Abstract
Aim
To synthetize studies assessing somatosensory deficits and alterations in cerebral responses evoked by somatosensory stimulation in individuals with cerebral palsy (CP) compared to typically developing individuals.
Method
A scoping review of the literature was performed in the MEDLINE, Embase, PsycInfo, CINAHL, Evidence‐Based Medicine Reviews, and Web of Science databases (last search carried out on 6th and 7th August 2020) with a combination of keywords related to CP and somatosensory functions. Somatosensory deficits were measured with clinical tests and alterations in cerebral responses were measured with functional magnetic resonance imaging, electroencephalography, and magnetoencephalography.
Results
Forty‐eight articles were included. Overall, 1463 participants with CP (mean [SD] age 13y 1mo [4y 11mo], range 1–55y; 416 males, 319 females, sex not identified for the remaining participants) and 1478 controls (mean [SD] age 13y 1mo [5y 8mo], range 1–42y; 362 males, 334 females, sex not identified for the remaining participants) were included in the scoping review. For tactile function, most studies reported registration (8 out of 13) or perception (21 out of 21) deficits in participants with CP. For proprioception, most studies also reported registration (6 out of 8) or perception (10 out of 15) deficits. Pain function has not been studied as much, but most studies reported registration (2 out of 3) or perception (3 out of 3) alterations. Neuroimaging findings (18 studies) showed alterations in the somatotopy, morphology, latency, or amplitude of cortical responses evoked by somatosensory stimuli.
Interpretation
Despite the heterogeneity in the methods employed, most studies reported somatosensory deficits. The focus has been mainly on tactile and proprioceptive function, whereas pain has received little attention. Future research should rigorously define the methods employed and include a sample that is more representative of the population with CP.
What this paper adds.
Most of the papers reviewed found tactile registration and perception deficits in the upper limbs.
Proprioceptive deficits were generally observed in cerebral palsy but results were heterogeneous.
Pain has received little attention compared to tactile and proprioceptive functions.
Neuroimaging studies supported behavioral observations.
Alterations were observed for both the most and least affected limb.
What this paper adds
Most of the papers reviewed found tactile registration and perception deficits in the upper limbs.
Proprioceptive deficits were generally observed in cerebral palsy but results were heterogeneous.
Pain has received little attention compared to tactile and proprioceptive functions.
Neuroimaging studies supported behavioral observations.
Alterations were observed for both the most and least affected limb.
Editor’s Choice
Cerebral palsy is essentially a sensorimotor disorder,1 but the clinical and research focus has almost exclusively been on motor aspects. My Editor's Choice for the December 2021 issue is this scoping review of clinical impairments of somatosensory function and functioning of the neural pathways in people with cerebral palsy. It highlights a useful body of findings on tactile and proprioceptive sensation and perception, and less good documentation of pain processing.
Reference 1. Dan B. Cerebral palsy is a sensorimotor disorder. Dev Med Child Neurol 2020; 62: 768.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Déficits somatosensoriales y correlatos cerebrales en la parálisis cerebral: una revisión del alcance
Objetivo
Sintetizar estudios que evalúen los déficits somatosensoriales y las alteraciones en las respuestas cerebrales provocadas por la estimulación somatosensorial en individuos con parálisis cerebral (PC) en comparación con individuos con desarrollo típico.
Método
Se realizó una revisión de alcance de la literatura en las bases de datos MEDLINE, Embase, PsycInfo, CINAHL, Evidence‐Based Medicine Reviews y Web of Science (última búsqueda realizada los días 6 y 7 de agosto de 2020) con una combinación de palabras clave relacionadas con la PC y funciones somatosensoriales. Los déficits somatosensoriales se midieron con pruebas clínicas y las alteraciones en las respuestas cerebrales se midieron con resonancia magnética funcional, electroencefalografía y magnetoencefalografía.
Resultados
Se incluyeron 48 artículos. En general, 1463 participantes con PC (edad media [DE] 13 años 1 mes [4 años 11 meses], rango 1‐55 años; 416 varones, 319 mujeres, sexo no identificado para los participantes restantes) y 1478 controles (edad media [DE] 13 años 1 mes [5 años y 8 meses], rango 1–42 años; 362 varones, 334 mujeres, sexo no identificado para los participantes restantes) se incluyeron en la revisión de alcance. Para la función táctil, la mayoría de los estudios informaron déficits de registro (8 de 13) o de percepción (21 de 21) en los participantes con PC. Para la propiocepción, la mayoría de los estudios también informaron déficits de registro (6 de 8) o de percepción (10 de 15). La función del dolor no se ha estudiado tanto, pero la mayoría de los estudios informaron alteraciones del registro (2 de 3) o de la percepción (3 de 3). Los hallazgos de neuroimagen (18 estudios) mostraron alteraciones en la somatotopía, morfología, latencia o amplitud de las respuestas corticales evocadas por estímulos somatosensoriales.
Interpretación
A pesar de la heterogeneidad en los métodos empleados, la mayoría de los estudios informaron déficits somatosensoriales. La atención se ha centrado principalmente en la función táctil y propioceptiva, mientras que el dolor ha recibido poca atención. Las investigaciones futuras deberían definir rigurosamente los métodos empleados e incluir una muestra más representativa de la población con PC.
Abstract
OBJETIVO
Sintetizar estudos avaliando déficits somatossensoriais e alterações nas respostas cerebrais evocadas por estimulação somatossensorial em indivíduos com paralisia cerebral (PC) comparados a indivíduos com desenvolvimento típico.
MÉTODO
Uma revisão de escopo da literatura foi realizada nas bases de dados MEDLINE, Embase, PsycInfo, CINAHL, Evidence‐Based Medicine Reviews e Web of Science (última pesquisa realizada em 6 e 7 de agosto de 2020) com uma combinação de palavras‐chave relacionadas a CP e funções somatossensoriais. Déficits somatossensoriais foram medidos com testes clínicos e alterações nas respostas cerebrais foram medidas com ressonância magnética funcional, eletroencefalografia e magnetoencefalografia.
RESULTADOS
Quarenta e oito artigos foram incluídos. No geral, 1.463 participantes com PC (média [SD] idade 13 anos 1 mês [4 anos 11 meses], intervalo 1¬–55 anos; 416 homens, 319 mulheres, sexo não identificado para os demais participantes) e 1.478 controles (média [SD] idade 13 anos 1 mês [5a 8 meses], intervalo de 1 a 42 anos; 362 homens, 334 mulheres, sexo não identificado para os demais participantes) foram incluídos na revisão de escopo. Para a função tátil, a maioria dos estudos relatou déficits de registro (8 de 13) ou percepção (21 de 21) em participantes com PC. Para propriocepção, a maioria dos estudos também relatou déficits de registro (6 em 8) ou percepção (10 em 15). A função da dor não foi muito estudada, mas a maioria dos estudos relatou alterações no registro (2 em 3) ou na percepção (3 em 3). Achados de neuroimagem (18 estudos) mostraram alterações na somatotopia, morfologia, latência ou amplitude das respostas corticais evocadas por estímulos somatossensoriais.
INTERPRETAÇÃO
Apesar da heterogeneidade nos métodos empregados, a maioria dos estudos relatou déficits somatossensoriais. O foco tem sido principalmente na função tátil e proprioceptiva, enquanto a dor tem recebido pouca atenção. Pesquisas futuras devem definir rigorosamente os métodos empregados e incluir uma amostra mais representativa da população com PC.
Abbreviations
- SEF
Somatosensory evoked field
- SEP
Somatosensory evoked potential
Cerebral palsy (CP) is a neurodevelopmental disorder caused by non‐progressive disturbances occurring in the fetal or infant brain and is characterized by permanent and non‐progressive motor, sensory, perceptive, cognitive, communicative, and/or behavioral deficits. 1 Historically, rehabilitation has focused mainly on motor deficits, but increasing attention has been paid to sensory impairments. 2 Across the various sensory impairments, the somatosensory system, which includes all peripheral and central components involved in the transmission and processing of sensory information arising from superficial or cutaneous receptors and/or from the musculoskeletal system, has received the most attention. This recent increase in interest for somatosensory deficits in CP is mainly explained by the fact that the somatosensory system is intimately related to the motor system. Therefore, somatosensory deficits are likely to partially explain and/or exacerbate motor impairments, 3 , 4 leading some authors to consider CP as a sensorimotor disorder. 5 , 6
A systematic review published in 2013 focused on somatosensory function in relation to precision grip control in children with unilateral CP and found that despite these children exhibiting significant impairments both at the sensory and motor level, there was no clear relationship between these two types of deficits. 7 At the somatosensory level, two main modalities were tested in children with unilateral CP: proprioception and tactile function, including stereognosis. 7 However, this review focused only on unilateral CP, which represents less than one‐third of children with CP (for a meta‐analysis, see Himpens et al. 8 ). Moreover, the absence of quality assessment of the articles included in the review limits the possibility to assess methodological bias and make conclusions about the severity of somatosensory deficits in children with unilateral CP. Finally, the use of somatosensory assessments has increased in the literature in the last decade due to renewed interest in somatosensory deficits in CP. For instance, important work has been done to categorize tactile deficits, assess psychometric properties of clinical tactile assessments, 9 and provide a framework to increase efficiency in tactile treatments. 9 , 10 To better understand alterations in tactile assessments, Auld et al. suggested dissociating between registration (stimulus detection by the somatosensory system) and perception (‘understand, interpret or give meaning to sensory stimuli’) to guide clinical practice and research. 9 Moreover, more attention has been paid to pain perception, 11 , 12 , 13 which is recognized as a serious issue in CP, 14 and proprioception. 15 , 16 For example, recent laboratory assessments have been used to assess proprioception in individuals with CP more precisely 16 and rehabilitation programs have focused on proprioception treatments in individuals with CP. 15 In addition, an increasing literature combines behavioral assessment and neuroimaging tools to objectivate somatosensory alterations. 17 , 18 , 19 Finally, somatosensory deficits in adults with CP are increasingly assessed 11 , 20 , 21 and might differ from those observed in children. 22
Therefore, the primary objective of this scoping review was to synthetize studies assessing somatosensory deficits in children and adults with CP compared to typically developing individuals. Since most somatosensory assessments depend on subjective reports, a secondary objective was to identify studies assessing alterations in cerebral responses evoked by somatosensory stimulation in individuals with CP compared to typically developing individuals. More specifically, this scoping review focuses on behavioral and neuroimaging studies.
