Abstract
A new cost‐effective NHS functionalized polyoxazoline (POx) loaded polymer with strong hemostatic properties has been developed. In this study, we investigate POx loaded hemostatic patches regarding hemostatic efficacy, local inflammatory reaction and wound‐healing, as compared to the non‐POx treated blanks and commercially available hemostatic products. Hundred and ten rats divided into 11 groups of 10 animals underwent partial liver lobe resection. Eight groups received experimental patches, two groups commercially available hemostatic patches (TachoSil® and Veriset™, positive controls), one group with gauzes (negative control). Each animal received twice a patch with a size 1.5 × 2.5 cm, on each partially resected lobe. Primary endpoint was time to hemostasis (TTH). The rats were sacrificed at different time points (1, 3, or 7 days) to measure local inflammatory response and early wound healing. Of the POx loaded patches, GFC NHS‐POx (TTH 20.4 s, p = .019) and GFC‐NHS‐POx1.5 (TTH 0.0 s, p = .003) showed significantly faster TTH compared to TachoSil® (TTH 95.4 s), and were comparable to Veriset™ (TTH 17.0 s). Three patches, GFC‐NHS‐POx 1.5 (TTH 0.0 s, p = .016), ORC NHS‐POx:NU‐POx (TTH 91.4 s, p = .033), and ORC‐PLGA NHS‐POx:NU‐POx (TTH 105.6 s, p = .04) had a lower TTH compared to their own blank carrier (TTH 74.9, 157.8, and 195.7 s, respectively). With regard to biocompatibility, all POx loaded patches showed results comparable to TachoSil® and Veriset™. NHS‐POx‐loaded hemostatic patch demonstrate fast and effective hemostasis, comparable or better than commercially available hemostatic patches, with similar early biocompatibility.
Keywords: biocompatibility, hemostatic patch, histology, liver surgery, polyoxazoline derivate
1. INTRODUCTION
Major surgical procedures and traumatic organ injury can cause bleeding of organs and tissues, which are difficult to stop permanently with stitches, clamps, electrical coagulation, or external pressure. These bleedings and the increased blood loss can result in longer operation time, prolonged hospital admission and cause serious complications such as hypovolemic shock, multi‐organ failure, and death. 1 , 2 , 3 When gauze tamponing, electrosurgery and vessel sealing do not offer a definite solution for bleedings in the abdominal and chest cavities, hemostatic sealants are used in the form of glues, sprays, powders, or patches. 4 , 5 , 6 , 7
To date, marketed products are mainly based on gelatin (gelfoam) or collagen (such as TUFT‐IT and Hemotese) 8 , 9 and oxidized regenerated cellulose (ORC) 10 , 11 with weak to moderate efficacy. Other products like TachoSil® use collagen in combination with human‐derived fibrinogen and thrombin which actively enhance the coagulation cascade. A third group of products use a N‐hydroxysuccinimide (NHS)‐activated polymer for coagulation cascade independent hemostasis (e.g., Veriset™). 4 , 12 These products are more effective but also expensive. Therefore, there is a need for a hemostatic patch with better cost‐effectiveness as compared to existing products and without the need of human and animal derived compounds.
We recently reported on the development of a new polymer that can be used as the active component of a patch to achieve hemostasis. 13 This polymer consists of a polyoxazoline (POx) base to which multiple NHS groups are linked, abbreviated NHS‐POx. These NHS groups bind to the NH2 groups of proteins in human tissues, including blood and proteins on organ surfaces, causing immediate clotting. With this polymer, a wide variety of possible formulations can be made including powders, glues, sprays, and patches. For this preclinical study, we developed new prototypes of hemostatic patches by adding POx polymers to a number of carriers that are (components of) products in clinical use for bleeding prevention or other applications. These new prototypes theoretically outperform 13 current products in clinical use for bleeding prevention with regards to time to hemostasis, reduction of blood loss and blood loss‐related complications. POx‐loaded hemostatic patches have three key features; enhanced adhesion to organ surfaces so that the product stays in place and may provide effective local tamponade, faster bleeding cessation by enhancing “passive” clotting with covalent gel formation upon contact with blood, and low‐cost as a result of a cost‐effective production process.
