Abstract
Pain is underdiagnosed and often not adequately treated, contributing to behavioral and psychological symptoms of dementia (BPSD). BPSD are treated with atypical antipsychotics that are associated with severe cerebrocardiovascular effects. Interestingly, treatment of pain may reduce agitation. Research is focusing on nonpharmacological treatment, such as aromatherapy, for pain and BPSD in dementia. This clinical study will assess the effect on agitation in severely demented elderly of BEO loaded in a nanotechnological odorless cream indistinguishable from placebo. This is a protocol for a randomized, double‐blind, placebo‐controlled trial (NCT04321889). A total of 134 patients aged ≥65 years with severe dementia (mini‐mental state examination <12) will be recruited and randomly allocated 1:1 to either BEO or placebo group. After baseline screening, BEO (80 mg) cream or placebo cream will be trans‐dermally applied on both arms twice a day for 4 weeks with a 4‐week follow‐up period. The effect on agitation will be the primary endpoint. Any adverse events will be reported. A double‐blind, clinical trial evaluating efficacy and safety of an essential oil endowed with strong analgesic properties has never been carried out before. This study could form the basis for a safer and more effective treatment of BPSD in severe dementia.
Keywords: agitation, aromatherapy, bergamot essential oil, BPSD, clinical trial, pain
1. INTRODUCTION
Currently, 50 million people in the world suffer from dementia and that is frequent in people aged ≥65, presenting comorbidities associated with nociceptive (e.g., osteoarthritis) and neuropathic pain (e.g., diabetic neuropathic pain) that are often under‐diagnosed. About 60–80% of patients affected by dementia resident in nursing homes experiences pain (see Achterberg et al., 2013). Behavioral and psychological symptoms of dementia (BPSD), such as agitation and aggression, are closely associated with pain when not adequately diagnosed (Sampson et al., 2015; Sengstaken & King, 1993) and, consequently, not relieved. Indeed, a stepwise protocol for pain treatment is demonstrated to reduce agitation of the 17% (Husebo, Ballard, Sandvik, Nilsen, & Aarsland, 2011). Interestingly, the model at the basis of the Describe‐Investigate‐Create‐Evaluate (“DICE”) approach suggests that the cause of BPSD consists in brain circuitry disruptions increasing vulnerability to triggers like pain, hence their assessment and modification takes on the utmost importance (Kales, Lyketsos, Miller, & Ballard, 2019). The treatment of BPSD currently relies on the use of atypical antipsychotics. These drugs show efficacy in the management of agitation mainly for short‐term (i.e., 6–12 weeks) treatment; furthermore, the control of symptoms is incomplete and an increased risk of mortality has been reported with antipsychotics (Schneider, Dagerman, & Insel, 2005). Therefore, pain treatment is fundamental for the management of agitation in patients with dementia, while atypical antipsychotics should be used if strictly necessary (Scuteri et al., 2017). Since there is no disease modifying intervention for AD and dementia, and the symptomatic pharmacological treatments have limited efficacy and considerable side effects, research is focusing on nonpharmacological treatment, including aromatherapy, for symptoms of dementia, in particular BPSD (Zucchella et al., 2018). The massage with Melissa officinalis essential oil improves the agitation score of the Cohen–Mansfield agitation inventory (CMAI) without significant side effects, as demonstrated in a placebo‐controlled study on 72 demented patients residing in nursing homes (Ballard, O'Brien, Reichelt, & Perry, 2002). A double‐blind parallel‐group placebo‐controlled randomized trial in England has not found superiority of aromatherapy with Melissa to placebo or donepezil in the treatment of agitation (Burns et al., 2011). Some efficacy of lavender in the control of agitation has been reported, too (Holmes et al., 2002; Lin, Chan, Ng, & Lam, 2007; Moorman Li et al., 2017), though not in all studies (O'Connor, Eppingstall, Taffe, & van der Ploeg, 2013; Zalomonson et al., 2019). The methodological difficulties of most aromatherapy trials have not allowed any definitive conclusion about the effectiveness of aromatherapy in dementia (Forrester et al., 2014). Due to the tight link between undertreated pain and agitation, aromatherapy can turn out to be a very useful approach in particular if an essential oil with powerful analgesic activity is used (Scuteri et al., 2019; Scuteri et al., 2019; Scuteri, Morrone, et al., 2017). The essential oil of bergamot (BEO), included in Farmacopea Ufficiale Italiana (Farmacopea, Ufficiale, & Italiana, 1991), has provided strong preclinical evidence of analgesic activity in inflammatory and neuropathic pain models (see Scuteri et al., 2021), being effective also for inhalation (Scuteri et al., 2018). The interval ranges of percentage of the main components of BEO are reported in Table 1.
