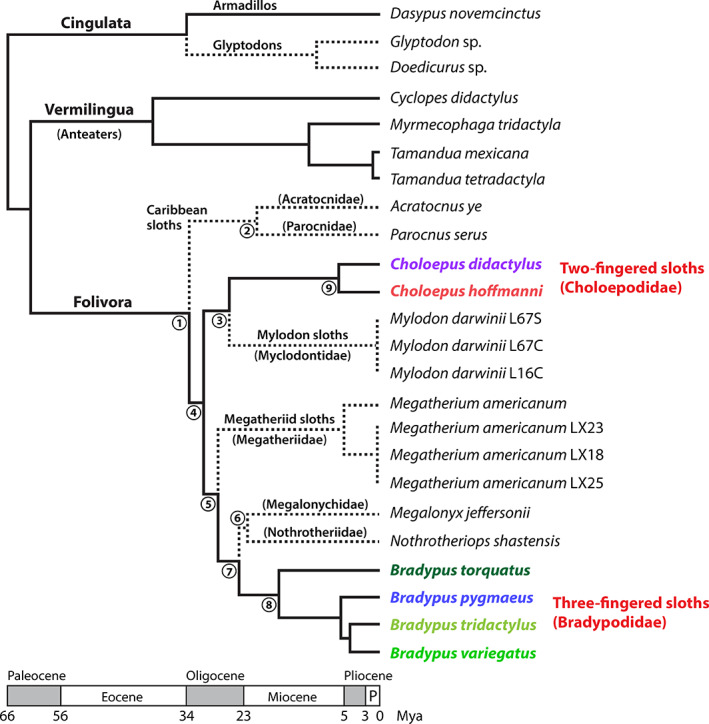
Phylogeny of sloths and their xenarthran relatives, anteaters and armadillos, with approximate timescales for branches. Dashed lines indicate extinct lineages or species. Main geological periods are shown (P = Pleistocene). Timescales are in millions of years (Mya). Synthesized from Delsuc et al. (2019) and Presslee et al. (2019), with indicated branch point timings being the averages of those reported by these two studies: (1) 33 Mya, (2) 21 Mya, (3) 26 Mya, (4) 31 Mya, (5) 27 Mya, (6) 22 Mya, (7) 24 Mya, (8) 17 Mya, and (9) 6 Mya. Recent molecular phylogenetic data suggests that Bradypus torquatus may be better assigned to a different genus (Scaeopus) and that Bradypus variegatus may represent two distinct species (trans‐Andean and cis‐Andean), although further studies are needed to clarify formal species distinctions (Ruiz‐García et al., 2020).
With a complex and largely self‐contained community of interacting epibionts defined by a boundary layer of fur, the sloth holobiont (host + associated biota) likely functions as an ecological unit in space (Jax, 2006). As sloths move slowly through the greater forest ecosystem, they can be considered ‘mobile ecosystems’ or dynamic, moving islands of biodiversity (cf. Heaney, Balete & Rickart, 2013; Borregaard et al., 2017). As such, they could represent unique systems to investigate questions in host–epibiont/host–microbiome ecology and coevolution within an unusual spatiotemporal/movement regime not typical of sessile organisms or fast‐moving animals.
We aim to highlight how studying sloths and their epibionts may be useful in addressing fundamental questions in microbial and metacommunity ecology, movement ecology, microbiome science, and the evolution of symbioses. We summarize what is known about the basic biology of sloths as it relates to their epibionts, and review evidence (or lack thereof) in support of several speculative conclusions that have accrued in the literature that have unfortunately led to misconceptions now canonized in the popular media (Meier, 2013; Greenwood, 2014; Lewis, 2014; Woollaston, 2014). We aim to challenge speculations that lack clear empirical support, articulate gaps in our understanding of the sloth as a mobile ecosystem (focused particularly on sloth fur as an ecosystem), and make suggestions for future directions in sloth research.
II. THE SLOTH AS A MODEL MOBILE ECOSYSTEM
All animals possess an assemblage of other species that live on or within them, the majority of which are microbial. When found within (as with gut microbiomes) these species often have a profound influence on host biology (McFall‐Ngai, 2015; Barko et al., 2017). As for other mammals, the gut microbiome of sloths is believed to play an important role in sloth health and be influenced by diet (Delsuc et al., 2014; Dill‐McFarland et al., 2016). However, it is the rich diversity of epibiotic symbionts on sloth fur that is most distinctive about the sloth holobiont and the focus of this review. Unlike the gut microbiome, which is shielded from the environment except through host‐driven dietary intake, the sloth fur ecosystem is open to the larger forest ecosystem through which the sloth moves. In addition to microbes, a variety of arthropods are an integral part of the fur multi‐trophic community (discussed in Section III). Similar to the pitcher plant (Boynton, 2012; Bittleston et al., 2018; Miller, Bradshaw & Holzapfel, 2018b ), which contains an elaborate food web of predators, prey, and detritivores that reside within a leafy ‘cup’ and is an entire ecosystem unto itself, sloth fur is a relatively self‐contained system of taxonomic diversity and trophic levels.
How a sloth's fur is colonized by this biodiversity is unknown but the process may be strongly influenced by the ecology of the skin/hair, endogenous host factors, and exogenous environmental factors as in humans (Grice & Segre, 2011). As with other host‐microbiome systems, the ‘extended phenotype’ (Dawkins, 1982) of the sloth could impose an ecological filter that shapes what and how epibionts assemble (Stagaman et al., 2017; Henry et al., 2019; Gilbert et al., 2020). The sloth fur ecosystem may have a ‘layered’ organization, similar to the canopy structure of a species‐rich grassland (Lane, Coffin & Lauenroth, 2000) or the stratified communities of microbial mats (Stolz, 2000) in which organisms are organized based on gradients in temperature or light penetration. The closer to hair follicles and skin, the warmer, dimmer, and more stable local conditions may be compared to those at the ends of hair tips that are more exposed to the elements. The sloth fur microbiome may be the foundation for recruiting and assembling taxa from higher trophic levels (discussed in Section IV.1) and may be fundamental to the well‐being of the sloth (discussed in Section IV.2). Like trees that are colonized by microbes in their phyllosphere (leaves) (Vacher et al., 2016) and by lichens in their dermosphere (bark) (Lambais, Lucheta & Crowley, 2014), slow‐moving sloths may be reservoirs of similar types of microbes that colonize substrates with low levels of movement‐based disturbance. Moreover, as sloths move from tree to ground and tree to tree in the forest canopy (Montgomery & Sunquist, 1975; Vaughan et al., 2007; see online Supporting Information, [Link], [Link]), they may acquire epibionts from interacting with hundreds of species of trees, each of which host unique phyllosphere, dermosphere, and rhizosphere communities (Lambais et al., 2014; Leff et al., 2015). As discussed further in Section IV.3, sloths may be vectors of dispersal unlike any other animal and provide a unique opportunity to understand ecological and evolutionary processes at a spatiotemporal scale that links microbes, arthropods, animal host movements, and three‐dimensional forest structure (cf. Prosser et al., 2007; Antwis et al., 2017; Shade et al., 2018).
(1). Unique sloth traits
Two‐ and three‐fingered sloths have many similar traits (Table 1), some that are hypothesized to have evolved convergently. Both groups of sloths have evolved suspensory posture, long, sharp claws for gripping branches and for territorial fights, and a modified skeletal structure to suit their slow, arboreal lifestyle (Miller, 1935; Montgomery & Sunquist, 1978; Mendel, 1981, 1985; Nyakatura & Fischer, 2011; Nyakatura, 2012; Pauli et al., 2016; Olson et al., 2018). Suspensory posture, and the many anatomical adaptations that have arisen for efficient suspensory locomotion in trees (see Supplementary Videos S3 and S4), are the most clearly convergent traits, given that no known fossil sloths were considered suspensory (Nyakatura, 2012). Evidence for a convergently evolved slow metabolism and diet is lacking, however. While it is not clear if ground sloths had cracked/grooved hair, this is a distinctive trait of all extant sloth species and has not been found on the closest relatives of sloths, armadillos and anteaters (Aiello, 1985; discussed further in Section III.1a), nor for any other mammal. The only other known mammals with epibiotic algal growth are polar bears in zoos (Lewin & Robinson, 1979) and manatees (Bledsoe et al., 2006), although they do not appear to have hair with crevices.
Sloths have diets consisting largely of leaves from trees, with three‐fingered sloths being almost exclusively folivorous. All sloths have low basal metabolic rates as is common for all arboreal folivores dependent on nutritionally poor food sources (McNab, 1978, 1986). Hoffmann's two‐fingered sloth, Choloepus hoffmanni, has the third slowest metabolism (234 kJ/day/kg) of all mammals, with a daily energy expenditure slightly greater than another folivorous mammal, the giant panda, Ailuropoda melanoleuca (185 kJ/day/kg) (Nie et al., 2015; Pauli et al., 2016). The brown‐throated three‐fingered sloth, Bradypus variegatus, has the slowest metabolism (162 kJ/day/kg; Pauli et al., 2016) and slowest rate of digestion of any mammal (Foley, Engelhardt & Charles‐Dominique, 1995).
(2). Geographical range, movement, and behaviour
Geographically, sloths are found throughout the neotropical forests of Central and South America, occupying a native range from Guatemala south through Peru and Brazil (Fig. 2). Although it is commonly thought that sloths are quite sedentary, they have been observed to move regularly throughout the forest canopy at rates of up to 0.5 km/h in short bursts (Sunquist & Montgomery, 1973). In one study based on radio‐telemetry observations, 54% of C. hoffmanni sloths were observed to move ≥38 m between daily locations (Sunquist & Montgomery, 1973) and <10% of observed sloths were found on the same tree on successive days. These two‐fingered sloths are nocturnal with the majority of their activity occurring in bouts lasting 2–6 h, with total activity averaging 7.6 h per day (Table 1). In a cacao agroecosystem, C. hoffmanni was found in 101 different tree species and eating from 34 species (Vaughan et al., 2007). By contrast, B. variegatus sloths tended to travel shorter distances, with 89% observed to travel <38 m per day (Sunquist & Montgomery, 1973). Unlike C. hoffmanni, these three‐fingered sloths are active throughout the day and night and for a greater proportion of the day (~10 h) but are four times more likely to remain on the same focal tree on successive days (Sunquist & Montgomery, 1973). This is consistent with observations of B. variegatus being found in and eating from fewer tree species than C. hoffmanni (71 and 15, respectively), and their preference to return more frequently to feed on specific trees, especially Cecropia spp. (Vaughan et al., 2007; Neam & Lacher, 2015; Garcés‐Restrepo, Peery & Pauli, 2019b ). B. variegatus is more likely to have bouts of activity of shorter duration than C. hoffmanni, with the majority being <1 h (Table 1). Like C. hoffmanni, however, bouts of activity lasting 2–6 h each account for the majority of the total time active during the day.
Fig 2.
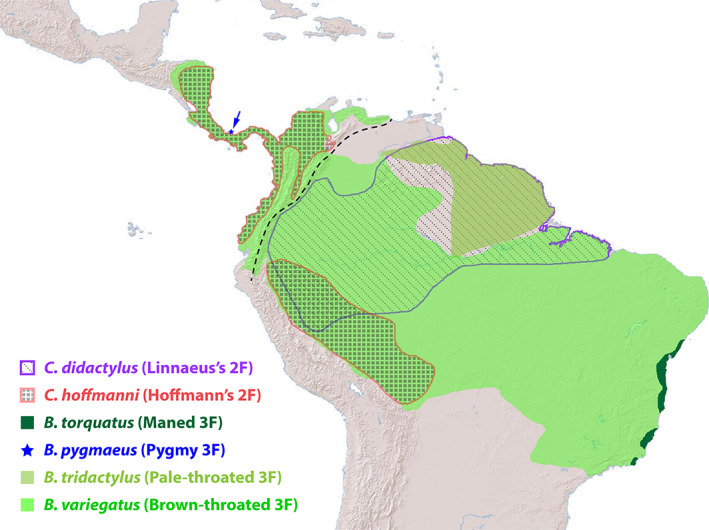
Distributional range of extant two‐fingered (2F) and three‐fingered (3F) sloth species across Central and South America. Synthesized from data of Chiarello & Plese (2014), Plese & Chiarello (2014), Chiarello & Moraes‐Barros (2014a , b ), Voirin et al. (2014) and Moraes‐Barros, Chiarello & Plese (2014) available at https://www.iucnredlist.org/. The population of trans‐Andean B. variegatus sloths located north and west of the Cordillera Oriental mountain range in Colombia (dashed line) is believed to represent a distinct species (Bradypus ephippiger) although this awaits formal confirmation (Ruiz‐García et al., 2020).
Home range sizes for sloths varies depending on species and sex, estimates of which are based largely on observations of C. hoffmanni and B. variegatus in mixed‐use landscapes centered around cacao plantations (see Table 1). Median home ranges for C. hoffmanni and B. variegatus sloths are estimated to be 4–7.5 and ~5 ha, respectively, which are generally within estimates predicted by a body size–space use scaling law for mammalian herbivores (Jetz et al., 2004; Table 1). However, individual home range observations are extremely variable, with a long‐tail distribution (and mode <2 ha) (Sunquist & Montgomery, 1973; Vaughan et al., 2007; Peery & Pauli, 2012); these data were typically acquired over a 1‐year period and it is possible that range estimates could increase (and variability decrease) with longer observation times (cf. Vaughan et al., 2007). Nonetheless, males appear to have larger home ranges than females in general.
Based on what we know about C. hoffmanni and B. variegatus, sloths move across the forest not just laterally, but up and down the canopy column. Sloths favour floristically diverse and structurally complex forest structures (vertically and horizontally), with dense canopy cover and variable tree heights (Montgomery & Sunquist, 1978; Mendoza et al., 2015; Neam & Lacher, 2015, 2018). As heterotherms, they prefer to rest and feed high up in the forest canopy (Neam & Lacher, 2015), moving in and out of canopy shade/sunlight and up and down trees to regulate their body temperatures as needed depending on ambient temperatures throughout the day (Montgomery & Sunquist, 1978; Pauli et al., 2016).
Sloths also exhibit the unusual behaviour of descending all the way down to the ground to defecate about once a week (Montgomery & Sunquist, 1975; Waage & Montgomery, 1976; Montgomery & Sunquist, 1978; Voirin et al., 2013). Many ideas have been proposed to explain this unusual defecation behaviour. It has been proposed that defecating on the ground (as opposed to letting dung drop from the canopy of trees) is a strategy that sloths use to remain undetected, since being quiet and hidden seems to be their predominant life strategy and defecating from the canopies of trees presumably may cause a disturbance that attracts predators (S. Trull, unpublished data). However, there is no evidence that descending to the base of the tree is risky to the sloth, especially since the majority of their predators, harpy eagles (Harpia harpyja), spectacled owls (Pulsatrix perspicillata), ocelots (Leopardus pardalis), and tayra (Eira barbara), can also detect and attack them from the tree canopy, often by knocking them to the ground where they proceed to eat them (Beebe, 1926; Izor, 1985; Bezerra et al., 2009; Voirin et al., 2009). Other theories include proposed benefits from fertilizing their most frequently used trees, communicating with other sloths through social latrines, trying to hide their scent from predators, sustaining a three‐way mutualism with moths and algae (discussed further in Section III.2c), or deriving nutritional benefits from consuming soil while on the ground (Beebe, 1926; Krieg, 1939; Goffart, 1971; Voirin et al., 2013). However, observational data suggest that three‐fingered sloths do not frequently eat soil (S. Trull, unpublished data), and no data exist in support of the other theories.