In this preclinical study, we investigate different prototypes of polyoxazoline loaded hemostatic patches for hemostatic efficacy in terms of time to hemostasis, early local inflammatory reaction and first signs of wound healing, in a rat partial liver resection model and compare the outcomes with the non‐POx treated blanks and commercially available and commonly used hemostatic patches in surgery of parenchymatous organs.
2. METHODS
2.1. Animals
Hundred and ten male Wistar (WU) rats weighing between 270 and 300 g (Charles Rivers, 's Hertogenbosch, Netherlands) were used. Rats were accommodated two per cage (eurostandard type III H), with water and standard food (containing 33%, 9%, and 58% of calories from proteins, fats, and carbohydrates, respectively) ad libitum (Ssniff R/M‐H, Bio Services BV, Uden). A standard 12 h light/dark cycle was maintained. All rats had a minimum 5‐day acclimatization period before surgery. The cages were enriched with a rat retreat and nesting material and were placed randomly on the shelves. All animals were checked at least twice daily. Humane endpoints were defined; animals were killed if they showed signs of severe discomfort (such as weight loss >20% compared to starting weight). The experiment was approved by the Dutch Animal Ethics Committee (CCD, number 2015‐0012) and performed in the Central Animal Laboratory of Radboud University. Experiments were performed according to Arrive guidelines. 14
2.2. NHS‐POx prototype production
Five different NHS‐POx‐loaded prototypes were developed with three different carriers. A Gelatin Fibrous Carrier (GFC, Gelita TUFT‐IT, origin: porcine, 5 × 7.5 cm, Gelita Medical, Germany), a Oxidized Regenerated Cellulose carrier (ORC, Ethicon, New Jersey, 7.5 × 5 cm) and a ORC‐poly(lactic‐co‐glycolic acid)carrier (PLGA, Ethicon, New Jersey, 7.5 × 5 cm). All three carriers were loaded with a powder containing NHS‐POx and nucleophilically activated polyoxazoline (NU‐POx), creating GFC NHS‐POx:NU‐POx, ORC NHS‐POx:NU‐POx and ORC PLGA NHS‐POx:NU‐POx. Furthermore, two other prototypes were made with the GFC, one loaded with NHS‐POx only (creating GFC NHS‐POx), and one with a powder containing gelatin and NHS‐POx (creating GFC NHS‐POx 1.5). All carriers were loaded with the combination EL‐POx NU‐POx to enhance internal cross‐linking to promote hemostasis, GFC already has amine groups due to the nature of gelatin, therefore also prototypes without NU‐POx were made. In addition, one prototype was made with additional gelatin to increase its bulk density.
GFC, ORC, and ORC‐PLGA carriers were also used as such (GFC‐blank, ORC‐blank and ORC‐PLGA‐blank) We chose the ORC or ORC‐PLGA carrier in order to have a fully synthetic hemostatic patch when combined with the POx polymers, the gelatin carriers because of good previous preliminary results. 13
Polymer description: All hemostatic patches were fabricated using NHS‐ester functionalized POx polymer P(EtOx‐c‐OH‐c‐NHS) 60‐20‐20, referred to as NHS‐POx. Where ORC or ORC‐PLGA carriers were tested also P(EtOx‐NH2) 80‐20, referred to as NU‐POx, was added on a 1:1 M ratio towards NHS‐POx. Gelatin carriers were combined with NHS‐POx or NHS‐POx:NU‐POx in a 1:0.5 M ratio.
Loading process and ratios: ORC‐PLGA samples were loaded via liquid deposition, dichloromethane (Sigma‐Aldrich) was used as solvent and 0.1 g/ml polymer solutions were applied starting with the loading of NU‐POx solution and after an overnight drying cycle at 40°C in the vacuum oven the NHS‐POx solution was loaded.
GFC and ORC carriers lost their structural integrity, when liquid deposition was used, therefore we used the Fibroline SL‐Preg LABORATORY MACHINE (Fibroline SA, Limonest, France) for the dry powder impregnation of the polymers. The following settings were used: 40 kV, 100 Hz, 20 s. Polymer powders were dosed gravimetric in a 3D PMMA array to ensure homogeneous distribution.
