Abstract
Background
Accelerated‐FEV1‐decline, defined as rate of decline in FEV1 > 64 ml/year, is a risk factor for asthma and chronic obstructive pulmonary disease in World Trade Center (WTC)‐exposed firefighters. Accelerated‐FEV1‐decline in this cohort is associated with elevated blood eosinophil concentrations, a mediator of Th‐2 response. We hypothesized that an association exists between Th‐2 biomarkers and FEV1 decline rate in those with accelerated‐FEV1‐decline.
Methods
Serum was drawn from Fire Department of the City of New York (FDNY) firefighters 1–6 months (early) (N = 816) and 12–13 years (late) (N = 983) after 9/11/2001. Th‐2 biomarkers IL‐4, IL‐13, and IL‐5 were assayed by multiplex Luminex. Individual FEV1 decline rates were calculated using spirometric measurements taken: (1) between 9/11/2001 and 9/10/2020 for the early biomarker group and (2) between late measurement date and 9/10/2020 for the late biomarker group. Associations of early and late Th‐2 biomarkers with subsequent FEV1 decline rates were analyzed using multivariable linear regression controlling for demographics, smoking status, and other potential confounders.
Results
In WTC‐exposed firefighters with accelerated‐FEV1‐decline, IL‐4, IL‐13, and IL‐5 measured 1–6 months post‐9/11/2001 were associated with greater FEV1 decline ml/year between 9/11/2001 and 9/10/2020 (−2.9 ± 1.4 ml/year per IL‐4 doubling; −8.4 ± 1.2 ml/year per IL‐13 doubling; −7.9 ± 1.3 ml/year per IL‐5 doubling). Among late measured Th‐2 biomarkers, only IL‐4 was associated with subsequent FEV1 decline rate (−4.0 ± 1.6 ml/year per IL‐4 doubling).
Conclusions
In WTC‐exposed firefighters with accelerated‐FEV1‐decline, elevated serum IL‐4 measured both 1–6 months and 12–13 years after 9/11 is associated with greater FEV1 decline/year. Drugs targeting the IL‐4 pathway may improve lung function in this high‐risk subgroup.
Keywords: cohort studies, FEV1 slope, firefighting, Th‐2 biomarkers, World Trade Center
1. INTRODUCTION
The World Trade Center (WTC) collapse on September 11, 2001 (9/11) released extremely high concentrations of dust and products of combustion into the air in lower Manhattan, causing lung injury in Fire Department of the City of New York (FDNY) rescue and recovery workers. 1 Twelve percent of WTC‐exposed firefighters developed a rate of decline in forced expiratory volume in 1 s (FEV1) that was more than twice the average rate of FEV1 decline of the cohort, >64 ml/year decline, defined as accelerated‐FEV1‐decline in previous investigations. 2 , 3 We observed that higher blood eosinophil concentrations, cells that mediate T‐helper 2 (Th‐2) immunity, were associated with accelerated‐FEV1‐decline in this population after controlling for covariates, in addition to being associated with wheeze and airflow obstruction in other WTC‐exposed cohorts. 2 , 4 Individuals in the accelerated‐FEV1‐decline subpopulation were more than four times as likely to have had incident airflow limitation as those who had expected age‐related FEV1 decline. 2 Accelerated‐FEV1‐decline also increased our patients' risk for asthma and asthma/chronic obstructive pulmonary disease (COPD) overlap syndrome by twofold, 5 and is associated with increased mortality in non‐WTC‐exposed individuals with smoking‐related COPD. 6
Inhaled corticosteroid and long‐acting beta agonist (ICS/LABA) combination therapy is a standard therapy for asthma and COPD with frequent exacerbations. 7 , 8 , 9 , 10 As time post‐9/11 has increased, ICS/LABA has been less effective in controlling WTC exposure‐associated respiratory symptoms. 11 The goal of the FDNY WTC Health Program's biomarker discovery program is to improve the prediction of disease risk and identification of inflammatory pathways to provide new therapeutic targets in those who are not responding to ICS/LABA treatment.