III. COMPONENTS OF THE MOBILE ECOSYSTEM
(1). Algae
The green hue of sloths arises from green algae that grow on sloth hair (Aiello, 1985; Suutari et al., 2010). Cyanobacteria may also contribute to this greenish hue, although only one species, Oscillatoria pilicola, has been identified to the species level to date (Table 2; Wujek & Lincoln, 1988). DNA sequences for red algae have also been found on sloths (Table 2; Suutari et al., 2010). For this review, we use the term ‘algae’ to refer broadly to eukaryotic algae and cyanobacteria unless specifically distinguished. It is not clear if algae are resident on all sloths in the wild. One study found that 73% of the 74 sampled sloths had visible algae on their fur identified via eye or microscope [Bradypus variegatus (N = 18), Bradypus tridactylus (N = 12), Bradypus pygmaeus (N = 12), Bradypus torquatus (N = 8), Choloepus hoffmanni (N = 22), Choloepus didactylus (N = 2)] (Suutari et al., 2010). However, neither sloth age, season of sampling, nor location were accounted for, and included in this analysis were captive sloths from zoos, which may lack native epibionts (likely due to being bred in captivity, bathed, or being kept in an enclosed habitat away from potential microbial symbionts in their native habitat). Given the limited and uneven sampling of this study, this value of 73% should not be interpreted as a definitive statistic. It is also generally overlooked that ‘brown’ sloths may actually host epibiotic algae even though not visibly green to the naked eye (Goffart, 1971): such algae may simply be in a dormant or non‐green state when moisture is limited. In fact, wetting of ‘brown’ sloth hair results in a rapid greening within seconds to minutes (Fig. 3), akin to what is observed with the wetting of desiccated biological soil crusts (Abed et al., 2014; Pietrasiak, 2014).
Table 2.
Known descriptions of algae found in sloth fur. Descriptions derived from Friedl (1995)a, Printz (1964)b, Schubert (2003)c, Suutari et al. (2010)d, Wujek & Timpano (1986)e, or AlgaeBase.org (Guiry & Guiry, 2019)
| Genus | Phylum | Class | Description |
|---|---|---|---|
| Trichophilus | Chlorophyta | Ulvophyceae | Small (3–13 μm) thick‐walled cells with numerous, small, discoid chloroplasts that lack pyrenoidsb,d |
| Trentepohlia | Chlorophyta | Ulvophyceae | Filamentous, orange in colour |
| Pseudendoclonium | Chlorophyta | Ulvophyceae | Filamentous, marine, cells with single parietal chloroplast and a pyrenoid |
| Trichosarcina | Chlorophyta | Ulvophyceae | Filamentous, cells with single parietal chloroplast and pyrenoid |
| Ulothrix | Chlorophyta | Ulvophyceae | Unbranched filaments with cells always closely adherent, uninucleated cylindrical cells |
| Printzina | Chlorophyta | Ulvophyceae | Filamentous, uninucleated cells, chloroplasts parietal and band‐shaped |
| Collinsiella | Chlorophyta | Ulvophyceae | Gelatinous, uninucleated cells, cup‐shaped chloroplasts |
| Asterochloris | Chlorophyta | Trebouxiophyceae | Found in association with fungus in lichen, single asteroid chloroplast in a crenulate, echinate, or lobed form |
| Chlorella | Chlorophyta | Trebouxiophyceae | Cells spherical, subspherical or ellipsoid, single or forming colonies, chloroplast single, parietal, pyrenoid present |
| Nannochloris | Chlorophyta | Trebouxiophyceae | Subspherical to subcylindrical, 0.8–4.5 μm in diameter unicells. May occur in pairs enclosed in mucilage, or in large numbers in a mucilage massc |
| Trebouxia | Chlorophyta | Trebouxiophyceae | Found in association with fungus in lichen, pyrenoid present |
| Stichococcus | Chlorophyta | Trebouxiophyceae | Unbranched filaments, cell walls thin, without gelatinous sheath, cells cylindrical and elongate, sometimes slightly oval |
| Myrmecia | Chlorophyta | Trebouxiophyceae | Coccoid cells, found in association with lichenous fungi; not to be confused with the genus of ants by the same namea |
| Dictyococcus | Chlorophyta | Chlorophyceae | Zoospores with a single parietal plastid nearly closed and lacking a pyrenoid, spherical cellse |
| Chlorococcum | Chlorophyta | Chlorophyceae | Uninucleated cells, ellipsoidal to spherical and varying in size, cell walls smooth, parietal chloroplast and with one or more pyrenoids |
| Planophila | Chlorophyta | Chlorophyceae | Uninucleated cells, spherical, solitary or tightly grouped in small (usually 2–8 cells) colonies, thin cell walls |
| Oscillatoria | Cyanobacteria | Cyanophyceae | Filamentous, trichomes blue‐green to brownish‐green, highly motile |
| Nostoc | Cyanobacteria | Cyanophyceae | Filamentous‐thallose, gelatinous, cells cylindrical, barrel‐shaped to almost spherical |
| Fischerella | Cyanobacteria | Cyanophyceae | Filamentous‐thallose, thallus usually felt‐like, usually barreliform cells |
| Rufusia | Rhodophyta | Stylonematophyceae | Branched‐filamentous, several parietal, discoidal to band‐shaped plastids with no pyrenoid, reddish to violet in colour |
Fig 3.
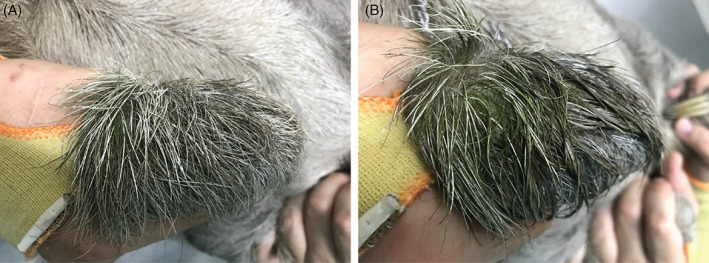
Dry and wet sloth hair. Hair on the back of the hand of (A) a dry Bradypus variegatus (brown‐throated three‐fingered) sloth, and (B) the same hand 10 s after wetting reveals a rapid greening and the presence of otherwise visually cryptic green algae/cyanobacteria.
(a). Sloth hair structure and algal growth
The morphology of sloth hair has the potential to influence the extent and composition of symbiotic growth. Three‐fingered sloth hair has transverse cracks that increase in quantity and depth as sloths age (Fig. 4; Aiello, 1985; Wujek & Cocuzza, 1986). The hairs swell considerably when wet, and it has been hypothesized that moisture that is retained within cracks sustains algal growth on the surface of the hairs (Aiello, 1985). It has also been hypothesized that this hair‐based absorption of water may help buffer changes in sloth body temperature (Pauli et al., 2016). It does not appear that the algae grow directly within the cracks, which would potentially limit access to photosynthetic radiation, but rather grow on the smooth outer surface of the hair (Aiello, 1985). It remains unknown whether algae directly colonize hair strands with pre‐existing cracks and/or if they contribute to hair crack development. By contrast, two‐fingered sloth hair has vertical grooves and does not appear to absorb as much water; algae appear only to be found within the grooves instead of coating the entire hair (Fig. 4B; Aiello, 1985; Wujek & Cocuzza, 1986). Differences in hair architecture may be responsible for the observed differences in fur amplicon sequencing surveys between the two genera of sloths (Aiello, 1985; Suutari et al., 2010). Although increased absorptive properties due to unusual hair structure are not limited to sloths (Kingdon et al., 2012), the unique cracked/grooved hair structure of sloths seems to facilitate symbiotic algal growth unlike any other mammal (Aiello, 1985). It is unknown whether algal and fungal species typically found on sloth hair are able to grow on texturally smooth hair. Whether such hair cracks/grooves co‐evolved with the associated microbes or is a convergently evolved trait among two‐ and three‐fingered sloths remains an open question.
Fig 4.

Scanning electron micrographs of sloth hairs. (A) Bradypus variegatus (brown‐throated three‐fingered sloth) hair at three different stages of development (scale bar = 0.6 mm). The bottom hair is from a young sloth in which transverse cracks are only beginning to develop. The middle hair is from an adult sloth displaying larger cracks. The top hair is from an old sloth and shows deep transverse cracks. (B) Choloepus hoffmanni (Hoffmann's two‐fingered sloth) hair showing longitudinal ribs or grooves, at 6× higher magnification than in A. Photographs reproduced from Aiello (1985) with permission (Smithsonian Institution Press).
(b). Identification of sloth algae
Morphological identification of sloth algae has yielded confusing results; in most cases, the sloth species from which algae have been derived was not recorded (Table 2). In a conference abstract by Thompson (1972), many sloth‐fur‐associated algae and cyanobacteria were listed, identified via morphology. However, algal and cyanobacterial species can be very hard to distinguish morphologically; DNA‐ and polyphasic‐based methods are typically required to make clear taxonomic assignments (Leliaert et al., 2014; Wilmotte et al., 2017). Unfortunately, no follow‐up confirmations of Thompson's (1972) identifications exist in the literature and Thompson did not specify from which sloth species these algae were obtained. Thompson identified two species of Oscillatoria and one of Nostoc, but it is not clear if either of these Oscillatoria are the same as the Oscillatoria pilicola identified and described by Wujek & Lincoln (1988) on both the fur of B. variegatus and C. hoffmanni. The genus Fischerella, three coccoid green algae (including Dictyococcus bradypodis and Chlorococcum choloepodis), three species of Trentepohlia, two of Stichococcus, and one of Nannochloris were identified (Table 2; Thompson, 1972; Wujek & Timpano, 1986). Rufusia, a red alga named by Wujek & Timpano (1986), was also identified on both B. variegatus and C. hoffmanni.
Trichophilus welckeri, the best‐known sloth green alga, was first identified on sloths in 1887 (Weber‐van Bosse, 1887). Trichophilus is in the class Ulvophyceae and is characterized by small (3–13 μm) thick‐walled cells with numerous, small, discoid chloroplasts that lack pyrenoids (Fig. 5; Table 2; Printz, 1964; Suutari et al., 2010). While solely morphology‐based taxonomic identifications must be taken cautiously, many of the aforementioned findings seem to be unknown to most modern readers of the sloth literature. Nonetheless, it appears that the diversity of green algae and cyanobacteria may be far greater than is suggested by more recent studies that focus on T. welckeri and its role in the sloth hair ecosystem (Pauli et al., 2014). Other species of algae should be taken into consideration, however, to understand how the community of photobionts is functioning and impacting its accompanying fungal and bacterial epibionts, arthropods, and the sloth itself.
Fig 5.

Morphology of green algal clusters, presumably of Trichophilus welckeri, found in sloth hair. (A) Trichophilus welckeri ‘fronds’ as described by Weber‐van Bosse (1887, fig. 15). s, sporangia; e, empty sporangial cells. (B, C) Trichophilus‐like alga from a hair of the pygmy three‐fingered sloth, Bradypus pygmaeus. (D) Hair with Trichophilus‐like alga from a Hoffmann's two‐fingered sloth, Choloepus hoffmanni. Modified from a figure in Suutari et al. (2010). Scale bars, 20 μm.
Amplicon metagenomic studies of sloth fur to date reveal a variable array of algae across and within different sloth species. Using amplicon‐based techniques, T. welckeri was found on the fur of B. variegatus, the pale‐throated sloth, Bradypus tridactylus, and the pygmy three‐fingered sloth, Bradypus pygmaeus (Suutari et al., 2010). No other green algal species were identified on these sloths from this 18S amplicon sequencing study (Suutari et al., 2010; Table 3) despite the prior observations by Thompson (1972), Wujek & Timpano (1986) and Wujek & Lincoln (1988). This discrepancy may be due in part to the use of captive sloths from zoos that potentially lack native epibionts (as mentioned earlier) and/or the intrinsic limitations of the use of 18S ribosomal DNA (rDNA) as a barcode for resolving algal taxa (Hall et al., 2010). T. welckeri has not yet been found environmentally (Suutari et al., 2010), although this may be a consequence of insufficient environmental sampling across the sloths’ geographical range and within the canopies of trees. The maned three‐fingered sloth, Bradypus torquatus, hosts a variety of algae belonging to genera known to be terrestrial, e.g. Trentepohlia and Myrmecia (Table 3; Suutari et al., 2010). C. hoffmanni, and B. tridactylus host Trichophilus spp. as well as terrestrial green algae from their surroundings (Table 3; Suutari et al., 2010).
Table 3.
Sloth species and associated algal epibionts identified to date. Those with an asterisk following the genus have thus far only been found on sloths and not yet on other environmental substrates. Data are from Suutari et al. (2010; clarified in some cases through personal correspondence with M. Suutari and J. Blomster). Cyanobacteria are indicated by superscript C. Eleven genera not listed in the table, Chlorococcum, Collinsiella, Dictyococcus, Fischerella C, Nannochloris, Nostoc C, Planophila, Pseudendoclonium, Stichococcus, Trichosarcina, and Ulothrix, were found on sloths, but are of an unidentified origin (Thompson, 1972; Wujek & Timpano, 1986). Note that Myrmecia below is a genus of green algae associated with lichens, not the genus of ants
| Sloth common name | Scientific name | Algal genera |
|---|---|---|
| Brown‐throated three‐fingered sloth | B. variegatus | Trichophilus*, Oscillatoria C, Rufusia |
| Pygmy three‐fingered sloth | B. pygmaeus | Trichophilus* |
| Pale‐throated three‐fingered sloth | B. tridactylus | Trichophilus* |
| Maned three‐fingered sloth | B. torquatus | Trentepohlia, Myrmecia, Asterochloris, Chlorella, Printzina, Trebouxia |
| Hoffmann's two‐fingered sloth | C. hoffmanni | Trichophilus*, Oscillatoria C, Rufusia, Trentepohlia |
| Linnaeus's two‐fingered sloth | C. didactylus | No data |
The 18S sequences for Trichophilus spp. found in association with B. variegatus, B. pygmaeus and B. tridactylus were found to cluster separately from Trichophilus sequences obtained from C. hoffmanni (Suutari et al., 2010). Trichophilus spp. from Bradypus and Choloepus differ in cell size, and B. variegatus and T. welckeri phylogenies are consistent with codivergence, which has led some to propose that B. variegatus and T. welckeri have coevolved (Suutari et al., 2010; Fountain et al., 2017). However, matching phylogenies is an insufficient demonstration of reciprocal coevolution (Janzen, 1980; Anderson, 2015). The differences in hair structure discussed earlier may impact differential colonization of sloth hair and the poorly characterized biogeography of environmental sources of sloth algae might explain the underlying phylogenetic concordance.
(c). Algal benefits
Several hypotheses have been proposed for how algae might benefit sloths, however, they all lack concrete empirical support, and in fact, it is not clear if algae provide any benefit to sloths. It is possible that it is simply a commensal relationship, and sloths may have so much algae in their fur because they do not have the means to clean themselves. Despite this, it is widely believed that fur algae provide a camouflage benefit to the sloth (Aiello, 1985; Suutari et al., 2010; Pauli et al., 2014), but no studies have been pursued to test this hypothesis. As discussed above, sloth fur coloration can change: they are primarily green during the rainy season when their hair is regularly wet (Fig. 6A), and in the dry season, many sloths lose their greenish hue and appear brown or grey (Britton, 1941; Gilmore et al., 2001). Direct observations of brown/grey sloths in their native canopy suggest that they are very well camouflaged with this colour scheme, blending in with the branches, trunks, and dead leaves of trees (Fig. 6C, D), as well as resembling ant and termite nests (Fig. 6B; Goffart, 1971).
Fig 6.

Colour and shape similarities of sloths. (A) A female Bradypus variegatus (brown‐throated three‐fingered sloth) with green fur coloration, taken during the wet season; (B) an Azteca ant carton nest that looks similar to a hanging sloth; (C) a dry B. variegatus sloth and (D) a dry Choloepus hoffmanni (Hoffmann's two‐fingered) sloth with similar coloration as the branches, vines, and bark of the trees they inhabit. Photograph of Azteca ant nest by Solar (2014) used with permission under Creative Commons License CC BY‐NC‐SA 2.0.