All samples were dried after loading at 40°C overnight in the vacuum oven. Samples were sealed under vacuum in Alu‐Alu pouches (LamiZip, Daklapack Europe, the Netherlands). A total of 0.1 g powder was loaded in each sample, except in sample GFC NHS‐POx 1.5 where a total of 0.3 g of a powder consisting of gelatin powder (Gelita Spon Powder, Gelita Medical) and NHS‐POx in a 1:1 ratio in weight were loaded manually in between the layers of the gelatin construct in a dry manner. Samples were sterilized using e‐beam in in a range from 25 to 35 kGy (Steris, Germany).
2.3. Experimental groups
The animals were divided into 11 groups of 10 animals: eight groups in which experimental patches were tested, two groups treated with commercially available hemostatic patches (TachoSil® (Takeda Austria GmbH, Linz, Austria) and Veriset™ (Covidien Inc., Mansfield, MA), and one group where hemostasis was achieved by applying pressure with gauzes.
The samples tested in this study are shown in Table 1. Each animal received the same patch twice during surgery at the wound area after partial liver resection. The rats were sacrificed at different time points (1, 3, or 7 days). A computer program randomized the allocation of the samples in rats.
TABLE 1.
Groups and baseline characteristics
| Hemostatic patch | TachoSil® | Veriset™ | GFC blank | GFC NHS‐POx | GFC NHS‐POx:NU‐POx | GFC NHS‐POx 1.5 | ORC blank | ORC NHS‐POx:NU‐POx | ORC‐PLGA blank | ORC‐PLGA NHS‐POx:NU‐POx | Gauzes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carrier | Gelatin fibrous carrier | ORC | ORC‐PLGA | ||||||||
| NHS‐POx (g) | 0 | 0.1 | 0.1 | 0.15 | 0 | 0.1 | 0 | 0.1 | |||
| NU‐POx (g) | 0 | 0 | 0.06 | 0 | 0 | 0.12 | 0 | 0.12 | |||
| Deposition | Dry | Dry | Dry | Dry | Liquid | ||||||
| NHS‐POx:NU‐POx molar ratio | 1:0 | 1:0.5 | 1:0 | 1:1 | 1:1 | ||||||
| Number of animals at start | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Weight, mean (SD) | 310.9 (12.3) | 315.8 (24.3) | 299.4 (13.5) | 302.2 (7.8) | 301.3 (14.4) | 319.9 (28.5) | 297.2 (12.2) | 300.1 (19.7) | 307.2 (26.4) | 311.7 (22.5) | 312.4 (18.1) |
| Gender | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male |
| Weight resected liver lobe 1 | 175.3 (39.1) | 194.5 (68.5) | 175.4 (32.6) | 167.8 (31.1) | 158.8 (28.2) | 175.9 (26.5) | 147.4 (21.8) | 168.8 (60.0) | 151.4 (39.0) | 156.3 (27.0) | 178.8 (39.8) |
| Weight resected liver lobe 2 | 158.5 (74.7) | 143.9 (31.7) | 149.7 (44.9) | 127.1 (19.3) | 130.9 (42.2) | 129.3 (22.2) | 119.9 (21.4) | 168.5 (142.0) | 121.5 (43.8) | 126.9 (20.4) | |
| Mortality | 1 a | 1 a | 1 a | 5 b | |||||||
Abbreviations: GFC, Gelatin fibrous carrier; ORC, oxidized regenerated Cellulose; PLGA: poly(lactic‐co‐glycolic acid).
Baseline: operative mortality due to anesthesia.
Operative mortality due to amount of blood loss.
2.4. Anesthesia and surgery
At least 15 min before the surgery, all rats were given 0.02 mg/kg buprenorphine subcutaneously as analgesia. Induction anesthesia was given by inhalation of 5% isoflurane in a 1:1 mix of pressurized air and oxygen in an induction chamber. The maintenance dose was 3.5% isoflurane (also in a 1:1 mix of pressurized air and oxygen) given through a snout mask. Rats were prepared by shaving, skin disinfection with iodine, and sterile covers. The rats were set on a 15° tilted table with their head higher than the tail. 15 Their body temperature was kept at 38°C with a heating pad and lamp. The rats were operated using strict aseptic techniques. The abdomen was opened by a 4–5 cm abdominal midline incision and the left lateral liver lobe was isolated. The lobe was placed on a piece of paper, and its distal section was excised using a scalpel, approximately 3 mm from the edge (see Figure 1). The resected part of the liver was put into a pre‐weighed tube and weighed afterwards. The bleeding was classified by two researchers. The researcher performing the surgery was blinded and was not aware which product was tested until after making the wound.