Elevated levels of eosinophils and of Th‐2 biomarkers IL‐4 and IgE measured within 6 months of 9/11 are risk factors for asthma/COPD overlap in WTC‐exposed firefighters. 5 Elevated IL‐4 is also a risk factor for WTC exposure‐associated asthma. 5 Conversely, higher levels of IFN‐γ, a Th‐1 cytokine, was associated with reduced risk of asthma and asthma/COPD overlap in WTC‐exposed firefighters. 5 Studies in populations with uncontrolled persistent asthma have shown that the monoclonal antibody dupilumab binds and inhibits the IL‐4/IL‐13 receptor, reducing asthma exacerbations, and improving asthma symptoms and FEV1. 12 , 13 , 14 In the current study, we hypothesized that an association exists between serum Th‐2 biomarkers and FEV1 decline rate in WTC‐exposed firefighters with accelerated‐FEV1‐decline.
2. METHODS
2.1. Study population
The source population included 9566 male firefighters who were actively employed by FDNY on 9/11, who first arrived to work at the WTC site between 9/11 and 9/24/2001, and who had ≥3 FEV1 measurements between 9/11/2001 and 9/10/2020 (Figure 1). The final study population was restricted to those who had serum Th‐2 biomarkers measured at either or both of the following time intervals: 1–6 months and 12–13 years post‐9/11 (N = 1686). Participants provided written informed consent, and the Albert Einstein College of Medicine Institutional Review Board approved this study.
Figure 1.
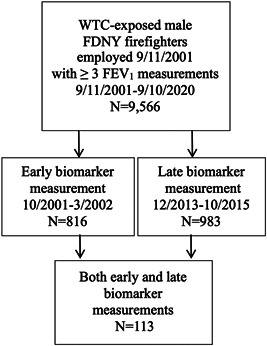
Flow diagram. Source population of World Trade Center (WTC)‐exposed Fire Department of the City of New York (FDNY) male firefighters who were actively employed on 9/11/2001 (9/11) and received ≥3 pulmonary function tests measuring forced expiratory volume in 1 s (FEV1) between 9/11 and 9/10/2020, and final study population including only those who had serum Th‐2 biomarkers measured during an “early” and/or “late” post‐9/11 time period
2.2. Baseline characteristics
We obtained demographic data from the FDNY employee database. Participants' height, weight, self‐reported smoking status, and time of initial arrival at the WTC site were assessed during routine medical monitoring examinations. Those who consistently self‐reported no cigarette smoking were classified as never‐smokers.
Serum biomarker concentrations were measured from blood drawn over two intervals: the first was between 1 and 6 months after 9/11, and the second took place 12–13 years post‐9/11. Serum was stored at −80°C. IL‐4, IL‐13, IL‐5, IFN‐γ, and TNF‐α were assayed with EMD Milipore HSTCMAG28SPMX21.
2.3. ICS/LABA treatment
Medication data were available via the FDNY electronic medical record and the FDNY WTC Health Program claims database. Participants in the late blood draw group were classified as having had ICS/LABA treatment if they had initiated the treatment before the late blood draw date.
2.4. Outcomes
FEV1 measurements were obtained from spirometric data collected during the FDNY medical monitoring examinations, as described in our previous studies. 1 , 2 , 3 Individual rates of FEV1 decline, and the corresponding standard error measurements, were estimated for each participant using linear regression analyses examining the effect of follow‐up time in years on FEV1 (ml). 2 Rates of FEV1 decline were calculated in two ways: (1) using participants' first post‐9/11 FEV1 measurement and all subsequent measurements through 9/10/2020 (overall FEV1 decline rate), and, (2) for participants with a late serum measurements, using participants' late blood draw date and all subsequent measurements through 9/10/2020 (late FEV1 decline rate). Individuals were classified as having accelerated‐FEV1‐decline if they experienced >64 ml/year decline in FEV1. 2 , 3
2.5. Statistical analyses
Characteristics of the source population and the final study population were assessed as proportions and means (SD). We estimated intraclass correlations between early and late biomarker concentrations in participants who had had serum drawn during both time intervals (N = 113) using average measures from linear mixed‐effects models with random effects. We then performed multivariable linear regression analyses to examine associations of early and late serum biomarker measurements with overall and late rates of FEV1 decline, respectively, using the standard errors of the individual FEV1 decline rate measurements for weighted least squares regression adjustment. Analyses in the early and late serum biomarker groups were stratified by FEV1 decline status (accelerated‐FEV1‐decline and FEV1 decline <64 ml/year), measured using participants' overall and late FEV1 decline rates, respectively. A sensitivity analysis repeated these analyses using the data of participants in the late biomarker group who had not previously initiated ICS/LABA therapy (N = 228/299 [76%] in the accelerated‐FEV1‐decline and N = 478/649 [74%] in the FEV1 decline ≤64 ml/year subgroups).