METHOD
The protocol for this review was registered with the PROSPERO network (https://www.crd.york.ac.uk/prospero/; registration number: CRD42020185046).
Data sources
The systematic literature search was conducted in six online databases: MEDLINE (Ovid); Embase (Ovid); PsycInfo (Ovid); CINAHL (EBSCOhost); Evidence‐Based Medicine Reviews (Ovid); and Web of Science (Science Citation Index Expanded, Social Sciences Citation Index, and Emerging Sources Citation Index). It was initially run on 4th July 2019 and then rerun on 6th August 2020 (MEDLINE, Embase, PsycInfo) and 7th August 2020 (CINAHL, Evidence‐Based Medicine Reviews, Web of Science). The search strategy was developed with a professional librarian. It explored two main concepts (‘cerebral palsy’ and ‘somatosensory function’) and used a combination of controlled vocabulary and free‐text terms. No language or date limit was applied. In accordance with the inclusion criteria, conference abstracts, dissertation abstracts, and books were excluded in the relevant databases. The full search strategy for each database is described in Table S1 (online supporting information). The bibliographical references of the studies included in the review were also searched for additional relevant articles.
Duplicates were removed by the librarian using EndNote (Clarivate Analytics, Philadelphia, PA, USA) according to the deduplication method suggested by Bramer et al. 23 Search results were exported to Covidence (https://www.covidence.org/), an online program optimized for review management. First, two review authors independently screened titles and abstracts to remove irrelevant articles. Then, they reviewed the full text of potentially relevant studies to determine their eligibility. Both authors completed the study selection and data extraction. In case of disagreement, studies were reassessed until consensus was reached. The process used to select the articles for this review follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.
Inclusion criteria
Studies were included if they met the following criteria: (1) they involved human participants (children or adults) with a diagnosis of CP; (2) they involved a control group composed of human participants (children or adults) with typical development and no other neurological conditions; (3) they assessed somatosensory function with behavioral measures (main objective) or assessed cerebral responses evoked by somatosensory stimulation (secondary objective); (4) they were research papers that included an original data set (single case studies, conference abstracts, and reviews were excluded) to increase objectivity and reduce the risk of including duplicate data sets; (5) they were published in a peer‐reviewed journal in English, French, or other languages that could be translated in a satisfactory manner with free online translation tools, such as Google Translate.
Data extraction
The following information was extracted from each study: (1) name of the first author and year of publication; (2) country of origin; (3) study’s setting and conflicts of interests; (3) aim/purpose of the study; (4) participant characteristics (number of participants, age, sex, CP etiology, Manual Ability Classification System and Gross Motor Function Classification System levels, type of CP [unilateral or bilateral and spastic or non‐spastic]). For the type of CP, the classification proposed by the Surveillance of Cerebral Palsy in Europe was used. 24 According to this scoping review, categorizing between unilateral/bilateral and spastic/non‐spastic is the best approach to absorb the large heterogeneity across studies when traditional classifications are used (motor, topography, functional level, or birth); 24 (5) tested body part(s); (6) subcategories of somatosensory function (see the next section for more details); (7) somatosensory tests and results. For neuroimaging studies, additional data were extracted: (1) neuroimaging method (e.g. electroencephalography [EEG], magnetoencephalography, functional magnetic resonance imaging [fMRI]); (2) types of measures (morphology, latency, amplitude, and/or somatotopy) and results.
As shown in Figure 1 and according to the International Classification of Functioning, Disability and Health validated for CP, 25 somatosensory function was categorized into three subtypes: tactile, proprioception, and pain. For each subtype, two domains were considered: registration (or sensation) and perception. 9 First, registration refers to the external or internal stimulus detection by the somatosensory receptor, that is, when the stimulus exceeds the receptor sensory threshold. For tactile and pain functions, registration can be subdivided according to mechanical (e.g. vibration threshold, tactile threshold with monofilaments, pain pressure threshold with pinprick) and thermal (e.g. cold/hot detection or pain threshold) stimuli. 9 , 26 For proprioception, registration refers to the detection of movement (e.g. identifying when a limb is passively moved or identifying the direction of movement). 27 Second, perception is to ‘understand, interpret or give meaning to sensory stimuli’. 9 For tactile and pain functions, perception gathers spatial (e.g. stereognosis, two‐point discrimination, graphesthesia, localization of painful stimulation), temporal (e.g. judgment of tactile temporal order, temporal summation), and modality‐specific components (e.g. texture discrimination, size of coins). 9 For proprioception, perception refers to the sense of limb position and movement (e.g. matching the position of one limb with the contralateral limb). 27
Figure 1.
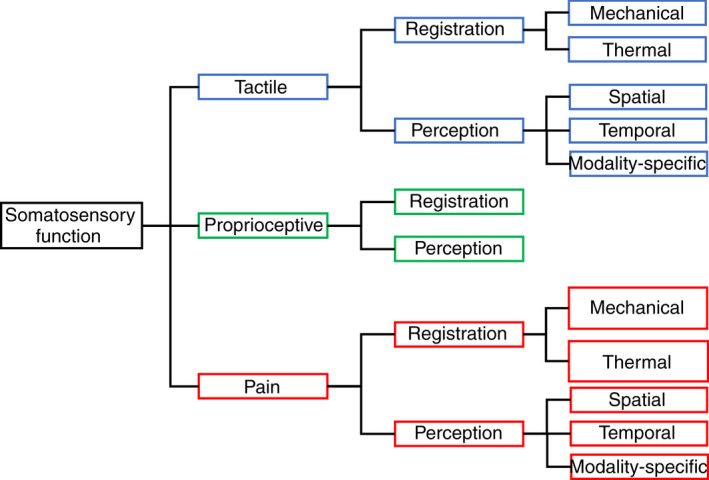
Classification of somatosensory function.
Quality assessment
The quality of the included studies was assessed with the Kmet quality assessment 28 to identify the strengths and weaknesses of each study included in this scoping review. The scale consists of 14 items rated from 0 to 2 (0, the paper does not meet the criterion; 1, the paper partially meets the criterion; 2, the paper meets the criterion) assessing the study objective and design, participant characteristics, measures and methods of analyses, results, and conclusions. Two authors independently assessed each article. Any discrepancy was resolved by consensus. The summary score for each paper was expressed as the percentage of the potential maximum score (total sum divided by the total possible sum). The total possible sum equaled 22 since items relative to interventional studies were not considered. A Cohen’s weighted κ score was used to determine the consensus interrater agreement for (1) each article and (2) each checklist item of the quality assessment tool.
RESULTS
Literature search results
A total of 9766 records were retrieved (including additional records identified through other sources). After discarding duplicates, the titles and abstracts of 4882 references were screened; 114 full‐text articles were assessed for eligibility and 66 were excluded because they were not original research 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 (n=19), they did not assess somatosensory processing 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 (n=18), they did not include a control group 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 (n=16), they were not in English or French and they could not be translated using free online tools 82 , 83 , 84 , 85 , 86 , 87 , 88 (n=7), they did not include individuals diagnosed with CP 89 , 90 , 91 (n=3), or their full text was not available 92 , 93 , 94 (n=3, all published before 1960). Therefore, 48 articles met the inclusion criteria and were included in this scoping review (Fig. S1, online supporting information).
Results of quality assessment
Interrater consensus agreement was very good for the summary score of the 48 studies according to the weighted Cohen’s κ (κ=0.81, SD=0.14, range 0.59–1) and good for each checklist item (κ=0.79, SD=0.11, range 0.65–1). See Table S2 (online supporting information) for detailed results.
Overall, the mean summary score was 14.5 out of 22 (SD=3.10; see Tables S3, –S6, online supporting information, for the details of the mean summary score for each study).
As shown in Table S2, most of the items of the Kmet quality assessment had an average score of approximately 1.5, meaning that the response was between ‘partial yes’ and ‘yes’, except for item 8. This item had an average score of 0.85 (response between ‘no’ and ‘partial yes’) and was relative to the outcomes (well defined and robust to measurements/misclassification bias).