FIGURE 1.

(A) Liver lobe on paper, (B) partial liver resection, (C) liver placed on patch (and fold around), (D) digital pressure for 1 min, (E) Check TTH, and (F) closing with suture
A 15 mm by 25 mm patch was gently folded around the wound and held for 60 s using digital pressure with a wet gauze. In order to have a direct comparison, all patches including the positive control were applied for 60 s even this is not in line with the instruction for use of the commercially available products (TachoSil® 3 min, Veriset™ 30 s). For the control group with gauzes only, gauzes were used with pressure against the wound (see Figure 1). After 60 s of pressure, time to hemostasis, starting from the moment the gauze was removed, was recorded by two researchers and the average time observed was recorded. Afterwards, the right part of the median liver lobe was visualized, a partial resection was made, and the same procedure and measurements were performed as for the left lobe, and using the same patch type and size. The liver was then repositioned, and the abdominal wall was closed by using a 3–0 Vicryl plus (Ethicon, Somerville, NJ). The skin was closed by using staples. Anesthesia was discontinued and the rat was placed back into the cage to recover from surgery. During the study, the rats were weighed and their discomfort was checked daily. They were scored for dehydration, fur, activity, breathing, wound, abdomen, and cleanliness of the nose.
After 1, 3, or 7 days, the rats were reopened by midline incision under anesthesia. Their abdominal cavity was inspected. Adhesions of omental tissue to patch where scored according to Zühlke. 16 Omental adhesions were analysed because this is the main “organ” involved in adhesion formation with liver surgery. The rat was killed by cervical dislocation. Liver tissue with the patch and adhered surroundings was excised and fixated using a 4% formaldehyde solution for histological analysis.
2.5. Histology
Specimens for histological analysis were infiltrated and embedded in paraffin, sectioned (4 μm) using a microtome, pasted on superfrost‐plus slides, and left to dry overnight in a stove at 56°C. Three different stains were used: HE (Hematoxylin and eosin), MSB (Martius Scarlet Blue) SR (Sirius Red Staining). All slides were digitalized using a Panoramic p250 scanner. Slides were randomly mixed for sequence and then scored by two different researchers using a digital scoring form. Parameters scored were the thickness of the patch, necrosis, inflammatory cells, and wound healing. The thickness of the patch was digitally measured using Pannoramic viewer. Necrosis was scored by observing signs of necrosis (y/n), and by digitally measuring the perimeter and surface area of the necrotic tissue using Pannoramic viewer. Signs of necrosis were defined as pieces of the liver with a clearly different structure than the healthy tissue (no nucleus of liver cells visible, “empty liver”). Inflammatory cells in the patch, the wound area and the liver under the wound were scored using the adjusted Ehrlich and Hunt scale. 17 Wound healing was scored by a general wound healing scale according to Shafer 18 and the amount of fibroblast in the new tissue. The scores of the two researchers were compared and differences were solved by consensus.
2.6. Statistical analysis
Data of efficacy is presented as means ± SE. Weight of the rats and resected pieces is presented by means ± SD. Because we measured twice within one rat, we needed to take the dependency between the two different bleedings into account in the analyses. Therefore, we used Linear Mixed Models for comparing efficacy in terms of TTH between groups. All analyses were performed using SPSS (version 25, IBM corporation). Differences in the incidence of adhesion formation (score 0–4; means ± SD) between groups were analyzed by Fisher's exact test or χ2 test. Inflammatory and healing parameters in biopsies were scored as numeric value and one score was made of three different stains. Comparison between groups was done in a descriptive way.
3. RESULTS
The NHS‐POx and NU‐POx amounts used in the prototypes and the crosslinker percentage are shown in Table 1. All groups started with 10 rats, with an average weight of 307 (SD 19.7) grams. Weight distribution between the groups was comparable. The weight of the first resected liver piece was slightly higher compared to the second, 168.2 (SD 39.8) versus 137.6 (SD 56.6) mg. Weight as a proxy of uniformity of injury and bleeding was comparable between groups.