Fur algae have also been proposed to serve as a nutritional food source for sloths based on remnants of green algal cells being found in their stomach contents (Pauli et al., 2014). However, there is little evidence to support the idea that sloths ‘farm’ algae on their fur that they then consume, or that they consume sufficient quantities of fur algae to be nutritionally beneficial (discussed further in Section III.2c). Other proposed hypotheses in the literature for how algae could benefit sloths include: (i) algae being a source of thermal insulation (Britton & Atkinson, 1938; Goffart, 1971; Montgomery & Sunquist, 1978; Aiello, 1985); (ii) algae providing some yet unidentified chemical benefit to overall sloth health (Aiello, 1985); (iii) algae producing exopolymeric substances to facilitate beneficial bacterial growth (Suutari et al., 2010); and (iv) algae acting as a sunscreen (Suutari et al., 2010). T. welckeri has been found to produce an UV‐absorbing mycosporine‐like amino acid believed to protect algae from UV radiation (Karsten et al., 2005); whether this protection extends to sloths with T. welckeri on their fur (beyond the intrinsic UV‐protection of fur itself) is unknown. To our knowledge, none of these hypotheses are supported by any evidence‐based rationale.
(2). Arthropods
(a). Biting arthropods
Sloths are also host to a wide range of arthropods living in their fur including parasitic, bloodsucking and biting arthropods such as mosquitoes and sandflies, triatomine bugs, lice, mites, and ticks (Gilmore et al., 2001). Six species of ticks have been found on two‐ and three‐fingered sloths, all from the genus Ambylomma, but only two species, Ambylomma geayi and Ambylomma varium, appear specialized for living on sloths as these ticks are rarely found on other hosts (Waage & Best, 1985). Tick infestation can be extremely high. At the Instituto Nacional de Pesquisas da Amazonia in Manaus (Brazil), 99% of three‐fingered and 86.7% of two‐fingered sloths carried Ambylomma spp. (Waage & Best, 1985). Nothing is known about how A. geayi or A. varium find a host sloth and no correlation has been found between the numbers of ticks at any life stage on a sloth or seasonal differences in rainfall (Gilmore et al., 2001). The blood‐sucking mites, Liponissus inheringi, Lobalges trouessarti, and Edentalges bradypus have been identified on three‐fingered sloths (Waage & Best, 1985) and the mite Edentalges choloepi has been found on Linnaeus's two‐fingered sloth, Choloepus didactylus (Fain, 1964). Whether ectoparasite loads impact the health of a sloth is an open question.
(b). Commensals and beetles
Many commensal arthropods are found in association with these slow‐moving mammals. It is quite possible that the algae on sloth fur serves as a food source for these commensal arthropods considering that mites and other insects display algophagy (Mckenna et al., 2015; Seniczak et al., 2016). Cockroaches have been found in sloth fur (Britton, 1941), although this may be quite rare (S. Trull, unpublished data). Adults of several scarab beetle species are frequently found in the fur of three‐fingered sloths (of which the beetle in Fig. 7 is an example), but have not been reported to be associated with Choloepus (Ratcliffe, 1980; Gilmore et al., 2001). The scarab beetles occur near the elbow or on the flanks behind the knees, buried deep inside the fur. The beetles found living on sloths are considered commensal because they are phoretic coprophages: the beetle larvae (and possibly adults) feed on sloth dung and they do not appear to harm the sloths (Ratcliffe, 1980; Gilmore et al., 2001). About a thousand such beetles (Trichillum adisi) have been found in the fur of a single brown‐throated three‐fingered sloth (B. variegatus) collected on Curari Island in the Central Amazon region (Waage & Best, 1985). Beetles of the genus Uroxys have been recorded from sloths in Bolivia, Brazil, Colombia and Panama (Waage & Best, 1985). Despite the ubiquity of beetle–sloth interactions, little is known about the dispersal and density fluctuations of these beetles on sloths, although in Panama, there seem to be higher numbers of beetles during the rainy season (Wolda & Estribi, 1985). It has been suggested that the beetles have dispersal flights at the beginning and end of the rainy season and that part of the population might enter reproductive diapause and disperse from the sloths to sites with some moisture; they presumably resume reproduction at the end of the dry season and return to the sloths (Wolda & Estribi, 1985). Just as there are no data to substantiate an effect of parasite load on sloths, no analysis has been performed to understand the effect of these suspected commensal arthropods or of total arthropod load on sloth health. Likewise, little is known of the potential role these beetles might play in the ecosystem. It is possible that beetles contribute to parasite suppression, secondary seed dispersal, and to nutrient cycling within the sloth fur ecosystem and the larger forest ecosystem (Nichols et al., 2008). It is also possible that some sloth‐associated arthropods play a protective and mutualistic role by preying on ectoparasites in sloth fur (cf. Ostlund‐Nilsson, Becker & Nilsson, 2005; Goedknegt, Welsh & Thieltges, 2012).
Fig 7.
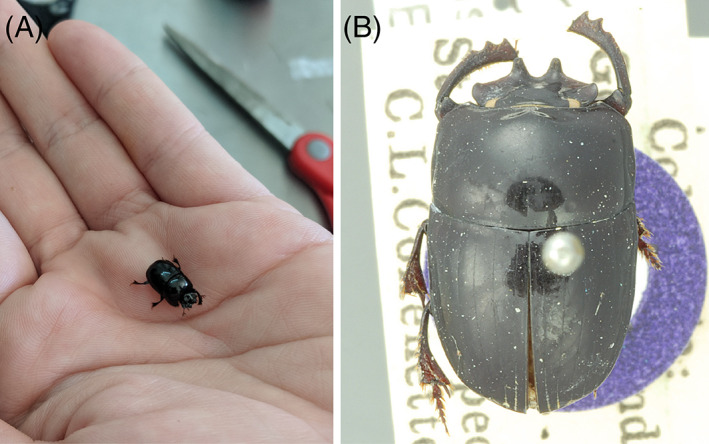
The sloth‐associated scarab beetle “Uroxys gorgon Arrow, 1933.” (A) Collected live from the fur of a Bradypus variegatus (brown‐throated three‐fingered sloth), and (B) a mounted specimen (Larsen, 2015), used with permission under Creative Commons License CC BY‐NC 3.0.
(c). Sloth moths
Sloth moths in the genus Cryptoses have received notable attention as a sloth epibiont. There is appreciable geographic sympatry amongst sloth‐associated moth species and several different species may coexist in the fur of a single sloth (Waage & Best, 1985). Various sloth moth species appear to be found on all species of sloths (Bradley, 1982; Waage & Best, 1985; Pauli et al., 2014). Cryptoses choloepi seems to be the most common moth found on B. variegatus and has been studied almost exclusively in relation to this sloth species (Fig. 8; Supplementary Videos S2 and S3). Female C. choloepi moths that live in B. variegatus fur have been observed to oviposit in the dung of the sloth as the sloth descends to the forest floor to defecate. Moth larvae in early stages spin silken threads between two or three pellets of dung, forming net‐like structures from which they feed (Waage & Montgomery, 1976). Upon maturation after 3–4 weeks, newly emerged moths presumably fly from the dung pile into the forest canopy to find a new sloth host (Waage & Montgomery, 1976). In addition to nutritional benefits that sloth moth larvae might receive from feeding on sloth dung, adult moths may consume sloth/algal secretions or microbes on sloth hair (Fig. 8); the sloth moth gut microbiome has yet to be explored but may provide evidence for this. Adult moths are believed to receive a transportation benefit as well as a protection benefit from living in sloth fur (Waage & Montgomery, 1976; Wolda, 1985). The amount of protection moths receive in association with sloths is questionable, however, since brown jays (Psilorhinus morio) have been observed to consume insects off sloth fur (Neam, 2015).
Fig 8.

The sloth moth, Cryptoses choloepi, on a Bradypus variegatus (brown‐throated three‐fingered sloth). (A) Moths often swarm the sloth's face, especially orifices such as the nose and eyes, and (B) appear well camouflaged on the sloth's grey‐brown fur.
Based on studies to date, it would appear that sloth moths have a commensal relationship with their sloth hosts. However, a three‐way mutualism has been proposed involving B. variegatus, their moths, and fur algae, specifically T. welckeri. According to this hypothesis, moths are portals for nutrients, increasing nitrogen levels in sloth fur through defecation, which is believed to promote algal growth (Pauli et al., 2014). T. welckeri‐like algae have been found (microscopically) in sloth stomach contents, which has led to the hypothesis that sloths consume these algae to augment their limited diet (see Section III.1c). With this set of observations, the proposal is that sloths are involved in an evolutionary trade‐off in which they risk their lives, descending to the ground to defecate, in order to preserve this sloth–moth–algae tripartite mutualism (Pauli et al., 2014). There are at least five potential problems with this hypothesis. First, the method of identifying the alga in this study as T. welckeri by morphology alone is not sufficiently rigorous, especially given that this taxon is often morphologically cryptic and under‐studied in general (Dudgeon et al., 2017). Second, while the main groups of bacteria that inhabit the gut microbiome of B. variegatus have been identified (Dill‐McFarland et al., 2016), no metagenomic study to date has been performed to characterize or confirm the eukaryotic/algal diversity in this species’ gastrointestinal tract. Sloths have been observed to lick and eat material off of branches and tree trunks, which may include lichens (Tirler, 1966; S. Trull, unpublished data), thus the algae observed in the stomachs of sloths may have derived from environmental substrates rather than from their fur. Third, thousands of hours of sloth behavioural research recorded during the day and night do not support the idea that sloths lick themselves (like cats) or eat epibiotic algae from their fur (Tirler, 1966; S. Trull, unpublished data). Fourth, only two B. variegatus individuals out of 12 sampled in one location in Costa Rica were identified as having Trichophilus spp. in their stomachs (Pauli et al., 2014). And lastly, if sloth tree‐descent and ground‐defecation is driven by a need to benefit moths via dung oviposition, one would expect there to be reciprocal fitness benefits provided to the sloth by the moths in order for this behaviour to have evolved or be maintained (Voirin et al., 2013); however, the implied and indirect benefits that sloths might obtain from moth‐influenced fur algal growth lack empirical support and may be quantitatively modest.
(3). Fungi
Fungi are known to be associated with sloth hair, but the roles they play in the community ecology of the sloth pelage and in the health of the sloth remain unexplored. A diverse group of Ascomycota and one Basidiomycete (Sporobolomyces subbrunneus) have been identified growing on sloth fur through sequencing and culture‐based methods (Suutari et al., 2010; Higginbotham et al., 2014). Only two species of fungi that have been found on sloths have also been found on the bark of trees in sloth habitats (Devriesia staurophora and Mycosphaerella pini; Suutari et al., 2010), although these results are from very limited sampling. These sloth‐associated fungi have been found in soil and plants (Arnold & Lutzoni, 2007; Wang et al., 2011), so it is possible that sloths are exposed to these fungi when they defecate on the ground or as they eat and interact with leaves and bark (Higginbotham et al., 2014). Nearly 35% of fungal isolates obtained from B. variegatus fur are identical to endophyte strains obtained from plants in the same region (Higginbotham et al., 2014). Given the taxonomic similarity between endolichenic and endophytic plant fungi in the same environments (U'ren et al., 2012), it is plausible that some sloth hair fungi may associate directly with green algae (Higginbotham et al., 2014). Previous studies support that fungi, and these taxa in particular, have intrinsic affinities for associating and forming mutualisms with algae, as seen in lichens [typically slow growing and commonly found on undisturbed trees (Hawksworth, 1988; Arnold et al., 2009)], and other similar symbioses (Hawksworth, 2000; Gareth Jones, Pang & Stanley, 2012; Hom & Murray, 2014; Du et al., 2019).
However, whether sloth‐associated fungi are commensals, parasitic, or mutualistic is not clear. Hair‐associated fungi from B. variegatus have been shown to display a broad range of inhibitory activities against parasites that cause malaria (Plasmodium falciparum) and Chagas disease (Trypanosoma cruzi), human breast cancer cells, and bacteria, particularly Gram‐negative bacteria (Higginbotham et al., 2014). The inhibitory activities of these fungi may thus provide benefits to the sloth as well. Some sloths have clear black fungal growth on their hair (Fig. 9A), which could potentially harm the sloth or outcompete other microbes in the sloth hair ecosystem. Others develop severe fungal infections on their skin that can be detrimental because the infections produce scabs, which then fall off, leaving bare skin that is susceptible to ectoparasites like ticks and mosquitos (Fig. 9C; Xavier et al., 2008); anecdotally, fungal infections generally correlate with sick sloths (S. Trull, unpublished data). It is unclear whether these infections derive from fungi that are already part of the sloth fur microbiome and/or whether they are a primary cause for sloths getting sick.
Fig 9.

Sloths and fungi. (A, B) The back of the heads of two Choloepus hoffmanni (Hoffmann's two‐fingered sloth) with visible growth on the fur of (A) black fungi and (B) algae. (C, D) Facial photographs of (C) a Bradypus variegatus (brown‐throated three‐fingered sloth) with a severe fungal infection that causes scabs of hair to fall off, and (D) a healthy B. variegatus sloth for comparison.
(4). Other symbionts
In addition to the algae, arthropods, and fungi that live and thrive within the pelage of sloths, other putative fur‐associated organisms have been identified through 18S amplicon sequencing; these include euglenozoans, amoebozoans, cercozoans, apicomplexans, dinoflagellates, and ciliates (Table 4; Suutari et al., 2010). To date, nothing is known about the role of these organisms within the sloth hair ecosystem. Apart from the sloth fur cyanobacteria mentioned above (Table 2), fur‐associated prokaryotes have not been well documented or sufficiently taxonomically resolved. Surprisingly, a 16S survey of the bacterial diversity on sloth fur has not yet been performed; it will be important to survey the prokaryotes present in the sloth fur ecosystem and to understand the inter‐kingdom interactions they may have with the sloth and other fur epibionts. Bacterial epibionts may influence the function of sloth‐associated fungi and algae, as they do for fungal endophytes associated with plants (Partida‐Martinez & Hertweck, 2005; Hoffman & Arnold, 2010) and lichens (Grube & Berg, 2009; Bates et al., 2011).
Table 4.
Other epibionts found in sloth fur. Species names were assigned based on the closest known matches in GenBank. Percentage similarity is to the closest match in GenBank. Data from Suutari et al. (2010). Given the low similarity for most matches and little taxonomic follow‐up, these species designations may not be correct
| Phylum | Taxon | Percentage similarity |
|---|---|---|
| Euglenozoa | Petalomonas cantuscygni | 82% |
| Amoebozoa | Lamproderma ovoideum | 85% |
| Cercozoa | Cercomonas plasmodialis | 99% |
| Apicomplexa | Eimeriidae spp. | 89–99% |
| Dynophyceae | Heterocapsaceae | 89–91% |
| Ciliophora | Bresslauidea discoideus | 97% |
| Campenella umbellaria | 87% | |
| Colepidae spp. | 95% | |
| Epistylis galea | 88–93% | |
| Opercularia microdiscum | 87–91% | |
| Peritrichia sp. | 87–91% | |
| Trithigmostoma steini | 90% |
Sloths are also carriers of a variety of arthropod‐associated viruses (‘arboviruses’; e.g. phleboviruses, flaviviruses, encephalitis viruses, and orthobunyaviruses) (Seymour, Peralta & Montgomery, 1983; Gilmore et al., 2001; Medlin et al., 2016; de Oliveira Filho et al., 2020) and insect‐borne protozoans (e.g. trypanosomes, such as Leishmania) (Shaw, 1985; Gilmore et al., 2001; Muñoz‐García et al., 2019). These pathogens may be transmitted by (free‐living or epibiotic) arthropod bites to blood but may also be found on the skin or within sloth fur. Phlebotomine sandflies that reside in the fur of sloths are known carriers of Leishmania, which causes leishmaniasis in humans (Arias & Freitas, 1978; Herrer & Christensen, 1980; Christensen et al., 1982). C. hoffmanni sloths likely become infected by these trypanosomes in their first few months of life and remain infected for a long time, but appear asymptomatic and do not show signs of pathology (Herrer & Christensen, 1980).