Study characteristics
Tables S3 to S6 present the summaries of the sample characteristics, methods, and results of each study included in this review according to the category of somatosensory function (tactile function, proprioception, pain function, and neuroimaging studies). Tables S7 and S8 (online supporting information) describe respectively the clinical tests and neuroimaging methods used in the studies included in this scoping review. No studies reported any conflicts of interest. Sources of funding, conflicts of interest, study setting, aim/purpose, and the country of origin of each study are reported in Table S9 (online supporting information). Of the 48 studies, 23 were conducted in North America, 21 in Europe, two in South America, one in Asia, and one in Oceania.
Overall, 1463 participants with CP (mean [SD] age 13y 1mo [4y 11mo], range 1–55y; 416 males, 319 females, sex not identified for the remaining participants) and 1478 controls (mean [SD] age 13y 1mo [5y 8mo], range 1–42y; 362 males, 334 females, sex not identified for the remaining participants) were included in this scoping review. One study did not report the age of its participants; of the 47 other studies, 38 involved only children, two only adults, and seven adults and children. To compare participants with CP to controls (i.e. typically developing individuals), eight studies only matched the age and 27 matched both the age and the sex between the two groups. The type of CP was reported in 41 studies. Of the 1463 participants with CP, 41.9% had unilateral CP and 41.4% had bilateral CP. Moreover, 42.7% of participants had spastic CP. The etiology of CP was reported in 14 studies. Only 11 studies reported Manual Ability Classification System and/or Gross Motor Function Classification System levels. Somatosensory assessments were preferentially performed on the distal part of the limb (30 studies); the upper and lower limbs were tested in 43 and 10 studies respectively. Most studies evaluated the more affected limb of participants with CP compared to the non‐dominant limb of controls (38 studies). For the other studies, the tested limb of individuals with CP and/or controls was not clearly reported (seven studies) or the most/least affected limb of individuals with CP was only compared to the dominant limb of the controls (three studies).
Various methods were employed to study somatosensory processing; most of the time, more than one test was used in each study. Tactile function was investigated in 26 studies, proprioception in 18, pain in five, and cortical responses evoked by somatosensory stimulation using neuroimaging in 18.
Study results
Tactile function
Table S3 details the results obtained for each study that included tactile function. 11 , 13 , 16 , 18 , 20 , 21 , 22 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113
Different components of tactile function were evaluated, including tactile registration (13 studies) and tactile perception (21 studies). Most studies reported the presence of tactile registration (eight studies) and tactile perception (21 studies) deficits in participants with CP. In 15 studies assessing tactile function, both the most and least affected limbs were tested and most showed similar results for both limbs.
Registration: mechanical stimuli
An increase in detection threshold was demonstrated for participants with CP in most studies, while a few studies reported no significant differences. In two studies, the light touch test was used in participants with bilateral CP, which might potentially account for the difference. In the other two studies, assessment of tactile registration was not the main focus of the study, methodological details were missing, and sample size was limited.
Registration: thermal stimuli
The detection threshold was reported as increasing in one study. Another study provided contrasting results, showing no significant differences between individuals with CP and typically developing individuals. However, this study was of lower quality since the clinical test employed was not standardized.
Perception: spatial component (including two‐point discrimination, graphesthesia, and stereognosis)
Various clinical tests in various CP populations were used in 20 studies encompassing a total of 37 clinical tests. All studies consistently demonstrated a decrease in spatial tactile perception in all clinical tests, except for two‐point discrimination. Indeed, 4 out of 11 studies using this test showed lack of significant differences compared to typically developing individuals, although these studies were of lower quality.
Modality‐specific perception
A similar agreement was observed. Studies focusing on the perception of texture stimuli showed deficits in individuals with CP. Only one study using a different test (roughness direction) showed no deficit.
In summary, deficits in tactile registration were observed in most studies and were mainly explained by a deficit in the registration of mechanical stimuli. Moreover, consistent results were observed for deficits in tactile perception (spatial component and modality‐specific) despite inconsistent results for the two‐point discrimination test.
Proprioception
Table S4 details the results of each study for the proprioceptive function. 16 , 20 , 22 , 78 , 96 , 98 , 99 , 102 , 105 , 106 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121
Different components of proprioception were evaluated, including proprioceptive registration (eight studies) and perception (15 studies). In 11 studies assessing proprioception, both the most and least affected limbs were tested; all studies, except for one, showed similar results between both limbs.
Registration: movement direction detection
Deficits were found in 5 out of 6 studies. Only one study demonstrated no significant difference but the sample size was limited compared to the other studies, raising the possibility of a type 2 error.
Registration: movement detection threshold
There was no consensus across the three available studies. This could be due to the heterogeneity in the populations tested since deficits were reported in studies with participants with bilateral CP but not unilateral CP.
Perception: position sense
This was altered in 10 out of 15 studies. The five studies showing no significant difference in position sense had lower sample sizes and Kmet quality scores; they used clinical tests that were not validated.
In summary, despite the heterogeneity of the populations included across studies, most of the studies demonstrated proprioceptive registration deficits. However, the results for perception were more variable across studies.
Pain function
Table S5 details the results obtained in each study assessing pain function. 13 , 20 , 22 , 102 , 122
Few studies assessed pain function in participants with CP compared to controls, focusing on either pain registration (three studies) or pain perception (three studies). These studies included participants with unilateral and bilateral CP but 83% of them had a spastic form. In 4 out of 5 studies, both the most and least affected limbs were tested; all studies showed similar results for both limbs.
Registration: nociceptive mechanical stimuli
All three studies found alterations in individuals with CP.
Registration: nociceptive thermal stimuli
This was assessed in one study and no deficit was found.
Modality‐specific perception
Perception was altered in all studies despite the variety of clinical tests used. One study reported impaired ability to discriminate between sharp and dull pin stimuli, signaling a deficit in sensory function. Other studies showed a gain in sensory function. Indeed, mechanical or pressure hyperalgesia was reported in about half of participants with CP, paradoxical heat sensation in about one‐third of participants, and allodynia in about one‐quarter, while thermal hyperalgesia or temporal summation was observed rarely. The last study focused specifically on individuals with CP and intellectual disability, who exhibited a stimulus response relationship to pressure stimulation for both pain ratings and facial expressions, with a larger increase in facial expressions along the increase in noxious stimulation compared to controls. 122
In summary, few studies assessed pain function. Registration deficits were characterized only by deficits for nociceptive thermal stimuli. Perception alterations were characterized either by a deficit (difficulty to discriminate between different textures) or a gain in sensory function (e.g. allodynia). Figure 2a depicts a summary of the results for tactile, proprioceptive, and pain functions.
Figure 2.
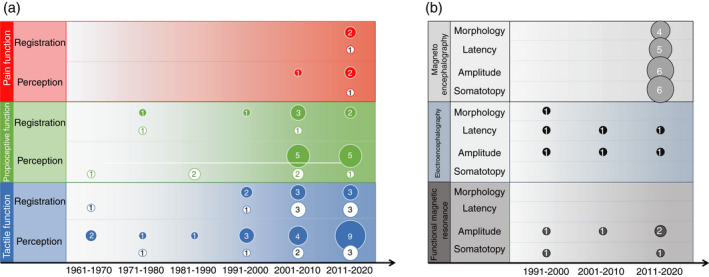
Representation of the distribution of studies included in this scoping review. (a) Studies assessing registration (R) or perception (P) of the different somatosensory functions (tactile, proprioception, and pain function). (b) Studies assessing cortical responses evoked by somatosensory stimulation. The numbers in each circle indicate the number of studies in each decade either reporting deficits (filled circles) or not (empty circles).
Cortical responses evoked by somatosensory stimulation
Table S6 details the results for each study assessing cortical responses evoked by somatosensory stimulation. 3 , 17 , 18 , 19 , 20 , 21 , 22 , 29 , 96 , 113 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130
Eight studies assessed somatotopy, five assessed morphology, eight measured latencies, and 12 evaluated the amplitude of cortical responses evoked by somatosensory stimuli using magnetoencephalography (eight studies), EEG (five studies), or fMRI (five studies).
In eight studies, somatosensory function was assessed with electrical stimulation, which is not specific to one sensory modality in the somatosensory system. However, electrical simulation preferentially activates large fibers and did not induce painful sensation in any of the studies. Tactile function was assessed in 12 studies, proprioception was assessed in one, and none of the studies evaluated pain function.
For all studies comparing the most and least affected limb to those of typically developing individuals, similar effects were observed on cortical responses evoked by somatosensory stimulation. Only two studies investigated the lower limbs.
Somatotopy: magnetoencephalography, EEG, and fMRI studies
Results showed some abnormalities in participants with unilateral and bilateral CP in 6 out of 7 studies. Half of the studies reported the occurrence of ipsilateral activations, or even absent activations, in response to stimulation in some participants with CP, while such results were not observed in typically developing individuals who had only contralateral activations. This heterogeneity in the results for participants with CP was not explained by the quality of the studies, methodological considerations, nor the type of CP (unilateral vs bilateral or spastic vs non‐spastic). Moreover, in the primary somatosensory cortex (S1), increased variability was observed in the somatosensory evoked field (SEF) contour map, with a shift in somatosensory digit and hand representation notably expressed by higher or lower Euclidean distance between digits in individuals with CP compared to typically developing individuals. However, this difference was not explained by the quality of the studies, the type of CP, or methodological considerations.
Morphology: magnetoencephalography and EEG studies
Results from all studies demonstrated attenuated, abnormal, or absent sharp deflections in some or all participants with CP for SEFs or somatosensory evoked potentials (SEPs), while SEFs and SEPs were robust in typically developing individuals.
