Abstract
Background
Neurocognitive impairments are common among brain tumor patients, and may impact patients’ awareness of performance in instrumental activities in daily life (IADL). We examined differences between patient- and proxy-reported assessments of the patient’s IADL, and whether the level of (dis)agreement is associated with neurocognitive impairments.
Methods
Brain tumor patients and their proxies completed the phase 3 version of the EORTC IADL-BN32 questionnaire measuring IADL, and patients completed six neurocognitive measures. Patient-proxy difference scores in IADL were compared between patients who were defined as neurocognitively impaired (≥2 neurocognitive measures ≥2.0 standard deviations below healthy controls) and non-neurocognitively impaired. With multinomial logistic regression analyses we examined if neurocognitive variables were independently associated with patient-proxy disagreement in IADL ratings.
Results
Patients (n = 81) did not systematically (P < .01) rate IADL outcomes different than their proxies. Proxies did report more problems on 19/32 individual items and all five scales. This effect was more apparent in dyads with a neurocognitively impaired patient (n = 37), compared to dyads with non-neurocognitively impaired patients (n = 44). Multinomial logistic regression analyses showed that several neurocognitive variables (e.g., cognitive flexibility and verbal fluency) were independently associated with disagreement between patients and proxies on different scales.
Conclusion
Neurocognitive deficits seem to play a role in the discrepancies between brain tumor patients and their proxies assessment of patient’s level of IADL. Although replication of our results is needed, our findings suggests that caution is warranted in interpreting self-reported IADL by patients with neurocognitive impairment, and that such self-reports should be supplemented with proxy ratings.
Keywords: brain tumor, daily functioning, IADL, instrumental activities of daily living, observer-reported, patient-reported
Neurocognitive impairments or reduced neurocognitive abilities are common in patients with a primary brain tumor or brain metastases, even before treatment.1 A brain tumor disrupts the functional networks of the brain, causing epileptic seizures and neurocognitive problems.2–4 Moreover, most anti-tumor treatments can have a positive or negative effect on a patient’s neurocognitive functioning. Although brain tumor treatments target brain cancer cells, healthy brain tissue may also be affected by the treatment. In addition, patients with brain metastases often undergo systemic therapy for the primary tumor which also could induce neurocognitive deficits. Chemotherapeutic drugs, for example, have been shown to induce cognitive deficits in several cognitive domains and is often described by cancer patients as a brain fog. This phenomenon is frequently referred to as “chemo-brain” and can persist for months after treatment.5 Therefore, neurocognitive impairments can affect brain tumor patients during the entire disease and treatment trajectory.
Intact neurocognitive functioning is important in all aspects of everyday life, particularly in complex activities of daily living that rely more heavily on higher order neurocognitive functioning6 such as managing personal finances, participating in traffic, using a mobile telephone, and caring for family members. Deterioration of neurocognitive functioning has been associated with more problems with such so-called instrumental activities of daily living (IADL) in the general7 and elderly6 population, and in patients prone to neurocognitive impairments, such as those suffering from dementia.8 Despite being a relevant outcome, IADL is not regularly measured in patients with brain tumors. An important reason for this is the paucity of valid and reliable IADL questionnaires designed specifically for this population.9 At present, a European Organisation for Research and Treatment of Cancer (EORTC)10 questionnaire to asses IADL in brain tumor patients is under development.
While the general consensus is that patients are the best and most appropriate source of ratings of their functioning and well-being,11 studies with dementia patients have suggested that neurocognitive impairments may limit patients’ ability to accurately rate their own level of daily functioning.11–13 Farias et al. (2005)11 compared self- and proxy-reported IADL impairments of four cognitive groups; elderly cognitively healthy controls, two groups of patients with mild cognitive impairments (MCI) (i.e. memory impaired and nonmemory impaired), and dementia patients. This study showed that, at the group level, proxies of dementia patients reported significantly more problems in IADL than the dementia patients themselves. For the other three groups, patients reported slightly more problems with everyday functioning than their proxies. This suggests that the severity of neurocognitive impairments is an important factor in the level of disagreement in the assessment of patient’s IADL between patients and their proxies. Previously, Sneeuw et al. (1997) showed that proxies of brain tumor patients tended to rate the patients as having a lower quality of life than the patients themselves, particularly in patients with more physical and cognitive impairments and those exhibiting mental confusion.14
Currently, it is still unclear whether brain tumor patients and their proxies differ in their reporting on patient’s level of IADL, and whether this is independently associated with the level of neurocognitive functioning of patients. The aim of this study, therefore, was to investigate if there were differences between patient- and proxy-reported assessments of brain tumor patients’ level of IADL, and if the differences are related to neurocognitive deficits.
Methods
Study Population
We used data collected during the development of an IADL questionnaire for brain tumor patients10 according to the EORTC procedures.15 The study sample consisted of an international group of adult patients with either a histologically confirmed grade II-IV glioma, or brain metastases from a histologically confirmed primary tumor, and their informal caregiver as proxy. For proxies, daily or weekly contact with the patient was a requirement to ensure reliable assessment of the patient’s daily functioning. Patients were consecutively recruited in both academic and non-academic outpatient clinics, irrespective of disease stage and treatment status. Only the data from patients and their proxies with available neurocognitive testing data was used. Ethical and research governance approvals were obtained at each participating center in accordance with local requirements, and both patients and proxies provided written informed consent before participation.
Measures
Both patients (patient-based version) and their proxies (proxy-based version) rated the 32 individual items of the phase 3 version of the EORTC IADL-BN32 questionnaire10 simultaneously at one time point (cross-sectional assessment). The phase 3 EORTC IADL-BN32 questionnaire showed favorable preliminary psychometric properties, and is available for general use.16 While the proxy-based version consisted of the same items as the patient-based version, it referred to the patient’s level of functioning (i.e. “Has he/she had difficulties with [..]”). Items were scored on a 4-point Likert-type scale ranging from “not at all” to “very much”. The EORTC IADL-BN32 comprises five multi-item and two single-item scales10 (see Supplementary Material 1). The provisional multi-item scales cover: Scale 1 (Domestic activities), Scale 2 (Activities requiring extended focus), Scale 3 (Modern devices and communication skills), Scale 4 (Administrative tasks), and Scale 5 (Social activities). The single-item scales cover difficulty doing your job (paid or voluntary) and difficulty managing own medication. Scale scores were calculated and linearly converted to a score ranging from 0 to 100 (i.e. score of 0 indicating no problems at all) in accordance with the EORTC IADL-BN32 manual.17 To compute a scale score, at least half of the items in a scale had to be answered.