IV. FUTURE DIRECTIONS
(1). Transmission of fur epibionts and coevolution
How are sloth fur epibionts acquired and transmitted? There are few to no data on the modes of transmission of the different sloth fur epibionts, or whether there are taxon‐specific differences in the degree of vertical versus horizontal transmission. To determine the extent of epibiont coevolution with sloth hosts, however, it is critical to determine the degree of vertical transmission of the epibiont community and reciprocal adaptation (Bordenstein & Theis, 2015; Meng et al., 2018; Rosenberg & Zilber‐Rosenberg, 2018; Roughgarden et al., 2018; Simon et al., 2019). Sloth algae, the most studied fur epibiont, is believed to be transmitted vertically from mother to baby (Beebe, 1926; Britton, 1941; Suutari et al., 2010) although this has not been tested directly. Horizontal transmission from environmental species pools to the sloth cannot be ruled out given the absence of data characterizing these pools. This is true for all fur epibionts that have been hypothesized to be specific to sloths. A mixed mode of epibiont transmission is likely, however, given that vertical and horizontal modes represent extreme cases (Rosenberg & Zilber‐Rosenberg, 2018). Obligate symbionts generally rely on vertical transmission (Rosenberg & Zilber‐Rosenberg, 2018), although there are also no compelling data on whether the algae on sloths are obligately or facultatively associated. It is tempting to imagine that the peculiar cracks/grooves of sloth hair may be a coevolved adaptation of the sloth with fur algae, but it is possible this may simply be a trait exapted by algae, given that cracked hair retains water that may aid sloths in thermoregulation (see Section III.1a).
The fact that over a third of sampled fungi from sloth fur were found to be identical to plant endophytic fungi from foliage in the same regional habitat and that other identified fungal genera can also be found in soil and with plants (Section III.3) suggests that fur microbes could in general be acquired from the environment. This may be particularly true for arthropods that are more mobile than microbes. These results highlight the importance of carefully characterizing environmental species pools to establish a null model that can be used to test hypotheses about vertical transmission or coevolution (cf. Fountain et al., 2017), efforts that have been lacking for sloth algal surveys to date. Although several arthropods appear to be specific to sloths, including the ticks A. geayi and A. varium (Waage & Best, 1985), the scarab beetle U. gorgon (Fig. 7), and sloth moth C. choloepi (Waage & Montgomery, 1976; Fig. 8), their transmission across generations remains unknown. The notion that sloth moths could have co‐evolved with B. variegatus and the green alga T. welckeri would require a high degree of partner fidelity (Archetti et al., 2011; Kaltenpoth et al., 2014). However, it is uncertain how after 3–4 weeks of maturation at a site of sloth defecation (Waage & Montgomery, 1976; Section III.2c) the moths emerge to find the original sloth host (rather than a different sloth) given the extent of sloth movement during this time (Section II.2).
In theory, horizontal transmission of epibionts could also occur between different sloths. However, sloths are considered solitary animals (Taube et al., 1999; Soares & Carneiro, 2002; S. Trull, unpublished data) and they generally do not interact with animals of other species, except for the occasional bird eating an insect off the sloth (Neam, 2015). On rare occasions, individuals from both two‐ and three‐fingered sloths have been observed to share the same or adjacent trees (Silva et al., 2013), including one extreme case of up to five B. variegatus sloths on a cacao farm feasting on the same Cecropia tree for over a month until the tree was completely defoliated (Vaughan et al., 2007), although this latter example may be far from representative of behaviour in the natural habitat. There is one brief and aggressive encounter documented in the literature between two males possibly related to defending mating territory (Greene, 1989), but this is consistent with sloths’ general preference to live alone. It is thus unlikely that sloth epibionts are typically transmitted through social contact with other sloths or other animals.
Sloths of the same species do, however, interact during two phases of sloth life history at which time fur epibionts could be transmitted: mating and early development. Sloths mate with the male on the back of the female or face‐to‐face, and can copulate for up to 7 min (Bezerra et al., 2008; Dias et al., 2009; Richard‐Hansen & Taube, 1997; S. Trull, unpublished data). Close physical contact during copulation could allow for the transmission of epibionts, especially mobile epibionts, such as arthropods, along with any microbes they might carry. Between the birth of young (gestational period of 5–10 months), sloths mate every 10–15 months for a total period of ~20 years (Taube et al., 2001); this amounts to approximately 10 matings over the life of a sloth, often with a different partner. The role of sex and the ‘reproductive microbiome’ – i.e. the microbiome associated with the reproductive system of parents that may inoculate offspring (Rowe et al., 2020) – on the transmission of fur epibionts between sloths remains to be elucidated.
Sloths give birth to their young in the canopies of trees, and newborn sloths immediately cling to the fur of the mother sloths’ abdomen for a continuous period of 5–7 months (Ramirez et al., 2011). Young sloths generally cling to the abdomen of their mother, not her back. However, juvenile sloths do climb onto the back and sides of the mother when she is stationary (Soares & Carneiro, 2002; S. Trull, unpublished data). It is not clear what microbes grow on the abdomen of sloths, since all sloth hair microbiome studies to date have sampled from the greenest parts of the sloth, generally the head, shoulder, or back (Suutari et al., 2010; Pauli et al., 2014). Since juvenile sloths remain on their mother for so many months, fur epibionts are likely vertically transmitted due to protracted close contact. At the very least, mothers dictate the exposure of their young to environmental species pools by the nature of their own movement throughout the forest canopy (Soares & Carneiro, 2002). Frequent sampling and analysis of sloth hair from a mother and her young throughout the period of maternal care and after the juvenile has separated from the mother would be very helpful towards resolving questions about epibiont transmission.
Sloths spend upwards of 70% of their waking hours resting in trees (Chiarello, 1998; Urbani & Bosque, 2007). They are often in direct contact with tree bark and leaves during their sleeping and resting hours, as they can be routinely found lying on branches or reclining against a branch or the trunk of a tree (S. Trull, unpublished data). Thus, transmission of biota from trees to sloths and vice versa is very likely, although there are few formal data to support this hypothesis. The phyllosphere is teeming with microorganisms, such as bacteria, archaea, fungi, and algae (Vacher et al., 2016), and by using metagenomic tools, one could in principle compare the structure and function of microbial communities on sloths and their surrounding canopy environment (Rastogi, Coaker & Leveau, 2013; Baldrian, 2017; Hassani, Durán & Hacquard, 2018). Sloths also interact with soil when they descend to the base of a tree to defecate once a week (Voirin et al., 2013; Pauli et al., 2014), where they could also acquire or disperse epibionts. The arthropods that reside in sloth fur may also be vectors that transmit microbial epibionts to and from sloths (Laroche, Raoult & Parola, 2019), especially for microbes that may have no apparent environmental species pool in the immediate surroundings of the sloth.
(2). Sloth fur ecosystem benefits
It is undeniable that sloth fur is an unusual and rich nexus of biodiversity, but does this fur ecosystem benefit the sloth in any way? Several ideas have been proposed for how algae could benefit the sloth (see Sections III.1c and III.2c); while all are problematic and/or lack empirical support, the notions that algae might provide some camouflage or chemical benefit to the sloth may be plausible. Based on these notions and inspired by the curious visual resemblance of hanging sloths to ant/termite nests (Fig. 6B), we suggest another hypothesis for how fur microbes could benefit the sloth. Azteca ants form a well‐known defensive mutualism with Cecropia trees (Janzen, 1969; Berg & Rosselli, 2005; Marting et al., 2018), a genus of trees that B. variegatus frequently use for food and refuge (Fig. 10; Vaughan et al., 2007; Ramirez et al., 2011; Neam & Lacher, 2015; Garcés‐Restrepo et al., 2019b ). Azteca ants fiercely defend these trees from herbivores (Schupp, 1986). While it is unknown whether these ants are effective at preventing sloths from eating the leaves of the Cecropia (Fig. 10D), anecdotal evidence suggests that sloths are not deterred by these notoriously aggressive biting ants (S. Trull & P. Marting, unpublished data). Given the broad abilities of microbial volatile organic compounds (mVOCs) to deter or modulate insect behaviour (Davis et al., 2013; Engl & Kaltenpoth, 2018), it is possible that semiochemicals produced by sloth hair microbes may act to repel Azteca ants. Such mVOCs may also play a role in scent‐based sloth–sloth recognition and mating (see Silva et al., 2013). While mVOCs from plants (Leach et al., 2017), bacteria, and fungi (Dickschat, 2017; Lemfack et al., 2017) have been investigated, the capacity for algae to produce such compounds has been little explored (Achyuthan et al., 2017; Lemfack et al., 2017). Given the prevalence of bacteria (e.g. Streptomyces and Myxobacteria) that produce mVOCs in addition to other diverse compounds (Audrain et al., 2015; Lemfack et al., 2017; Veselova, Plyuta & Khmel, 2019) and unexplored algal and fungal mVOCs, the sloth fur microbiome may be a reservoir for novel mVOC‐producing microbes.
Fig 10.

Photographs showing the (A) canopy of a Cecropia obtusifolia tree, (B) mutualistic ants, Azteca constructor, harvesting food bodies from a Cecropia petiole/stalk, (C) Azteca ants attacking an encroaching vine to protect a Cecropia tree, and (D) a brown‐throated three‐fingered sloth, Bradypus variegatus, eating fruit from a Cecropia tree, seemingly unbothered by ants. A, B, and C reproduced from Marting et al. (2018) with permission under Creative Commons License CC BY 4.0.
Microbes are known to play a fundamental role in the development and immune function of most animals (McFall‐Ngai, 2014, 2015; Bosch, Guillemin & McFall‐Ngai, 2019) and this may also be true for sloths. Sloths in captivity face many health challenges (de Stefani Munaó Diniz & Oliveira, 1999); misinformed practices at sloth rehabilitation facilities and zoos, such as bathing sloths without a specific need, could be ridding them of beneficial fur epibionts and disrupting fur ecosystem balance in a manner that negatively impacts sloth well‐being (cf. Hooks & O'Malley, 2017; Levy et al., 2017). The fungal infections afflicting wild sloths (Section III.3; Fig. 9) may serve as a practical target for understanding the basis of sloth disease regarding the normal functions and composition of the sloth fur ecosystem. Because symbiotic interactions can be context dependent and mutualists/commensals can potentially become parasites (Bronstein, 1994; Kogel, Franken & Hückelhoven, 2006; Leung & Poulin, 2008; Jones et al., 2015; Akçay, 2017; Vostinar & Ofria, 2019), it is possible that these fungal infections stem from resident or dormant members that become pathogenic due to environmental shifts or fur ecosystem imbalance. It is also possible that the sloth fur ecosystem endows sloths with a resilience against external pathogens.
(3). Access to a unique ecological regime in time and space
It is becoming evident that explicit consideration of spatial, temporal, and phylogenetic scales (specifically ideas of granularity and extent) along with system nestedness will be critical to elucidating both the patterns and mechanisms of community assembly in ecosystems in which microbes play a foundational role (Addicott et al., 1987; Wiens, 1989; Wang & Loreau, 2014; Shade et al., 2018; Ladau & Eloe‐Fadrosh, 2019). For sloths that are effectively foci of epibiont biodiversity, it may be fruitful to consider how much of the sloth fur community could be understood from the perspective of island biogeography (MacArthur & Wilson, 1967; Bell et al., 2005; Peay et al., 2007; Wilson, 2010; Belisle, Peay & Fukami, 2012; Glassman et al., 2017; Proctor & Relman, 2017). Sloths may be a good model for examining interactions at different hierarchical levels whereby biotic feedbacks between microbes and higher trophic levels of an ecosystem are explicitly considered in understanding how host ecosystems are shaped and structured (Leibold et al., 2004; Carmona et al., 2016; Seibold et al., 2018; Miller, Svanbäck & Bohannan, 2018a ; Leibold & Chase, 2019; Liu et al., 2019).
We have referred to the sloth as a ‘mobile ecosystem’ to highlight the fact that sloths experience life and movement within an unusual regime of time and space, unlike most other macro‐organisms. They may serve as genetic and biotic ‘mobile links’ within neotropical forests (Lundberg & Moberg, 2003). The slow movements of sloths through their geographical range and the vertical column of the forest canopy may allow us to examine an ecological and spatiotemporal regime not typically experienced by sessile (e.g. plants/trees) or significantly more mobile organisms of comparable size. Sloths may thus provide unique insights into ecological connectivity and movement ecology of wild, free‐ranging animals (see Jacoby & Freeman, 2016; Schlägel et al., 2020). As discussed herein, sloths can travel ≥38 m per day but typically do so in <1 h bursts of activity and commonly within a range of <2 ha (Section II.2); they can be found at various vertical heights between the forest canopy and the ground, to which they descend once a week to defecate. The curious abundance and diversity of microbes and arthropods that take up residence within the sloth pelage raises the question as to whether the uniquely slow timescales at which sloths move, their intermittent patterns of lateral movement, together with their vertical migrations, might facilitate this phenomenon. Is there some sort of temporal resonance of ecosystem processes with sloth movement dynamics that facilitates the striking biodiversity on sloth fur? How does community diversity change as a function of the characteristic timescales of underlying assembly/dispersal processes, animal movement, and forest structure?
The movement of sloths throughout their range and up and down the canopy column may connect and disperse fur epibionts between very different ecological niches. As sloths are scattered across the tree canopy, finding, catching, and studying sloths can be experimentally challenging. However, with recent advances in Global Positioning System (GPS) tracking and remote‐sensing/monitoring technology (Kays et al., 2015; Lennox et al., 2017; Neethirajan, 2017; Taylor et al., 2017; Hughey et al., 2018; Shipley et al., 2018; Williams et al., 2019; Ripperger et al., 2020), it may now be easier and more feasible to pursue continuous monitoring studies of sloths that are otherwise difficult to investigate by traditional search‐and‐catch methods. These capabilities may make sloths – along with their entourage of microbial and arthropod epibionts – a tractable model for exploring questions of context dependency given habitat differences across their large geographical range. Accurate time‐resolved data of sloth movements in three dimensions (latitude, longitude, and altitude/elevation) are currently lacking, limiting a deeper understanding about how sloths move through the forest, their interactions with their environment and other animals, and their responses to habitat degradation or change (Pool et al., 2016; Santos et al., 2016; Brandão et al., 2019; Garcés‐Restrepo, Pauli & Peery, 2019a ). Data on social and habitat connectivity are critical for understanding the sources and modes of symbiont transmission. Coupling movement (spatial geo‐tracking) data with real‐time local environmental sensing (or time‐series data) of temperature, humidity, light, etc., and with periodic biodiversity surveys of sloth fur, would provide invaluable insights into the degree of variation and environmental conditions that a sloth experiences regarding how the fur ecosystem is structured and changes.
(4). The nature and network of sloth–epibiont interactions
Thus far, few studies have even attempted to simply determine the nature of the interactions between sloths and their fur epibionts. Building upon this limited knowledge, future efforts should aim to identify the symbiotic traits of each interacting organism and the selective pressures acting on those traits. The ecosystem functions of sloths within their native habitat are largely unknown, although they are believed to be an important source of long‐term stable nutrients at the base of trees where they defecate (Montgomery & Sunquist, 1975). It will be important to determine through environmental sampling if algae like T. welckeri are generally limited to growth on sloths or if they can grow independently on other environmental substrates within the sloth habitat. If found environmentally, it would provide support for a model in which sloths acquire algae from the environment, and will provide a proper null model by which to assess sloth–algae coevolution as discussed in Section III.1. Other organisms are found in sloth fur, including bacteria, euglenozoans, amoebozoans, cercozoans, and alveolates (Table 4; Wujek & Lincoln, 1988; Suutari et al., 2010), many of which appear not to be present in the environment around sloths (Suutari et al., 2010). The functions of these organisms in the sloth hair ecosystem are unknown but have the potential to directly impact sloth health. Sloths appear to be carriers for several arthropod‐borne viruses and parasites and understanding the basis for why sloths seem not to be burdened by such pathogens may be of relevance to human health. Also unclear is the role that microbial epibionts have in facilitating host defence against pathogens in general, which has been well demonstrated in plant and pollinator systems (Liu et al., 2019).