Latency: magnetoencephalography and EEG studies
Only early components of SEFs and SEPs were assessed using electrical stimulation or air pressure pulses. Increased latencies in individuals with CP compared to typically developing individuals were observed in five studies, while no difference was observed in three studies. However, it is important to note that for the studies showing no difference, SEFs and SEPs were not always present in participants with CP and sample size was limited.
SEF and SEP amplitudes: magnetoencephalography and EEG studies
Amplitude was decreased in participants with CP compared to typically developing individuals in half of the studies, while in the other studies no difference between groups was observed. As mentioned earlier for latencies, in studies showing no difference, SEFs and SEPs were not always present and sample size was limited.
Spectral power: magnetoencephalography and EEG studies
Results of various cerebral waves were heterogeneous and not related to the type of somatosensory stimulation (electrical or air pressure pulse), site (upper vs lower limb), nor the quality of the studies. Beta band magnitude (related to the processing of somatosensory inputs) in the primary somatosensory cortex (S1) was increased in bilateral CP while it was unaltered in unilateral CP. Gamma suppression was observed in unilateral CP, indicating abnormal S1 activity in response to somatosensory stimulation. However, for the theta‐alpha band (excitability of S1), similar amplitude in participants with bilateral CP and typically developing individuals was found in two studies. A decreased amplitude in somatosensory gating for the 10Hz to 75Hz band was found, which was more attenuated for the second stimulation (i.e. hypergating) in participants with bilateral CP compared to typically developing individuals.
Amplitude: fMRI studies
A decreased activation evoked by somatosensory stimulation in several cortical regions for participants with either unilateral or bilateral CP was observed. For tactile function, a decreased postcentral gyrus activation evoked by stimulation, except for brushing, was observed in individuals with CP compared to typically developing individuals. Interestingly, a decreased response amplitude was also observed in areas involved in somatosensory perception (posterior parietal cortex) and motor function. However, no difference in brain activation amplitude was observed for the proprioceptive function.
In summary, somatotopy was altered in most of the studies characterized by ipsilateral or no activation, a shift and increased variability in S1. Morphology analyses revealed that SEFs and SEPs were less robust in individuals with CP compared to typically developing individuals. In studies where SEFs and SEPs were present in all participants with CP, latencies and amplitude were decreased compared to typically developing individuals. Spectral power analyses showed inconsistent results, while a decreased amplitude in fMRI studies was observed for the tactile function. Figure 2b depicts a summary of the results for neuroimaging studies.
DISCUSSION
Historically, attention has been paid mainly to motor function in CP. A growing interest for somatosensory function has been observed, mainly over the last decade (56% of the included studies were published after 2010). Recently, it has been highlighted that somatosensory registration and perception might play a central role in impairments resulting from CP, leading some authors to consider CP as a sensorimotor disorder. 5 This scoping review shows that tactile function has been the most studied function through the last decades and suggests deficits for both tactile registration and perception. Proprioception function has been investigated mostly in the last 20 years and results indicate deficits in perception, while they are more conflicting for the presence of registration deficits. Studies on pain function are the most recent (in the last 10 years) and are still limited in number compared to tactile and proprioceptive function. Neuroimaging studies performed over the last three decades support the behavioral results for tactile function but there are still few or no neuroimaging studies for proprioceptive and pain function. Moreover, studies have focused mainly on children and upper limbs; the type of CP (spastic vs non‐spastic and unilateral vs bilateral) is very heterogeneous across studies. Finally, the methods employed (particularly for behavioral measures) are poorly detailed in most studies.
Registration refers to external or internal stimulus detection, that is, when the stimulus exceeds the receptor sensory threshold and then reaches the primary somatosensory cortex (S1). 9 , 131 Registration deficits in individuals with CP were mainly characterized by hypoesthesia, deficits in movement and direction detection, and a lowered pain threshold. These deficits are confirmed by neuroimaging studies (mostly for tactile function and the upper limbs), which provide some insight into the underlying mechanisms. First, the representations of body segments in S1 are blurred, as demonstrated by an increased variability of SEF contour maps 123 and a shift in S1 representations. 17 , 18 , 21 , 125 Moreover, ipsilateral activation was found in some studies, 21 , 123 , 125 , 128 although not systematically, 17 , 19 , 132 similar to what has been observed for corticospinal projections. 125 Second, an increase in early SEP and SEF latencies, a decrease in SEP and SEF amplitudes, and alterations in oscillatory activity were observed, suggesting alterations in sensory pathways. These results are consistent with the observation of structural alterations along the thalamocortical pathway and in the thalamus in children with CP using diffusion tensor imaging. 98 , 133 , 134 , 135 In addition, peripheral somatosensory alterations have also been observed that are characterized by alterations in afferent neural spinal transmission, 63 partial demyelination of sensory nerves, 65 and alterations in muscle spindles. 136 Therefore, deficits of somatosensory registration might be explained by alterations at different levels (peripheral, subcortical, or cortical) of somatosensory afferences. Reduced or altered registration in turn undoubtedly contributes to alteration in perception. 9
Perception refers to understanding, interpreting, or giving meaning to sensory stimuli. 9 Deficits in perception can result from either alterations in registration or alterations in associative cortices, mainly the posterior parietal cortex. 131 This scoping review shows that alterations in tactile perception were almost systematic, while alterations in proprioception and pain were more heterogeneous across participants with CP.
Deficits in proprioception were characterized by alterations in joint position sense. It is important to note that position sense was altered in studies using a matching task (bilateral task) whereas fewer deficits were observed in studies using unilateral assessment (e.g. to reproduce a position from memory), suggesting a bias in methodological assessment in proprioception, which is a recurrent issue in proprioception. 27 Larger deficits in bilateral tasks could be explained by the presence of motor deficits causing difficulties to reproduce the position with the contralateral limb, especially given that 54% of participants had bilateral motor deficits and that even in unilateral CP the least affected limb also presented with somatosensory deficits.
Tactile perception deficits are mainly characterized by deficits in stereognosis, graphesthesia, and two‐point discrimination. Two studies assessed cortical responses with fMRI during tactile perception (shape and grating discrimination tests) and showed a decreased activation in frontal and parietal cortices in participants with CP compared to controls. 113 , 129 Notably, decreased activations in the frontal cortex were located in areas not typically associated with tactile functions but rather motor functions, such as the primary motor cortex, the supplementary motor area, and the premotor cortex. 113 , 129 It is important to note that during the tasks, participants did not actively move their fingers to discriminate between shapes and grating. In typically developing individuals, motor cortices have been shown to be involved in somatosensory perception. 137 , 138 In addition to the alterations in the anterior parietal cortex mentioned earlier in registration deficits (S1), alterations in the posterior parietal cortex have also been observed (posterior parietal operculum, superior and inferior intraparietal lobules). 113 , 129 The posterior parietal cortex is involved in internal models of motor control and in multisensory integration, which allow individuals to have a unified representation of the body (body schema). 138 Alterations in the internal models of motor control have been suggested 139 , 140 and disturbances in body schema have been observed in individuals with CP. 79 , 141 , 142 , 143 , 144 Body schema disorders are characterized by altered motor imagery, 141 , 143 , 144 a decreased perceived length of the hemiparetic arm, 142 and altered lower back perception. 79 Interestingly, in the latest study, alteration was more pronounced in individuals with CP with lower back pain than in individuals with CP without pain, 79 which is in line with studies showing that people with pain have strong alterations in somatosensory registration and perception. 145 , 146 , 147 However, this scoping review reveals that pain has received very little consideration in the literature on somatosensory functions in CP. Indeed, the pain characteristics of the sample are rarely described despite the fact that the presence of pain might interfere with tactile and proprioceptive functions.
While pain is recognized as a serious issue in CP, 14 affecting 77% of children with CP, 148 only 5 out of the 48 studies included in this scoping review (approximately 10% of the studies) assessed pain registration and perception. A potential explanation for the paucity of studies is the fact that standardized measurements of pain applicable to the population with CP are needed. 14 In the literature, pain in individuals with CP is mainly considered as musculoskeletal and highly related to motor disorders (e.g. muscle tone, immobilization, deformities, spasticity). 57 However, this scoping review shows that deficits in pain function are characterized by hyperalgesia and allodynia for both the most and least affected limb. Hyperalgesia and allodynia commonly characterize neuropathic pain, which can be defined as a ‘pain caused by a lesion or disease of the somatosensory nervous system’. 149 Few studies specifically aim to assess neuropathic pain in individuals with CP; 13 , 150 therefore, further studies are needed to assess these two types of pain (musculoskeletal vs neuropathic) separately to manage pain in individuals with CP more efficiently.