Patients also underwent neurocognitive testing using a neuropsychological test battery. This battery, commonly used in EORTC brain tumor studies,18–23 consists of 3 neurocognitive tests comprising 6 different neurocognitive measures: the Hopkins Verbal Learning Test–Revised (HVLT-R)24 (direct recall, delayed recall and recognition discrimination [number of true positives – number of false positives]), the Trail Making Test (A+B)25 (information processing speed [TMT-A] and cognitive flexibility [TMT-B]) and the Controlled Oral Word Association Test (COWAT)26 (verbal fluency). The six neurocognitive measure z-scores were calculated based on norm scores.24,27,28 The more stringent z-score of ≥2.0 standard deviation (SD) below the norm scores was used to indicate presence of cognitive impairment.29 Patients with ≥2 impaired neurocognitive measures were considered neurocognitively impaired. In addition, the patients also completed a patient-reported subjective neurocognitive complaints questionnaire (as measured with the Medical Outcomes Study Cognitive Functioning Scale–Revised (MOS-COG–R)30).
Statistical Analysis
Descriptive statistics were used to describe the sociodemographic and clinical characteristics of the participants. As the EORTC IADL-BN32 does not have an overall sum score, all analyses were done at scale or individual item level.
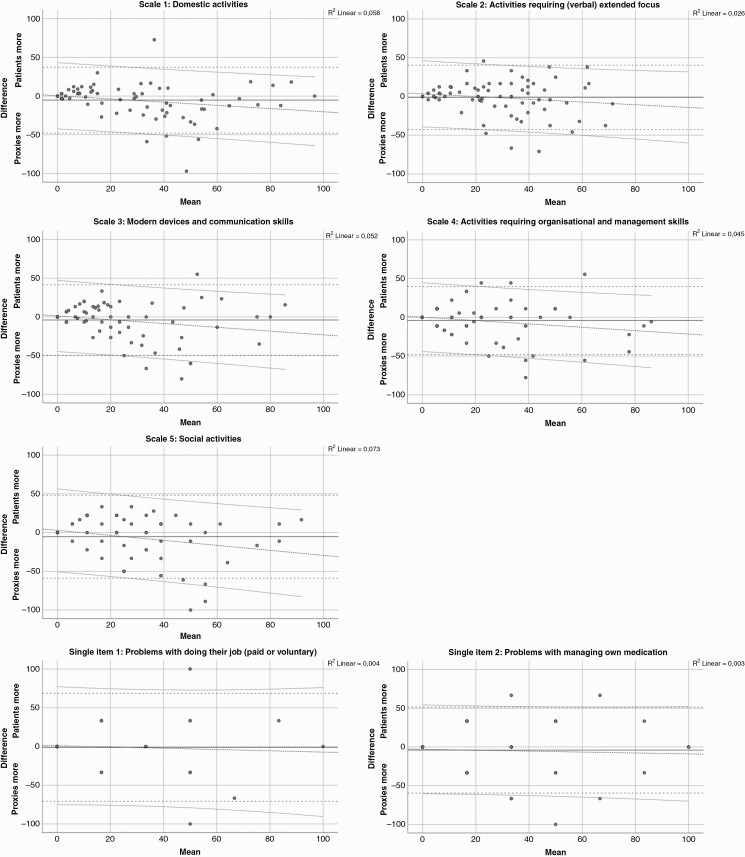
At the group level, differences between patients and proxies were examined for each of the 32 individual items and for each scale using nonparametric paired samples tests to examine the possible presence systematic response biases. The magnitude of any observed systematic bias was examined with the effect size for paired observations according to Cohen’s classification, with d = 0.2 as being a small effect size, d = 0.5 as moderate and d = 0.8 as large. To analyze each patient and proxy difference, patient-proxy difference scores were computed by subtracting the patient’s scores from their proxy’s scores. The difference could range from –100 to 100. Positive differences indicated that patients reported more problems on that scale, while negative differences indicated that proxies reported more problems. Larger differences suggest a larger discrepancy between patients and proxies. The level of agreement between patient and proxy scores was evaluated using Bland-Altman plots, including the calculation of the mean difference between patients and proxies and the 95% confidence interval (CI) limits of the differences, and a regression line with 95% CI limits.
Subsequently, Mann-Whitney U tests were performed to identify any significant differences between neurocognitively impaired patients and non-neurocognitively impaired patients in patient-proxy difference scores for each of the 32 individual items as well as at the scale level. Patient-proxy differences were then categorized into “(dis)agreement groups”. For multi-item scales, patient-proxy dyads were 1) in agreement (defined as ≤10% under or above perfect agreement), 2) patients reporting more problems than their proxies, and 3) proxies reporting more problems than the patients. For the single-item scales, classifications were based on 1) in agreement, 2) patients reporting more problems, and 3) proxies reporting more problems. Pearson chi-square tests were used to determine if there were significant differences in neurocognitive status between the three (dis)agreement groups.
Lastly, independent associations between neurocognitive variables and the (dis)agreement groups were evaluated, specifically if neurocognitive variables were related to proxies reporting more problems, using univariable and multivariable multinomial logistic regression analyses (with the in agreement group as reference group). Relevant variables with P < .10 in the univariable analyses were included in the multivariable analyses (entry and removal likelihood ratios were set at P < .05). The univariable analyses included neurocognitive variables as well as sociodemographic and clinical variables to examine how they related to the two (dis)agreement groups. The neurocognitive variables included the number of impaired neurocognitive measures to evaluate the extent of the neurocognitive impairment, as well as the score on specific neurocognitive measures separately. The sociodemographic variables included patients’ age, sex, and level of education (low [score 1–4] vs. high [score 5–8]; according to the International Standard Classification of Education (ISCED)31), proxy type (partner vs. other) and duration of relationship in years. Clinical variables included tumor type (primary vs. metastatic brain tumor), tumor location (focal vs. diffuse), Karnofsky Performance Status (KPS) (able [KPS > 70] or unable [KPS ≤ 70] to perform normal activities), active tumor treatment (no current active tumor treatment vs. current active tumor treatment) and patients with and without “above average” (i.e. z-score of ≥1.0 SD below the norm scores29) patient-reported subjective neurocognitive complaints. Multivariable backward stepwise multinomial logistic regression analyses were performed for each of the five multi-item and the two single-item scales to determine which variables were independently associated with the two (dis)agreement groups, i.e. patients reporting more problems and proxies reporting more problems. Before performing the multivariable analyses, all relevant variables resulting from the univariable analyses were first checked for potential multicollinearity using multiple linear regression collinearity. Variables with multicollinearity (i.e. VIF < 10 or Tolerance >0.1) were excluded. In addition, sensitivity analyses were performed with stricter (≤15% under or above perfect agreement) and less strict (≤5% under or above perfect agreement) scale difference scores (Supplementary Material 3). For all statistical analyses, IBM SPSS version 26.032 was used. As this was an exploratory study, no multiple testing correction was implemented. However, a more stringent P-value of <.01 was used as statically significant when analyzing patient-proxy differences on the multiple scales. For the multivariable multinomial logistic regression analyses a P-value of <.05 was used.