As discussed above, the slow but unusual movement dynamics of sloths through the ‘volume’ of forest canopy may enable them to be hotspots of biodiversity. The stop‐and‐go pattern of movement of sloths and their migrations of over several m/day at relatively slow speeds may make them attractive for arthropod occupancy and dispersal via hitchhiking. The slow movements of sloths may also enable them to be colonized more easily by biota like lichenous fungi (being more similar to a tree than many fast‐moving animals; Supplementary Videos S1 and S2) that require low‐levels of movement disturbance. Interestingly, epizoic lichens, fungi, and/or cyanobacteria have been found to grow on arthropods, specifically two species of leaf mantis in the genus Choeradodis (Lücking, Mata‐Lorenzen & Dauphin, 2010) and various harvestmen arachnids (within small pits) (Machado & Vital, 2001; Proud et al., 2012; Young, Moore & Townsend Jr., 2018). Whether any of the sloth‐associated arthropods carry these taxa epibiotically is unknown, but fungal–algal associations in sloth fur could potentially link sloths to arthropods and bacteria.
As primary producers, photoautotrophic algae are at the base of the food web in many ecosystems (Polis & Hurd, 1995; Segovia et al., 2015; Kohlbach et al., 2016; Brocks et al., 2017), and are likely to serve as the base of the sloth fur ecosystem as well. Algae may be dependent on nitrogen from resident fur arthropods (via faeces) or nitrogen‐fixing bacteria, but there are no data available on this. It is also unclear how algal growth influences the composition of the rest of the microbiome and if arthropods farm and/or consume the algae. Microbial epibionts in sloth fur may provide supporting services, including producing ‘pioneer’ metabolite products that provide a foundation for community development, biofilm formation, nutrient cycling, and a thriving ecosystem (McKenney et al., 2018). As a poorly studied reservoir for potentially novel microbial and genetic diversity, these hair algae/microbes may produce specialized or secondary metabolites that prevent infections, or volatiles that repel ectoparasites/predators or attract arthropods in a similar manner to how plants use volatiles to attract or repel pollinators and predators (Kessler & Baldwin, 2001; Pichersky & Gershenzon, 2002). In so doing, these natural products may play a vital role in the chemical ecology of the fur ecosystem, in shaping epibiont community structure, and in modulating sloth scent in a beneficial manner. Microbes associated with insects are known to be a source of bioactive compounds and enzymes that have biotechnological potential (Berasategui et al., 2016) and sloth microbes may ultimately be of relevance to human health and agriculture.
V. CONCLUSIONS
Sloths are slow‐moving, tree‐dwelling, leaf‐eaters in Central and South America that experience life and movement within an unusual regime of time and space, unlike any other macro‐organism. With a taxonomically diverse epibiotic community residing in their fur, sloths can be considered ‘mobile ecosystems’ or moving islands of biodiversity. They are unique models for investigating ecological and evolutionary processes linking microbes, arthropods, animal host movement, and neotropical forest resources and structure.
Sloth hair is unusual in having cracks or grooves that absorb water and support the growth of epibiotic algae, which is the basis for the distinct coloration of sloths in the wild. These primary producer algae are part of a rich multi‐trophic community of microbes (including bacteria, fungi, and protists) and arthropods (including moths, beetles, sandflies, triatomine bugs, lice, mites, and ticks). Sloths are also carriers of a variety of arthropod‐associated viruses (‘arboviruses’). To date, how the fur ecosystem assembles, functions, and the benefits it provides the sloth host are unclear at best.
It is not known whether sloths have coevolved with their fur epibionts nor how these epibionts are transmitted across generations of sloths. Incomplete studies to date suggest that sloths may host unique species of algae (Trichophilus welckeri), ticks (Ambylomma geayi and Ambylomma varium), and moths (Cryptoses choloepi) that rarely if ever are found on other hosts, consistent with the idea of vertical epibiont transmission. However, this hypothesis has yet to be tested empirically, and the absence of data on environmental species pools (which would support modes of horizontal transmission) is not data of absence. Sloth‐associated fungi have been found in soil and plants and over a third of fungal isolates obtained from sloth fur are identical to endophytes obtained from plants in the area, suggesting that horizontal transmission of at least some microbial epibionts may be likely.
Accurate animal tracking of sloths in four dimensions (latitude, longitude, altitude/elevation, and time) as they move through up, down, and across the tree canopy would provide extremely useful data for understanding the movement ecology of these mobile ecosystems as they interact with different components of the neotropical forest. Coupling movement data with real‐time environmental sensing of temperature, humidity, and light (with periodic biodiversity surveys of sloth fur) would provide particularly valuable insights.
Experimental efforts should focus on elucidating sloth fur ecosystem functions, the nature of epibiont–epibiont and sloth–epibiont interactions, and selective forces that shape this ecosystem. Understanding how fur ecosystem diversity and resilience impacts sloth health is an important practical target that could inform best practices on caring for sloths at rehabilitation facilities and zoos. Like other mammalian microbiomes, sloth fur microbiota may play an important part in immune function, and may also play a vital role in the chemical ecology of the fur ecosystem and of sloths with other individuals.
Supporting information
Video S1. A male Bradypus variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released following a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica.
Video S2. A female B. variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released following a health check‐up (at The Sloth Institute in Manuel Antonio, Costa Rica) on her and her baby, seen clinging to her abdomen. Video by Carole Moncoquet (used with author's permission).
Video S3. A female B. variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released at night following a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica. Note the green coloration on her shoulders as well as the sloth moths swarming her back.
Video S4. A female Choloepus hoffmanni (Hoffmann's two‐fingered sloth) climbs relatively quickly up a vine (compared to a brown‐throated three‐fingered sloth) after a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica.
VI. ACKNOWLEDGEMENTS
We thank Michael Clear, Xia Li, Amber Horning, Margaret Campbell, and Thomas Collins for their insightful comments on early drafts. We also thank Rob Carmichael, Steve Reichling, Susan Balenger, Colin Jackson, Victoria Calatrava, and Kenny Coogan for providing feedback, Peter Marting for helpful discussions about Azteca ant and Cecropia ecology, Paul Lago and Bert Kohlmann for help identifying the scarab beetle commonly associated with sloths, Carole Moncoquet for permission to use one of her sloth videos as supplementary material, and Julio Bustamante of the Sistema Nacional de Áreas de Conservación (Costa Rica) for issuing permit ACOPAC‐INV‐001‐17 to S.T. Finally, we thank Judie Bronstein and other anonymous reviewers for their very constructive comments. This work was supported in part by the National Science Foundation under grants DEB‐1541538 (GoLife) and DEB‐1846376 (CAREER), and a sabbatical fellowship to E.F.Y.H. at the Deutsches Zentrum für Integrative Biodiversitätsforschung (iDiv) of Halle‐Jena‐Leipzig, Germany under grant DFG–FZT 118, 202548816 (German Research Foundation). This is the UM Center for Biodiversity and Conservation Research publication No. 012.
REFERENCES
- Abed, R. M. M. , Polerecky, L. , Al‐Habsi, A. , Oetjen, J. , Strous, M. & de Beer, D. (2014). Rapid recovery of cyanobacterial pigments in desiccated biological soil crusts following addition of water. PLoS One 9, e112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achyuthan, K. E. , Harper, J. C. , Manginell, R. P. & Moorman, M. W. (2017). Volatile metabolites emission by in vivo microalgae—an overlooked opportunity? Metabolites 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addicott, J. F. , Aho, J. M. , Antolin, M. F. , Padilla, D. K. , Richardson, J. S. & Soluk, D. A. (1987). Ecological neighborhoods: scaling environmental patterns. Oikos 49, 340–346. [Google Scholar]
- Aiello, A. (1985). Sloth hair: unanswered questions. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas (ed. Montgomery G. G.), pp. 213–218. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Akçay, E. (2017). Population structure reduces benefits from partner choice in mutualistic symbiosis. Proceedings of the Royal Society B: Biological Sciences 284, 20162317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, B. (2015). Coevolution in mutualisms. In Mutualism (ed. Bronstein J. L.), pp. 107–130. Oxford University Press, Oxford. [Google Scholar]
- Anderson, R. P. & Handley, C. O. (2001). A new species of three‐toed sloth (Mammalia: Xenarthra) from Panama, with a review of the genus Bradypus . Proceedings, Biological Society of Washington 114, 1–33. [Google Scholar]
- Antwis, R. E. , Griffiths, S. M. , Harrison, X. A. , Aranega‐Bou, P. , Arce, A. , Bettridge, A. S. , Brailsford, F. L. , de Menezes, A. , Devaynes, A. , Forbes, K. M. , Fry, E. L. , Goodhead, I. , Haskell, E. , Heys, C. , James, C. , et al. (2017). Fifty important research questions in microbial ecology. FEMS Microbiology Ecology 93, fix044. [DOI] [PubMed] [Google Scholar]
- Archetti, M. , Scheuring, I. , Hoffman, M. , Frederickson, M. E. , Pierce, N. E. & Yu, D. W. (2011). Economic game theory for mutualism and cooperation. Ecology Letters 14, 1300–1312. [DOI] [PubMed] [Google Scholar]
- Arias, J. R. & Freitas, R. A. (1978). Sobre os vetores de leishmaniose cutânea na Amazônia Central do Brasil. 2: Incidência de flagelados em flebotomos selváticos. Acta Amazonica 8, 387–396. [Google Scholar]
- Arnold, A. E. & Lutzoni, F. (2007). Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88, 541–549. [DOI] [PubMed] [Google Scholar]
- Arnold, A. E. , Miadlikowska, J. , Higgins, K. L. , Sarvate, S. D. , Gugger, P. , Way, A. , Hoffstetter, V. , Kauff, F. & Lutzoni, F. (2009). A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification? Systematic Biology 58, 283–297. [DOI] [PubMed] [Google Scholar]
- Audrain, B. , Farag, M. A. , Ryu, C. M. & Ghigo, J. M. (2015). Role of bacterial volatile compounds in bacterial biology. FEMS Microbiology Reviews 39, 222–233. [DOI] [PubMed] [Google Scholar]
- Baldrian, P. (2017). Forest microbiome: diversity, complexity, and dynamics. FEMS Microbiology Reviews 41, 109–130. [DOI] [PubMed] [Google Scholar]
- Barko, P. C. , McMichael, M. A. , Swanson, K. S. & Williams, D. A. (2017). The gastrointestinal microbiome: a review. Journal of Veterinary Internal Medicine 32, 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, S. T. , Cropsey, G. W. G. , Caparaso, J. G. , Knight, R. & Fierer, N. (2011). Bacterial communities associated with the lichen symbiosis. Applied and Environmental Microbiology 77, 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe, W. (1926). The three‐toed sloth Bradypus cuculliger cuculliger Wagler. Zoologica 7, 1–67. [Google Scholar]
- Belisle, M. , Peay, K. G. & Fukami, T. (2012). Flowers as islands: spatial distribution of nectar‐inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird‐pollinated shrub. Microbial Ecology 63, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, T. , Ager, D. , Song, J. I. , Newman, J. A. , Thompson, I. P. , Lilley, A. K. & van der Gast, C. J. (2005). Larger islands house more bacterial taxa. Science 308, 1884. [DOI] [PubMed] [Google Scholar]
- Berasategui, A. , Shukla, S. , Salem, H. & Kaltenpoth, M. (2016). Potential applications of insect symbionts in biotechnology. Applied Microbiology and Biotechnology 100, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, C. C. & Rosselli, P. F. (2005). Cecropia (Flora Neotropica Monograph No. 94). New York Botanical Garden, New York. [Google Scholar]
- Bezerra, B. M. , Barnett, A. A. , Souto, A. & Jones, G. (2009). Predation by the tayra on the common marmoset and the pale‐throated three‐toed sloth. Journal of Ethology 27, 91–96. [Google Scholar]
- Bezerra, B. M. , Souto, A. , Halsey, L. G. & Schiel, N. (2008). Observation of brown‐throated three‐toed sloths: mating behaviour and the simultaneous nurturing of two young. Journal of Ethology 26, 175–178. [Google Scholar]
- Bittleston, L. S. , Wolock, C. J. , Yahya, B. E. , Chan, X. Y. , Chan, K. G. , Pierce, N. E. & Pringle, A. (2018). Convergence between the microcosms of Southeast Asian and North American pitcher plants. eLife 7, e36741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe, E. L. , Harr, K. E. , Cichra, M. F. , Phlips, E. J. , Bonde, R. K. & Lowe, M. (2006). A comparison of biofouling communities associated with free‐ranging and captive Florida manatees (Trichechus manatus latirostris). Marine Mammal Science 22, 997–1003. [Google Scholar]
- Bordenstein, S. R. & Theis, K. R. (2015). Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biology 13, e1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard, M. K. , Amorim, I. R. , Borges, P. A. V. , Cabral, J. S. , Fernández‐Palacios, J. M. , Field, R. , Heaney, L. R. , Kreft, H. , Matthews, T. J. , Olesen, J. M. , Price, J. , Rigal, F. , Steinbauer, M. J. , Triantis, K. A. , Valente, L. , et al. (2017). Oceanic Island biogeography through the lens of the general dynamic model: assessment and prospect. Biological Reviews 92, 830–853. [DOI] [PubMed] [Google Scholar]
- Bosch, T. C. G. , Guillemin, K. & McFall‐Ngai, M. (2019). Evolutionary “experiments” in symbiosis: the study of model animals provides insights into the mechanisms underlying the diversity of host‐microbe interactions. BioEssays 41, e1800256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton, P. J. (2012). Ecological patterns and processes in Sarracenia carnivorous pitcher plant fungi. Doctoral dissertation, Harvard University.