Each biomarker was assessed separately, and all multivariable models controlled for age on 9/11/2001, race, smoking (ever vs. never), WTC exposure level, height, and FEV1% predicted at time of blood draw. Biomarker concentrations were log2‐transformed to improve skewness and kurtosis. All data analyses were performed using SAS version 9.4.
3. RESULTS
The study population consisted of 1686 firefighters with early serum biomarkers measured between 10/2001 and 3/2002 (N = 816) and/or late serum biomarkers measured between 12/2013 and 10/2015 (N = 983); 113 individuals had biomarkers measured at both times. The median first post‐9/11 blood draw date was 12/4/2001 (interquartile range: 11/14/2001 to 1/3/2002). The median second post‐9/11 blood draw date was 9/23/2014 (interquartile range: 3/27/2014 to 12/14/2014). Compared with the source population, the population with early serum biomarker measurements was similar in age, race distribution, WTC exposure profile, FEV1% predicted at first post‐9/11 monitoring (baseline) exam, and had similar FEV1 decline rate (ml/year) over longitudinal follow‐up. There were, however, a smaller proportion of ever‐smokers. The population with late serum biomarker measurements was similar to the source population in age, race distribution, smoking status, and WTC exposure profile, but had a lower baseline FEV1% predicted and greater overall FEV1 decline rate (Table 1).
Table 1.
Cohort characteristics
| Biomarkers 10/2001 to 3/2002 | Biomarkers 12/2013 to 10/2015 | Source population | |
|---|---|---|---|
| N = 816 | N = 983 | N = 9566 | |
| Age on 9/11 | 41.4 ± 7.5 | 42.0 ± 7.8 | 40.2 ± 7.4 |
| Race, n (%) | |||
| White | 780 (95.6) | 904 (92.0) | 9012 (94.2) |
| African American | 12 (1.5) | 39 (4.0) | 223 (2.3) |
| Hispanic | 23 (2.8) | 36 (3.7) | 304 (3.2) |
| Other | 1 (0.1) | 4 (0.4) | 27 (0.3) |
| Smoking status, n (%) | |||
| Never | 680 (83.3) | 615 (62.6) | 6406 (67.0) |
| Ever | 136 (16.7) | 368 (37.4) | 3160 (33.0) |
| World Trade Center | arrival time, n (%) | ||
| Morning of 9/11 | 130 (15.9) | 174 (17.7) | 1560 (16.3) |
| Afternoon of 9/11 to 9/12 | 626 (76.7) | 697 (70.9) | 6849 (71.6) |
| 9/13/2001 or later | 60 (7.4) | 112 (11.4) | 1157 (12.1) |
| FEV1% predicteda | 98.6 ± 14.5 | 97.2 ± 14.6 | 97.0 ± 13.7 |
| FEV1 decline ml/yearb | 38.4 ± 26.6 | 36.1 ± 23.4 | 37.3 ± 28.9 |
First post‐9/11 measurement.
Between 9/11/2001 and 9/10/2020.
We examined the longitudinal constancy of serum biomarker concentrations by estimating the intraclass correlations between early and late biomarkers in the 113 individuals with measurements at both times. The early and late measurements of the Th‐2 biomarkers IL‐5, IL‐13 and IL‐4 were correlated (IL‐5 intraclass correlation coefficient [ICC]: 0.527, p < 0.001; IL‐13 ICC: 0.568, p < 0.001; and IL‐4 ICC: 0.468, p < 0.001; Table 2). There was also correlation between early and late levels of IFN‐γ, a Th‐1 cytokine (ICC: 0.802, p < 0.001). A different pattern emerged for TNF‐α, a cytokine that mediates acute inflammation. There was no significant correlation between early and late TNF‐α concentrations (ICC: 0.209; p = 0.104).
Table 2.
Intraclass correlations between early and late serum biomarkers in individuals with measurement at both time pointsa
| Serum cytokine | ICCb | 95% Confidence interval | p value |
|---|---|---|---|
| IL‐4 | 0.468 | 0.226, 0.629 | <0.001 |
| IL‐5 | 0.527 | 0.318, 0.672 | <0.001 |
| IL‐13 | 0.568 | 0.375, 0.701 | <0.001 |
| IFN‐γ | 0.802 | 0.705, 0.867 | <0.001 |
| TNF‐α | 0.209 | −0.140, 0.451 | 0.104 |
Abbreviation: ICC, intraclass correlation coefficient.
