Abstract
Autoimmune conditions affect 23 million Americans or 7% of the US population. There are more than 100 autoimmune disorders, affecting every major organ system in humans. This chapter aims to further explain Treg dysfunction autoimmune disorders, including monogenic primary immune deficiency such as immune dysregulation polyendocrinopathy, enteropathy, X-linked inheritance (IPEX) syndrome, and polygenic autoimmune diseases with Treg dysfunction such as multiple sclerosis (MS), inflammatory bowel disease (IBD), and food allergy. These conditions are associated with an abnormal small intestinal and colonic microbiome. Some disorders clearly improve with therapies aimed at microbial modification, including probiotics and fecal microbiota transplantation (FMT). Approaches to prevent and treat these disorders will need to focus on the acquisition and maintenance of a healthy colonic microbiota, in addition to more focused approaches at immune suppression during acute disease exacerbations.
Keywords: Probiotic, Dysbiosis, Immunodeficiency, Multiple sclerosis, Inflammatory bowel disease
10.1. Tregs Are Shaped by the Gut Microbiota
The gut microbiota is essential for the development and maturation of the immune system; reciprocally, the microbial community is also profoundly affected by the complex host immune system. The gut epithelium spatially segregates the lamina propria (LP), rich in lymphocytes, plasma cells, dendritic cells (DCs), macrophages, neutrophils, and scaffolding myofibroblasts, from the luminal contents, particularly the gut microbiome (bacteria, fungi, viruses, archaea, and phage) and a variety of food antigens. One of the most fascinating observations is that the subepithelial immune network can exert pressure against selective microbes by elaborating cytokines, antimicrobial peptides, nutrients, and mucus (Zhou and Sonnenberg 2018). Likewise, immune cells can “welcome” certain organisms. T cells in the LP are the crucial source of anti-inflammatory cytokine IL-10. To exemplify microbial induction of immune tolerance, luminal Bacillus fragilis produces polysaccharide antigen A (PSA), which acts on DCs, resulting in TGF-β and IL-10 production, which supports the development of gut anti-inflammatory cells, especially regulatory T cells (Tregs). Consequently, Tregs produce IL-10 that induces in macrophages a tolerogenic phenotype. However, even in the absence of IL-10 or in hosts with IL-10 receptor mutations, an unusual condition in humans with very early onset of inflammatory bowel disease (IBD), different Lactobacillus species can effectively prevent or attenuate the colitis that develops (Madsen et al. 1999).
A seminal observation related to luminal microbial impact on the immune system was that LP CD4+ (helper) T cells were shifted toward an inflammatory TH17 phenotype when mice were colonized with segmented filamentous bacteria (SFB). SFB are interesting coiled microbes which penetrate the mucus gel in the ileum and directly attach like leeches to the epithelial cells (Ivanov et al. 2009). However, Clostridia species and other microbes produced short-chain fatty acids (SCFAs) via the metabolism of dietary fiber, resulting in colonic Tregs development and reduced inflammation in this model, via a mechanism involving G-protein-coupled receptor GPCR43 (Smith et al. 2013). In humans with IBD (to be discussed below), certain colonic microbes have been clearly associated with active diseases, such as Ruminococcus gnavus (Schirmer et al. 2019) and oral cavity inhabitants (Veillonella dispar, Aggregatibacter, Lachnospiraceae, and Haemophilus parainfluenzae) (Schirmer et al. 2018a), whereas other organisms such as Faecalibacterium prausnitzii, which produces the SCFA butyrate, are clearly associated with disease remission (Schirmer et al. 2018b).
10.2. Gut Dysbiosis-Related Autoimmune Disorders Involve Tregs
Autoimmune disorders prevalent in humans include disorders of monogenic primary immune deficiency and polygenic autoimmune diseases including diseases of the central nervous system (CNS) (multiple sclerosis, transverse myelitis, and Guillain Barre syndrome); the gastrointestinal tract (inflammatory bowel disease, autoimmune hepatitis, and celiac disease); and autoimmune conditions of the skin (including psoriasis, eczema, and scleroderma).
10.2.1. Monogenic Primary Autoimmune Disorders Related to Tregs Are Associated with Gut Microbial Dysbiosis
Monogenic autoimmune disorders are due to a single-gene defect and are generally quite rare. These mutations cause impairment in one of the principal mechanisms controlling adaptive immunity, such as loss of control of inflammatory effector T cells (TH1/TH2) caused by deficiency of Foxp3+Tregs. Foxp3+Treg deficiency can be due to a Foxp3 gene mutation or deletion, resulting clinically in a condition characterized by immune dysregulation polyendocrinopathy, enteropathy, and X-linked inheritance, called IPEX syndrome and in mice the scurfy (SF) phenotype. A recent review (Chervonsky 2013) stated that monogenic diseases including IPEX (Chinen et al. 2010) and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, due to mutations in the transcription factor autoimmune regulator (AIRE)) (Gray et al. 2007) are insensitive to commensal microbial regulation, because these diseases occur in germ-free (GF) animals. The authors suggested that autoimmune T cells are activated in the absence of the innate-adaptive connection. A critical role for Treg-mediated control of inflammation was studied using GF mice compared with specific pathogen-free (SPF) mice and demonstrated that Treg development and suppressive function were not dependent on gut microbiota. However, in a Treg-depleted model (Foxp3-DTR), inflammation in the small intestine of SPF mice was more severe than in GF mice, as shown by significantly increased gut lymphocyte infiltration, decreased body weight, and increased percentage of IFN-γ-producing T helper cells, indicating that Treg deficiency-induced intestinal inflammation is indeed influenced by the composition of the gut microbiota (Chinen et al. 2010).