TABLE 1.
Percentages of the most representative and abundant components in BEO
| Chemical substance | Interval ranges (%) |
|---|---|
| α‐Pinene | 0.7–2.0 |
| Sabinene | 0.5–2.0 |
| β‐Pinene | 5.0–10.0 |
| Limonene | 30.0–50.0 |
| γ‐Terpinene | 6.0–18.5 |
| Linalool | 6.0–15.0 |
| Linalyl acetate | 23.0–35.0 |
| Geranial | <0.5 |
| Geranyl acetate | 0.1–0.7 |
| Cariophyllene | 0.2–0.5 |
BEO (Citrus bergamia Risso et Poiteau) is able to modulate endogenous peripheral and central opioid system, as demonstrated by the finding that pretreatment with naloxone methiodide, an opioid antagonist that cannot cross blood brain barrier, reduces BEO‐induced analgesia (see Scuteri, Rombola, Morrone, et al., 2019). Moreover, BEO enhances morphine analgesic effect, both locally and systemically (see Scuteri, Rombola, Morrone, et al., 2019). BEO can play a pivotal role in the modulation of synaptic glutamate (Morrone et al., 2007), thus contributing to the processes of sensitization and autophagy (Pereira et al., 2017), known to be dysregulated in neuropathic pain. It is also endowed with anxiolytic‐like activity, devoid of sedation contrary to benzodiazepines (Rombolà et al., 2019). Therefore, the rationale of this clinical trial relies on three main points:
BEO is endowed with analgesic properties in models of inflammatory and neuropathic pain.
Essential oils used in aromatherapy for the control of agitation have not provided wide evidence of analgesic activity, fundamental considering the close association between pain and agitation in dementia.
The strength of essential oils aroma has prevented a real double‐blind design of clinical trials up to now. Indeed, either the patient receiving the essential oil, though often anosmic, or the operator administering it could distinguish it from placebo because of the scent.
2. MATERIALS AND METHODS
2.1. Study design
This trial is designed to study the efficacy of aromatherapy with BEO on agitation in patients affected with severe dementia. The active treatment will consist in furocoumarin‐free BEO loaded in a nanotechnology‐based delivery system in the form of an odorless cream indistinguishable from placebo empty cream. This novel formulation, named NanoBEO, is under patent consideration (Scuteri et al., 2021). The pharmaceutical formulation is furocoumarin‐free to avoid phototoxicity, according to the assessment report of the European Medicine Agency (EMA) (September 13, 2011 EMA/HMPC/56155/2011 Committee on Herbal Medicinal Products [HMPC]), and contains α‐tocopheryl stearate as antioxidant to favor chemical stability. The primary outcome consists in the assessment of the clinical effectiveness of NanoBEO in the treatment of agitation in patients suffering from severe dementia. The secondary outcomes are further 4‐week follow‐up of agitation after the end of the intervention; evaluation of clinical efficacy of NanoBEO on pain in severely demented patients. In particular, the primary outcome measure is a 30% change of the CMAI during the treatment (assessed every week). The secondary outcome measures are change of the CMAI score during follow‐up; change of the pain score according to the Mobilization–Observation–Behavior–Intensity–Dementia (MOBID)‐2.