N = 113.
Two‐way random effects model average measure.
We then estimated the associations between early serum biomarker concentrations and overall FEV1 decline rates in 117 individuals in the early measurement group with accelerated‐FEV1‐decline (>64 ml/year decline in FEV1 from 9/11/2001 to 9/10/2020). IL‐5, IL‐13, and IL‐4 concentrations were significantly associated with greater post‐9/11 FEV1 decline (−7.9 ± 1.3 ml/year per doubling of IL‐5, p < 0.001; −8.4 ± 1.2 ml/year per doubling of IL‐13, p < 0.001; and −2.9 ± 1.4 ml/year per doubling of IL‐4, p = 0.04) controlling for age, race, WTC exposure level, smoking status, height, and first post‐9/11 FEV1 percent predicted (Table 3). IFN‐γ and TNF‐α, on the contrary, were both significantly associated with less post‐9/11 FEV1 decline (13.9 ± 2.3 ml/year [i.e., reduced FEV1 decline] per doubling of IFN‐γ, p < 0.001 and 16.7 ± 4.1 ml/year per doubling of TNF‐α, p < 0.001) in this subgroup. In the subgroup of 699 firefighters with FEV1 decline ≤64 ml/year between 9/11/2001 and 9/10/2020, only IL‐5 was associated with greater FEV1 decline (−1.3 ± 0.5 ml/year per doubling of IL‐5, p = 0.008; Table 4).
Table 3.
Multivariable linear regression models examining associations between early serum biomarker concentrations and subsequent FEV1 decline (ml/year) in those with accelerated‐FEV1‐declinea
| Unstandardized coefficientsb | Standardized coefficientsb | |||
|---|---|---|---|---|
| β | SE | β | p value | |
| Doubling IL‐4c | −2.88 | 1.38 | −0.15 | 0.039 |
| Doubling IL‐5c | −7.94 | 1.32 | −0.38 | <0.001 |
| Doubling IL‐13c | −8.42 | 1.17 | −0.45 | <0.001 |
| Doubling IFN‐γc | 13.9 | 2.3 | 0.45 | <0.001 |
| Doubling TNF‐αc | 16.7 | 4.1 | 0.26 | <0.001 |
Abbreviation: WTC, World Trade Center.
N = 117.
Controlling for age, smoking status, race, WTC exposure level, height, and FEV1% predicted at time of biomarker measurement in weighted least squares regression using 1/SE of individual FEV1 decline rates.
The number of biomarker concentration doubling from the 10th to 90th percentile: 3.5 for IL‐4, 2.8 for IL‐5, 3.2 for IL‐13, 1.8 for IFN‐γ, and 2.0 for TNF‐α.
Table 4.
Multivariable linear regression models examining associations between early serum biomarker concentrations and subsequent FEV1 decline (ml/year) in those with ≤64 ml/year FEV1‐declinea
| Unstandardized coefficientsb | Standardized coefficientsb | |||
|---|---|---|---|---|
| β | SE | β | p value | |
| Doubling IL‐4c | 0.04 | 0.28 | 0.006 | 0.877 |
| Doubling IL‐5c | −1.34 | 0.50 | −0.10 | 0.008 |
| Doubling IL‐13c | 0.17 | 0.43 | 0.014 | 0.690 |
| Doubling IFN‐γc | −0.53 | 0.66 | −0.03 | 0.422 |
| Doubling TNF‐αc | −0.09 | 1.03 | −0.003 | 0.932 |
Abbreviation: WTC, World Trade Center.
N = 699.
Controlling for age, smoking status, race, WTC exposure level, height, and FEV1% predicted at time of biomarker measurement in weighted least squares regression using 1/SE of individual FEV1 decline rates.
The number of biomarker concentration doubling from the 10th to 90th percentile: 3.5 for IL‐4, 2.8 for IL‐5, 3.2 for IL‐13, 1.8 for IFN‐γ, and 2.0 for TNF‐α.