Additional experiments provide evidence that a single-gene immune-related mutation is linked to intestinal dysbiosis. Dysbiosis is defined as atypical microbial populations, which carry proinflammatory characteristics. It is not surprising that the feces of individuals with autoimmune disorders harbor skewed populations of microbes that differ from those of healthy controls. We observed in Foxp3-deficient scurfy mice that development of autoimmunity was accompanied by a progressive gut microbial dysbiosis over the first 22 days of life (He et al. 2017b). We demonstrated reduced bacterial diversity and altered bacterial composition. Altered gut microbiota has also been shown in immunodeficient mice lacking B cells (Ighm−/−), T cells (Cd3e−/−), or both B and T cells (Rag1−/−), whereas administration of Foxp3+Tregs to T-cell-deficient mice restored bacterial diversity (Kawamoto et al. 2014). A detailed study compared Rag1+/+ and Rag1−/−mice of the same genetic background and eliminated cage effects. Investigators sought to determine the effect of Rag1 and the adaptive immune system on gut microbiota. They demonstrated that Rag1 status is a source of variation in community structure of gut microbiota (Zhang et al. 2015). A recent study of individuals with APECED also indicated that their microbiota composition is altered. These patients developed early and sustained responses to gut microbial antigens, and abnormal immune recognition of gut commensals was linked to enteropathy, the development of defensin-specific T cells and anti-defensin antibodies, and production of anti-Saccharomyces cerevisiae antibodies (ASCA). ASCA level was highly correlated with the depletion of gut-associated Tregs (r = 0.7, P < 0.01); and it is worthy to note that ASCA has been linked to Crohn’s disease in humans, indicating that transcription factor AIRE is an important regulator of intestinal homeostasis (Dobes et al. 2015; Hetemaki et al. 2016). Thus, overall evidence leads us to conclude that host adaptive immunity in diseases with single-gene mutations alters gut microbiota.
Our team has studied the previously mentioned Foxp3-deficient mouse SF mouse. We observed dynamic changes of autoimmunity and gut microbial dysbiosis in this disease. We noticed that microbial beta-diversity in SF mice was successfully shifted by orally administering a probiotic or health-promoting bacteria (Lactobacillus reuteri DSM 17938, LR 17938) to produce a distinct microbial signature and a concomitant reduction in disease severity (He et al. 2017b).
Metabolites produced by bacteria may be major mediators of immune tolerance. We discovered that the adenosine metabolite inosine is reduced in plasma of SF mice and is restored by LR 17938 treatment. Orally gavaging this nucleoside to SF mice, similar to gavaging the probiotic LR itself, prolonged the survival of SF mice and reduced inflammation in multiple organs such as liver and lung. Inosine and LR 17938 each controlled inflammation by acting as a ligand to the adenosine receptor 2A (A2A), as evidenced by the lack of inhibition of naïve CD4+ T-cell differentiation in mice with genetically deleted adenosine receptor A2A (A2A−/− mice). Inosine and LR 17938 were ineffective in mice with mutations of three other adenosine receptors (A1, A2B, and A3) (He et al. 2017b). We concluded that A2A is required for the protection by this probiotic (He et al. 2017a).
10.2.2. Polygenic Autoimmune Diseases with Treg Dysfunction Are Associated with Gut Microbial Dysbiosis
Polygenic autoimmune diseases generally develop gradually, with incomplete penetration of the disease, meaning that many individuals expected to develop symptoms do not become sick. The conditions eventually occur because there is a “perfect storm” of environmental insults, even in some cases emotional trauma, coupled with aberrant microbial population in the gut which predisposes to systemic inflammation.
The intestine harbors ~1014 microbes (more than our own cell population) and approximately 1000 different taxa, accounting for 3% of our total body mass. In almost every autoimmune disorder, an abnormal population of enteric microbes has been identified, called dysbiosis. However, it is remarkable that the microbial populations segregate differentially with respect to each other, as well as segregating differently from controls when analyzed by principal components analysis. Investigators recently showed that when looking at stool 16S rDNA sequences of patients with different autoimmune disorders, the microbial beta diversity was distinct for Sjogren syndrome, compared with systemic lupus erythematosus (SLE) and primary phospholipid syndrome (PPLS), and these were distinct from controls not having autoimmune disease (Fig. 10.1).
Fig. 10.1.
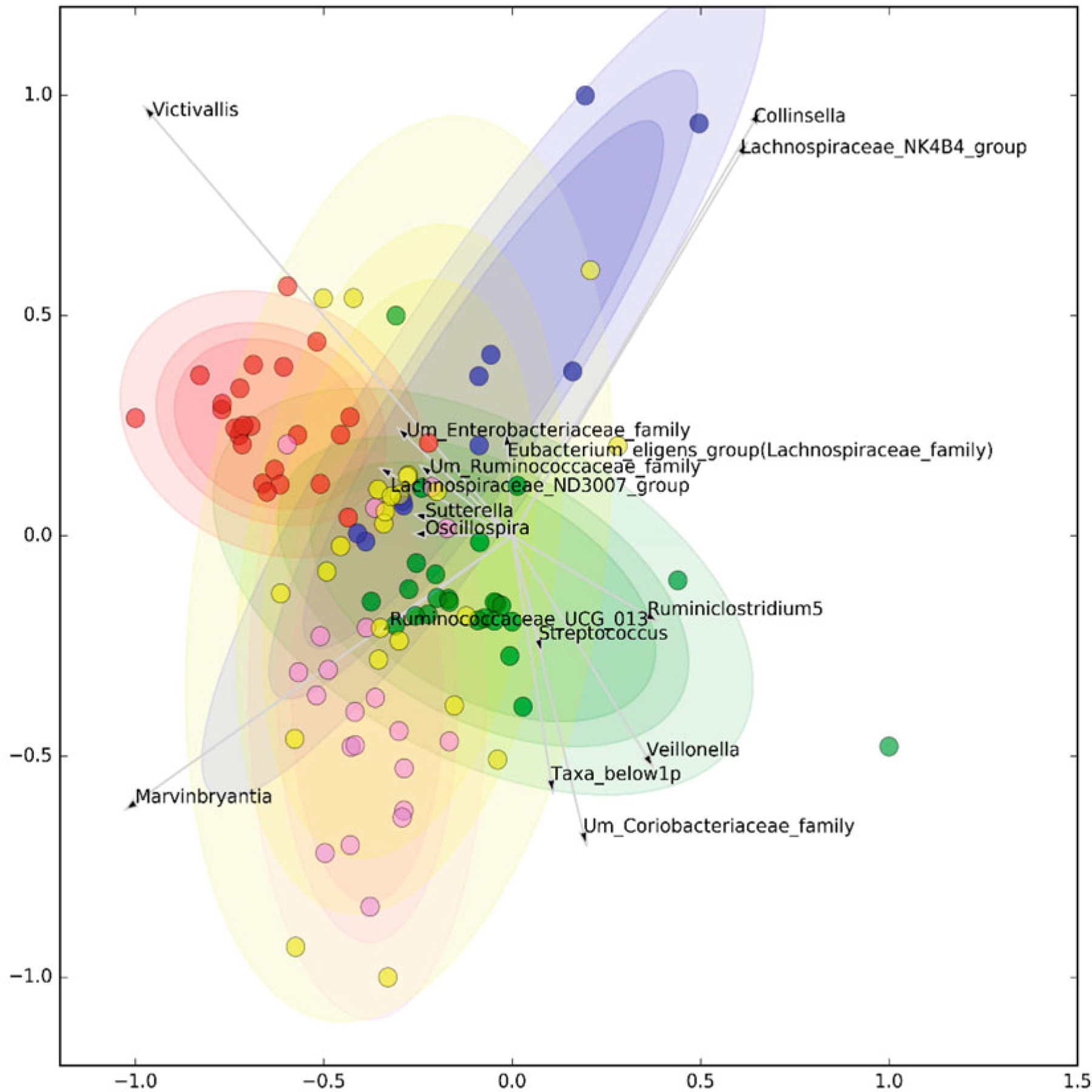