2.2. Eligibility criteria
Consecutive patients, admitted to the service for neurodegenerative diseases of Institute Sant'Anna, Crotone and with diagnosis of Dementia will be enrolled, accordingly with the following criteria:
Mini‐mental state examination (MMSE) <12
Informed consent by a legal representative
The administration of authorized concurrent therapies for the treatment of agitation (i.e., risperidone for aggressive behaviors) is allowed and any change in their prescriptions will be monitored; patients have to be free from other psychotropic medications (i.e., other neuroleptics, antidepressants, and benzodiazepines) for at least 2 weeks. Therapies for the treatment of chronic comorbidities due to the age of the patients (e.g., drugs for the treatment of hypertension and diabetes, as well as drugs for gastric protection, antiinflammatories, antibiotics, and chemotherapy) are allowed.
Exclusion criteria:
Patients with previous clinical history positive to prior disabling neurologic or psychiatric diseases, for example, Parkinson's disease, stroke, cerebral hemorrhage, delirium, and psychosis, will be excluded from enrollment.
The enrollment will last 12–18 months. The informed consent will be obtained by healthcare operators. A legal representative of the patient will be informed about the study and provided a consent form.
2.3. Intervention timeline
The patients fitting the inclusion criteria will be enrolled in the study and randomized in two groups: active intervention and placebo. After the randomization (T0), all patients in both groups will be administered active or placebo cream on both arms twice a day for 4 weeks. The procedure will take about 2 min for the administration on each arm. The active intervention will consist in furocoumarin‐free BEO loaded in a nanotechnology‐based delivery system in the form of an odorless cream indistinguishable from placebo cream. The formulation will be distributed through an airless system that will allow the quantitative definition of the dose, which must contain 80 mg of BEO. The same dose of placebo will be applied. Since this study involves noncommunicative patients residing in a nursing home, the interventions will be topically administered by healthcare operators. Therefore, there is no possibility of lack of adherence to interventions. Anyway, the healthcare operators will be asked to save the empty dispenser to demonstrate complete adherence of the participants to the treatment.
2.4. Schedule of assessment
After the signature of the informed consent form (see Supplementary material) to take part to the study, at T0, the patients will undergo the following baseline clinical evaluations: MMSE; CMAI; MOBID‐2 (for tables, see Supplementary material). Furthermore, a complete anamnestic collection will be reported including: significant medical events in the last 30 days; drugs taken; complementary therapies, that is, neurorehabilitation procedures. CMAI and MOBID‐2 will be repeated weekly for 4 weeks (T1, end of cream delivery) and again weekly for further 4 weeks (T2, follow‐up). The schedule is reported in Figure 1.
FIGURE 1.
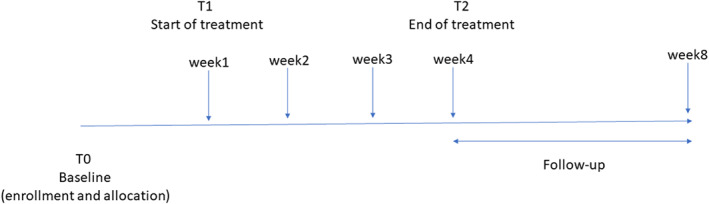
Schedule of treatment and assessment of primary and secondary outcome measures [Colour figure can be viewed at wileyonlinelibrary.com]
At T1 and T2, MMSE and the anamnestic collection will be also repeated. In particular, the following parameters will be highlighted: concurrent treatments, adverse events; discontinuations of treatment and reasons. In case of discontinuation of the treatment, the patient will be excluded from the study. A follow‐up period of 4 weeks will be carried out to monitor potentially delayed side effects.
2.5. Sample size
The calculation of the sample power is based on literature data to achieve a 30% improvement in agitation measured by the CMAI score (Ballard et al., 2002). The formula for calculating the sample size is given, for two arms (treatment and placebo) with the same size, as it follows:
Therefore, considering a 95% confidence level and a study power of 80%, the result will be of n = 67 per arm. Since we have two arms, that is, active cream and placebo, patients enrolled will be 134.