Given the correlation between early and late Th‐2 biomarker concentrations, we also assessed the associations between late biomarker concentrations and late FEV1 decline rate. In 274 participants who experienced accelerated‐FEV1‐decline after the date of their late serum biomarker measurement (defined as having >64 ml/year decline in FEV1 from date of late measurement to 9/10/2020), only IL‐4 was associated with subsequent FEV1 decline (−4.0 ± 1.6 ml/year per doubling of IL‐4, p = 0.01; Table 5). There was no association between late IL‐5, IL‐13, IFN‐γ, or TNF‐α concentration and late FEV1 decline rate in these individuals. In the 611 participants with late FEV1 decline ≤64 ml/year, we did not observe associations between late IL‐5, IL‐13, IL‐4, IFN‐γ, or TNF‐α concentrations and late FEV1 decline rate (Table 6).
Table 5.
Multivariable linear regression models examining associations between late serum biomarker concentrations and subsequent FEV1 decline (ml/year) in those with accelerated‐FEV1‐declinea
| Unstandardized coefficientsb | Standardized coefficientsb | |||
|---|---|---|---|---|
| β | SE | β | p value | |
| Doubling IL‐4c | −4.00 | 1.58 | −0.16 | 0.01 |
| Doubling IL‐5c | 0.02 | 1.02 | 0.001 | 0.98 |
| Doubling IL‐13c | 0.41 | 0.83 | 0.03 | 0.62 |
| Doubling IFN‐γc | −2.33 | 1.78 | −0.08 | 0.191 |
| Doubling TNF‐αc | −2.25 | 2.13 | −0.07 | 0.291 |
Abbreviation: WTC, World Trade Center.
N = 274 due to missing covariates.
Controlling for age, smoking status, race, WTC exposure level, height, and FEV1% predicted at time of biomarker measurement in weighted least squares regression using 1/SE of individual FEV1 decline rates.
The number of biomarker concentration doubling from the 10th to 90th percentile: 2.5 for IL‐4, 4.5 for IL‐5, 6 for IL‐13, 2.5 for IFN‐γ, and 1.8 for TNF‐α.
Table 6.
Multivariable linear regression models examining associations between late serum biomarker concentrations and subsequent FEV1 decline (ml/year) in those with ≤64 ml/year FEV1 declinea
| Unstandardized coefficientsb | Standardized coefficientsb | |||
|---|---|---|---|---|
| β | SE | β | p value | |
| Doubling IL‐4c | −0.27 | 1.08 | −0.01 | 0.807 |
| Doubling IL‐5c | −0.38 | 0.65 | −0.02 | 0.559 |
| Doubling IL‐13c | −0.25 | 0.56 | −0.02 | 0.660 |
| Doubling IFN‐γc | −0.67 | 1.03 | −0.03 | 0.515 |
| Doubling TNF‐αc | −0.61 | 1.77 | −0.01 | 0.729 |
Abbreviation: WTC, World Trade Center.
N = 611 due to missing covariates.
Controlling for age, smoking status, race, WTC exposure level, height, and FEV1% predicted at time of biomarker measurement in weighted least squares regression using 1/SE of individual FEV1 decline rates.
The number of biomarker concentration doubling from the 10th to 90th percentile: 2.5 for IL‐4, 4.5 for IL‐5, 6 for IL‐13, 2.5 for IFN‐γ, and 1.8 for TNF‐α.
Sensitivity analyses conducted in the subset of the late biomarker measurement population who had not received ICS/LABA treatment yielded similar results to those shown above; higher IL‐4 concentration was associated with a larger FEV1 decline (−3.3 ± 1.5 ml/year per doubling of IL‐4, p = 0.03), only in participants with accelerated‐FEV1‐decline (N = 228).