Each specific disorder has microbial signature which segregated the disease via its own microbial signature. Clustering of selected microbiome genera. Graphical representation is optimized to maximize the compactness and separation of clusters. Red = healthy controls, purple = systemic lupus erythematosus, green = Sjogren’s syndrome, blue = primary antiphospholipid syndrome, and yellow = undifferentiated connective tissue disease. Reproduced with permission from Bellocchi et al. (2019). The association between autoimmunity and gut microbiota would be discussed in three polygenic autoimmune diseases as examples, multiple sclerosis, inflammatory bowel diseases, and food allergy
10.2.3. Autoimmune Diseases in the Central Nervous System (CNS)
Multiple sclerosis (MS).
A polygenic autoimmune disorder associated with microbial dysbiosis is multiple sclerosis (MS). This disease affects >1 million Americans and is characterized by autoimmune cells invading the CNS, producing impaired vision, weakness, loss of sensation, chronic pain, and declining cognition (Wallin et al. 2019). Although there is no cure, the major thrust of treatment is immunosuppression. T cells play a major role in the pathogenesis of MS, with TH1- and TH17-associated cytokines being elevated, along with reported major abnormalities in the function of Tregs (Danikowski et al. 2017). Gut bacteria have an important influence on the development of this disease. In MS and its mouse model called experimental autoimmune encephalomyelitis (EAE), antibodies are produced that react to myelin. In the laboratory, to induce EAE, myelin oligodendrocyte (MOG) peptides with Freund’s adjuvant are injected in the presence of pertussis toxin to produce the gradual onset of varying degrees of paralysis. MOG peptides presented by antigen-presenting cells subsequently activate T cells, which migrate to the CNS. Unlike conventional mice, germ-free mice are protected from paralysis, whereas those inoculated with a single organism SFB (as discussed above) develop the symptoms (Lee et al. 2011). In these studies, SFBs were shown to induce IL-17A-producing CD4+ T cells (TH17) in the CNS.
When peripheral blood leukocytes are examined from individuals with MS, many have been found to reduce Foxp3 expression, as well as reduced numbers of Foxp3-negative regulatory T cells or Tr1 cells. About 30% of the brain lesions in MS patients have no Foxp3 expression. Not surprisingly, many but not all treatments for MS are associated with the ability to increase Tregs. Notably, indoleamine 2,3-dioxygenase (IDO), a product of the metabolism of tryptophan (now well characterized as a product of gut microbes), when administered parenterally ameliorates experimental EAE (Yan et al. 2010). Specific chemokines, such as CCR4 and dendritic cell-derived CCL17 are required for Treg recruitment to the CSF, whereas CXCR3 is important for the transmigration of Tregs and Tr1 cells across the blood–brain barrier to combat the neuroinflammation (Danikowski et al. 2017).
We discovered that the probiotic LR 17938 when given enterally to mice with EAE was able to reduce the severity of clinical disease, measured by the extent of paralysis, as well as inflammation in the spinal cord, as measured by hematoxylin and eosin staining and CD3+ T-cell staining. LR 17938 also reduced the number of circulating TH1/TH17 cells and their associated cytokines IFN-γ/IL-17. We also showed that the loss of diversity of gut microbiota induced by EAE was largely reversed by LR 17938 treatment (He et al. 2019). Taxonomy-based analysis of gut microbiota showed that three “beneficial” genera Bifidobacterium, Prevotella, and Lactobacillus were negatively correlated with EAE clinical severity. Notably Prevotella was previously shown to be reduced in humans with MS (Schepici et al. 2019). Conversely, genera Anaeroplasma, Rikenellaceae, and Clostridium were positively correlated with disease severity. Remarkably, LR 17938 treatment coordinately altered the relative abundance of these EAE-associated genera. These studies suggested that LR 17938 might represent a novel adjunctive treatment in future studies to modify the severity of MS.
10.2.4. Gastrointestinal Autoimmune Disorders
Inflammatory bowel disease (IBD).
IBD is the most common chronic gastrointestinal illness of children and young adults, affecting 100–200 children per 100,000 in the US (or ~70,000 children) (Rosen et al. 2015). The two most prevalent conditions are Crohn’s disease (CD), which can cause ulceration in any part of the gastrointestinal tract, especially the ileum and proximal colon, and ulcerative colitis (UC), which involves circumferential ulceration of the colon only. Symptoms include diarrhea, abdominal pain, fever, weight loss, arthritis, mucositis, perianal disease, bowel obstruction, and fistula development. The most accepted working hypothesis for the pathogenesis of IBD is that a dysfunctional immune system interacts with an abnormal microbiota, with exacerbating environmental influences to induce disease. Contributing factors, such as antibiotics, high animal protein intake, cigarette smoke, and high linoleic acid intake, all may result in an imbalance between pro- and anti-inflammatory cells in the gastrointestinal mucosa (Bernstein 2017).