Results
Data from 81 primary or metastatic brain tumor patients with neurocognitive data available and their proxies were analyzed to gain preliminary insight in the relation between neurocognitive deficits and differences in IADL assessment between patients and their proxies. Participants’ sociodemographic and clinical characteristics are described in Table 1.
Table 1.
Participants’ Sociodemographic, Clinical, and Neurocognitive Characteristics
| Patients | Proxies | |
|---|---|---|
| Participants, N | 81 | 81 |
| Sex (male), N (%) | 32 (39.5%) | 32 (39.5%) |
| Age, mean (SD) | 58.2 (11.7) | 57.7 (11.7) |
| Level of education [1–8], median [range] | 3 [1–8] | 4 [1–7] |
| Type of proxy, N (%) | ||
| Partner | 60 (74.1%) | |
| Other (e.g. parent/child/sibling) | 21 (25.9%) | |
| Duration relationship (in yrs), mean (SD) | 32.9 (14.20) | |
| Tumor type, N (%) | ||
| Glioma | 43 (53.1%) | |
| Diffuse astrocytoma, IDH-mutant + 1p/19q noncodeleted | 2 (4.7%) | |
| Diffuse astrocytoma, IDH-mutant + unknown 1p/19q-codeletion | 2 (4.7%) | |
| Oligodendroglioma, IDH-mutant + 1p/19q-codeleted | 2 (4.7%) | |
| Diffuse astrocytoma, IDH-wildtype | 1 (2.3%) | |
| Diffuse astrocytoma, NOS | 11 (25.6%) | |
| Anaplastic astrocytoma, IDH-mutant | 1 (2.3%) | |
| Anaplastic oligodendroglioma, 1p/19p-codeleted | 4 (9.3%) | |
| Anaplastic glioma, NOS | 2 (4.7%) | |
| Glioblastoma, IDH-mutant | 1 (2.3%) | |
| Glioblastoma, IDH-wildtype | 10 (23.3%) | |
| Glioblastoma, NOS | 7 (16.3%) | |
| Brain metastases | 38 (46.9%) | |
| 1-3 brain metastases | 21 (25.9%) | |
| >3 brain metastases | 17 (21.0%) | |
| Tumor location, N (%) | ||
| Frontal | 24 (29.6%) | |
| Temporal | 12 (14.8%) | |
| Occipital | 5 (6.2%) | |
| Parietal | 8 (9.9%) | |
| Multiple | 29 (35.8%) | |
| Other | 2 (2.5%) | |
| Unreported | 1 (1.2%) | |
| KPS score, median [range] | 80 [40-100] | |
| Subjective neurocognitive complaints (MOS COG–R), median [range] | 29 [6-36] | |
| Above average subjective neurocognitive complaints (MOS COG–R), N (%) | 56 (69.1%) | |
| Treatment status (active anti-tumor treatment), N (%) | 24 (29.6%) | |
| Neurocognitively impaired, N (%) | 37 (45.7%) | |
| Direct recall impaired, N (%) | 21 (25.9%) | |
| Delayed recall impaired, N (%) | 25 (30.9%) | |
| Recognition discrimination impaired, N (%) | 8 (9.9% | |
| Information processing speed impaired, N (%) | 22 (27.2%) | |
| Cognitive flexibility impaired, N (%) | 45 (55.6%) | |
| Verbal fluency impaired, N (%) | 18 (22.2%) |
N, number; SD, standard deviation; yrs, years; LGG, low grade glioma; HGG, high grade glioma; KPS, Karnofsky Performance Score; MOS COG–R, Medical Outcomes Study Cognitive Functioning Scale–Revised.
(Dis)agreement Between Patient- and Proxy-Reported Level of IADL
At the group level, proxies reported more problems on 27/32 individual IADL items and all multi-item scales than the patients themselves, although not on a statistically significant level. Only one individual item showed a statistically significant systematic response bias of P < .01 with a small effect size (i.e. “difficulty performing your daily activities without help of others”, patients M = 1.58 vs. proxies M = 1.86, P < .01, d = 0.3) (see Supplementary Material 2 for patient-proxy differences and effect sizes). Review of the Bland-Altman plots (Figure 1) revealed that there was a good deal of variation between patient-proxy dyads’ assessment of patient’s level of IADL, with 95% confidence interval limits being approximately 50 and –50 (on a range between –100 to 100). For Scale 1 (Domestic activities), Scale 3 (Modern devices and communication skills), and Scale 5 (Social activities), there was a significant deviation from a linear association between the assessment of patients and proxies, with proxies reporting more problems than patients. This trend was also observed for the other two scales and the two single-item scales, although not significant.
Figure 1.
Bland-Altman plots for each multi-item scale and the two single-item scales depicting the mean with confidence interval limits, as well as a regression line with confidence interval limits.