From a research perspective, this scoping review highlights several recommendations for future studies. First, outcomes were often poorly defined (including in the most recent studies) and information about the psychometric properties of the clinical tests was often missing, which calls into question the validity of the results. Auld et al. 9 reviewed the psychometric properties of clinical tactile tests in children with CP and made recommendations for tactile registration and perception assessments. Unfortunately, no such recommendations have been made for proprioceptive and pain function in individuals with CP, an aspect that needs to be investigated further. In the absence of psychometric properties, authors should at least present in detail the methodology of clinical assessments (e.g. number and order of trials, order of tests, how the final score is calculated) to allow replication and comparisons across studies. Second, previous studies focused mainly on the upper limbs and on children. Indeed, few studies included adult participants (7 out of 48). Somatosensory deficits in adults need to be investigated further since deficits in somatosensory function differ across ages. 22 Third, assessment of somatosensory functions requires understanding and performing the task; therefore, results are limited to children with no to mild cognitive impairments. Blankenburg et al. 13 reported that only 17% of their sample (total sample n=176) could perform quantitative sensory testing, suggesting that the somatosensory deficits observed in this review reflect only a limited part of the population. Fourth, clinical characteristics are generally poorly described. For example, etiology, including preterm birth, was mentioned in only 29.2% of the studies. However, it is well known that preterm birth leads to a different clinical profile since, for example, a higher prevalence of spasticity and bilateral deficits are observed in children born preterm compared to children born at term. 8 Fifth, the type of CP (spastic vs non‐spastic and bilateral vs unilateral) was not representative of the general population with CP. A meta‐analysis demonstrated that more than three‐quarters of the population with CP have a spastic form and more than two‐thirds have bilateral disorders 8 while in our review approximately 40% had spastic CP and approximately 40% had bilateral CP. Therefore, in future studies, population characteristics need to be better defined and explored.
From a rehabilitation perspective, this scoping review emphasizes the need to carefully consider somatosensory deficits in CP. Unfortunately, somatosensory function is not systematically assessed by therapists. 151 Some rehabilitation programs focusing on tactile and proprioceptive functions have shown the beneficial effects of such interventions but the number of studies is limited. 15 , 152 Future work is needed to develop efficient somatosensory therapies in CP 10 by considering efficient therapies that are already developed in patients with stroke. 152
Several limitations that impact on the external validity of the present findings need to be highlighted. First, the major limitation of this review concerns restrictions about the language and type of publication and study designs; therefore, publication bias might be present. However, five of the seven articles were excluded because they were not in English or French and published before 1990, and it was not possible to translate them in a satisfactory manner with a free online translation tool. Second, this scoping review does not allow us to draw a definitive conclusion about the presence of somatosensory deficits since no formal quality assessment with an appropriate risk‐of‐bias grid was made.
To conclude, this scoping review shows that somatosensory deficits in CP have been investigated in a large number of studies, mostly in the last 10 years, but to a different extent across somatosensory functions. Tactile and proprioceptive functions were the most studied, while pain has received little attention (both in behavioral and neuroimaging studies). While a scoping review does not allow a definitive conclusion about the presence of somatosensory deficits in CP, this review suggests that such deficits might be present in tactile, proprioceptive, and pain functions. Additional work is needed to confirm these results and future research should rigorously define the methods employed and include samples that are more representative of the population with CP.
Supporting information
Table S1: Full search strategy for each database
Table S2: Assessment of study quality for each article and inter‐agreement
Table S3: Characteristics and results for each included study assessing tactile function
Table S4: Characteristics and results for each included study assessing proprioception
Table S5: Characteristics and results for each included study assessing pain function
Table S6: Characteristics and results for each included study assessing cortical responses evoked by somatosensory stimulation
Table S7: Description of clinical tests used in the studies included in this scoping review
Table S8: Methods to assess cortical responses evoked by somatosensory stimulation
Table S9: Source of funding and conflict of interest for each study included in the scoping review
Figure S1: Flow diagram of the bibliographical search
Acknowledgments
The study was supported by the Laval University Cerebral Palsy Research Chair of which Catherine Mercier is the holder. Catherin Mercier is supported by an Emeritus researcher salary award from Fonds de Recherche Québec‐Santé. There is no mitigation or influence of the funders on this scoping review.
REFERENCES
- 1. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy. Dev Med Child Neurol Suppl 2007; 109: 8–14. [PubMed] [Google Scholar]
- 2. Clayton K, Fleming JM, Copley J. Behavioral responses to tactile stimuli in children with cerebral palsy. Phys Occup Ther Pediatr 2003; 23: 43–62. [PubMed] [Google Scholar]
- 3. Kurz MJ, Becker KM, Heinrichs‐Graham E, Wilson TW. Children with cerebral palsy have uncharacteristic somatosensory cortical oscillations after stimulation of the hand mechanoreceptors. Neuroscience 2015; 305: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frisk RF, Jensen P, Kirk H, Bouyer LJ, Lorentzen J, Nielsen JB. Contribution of sensory feedback to plantar flexor muscle activation during push‐off in adults with cerebral palsy. J Neurophysiol 2017; 118: 3165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dan B. Cerebral palsy is a sensorimotor disorder. Dev Med Child Neurol 2020; 62: 768. [DOI] [PubMed] [Google Scholar]
- 6. Auld ML, Boyd RN, Moseley GL, Ware RS, Johnston LM. Impact of tactile dysfunction on upper‐limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehabil 2012; 93: 696–702. [DOI] [PubMed] [Google Scholar]
- 7. Bleyenheuft Y, Gordon AM. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: a systematic review. Res Dev Disabil 2013; 34: 3014–28. [DOI] [PubMed] [Google Scholar]
- 8. Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta‐analytic review. Dev Med Child Neurol 2008; 50: 334–40. [DOI] [PubMed] [Google Scholar]
- 9. Auld ML, Boyd RN, Moseley GL, Johnston LM. Tactile assessment in children with cerebral palsy: a clinimetric review. Phys Occup Ther Pediatr 2011; 31: 413–39. [DOI] [PubMed] [Google Scholar]
- 10. Auld ML, Johnston LM. Perspectives on tactile intervention for children with cerebral palsy: a framework to guide clinical reasoning and future research. Disabil Rehabil 2018; 40: 1849–54. [DOI] [PubMed] [Google Scholar]
- 11. Riquelme I, Cifre I, Montoya P. Age‐related changes of pain experience in cerebral palsy and healthy individuals. Pain Med 2011; 12: 535–45. [DOI] [PubMed] [Google Scholar]
- 12. Riquelme I, Zamorano A, Montoya P. Reduction of pain sensitivity after somatosensory therapy in adults with cerebral palsy. Front Hum Neurosci 2013; 7: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blankenburg M, Junker J, Hirschfeld G, et al. Quantitative sensory testing profiles in children, adolescents and young adults (6–20 years) with cerebral palsy: hints for a neuropathic genesis of pain syndromes. Eur J Paediatr Neurol 2018; 22: 470–81. [DOI] [PubMed] [Google Scholar]
- 14. Ostojic K, Paget SP, Morrow AM. Management of pain in children and adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol 2019; 61: 315–21. [DOI] [PubMed] [Google Scholar]
- 15. Yardımcı‐Lokmanoğlu BN, Bingöl H, Mutlu A. The forgotten sixth sense in cerebral palsy: do we have enough evidence for proprioceptive treatment? Disabil Rehabil 2020; 42: 3581–90. [DOI] [PubMed] [Google Scholar]
- 16. Kuczynski AM, Dukelow SP, Semrau JA, Kirton A. Robotic quantification of position sense in children with perinatal stroke. Neurorehabil Neural Repair 2016; 30: 762–72. [DOI] [PubMed] [Google Scholar]
