Abstract
This study aimed to explore the changes in functional connections between cerebral hemispheres and local brain regions functional activities in patients with acute ischemic stroke (AIS) treated with International Standard Scalp Acupuncture (ISSA). Thirty patients with middle cerebral artery AIS in the dominant hemisphere were selected and randomly divided into two groups such as the control group and the scalp acupuncture group, with 15 patients in each group. Patients in the control group were treated with conventional Western medicine, while patients in the scalp acupuncture group received ISSA (acupuncture at the parietal midline [MS5], acupuncture at the left anterior parietotemporal oblique line [MS6] and acupuncture at the left posterior parietotemporal oblique line [MS7]) for one course of treatment. All patients were evaluated for treatment efficacy and received whole brain resting state functional magnetic resonance imaging (Rs‐fMRI) scan before and after treatment. The observational indicators included: (a) the National Institutes of Health Stroke Scale (NIHSS) scores and the simplified Fugl‐Meyer Assessment (SFMA) scores; (b) analyses of the amplitude of low‐frequency fluctuation (ALFF), regional homogeneity (ReHo) and voxel‐mirrored homotopic connectivity (VMHC). The results showed a significant difference in the NIHSS scores before and after treatment in the scalp acupuncture group compared with the control group (p < .05), indicating that patients improved better after scalp acupuncture treatment. Compared with the control group, the VMHC, ALFF and ReHo values in the scalp acupuncture group increased after treatment. The VMHC values increased in the brain regions dominated by bilateral BA6 and BA8; the ALFF values increased in the left BA39 and the adjacent superior temporal gyrus and middle temporal gyrus; and the ReHo values increased in the brain regions extending from left middle temporal gyrus (including BA21) to BA37, and the brain regions extending from the left BA40 and angular gyrus to BA7. The present study indicated that scalp acupuncture can specifically strengthen the functional activities of the brain regions related to sensory integration, language processing and motor coordination in the middle aged and elderly patients with AIS of the dominant cerebral hemisphere, and can strengthen bilateral frontal lobe motor control. This study may provide a scientific basis for the clinical application of ISSA treatment in patients with AIS, and may also provide a preliminary research basis for further animal experiments.
Keywords: acute ischemic stroke, amplitude of low‐frequency fluctuation (ALFF), international standard scalp acupuncture, regional homogeneity (ReHo), resting state functional magnetic resonance imaging (Rs‐fMRI), voxel‐mirrored homotopic connectivity (VMHC)
Abstract
本研究为探讨急性期缺血性脑卒中功能障碍患者国际标准头针疗程干预后脑区的脑半球间功能连接和局部功能活动性变化,纳入30名优势半球大脑中动脉供血区急性缺血性脑卒中(Acute ischemic stroke,AIS)患者,随机分为头针组和对照组各15例。对照组采用西医药物常规治疗,头针组在对照组基础上,结合国际标准头针(顶中线、左侧顶颞前斜线和左侧顶颞后斜线),分别治疗1个疗程。疗程治疗前后,2组患者均进行疗效评定,并接受Rs‐fMRI扫描。观察指标包括(1)NIHSS评分及SFMA评分;(2)低频震荡振幅(ALFF)、局部一致性(ReHo)、体素‐镜像同伦连接(VMHC)。结果显示头针组治疗前后NIHSS评分差值比对照组有显著改善(P<0.05)。与对照组相比,头针组治疗后的VMHC、ALFF、ReHo值均有增强:以双侧BA6、BA8为主的脑区VMHC值增强;以左侧BA39为主,向邻近的颞上回和颞中回延伸的脑区ALFF值增强;以左侧颞中回(含BA21)为主,向BA37延伸的脑区,及以左侧BA40、角回为主,向BA7延伸的脑区ReHo值增强。本研究初步发现,国际标准头针组穴针刺能特异性加强优势大脑半球中动脉急性梗死的中老年患者感觉整合、语言加工、运动协调相关脑区的局域性功能活动,同时强化双侧额叶运动调控相关脑区的连通性,为国际标准头针治疗急性期缺血性脑卒中的临床应用提供科学依据,也为进一步地动物实验提供前期研究基础。
Abbreviations
- AIS
acute ischemic stroke
- ALFF
amplitude of low‐frequency fluctuation
- BOLD
blood oxygen level dependent
- fMRI
functional magnetic resonance imaging
- ISSA