There are >250 different mutations that have now been linked to CD. Some mutations affect T cells, but others involve defective phagocytic bacterial killing (such as the NOD1 mutation) or may impair more global cellular protective processes such as autophagy (e.g., the ATG16L1 mutation) (Bianco et al. 2015). When one begins to look at genetic mutations in IBD, it becomes evident that IBD represents a common “denominator” of many different abnormalities in immune cell function and microbial homeostasis.
Reproducible findings of T-cell functional abnormalities seen in individuals with IBD have led to the hypothesis that unregulated inflammation must be related to an imbalance between helper T-cell subsets, specifically the balance between Tregs and TH1 and TH17 cells (Packey and Sartor 2008). The important roles of both TH1 and TH2 cells in UC and TH1 and TH17 cells in CD have been described, but the role of Tregs is complex. For example, in pediatric IBD, two types of Tregs are actually elevated. Tr1 cells are more abundant than normal in the peripheral circulation of children with ulcerative colitis and Crohn’s disease, whereas in UC, circulating Foxp3 + Treg cells are increased at the time of diagnosis but dwindle during remission (Vitale et al. 2020).
Animal models uncover a complex and some-what paradoxical role of Tregs in IBD. For example, Tbet (the transcription factor associated with TH1 cells) can also be expressed on Tregs (which are characterized by Foxp3 expression) in the initial stages of colitis in humans. Knockout of Tbet on Foxp3-positive Tregs reduces colitis severity, implicating a pro-inflammatory role not previously recognized for this group of Treg cells. In a mouse model of colitis induced by dextran sodium sulfate (DSS), there was an influx into the gut epithelium of cells positive for Tbet, interferon-gamma, and Foxp3 occurring prior to the accumulation of classic TH1 cells into the mucosa of the colon that was preventable by a conditional knockout of Tbet on Tregs (Di et al. 2019).
Clearly, T cells and microbes play a central role in the regulation of IBD. Biologic therapies that are widely used include immunosuppression by the anti-tumor necrosis factor (TNF) antibodies infliximab and adalimumab and administration of anti-integrin alpha7beta4 (α7β4) antibodies such as vedolizumab, which prevents homing of leukocytes to the gastrointestinal (GI) tract (Jeong et al. 2019).
In taking an “outside the box” approach to severe IBD, Treg infusion therapy was found to have only limited success in humans with CD. In 2012, investigators participated in an 8-week open-label dose-ranging trial of human Tregs for 20 adults with CD. Tregs were isolated from patients’ peripheral blood mononuclear cells, exposed to chicken ovalbumin, and administered intravenously (ova-Tregs); subsequently, a meringue cake diet (rich in ovalbumin) stimulated Treg proliferation in the GI tract. There was an improvement in Crohn’s disease activity (CDA) in 40% of patients, but only minimal improvement in circulating inflammatory markers (CRP and fecal calprotectin), without an increase in Treg numbers in the blood; and of the four doses tested, only the lowest dose appeared to be effective (Desreumaux et al. 2012). No significant human trials of Treg isolation, in vitro expansion, and administration have been subsequently published for patients with IBD.
A review pointed out that the persistence of inflammation in patients with IBD in the face of high levels of Foxp3+ Tregs in the affected tissues suggests that the condition is resistant to Tregs, perhaps due to other effector cell activation resistant to Tregs or tissue/extracellular matrix factors unsupportive of their inhibitory action (Lord 2015).
Recently, interest in Tr1 cells which do not express Foxp3 has arisen as a potential therapeutic target (Huang et al. 2017). These cells secrete IL-10 and IL-22, both with anti-inflammatory properties, and Tr1 cells were shown to reduce the proliferation of effector T cells and the secretion of cytokines by myeloid cells. These cells also protected barrier function of cultured T84 cells in vitro (Cook et al. 2019). Extensive research has also shown that commensal microbes produce SCFAs that interact with cell surface G-protein-coupled receptors GPR43 and GPR41 on myeloid cells to subsequently induce regulatory phenotype and IL-10 secretion by Tr1 cells (Sun et al. 2018). Commensals can also act on monocytes (Ly6Chi cells) via their SCFA, resulting in prostaglandin E2 production that reduces the activation level of neutrophils (Grainger et al. 2013).
Food allergy.
Given the importance of Tregs in maintaining tolerance to endogenous and exogenous stimuli, it is not surprising that Tregs are localized predominantly in the intestines and skin, two tissues with the largest surface area exposing to the external environment (Harrison and Powrie 2013). It is know that the skin is more sensitizing, while the gut is more tolerizing. In murine model of allergy, the skin is used to create an allergic immune development. If the mice are fed with the allergen, for example, ovalbumin, prior to the skin sensitization, inducing the allergy is more difficult. This observation indicates that the intestinal microenvironment is designed to be more immune tolerant. Previously, the American Academy of Pediatrics (AAP) had recommended avoiding early introduction of highly allergenic foods to infants until after 1 year of age. Ironically, over 10 years later, the incidence of food allergy continues to rise. After a pivotal clinical trial of early introduction of peanuts in infants had demonstrated a significant reduction in the development of peanut allergy, the new AAP guidelines now recommend introduction of highly allergenic foods to infants as early as 4–-6 months of age (Du et al. 2015).
These epidemiologic and clinical studies have demonstrated the importance of the intestines in regulating food allergy development. However, the mechanisms involved in tolerance induction are quite complex. Tregs clearly play a critical role in controlling food allergy development, since patients with IPEX syndrome have a higher incidence of severe food allergy (Torgerson et al. 2007). Clinical studies using oral immunotherapy (OIT) for the prevention or treatment of food allergy have shown an increase in Tregs, particularly allergen-induced Tregs, throughout the therapy (Hardy et al. 2019). Interestingly, food allergy is a problem in developed countries, supporting the hygiene hypothesis (Platts-Mills 2015). The western diet, sedentary lifestyle, cleanliness, and indoor living have altered the microbiomes on the skin, mucosa, and intestines as well as reduction in vitamin D level (Du et al. 2016). In atopic infants living in this modernized environment, skin exposure to allergenic food proteins might induce a more sensitizing immune response, since the immune system might be regulated differently. In contrast, those in developing or underdeveloped countries, the immune system might be more tolerant since its focus would be on controlling the various microbes on the skin, mucosa, and intestines. An analogy would be in the time of war, people concentrate more attention on survival.