Patient-Proxy Difference Scores Between Neurocognitively Impaired and Non-Neurocognitively Impaired Patients
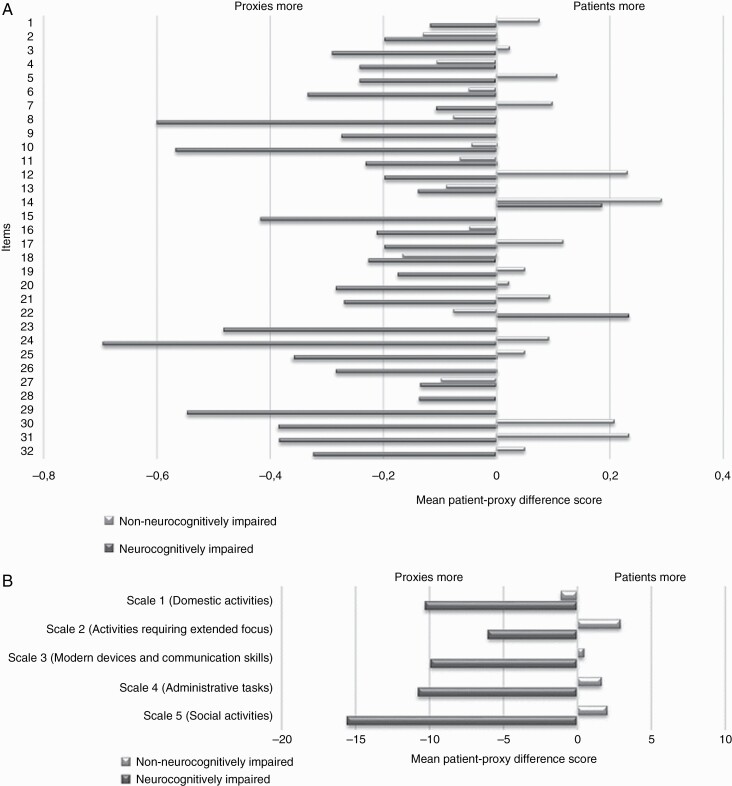
Figure 2A,B shows a comparison of patient-proxy difference scores of neurocognitively impaired and non-neurocognitively impaired patients, respectively. Although no significant (P < .01) differences were found on either the individual item or scale level, neurocognitively impaired patients reported more problems than their proxies on only 2/32 individual items (i.e. “difficulty having a one-on-one conversation in noisy surroundings” and “difficulty reading”), whereas non-neurocognitively impaired patients reported more problems compared to their proxies on almost half of the individual items (15/32). Similarly, at the scale level, proxies of neurocognitively impaired patients reported more problems than the patients themselves on all five scales, while non-neurocognitively impaired patients reported more problems compared to their proxies on 4/5 scales. The largest patient-proxy difference score discrepancies between neurocognitively impaired and non-neurocognitively impaired patients were for the individual items “difficulty learning new things”, “difficulty performing your tasks at work”, and “difficulty participating in a group conversation”.
Figure 2.
Average mean difference scores between patients and proxies for each of the 32 individual items separately (A) and for the five multi-item scales (B), presented separately for neurocognitively impaired and non-neurocognitively impaired patients.
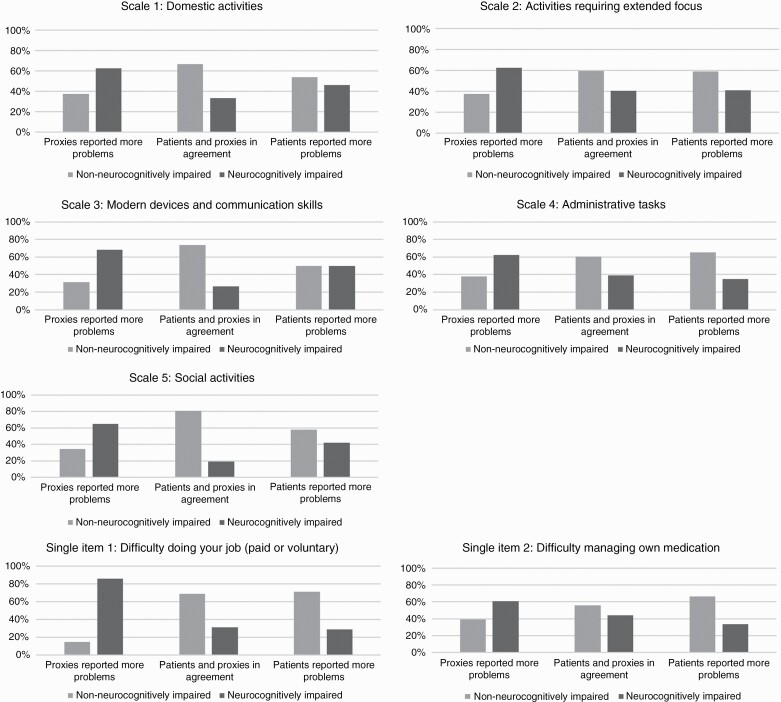
The association between neurocognitive status and the patient-proxy agreement groups is depicted in Figure 3. Depending on the scale, between 34.7% and 58.9% of the patient-proxy dyads were in the in agreement group, between 16.4–34.7% in the patients reported more problems group, and between 20.0–37.7% in the proxies reported more problems group. The in agreement and patients reported more problems group reflected most often non-neurocognitively impaired patients, while the proxies reported more problems group reflected most often neurocognitively impaired patients. Significant differences between the three subgroups were found for Scale 3 (Modern devices and communication skills; χ = 9.73, P = .01), Scale 5 (Social activities; χ = 10.66, P = .01), and Single item 1 (Difficulty doing your job; χ = 6.69, P = .04).
Figure 3.
Percentage of patient-proxy dyads in each (dis)agreement group (i.e. no agreement, patients reported more problems or proxies reported more problems) per neurocognitive status.
Association of Sociodemographic, Clinical, and Neurocognitive Variables With Patient-Proxy (Dis)agreement Groups
None of the sociodemographic variables had a statistically significant association with either the patients- or proxies reporting more problems (dis)agreement group. The univariable multinomial logistic regressions did show an association between various neurocognitive and clinical variables and the (dis)agreement groups on the assessed scales (Table 2).
Table 2.