2.6. Allocation, concealment, and blinding
Patients will be randomly allocated to BEO cream group or placebo group in a 1:1 allocation ratio. Since there are not differences induced by the gender of the participants (McDonnell & Newcomb, 2019; Straface et al., 2020; Xiong et al., 2018) or other parameters, no factors for stratification will be considered. No blocking, for example, incomplete randomization, will occur. The randomization codes of allocation will be generated using Random Number selection Microsoft Office Excel 2010 (Microsoft, Milan, Italy). No member of the trial who will administer intervention and placebo or analyse data will have access to the codes up to the end of the trial. The creams packaging will be identical and the creams will be indistinguishable in appearance, texture, and scent. Three different researchers/operators will independently recruit patients, generate the allocation sequence and who assign participants to interventions. Healthcare personnel, patients, outcome assessors, and data analysts will be blinded after assignment to interventions and also about the nature of the interventions. Data analysts will be blinded about the assignment to interventions.
2.7. Data management and statistical analysis
All the healthcare operators assessors will be provided a training period to guarantee the correct execution of the protocol and inter‐rater reliability. A description of the used study instruments, that is, CMAI and MOBID‐2, concerning various aspects of validity and reliability, will be given. The same rater will perform baseline assessment and evaluations during the administration of the active intervention and of the placebo, as well as follow‐up measurements. To guarantee security and data quality, double data entry will be performed by two independent researchers. Only the responsible secretariat of the clinical center will collect and maintain personal information about patients, in order to protect confidentiality before, during and after the trial. The obtained results will undergo statistical analysis according to their distribution, considering p < 0.05 significant. According to the statistical analysis plan, for the comparison of the characteristics between the two groups, χ2 and Mann–Whitney U tests will be used. Differences in the primary and secondary outcome measures will be evaluated through χ2, Wilcoxon test, and Mann–Whitney U tests. Differences in mean and standard error (SE) of the mean of the outcome measures will be analysed using Student's t test. The repeated measures analysis of covariance (ANCOVA) will be carried out for the primary and the secondary outcomes. Analyses will be carried out using SPSS statistics software (Chicago, Illinois). Since there are only two groups, no subgroup analyses will be performed. Interim results of safety will be evaluated by the chief investigator to decide if it is necessary to terminate the trial.
3. RESULTS AND DISCUSSION
This study will be the first clinical trial assessing the effectiveness of BEO on agitation in severe dementia. Moreover, it will represent the first study to evaluate aromatherapy with a very rigorous blinded design. Indeed, the pharmaceutical formulation used in this study and currently under patent evaluation is the first nanotechonological‐based delivery system of an essential oil loaded into solid lipid nanoparticles and presented in the final form of an odorless cream indistinguishable from placebo enriched with α‐tocopheryl stearate as antioxidant to favor stability of the components. Indeed, reproducibility of the dose is guaranteed. NanoBEO allows an efficient transdermal release of the oil and feasibility of administration through an airless dispenser. A rigorous assessment of the efficacy and safety of BEO on agitation and pain in demented patients is fundamental because BPSD are treated only with antipsychotics that are used off‐label in this population. Antipsychotics are safe only for 6–12 weeks of treatment, but it has been reported that they are used also for 2.2 years on average (Harrison et al., 2020). Therefore, alternative treatments, if effective, should be the first‐line treatment for BPSD. In this context, aromatherapy with an essential oil able to induce strong analgesia, thus reducing agitation, like BEO, could be a safe and effective approach. In fact, chronic pain, mainly of neuropathic nature is not adequately diagnosed and treated in these patients (Scuteri et al., 2017; Scuteri et al., 2018; Scuteri et al., 2021; Scuteri, Corasaniti, Tonin, & Bagetta, 2019) and this occurs also for migraine, since novel effective therapies with rapid action are not even tested in this population (Scuteri, Corasaniti, Tonin, & Bagetta, 2019). The situation is worsened by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic (Scuteri et al., 2020). For all these reasons, a rigorous randomized placebo‐controlled clinical trial is needed to establish the possible role of BEO in agitation. BEO could also prevent or reduce the use of other psychotropic drugs, with side effects in dementia and even less supported efficacy, but often prescribed instead of antipsychotics because of their proven toxicity (see Kales, Gitlin, & Lyketsos, 2019).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed equally to this study. All authors read and approved the final manuscript.