4. DISCUSSION
A subset of WTC‐exposed rescue/recovery workers have accelerated‐FEV1‐decline, a strong risk factor for asthma, COPD and asthma/COPD overlap syndrome. 2 , 5 Unless effective therapy can improve lung function trajectories in the accelerated‐FEV1‐decline subgroup, many will progress to COPD‐related impairment. 2 Accelerated‐FEV1‐decline has also been associated with increased mortality in non‐WTC‐exposed individuals with smoking‐related COPD. 6 Cigarette smoking, heterozygosity for alpha‐1‐antitrypsin deficiency, and elevated blood eosinophil concentrations are all risk factors for accelerated‐FEV1‐decline in WTC‐exposed firefighters. 2 , 3 , 15 Prolonged longitudinal follow‐up is needed to identify biomarkers and define phenotypes to improve therapy for individuals with accelerated‐FEV1‐decline who are not responding to standard treatment. In this investigation, we found that greater concentrations of the serum Th‐2 biomarkers IL‐4, IL‐13, and IL‐5 measured soon after WTC exposure were risk factors for greater annual FEV1 decline in the subgroup with post‐9/11 accelerated‐FEV1‐decline (9/11/2001 to 9/10/2020), while greater concentration of IFN‐γ, a Th‐1 cytokine, was associated with a slower rate of FEV1 decline. Surprisingly, TNF‐α, a marker of acute inflammation, 16 was also protective against FEV1 decline in this subgroup. Finally, we found that IL‐4 concentration measured from serum drawn 12–13 years after WTC exposure was also associated with greater FEV1 decline rate in those who experienced accelerated‐FEV1‐decline between the later blood draw date and 9/10/2020.
Individuals' early and late serum IL‐4, IL‐13, IL‐5, and IFN‐γ concentrations, measured during two time periods over 10 years apart, were correlated; however, early and late measurements of TNF‐α, a cytokine marking acute inflammation, 16 were not significantly correlated. The relative stability of Th‐1 and Th‐2 biomarkers is consistent with individuals having characteristic set points for T‐cell‐mediated innate or adaptive inflammation that persists over longitudinal follow‐up. Similarly, in WTC‐exposed firefighters with non‐resolving chronic rhinosinusitis, blood eosinophil levels were consistently elevated throughout longitudinal follow‐up. 17 In our cohort and in other WTC‐exposed individuals, elevated eosinophil levels were associated with FEV1 decline, airflow obstruction, and wheezing. 2 , 4 The association between persistently elevated Th‐2 biomarkers and greater FEV1 decline in those with accelerated‐FEV1‐decline suggests an intrinsic predisposition to Th‐2 inflammation, which, in the context of an acute irritant injury, produces non‐resolving inflammation and end organ damage. 18
Members of the accelerated decline subgroup likely suffer from irritant‐induced occupational asthma, caused by injury to the airway epithelium from WTC dust and products of combustion. 5 , 19 The Th‐2 innate response is induced by injured epithelium releasing alarmins such as IL‐33 or thymic stromal lymphopoietin (TSLP), which lead to Th‐2 cytokine production by Group 2 innate lymphoid cells (ILC2s) in the lung. 20 , 21 , 22 Conversely, IFN‐γ inhibits ILC2 function, 23 and TNF‐α induces the RelB, an inhibitor of ILC2. 24 , 25 The associations of multiple Th‐2 cytokines measured soon after WTC exposure with FEV1 decline rate likely represent an acute inflammatory response to dust and products of combustion at the WTC site. The persistence of the association of IL‐4 with subsequent FEV1 decline, even when measured 12–13 years after WTC exposure, is consistent with IL‐4 mediating chronic post‐injury inflammation. Prolonged alteration of IL‐4 concentration in serum could be a result of “trained immunity,” a form of innate immune memory produced by covalent histone modification in lung ILC2 cells in patients with severe asthma. 26 Notably, ILC2 cells are steroid‐resistant, which may partly explain why inhaled steroids fail to control respiratory symptoms in many WTC‐exposed firefighters. 11 , 27 , 28 , 29 Future research should assess associations of accelerated‐FEV1‐decline with upstream mediators of chronic airway inflammation that modulate ILC2 activity such as TSLP and IL‐33.
There are several limitations to this study. Serum biomarkers were available for only a subset of the WTC‐exposed firefighter cohort, raising the potential of selection bias; however, the study populations are similar to the source population, and samples were drawn during routine monitoring exams, thereby reducing this potential bias. Atopy is likely underrepresented in this population, as asthma at time of pre‐employment medical evaluation precludes work as a FDNY firefighter, but unmeasured confounding could still be present and would particularly be of concern in the late biomarker measurement analyses. By the time of the late blood draw, some WTC‐exposed firefighters had received ICS/LABA therapy that could have altered serum cytokine levels. A sensitivity analysis excluding individuals who initiated ICS/LABA treatment before time of late blood draw, however, did not alter the association between IL‐4 and subsequent FEV1 decline. Lastly, this investigation did not examine other potential risk factors such as heterozygosity for alpha‐1‐antitrypsin deficiency. 15 Despite these limitations, the FDNY WTC Health Program is a valuable resource for understanding irritant‐induced conditions such as accelerated‐FEV1‐decline in an occupational setting where there is otherwise little available data. Even with the unique aspects of the FDNY WTC‐exposed cohort, findings in this population have been replicated in other WTC‐exposed cohorts. 30 , 31 , 32 , 33 , 34
A strength of our study was that individual rates of FEV1 decline were calculated using spirometric measurements taken after participants' blood draw dates, reducing the potential for “reverse causation.” While there is no evidence that greater FEV1 decline rate is associated with atopy commonly found in allergic asthma, allergen‐induced adaptive immunity cannot be excluded.