It is important to note that there are two major subsets of Tregs: thymic Tregs (tTregs) and peripheral induced Tregs (pTregs) (Horwitz et al. 2008; Zhang et al. 2020). tTregs are developed in the thymus with a stable expression of Foxp3 due to high demethylation of the Foxp3 promoter region called Treg-specific demethylated region (TSDR). pTregs are generated in the peripheral tissues through the induction of Foxp3 expression in CD4+ T cells. These pTregs have greater plasticity and significantly lower demethylation in the TSDR. Current data support that tTregs are important in controlling intrinsic autoimmunity, while pTregs help to regulate extrinsic immune response (Shevach and Thornton 2014). pTregs are essential for maternal–fetal tolerance. In the absence of pTregs, using a specific murine model deficient in conserved noncoding sequence 1 (CNS1) of the Foxp3 enhancer region, there is increased fetal resorption, resulting in a significant reduction in live birth (Samstein et al. 2012). Another important insight using this pTreg-deficient murine model is the importance of pTregs in maintaining intestinal microbial communities by modulating immune defense mechanisms that can lead to dysbiosis (Campbell et al. 2018). The microbiome is vital in contributing to immune homeostasis through its interaction with Tregs. Perturbation in the microbiome can lead to dysbiosis, resulting in aberrant immune responses such as food allergy (Bunyavanich and Berin 2019). Technologies continue to advance rapidly; and so it would be challenging to change people’s lifestyle to living that is more rural. Therefore, the focus would be on developing natural therapies and diet that would promote Treg homeostasis and prevent dysbiosis.
10.3. Resetting Microbiota and Tregs in Diseases
Probiotics.
Probiotics (as mentioned above) are “live organisms that when administered in adequate doses confer a health benefit to the host”. They are provided as fermentable foods, pills, powders, beverages, and liquid drops. Common probiotics are available in pharmacies, groceries, and online. They include but are not limited to Lactobacilli, Bacillus species, several strains of Bifidobacteria, Streptococcus thermophilus, Escherichia coli strain Nissle 1917, and yeasts, including Saccharomyces boulardii and Saccharomyces cerevisiae. Many commercially available probiotics contain mixtures of two or more individual species.
Although not discussed earlier in this review, a major medical condition for which probiotics are being studied is neonatal necrotizing enterocolitis (NEC), the leading cause of intestinal failure in premature babies. We have found in mouse and rat models of NEC that probiotic administration in the setting of experimental neonatal gut hypoxia reset the balance between inflammatory effector T cells and their Treg counterparts, reducing both the incidence and severity of NEC (Liu et al. 2013, 2014). Our results revealed that there were low numbers of Tregs in the terminal ileum of newborn rats with NEC. Adoptive transfer of Tregs significantly improved weight loss, improved survival and reduced the incidence of NEC (Dingle et al. 2013). Our findings suggested that while Tregs are present in the newborn intestine, their numbers might be insufficient to dampen the excessive inflammatory state in NEC.
We have not directly tested the impact of probiotics on the microbiota on fecal community composition in the NEC model, but in subsequent investigations in healthy newborn mice, we showed that LR 17938 increased the proportion of Foxp3+ Tregs and also increased bacterial diversity (Liu et al. 2019). LR 17938 was associated with increased relative abundance of phylum Firmicutes, families Lachnospiraceae and Ruminococcaceae, and genera Clostridium and Candidatus arthromitus. LR 17938 concomitantly decreased the relative abundance of phylum Bacteroidetes, families Bacteroidaceae and Verrucomicrobiaceae, and genera Bacteroides, Ruminococcus, Akkermansia, and Sutterella. Finally, LR 17938 also exerted a major impact on the plasma metabolome, upregulating amino acid metabolites formed via the urea, tricarboxylic acid, and methionine cycles, and simultaneously increasing tryptophan metabolism. Of significance, LR 17938 feeding increased the levels of key tryptophan metabolites indolepropionate and indoleacrylate and purine nucleosides (adenosine and inosine), each of which is known to enhance tolerance to inflammatory stimuli (Liu et al. 2019).
The mechanisms by which probiotics induce Tregs in different diseases have been demonstrated to be variable and strain specific, as summarized in a review article by Dwivedi et al. (2016) and Fig. 10.2. Probiotics and probiotic-modulated beneficial gut microbiota induce intestinal Tregs through direct microbial–immune cell or microbial-intestinal epithelial cell interactions, for example, via stimulation of Toll-like receptors (TLRs 2, 3, 4, or 9) to activate mucosal tolerogenic DCs to produce IL10 and/or Treg-interacting molecules (IL10 or TGFβ) (Dwivedi et al. 2016). In addition, several studies have revealed a complex relationship between Tregs and microbial metabolism, for example, bacterial production of different metabolites (SCFA, tryptophan derivatives, and adenosine/inosine). Probiotics or probiotic-modulated beneficial gut bacteria produce SCFAs (acetate, propionate, and butyrate) through fermentation of dietary fibers, which maintain immune homeostasis. Studies have indicated the significant alteration in the number of butyrate-producing bacteria in colon of patients with inflammatory disorders (Frank et al. 2007; Wang et al. 2012). SCFAs may induce intestinal Treg cells by several mechanisms: (a) by interacting with specific receptors including G-protein-coupled receptors (GPCRs) GPR43 and GPR109A (also known as hydroxycarboxylic acid receptor 2 or HCA2) expressed in colonic epithelium, adipose tissue, and immune cells (Blad et al. 2012; Ganapathy et al. 2013); (b) by inhibiting histone deacetylases (HDACs), resulting in increased Foxp3 gene expression and the suppressive function of FoxP3+ Tregs (Arpaia et al. 2013; Li et al. 2007; Tao et al. 2007); or (c) by enhancing production of prostaglandin E2 (PGE2), which has been shown to promote the development of Tregs in humans and in mice (Mahic et al. 2006; Sharma et al. 2005). Interestingly, PGE2 is also involved in mediating the suppressive activity of Tregs via IL-10 and reduced production of multiple proinflammatory cytokines (Baratelli et al. 2005). Microbial tryptophan metabolites including indoleacetic acid (IAA), indolealdehyde (IAId), indoleacrylic acid (IA), and indolelactic acid (ILA) act on the aryl hydrocarbon receptor (AHR) found in intestinal immune cells, and thereby mitigate innate and adaptive immune responses in a ligand-specific fashion to promote Treg function and/or produce anti-inflammatory IL22 (Roager and Licht 2018).