international standard scalp acupuncture
- M1
primary motor cortex
- NIHSS
National Institute of Health stroke scale
- PMA
premotor cortex area
- ReHo
regional homogeneity
- Rs‐fMRI
resting‐state functional magnetic resonance imaging
- SFMA
simplified Fugl‐Meyer Assessment
- VMHC
voxel‐mirrored homotopic connectivity
1. INTRODUCTION
Acupuncture, an important part of traditional Chinese medicine (TCM) has been used as an alternative and complementary treatment for different diseases (NIH Consens Statement, 1997). In 1980, acupuncture was recommended by the World Health Organization (WHO) as an alternative treatment for 43 different disorders, including stroke. Several randomized controlled clinical trials (Bao et al., 2021; Hu et al., 1993; Johansson et al., 2001; Kjendahl, Salistrom, Osten, Stanghelle, & Borchgrevink, 1997; Sallstrom, Kjendahl, Osten, et al., 1995) have demonstrated that acupuncture can improve the motor, sensory, speech and other nervous system dysfunctions in stroke patients (World Health Organization, 2002). The therapeutic effect of acupuncture was further confirmed by the relevant evaluation methods including the Cochrane Library System Evaluation Database in 2018. Acupuncture has also been shown to improve the symptoms of acute stroke significantly with no obvious adverse effects (Xu, Li, & Zhang, 2018).
The International Standard Scalp Acupuncture (ISSA) therapy is one of the common acupuncture methods for the treatment of acute stroke. The ISSA is developed based on the traditional acupuncture science, modern anatomy, neurophysiology and bioholographic theory (Liu et al., 2012). Two systematic reviews have confirmed a significant improvement in the neurological symptoms such as, motor dysfunction in patients with acute ischemic stroke (AIS) after scalp acupuncture treatment (Wang et al., 2012; You et al., 2018). Moreover, scalp acupuncture has also been demonstrated to have a lasting therapeutic effect on the recovery of sensory and motor functions in stroke patients (Hegyi & Szigeti, 2012). However, the mechanism of scalp acupuncture is not clear, which hinders its promotion and application in clinical practice. At present, the treatment methods for ischemic stroke in the acute and sub‐acute phases are mainly the use of Western medicine and rehabilitation training (Jorgensen, Nakayama, Raaschou, et al., 1995). However, ischemic stroke patients who miss the best time or have contraindications for thrombolytic therapy tend to suffer from dyskinesia and other sequelae. The risks of mortality and intracranial hemorrhage also increased with the use of thrombolytic agents (Wardlaw et al., 2014). Therefore, scalp acupuncture is considered as one of the important alternative treatment methods for AIS. Several clinical trials have been conducted to promote the application of ISSA treatment for stroke and other brain diseases (Wang et al., 2012). This may help to explore the mechanism of scalp acupuncture in the central nervous system diseases.
In recent years, the functional reorganization theory has become a central point in the research of motor function recovery after stroke. The recovery of motor function after a stroke includes not only the recovery of brain function in the lesion and its surrounding motor‐related brain areas, but also the recovery of brain function in the remote and structurally unaffected motor‐related brain areas (Chen et al., 2020). A breakthrough point to evaluate the mechanism of scalp acupuncture is to stimulate the local brain regions through acupuncture and to observe the functional reorganization and motor function recovery after stroke.