- 17. Papadelis C, Ahtam B, Nazarova M, et al. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neurosci 2014; 8: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papadelis C, Butler EE, Rubenstein M, et al. Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts. Neuroimage Clin 2017; 17: 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pihko E, Nevalainen P, Vaalto S, et al. Reactivity of sensorimotor oscillations is altered in children with hemiplegic cerebral palsy: a magnetoencephalographic study. Hum Brain Mapp 2014; 35: 4105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riquelme I, Padrón I, Cifre I, González‐Roldán AM, Montoya P. Differences in somatosensory processing due to dominant hemispheric motor impairment in cerebral palsy. BMC Neurosci 2014; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiori S, Biagi L, Cecchi P, et al. Potentials of ultrahigh‐field MRI for the study of somatosensory reorganization in congenital hemiplegia. Neural Plast 2018; 2018: 8472807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurophysiol 2010; 121: 1314–20. [DOI] [PubMed] [Google Scholar]
- 23. Bramer WM, Giustini D, de Jonge GB , Holland L, Bekhuis T. De‐duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104: 240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Surveillance of Cerebral Palsy in Europe . Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000; 42: 816–24. [DOI] [PubMed] [Google Scholar]
- 25. Schiariti V, Longo E, Shoshmin A, et al. Implementation of the International Classification of Functioning, Disability, and Health (ICF) core sets for children and youth with cerebral palsy: global initiatives promoting optimal functioning. Int J Environ Res Public Health 2018; 15: 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nijs J, Paul van Wilgen C, Van Oosterwijck J, van Ittersum M , Meeus M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: practice guidelines. Man Ther 2011; 16: 413–8. [DOI] [PubMed] [Google Scholar]
- 27. Fortier S, Basset FA. The effects of exercise on limb proprioceptive signals. J Electromyogr Kinesiol 2012; 22: 795–802. [DOI] [PubMed] [Google Scholar]
- 28. Kmet LM, Lee RC, Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Edmonton, AB, Canada: Alberta Heritage Foundation for Medical Research, 2004. [Google Scholar]
- 29. Kurz M, Groff B, Coolidge N, Wiesman A, Wilson T. Children with cerebral palsy display abnormal somatosensory cortical activity during a haptic exploration task. Dev Med Child Neurol 2018; 60(Suppl. 3): 15 (abstract). [Google Scholar]
- 30. Kurz M, Wiesman AI, Heinrichs‐Graham E, Wilson T. Children with cerebral palsy display uncharacteristic somatosensory cortical gating for peripheral stimulations applied to the foot. Dev Med Child Neurol 2017; 59(Suppl): 27 (abstract). [Google Scholar]
- 31. Papadelis C, Butler E, Rubenstein M, Snyder B, Grant P. Reorganization of the somatosensory cortex in children with cerebral palsy due to impaired thalamocortical sensory tracts. Dev Med Child Neurol 2016; 58(Suppl. 5): 68–9 (abstract). [Google Scholar]
- 32. Gehringer J, Arpin D, Wilson T, Kurz M. Self‐reported pain levels are related with the aberrant somatosensory activity in children with cerebral palsy. Dev Med Child Neurol 2018; 60(Suppl. 3): 98 (abstract). [Google Scholar]
- 33. Maitre NL, Key AP. Quantitative assessment of cortical auditory‐tactile processing in children with disabilities. J Vis Exp 2014; 83: e51054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson JJ, Wilson BC. Some sensory and perceptual deficits in cerebral palsied children. Dev Med Child Neurol 1965; 7: 90. [Google Scholar]
- 35. Wilson BC, Wilson JJ. Sensory and perceptual functions in the cerebral palsied. I. Pressure thresholds and two‐point discrimination. J Nerv Ment Dis 1967; 145: 53–60. [DOI] [PubMed] [Google Scholar]
- 36. Wilson B, Wilson JJ. Sensory and perceptual functions in the cerebral palsied. II. Stereognosis. J Nerv Ment Dis 1967; 145: 61–8. [DOI] [PubMed] [Google Scholar]
- 37. Kuczynski A, Dukelow S, Yajure J, Roe J, Kirton A. Robotic quantification of proprioceptive dysfunction in children with perinatal stroke. Stroke 2014; 45: O2.04 (abstract). [Google Scholar]
- 38. Dufresne D, Dagenais L, Shevell MI, Consortium R. Prevalence and characteristics of severe sensory impairment in a population‐based cohort of children with cerebral palsy: CS‐10. Ann Neurol 2012; 72(Suppl.): S186 (abstract). [Google Scholar]
- 39. Takakura H, Kihara K, Takeshita K. A study of topographical somatosensory evoked‐potentials after median nerve‐stimulation in cerebral palsy cases with spastic diplegia. Brain Dev 1987; 9: 152. [Google Scholar]
- 40. Kukke S, Sanger TD. Is childhood secondary dystonia a sensory disorder: Children with arm dystonia and cerebral palsy have a deficit of tactile sensory discrimination. Mov Disord 2004; 19(Suppl. 9): P277 (abstract). [Google Scholar]
- 41. Lewis C, Goble D, Hurvitz E, Brown S. Proprioceptive acuity and multi‐joint coordination in hemiplegic cerebral palsy. Neurorehabil Neural Repair 2005; 19: 381 (abstract). [Google Scholar]
- 42. Kirton A, Yajure J, Leah I, Roe J, Scott S, Dukelow S. Robotic Assessment of proprioceptive dysfunction in children with perinatal stroke. Stroke 2013; 44: TMP102 (abstract). [Google Scholar]
- 43. Wingert JR. Tactile and proprioception abnormalities in cerebral palsy: sensory testing and functional MRI. St. Louis, MO: Washington University, 2007. [Google Scholar]
- 44. Yekutiel M, Jariwala M, Stretch P. Sensory deficit in the hands of children with cerebral palsy: a new look at assessment and prevalence. Dev Med Child Neurol 1994; 36: 619–24. [DOI] [PubMed] [Google Scholar]
- 45. Bernstein V. Neuropsychological assessment in children with cerebral palsy and dual sensory impairment. Arch Clin Neuropsychol 2000; 15: 791 (abstract). [Google Scholar]
- 46. Tardieu G, Monfraix C, Tabary JC, Tarbieu C. Disorders of manual recognition of shapes in the child with cerebral motor infirmity. [Article in French]. Rev Neurol (Paris) 1961; 105: 480–8. [PubMed] [Google Scholar]
- 47. Tardieu G. Some questions and answers regarding astereognosis in cerebral palsy. Clin Orthop Relat Res 1966; 46: 53–4. [PubMed] [Google Scholar]
- 48. Woodward KE, Carlson HL, Kuczynski A, Saunders J, Hodge J, Kirton A. Sensory‐motor network functional connectivity in children with unilateral cerebral palsy secondary to perinatal stroke. Neuroimage Clin 2019; 21: 101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sgandurra G, Biagi L, Fogassi L, et al. Reorganization of the action observation network and sensory‐motor system in children with unilateral cerebral palsy: an fMRI study. Neural Plast 2018; 2018: 6950547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tomhave WA, Van Heest AE, Bagley A, James MA. Affected and contralateral hand strength and dexterity measures in children with hemiplegic cerebral palsy. J Hand Surg Am 2015; 40: 900–7. [DOI] [PubMed] [Google Scholar]
- 51. Pavão SL, Rocha NACF. Sensory processing disorders in children with cerebral palsy. Infant Behav Dev 2017; 46: 1–6. [DOI] [PubMed] [Google Scholar]
- 52. Kurz MJ, Heinrichs‐Graham E, Arpin DJ, Becker KM, Wilson TW. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophysiol 2014; 111: 573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duque J, Thonnard J‐L, Vandermeeren Y, Sébire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 2003; 126: 732–47. [DOI] [PubMed] [Google Scholar]
- 54. Burton H, Dixit S, Litkowski P, Wingert JR. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res 2009; 26: 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinnucan E, Van Heest A, Tomhave W. Correlation of motor function and stereognosis impairment in upper limb cerebral palsy. J Hand Surg Am 2010; 35: 1317–22. [DOI] [PubMed] [Google Scholar]
- 56. Dinomais M, Groeschel S, Staudt M, Krägeloh‐Mann I, Wilke M. Relationship between functional connectivity and sensory impairment: red flag or red herring? Hum Brain Mapp 2012; 33: 628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jahnsen R, Villien L, Aamodt G, Stanghelle JK, Holm I. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J Rehabil Med 2004; 36: 78–84. [DOI] [PubMed] [Google Scholar]
- 58. Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol 2001; 49: 320–7. [PubMed] [Google Scholar]
- 59. Howard EM, Henderson SE. Perceptual problems in cerebral‐palsied children: a real‐world example. Hum Mov Sci 1989; 8: 141–60. [Google Scholar]
- 60. Dolphin JE, Cruickshank WM. Tactual motor perception of children with cerebral palsy. J Pers 1952; 20: 466–71. [DOI] [PubMed] [Google Scholar]
- 61. Nelson TM. A study comparing visual and visual‐motor perceptions of unimpaired, defective and spastic cerebral palsied children. J Genet Psychol 1962; 101: 299–332. [DOI] [PubMed] [Google Scholar]
- 62. Birch H, Bortner M. Stimulus competition and concept utilization in brain damaged children. Dev Med Child Neurol 1967; 9: 402–10. [DOI] [PubMed] [Google Scholar]
- 63. Achache V, Roche N, Lamy J‐C, et al. Transmission within several spinal pathways in adults with cerebral palsy. Brain 2010; 133: 1470–83. [DOI] [PubMed] [Google Scholar]
- 64. Curry J, Exner C. Comparison of tactile preferences in children with and without cerebral palsy. Am J Occup Ther 1988; 42: 371–7. [DOI] [PubMed] [Google Scholar]
- 65. Frascarelli M, Frascarelli F, Gentile MG, et al. Entrapment neuropathy in patients with spastic cerebral palsy. Acta Neurol Scand 2005; 112: 178–82. [DOI] [PubMed] [Google Scholar]