Fig. 10.2.
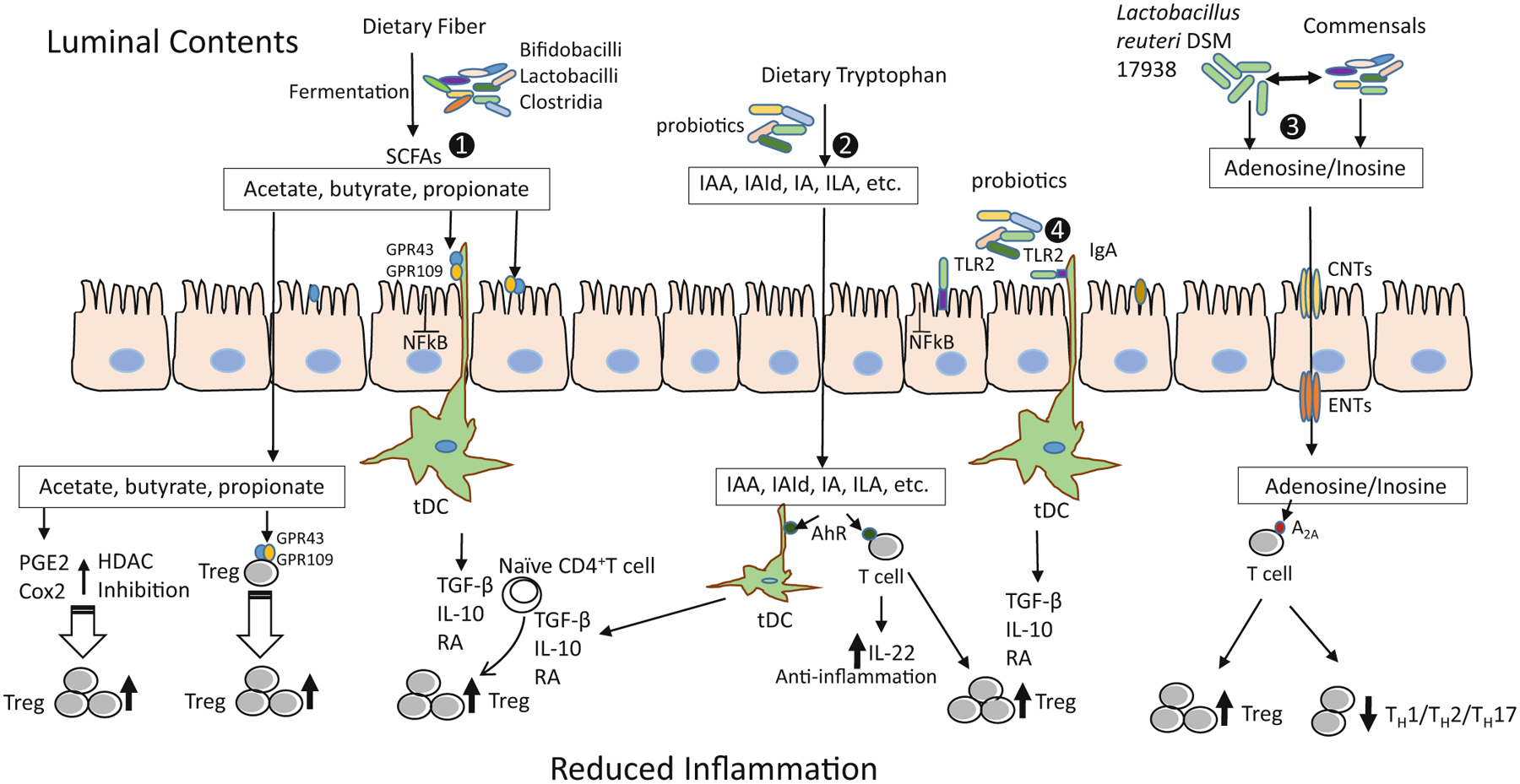
Different mechanisms of Treg induction by probiotics to reduce inflammation. (1) Probiotics metabolize the dietary fibers such as resistant starch, inulin, FOS, β-GOS to release SCFAs: acetate, butyrate and propionate. These SCFAs activate the GPR43 and GPR109A: on intestinal epithelial cells to inhibit the NF-κB pathway to reduce inflammation; on DCs to generate tDCs producing TGFβ, IL-10, and RA; and on Treg cells. This interaction of SCFAs and GPCRs results into induction and expansion of Tregs capable of suppressing the inflammatory state. GPR43 is activated by all three SCFAs; however, GPR109A is activated only by butyrate. SCFA induction also involves inhibition of HDACs which in turn increases the production and suppressive function of FoxP3+Tregs by promoting the acetylation of Foxp3 protein. In addition, SCFAs modulate the production of PGE2 and COX2 (butyrate in particular), promoting expansion of pre-existing Tregs. (2) Microbial tryptophan metabolites including IAA, IAId, IA, and ILA interact with the AHR on DCs to generate tDCs and AHR on T cells to promote Treg function and/or produce anti-inflammatory IL-22. (3) Probiotic Lactobacillus reuteri DSM 17938-mediated production of adenosine and its metabolite inosine, by serving as an A2A agonist, inhibits TH1/TH2/TH17 cells and promotes Treg-mediated immunoregulation. (4) Probiotics interact with TLR2 expressed on intestinal epithelial cells and immune cells to induce tDCs and subsequently to induce Tregs (Illustration by Yuying Liu). Treg regulatory T cell, FOS fructooligosaccharides, GOS galactooligosaccharide, SCFA short-chain fatty acid, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, DCs dendritic cells, tDCs tolerogenic dendritic cells, GPR43 and GPR109A G-protein-coupled receptors (GPCRs), TGFβ transforming growth factor beta, IL interleukin, RA retinoic acid, HDACs histone deacetylases, PGE2 prostaglandin E2, COX-2 cyclooxygenase-2, IAA indoleacetic acid, IAId indolealdehyde, IA indoleacrylic acid, ILA indolelactic acid, AHR aryl hydrocarbon receptor, A2A adenosine receptor 2A, TH cell T helper cell, TLR2 toll-like receptor 2, CNT concentrative nucleoside transporters, ENT equilibrative nucleoside transporters
A subset of iTreg, expressing ectonucleotidases CD39 and CD73 is able to hydrolyze ATP to 5′-AMP, generate adenosine, and thus mediate suppression of inflammatory T cells while promoting Foxp3 expression of Tregs via interaction with adenosine receptors. These immune cells express predominately adenosine receptor 2A (A2A) (Su et al. 2019). As mentioned above, one of our previous studies showed that probiotic LR 17938-mediated production of adenosine metabolite inosine, as an A2A agonist, inhibited TH1 and TH2 cells and their associated cytokines during of Treg-deficiency-induced autoimmunity (He et al. 2017a, b).