Three animals prematurely died during the surgical procedure, one each in the group TachoSil®, GFC blank and GFC NHS‐POx:NU‐POx. They stopped breathing during the operation and attempts to resuscitate failed. This may have had an anesthetic cause or was due to blood loss or a combination. Five rats in the group “gauze hemostasis” were taken out of the experiment during time to hemostasis measurement as a results of excessive blood loss. The bleedings in these rats restarted every time the gauzes were removed.
No rats reached a humane endpoint and no animals showed other than for this procedure expected symptoms.
3.1. Efficacy
The group treated with gauzes showed the highest average time to hemostasis (1302 s, Figure 2). The new prototypes GFC NHS‐POx (TTH = 20.4 s, p = 0.019) and GFC‐NHS‐POx1.5 (TTH = 0.0 s, p = 0.003) showed significant faster TTH as compared to TachoSil® (TTH = 95.4 s). The only product that stopped all bleedings immediately was GFC NHS‐POx 1.5 (TTH = 0.0 s). Veriset™ (TTH 17.0 s) showed also a significant faster TTH compared to TachoSil®. Performances of GFC blank (TTH = 74.9 s), GFC NHS‐POx:NU‐POx (TTH = 37.2 s), ORC NHS‐POx:NU‐POx (TTH = 91.4 s) and ORC PLGA NHS‐POx:NU‐POx (TTH = 105.6 s) were comparable to that of TachoSil® (TTH = 95.4 s). ORC blank (TTH =157.8 s) and ORC PLGA blank (TTH = 195.7 s) showed longer time to hemostasis (p = .045 and p = .002, respectively) compared to TachoSil® (TTH = 95.4 s).
FIGURE 2.
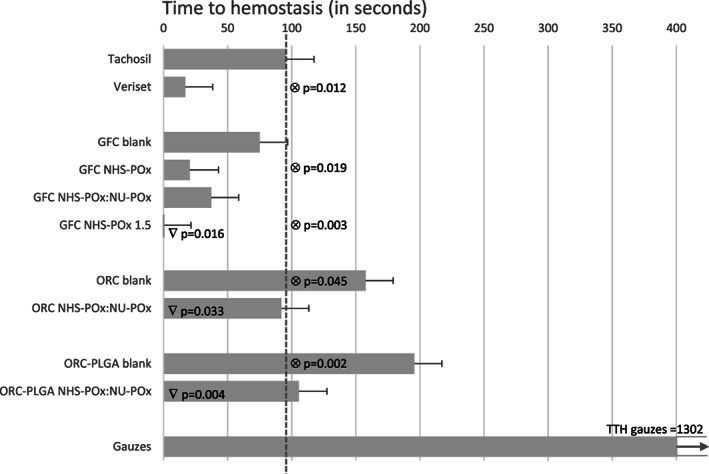
Time to hemostasis in seconds (bars represent average with standard error). ⊗ significantly different with TachoSil® (─ ─ ). ∇ significantly different with their own blank
When comparing the prototypes to their own blanks for determining the influence of the added polymer, GFC NHS‐POx 1.5, ORC NHS‐POx, and ORC PLGA performed better than their blanks.
3.2. Histology
The inflammatory cells were scored in the wound area at day 1, 3, and 7. Veriset™, GFC NHS‐POx 1.5, and ORC PLGA NHS‐POx:NU‐POx demonstrated more high scores 3 and 4 compared to Tachosil® at day 3. At day 7 Veriset™ and ORC PLGA NHS‐POx:NU‐POx remained highest. For the other groups no clear differences were found in amount of inflammatory cells and when compared to TachoSil®.
The number of fibroblasts were scored as a sign of wound healing. An increase of the amount of fibroblasts is observed over time. No differences were found between the prototype and control patches.
As assessed by scores derived by the three stains the parameters thickness of the patch, area of necrosis, inflammatory cells in patch, inflammatory cells in liver under the wound and general wound healing did not reveal clear differences between patches. Furthermore, we were not able to stain poly‐oxazoline. Average adhesion scores, (omentum to patch), were lowest in GFC NHS‐Pox (1.25, SD 1.3) and highest in GFC NHS‐PO 1.5 (2,20, SD 0.94), without differences between the prototypes and compared to commercial products.