- 66. Mailleux L, Simon‐Martinez C, Radwan A, et al. White matter characteristics of motor, sensory and interhemispheric tracts underlying impaired upper limb function in children with unilateral cerebral palsy. Brain Struct Funct 2020; 225: 1495–509. [DOI] [PubMed] [Google Scholar]
- 67. Zarkou A, Lee SCK, Prosser LA, Jeka JJ. Foot and ankle somatosensory deficits affect balance and motor function in children with cerebral palsy. Front Hum Neurosci 2020; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ryu HJ, Song G‐B. Differences in proprioceptive senses between children with diplegic and children with hemiplegic cerebral palsy. J Phys Ther Sci 2016; 28: 658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Langan J, Kern KL, Hurvitz EA, Brown SH. Upper‐limb position sense deficits in adults with cerebral palsy. Am J Phys Med Rehabil 2014; 93: 774–81. [DOI] [PubMed] [Google Scholar]
- 70. Guzzetta A, Bonanni P, Biagi L, et al. Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol 2007; 118: 1110–21. [DOI] [PubMed] [Google Scholar]
- 71. Lee D, Turnbull J, Cook ML. Disorders of felt position of parts of the body in hemiparetic cerebral palsied children. Int J Rehabil Res 1989; 12: 90–4. [Google Scholar]
- 72. Arnould C, Penta M, Thonnard J‐L. Hand impairments and their relationship with manual ability in children with cerebral palsy. J Rehabil Med 2007; 39: 708–14. [DOI] [PubMed] [Google Scholar]
- 73. Upton AR, Cooper IS. Some neurophysiological effects of cerebellar stimulation in man. Can J Neurol Sci 1976; 3: 237–54. [DOI] [PubMed] [Google Scholar]
- 74. Guzzetta A, Biagi L, Bonanni P, et al. Reorganization of the somatosensory system after early brain damage: an fMRI and SEP study. Dev Med Child Neurol 2006; 48(Suppl): 9 (abstract). [Google Scholar]
- 75. Koweek R, Rosenstein SN. Comparison of oral stereognosis ability between cerebral palsy patients with head and neck involvement and those without this involvement. J Dent Guid Counc Handicap 1976; 15: 3–7. [PubMed] [Google Scholar]
- 76. Lang AH, Sillanpää M, Hynninen P. Asymmetric function of peripheral nerves in children with cerebral palsy. Acta Neurol Scand 1983; 67: 108–13. [DOI] [PubMed] [Google Scholar]
- 77. Lemée J‐M, Chinier E, Ali P, Labriffe M, Ter Minassian A, Dinomais M. (Re)organisation of the somatosensory system after early brain lesion: a lateralization index fMRI study. Ann Phys Rehabil Med 2019; 63: 416–21. [DOI] [PubMed] [Google Scholar]
- 78. Manikowska F, Chen BP, Jóźwiak M, Lebiedowska MK. The role of exaggerated patellar tendon reflex in knee joint position sense in patients with cerebral palsy. Res Dev Disabil 2015; 45–46: 253–60. [DOI] [PubMed] [Google Scholar]
- 79. Yamashita H, Nishigami T, Mibu A, et al. Perceived body distortion rather than actual body distortion is associated with chronic low back pain in adults with cerebral palsy: a preliminary investigation. Pain Pract 2019; 19: 826–35. [DOI] [PubMed] [Google Scholar]
- 80. Bleyenheuft Y, Thonnard J‐L. Tactile spatial resolution in unilateral brain lesions and its correlation with digital dexterity. J Rehabil Med 2011; 43: 251–6. [DOI] [PubMed] [Google Scholar]
- 81. Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. J Hand Surg Am 1993; 18: 278–81. [DOI] [PubMed] [Google Scholar]
- 82. Lesný I. 2‐point discrimination disorders in cerebral palsy. [Article in Czech] Cesk Pediatr 1970; 25: 138–42. [PubMed] [Google Scholar]
- 83. Lesný I. Additional results from studies on the discrimination of 2 points in children with cerebral palsy. [Article in Czech]. Cesk Psychiatr 1970; 66: 193–202. [PubMed] [Google Scholar]
- 84. Lesný I, Havlícek I. Results of 2‐point‐discrimination analysis in various forms of children’s cerebral paralysis. [Article in Czech]. Cesk Pediatr 1973; 28: 587–90. [PubMed] [Google Scholar]
- 85. Koterazawa K, Shimogaki K, Nabetani M, Miyata H, Kodama S. A study on VEP, SSEP in spastic diplegia with visual impairment. [Article in Japanese]. No to Hattatsu 1996; 28: 261–3. [PubMed] [Google Scholar]
- 86. Kalantari M, Hasani M, Hasani K, Taghizade G. Evaluation of hand stereognosis level in 3–6 years old children with spastic hemiplegia and diplegia. J Rehabil 2013; 14: 93–101. [Google Scholar]
- 87. Hirayama Y, Takahashi H, Yasuhara A, Ochi A. Somatosensory evoked potential (SEP) to posterior tibial nerve stimulation in children with cerebral palsy. [Article in Japanese]. Rinsho Byori 1999; 47: 76–82. [PubMed] [Google Scholar]
- 88. Thomas D, Shabalov VA. Somatosensory disorders in patients with extrapyramidal pathology: the effect of a stereotaxic intervention. Zh Vopr Neirokhir Im N N Burdenko 1990; 1: 27–9. [PubMed] [Google Scholar]
- 89. Whitehead K, Jones L, Laudiano‐Dray MP, Meek J, Fabrizi L. Altered cortical processing of somatosensory input in pre‐term infants who had high‐grade germinal matrix‐intraventricular haemorrhage. Neuroimage Clin 2020; 25: 102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Law S‐H, Lo SK, Chow S, Cheing GLY. Grip force control is dependent on task constraints in children with and without developmental coordination disorder. Int J Rehabil Res 2011; 34: 93–9. [DOI] [PubMed] [Google Scholar]
- 91. Adams ILJ, Ferguson GD, Lust JM, Steenbergen B, Smits‐Engelsman BC. Action planning and position sense in children with developmental coordination disorder. Hum Mov Sci 2016; 46: 196–208. [DOI] [PubMed] [Google Scholar]
- 92. Crothers B. Sensory disorders in cerebral palsy. J Nerv Ment Dis 1953; 118: 455. [Google Scholar]
- 93. Weidenbacker R. Sensory discrimination of children with cerebral palsy. Am Psychol 1952; 7: 319. [DOI] [PubMed] [Google Scholar]
- 94. Weidenbacker R, Sandry M, Moed G. Sensory discrimination of children with cerebral palsy: pressure/pain thresholds on the foot. Percept Mot Skills 1963; 17: 603–10. [DOI] [PubMed] [Google Scholar]
- 95. Auld ML, Boyd R, Moseley GL, Ware R, Johnston LM. Tactile function in children with unilateral cerebral palsy compared to typically developing children. Disabil Rehabil 2012; 34: 1488–94. [DOI] [PubMed] [Google Scholar]
- 96. Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. J Child Neurol 1995; 10: 300–9. [DOI] [PubMed] [Google Scholar]
- 97. Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol 1999; 41: 586–91. [DOI] [PubMed] [Google Scholar]
- 98. Hoon AH Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 2009; 51: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kenney WE. Certain sensory defects in cerebral palsy. Clin Orthop Relat Res 1963; 27: 193–5. [PubMed] [Google Scholar]
- 100. Krumlinde‐Sundholm L, Eliasson A‐C. Comparing tests of tactile sensibility: Aspects relevant to testing children with spastic hemiplegia. Dev Med Child Neurol 2002; 44: 604–12. [DOI] [PubMed] [Google Scholar]
- 101. Kurtaran A, Selçuk B, Kumbara F, Yalçin E, Ersoz M, Akyüz M. Evaluation of hand sensation and function in children with cerebral palsy. Neurosurg Q 2014; 25: 145–8. [Google Scholar]
- 102. Mclaughlin JF, Felix SD, Nowbar S, Ferrel A, Bjornson K, Hays RM. Lower extremity sensory function in children with cerebral palsy. Pediatr Rehabil 2005; 8: 45–52. [DOI] [PubMed] [Google Scholar]
- 103. Bolanos AA, Bleck EE, Firestone P, Young L. Comparison of stereognosis and two‐point discrimination testing of the hands of children with cerebral palsy. Dev Med Child Neurol 1989; 31: 371–6. [DOI] [PubMed] [Google Scholar]
- 104. Guedin N, Fluss J, Thevenot C. Dexterity and finger sense: a possible dissociation in children with cerebral palsy. Percept Mot Skills 2018; 125: 718–31. [DOI] [PubMed] [Google Scholar]
- 105. Kuczynski AM, Semrau JA, Kirton A, Dukelow SP. Kinesthetic deficits after perinatal stroke: robotic measurement in hemiparetic children. J Neuroeng Rehabil 2017; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kuczynski AM, Carlson HL, Lebel C, et al. Sensory tractography and robot‐quantified proprioception in hemiparetic children with perinatal stroke. Hum Brain Mapp 2017; 38: 2424–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lesný I. Disturbance of two ‐point discrimination sensitivity in different forms. Dev Med Child Neurol 1971; 13: 330–4. [DOI] [PubMed] [Google Scholar]
- 108. Lesný I, Stehlík A, Tomásek J, Tománková A, Havlíĉek I. Sensory disorders in cerebral palsy: two‐point discrimination. Dev Med Child Neurol 1993; 35: 402–5. [DOI] [PubMed] [Google Scholar]
- 109. Monfraix C, Tardieu G, Tardieu C. Disturbances of manual perception in children with cerebral palsy. Cereb Palsy Bull 1961; 3: 544–52. [DOI] [PubMed] [Google Scholar]
- 110. Ocarino JM, Fonseca ST, Silva PLP, Gonçalves GGP, Souza TR, Mancini MC. Dynamic touch is affected in children with cerebral palsy. Hum Mov Sci 2014; 33: 85–96. [DOI] [PubMed] [Google Scholar]
- 111. Sanger TD, Kukke SN. Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol 2007; 22: 289–93. [DOI] [PubMed] [Google Scholar]
- 112. Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL. Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol 2008; 50: 832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wingert JR, Sinclair RJ, Dixit S, Damiano DL, Burton H. Somatosensory‐evoked Cortical Activity in Spastic Diplegic Cerebral Palsy. Hum Brain Mapp 2010; 31: 1772–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chrysagis NK, Skordilis EK, Koutsouki D, Evans E. Kinesthetic ability in children with spastic hemiplegia. Adapt Phys Activ Q 2007; 24: 332–51. [DOI] [PubMed] [Google Scholar]