As a non‐invasive method, resting state‐functional magnetic resonance imaging (Rs‐fMRI) can reflect the spontaneous neural activity of the brain and the continuous therapeutic effect of acupuncture. Rs‐fMRI is an important method to explore the brain function reorganization in stroke patients after scalp acupuncture treatment in vivo. The voxel‐mirrored homotopic connectivity (VMHC), amplitude of low‐frequency fluctuation (ALFF) and regional homogeneity (ReHo) are three different analyses methods of Rs‐fMRI. VMHC is related to the synchronization of the spontaneous neural function activities between the symmetrical areas of the bilateral hemispheres, ALFF is related to the intensity of the focal neuron activity in the brain area, and ReHo is related to the synchronization of neuronal activity between focal brain regions and adjacent regions. Previous studies have shown that reduced VMHC, ALFF and ReHo values in stroke patients with motor dysfunction (Chen et al., 2019; Chen et al., 2020; Jiang, Yi, Cai, et al., 2019) were mainly related to the precentral gyrus. Based on the theory of functional reorganization, we speculated that changes in the VMHC value may be due to the changes in the local brain regions. This study put forward the hypothesis that the mechanism of scalp acupuncture for motor dysfunction after stroke may be related to the activation of local brain regions and the connections between the cerebral hemispheres.
The present study evaluated the role of ISSA treatment in patients with AIS, and analyzed the changes in the functional connections between cerebral hemispheres and local brain regions by using the VMHC, ALFF and ReHo. The study may help to evaluate the mechanism of ISSA treatment in patients with AIS.
2. MATERIALS AND METHODS
2.1. Participants
The present study is a randomized controlled trial, approved by the Ethics Committee of the China‐Japan Union Hospital (Jilin University, China) (No.: 2016ks043) and registered at the Chinese Clinical Trial Registry (No.: ChiCTR‐IOR‐15007672). Based on the complexity and particularity of Rs‐fMRI image acquisition and data statistics of stroke patients, this paper estimates the sample size of the Rs‐fMRI study on the acupuncture effect mechanism. Referring to Desmond and Glover (2002) and the imaging literature of acupuncture improving cognitive function or motor function of stroke patients at home and abroad, and considering the 20% drop‐off rate, 15 patients in each group were included in this study. Thirty patients with middle cerebral artery AIS of the dominant hemisphere were recruited from the China–Japan Union Hospital (Jilin University, China) between January 2017 and December 2017. The patients were randomly assigned by using the random number table method. The ratio of the scalp acupuncture group to the control group was 1:1, with 15 patients in each group.
Diagnostic criteria: Patients were diagnosed based on the Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke in China, 2014 (Chinese Society of Neurology, Cerebrovascular Disease Group of Chinese Society Neurology, 2015).
Inclusion criteria: (a) patients with typical right sided motor and sensory disorders symptoms were diagnosed with left middle cerebral artery AIS as examined by CT or MRI scans; (b) patients with relatively stable disease; (c) aged 40–70 years; (d) patients who received conventional Western medicine treatment (except for drugs that promote collateral circulation like Ureklin and Butylphthalide); (e) diet, sleep and weight were basically normal, and patients were not addicted to tobacco, alcohol, tea, and coffee; (f) right‐handed; (g) informed consent was obtained from all patients before recruiting them in this trial.
Exclusion criteria: (a) patients with a course of disease longer than 72 hr; (b) patients with newly onset of cerebral hemorrhage as examined by CT or MRI scans; (c) patients with cerebrovascular pathological variation; (d) patients with disturbance of consciousness, sensory aphasia, mixed aphasia, claustrophobia or dementia, which may affect the communication and operation during the experiment; (e) patients with heart, kidney, liver diseases or tumors that may affect the test results; (f) patients with a history of acupuncture treatment within 1 month before the trial; (g) pregnant or breastfeeding women; (h) patients who had metal substances in the body; (i) patients who received transcranial magnetic stimulation, or transcranial direct current treatment, or electroencephalography (EEG) biofeedback treatment during the same period; (j) Patients with acupuncture contraindications such as hemophilia.