4. DISCUSSION
The two NHS‐POx loaded patches with a gelatin backing have superior hemostatic efficacy compared to the commercially available TachoSil® and perform similar to Veriset™ in experimental liver resection. The GFC‐NHS‐POx1.5 patch was the only patch that immediately stopped the bleeding from the wound surface. Patches containing the NHS‐POx polymers showed faster hemostasis compared to their blank carriers, indicating added value of the polymer to the hemostatic patch.
In an earlier study, we showed that NHS‐POx polymers are excellent candidates for the development of hemostatic patches 13 which is confirmed by results of this preclinical study. A NHS functionalized Polyethylene glycol (PEG) is a polymer that is used in several different hemostatic devices for instance Veriset™. Veriset™ has proven faster hemostasis as compared to TachoSil®, which findings are echoed by this study. 4 , 19 POx theoretically has a better hemostatic potential compared to PEG, because POx has more NHS functionalized side groups for binding proteins. The GFC NHS‐POx 1.5 prototype indeed stopped the bleeding immediately in all instances whereas most Veriset™ patches showed continued but limited bleeding after relieving the 60 s of pressure. GFC NHS‐POx 1.5 contains more NHS‐groups and more gelatin then GFC NHS‐POx. The combination of the NHS‐POx with gelatin is possibly working so well because gelatin contains amines, which bind to NHS groups from NHS‐POx polymer and create an even stronger adhesion. Also hydrophilicity of the gelatin may enhance the initial tissue affinity of the patch in comparison to patches where more hydrophobic carriers are used.
The control group treated with gauzes took on average 22 min to achieve hemostasis with 50% animals having unstoppable bleeding after repeated attempts of gauze removal and gauze pressure. These findings indicate a challenging and clinically relevant model of parenchymatous bleeding for testing new hemostatic materials. Notably, no electrocautery, vessel sealing, sutures or clips were used in this model, which often are primary choices of hemostasis in liver surgery. Were other studies used punch biopsy for liver bleeding in rat, 15 , 20 we choose for a partial rat liver resection in order to have a more challenging and clinical relevant model like Schmiedt and colleagues did. 21
The welfare and weight recovery data and histology results of the polymer‐loaded prototypes correspond with those of the commercially available and clinically safe hemostatic patches TachoSil® and Veriset™. Tissue necrosis was generally limited and necrosis in the patch was mainly remnants of blood. Inflammation in the wound area seemed to decrease in time of most NHS‐POx loaded patches, but remained considerable in a few, of which Veriset™ was one, at 1 week. This finding, however, is not a drawback per se because it may indicate early breakdown of the bioresorbable patch. Wound healing as expressed by fibroblast density increases in time with at day 7 confluent cells and fibers in most patches, particularly the gelatin patches with the POx polymers and Veriset™. Food intake as a potential factor affecting inflammation and wound healing 22 , 23 is unlikely and cannot explain differences between groups because all groups had the same food. Furthermore, the food contained low fat percentage, which is the main trigger for an inflammatory response, and surgical trauma and implantation of an biomaterial probably elicited a higher inflammatory response compared to food. Due to the short duration of the experiment we cannot comment on the long term consequences of the scar formation at the liver surface and adhesions to other organs and tissues. In most animals adhesions were present at the patch area at sacrifice which, however, is a common finding in rat abdominal procedures also in the absence of foreign material 24 As expected, most common adhesion formation location was between omental tissue and liver patch and no differences in adhesion formation were found between groups.
We included safety endpoints in our study, for example, welfare, weight and inflammatory response on the POx polymers for identifying early adverse events and biocompatibility issues. The finding of comparable results for the POx loaded patches and the blank carrier patches suggests no additional tissue effect of the polymers. However, this should be interpreted with caution because late effects, after 7 days, were not part of this study and we did not power this study for secondary endpoints such as histology.