- 115. Jones B. The perception of passive joint‐movements by cerebral‐palsied children. Dev Med Child Neurol 1976; 18: 25–30. [DOI] [PubMed] [Google Scholar]
- 116. Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL, Joint‐position DDL. Joint‐position sense and Kinesthesia in Cerebral Palsy. Arch Phys Med Rehabil 2009; 90: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Goble DJ, Hurvitz EA, Brown SH. Deficits in the ability to use proprioceptive feedback in children with hemiplegic cerebral palsy. Int J Rehabil Res 2009; 32: 267–9. [DOI] [PubMed] [Google Scholar]
- 118. Hall J, Gardner MC. Kinesthetic sensitivity of cerebral palsied individuals. Am Correct Ther J 1981; 35: 6–10. [PubMed] [Google Scholar]
- 119. Smorenburg ARP, Ledebt A, Deconinck FJA, Savelsbergh GJP. Deficits in upper limb position sense of children with spastic hemiparetic cerebral palsy are distance‐dependent. Res Dev Disabil 2012; 33: 971–81. [DOI] [PubMed] [Google Scholar]
- 120. de Andrade E , Souza Mazuchi F, Mochizuki L, Hamill J, et al. Joint‐position sense accuracy is equally affected by vision among children with and without cerebral palsy. J Mot Behav 2021; 53: 209–16. [DOI] [PubMed] [Google Scholar]
- 121. Tardieu G, Tardieu C, Lespargot A, Roby A, Bret MD. Can vibration‐induced illusions be used as a muscle perception test for normal and cerebral‐palsied children? Dev Med Child Neurol 1984; 26: 449–56. [DOI] [PubMed] [Google Scholar]
- 122. Benromano T, Pick CG, Merick J, Defrin R. Physiological and behavioral responses to calibrated noxious stimuli among individuals with cerebral palsy and intellectual disability. Pain Med 2017; 18: 441–53. [DOI] [PubMed] [Google Scholar]
- 123. Guo X, Xiang J, Mun‐bryce S, Mun‐Bryce S, et al. Aberrant high‐gamma oscillations in the somatosensory cortex of children with cerebral palsy: a meg study. Brain Dev 2012; 34: 576–83. [DOI] [PubMed] [Google Scholar]
- 124. Kurz MJ, Wilson TW. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett 2011; 490: 1–5. [DOI] [PubMed] [Google Scholar]
- 125. Nevalainen P, Pihko E, Mäenpää H, Valanne L, Nummenmaa L, Lauronen L. Bilateral alterations in somatosensory cortical processing in hemiplegic cerebral palsy. Dev Med Child Neurol 2012; 54: 361–7. [DOI] [PubMed] [Google Scholar]
- 126. Kulak W, Sobaniec W, Sołowiej E, et al. Somatosensory and visual evoked potentials in children with cerebral palsy: correlations and discrepancies with MRI findings and clinical picture. Pediatr Rehabil 2006; 9: 201–9. [DOI] [PubMed] [Google Scholar]
- 127. Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E, Tsikoulas I. Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatr Neurol 2011; 44: 177–82. [DOI] [PubMed] [Google Scholar]
- 128. Chu D, Huttenlocher PR, Levin DN, Towle VL. Reorganization of the hand somatosensory cortex following perinatal unilateral brain injury. Neuropediatrics 2000; 31: 63–9. [DOI] [PubMed] [Google Scholar]
- 129. Van de Winckel A, Verheyden G, Wenderoth N, et al. Does somatosensory discrimination activate different brain areas in children with unilateral cerebral palsy compared to typically developing children? An fMRI study. Res Dev Disabil 2013; 34: 1710–20. [DOI] [PubMed] [Google Scholar]
- 130. Van de Winckel A, Klingels K, Bruyninckx F, et al. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res Dev Disabil 2013; 34: 183–97. [DOI] [PubMed] [Google Scholar]
- 131. Klingner CM, Witte OW. Somatosensory deficits. In: Vallar G, Branch Coslett H, editors. Handbook of Clinical Neurology, 1st ed. San Diego, CA: Elsevier Science & Technology, 2018: 185–206. [DOI] [PubMed] [Google Scholar]
- 132. Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW. Children with cerebral palsy hyper‐gate somatosensory stimulations of the foot. Cereb Cortex 2018; 28: 2431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yoshida S, Hayakawa K, Yamamoto A, et al. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Dev Med Child Neurol 2010; 52: 935–40. [DOI] [PubMed] [Google Scholar]
- 134. Yoshida S, Hayakawa K, Oishi K, et al. Athetotic and spastic cerebral palsy: anatomic characterization based on diffusion‐tensor imaging. Radiology 2011; 260: 511–20. [DOI] [PubMed] [Google Scholar]
- 135. Scheck SM, Boyd RN, Rose SE. New insights into the pathology of white matter tracts in cerebral palsy from diffusion magnetic resonance imaging: a systematic review. Dev Med Child Neurol 2012; 54: 684–96. [DOI] [PubMed] [Google Scholar]
- 136. Fukuhara T, Namba Y, Yamadori I. Peripheral sensory neuropathy observed in children with cerebral palsy: is chronic afferent excitation from muscle spindles a possible cause? Childs Nerv Syst 2010; 26: 751–4. [DOI] [PubMed] [Google Scholar]
- 137. Naito E, Kochiyama T, Kitada R, et al. Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci 2002; 22: 3683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dijkerman HC, de Haan EHF . Somatosensory processes subserving perception and action. Behav Brain Sci 2007; 30: 189–201. [DOI] [PubMed] [Google Scholar]
- 139. Ritterband‐Rosenbaum A, Christensen MS, Kliim‐Due M, Petersen LZ, Rasmussen B, Nielsen JB. Altered sense of agency in children with spastic cerebral palsy. BMC Neurol 2011; 11: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nielsen JB, Christensen MS, Farmer SF, Lorentzen J. Spastic movement disorder: should we forget hyperexcitable stretch reflexes and start talking about inappropriate prediction of sensory consequences of movement? Exp Brain Res 2020; 238: 1627–36. [DOI] [PubMed] [Google Scholar]
- 141. Butti N, Montirosso R, Giusti L, Piccinini L, Borgatti R, Urgesi C. Early brain damage affects body schema and person perception abilities in children and adolescents with spastic diplegia. Neural Plast 2019; 2019: 1678984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nuara A, Papangelo P, Avanzini P, Fabbri‐Destro M. Body representation in children with unilateral cerebral palsy. Front Psychol 2019; 10: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fontes PLB, Cruz TKF, Souto DO, Moura R, Haase VG. Body representation in children with hemiplegic cerebral palsy. Child Neuropsychol 2017; 23: 838–63. [DOI] [PubMed] [Google Scholar]
- 144. Steenbergen B, Jongbloed‐Pereboom M, Spruijt S, Gordon AM. Impaired motor planning and motor imagery in children with unilateral spastic cerebral palsy: challenges for the future of pediatric rehabilitation. Dev Med Child Neurol 2013; 55(Suppl. 4): 43–6. [DOI] [PubMed] [Google Scholar]
- 145. Palmer S, Bailey J, Brown C, Jones A, McCabe CS. Sensory function and pain experience in arthritis, complex regional pain syndrome, fibromyalgia syndrome, and pain‐free volunteers: a cross‐sectional study. Clin J Pain 2019; 35: 894–900. [DOI] [PubMed] [Google Scholar]
- 146. Brun C, Giorgi N, Pinard A‐M, Gagné M, McCabe CS, Mercier C. Exploring the relationships between altered body perception, limb position sense, and limb movement sense in complex regional pain syndrome. J Pain 2019; 20: 17–27. [DOI] [PubMed] [Google Scholar]
- 147. Lotze M, Moseley GL. Role of distorted body image in pain. Curr Rheumatol Rep 2007; 9: 488–96. [DOI] [PubMed] [Google Scholar]
- 148. Parkinson KN, Dickinson HO, Arnaud C, Lyons A, Colver A. Pain in young people aged 13 to 17 years with cerebral palsy: cross‐sectional, multicentre European study. Arch Dis Child 2013; 98: 434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014; 348: f7656. [DOI] [PubMed] [Google Scholar]
- 150. Lauder GR, White MC. Neuropathic pain following multilevel surgery in children with cerebral palsy: a case series and review. Paediatr Anaesth 2005; 15: 412–20. [DOI] [PubMed] [Google Scholar]
- 151. Auld ML, Johnston LM. Getting inTOUCH: outcomes of a knowledge translation intervention for tactile assessment knowledge, barriers, and practice in paediatric therapists working with children with cerebral palsy. Disabil Rehabil 2019; 41: 2350–8. [DOI] [PubMed] [Google Scholar]
- 152. Auld ML, Russo R, Moseley GL, Johnston LM. Determination of interventions for upper extremity tactile impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2014; 56: 815–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Full search strategy for each database
Table S2: Assessment of study quality for each article and inter‐agreement
Table S3: Characteristics and results for each included study assessing tactile function
Table S4: Characteristics and results for each included study assessing proprioception
Table S5: Characteristics and results for each included study assessing pain function
Table S6: Characteristics and results for each included study assessing cortical responses evoked by somatosensory stimulation
Table S7: Description of clinical tests used in the studies included in this scoping review
Table S8: Methods to assess cortical responses evoked by somatosensory stimulation
Table S9: Source of funding and conflict of interest for each study included in the scoping review
Figure S1: Flow diagram of the bibliographical search