4.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENT
DS is a researcher in the frame of the project supported by the Italian Ministry of Health: NET‐2016‐02361805 (WP 5). Open Access Funding provided by Universita della Calabria within the CRUI‐CARE Agreement. [Correction added on 23 June 2022, after first online publication: CRUI funding statement has been added.]
Funding information
No funding has been received for this trial. This is a nonprofit study, in which no form of remuneration has been foreseen for study participants and for the chief investigator and all the staff involved.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Achterberg, W. P. , Pieper, M. J. , van Dalen‐Kok, A. H. , de Waal, M. W. , Husebo, B. S. , Lautenbacher, S. , … Corbett, A. (2013). Pain management in patients with dementia. Clinical Interventions in Aging, 8, 1471–1482. 10.2147/CIA.S36739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, C. G. , O'Brien, J. T. , Reichelt, K. , & Perry, E. K. (2002). Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: The results of a double‐blind, placebo‐controlled trial with Melissa. The Journal of Clinical Psychiatry, 63(7), 553–558. 10.4088/jcp.v63n0703 [DOI] [PubMed] [Google Scholar]
- Burns, A. , Perry, E. , Holmes, C. , Francis, P. , Morris, J. , Howes, M. J. , … Ballard, C. (2011). A double‐blind placebo‐controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 31(2), 158–164. 10.1159/000324438 [DOI] [PubMed] [Google Scholar]
- Farmacopea, Ufficiale, & Italiana . (1991). Droghe Vegetali e Preparazioni (IX Edizione) .
- Forrester, L. T. , Maayan, N. , Orrell, M. , Spector, A. E. , Buchan, L. D. , & Soares‐Weiser, K. (2014). Aromatherapy for dementia. Cochrane Database of Systematic Reviews, 2, CD003150. 10.1002/14651858.CD003150.pub2 [DOI] [PubMed] [Google Scholar]
- Harrison, F. , Cations, M. , Jessop, T. , Aerts, L. , Chenoweth, L. , Shell, A. , … Brodaty, H. (2020). Prolonged use of antipsychotic medications in long‐term aged care in Australia: A snapshot from the HALT project. International Psychogeriatrics, 32, 335–345. 10.1017/S1041610219002011 [DOI] [PubMed] [Google Scholar]
- Holmes, C. , Hopkins, V. , Hensford, C. , MacLaughlin, V. , Wilkinson, D. , & Rosenvinge, H. (2002). Lavender oil as a treatment for agitated behaviour in severe dementia: A placebo controlled study. International Journal of Geriatric Psychiatry, 17(4), 305–308. 10.1002/gps.593 [DOI] [PubMed] [Google Scholar]
- Husebo, B. S. , Ballard, C. , Sandvik, R. , Nilsen, O. B. , & Aarsland, D. (2011). Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: Cluster randomised clinical trial. BMJ, 343, d4065. 10.1136/bmj.d4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales, H. C. , Gitlin, L. N. , & Lyketsos, C. G. (2019). When less is more, but still not enough: Why focusing on limiting antipsychotics in people with dementia is the wrong policy imperative. Journal of the American Medical Directors Association, 20(9), 1074–1079. 10.1016/j.jamda.2019.05.022 [DOI] [PubMed] [Google Scholar]
- Kales, H. C. , Lyketsos, C. G. , Miller, E. M. , & Ballard, C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer's disease: An international Delphi consensus. International Psychogeriatrics, 31(1), 83–90. 10.1017/s1041610218000534 [DOI] [PubMed] [Google Scholar]
- Lin, P. W. , Chan, W. C. , Ng, B. F. , & Lam, L. C. (2007). Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: A cross‐over randomized trial. International Journal of Geriatric Psychiatry, 22(5), 405–410. 10.1002/gps.1688 [DOI] [PubMed] [Google Scholar]
- McDonnell, B. , & Newcomb, P. (2019). Trial of essential oils to improve sleep for patients in cardiac rehabilitation. Journal of Alternative and Complementary Medicine, 25(12), 1193–1199. 10.1089/acm.2019.0222 [DOI] [PubMed] [Google Scholar]