A rat has a comparable coagulation cascade to a human and parenchymal organ surfaces are similar regarding patch adherence. 25 , 26 Drawback is the small size of the liver demanding using only small patches. And although small pieces cut from full size patches were applied, we cannot rule out a disproportion which might have resulted in an overestimate of the hemostatic efficacy of experimental and control patches. Notably, cutting, placing and wrapping control patches for human use seemed not different from experimental patches when applying these patches with wet gauze. An advantage of the small liver is that the hemostatic patches needs to be flexible and pliable for application around the liver of a rat. Therefore, the model will distinguish between feasibility of patches in terms of flexibility and pliability. From a toxicological point of view, this disproportion is considered a worst case scenario. The implanted dose (~600 mg/kg) in these rats is much higher than the maximum dose recommended in marketed hemostatic patches (~16 mg/kg, corresponding to 10 patches/adult, each patch having a total surface of 50 cm2). Patches the size that are used in humans are currently studied in a pig liver resection model. This model also allows us to investigate hemostatic properties of the POx polymer patches under clinically relevant and challenging circumstances of hemostasis, for example, coagulation disorders, anti‐coagulant medication. 27
NHS‐POx‐loaded polymer patches, particularly combined with a gelatin carrier, demonstrate fast and effective hemostasis similar or better compared to clinically and commonly used commercial hemostatic products in a rat liver resection model.
CONFLICT OF INTEREST
There are no conflicts of interest to declare. R.P. Félix Lanao and E.A. Roozen are currently fulltime employed at GATT Technologies BV.
AUTHOR CONTRIBUTIONS
Edwin A. Roozen: study design, data collections, data analysis, writing. Michiel C. Warlé: data analysis, writing. Roger M.L.M. Lomme: study design, data collections, data analysis, writing. Rosa P. Félix Lanao: study design, data collections, writing. Harry Van Goor: study design, data analysis, writing.
ACKNOWLEDGMENTS
The author thanks N. Calon for her assistance with the histological processing of liver specimens.
Examples of histological pictures of HE staining of liver resections treated with GFC NHS‐POx prototypes after 1 day (Figure A1) and 7 days (Figure A2).
FIGURE A1.

(A) Normal liver, (B) patch, (C) wound area, (D) necrosis, (E) blood cloth, and (F) inflammatory cells
FIGURE A2.

(A) Normal liver, (B) patch, (C) wound area, and (D) new (granulation) tissue
Roozen, E. A. , Warlé, M. C. , Lomme, R. M. L. M. , Félix Lanao, R. P. , & van Goor, H. (2021). New polyoxazoline loaded patches for hemostasis in experimental liver resection. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 110(3), 597–605. 10.1002/jbm.b.34938
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Edwin Roozen, upon reasonable request.
REFERENCES
- 1. Hilal MA, Underwood T, Taylor MG, Hamdan K, Elberm H, Pearce NW. Bleeding and hemostasis in laparoscopic liver surgery. Surg Endosc. 2010;24(3):572‐577. [DOI] [PubMed] [Google Scholar]
- 2. Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(4):540‐548. [DOI] [PubMed] [Google Scholar]
- 3. Boonstra EA, Molenaar IQ, Porte RJ, de Boer MT. Topical haemostatic agents in liver surgery: do we need them? HPB. 2009;11(4):306‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Öllinger R, Mihaljevic AL, Schuhmacher C, et al. A multicentre, randomized clinical trial comparing the Veriset™ haemostatic patch with fibrin sealant for the management of bleeding during hepatic surgery. HPB. 2013;15(7):548‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna EM, Martinie JB, Swan RZ, Iannitti DA. Fibrin sealants and topical agents in hepatobiliary and pancreatic surgery: a critical appraisal. Langenbecks Arch Surg. 2014;399(7):825‐835. [DOI] [PubMed] [Google Scholar]
- 6. Emilia M, Luca S, Francesca B, et al. Topical hemostatic agents in surgical practice. Transfus Apher Sci. 2011;45(3):305‐311. [DOI] [PubMed] [Google Scholar]