In summary, our longitudinal biomarker data suggest that accelerated‐FEV1‐decline patients are biologically different in one or more Th‐2 pathways. Accelerated‐FEV1‐decline patients are likely predisposed to exaggerated inflammation and/or poor counter‐regulatory responses to inflammation. Targeting Th‐2 inflammatory pathway(s) for intervention may yield more effective therapies. Studies with IL‐4/IL‐13 blocking drugs such as dupilumab are needed to assess whether targeting elevated IL‐4 improves FEV1 trajectories in those with accelerated‐FEV1‐decline. 12 , 13
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
DISCLOSURE BY AJIM EDITOR OF RECORD
Steven Markowitz declares that he has no conflict of interest in the review and publication decision regarding this article.
AUTHOR CONTRIBUTIONS
Conception or design of the work: Michael D. Weiden, Rachel Zeig‐Owens, David G. Goldfarb, and Barbara Putman. Acquisition, analysis, and interpretation of data: Michael D. Weiden, David G. Goldfarb, Ankura Singh, Barbara Putman, Rachel Zeig‐Owens, Theresa Schwartz, C. B. H., David J. Prezant. Drafting the work or revising it critically for important intellectual content: Michael D. Weiden, Ankura Singh, Rachel Zeig‐Owens, Hillel W. Cohen, David G. Goldfarb, David J. Prezant. Final approval of the version to be published and agreement to be accountable for all aspects of the work: Michael D. Weiden.
ETHICS APPROVAL AND INFORMED CONSENT
The Albert Einstein College of Medicine Institutional Review Board approved this study (IRB #2019‐10309). All participants provided written informed consent.
ACKNOWLEDGMENT
This study was funded by the National Institute for Occupational Safety and Health (Cooperative agreement number: U01 OH011682).
Weiden MD, Singh A, Goldfarb DG, et al. Serum Th‐2 cytokines and FEV1 decline in WTC‐exposed firefighters: a 19‐year longitudinal study. Am J Ind Med. 2021;64:845‐852. 10.1002/ajim.23276
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Aldrich TK, Gustave J, Hall CB, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeig‐Owens R, Singh A, Aldrich TK, et al. Blood leukocyte concentrations, FEV1 decline, and airflow limitation. A 15‐year longitudinal study of World Trade Center‐exposed firefighters. Ann Am Thorac Soc. 2018;15(2):173‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldrich TK, Vossbrinck M, Zeig‐Owens R, et al. Lung function trajectories in World Trade Center‐exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kazeros A, Maa MT, Patrawalla P, et al. Elevated peripheral eosinophils are associated with new‐onset and persistent wheeze and airflow obstruction in world trade center‐exposed individuals. J Asthma. 2013;50(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh A, Liu C, Putman B, et al. Predictors of asthma/COPD overlap in FDNY firefighters with World Trade Center dust exposure: a longitudinal study. Chest. 2018;154(6):1301‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Lung function trajectories leading to chronic obstructive pulmonary disease as predictors of exacerbations and mortality. Am J Respir Crit Care Med. 2020;202(2):210‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bateman ED, Boushey HA, Bousquet J, et al. Can guideline‐defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med. 2004;170(8):836‐844. [DOI] [PubMed] [Google Scholar]
- 8. Pauwels RA, Löfdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337(20):1405‐1411. [DOI] [PubMed] [Google Scholar]
- 9. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention (2017 Update). Vancouver, USA: Global Initiative for Asthma; 2017. [Google Scholar]
- 10. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557‐582. [DOI] [PubMed] [Google Scholar]
- 11. Putman B, Lahousse L, Singh A, et al. Dyspnea and inhaled corticosteroid and long‐acting β‐agonist therapy in an occupational cohort: a longitudinal study. Ann Am Thorac Soc. 2020;17(6):770‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 13. Corren J, Castro M, O'Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate‐to‐severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516‐526. [DOI] [PubMed] [Google Scholar]