Univariable and Multivariable Backward Stepwise Multinomial Logistic Regressions
| Univariable multinomial logistic regressions | Multivariable multinomial logistic regressions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients>Proxies | Proxies>Patients | Patients>Proxies | Proxies>Patients | |||||||||
| B (SE) | ORR [95% CI] | P | B (SE) | ORR [95% CI] | P | B (SE) | ORR [95% CI] | P | B (SE) | ORR [95% CI] | P | |
| Scale 1: Domestic activities (n = 79) Patients>Proxies (n = 13) & Proxies>Patients (n = 24) | ||||||||||||
| KPS score ≤70 | 2.83 (0.83) | 16.92 [3.31–86.50] | <.01 | 0.39 (1.29) | 1.47 [0.12–18.58] | .77 | 2.43 (0.87) | 11.39 [2.06–63.02] | <.01 | |||
| Above average MOS COG–R score | 1.15 (0.67) | 3.14 [0.84–11.72] | .09 | |||||||||
| Number of neurocognitive measures impaired | 0.48 (0.17) | 1.62 [1.16–2.24] | <.01 | |||||||||
| Impaired HVLT-R direct recall | 1.46 (0.61) | 4.29 [1.31–14.03] | .02 | |||||||||
| Impaired HVLT-R delayed recall | 1.28 (0.57) | 3.60 [1.18–10.94] | .02 | |||||||||
| Impaired HVLT-R recognition discrimination | 1.79 (0.98) | 6.00 [0.88–40.87] | .07 | |||||||||
| Impaired TMT-A | 1.44 (0.80) | 4.22 [0.88–20.19] | .07 | 2.25 (0.67) | 9.50 [2.58–35.02] | <.01 | 1.12 (0.86) | 3.07 [0.57–16.44] | .19 | 1.83 (0.72) | 6.25 [1.51–25.81] | .01 |
| Impaired COWAT | 1.49 (0.64) | 4.44 [1.28–15.45] | .02 | |||||||||
| Scale 2: Activities requiring extended focus (n = 80) Patients>Proxies (n = 22) & Proxies>Patients (n = 16) | ||||||||||||
| KPS score ≤70 | 1.75 (0.69) | 5.76 [1.48–22.41] | .01 | |||||||||
| Above average MOS COG–R score | –1.08 (0.59) | 2.94 [0.93–9.26] | .07 | 1.20 (0.64) | 3.31 [0.95–11.57] | .06 | ||||||
| Number of neurocognitive measures impaired | 0.42 (0.17) | 1.52 [1.09–2.12] | .01 | |||||||||
| Impaired HVLT-R direct recall | 1.36 (0.65) | 3.89 [1.08–13.96] | .04 | |||||||||
| Impaired HVLT-R delayed recall | 1.16 (0.62) | 3.20 [0.95–10.73] | .06 | |||||||||
| Impaired COWAT | 1.61 (0.65) | 5.00 [1.40–17.85] | .01 | –1.39 (1.11) | 0.25 [0.03–2.18] | .21 | 1.61 (0.65) | 5.00 [1.40–17.85] | .01 | |||
| Scale 3: Modern devices and communication skills (n = 77) Patients>Proxies (n = 20) & Proxies>Patients (n = 19) | ||||||||||||
| KPS score ≤70 | 2.20 (1.16) | 9.00 [0.93–87.03] | .06 | 3.27 (1.12) | 26.18 [2.94–232.95] | <.01 | 2.12 (1.17) | 8.30 [0.85–81.42] | .07 | 3.00 (1.15) | 20.16 [2.12–191.72] | <.01 |
| Active treatment | 1.22 (0.61) | 3.38 [1.03–11.11] | <.05 | |||||||||
| Number of neurocognitive measures impaired | 0.46 (0.17) | 1.59 [1.13–2.23] | <.01 | 0.23 (0.20) | 1.26 [0.85–1.87] | .26 | 0.50 (0.21) | 1.65 [1.10–2.49] | .02 | |||
| Impaired HVLT-R direct recall | 1.14 (0.65) | 3.11 [0.87–11.15] | .08 | |||||||||
| Impaired HVLT-R delayed recall | 1.22 (0.61) | 3.38 [1.03–11.11] | <.05 | |||||||||
| Impaired TMT-A | 1.57 (0.64) | 4.80 [1.37–16.81] | .01 | |||||||||
| Impaired COWAT | 1.57 (0.67) | 4.80 [1.30–17.78] | .02 | |||||||||
| Scale 4: Administrative tasks (n = 77) Patients>Proxies (n = 20) & Proxies>Patients (n = 20) | ||||||||||||
| Active treatment | 1.53 (0.67) | 4.60 [1.25–16.97] | .02 | 1.60 (0.69) | 4.94 [1.28–19.03] | .02 | 0.24 (0.68) | 1.27 [0.33–4.83] | .73 | |||
| Number of neurocognitive measures impaired | 0.42 (0.17) | 1.52 [1.09–2.12] | .01 | –0.10 (0.22) | 0.91 [0.60–1.39] | .66 | 0.40 (0.17) | 1.49 [1.07–2.07] | .02 | |||
| Impaired HVLT-R direct recall | 1.03 (0.62) | 2.81 [0.83–9.56] | <.10 | |||||||||
| Impaired HVLT-R delayed recall | 1.09 (0.59) | 2.98 [0.93–9.52] | .07 | |||||||||
| Impaired TMT-A | 1.58 (0.66) | 4.88 [1.35–17.65] | .02 | |||||||||
| Impaired TMT-B | 0.94 (0.55) | 2.56 [0.87–7.57] | .09 | |||||||||
| Scale 5: Social activities (n = 75) Patients>Proxies (n = 26) & Proxies>Patients (n = 23) | ||||||||||||
| KPS score ≤70 | 2.39 (1.12) | 10.94 [1.23–97.46] | .03 | |||||||||
| Primary brain tumor | –1.25 (0.60) | 0.27 [0.09–0.93] | .04 | –1.01 (0.63) | 0.37 [0.11–1.25] | .11 | –1.85 (0.70) | 0.16 [0.04–0.63] | <.01 | |||
| Number of neurocognitive measures impaired | 0.58 (0.21) | 1.79 [1.19–2.70] | <.01 | |||||||||
| Impaired HVLT-R direct recall | 1.67 (0.85) | 5.33 [1.01–28.21] | <.05 | 1.66 (0.86) | 5.25 [0.97–28.57] | .06 | ||||||
| Impaired TMT-A | 1.95 (0.74) | 7.03 [1.64–30.11] | <.01 | |||||||||
| Impaired TMT-B | 1.68 (0.63) | 5.35 [1.56–18.36] | <.01 | 0.99 (0.62) | 2.69 [0.80–9.02] | .11 | 2.19 (0.72) | 8.94 [2.20–36.38] | <.01 | |||
| Impaired COWAT | 1.41 (0.75) | 1.09 [0.93–17.92] | .06 | |||||||||
| Single item 1: Difficulty doing your job (paid or voluntary) (n = 30) Patients>Proxies (n = 7) & Proxies>Patients (n = 7) * | ||||||||||||
| Primary brain tumor | –2.02 (1.02) | 0.13 [0.02–0.98] | <.05 | –2.02 (1.02) | 0.13 [0.02–0.98] | <.05 | ||||||
| Active treatment | 2.02 (1.02) | 7.50 [1.02–55.00] | <.05 | |||||||||
| Number of neurocognitive measures impaired | 0.79 (0.36) | 2.21 [1.09–4.47] | .03 | |||||||||
| Impaired TMT-A | 2.23 (1.08) | 9.33 [1.14–76.69] | .04 | |||||||||
| Impaired COWAT | 1.75 (1.00) | 5.78 [0.82–40.76] | .08 | |||||||||
| Single item 2: Difficulty managing own medication (n = 73) Patients>Proxies (n = 18) & Proxies>Patients (n = 12) | ||||||||||||
| Impaired HVLT-R direct recall | 1.19 (0.64) | 3.27 [0.94–11.38] | .06 |
All variables P < .10 in the univariable analyses were included. Reference category = “Patients and proxies in agreement”.