Withdrawal and drop‐off criteria: The intervention was terminated in patients who could not continue to be observed because of the deterioration of the disease. Drop‐off cases were defined as patients who were unable to complete the course of treatment or those with incomplete Rs‐fMRI data.
2.2. Interventional method
2.2.1. Western medicine treatment
Patients in both groups were given conventional Western medicine treatment, such as anti‐platelet, lipid‐lowering and stabilizing agents, and blood pressure medications. The physician made a basic treatment plan based on the patient's specific condition and recorded the medications used in detail.
2.2.2. International standard scalp acupuncture treatment
According to the International Standardization Scheme for Scalp Acupuncture Points (Min et al., 2007; World Health Organization, 1991; Zhongguo biao zhun chu ban she, 2008), acupuncture was performed at the midline of vertex (MS5), the left anterior oblique line of vertex‐temporal (MS6) and the left posterior oblique line of vertex‐temporal (MS7). After disinfection, the stainless steel needles (0.3 mm × 40 mm) were inserted at the angles of 15°–30° with respect to the epicranial aponeurosis. When the participants reported that the needles had elicited Deqi sensation, the needles were twisted at a frequency of 200 turns per minute, causing a distending sensation. Twisting was repeated at 10‐min interval during the 30‐min period. Two times a day for 6 days as one course, the trial was finished after a course of treatment.
Patients in the scalp acupuncture group were treated by the TCM practitioner who has been engaged in acupuncture clinical work for more than 3 years.
2.3. MRI acquisition
T1‐weighted magnetization‐prepared rapid acquisition of gradient‐echo and fMRI‐blood oxygen level‐dependent (BOLD) echo‐planar images was conducted. Brain scans were acquired using the MRI system (Siemens 3.0 T, Siemens Healthineers; Germany) before and after the treatment course. The subjects were laid supine on the MRI scan bed under the guidance of the physician and the head was fixed with a foam pad during the scan. The subjects stayed awake throughout the scanning and their eyes were covered with black goggles and ears were blocked with sponge earplugs.
The fMRI‐BOLD scanning parameters were as follows: pulse repetition time (TR) = 2000 ms, echo time (TE) = 30 ms; flip angle = 90°, thickness = 3.5 mm, gap = 0.7 mm, voxel size = 3.5 × 3.5 × 3.5 mm3, matrix = 64 × 64, axial slices = 37 layers, field of view (FOV) = 224 mm × 224 mm, repetitions (phases per location) = 240, total scan 8 min.
2.4. Test information
2.4.1. Main indicators
Based on the Matlab 2012a platform, the DPABI tool (http://rfmri.org/dpabi) was used to start the statistical parameter map (SPM 12), and the values of the ALFF, ReHo and VMHC were obtained for each patient by time calibration, spatial correction, normalization, smoothness, linear drift elimination, covariate elimination of white matter and cerebrospinal fluid and image filtering on the image data of the subject's MRI scan.
2.4.2. Minor indicators
The National Institute of Health stroke scale (NIHSS) score was used to assess the patient's neurological deficits before and after treatment. At the same time, the simplified Fugl‐Meyer Assessment (SFMA) score was used to evaluate the motor function recovery.
2.5. Imaging processing and statistical analysis
Based on the Matlab 2012a platform, the REST1.8 tool (http://www.restfmri.net/forum/REST_V1.8) was used for the independent sample t‐test analysis of the data model of ALFF, ReHo and VMHC of the two groups of subjects to draw statistical parameter graph with gender, age, disease course, systolic blood pressure, diastolic blood pressure and head movement as covariates. By identifying and correcting the test data, the anatomical position and activation intensity of the brain area with changes in the ALFF, ReHo and VMHC values between the two groups were obtained. Finally, an experienced neurologist corrected the data based on the anatomical knowledge and clinical experience. In addition, the χ 2 test was used to access the gender data and the t‐test was used to determine the measurement data, such as age and NIHSS score. SPSS 20.0 software was used for statistical analysis.