- 7. Brustia R, Granger B, Scatton O. An update on topical haemostatic agents in liver surgery: systematic review and meta analysis. J Hepatobiliary Pancreat Sci. 2016;23(10):609‐621. [DOI] [PubMed] [Google Scholar]
- 8. Hajosch R, Suckfuell M, Oesser S, Ahlers M, Flechsenhar K, Schlosshauer B. A novel gelatin sponge for accelerated hemostasis. J Biomed Mater Res B Appl Biomater. 2010;94(2):372‐379. [DOI] [PubMed] [Google Scholar]
- 9. Kabiri M, Emami SH, Rafinia M, Tahriri M. Preparation and characterization of absorbable hemostat crosslinked gelatin sponges for surgical applications. Curr Appl Phys. 2011;11(3):457‐461. [Google Scholar]
- 10. Lewis KM, Spazierer D, Urban MD, Lin L, Redl H, Goppelt A. Comparison of regenerated and non‐regenerated oxidized cellulose hemostatic agents. Eur Surg. 2013;45:213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, He J, Cheng W, et al. Oxidized regenerated cellulose‐based hemostat with microscopically gradient structure. Carbohydr Polym. 2012;88(3):1023‐1032. [Google Scholar]
- 12. Lewis KM, Spazierer D, Slezak P, Baumgartner B, Regenbogen J, Gulle H. Swelling, sealing, and hemostatic ability of a novel biomaterial: a polyethylene glycol‐coated collagen pad. J Biomater Appl. 2014;29(5):780‐788. [DOI] [PubMed] [Google Scholar]
- 13. Boerman MA, Roozen E, Sanchez‐Fernandez MJ, et al. Next generation hemostatic materials based on NHS‐Ester functionalized poly(2‐oxazoline)s. Biomacromolecules. 2017;18(8):2529‐2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murakami Y, Yokoyama M, Nishida H, Tomizawa Y, Kurosawa H. A simple hemostasis model for the quantitative evaluation of hydrogel‐based local hemostatic biomaterials on tissue surface. Colloids Surf B Biointerfaces. 2008;65(2):186‐189. [DOI] [PubMed] [Google Scholar]
- 16. Zühlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990;1:1009‐1016. [PubMed] [Google Scholar]
- 17. Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin a on the healing of intestinal anastomoses. Am J Surg. 1992;163(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 18. Deyhimi P, Khademi H, Birang R, Akhoondzadeh M. Histological evaluation of wound healing process after photodynamic therapy of rat Oral mucosal ulcer. J Dent (Shiraz). 2016;17(1):43‐48. [PMC free article] [PubMed] [Google Scholar]
- 19. Glineur D, Hendrikx M, Krievins D, et al. A randomized, controlled trial of Veriset hemostatic patch in halting cardiovascular bleeding. Med Dev. 2018;11:65‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Csukas D, Urbanics R, Moritz A, Ellis‐Behnke R. AC5 surgical hemostat as an effective hemostatic agent in an anticoagulated rat liver punch biopsy model. Nanomedicine. 2015;11(8):2025‐2031. [DOI] [PubMed] [Google Scholar]
- 21. Schmiedt CW, Köhler R, Brainard BM. Use of topical bovine thrombin in an anti‐coagulated rat model of hepatic injury. Res Vet Sci. 2012;93(3):1498‐1503. [DOI] [PubMed] [Google Scholar]
- 22. Saha AK, Mousavi M, Dallo SF, Evani SJ, Ramasubramanian AK. Influence of membrane cholesterol on monocyte chemotaxis. Cell Immunol. 2018;324:74‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saha AK, Osmulski P, Dallo SF, Gaczynska M, Huang TH, Ramasubramanian AK. Cholesterol regulates monocyte rolling through CD44 distribution. Biophys J. 2017;112(7):1481‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaturvedi AA, Lomme RM, Hendriks T, van Goor H. Prevention of postsurgical adhesions using an ultrapure alginate‐based gel. Br J Surg. 2013;100(7):904‐910. [DOI] [PubMed] [Google Scholar]
- 25. Weber B, Lackner I, Haffner‐Luntzer M, et al. Modeling trauma in rats: similarities to humans and potential pitfalls to consider. J Transl Med. 2019;17(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lewis JH, Van Thiel DH, Hasiba U, Spero JA, Gavaler J. Comparative hematology and coagulation: studies on rodentia (rats). Comp Biochem Physiol A Physiol. 1985;82(1):211‐215. [DOI] [PubMed] [Google Scholar]
- 27. MacDonald MH, Wang AY, Clymer JW, Hutchinson RW, Kocharian R. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Dev. 2017;10:273‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Edwin Roozen, upon reasonable request.