*Sample size too small for multivariate multinomial logistic regression.
KPS, Karnofsky Performance Score; MOS COG–R, Medical Outcomes Study Cognitive Functioning Scale–Revised; HVLT-R, Hopkins Verbal Learning Test–Revised; TMT, Trail Making Test; COWAT, Controlled Oral Word Association Test.
The multivariable analyses revealed which clinical variables and neurocognitive measures were independently associated with which (dis)agreement group (also see Table 2). Neurocognitive variables showed independent associations with proxies reporting more problems; namely impaired information processing speed for Scale 1 (Domestic activities), impaired verbal fluency for Scale 2 (Activities requiring extended focus), impaired cognitive flexibility for Scale 5 (Social activities), and a higher number of impaired neurocognitive measures for Scale 3 (Modern devices and communication skills) and Scale 4 (Administrative tasks). In addition, clinical variables independently associated with proxies reporting more problems were found for scales 1 and 3 with respect to a KPS score ≤70 and having a metastatic brain tumor for scale 5. No neurocognitive variables were found to be independently associated with patients reporting more problems. In this group, the only significant independent association was found between undergoing active tumor treatment and scale 4.
With regards to the single-item scales, the sample size that could rate Single item 1: Difficulty doing your job (paid or voluntary) was too small (n = 30) to perform a reliable multivariable regression analysis (many patients were not employed), and for Single item 2: Difficulty managing own medication, no variable was significantly associated with either (dis)agreement group.
Sensitivity analyses with more and less strict cutoffs for agreement had an impact on which outcomes were associated, although they were all related to neurocognitive or clinical functioning (Supplementary Material 3). Indeed, the type of neurocognitive variable varied, or additional neurocognitive or clinical (i.e. above average subjective neurocognitive complaints or a KPS score of ≤70) variables were found to be independently associated with either the patients- or proxies reporting more problems (dis)agreement group for the different scales.
Discussion
These first analyses towards a better insight in the potential effects neurocognitive deficits could have on self-assessment of IADL in brain tumor patients suggests that patients and their proxies differ in their assessment of the patient’s instrumental daily functioning, particularly when patients are cognitively impaired. In general, patients with neurocognitive impairments reported fewer problems with IADL compared to their proxies. In patients without extensive neurocognitive impairments, there tended to be better agreement between patient- and proxy-rated IADL problems, with patients even reporting more problems with IADL than their proxy in almost half the individual items.
The multivariable multinomial logistic regressions analyses revealed that neurocognitive variables were the variables most strongly associated with the proxies reporting more problems group, although the specific variables differed per scale (e.g. associations with either information processing speed, verbal fluency, or cognitive flexibility). This is not unexpected in that the different scales may assess IADLs that rely on different neurocognitive domains. Hall et al. (2011),33 for example, found that even at the individual item level, intact executive functioning predicted independence in medication management, transportation, laundry, and housekeeping, while intact memory and learning capacity predicted independence in financial management, shopping, and telephone use in patients with mild Alzheimer’s disease. Further, the effects of neurocognitive impairments on the ability to assess one’s own level of functioning is complex. The mere presence of neurocognitive impairments does not automatically mean that the patient has decreased awareness of his/her level of functioning,34 but perhaps decreased awareness is related to damage to specific brain regions associated with meta-cognition, self-reflection, and/or memory.35 This might also explain the variation in agreement observed among patient-proxy dyads, even when patients have similar levels of neurocognitive functioning. Nevertheless, on a group level patents and proxies did not seem to differ, which is in contrast to findings in other patient populations,11–13 suggesting that multiple brain functions are involved. Indeed, many cognitive problems cannot be explained by tumor location or volume alone,36–38 and presumably also problems with IADL, and future studies should therefore address the relation between tumor location, volume, and functional brain networks, and problems with IADL.
The multivariable multinomial logistic regression analyses also showed an independent association between having a metastatic brain tumor and proxies reporting more problems on the Social activity scale, in addition to cognitive flexibility, but not for the other scales. More in-depth research should be performed to determine the interplay between tumor-induced and treatment-induced neurocognitive deficits (e.g. “chemo-brain”) and the difference in the assessment of IADL between patients and their proxies.
This study has some limitations with respect to the sample size and population, and lack of inclusion of possible confounding variables. First, no variables were found to be clearly independently associated with the IADL scales in the patients reporting more problems group. Even variables such as patients’ self-reported subjective cognitive complaints were not found to be independently associated with this (dis)agreement group. This could be related to the relatively small sample and the heterogeneous brain tumor population, as this study included a small population (n = 81) of both primary and metastatic brain tumor patients in all stages of their disease. As has been reported in previous research,3,10 none of the sociodemographic variables and only some of the clinical variables were associated with either patients or proxies reporting more IADL problems. However, perhaps other potentially relevant variables not considered in this study, such as patients’ and proxies’ personalities, mental state (i.e. proxy’s level of psychological distress or perceived burden) during the assessment and other psychological factors (i.e. mood disorders), could have played a role in the patient-proxy disagreement. It could be argued though, that these factors may have a larger effect on more subjective measures, such as pain or feelings of anxiety, than on the more observable daily functioning activities.39,40 In addition, the norm data used in this study to assess the neuropsychological tests are somewhat outdated, but it is expected that the categorization of patients into impaired or nonimpaired is not impacted.