- Moorman Li, R. , Gilbert, B. , Orman, A. , Aldridge, P. , Leger‐Krall, S. , Anderson, C. , & Hincapie Castillo, J. (2017). Evaluating the effects of diffused lavender in an adult day care center for patients with dementia in an effort to decrease behavioral issues: A pilot study. Journal of Drug Assess, 6(1), 1–5. 10.1080/21556660.2016.1278545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone, L. A. , Rombolà, L. , Pelle, C. , Corasaniti, M. T. , Zappettini, S. , Paudice, P. , … Bagetta, G. (2007). The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: Implication of monoterpene hydrocarbons. Pharmacological Research, 55(4), 255–262. 10.1016/j.phrs.2006.11.010 [DOI] [PubMed] [Google Scholar]
- O'Connor, D. W. , Eppingstall, B. , Taffe, J. , & van der Ploeg, E. S. (2013). A randomized, controlled cross‐over trial of dermally‐applied lavender (Lavandula angustifolia) oil as a treatment of agitated behaviour in dementia. BMC Complementary and Alternative Medicine, 13, 315. 10.1186/1472-6882-13-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, G. J. , Antonioli, M. , Hirata, H. , Ureshino, R. P. , Nascimento, A. R. , Bincoletto, C. , … Smaili, S. S. (2017). Glutamate induces autophagy via the two‐pore channels in neural cells. Oncotarget, 8(8), 12730–12740. 10.18632/oncotarget.14404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombolà, L. , Scuteri, D. , Adornetto, A. , Straface, M. , Sakurada, T. , Sakurada, S. , … Morrone, L. A. (2019). Anxiolytic‐like effects of bergamot essential oil are insensitive to flumazenil in rats. Evidence‐Based Complementary and Alternative Medicine, 6, 2156873. 10.1155/2019/2156873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson, E. L. , White, N. , Lord, K. , Leurent, B. , Vickerstaff, V. , Scott, S. , & Jones, L. (2015). Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: A longitudinal cohort study. Pain, 156(4), 675–683. 10.1097/j.pain.0000000000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, L. S. , Dagerman, K. S. , & Insel, P. (2005). Risk of death with atypical antipsychotic drug treatment for dementia: Meta‐analysis of randomized placebo‐controlled trials. JAMA, 294(15), 1934–1943. 10.1001/jama.294.15.1934 [DOI] [PubMed] [Google Scholar]
- Scuteri, D. , Cassano, R. , Trombino, S. , Russo, R. , Mizoguchi, H. , Watanabe, C. , … Bagetta, G. (2021). Development and translation of NanoBEO, a nanotechnology‐based delivery system of bergamot essential oil deprived of furocumarins, in the control of agitation in severe dementia. Pharmaceutics, 13(3). 10.3390/pharmaceutics13030379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Corasaniti, M. T. , Tonin, P. , & Bagetta, G. (2019). Eptinezumab for the treatment of migraine. Drugs Today (Barc), 55(11), 695–703. 10.1358/dot.2019.55.11.3069864 [DOI] [PubMed] [Google Scholar]
- Scuteri, D. , Crudo, M. , Rombola, L. , Watanabe, C. , Mizoguchi, H. , Sakurada, S. , … Bagetta, G. (2018). Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia, 129, 20–24. 10.1016/j.fitote.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Scuteri, D. , Garreffa, M. R. , Esposito, S. , Bagetta, G. , Naturale, M. D. , & Corasaniti, M. T. (2018). Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regeneration Research, 13(9), 1619–1621. 10.4103/1673-5374.237125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Hamamura, K. , Sakurada, T. , Watanabe, C. , Sakurada, S. , Morrone, L. A. , … Corasaniti, M. T. (2021). Efficacy of essential oils in pain: A systematic review and meta‐analysis of preclinical evidence. Frontiers in Pharmacology, 12, 640128. 