Fecal microbiota transplantation (FMT).
The use of processed stool cultures from highly screened healthy donors for humans with C. difficile infection (CDI) has been shown to be lifesaving. These FMTs are generally given by a series of colonoscopies with infusions into the colon through the biopsy channel. Preclinical work has shown that FMT is beneficial in mouse models of autoimmune diseases, including myocarditis (Hu et al. 2019), myasthenia gravis (Zheng et al. 2019), and experimental autoimmune uveitis (Ye et al. 2018). In humans, only preliminary studies are available for autoimmune diseases, but efficacy has been suggested for a plethora of conditions, including ulcerative colitis (Dang et al. 2020), acute graft versus host disease (Cohen and Maharshak 2017), and a variety of other conditions which include pouchitis, multiple sclerosis, metabolic syndrome, autism, and irritable bowel syndrome (Cohen and Maharshak 2017). In severe cases of inflammatory bowel disease, FMT has been undertaken with partial success (Hirten et al. 2019). In a mouse model of colitis-associated colon cancer (CAC), FMT improved the severity of colitis. Foxp3+ Tregs were dramatically upregulated among splenic, mesenteric lymph node, and lamina propia lymphocytes of CAC mice after FMT (Wang et al. 2019). Ongoing studies are determining the safety of FMT and duration of response in IBD.
FMT has many common side effects, including bloating, discomfort, low-grade fevers, and flatulence (Dailey et al. 2019). More severe delayed effects may also be seen; some fatal cases may have resulted from transmission of antibiotic-resistant microbial sepsis, such as that due to E. coli (DeFilipp et al. 2019). Transmission of viruses is often seen (Chehoud et al. 2016), leading to theoretical concerns about performing this procedure during the coronavirus era (Ianiro et al. 2020). One must consider that in some of these diseases, probiotics may be equally effective (Dang et al. 2020). New approaches that may carry less risk included targeted-bacterial therapy and administration of health-promoting bacterial-derived metabolites (postbiotics). Nevertheless, the FMT story testifies to the strong relationship between the gut microbiome and host autoimmunity.
Contributor Information
Yuying Liu, Department of Pediatrics, Division of Gastroenterology, The University of Texas Health Science Center at Houston McGovern Medical School, Houston, TX, USA.
Dat Q. Tran, Department of Pediatrics, Division of Gastroenterology, The University of Texas Health Science Center at Houston McGovern Medical School, Houston, TX, USA
John William Lindsey, Department of Neurology, Division of Multiple Sclerosis and Neuroimmunology, The University of Texas Health Science Center at Houston McGovern Medical School, Houston, TX, USA.
Jon Marc Rhoads, Department of Pediatrics, Division of Gastroenterology, The University of Texas Health Science Center at Houston McGovern Medical School, Houston, TX, USA.
References
- Arpaia N, Campbell C, Fan X et al. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504 (7480):451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L et al. (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175 (3):1483–1490 [DOI] [PubMed] [Google Scholar]
- Bellocchi C, Fernandez-Ochoa A, Montanelli G et al. (2019) Identification of a shared microbiomic and metabolomic profile in systemic autoimmune diseases. J Clin Med 8(9):1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN (2017) Review article: changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment Pharmacol Ther 46(10):911–919 [DOI] [PubMed] [Google Scholar]
- Bianco AM, Girardelli M, Tommasini A (2015) Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J Gastroenterol 21 (43):12296–12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blad CC, Tang C, Offermanns S (2012) G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov 11(8):603–619 [DOI] [PubMed] [Google Scholar]
- Bunyavanich S, Berin MC (2019) Food allergy and the microbiome: current understandings and future directions. J Allergy Clin Immunol 144(6):1468–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, Dikiy S, Bhattarai SK et al. (2018) Extrathymically generated regulatory T cells establish a niche for intestinal border-dwelling Bacteria and affect physiologic metabolite balance. Immunity 48 (6):1245–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehoud C, Dryga A, Hwang Y et al. (2016) Transfer of viral communities between human individuals during fecal microbiota transplantation. MBio 7(2):e00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky AV (2013) Microbiota and autoimmunity. Cold Spring Harb Perspect Biol 5(3):a007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen T, Volchkov PY, Chervonsky AV et al. (2010) A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med 207(11):2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NA, Maharshak N (2017) Novel indications for fecal microbial transplantation: update and review of the literature. Dig Dis Sci 62(5):1131–1145 [DOI] [PubMed] [Google Scholar]
- Cook L, Stahl M, Han X et al. (2019) Suppressive and gut-reparative functions of human type 1 T regulatory cells. Gastroenterology 157(6):1584–1598 [DOI] [PubMed] [Google Scholar]
- Dailey FE, Turse EP, Daglilar E et al. (2019) The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol 49:29–33 [DOI] [PubMed] [Google Scholar]
- Dang X, Xu M, Liu D et al. (2020) Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One 15(3):e0228846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danikowski KM, Jayaraman S, Prabhakar BS (2017) Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 14(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilipp Z, Bloom PP, Torres SM et al. (2019) Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 381 (21):2043–2050 [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Foussat A, Allez M et al. (2012) Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143(5):1207–1217 [DOI] [PubMed] [Google Scholar]
- Di GM, Rizzo A, Franze E et al. (2019) Tbet expression in regulatory T cells is required to initiate Th1-mediated colitis. Front Immunol 10:2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle BM, Liu Y, Fatheree NY et al. (2013) FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One 8(12):e82963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobes J, Neuwirth A, Dobesova M et al. (2015) Gastrointestinal autoimmunity associated with loss of central tolerance to enteric alpha-Defensins. Gastroenterology 149(1):139–150 [DOI] [PubMed] [Google Scholar]
- Du TG, Roberts G, Sayre PH et al. (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372(9):803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du TG, Tsakok T, Lack S et al. (2016) Prevention of food allergy. J Allergy Clin Immunol 137(4):998–1010 [DOI] [PubMed] [Google Scholar]
- Dwivedi M, Kumar P, Laddha NC et al. (2016) Induction of regulatory T cells: a role for probiotics and prebiotics to suppress autoimmunity. Autoimmun Rev 15(4):379–392 [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA et al. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104 (34):13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD et al. (2013) Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 13(6):869–874 [DOI] [PubMed] [Google Scholar]