The findings in this study raise the question who can best rate the brain tumor patients’ level of IADL, particularly when significant neurocognitive impairments are present. Objective IADL performance assessments, not included in the present study, might be necessary in determining who can most accurately rate the patient’s IADL and what role neurocognitive impairments play, as they assess IADL in a standardized context and typically are precise and sensitive to changes. However, studies comparing objective and subjective assessments of everyday activities have not always found strong associations. Sadek et al. (2011)41 did find significant correlations of moderate strength between a performance-based battery of daily living skills, and the self- and informant-reported version of the Functional Activities Questionnaire (FAQ) in stroke patients. However, other studies in patients with multiple sclerosis,42 patients with Parkinson’s disease,43 and cognitively healthy older adults44 did not observe a significant association between performance-based and self-reported IADL. The correlation between a performance-based and self- and proxy-reported IADL score in older adults with MCI was found to be significant, but weak.45 Despite the seemingly inconsistent results between subjective and objective IADL measures in other patient groups, future research is needed to determine the concurrent validity between performance-based IADL and the corresponding self-reported and proxy-reported IADL, to evaluate which type of assessment is more informative to assess IADL in brain tumor patients. The downside to objective assessments of IADL performance is that they often assess a limited number of aspects of IADL (e.g. cooking, grocery shopping or managing finances) and are an assessment of a patient’s ability on that particular day. Moreover, there are often no premorbid assessments and it is, therefore, difficult to gain insight in potential subtle changes in abilities over time, which could have been observable to proxies as they typically know how the patient functioned before the disease onset. Lastly, in terms of practicality, performance-based measures take time which may be perceived as a barrier in busy clinics. Nevertheless, it remains to be investigated to what extent the EORTC IADL-BN32 questionnaire correlates with performance-based measures. Currently, new technologies are being explored to objectively assess IADL performance. Virtual Reality46 and smart homes47 seem promising methods for objective assessment of IADL performance in patients with mild cognitive impairments.
Our results suggest that patients with a brain tumor and their proxies differ in their assessment of patient’s level of IADL and that neurocognitive impairments seem to be associated with this patient-proxy (dis)agreement. Future studies should address the exact role between neurocognitive impairments and neurocognitive decline over time, and the level of patient-proxy (dis)agreement, focusing on the associated neurocognitive domains and underlying functional networks. Data from the phase IV validation study may be used to (partly) answer this question. Whether the patient or proxy evaluation of patient’s level of IADL is most accurate remains to be determined, but it does appear that caution is warranted with the self-reported IADL by brain tumor patients with neurocognitive impairments. Therefore patient-reported IADL should be supplemented with the proxy-reported assessments in cases when neurocognitive impairments are apparent to gain a better picture of the patients IADL functioning. Future efforts in the development of a reliable and valid IADL instrument includes establishing the concurrent validity between objective assessment of IADL performance and self-reported IADL in brain tumor patients.
Supplementary Material
Contributor Information
Quirien Oort, Department of Neurology and Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Linda Dirven, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Sietske A M Sikkes, Department of Epidemiology and Biostatistics, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Alzheimer Center, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Vrije Universiteit Amsterdam, Faculty of Behavioural and Movement Sciences (FGB), Department of Clinical Developmental & Clinical Neuropsychology, Amsterdam, The Netherlands.
Neil Aaronson, Division of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Florien Boele, Leeds Institute of Medical Research, St James’s University Hospital, Leeds, UK; Leeds Institute of Health Sciences, Faculty of Medicine and Health, University of Leeds, Leeds, UK.
Christine Brannan, East & North Hertfordshire NHS Trust incorporating Mount Vernon Cancer Centre, Northwood, UK.
Jonas Egeter, Department for Psychiatry, Psychotherapy and Psychosomatics, University Hospital of Psychiatry II, Medical University of Innsbruck, Innsbruck, Austria.
Robin Grant, Department of Clinical Neurosciences, Western General Hospital, Edinburgh, UK.
Martin Klein, Department of Medical Psychology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Irene M Lips, Department of Radiation Oncology, Leiden University Medical Center, Leiden, The Netherlands.
Yoshitaka Narita, Department of Neurosurgery and Neuro-Oncology, National Cancer Center, Tokyo, Japan.
Hitomi Sato, Department of Neurosurgery and Neuro-Oncology, National Cancer Center, Tokyo, Japan; Department of Nursing, Teikyo Heisei University, Tokyo, Japan.
Monika Sztankay, Department for Psychiatry, Psychotherapy and Psychosomatics, University Hospital of Psychiatry II, Medical University of Innsbruck, Innsbruck, Austria.
Günther Stockhammer, Department of Neurology, Innsbruck Medical University, Innsbruck, Austria.
Andrea Talacchi, Department of Neurosurgery, Azienda Ospedaliera San Giovanni Addolorata, Roma, Italy.
Bernard M J Uitdehaag, Department of Neurology and Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Jaap C Reijneveld, Department of Neurology and Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Neurology, Stichting Epilepsie Instellingen Nederland (SEIN), Heemstede, The Netherlands.
Martin J B Taphoorn, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Funding
This study was funded by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Group. QLG Project Number (grant number): 004/2014.
Conflict of interest statement. None of the authors declares a conflict of interest.
References
- 1. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000; 47(2): 324–33-; discussion 33–34. [DOI] [PubMed] [Google Scholar]
- 2. Bartolomei F, Bosma I, Klein M, et al. How do brain tumors alter functional connectivity? A magnetoencephalography study. Ann Neurol. 2006;59(1):128–138. [DOI] [PubMed] [Google Scholar]
- 3. Bosma I, Reijneveld JC, Klein M, et al. Disturbed functional brain networks and neurocognitive function in low-grade glioma patients: a graph theoretical analysis of resting-state MEG. Nonlinear Biomed Phys. 2009;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Derks J, Reijneveld JC, Douw L. Neural network alterations underlie cognitive deficits in brain tumor patients. Curr Opin Oncol. 2014;26(6):627–633. [DOI] [PubMed] [Google Scholar]
- 5. Kovalchuk A, Kolb B. Chemo brain: from discerning mechanisms to lifting the brain fog—an aging connection. Cell Cycle. 2017;16(14):1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 7. Passler JS, Kennedy RE, Clay OJ, et al. The relationship of longitudinal cognitive change to self-reported IADL in a general population. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2019;27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koster N, Knol DL, Uitdehaag BM, Scheltens P, Sikkes SA. The sensitivity to change over time of the Amsterdam IADL Questionnaire((c)). Alzheimers Dement. 2015;11(10):1231–1240. [DOI] [PubMed] [Google Scholar]
- 9. Oort Q, Taphoorn MJB, Sikkes SAM, et al. Evaluation of the content coverage of questionnaires containing basic and instrumental activities of daily living (ADL) used in adult patients with brain tumors. J Neurooncol. 2019;143(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oort Q, Dirven L, Sikkes SAM, et al. ; EORTC Quality of Life Group. Development of an EORTC questionnaire measuring instrumental activities of daily living (IADL) in patients with brain tumours: phase I-III. Qual Life Res. 2021;30(5):1491–1502. doi: 10.1007/s11136-020-02738-5. Epub 2021 Jan 26. PMID: 33496902; PMCID: PMC8068708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20(9):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ediebah DE, Reijneveld JC, Taphoorn MJ, et al. Impact of neurocognitive deficits on patient-proxy agreement regarding health-related quality of life in low-grade glioma patients. Qual Life Res. 2017;26(4):869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiyak HA, Teri L, Borson S. Physical and functional health assessment in normal aging and in alzheimer’s disease: self-reports vs family reports. Gerontologist. 1994;34(3):324–330. [DOI] [PubMed] [Google Scholar]
- 14. Sneeuw KC, Aaronson NK, Osoba D, et al. The use of significant others as proxy raters of the quality of life of patients with brain cancer. Med Care. 1997;490:506. [DOI] [PubMed] [Google Scholar]
- 15. EORTC QLQ-C30 Scoring Manual. EORTC QOL Group; 2001. [Google Scholar]
- 16. EORTC Quality of Life Group Modules. https://qol.eortc.org/modules/.