3. RESULTS
3.1. Basic data
According to the diagnostic, inclusion and exclusion criteria, 30 patients with middle cerebral artery AIS of the dominant hemisphere were selected from 1983 suspected AIS patients. During the study, using the Rs‐fMRI data collection method as a guideline, four patients discontinued the trial due to aggravation of the condition, and 13 patients withdraw from the trial due to incomplete BOLD signal (Figure 1). The general information and clinical efficacy data of all 30 patients were retained. During the treatment, three patients in the scalp acupuncture group experienced punctate hemorrhage after withdrawing the needles (the bleeding was stopped when pressed with the sterile dry cotton for 5–10 s). No serious adverse events were reported by the patients in the two groups. There were no statistically significant differences in gender, age, course of disease, systolic blood pressure and diastolic blood pressure among the 30 patients (p > .05), (Table 1).
FIGURE 1.
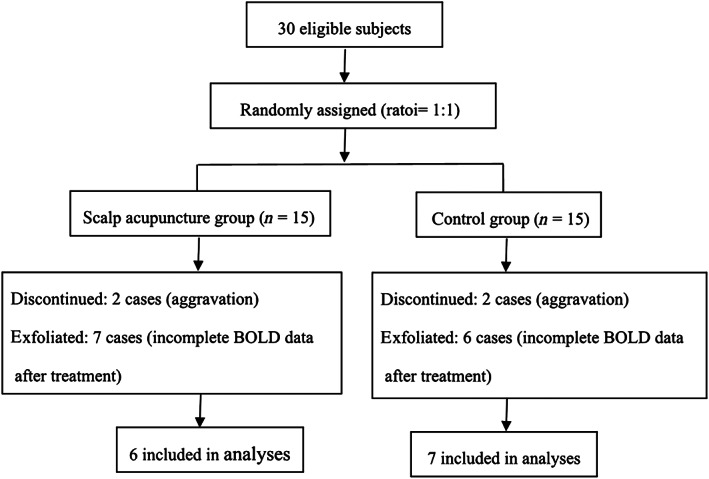
The inclusion criteria of patients
TABLE 1.
The basic experimental data of patients
| Item | Scalp acupuncture group | Control group | Statistic | p |
|---|---|---|---|---|
| Gender (male/female, n) | 12/3 | 10/5 | χ 2 = 0.26 | .61 |
| Age (years) | 56.47 ± 8.25 | 61.73 ± 7.79 | t = −1.59 | .12 |
| Last scan time (day) | 10.00 ± 1.07 | 9.77 ± 1.64 | t = 0.46 | .65 |
| Systolic pressure (mmHg) | 144.87 ± 13.44 | 148.73 ± 13.97 | t = −0.77 | .45 |
| Diastolic pressure (mmHg) | 84.53 ± 9.46 | 88.40 ± 11.40 | t = −1.01 | .32 |
Note: The last scan time is the interval between the onset time and the scan after treatment.
3.2. Comparison of NIHSS scores and SFMA scores between the two groups before and after treatment
There was a significant difference in the NIHSS scores before and after treatment in the scalp acupuncture group, compared with the control group (p < .05). However, no significant difference was observed in the SFMA scores before and after treatment in the scalp acupuncture group, compared with the control group (Table 2).
TABLE 2.
The difference of NIHSS scores and SFMA scores in the two groups before and after treatment
| Group | Cases (n) | NIHSSpre‐post | SFMApre‐post |
|---|---|---|---|
| Scalp acupuncture group | 15 | 3.20 ± 2.46 | 10.27 ± 1.96 |
| Control group | 15 | 1.67 ± 1.05 | 7.73 ± 1.76 |
| t | 2.23 | 0.96 | |
| p | 0.04 | 0.34 |
Abbreviations: NIHSS, National Institute of Health stroke scale; pre‐post, changes between post treatment and baseline; SFMA, simplified Fugl‐Meyer Assessment.