10.3389/fphar.2021.640128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Matamala‐Gomez, M. , Bottiroli, S. , Corasaniti, M. T. , De Icco, R. , Bagetta, G. , & Tonin, P. (2020). Pain assessment and treatment in dementia at the time of coronavirus disease COVID‐19. Frontiers in Neurology, 11, 890. 10.3389/fneur.2020.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Morrone, L. A. , Rombola, L. , Avato, P. R. , Bilia, A. R. , Corasaniti, M. T. , … Bagetta, G. (2017). Aromatherapy and aromatic plants for the treatment of Behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: Clinical evidence and possible mechanisms. Evidence‐Based Complementary and Alternative Medicine, 8, 9416305. 10.1155/2017/9416305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Piro, B. , Morrone, L. A. , Corasaniti, M. T. , Vulnera, M. , & Bagetta, G. (2017). The need for better access to pain treatment: Learning from drug consumption trends in the USA. Functional Neurology, 22(4), 229–230. 10.11138/fneur/2017.32.4.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Rombola, L. , Morrone, L. A. , Bagetta, G. , Sakurada, S. , Sakurada, T. , … Corasaniti, M. T. (2019). Neuropharmacology of the neuropsychiatric symptoms of dementia and role of pain: Essential oil of bergamot as a novel therapeutic approach. International Journal of Molecular Sciences, 20(13). 10.3390/ijms20133327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri, D. , Rombola, L. , Tridico, L. , Mizoguchi, H. , Watanabe, C. , Sakurada, T. , … Morrone, L. A. (2019). Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain‐related BPSDs. Current Medicinal Chemistry, 26(20), 3764–3774. 10.2174/0929867325666180307115546 [DOI] [PubMed] [Google Scholar]
- Scuteri, D. , Vulnera, M. , Piro, B. , Bossio, R. B. , Morrone, L. A. , Sandrini, G. , … Corasaniti, M. T. (2021). Pattern of treatment of behavioural and psychological symptoms of dementia and pain: Evidence on pharmacoutilization from a large real‐world sample and from a Centre for cognitive disturbances and dementia. European Journal of Clinical Pharmacology, 77(2), 241–249. 10.1007/s00228-020-02995-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengstaken, E. A. , & King, S. A. (1993). The problems of pain and its detection among geriatric nursing home residents. Journal of the American Geriatrics Society, 41(5), 541–544. [DOI] [PubMed] [Google Scholar]
- Straface, M. , Makwana, R. , Palmer, A. , Rombola, L. , Aleong, J. C. , Morrone, L. A. , & Sanger, G. J. (2020). Inhibition of neuromuscular contractions of human and rat colon by bergamot essential oil and linalool: Evidence to support a therapeutic action. Nutrients, 12(5). 10.3390/nu12051381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, M. , Li, Y. , Tang, P. , Zhang, Y. , Cao, M. , Ni, J. , & Xing, M. (2018). Effectiveness of aromatherapy massage and inhalation on symptoms of depression in Chinese community‐dwelling older adults. Journal of Alternative and Complementary Medicine, 24(7), 717–724. 10.1089/acm.2017.0320 [DOI] [PubMed] [Google Scholar]
- Zalomonson, S. , Freud, T. , Punchik, B. , Samson, T. , Lebedinsky, S. , & Press, Y. (2019). The results of a crossover placebo‐controlled study of the effect of lavender oil on behavioral and psychological symptoms of dementia. Rejuvenation Research, 22(3), 246–253. 10.1089/rej.2018.2123 [DOI] [PubMed] [Google Scholar]
- Zucchella, C. , Sinforiani, E. , Tamburin, S. , Federico, A. , Mantovani, E. , Bernini, S. , … Bartolo, M. (2018). The multidisciplinary approach to Alzheimer's disease and dementia. A narrative review of non‐pharmacological treatment. Frontiers in Neurology, 9, 1058. 10.3389/fneur.2018.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