- Bradley, J. D. (1982). Two new species of moths (Lepidoptera, Pyralidae, Chrysauginae) associated with the three‐toed sloth (Bradypus spp.) in South America. Acta Amazonica 12, 649–656. [Google Scholar]
- Brandão, M. L. , Furtado, M. C. , Duarte de Albuquerque, D. , Cordeiro, J. L. P. , Lourenço, M. C. & Figueiredo, F. B. (2019). Management of wild sloths in an anthropized area at Atlantic forest. Oecologia Australis 23, 644–651. [Google Scholar]
- Britton, S. W. (1941). Form and function in the sloth. The Quarterly Review of Biology 16, 13–34. [Google Scholar]
- Britton, S. W. & Atkinson, W. E. (1938). Poikilothermism in the sloth. Journal of Mammalogy 19, 94–99. [Google Scholar]
- Brocks, J. J. , Jarrett, A. J. , Sirantoine, E. , Hallmann, C. , Hoshino, Y. & Liyanage, T. (2017). The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581. [DOI] [PubMed] [Google Scholar]
- Bronstein, J. L. (1994). Conditional outcomes in mutualistic interactions. Trends in Ecology & Evolution 9, 214–217. [DOI] [PubMed] [Google Scholar]
- Bronstein, J. L. (2015). Mutualism. Oxford University Press, New York. [Google Scholar]
- Carmona, C. P. , de Bello, F. , Mason, N. W. H. & Lepš, J. (2016). Traits without borders: integrating functional diversity across scales. Trends in Ecology & Evolution 31, 382–394. [DOI] [PubMed] [Google Scholar]
- Chiarello, A. G. (1998). Activity budgets and ranging patterns of the Atlantic forest maned sloth Bradypus torquatus (Xenarthra: Bradypodidae). Journal of Zoology 246, 1–10. [Google Scholar]
- Chiarello, A. G. (2008). Sloth ecology: an overview of field studies. In The Biology of the Xenarthra (eds Vizcaíno S. F. and Loughry W. J.), pp. 269–280. University Press of Florida, Gainesville. [Google Scholar]
- Chiarello, A. & Moraes‐Barros, N. (2014a). Bradypus torquatus. The IUCN Red List of Threatened Species 2014: e.T3036A47436575. 10.2305/IUCN.UK.2014-1.RLTS.T3036A47436575.en . [DOI]
- Chiarello, A. & Moraes‐Barros, N. (2014b). Bradypus tridactylus. The IUCN Red List of Threatened Species 2014: e.T3037A47436865. 10.2305/IUCN.UK.2014-1.RLTS.T3037A47436865.en . [DOI]
- Chiarello, A. & Plese, T. (2014). Choloepus didactylus. The IUCN Red List of Threatened Species 2014: e.T4777A47439542. 10.2305/IUCN.UK.2014-1.RLTS.T4777A47439542.en . [DOI]
- Christensen, H. A. , Arias, J. R. , de Vasquez, A. M. & de Freitas, R. A. (1982). Hosts of sandfly vectors of Leishmania braziliensis guyanensis in the Central Amazon of Brazil. The American Journal of Tropical Medicine and Hygiene 31, 239–242. [DOI] [PubMed] [Google Scholar]
- Davis, T. S. , Crippen, T. L. , Hofstetter, R. W. & Tomberlin, J. K. (2013). Microbial volatile emissions as insect semiochemicals. Journal of Chemical Ecology 39, 840–859. [DOI] [PubMed] [Google Scholar]
- Dawkins, R. (1982). The Extended Phenotype: The Long Reach of the Gene. Oxford University Press, New York. [Google Scholar]
- de Oliveira Filho, E. F. , Moreira‐Soto, A. , Fischer, C. , Rasche, A. , Sander, A. ‐L. , Avey‐Arroyo, J. , Arroyo‐Murillo, F. , Corrales‐Aguilar, E. & Drexler, J. F. (2020). Sloths host Anhanga virus‐related phleboviruses across large distances in time and space. Transboundary and Emerging Diseases 67, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stefani Munaó Diniz, L. & Oliveira, P. M. A. (1999). Clinical problems of sloths (Bradypus sp. and Choloepus sp.) in captivity. Journal of Zoo and Wildlife Medicine 30, 76–80. [PubMed] [Google Scholar]
- Delsuc, F. , Kuch, M. , Gibb, G. C. , Karpinski, E. , Hackenberger, D. , Szpak, P. , Martínez, J. G. , Mead, J. I. , McDonald, H. G. , MacPhee, R. D. E. , Billet, G. , Hautier, L. & Poinar, H. N. (2019). Ancient mitogenomes reveal the evolutionary history and biogeography of sloths. Current Biology 29, 2031–2042. [DOI] [PubMed] [Google Scholar]
- Delsuc, F. , Metcalf, J. L. , Wegener Parfrey, L. , Song, S. J. , González, A. & Knight, R. (2014). Convergence of gut microbiomes in myrmecophagous mammals. Molecular Ecology 23, 1301–1317. [DOI] [PubMed] [Google Scholar]
- Dias, B. B. , dos Santos, L. A. D. , Lara‐Ruiz, P. , Cassano, C. R. , Pinder, L. & Chiarello, A. G. (2009). First observation on mating and reproductive seasonality in maned sloths Bradypus torquatus (Pilosa: Bradypodidae). Journal of Ethology 27, 97–103. [Google Scholar]
- Dickschat, J. S. (2017). Fungal volatiles–a survey from edible mushrooms to moulds. Natural Product Reports 34, 310–328. [DOI] [PubMed] [Google Scholar]
- Dill‐McFarland, K. A. , Weimer, P. J. , Pauli, J. N. , Peery, M. Z. & Suen, G. (2016). Diet specialization selects for an unusual and simplified gut microbiota in two‐and three‐toed sloths. Environmental Microbiology 18, 1391–1402. [DOI] [PubMed] [Google Scholar]
- Du, Z. Y. , Zienkiewicz, K. , Vande Pol, N. , Ostrom, N. E. , Benning, C. & Bonito, G. M. (2019). Algal‐fungal symbiosis leads to photosynthetic mycelium. eLife 8, e47815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudgeon, S. , Kübler, J. E. , West, J. A. , Kamiya, M. & Krueger‐Hadfield, S. A. (2017). Asexuality and the cryptic species problem. Perspectives in Phycology 4, 47–59. [Google Scholar]
- Engl, T. & Kaltenpoth, M. (2018). Influence of microbial symbionts on insect pheromones. Natural Product Reports 35, 386–397. [DOI] [PubMed] [Google Scholar]
- Falconi, N. , Vieira, E. M. , Baumgarten, J. , Faria, D. & Giné, G. A. F. (2015). The home range and multi‐scale habitat selection of the threatened maned three‐toed sloth (Bradypus torquatus). Mammalian Biology 80, 431–439. [Google Scholar]
- Fain, A. (1964). Edentalges choloepi sp. n. acarien parasite cuticole du paresseux didactyle Choloepus didactylus (L.) (Psoroptidae: Sarcoptiformes). Zeitschrift für Parasitenkunde 25, 103–107. [DOI] [PubMed] [Google Scholar]
- Feldhamer, G. A. , Drickamer, L. C. , Vessey, S. H. , Merritt, J. F. & Krajewski, C. (2015). Mammalogy: Adaptation, Diversity, Ecology, pp. 772–774. Johns Hopkins University Press, 4th Edition, Baltimore. [Google Scholar]
- Foley, W. J. , Engelhardt, W. V. & Charles‐Dominique, P. (1995). The passage of digesta, particle size, and in vitro fermentation rate in the three‐toed sloth Bradypus tridactylus (Edentata: Bradypodidae). Journal of Zoology 236, 681–696. [Google Scholar]
- Fountain, E. D. , Pauli, J. N. , Mendoza, J. E. , Carlson, J. & Peery, M. Z. (2017). Cophylogenetics and biogeography reveal a coevolved relationship between sloths and their symbiont algae. Molecular Phylogenetics and Evolution 110, 73–80. [DOI] [PubMed] [Google Scholar]
- Friedl, T. (1995). Inferring taxonomic positions and testing genus level assignments in coccoid green lichen algae: a phylogenetic analysis of 18S ribosomal RNA sequences from Dictyochloropsis reticulata and from members of the genus Myrmecia (Chlorophyta, Trebouxiophyceae cl. nov.). Journal of Phycology 31, 632–639. [Google Scholar]
- Garcés‐Restrepo, M. F. , Pauli, J. N. & Peery, M. Z. (2019a). Natal dispersal of tree sloths in a human‐dominated landscape: implications for tropical biodiversity conservation. Journal of Applied Ecology 55, 2253–2262. [Google Scholar]
- Garcés‐Restrepo, M. F. , Peery, M. Z. & Pauli, J. N. (2019b). The demography of a resource specialist in the tropics: Cecropia trees and the fitness of three‐toed sloths. Proceedings of the Royal Society B: Biological Sciences 286, 20182206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareth Jones, E. B. , Pang, K.‐L. & Stanley, S. J. (2012). Fungi from marine algae. In Marine Fungi and Fungal‐Like Organisms (eds Gareth Jones E. B. and Pang K.‐L.), pp. 329–344. Walter De Guyter, Berlin. [Google Scholar]
- Gilbert, K. J. , Bittleston, L. S. , Tong, W. & Pierce, N. E. (2020). Tropical pitcher plants (Nepenthes) act as ecological filters by altering properties of their fluid microenvironments. Scientific Reports 10, 4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, D. P. , da Da Costa, C. P. & Duarte, D. P. F. (2001). Sloth biology: an update on their physiological ecology, behavior and role as vectors of arthropods and arboviruses. Brazilian Journal of Medical and Biological Research 34, 9–25. [DOI] [PubMed] [Google Scholar]
- Glassman, S. I. , Lubetkin, K. C. , Chung, J. A. & Bruns, T. D. (2017). The theory of Island biogeography applies to ectomycorrhizal fungi in subalpine tree “islands” at a fine scale. Ecosphere 8, e01677. [Google Scholar]
- Goedknegt, A. , Welsh, J. & Thieltges, D. W. (2012). Parasites as prey. eLS, Volume 5, pp. 1–8. John Wiley & Sons, Ltd., New Jersey. 10.1002/9780470015902.a0023604. [DOI] [Google Scholar]
- Goffart, M. (1971). Function and Form in the Sloth. Pergamon Press, Oxford. [Google Scholar]
- Goodwin, E. R. & Ayres, M. P. (2014). Behavioral thermoregulation in facultatively poikilothermic sloths (Bradypus Variegatus and Choloepus hoffmanni). Dartmouth Studies in Tropical Ecology 24, 99–104. [Google Scholar]
- Greene, H. W. (1989). Agonistic behavior by three‐toed sloths, Bradypus variegatus . Biotropica 21, 369–372. [Google Scholar]
- Greenwood, V . (2014) The Mystery of Sloth Poop: One More Reason to Love Science. http://science.time.com/2014/01/22/the-mystery-of-sloth-poop-one-more-reason-to-love-science/ .
- Grice, E. A. & Segre, J. A. (2011). The skin microbiome. Nature Reviews Microbiology 9, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube, M. & Berg, G. (2009). Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biology Reviews 23, 72–85. [Google Scholar]
- Guiry, M. D. & Guiry, G. M. (2019). AlgaeBase. National University of Ireland, Galway. http://www.algaebase.org [searched on 16.8.2019]. [Google Scholar]
- Hall, J. D. , Fučíková, K. , Lo, C. , Lewis, L. A. & Karol, K. G. (2010). An assessment of proposed DNA barcodes in freshwater green algae. Cryptogamie Algologie 31, 529–555. [Google Scholar]
- Hassani, M. A. , Durán, P. & Hacquard, S. (2018). Microbial interactions with the plant holobiont. Microbiome 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth, D. L. (1988). The variety of fungal‐algal symbioses, their evolutionary significance, and the nature of lichens. Botanical Journal of the Linnean Society 96, 3–20. [Google Scholar]
- Hawksworth, D. L. (2000). Freshwater and marine lichen‐forming fungi. Fungal Diversity 5, 1–7. [Google Scholar]
- Heaney, L. R. , Balete, D. S. & Rickart, E. A. (2013). Models of oceanic Island biogeography: changing perspectives on biodiversity dynamics in archipelagoes. Frontiers of Biogeography 5, 249–257. [Google Scholar]
- Henry, L. P. , Bruijning, M. , Forsberg, S. K. G. & Ayroles, J. F. (2019). Can the microbiome influence host evolutionary trajectories? bioRxiv 2019, 700237. 10.1101/700237. [DOI] [Google Scholar]
- Herrer, A. & Christensen, H. A. (1980). Leishmania braziliensis in the Panamanian two‐toed sloth, Choloepus hoffmanni . The American Journal of Tropical Medicine and Hygiene 29, 1196–1200. [DOI] [PubMed] [Google Scholar]
- Higginbotham, S. , Wong, W. R. , Linington, R. G. , Spadafora, C. , Iturrado, L. & Arnold, A. E. (2014). Sloth hair as a novel source of fungi with potent anti‐parasitic, anti‐cancer and anti‐bacterial bioactivity. PLoS One 9, e84549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, M. T. & Arnold, A. E. (2010). Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Applied and Environmental Microbiology 76, 4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom, E. F. Y. & Murray, A. W. (2014). Niche engineering demonstrates a latent capacity for fungal‐algal mutualism. Science 345, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks, K. B. & O'Malley, M. A. (2017). Dysbiosis and its discontents. mBio 8, e01492‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey, L. F. , Hein, A. M. , Strandburg‐Peshkin, A. & Jensen, F. H. (2018). Challenges and solutions for studying collective animal behaviour in the wild. Philosophical Transactions of the Royal Society B 373, 20170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izor, R. J. (1985). Sloths and other mammalian prey of the harpy eagle. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas (ed. Montgomery G. G.), pp. 343–346. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Jacoby, D. M. P. & Freeman, R. (2016). Emerging network‐based tools in movement ecology. Trends in Ecology & Evolution 31, 301–314. [DOI] [PubMed] [Google Scholar]
- Janzen, D. H. (1969). Allelopathy by myrmecophytes: the ant Azteca as an allelopathic agent of Cecropia . Ecology 50, 147–153. [Google Scholar]
- Janzen, D. H. (1980). When is it coevolution? Evolution 34, 611–612. [DOI] [PubMed] [Google Scholar]
- Jax, K. (2006). Ecological units: definitions and applications. The Quarterly Review of Biology 81, 237–258. [DOI] [PubMed] [Google Scholar]
- Jetz, W. , Carbone, C. , Fulford, J. & Brown, J. H. (2004). The scaling of animal space use. Science 306, 266–268. [DOI] [PubMed] [Google Scholar]
- Jones, E. I. , Afkhami, M. E. , Akçay, E. , Bronstein, J. L. , Bshary, R. , Frederickson, M. E. , Heath, K. D. , Hoeksema, J. D. , Ness, J. H. , Pankey, M. S. , Porter, S. S. , Sachs, J. L. , Scharnagl, K. & Friesen, M. L. (2015). Cheaters must prosper: reconciling theoretical and empirical perspectives on cheating in mutualism. Ecology Letters 18, 1270–1284. [DOI] [PubMed] [Google Scholar]
- Kaltenpoth, M. , Roeser‐Mueller, K. , Koehler, S. , Peterson, A. , Nechitaylo, T. Y. , Stubblefield, J. W. , Herzner, G. , Seger, J. & Strohm, E. (2014). Partner choice and fidelity stabilize coevolution in a Cretaceous‐age defensive symbiosis. Proceedings of the National Academy of Sciences of the United States of America 111, 6359–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten, U. , Friedl, T. , Schumann, R. , Hoyer, K. & Lembcke, S. (2005). Mycosporine‐like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). Journal of Phycology 41, 557–566. [Google Scholar]
- Kays, R. , Crofoot, M. C. , Jetz, W. & Wikelski, M. (2015). Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478. [DOI] [PubMed] [Google Scholar]
- Kessler, A. & Baldwin, I. T. (2001). Defensive function of herbivore‐induced plant volatile emissions in nature. Science 291, 2141–2144. [DOI] [PubMed] [Google Scholar]
- Kingdon, J. , Agwanda, B. , Kinnaird, M. , O'Brien, T. , Holland, C. , Gheysens, T. , Boulet‐Audet, M. & Vollrath, F. (2012). A poisonous surprise under the coat of the African crested rat. Proceedings of the Royal Society B: Biological Sciences 279, 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel, K.‐H. , Franken, P. & Hückelhoven, R. (2006). Endophyte or parasite–what decides? Current Opinion in Plant Biology 9, 358–363. [DOI] [PubMed] [Google Scholar]
- Kohlbach, D. , Graeve, M. , Lange, B. A. , David, C. , Peeken, I. & Flores, H. (2016). The importance of ice algae‐produced carbon in the central Arctic Ocean ecosystem: food web relationships revealed by lipid and stable isotope analyses. Limnology and Oceanography 61, 2027–2044. [Google Scholar]
- Krieg, H. (1939). Begegnungen mit Ameisenbären und Faultieren in freier Wildbahn. Ethology 2, 282–292. [Google Scholar]
- Ladau, J. & Eloe‐Fadrosh, E. A. (2019). Spatial, temporal, and phylogenetic scales of microbial ecology. Trends in Microbiology 27, 662–669. [DOI] [PubMed] [Google Scholar]
- Lambais, M. R. , Lucheta, A. R. & Crowley, D. E. (2014). Bacterial community assemblages associated with the phyllosphere, dermosphere, and rhizosphere of tree species of the Atlantic forest are host taxon dependent. Microbial Ecology 68, 567–574. [DOI] [PubMed] [Google Scholar]
- Lane, D. R. , Coffin, D. P. & Lauenroth, W. K. (2000). Changes in grassland canopy structure across a precipitation gradient. Journal of Vegetation Science 11, 359–368. [Google Scholar]
- Laroche, M. , Raoult, D. & Parola, P. (2019). Insects and the transmission of bacterial agents. In Microbial Transmission (eds Baquero F., Bouza E., Gutiérrez‐Fuentes J. A. and Coque T. M.), pp. 195–202. ASM Press, Washington, DC. [Google Scholar]
- Larsen, T . (2015). Uroxys gorgon (Scarabaeinae). http://scarabaeinae.myspecies.info/taxonomy/term/18868 .