3.3. The differences in VMHC, ALFF and ReHo values between the two groups after treatment
Compared with the control group, there were significant differences in the VMHC, ALFF and REHO values in the scalp acupuncture group after treatment in different brain regions and their localization were as follows (Table 3): (a) The VMHC values increased at the bilateral BA6 and BA8 (Figure 2); (b) The ALFF values increased at the left BA39 extending to the adjacent superior temporal gyrus and middle temporal gyrus (Figure 3); and (c) The ReHo values increased at the left middle temporal gyrus (including BA21) extending to the BA37. The ReHo values also increased at the left BA40 and angular gyrus extending to the BA7 (Figure 4).
TABLE 3.
Brain areas with differences in VMHC values between the two groups after treatment
| Item | Effect | Brain region | MiNi coordinate | Intensity (T‐value) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| VMHC | Enhanced | Right BA6, BA8 | 27 | 21 | 57 | 22.39 |
| Enhanced | Left BA6, BA8 | −27 | 21 | 57 | 22.39 | |
| ALFF | Enhanced | Left BA39, superior temporal gyrus, middle temporal gyrus | −48 | −63 | 18 | 12.53 |
| ReHo | Enhanced | Left middle temporal gyrus (include BA21), BA37 | −57 | −48 | 0 | 21.48 |
| Enhanced | Left BA40, angular gyrus, BA7 | −39 | −54 | 51 | 13.69 | |
Note: The letters X and Y and Z to represent the brain space positioning axis. The letter X represents the coordinate position of the left and right orientation in the MiNi standardized spatial coordinate system. The letter Y represents the coordinate position of the front and rear azimuth in the MiNi standardized space coordinate system. The letter Z indicates the coordinate position of the upper and lower positions in the MiNi standardized space coordinate system.
FIGURE 2.
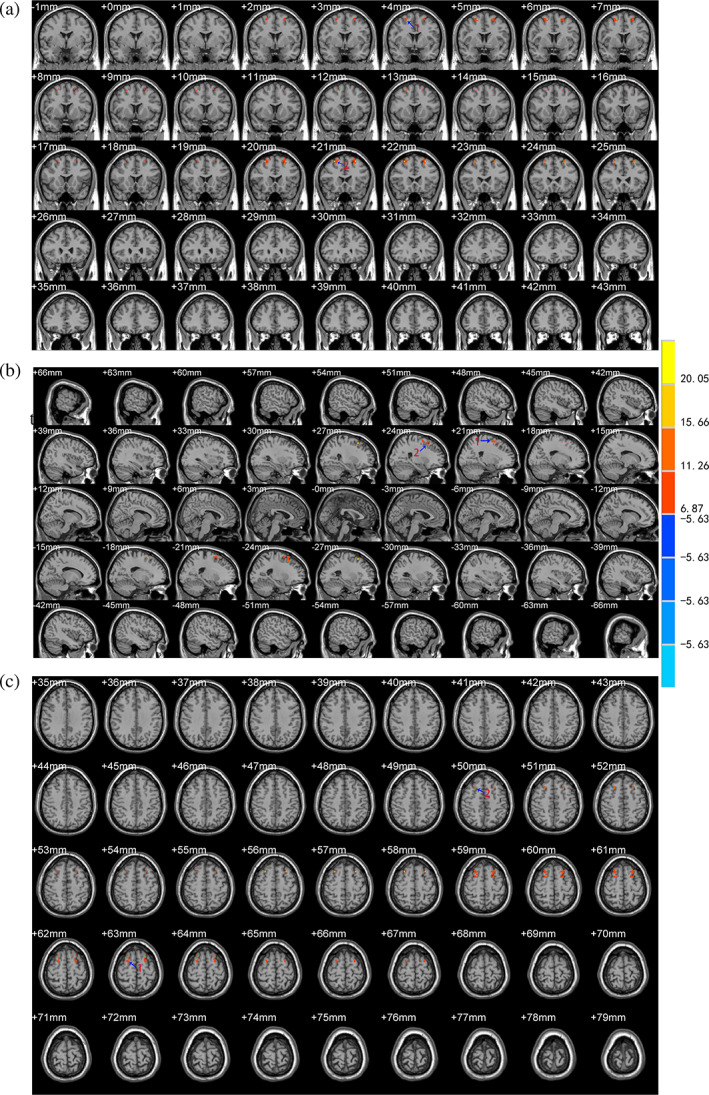
Brain areas with differences in the VMHC values between the two groups after treatment. The letter (a) represents the coronal position of the VMHC analysis results, (b) represents the sagittal position, and (c) represents the horizontal position. The color bar on the right side of the figure represents the intensity of activation in the brain area. The numbers represent different brain regions for VMHC: 1. BA6, 2. BA8
FIGURE 3.