- Grainger JR, Wohlfert EA, Fuss IJ et al. (2013) Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 19(6):713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DH, Gavanescu I, Benoist C et al. (2007) Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci U S A 104(46):18193–18198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy LC, Smeekens JM, Kulis MD (2019) Biomarkers in food allergy immunotherapy. Curr Allergy Asthma Rep 19(12):61. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Powrie FM (2013) Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol 5(7):a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hoang TK, Tran DQ et al. (2017a) Adenosine A2A receptor deletion blocks the beneficial effects of lactobacillus reuteri in regulatory T-deficient scurfy mice. Front Immunol 8:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hoang TK, Wang T et al. (2017b) Resetting microbiota by lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med 214(1):107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hoang TK, Tian X et al. (2019) Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol 10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetemaki I, Jarva H, Kluger N et al. (2016) Anticommensal responses are associated with regulatory T cell defect in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. J Immunol 196 (7):2955–2964 [DOI] [PubMed] [Google Scholar]
- Hirten RP, Grinspan A, Fu SC et al. (2019) Microbial engraftment and efficacy of fecal microbiota transplant for Clostridium Difficile in patients with and without inflammatory bowel disease. Inflamm Bowel Dis 25 (6):969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz DA, Zheng SG, Gray JD (2008) Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol 29(9):429–435 [DOI] [PubMed] [Google Scholar]
- Hu XF, Zhang WY, Wen Q et al. (2019) Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res 139:412–421 [DOI] [PubMed] [Google Scholar]
- Huang W, Solouki S, Koylass N et al. (2017) ITK signalling via the Ras/IRF4 pathway regulates the development and function of Tr1 cells. Nat Commun 8:15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiro G, Mullish BH, Kelly CR et al. (2020) Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol 5(5):430–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139(3):485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong DY, Kim S, Son MJ et al. (2019) Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun Rev 18 (5):439–454 [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Maruya M, Kato LM et al. (2014) Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41(1):152–165 [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y et al. (2011) Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 108(Suppl):14615–14622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X et al. (2007) FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A 104(11):4571–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fatheree NY, Dingle BM et al. (2013) Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 8(2):e56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tran DQ, Fatheree NY et al. (2014) Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 307(2):G177–G186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tian X, He B et al. (2019) Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am J Physiol Gastrointest Liver Physiol 317(6):G824–G838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JD (2015) Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World J Gastroenterol 21(40):11236–11245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Doyle JS, Jewell LD et al. (1999) Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116(5):1107–1114 [DOI] [PubMed] [Google Scholar]
- Mahic M, Yaqub S, Johansson CC et al. (2006) FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol 177(1):246–254 [DOI] [PubMed] [Google Scholar]
- Packey CD, Sartor RB (2008) Interplay of commensal and pathogenic bacteria, genetic mutations, and immuno-regulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med 263(6):597–606 [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA (2015) The allergy epidemics: 1870–2010. J Allergy Clin Immunol 136(1):3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roager HM, Licht TR (2018) Microbial tryptophan catabolites in health and disease. Nat Commun 9 (1):3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Dhawan A, Saeed SA (2015) Inflammatory bowel disease in children and adolescents. JAMA Pediatr 169(11):1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A et al. (2012) Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150 (1):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepici G, Silvestro S, Bramanti P et al. (2019) The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 28(12):1507–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Denson L, Vlamakis H et al. (2018a) Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 24(4):600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Franzosa EA, Lloyd-Price J et al. (2018b) Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol 3 (3):337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Garner A, Vlamakis H et al. (2019) Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol 17(8):497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Yang SC, Zhu L et al. (2005) Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res 65 (12):5211–5220 [DOI] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259 (1):88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N et al. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341 (6145):569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Chen X, Zhu W et al. (2019) The cAMP-adenosine feedback loop maintains the suppressive function of regulatory T cells. J Immunol 203(6):1436–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Wu W, Chen L et al. (2018) Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 9 (1):3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E et al. (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 13(11):1299–1307 [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Linane A, Moes N et al. (2007) Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology 132(5):1705–1717 [DOI] [PubMed] [Google Scholar]
- Vitale A, Strisciuglio C, Vitale S et al. (2020) Increased frequency of regulatory T cells in pediatric inflammatory bowel disease at diagnosis: a compensative role? Pediatr Res 87(5):853–861 [DOI] [PubMed] [Google Scholar]
- Wallin MT, Culpepper WJ, Campbell JD et al. (2019) The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92 (10):e1029–e1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cai G, Qiu Y et al. (2012) Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 6(2):320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hua W, Li C et al. (2019) Protective role of fecal microbiota transplantation on colitis and colitis-associated colon cancer in mice is associated with Treg cells. Front Microbiol 10:2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhang GX, Gran B et al. (2010) IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol 185(10):5953–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhang N, Wu C et al. (2018) A metagenomic study of the gut microbiome in Behcet’s disease. Microbiome 6(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sparks JB, Karyala SV et al. (2015) Host adaptive immunity alters gut microbiota. ISME J 9 (3):770–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Olsen N, Zheng SG (2020) The progress and prospect of regulatory T cells in autoimmune diseases. J Autoimmun 111:102461. [DOI] [PubMed] [Google Scholar]
- Zheng P, Li Y, Wu J et al. (2019) Perturbed microbial ecology in myasthenia gravis: evidence from the gut microbiome and fecal metabolome. Adv Sci (Weinh) 6 (18):1901441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sonnenberg GF (2018) Essential immunologic orchestrators of intestinal homeostasis. Sci Immunol 3(20):eaao1605 [DOI] [PMC free article] [PubMed] [Google Scholar]