- Leach, J. E. , Triplett, L. R. , Argueso, C. T. & Trivedi, P. (2017). Communication in the phytobiome. Cell 169, 587–596. [DOI] [PubMed] [Google Scholar]
- Leff, J. W. , Del Tredici, P. , Friedman, W. E. & Fierer, N. (2015). Spatial structuring of bacterial communities within individual Ginkgo biloba trees. Environmental Microbiology 17, 2352–2361. [DOI] [PubMed] [Google Scholar]
- Leibold, M. A. & Chase, J. M. (2019). Metacommunity Ecology. Princeton University Press, Princeton. [Google Scholar]
- Leibold, M. A. , Holyoak, M. , Mouquet, N. , Amarasekare, P. , Chase, J. M. , Hoopes, M. F. , Holt, R. D. , Shurin, J. B. , Law, R. , Tilman, D. , Loreau, M. & Gonzalez, A. (2004). The metacommunity concept: a framework for multi‐scale community ecology. Ecology Letters 7, 601–613. [Google Scholar]
- Leliaert, F. , Verbruggen, H. , Vanormelingen, P. , Steen, F. , López‐Bautista, J. M. , Zuccarello, G. C. & De De Clerck, O. (2014). DNA‐based species delimitation in algae. European Journal of Phycology 49, 179–196. [Google Scholar]
- Lemfack, M. C. , Gohlke, B.‐O. , Toguem, S. M. T. , Preissner, S. , Piechulla, B. & Preissner, R. (2017). mVOC 2.0: a database of microbial volatiles. Nucleic Acids Research 46, D1261–D1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox, R. J. , Aarestrup, K. , Cooke, S. J. , Cowley, P. D. , Deng, Z. D. , Fisk, A. T. , Harcourt, R. G. , Heupel, M. , Hinch, S. G. , Holland, K. N. , Hussey, N. E. , Iverson, S. J. , Kessel, S. T. , Kocik, J. F. , Lucas, M. C. , et al. (2017). Envisioning the future of aquatic animal tracking: technology, science, and application. BioScience 67, 884–896. [Google Scholar]
- Leung, T. L. F. & Poulin, R. (2008). Parasitism, commensalism, and mutualism: exploring the many shades of symbioses. Vie et Milieu 58, 107–115. [Google Scholar]
- Levy, M. , Kolodziejczyk, A. A. , Thaiss, C. A. & Elinav, E. (2017). Dysbiosis and the immune system. Nature Reviews Immunology 17, 219–232. [DOI] [PubMed] [Google Scholar]
- Lewin, R. A. & Robinson, P. T. (1979). The greening of polar bears in zoos. Nature 278, 445–447. [DOI] [PubMed] [Google Scholar]
- Lewis, T. (2014) Why sloths leave the trees to defecate. https://www.scientificamerican.com/article/why-sloths-leave-the-trees-to-defecate/.
- Liu, H. , Macdonald, C. A. , Cook, J. , Anderson, I. C. & Singh, B. K. (2019). An ecological loop: host microbiomes across multitrophic interactions. Trends in Ecology & Evolution 34, 1118–1130. [DOI] [PubMed] [Google Scholar]
- Lücking, R. , Mata‐Lorenzen, J. & Dauphin, L. G. (2010). Epizoic liverworts, lichens and fungi growing on Costa Rican Shield Mantis (Mantodea: Choeradodis). Studies on Neotropical Fauna and Environment 45, 175–186. [Google Scholar]
- Lundberg, J. & Moberg, F. (2003). Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6, 87–98. [Google Scholar]
- MacArthur, R. H. & Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton University Press, Princeton. [Google Scholar]
- Machado, G. & Moreira Vital, D. (2001). On the occurrence of epizoic cyanobacteria and liverworts on a neotropical harvestman (Arachinida: Opiliones). Biotropica 33, 535–538. [Google Scholar]
- Marting, P. R. , Kallman, N. M. , Wcislo, W. T. & Pratt, S. C. (2018). Ant‐plant sociometry in the Azteca‐Cecropia mutualism. Scientific Reports 8, 17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall‐Ngai, M. J. (2014). The importance of microbes in animal development: lessons from the squid‐vibrio symbiosis. Annual Review of Microbiology 68, 177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall‐Ngai, M. J. (2015). Giving microbes their due—animal life in a microbially dominant world. Journal of Experimental Biology 218, 1968–1973. [DOI] [PubMed] [Google Scholar]
- McKenna, D. D. , Farrell, B. D. , Caterino, M. S. , Farnum, C. W. , Hawks, D. C. , Maddison, D. R. , Seago, A. E. , Short, A. E. Z. , Newton, A. F. & Thayer, M. K. (2015). Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Systematic Entomology 40, 35–60. [Google Scholar]
- McKenney, E. A. , Koelle, K. , Dunn, R. R. & Yoder, A. D. (2018). The ecosystem services of animal microbiomes. Molecular Ecology 27, 2164–2172. [DOI] [PubMed] [Google Scholar]
- McNab, B. K. (1978). Energetics of arboreal folivores: physiological problems and ecological consequences of feeding on an ubiquitous food supply. In The Ecology of Arboreal Folivores (ed. Montgomery G. G.), pp. 153–162. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- McNab, B. K. (1986). The influence of food habits on the energetics of eutherian mammals. Ecological Monographs 56, 1–19. [Google Scholar]
- Medlin, S. , Deardorff, E. R. , Hanley, C. S. , Vergneau‐Grosset, C. , Siudak‐Campfield, A. , Dallwig, R. , da Rosa, A. T. , Tesh, R. B. , Martin, M. P. , Weaver, S. C. , Vaughan, C. , Ramirez, O. , Sladky, K. K. & Paul‐Murphy, J. (2016). Serosurvey of selected arboviral pathogens in free‐ranging, two‐toed sloths (Choloepus hoffmanni) and three‐toed sloths (Bradypus variegatus) in Costa Rica, 2005‐07. Journal of Wildlife Diseases 52, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, A . (2013) Curious Fact of the Week: The Remarkable Reason Algae Grows on Sloths. https://www.atlasobscura.com/articles/curious-fact-of-the-week-algae-on-sloths .
- Mendel, F. C. (1981). Use of hands and feet of two‐toed sloths (Choloepus hoffmanni) during climbing and terrestrial locomotion. Journal of Mammalogy 62, 413–421. [Google Scholar]
- Mendel, F. C. (1985). Use of hands and feet of three‐toed sloths (Bradypus variegatus) during climbing and terrestrial locomotion. Journal of Mammalogy 66, 359–366. [Google Scholar]
- Mendoza, J. E. , Peery, M. Z. , Gutiérrez, G. A. , Herrera, G. & Pauli, J. N. (2015). Resource use by the two‐toed sloth (Choloepus hoffmanni) and the three‐toed sloth (Bradypus variegatus) differs in a shade‐grown agro‐ecosystem. Journal of Tropical Ecology 31, 49–55. [Google Scholar]
- Meng, A. , Marchet, C. , Corre, E. , Peterlongo, P. , Alberti, A. , da Silva, C. , Wincker, P. , Pelletier, E. , Probert, I. , Decelle, J. , Le Crom, S. , Not, F. & Bittner, L. (2018). A de novo approach to disentangle partner identity and function in holobiont systems. Microbiome 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. T. , Svanbäck, R. & Bohannan, B. J. M. (2018a). Microbiomes as metacommunities: understanding host‐associated microbes through metacommunity ecology. Trends in Ecology & Evolution 33, 926–935. [DOI] [PubMed] [Google Scholar]
- Miller, R. A. (1935). Functional adaptations in the forelimb of the sloths. Journal of Mammalogy 16, 38–51. [Google Scholar]
- Miller, T. E. , Bradshaw, W. E. & Holzapfel, C. M. (2018b). Pitcher‐plant communities as model systems for addressing fundamental questions in ecology and evolution. In Carnivorous Plants: Physiology, Ecology, and Evolution (eds Ellison A. M. and Adamec L.), pp. 333–348. Oxford University Press, Oxford. [Google Scholar]
- Montgomery, G. G. & Sunquist, M. E. (1975). Impact of sloths on Neotropical forest energy flow and nutrient cycling. In Tropical Ecological Systems (eds Golley F. B. and Medina E.), pp. 69–98. Springer, Berlin. [Google Scholar]
- Montgomery, G. G. & Sunquist, M. E. (1978). Habitat selection and use by two‐toed and three‐toed sloths. In The Ecology of Arboreal Folivores (ed. G. G. Montgomery), pp. 329–359. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Moraes‐Barros, N. , Chiarello, A. & Plese, T. (2014). Bradypus variegatus. The IUCN Red List of Threatened Species 2014: e.T3038A47437046. 10.2305/IUCN.UK.2014-1.RLTS.T3038A47437046.en . [DOI]
- Muñoz‐García, C. I. , Sánchez‐Montes, S. , Villanueva‐García, C. , Romero‐Callejas, E. , Díaz‐López, H. M. , Gordillo‐Chávez, E. J. , Martínez‐Carrasco, C. , Berriatua, E. & Rendón‐Franco, E. (2019). The role of sloths and anteaters as Leishmania spp. reservoirs: a review and a newly described natural infection of Leishmania mexicana in the northern anteater. Parasitology Research 118, 1095–1101. [DOI] [PubMed] [Google Scholar]
- Neam, K. D. (2015). The odd couple: interactions between a sloth and a brown jay. Frontiers in Ecology and the Environment 13, 170–171. [Google Scholar]
- Neam, K. D. & Lacher, T. E. (2015). Spatial distribution, resource use, and behavior of brown‐throated sloths (Bradypus variegatus) in a multi‐use landscape. Edentata 16, 46–56. [Google Scholar]
- Neam, K. D. & Lacher Jr, T. E. (2018). Multi‐scale effects of habitat structure and landscape context on a vertebrate with limited dispersal ability (the brown‐throated sloth, Bradypus variegatus). Biotropica 50, 684–693. [Google Scholar]
- Neethirajan, S. (2017). Recent advances in wearable sensors for animal health management. Sensing and Bio‐Sensing Research 12, 15–29. [Google Scholar]
- Nichols, E. , Spector, S. , Louzada, J. , Larsen, T. , Amezquita, S. , Favila, M. E. & The Scarabaeinae Research Network. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation 141, 1461–1474. [Google Scholar]
- Nie, Y. , Speakman, J. R. , Wu, Q. , Zhang, C. , Hu, Y. , Xia, M. , Yan, L. , Hambly, C. , Wang, L. , Wei, W. , Zhang, J. & Wei, F. (2015). Exceptionally low daily energy expenditure in the bamboo‐eating giant panda. Science 349, 171–174. [DOI] [PubMed] [Google Scholar]
- Nyakatura, J. A. (2012). The convergent evolution of suspensory posture and locomotion in tree sloths. Journal of Mammalian Evolution 19, 225–234. [Google Scholar]
- Nyakatura, J. A. & Fischer, M. S. (2011). Functional morphology of the muscular sling at the pectoral girdle in tree sloths: convergent morphological solutions to new functional demands? Journal of Anatomy 219, 360–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, R. A. , Glenn, Z. D. , Cliffe, R. N. & Butcher, M. T. (2018). Architectural properties of sloth forelimb muscles (Pilosa: Bradypodidae). Journal of Mammalian Evolution 25, 573–588. [Google Scholar]
- Östlund‐Nilsson, S. , Becker, J. H. & Nilsson, G. E. (2005). Shrimps remove ectoparasites from fishes in temperate waters. Biological Letters 1, 454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida‐Martinez, L. P. & Hertweck, C. (2005). Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437, 884–888. [DOI] [PubMed] [Google Scholar]
- Pauli, J. N. , Mendoza, J. E. , Steffan, S. A. , Carey, C. C. , Weimer, P. J. & Peery, M. Z. (2014). A syndrome of mutualism reinforces the lifestyle of a sloth. Proceedings of the Royal Society B: Biological Sciences 281, 20133006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli, J. N. & Peery, M. Z. (2012). Unexpected strong polygyny in the brown‐throated three‐toed sloth. PLoS One 7, e51389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli, J. N. , Peery, M. Z. , Fountain, E. D. & Karasov, W. H. (2016). Arboreal folivores limit their energetic output, all the way to slothfulness. The American Naturalist 188, 196–204. [DOI] [PubMed] [Google Scholar]
- Peay, K. G. , Bruns, T. D. , Kennedy, P. G. , Bergemann, S. E. & Garbelotto, M. (2007). A strong species‐area relationship for eukaryotic soil microbes: Island size matters for ectomycorrhizal fungi. Ecology Letters 10, 470–480. [DOI] [PubMed] [Google Scholar]
- Peery, M. Z. & Pauli, J. N. (2012). The mating system of a ‘lazy’ mammal, Hoffmann's two‐toed sloth. Animal Behaviour 84, 555–562. [Google Scholar]
- Pichersky, E. & Gershenzon, J. (2002). The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology 5, 237–243. [DOI] [PubMed] [Google Scholar]
- Pietrasiak, N. (2014). Field Guide to Classify Biological Soil Crusts for Ecological Site Evaluation. USDA Natural Resources Conservation Service Technical Reference, Washington, D.C. https://www.nrcs.usda.gov/wps/PA_NRCSConsumption/download?cid=stelprdb1263524&ext=pdf. [Google Scholar]
- Plese, T. & Chiarello, A. (2014). Choloepus hoffmanni. The IUCN Red List of Threatened Species 2014: e.T4778A47439751. 10.2305/IUCN.UK.2014-1.RLTS.T4778A47439751.en . [DOI]
- Polis, G. A. & Hurd, S. D. (1995). Extraordinarily high spider densities on islands: flow of energy from the marine to terrestrial food webs and the absence of predation. Proceedings of the National Academy of Sciences of the United States of America 92, 4382–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool, M. , Boateng, R. , Ako‐Adounvo, A.‐M. , Allen‐McFarlane, R. , Elizondo, D. , Paturault, H. , Alhawas, H. & Middendorf, G. (2016). Sloths in the city: unexpectedly high density of pale‐throated three‐toed sloths (Bradypus tridactylus) found in an urban forest patch in Paramaribo, Suriname. Edentata 17, 25–33. [Google Scholar]
- Presslee, S. , Slater, G. J. , Pujos, F. , Forasiepi, A. M. , Fischer, R. , Molloy, K. , Mackie, M. , Olsen, J. V. , Kramarz, A. , Taglioretti, M. , Scaglia, F. , Lezcano, M. , Lanata, J. L. , Southon, J. , Feranec, R. , Bloch, J. , et al. (2019). Palaeoproteomics resolves sloth relationships. Nature Ecology & Evolution 3, 1121–1130. [DOI] [PubMed] [Google Scholar]
- Printz, H. (1964). Die Chaetophoralen der Binnengewässer. Hydrobiologia 24, 1–376. [Google Scholar]
- Proctor, D. M. & Relman, D. A. (2017). The landscape ecology and microbiota of the human nose, mouth, and throat. Cell Host & Microbe 21, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser, J. I. , Bohannan, B. J. M. , Curtis, T. P. , Ellis, R. J. , Firestone, M. K. , Freckleton, R. P. , Green, J. L. , Green, L. E. , Killham, K. , Lennon, J. J. , Osborn, A. M. , Solan, M. , van der Gast, C. J. & Young, J. P. W. (2007). The role of ecological theory in microbial ecology. Nature Reviews Microbiology 5, 384–392. [DOI] [PubMed] [Google Scholar]
- Proud, D. N. , Wade, R. R. , Rock, P. , Townsend Jr, V. R. & Chavez, D. J. (2012). Epizoic cyanobacteria associated with a Neotropical harvestman (Opiliones: Sclerosomatidae) from Costa Rica. The Journal of Arachnology 40, 259–261. [Google Scholar]
- Ramirez, O. , Vaughan, C. , Herrera, G. & Guries, R. (2011). Temporal and spatial resource use by female three‐toed sloths and their young in an agricultural landscape in Costa Rica. Revista de Biologia Tropical 59, 1743–1755. [PubMed] [Google Scholar]
- Rastogi, G. , Coaker, G. L. & Leveau, J. H. (2013). New insights into the structure and function of phyllosphere microbiota through high‐throughput molecular approaches. FEMS Microbiology Letters 348, 1–10. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, B. C. (1980). New species of coprini (Coleoptera: Scarabaeidae: Scarabaeinae) taken from the pelage of three toed sloths (Bradypus tridactylus L.) (Edentata: Bradypodidae) in central Amazonia with a brief commentary on scarab‐sloth relationships. The Coleopterists Bulletin 34, 337–350. [Google Scholar]