- 17. EORTC Quality of Life Group. https://qol.eortc.org/.
- 18. Weller J, Tzaridis T, Mack F, et al. Health-related quality of life and neurocognitive functioning with lomustine–temozolomide versus temozolomide in patients with newly diagnosed, MGMT-methylated glioblastoma (CeTeG/NOA-09): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2019;20(10):1444–1453. [DOI] [PubMed] [Google Scholar]
- 19. Hassler M, Elandt K, Preusser M, et al. Neurocognitive training in patients with high-grade glioma: A pilot study. J Neurooncol. 2009;97:109–115. [DOI] [PubMed] [Google Scholar]
- 20. Haldbo-Classen L, Amidi A, Lukacova S, et al. Cognitive impairment following radiation to hippocampus and other brain structures in adults with primary brain tumors. Radiother Oncol. 2020;148:1–7. [DOI] [PubMed] [Google Scholar]
- 21. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. [DOI] [PubMed] [Google Scholar]
- 23. Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 24. Brandt J, Benedict RHB.. Hopkins Verbal Learning Test—Revised. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 25. Reitan RM, Wolfson D. The Halstead–Reitan neuropsychological test battery: research findings and clinical application. In: Kaufman AS, Kaufman NL, eds. Specific Learning Disabilities and Difficulties in Children and Adolescents: Psychological Assessment and Evaluation. Cambridge University Press; 2001:309–346. [Google Scholar]
- 26. Benton AL, Hamsher K, Sivan AB.. Multilingual aphasia examination. Iowa City: University of Iowa;1976. [Google Scholar]
- 27. Mitrushina M, Boone KB, Razani J, D’Elia LF.. Handbook of normative data for neuropsychological assessment. Oxford University Press; 2005. [Google Scholar]
- 28. Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301. [DOI] [PubMed] [Google Scholar]
- 29. Lezak MD, Howieson DB, Loring DW.. Neuropsychological assessment. New York: Oxford Univer. Press Google Scholar;1995. [Google Scholar]
- 30. Hays RD, Sherbourne CD, Mazel R.. User’s manual for the medical outcomes study (mos) core measures of health-related quality of life. Santa Monica, CA: RAND Corporation;1995. [Google Scholar]
- 31. UNESCO Institute for Statistics. International Standard Classification of Education: ISCED 2011; 2012. [Google Scholar]
- 32. IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. 2016. [Google Scholar]
- 33. Hall JR, Vo HT, Johnson LA, Barber RC, O’Bryant SE. The link between Cognitive Measures and ADLs and IADL Functioning in Mild Alzheimer’s: what has gender got to do with it? Int J Alzheimers Dis. 2011;2011:276734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caramanna I, Bottomley A, Drijver AJ, et al. Objective neurocognitive functioning and neurocognitive complaints in patients with high-grade glioma: evidence of cognitive awareness from the European Organisation for Research and Treatment of Cancer brain tumor clinical trials. Eur J Cancer. 2021;144:162–168. [DOI] [PubMed] [Google Scholar]
- 35. Lyons KE, Zelazo PD. Monitoring, metacognition, and executive function: Elucidating the role of self-reflection in the development of self-regulation. Adv Child Develop Behav 2011;40:379–412. [DOI] [PubMed] [Google Scholar]
- 36. Dallabona M, Sarubbo S, Merler S, et al. Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neuro-Oncol Pract 2017;4(4):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Habets EJJ, Hendriks EJ, Taphoorn MJB, et al. Association between tumor location and neurocognitive functioning using tumor localization maps. J Neurooncol. 2019;144(3):573–582. [DOI] [PubMed] [Google Scholar]
- 38. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumors. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 39. Essen L. Proxy ratings of patient quality of life Factors related to patient–proxy agreement. Acta Oncol. 2004;43(3):229–234. [DOI] [PubMed] [Google Scholar]
- 40. Sneeuw KCA, Aaronson NK, Sprangers MAG, et al. Evaluating the quality of life of cancer patients: assessments by patients, significant others, physicians and nurses. Br J Cancer. 1999;81(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sadek JR, Stricker N, Adair JC, Haaland KY. Performance-based everyday functioning after stroke: relationship with IADL questionnaire and neurocognitive performance. J Int Neuropsychol Soc. 2011;17(5):832–840. [DOI] [PubMed] [Google Scholar]
- 42. Goverover Y, Kalmar J, Gaudino-Goering E, et al. The relation between subjective and objective measures of everyday life activities in persons with multiple sclerosis. Arch Phys Med Rehabil. 2005;86(12):2303–2308. [DOI] [PubMed] [Google Scholar]
- 43. Shulman LM, Pretzer-Aboff I, Anderson KE, et al. Subjective report versus objective measurement of activities of daily living in Parkinson’s disease. Mov Disord. 2006;21(6):794–799. [DOI] [PubMed] [Google Scholar]
- 44. Schmitter-Edgecombe M, Parsey C, Cook DJ. Cognitive correlates of functional performance in older adults: comparison of self-report, direct observation, and performance-based measures. J Int Neuropsychol Soc. 2011;17(5):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology 2009;55(5):570–581. [DOI] [PubMed] [Google Scholar]
- 46. Atkins AS, Khan A, Ulshen D, et al. Assessment of instrumental activities of daily living in older adults with subjective cognitive decline using the Virtual Reality Functional Capacity Assessment Tool (VRFCAT). J Prev Alzheimer’s Dis. 2018;5(4):216–234. [DOI] [PubMed] [Google Scholar]
- 47. Rawtaer I, Abdul Jabbar K, Liu X, et al. Performance-based IADL evaluation of older adults with cognitive impairment within a smart home: a feasibility study. Alzheimers Dement (N Y). 2021;7(1): e12152-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.