- 14. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet (London, England). 2016;388(10039):31‐44. [DOI] [PubMed] [Google Scholar]
- 15. Banauch GI, Brantly M, Izbicki G, et al. Accelerated spirometric decline in New York City firefighters with α1‐antitrypsin deficiency. Chest. 2010;138(5):1116‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwon S, Putman B, Weakley J, et al. Blood eosinophils and World Trade Center exposure predict surgery in chronic rhinosinusitis. A 13.5‐year longitudinal study. Ann Am Thorac Soc. 2016;13(8):1253‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeig‐Owens R, Nolan A, Putman B, Singh A, Prezant DJ, Weiden MD. Biomarkers of patient intrinsic risk for upper and lower airway injury after exposure to the World Trade Center atrocity. Am J Ind Med. 2016;59(9):788‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarlo SM, Lemiere C. Occupational asthma. N Engl J Med. 2014;370(7):640‐649. [DOI] [PubMed] [Google Scholar]
- 20. Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle‐treated human bronchial epithelial cells upregulate Jagged‐1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol (Baltimore, Md: 1950). 2010;185(11):6636‐6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Wang W, Lv Z, et al. Elevated expression of IL‐33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol (Baltimore, Md: 1950). 2018;200(7):2253‐2262. [DOI] [PubMed] [Google Scholar]
- 22. Weng CM, Wang CH, Lee MJ, et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium‐derived cytokines expression in severe allergic asthma. Allergy. 2018;73(11):2192‐2204. [DOI] [PubMed] [Google Scholar]
- 23. Moro K, Kabata H, Tanabe M, et al. Interferon and IL‐27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nature Immunol. 2016;17(1):76‐86. [DOI] [PubMed] [Google Scholar]
- 24. Wang J, Ferreira R, Lu W, et al. TNFR2 ligation in human T regulatory cells enhances IL2‐induced cell proliferation through the non‐canonical NF‐κB pathway. Sci Rep. 2018;8(1):12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L, Ying Y, Chen S, et al. The transcription factor RelB restrains group 2 innate lymphoid cells and type 2 immune pathology in vivo. Cell Mol Immunol. 2021;18(1):230‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stadhouders R, Li BWS, de Bruijn MJW, et al. Epigenome analysis links gene regulatory elements in group 2 innate lymphocytes to asthma susceptibility. J Allergy Clin Immunol. 2018;142(6):1793‐1807. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van der Ploeg EK, Golebski K, van Nimwegen M, et al. Steroid‐resistant human inflammatory ILC2s are marked by CD45RO and elevated in type 2 respiratory diseases. Sci Immunol. 2021;6:eabd3489. [DOI] [PubMed] [Google Scholar]
- 29. Wang L, Netto KG, Zhou L, et al. Single‐cell transcriptomic analysis reveals the immune landscape of lung in steroid‐resistant asthma exacerbation. Proc Natl Acad Sci U S A. 2021;118(2):e2005590118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brite J, Friedman S, de la Hoz RE, Reibman J, Cone J. Mental health, long‐term medication adherence, and the control of asthma symptoms among persons exposed to the WTC 9/11 disaster. J Asthma. 2020;57(11):1253‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman SM, Farfel MR, Maslow C, et al. Risk factors for and consequences of persistent lower respiratory symptoms among World Trade Center health registrants 10 years after the disaster. Occup Environ Med. 2016;73(10):676‐684. [DOI] [PubMed] [Google Scholar]
- 32. Kim H, Herbert R, Landrigan P, et al. Increased rates of asthma among World Trade Center disaster responders. Am J Ind Med. 2012;55(1):44‐53. [DOI] [PubMed] [Google Scholar]
- 33. Reibman J, Caplan‐Shaw C, Wu Y, et al. Characterization of persistent uncontrolled asthma symptoms in community members exposed to World Trade Center dust and fumes. Int J Environ Res Public Health. 2020;17(18):6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wisnivesky JP, Teitelbaum SL, Todd AC, et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: a cohort study. Lancet (London, England). 2011;378(9794):888‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