- Richard‐Hansen, C. & Taube, E. (1997). Note on the reproductive behavior of the three‐toed sloth, Bradypus tridactylus, in French Guiana. Mammalia 61, 259–263. https://www.researchgate.net/profile/Erica-Taube/publication/231447659_Note_on_the_reproductive_behavior_of_the_three-toed_sloth_Bradypus_tridactylus_in_French_Guiana/links/0f317531045aa10b99000000/Note-on-the-reproductive-behavior-of-the-three-toed-sloth-Bradypus-tridactylus-in-French-Guiana.pdf. [Google Scholar]
- Ripperger, S. P. , Carter, G. G. , Page, R. A. , Duda, N. , Koelpin, A. , Weigel, R. , Hartmann, M. , Nowak, T. , Thielecke, J. , Schadhauser, M. , Robert, J. , Herbst, S. , Meyer‐Wegener, K. , Wägemann, P. , Schröder‐Preikschat, W. , et al. (2020). Thinking small: next‐generation sensor networks close the size gap in vertebrate biologging. PLoS Biology 18, e3000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, E. & Zilber‐Rosenberg, I. (2018). The hologenome concept of evolution after 10 years. Microbiome 6, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughgarden, J. , Gilbert, S. F. , Rosenberg, E. , Zilber‐Rosenberg, I. & Lloyd, E. A. (2018). Holobionts as units of selection and a model of their population dynamics and evolution. Biological Theory 13, 44–65. [Google Scholar]
- Rowe, M. , Veerus, L. , Trosvik, P. , Buckling, A. & Pizzari, T. (2020). The reproductive microbiome: an emerging driver of sexual selection, sexual conflict, mating systems, and reproductive isolation. Trends in Ecology & Evolution 35, 220–234. [DOI] [PubMed] [Google Scholar]
- Ruiz‐García, M. , Chacón, D. , Plese, T. & Shostell, J. M. (2020). Molecular Phylogenetics of Bradypus (Three‐Toed Sloth, Pilosa: Bradypodidae, Mammalia) and Phylogeography of Bradypus variegatus (Brown‐Throated Three‐Toed Sloth) with Mitochondrial Gene Sequences. Journal of Mammalian Evolution 27, 461–482. [Google Scholar]
- Santos, P. M. , Chiarello, A. G. , Ribeiro, M. C. , Ribeiro, J. W. & Paglia, A. P. (2016). Local and landscape influences on the habitat occupancy of the endangered maned sloth Bradypus torquatus within fragmented landscapes. Mammalian Biology 81, 447–454. [Google Scholar]
- Schlägel, U. E. , Grimm, V. , Blaum, N. , Colangeli, P. , Dammhahn, M. , Eccard, J. A. , Hausmann, S. L. , Herde, A. , Hofer, H. , Joshi, J. , Kramer‐Schadt, S. , Litwin, M. , Lozada‐Gobilard, S. D. , Müller, M. E. H. , Müller, T. , et al. (2020). Movement‐mediated community assembly and coexistence. Biological Reviews 95, 1073–1096. [DOI] [PubMed] [Google Scholar]
- Shubert, L. E. (2003). Nonmotile coccoid and colonial green algae. In Freshwater Algae of North America (eds. Wehr J., Sheath R. and Kociolek J. P..), pp. 253–309. Academic Press, San Diego, CA. [Google Scholar]
- Schupp, E. W. (1986). Azteca protection of Cecropia: ant occupation benefits juvenile trees. Oecologia 70, 379–385. [DOI] [PubMed] [Google Scholar]
- Segovia, B. T. , Pereira, D. G. , Bini, L. M. , de Meira, B. R. , Nishida, V. S. , Lansac‐Tôha, F. A. & Velho, L. F. M. (2015). The role of microorganisms in a planktonic food web of a floodplain lake. Microbial Ecology 69, 225–233. [DOI] [PubMed] [Google Scholar]
- Seibold, S. , Cadotte, M. W. , Maclvor, J. S. , Thorn, S. & Müller, J. (2018). The necessity of multitrophic approaches in community ecology. Trends in Ecology & Evolution 33, 754–764. [DOI] [PubMed] [Google Scholar]
- Seniczak, A. , Ligocka, A. , Seniczak, S. & Paluszak, Z. (2016). Effects of green algae and napa cabbage on life‐history parameters and gut microflora of Archegozetes longisetosus (Acari: Oribatida) under laboratory conditions. Biological Letters 53, 67–78. [DOI] [PubMed] [Google Scholar]
- Seymour, C. , Peralta, P. H. & Montgomery, G. G. (1983). Viruses isolated from Panamanian sloths. The American Journal of Tropical Medicine and Hygiene 32, 1435–1444. [DOI] [PubMed] [Google Scholar]
- Shade, A. , Dunn, R. R. , Blowes, S. A. , Keil, P. , Bohannan, B. J. M. , Herrmann, M. , Küsel, K. , Lennon, J. T. , Sanders, N. J. , Storch, D. & Chase, J. (2018). Macroecology to unite all life, large and small. Trends in Ecology & Evolution 33, 731–744. [DOI] [PubMed] [Google Scholar]
- Shaw, J. J. (1985). The hemoflagellates of sloths, vermilinguas (anteaters), and armadillos. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas (ed. Montgomery G. G.), pp. 279–292. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Shipley, J. R. , Kapoor, J. , Dreelin, R. A. & Winkler, D. W. (2018). An open‐source sensor‐logger for recording vertical movement in free‐living organisms. Methods in Ecology and Evolution 9, 465–471. [Google Scholar]
- Silva, S. M. , Clozato, C. L. , Moreas‐Barros, N. & Morgante, J. S. (2013). Towards a standard framework to describe behaviours in the common‐sloth (Bradypus variegatus Schinz, 1825): novel interactions data observed in distinct fragments of the Atlantic forest, Brazil. Brazilian Journal of Biology 73, 527–531. [DOI] [PubMed] [Google Scholar]
- Simon, J.‐C. , Marchesi, J. R. , Mougel, C. & Selosse, M.‐A. (2019). Host‐microbiota interactions: from holobiont theory to analysis. Microbiome 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, G. J. , Cui, P. , Forasiepi, A. M. , Lenz, D. , Tsangaras, K. , Voirin, B. , de Moraes‐Barros, N. , MacPhee, R. D. E. & Greenwood, A. D. (2016). Evolutionary relationships among extinct and extant sloths: the evidence of mitogenomes and retroviruses. Genome Biology and Evolution 8, 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, C. A. & Carneiro, R. S. (2002). Social behavior between mothers × young of sloths Bradypus variegatus Schinz, 1825 (Xenarthra: Bradypodidae). Brazilian Journal of Biology 62, 249–252. [DOI] [PubMed] [Google Scholar]
- Solar, R . (2014) Azteca ant carton nest. https://www.flickr.com/photos/bob_solar/12187831725/in/photolist-9EEHqj-jyZPKK-bVzU8N-bVzU73-ouoTBW-xrN7gd .
- Stagaman, K. , Burns, A. R. , Guillemin, K. & Bohannan, B. J. M. (2017). The role of adaptive immunity as an ecological filter on the gut microbiota in zebrafish. ISME Journal 11, 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz, J. F. (2000). Structure of microbial mats and biofilms. In Microbial Sediments (eds Riding R. E. and Awramik S. M.), pp. 1–8. Springer Verlag, Berlin. [Google Scholar]
- Sunquist, M. E. & Montgomery, G. G. (1973). Activity patterns and rates of movement of two‐toed and three‐toed sloths (Choloepus hoffmanni and Bradypus infuscatus). Journal of Mammalogy 54, 946–954. [PubMed] [Google Scholar]
- Suutari, M. , Majaneva, M. , Fewer, D. P. , Voirin, B. , Aiello, A. , Friedl, T. , Chiarello, A. G. & Blomster, J. (2010). Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae). BMC Evolutionary Biology 10, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube, E. , Vié, J.‐C. , Fournier, P. , Genty, C. & Duplantier, J.‐M. (1999). Distribution of Two Sympatric Species of Sloths (Choloepus didactylus and Bradypus tridactylus) along the Sinnamary River, French Guiana. Biotropica 31, 686–691. [Google Scholar]
- Taube, E. , Keravec, J. , Vié, J.‐C. & Duplantier, J.‐M. (2001). Reproductive biology and postnatal development in sloths, Bradypus and Choloepus: review with original data from the field (French Guiana) and from captivity. Mammal Review 31, 173–188. [Google Scholar]
- Taylor, P. D. , Crewe, T. L. , Mackenzie, S. A. , Lepage, D. , Aubry, Y. , Crysler, Z. , Finney, G. , Francis, C. M. , Guglielmo, C. G. , Hamilton, D. J. , Holberton, R. L. , Loring, P. H. , Mitchell, G. W. , Norris, D. R. , Paquet, J. , et al. (2017). The Motus Wildlife Tracking System: a collaborative research network. Avian Conservation and Ecology 12, 8. [Google Scholar]
- Thompson, R. H. (1972). Algae from the hair of the sloth Bradypus . Journal of Phycology 8(s2), 8. [Google Scholar]
- Tirler, H. (1966). A Sloth in the Family. The Harvill Press, London. [Google Scholar]
- Urbani, B. & Bosque, C. (2007). Feeding ecology and postural behaviour of the three‐toed sloth (Bradypus variegatus flaccidus) in northern Venezuela. Mammalian Biology 72, 321–329. [Google Scholar]
- U'Ren, J. M. , Lutzoni, F. , Miadlikowska, J. , Laetsch, A. D. & Arnold, A. E. (2012). Host and geographic structure of endophytic and endolichenic fungi at a continental scale. American Journal of Botany 99, 898–914. [DOI] [PubMed] [Google Scholar]
- Vacher, C. , Hampe, A. , Porté, A. J. , Sauer, U. , Compant, S. & Morris, C. E. (2016). The phyllosphere: microbial jungle at the plant‐climate interface. Annual Review of Ecology, Evolution, and Systematics 47, 1–24. [Google Scholar]
- Vaughan, C. , Ramírez, O. , Herrera, G. & Guries, R. (2007). Spatial ecology and conservation of two sloth species in a cacao landscape in Limón, Costa Rica. Biodiversity and Conservation 16, 2293–2310. [Google Scholar]
- Veselova, M. A. , Plyuta, V. A. & Khmel, I. A. (2019). Volatile compounds of bacterial origin: structure, biosynthesis, and biological activity. Microbiology 88, 261–274. [Google Scholar]
- Voirin, J. B. , Kays, R. , Lowman, M. D. & Wikelski, M. (2009). Evidence for three‐toed sloth (Bradypus variegatus) predation by spectacled owl (Pulsatrix perspicillata). Edentata 10, 15–20. [Google Scholar]
- Voirin, B. , Kays, R. , Wikelski, M. & Lowman, M. (2013). Why do sloths poop on the ground? In Treetops at Risk (eds Lowman M., Devy S. and Ganesh T.), pp. 195–199. Springer, New York. [Google Scholar]
- Voirin, B. , Smith, D. , Chiarello, A. & Moraes‐Barros, N. (2014). Bradypus pygmaeus. The IUCN Red List of Threatened Species 2014: e.T61925A47444229. 10.2305/IUCN.UK.2014-1.RLTS.T61925A47444229.en . [DOI]
- Vostinar, A. E. & Ofria, C. (2019). Spatial structure can decrease symbiotic cooperation. Artificial Life 24, 229–249. [DOI] [PubMed] [Google Scholar]
- Waage, J. K. & Best, R. C. (1985). Arthropod associates of sloths. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas (ed. Montgomery G. G.), pp. 297–311. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Waage, J. K. & Montgomery, G. G. (1976). Cryptoses choloepi: a coprophagous moth that lives on a sloth. Science 193, 157–158. [DOI] [PubMed] [Google Scholar]
- Wang, P. , Liu, Y. , Yin, Y. , Jin, H. , Wang, S. , Xu, F. , Zhao, S. & Geng, X. (2011). Diversity of microorganisms isolated from the soil sample surround Chroogomphus rutilus in the Beijing region. International Journal of Biological Sciences 7, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. & Loreau, M. (2014). Ecosystem stability in space: α, β, and γ variability. Ecology Letters 17, 891–901. [DOI] [PubMed] [Google Scholar]
- Weber‐van Bosse, A. (1887). Étude sur les algues parasites des paresseux. De Erven Loosjes, Haarlem. [Google Scholar]
- Wetzel, R. M. (1985). The identification and distribution of recent Xenarthra (= Edentata). In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas (ed. Montgomery G. G.), pp. 5–21. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology 3, 385–397. [Google Scholar]
- Williams, H. J. , Taylor, L. A. , Benhamou, S. , Bijleveld, A. I. , Clay, T. A. , de Grissac, S. , Demšar, U. , English, H. M. , Franconi, N. , Gómez‐Laich, A. , Griffiths, R. C. , Kay, W. P. , Morales, J. M. , Potts, J. R. , Rogerson, K. F. , et al. (2019). Optimizing the use of biologgers for movement ecology research. Journal of Animal Ecology 89, 186–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmotte, A. , Laughinghouse, H. D. IV , Capelli, C. , Rippka, R. & Salmaso, N. (2017). Taxonomic identification of cyanobacteria by a polyphasic approach. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria (eds Kurmayer R., Sivonen K., Wilmotte A. and Salmaso N.), pp. 79–119. John Wiley and Sons, Hoboken. [Google Scholar]
- Wilson, E. O. (2010). Island biogeography in the 1960s. In The Theory of Island Biogeography Revisited (eds Losos J. B. and Ricklefs R. E.), pp. 1–12. Princeton University Press, Princeton. [Google Scholar]
- Wolda, H. (1985). Seasonal distribution of sloth moths Cryptoses choloepi Dyar (Pyralidae; Chrysauginae) in light traps in Panama. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas (ed. Montgomery G. G.), pp. 313–318. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Wolda, H. & Estribi, M. A. (1985). Seasonal distribution of the large sloth beetle Uroxys gorgon Arrow (Scarabaeidae; Scarabaeinae) in light traps in Panama. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas (ed. Montgomery G. G.), pp. 319–322. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Woollaston, V . (2014) Sloths move so slowly that algae grows on their fur – and it can be used as camouflage and food. https://www.dailymail.co.uk/sciencetech/article-2532635/Sloths-slowly-ALGAE-grows-fur-used-camouflage-food.html .
- Wujek, D. E. & Cocuzza, J. M. (1986). Morphology of hair of two‐ and three‐toed sloths (Edentata: Bradypodidae). Revista de Biologia Tropical 34, 243–246. [PubMed] [Google Scholar]
- Wujek, D. E. & Lincoln, T. A. (1988). Ultrastructure and taxonomy of Oscillatoria pilicola, a new species of bluegreen alga from sloth hair. Brenesia 29, 1–6. [Google Scholar]
- Wujek, D. & Timpano, P. (1986). Rufusia (Porphyridiales, Phragmonemataceae), a new red alga from sloth hair. Brenesia 25–26, 163–168. [Google Scholar]
- Xavier, G. A. A. , da Silva, L. B. G. , da Silva, D. R. , de Moraes Peixoto, R. , Lino, G. C. & Mota, R. A. (2008). Dermatophytosis caused by Microsporum canis and Microsporum gypseum in free‐living Bradypus variegatus (Schinz, 1825) in the state of Pernambuco, Brazil. Brazilian Journal of Microbiology 39, 508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, V. A. , Moore, M. K. & Townsend, V. R. Jr. (2018). Epizoic cyanobacteria associated with harvestmen (Arachnida: Opiliones) from Tobago, West Indies. Living World, Journal of the Trinidad and Tobago Field Naturalists’ Club 2018, 94–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. A male Bradypus variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released following a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica.
Video S2. A female B. variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released following a health check‐up (at The Sloth Institute in Manuel Antonio, Costa Rica) on her and her baby, seen clinging to her abdomen. Video by Carole Moncoquet (used with author's permission).
Video S3. A female B. variegatus (brown‐throated three‐fingered sloth) climbs up a tree after being released at night following a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica. Note the green coloration on her shoulders as well as the sloth moths swarming her back.
Video S4. A female Choloepus hoffmanni (Hoffmann's two‐fingered sloth) climbs relatively quickly up a vine (compared to a brown‐throated three‐fingered sloth) after a health check‐up at The Sloth Institute in Manuel Antonio, Costa Rica.