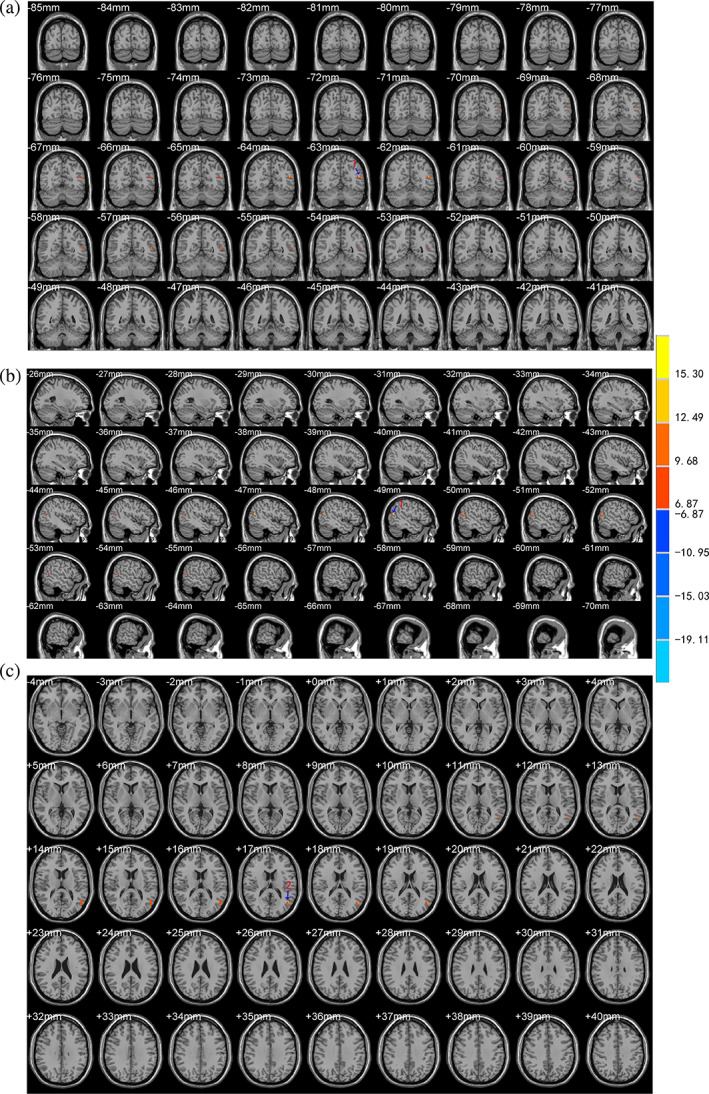
Brain areas with differences in the ALFF values between the two groups after treatment. The letter (a) represents the coronal position of the ALFF analysis results, (b) represents the sagittal position, and (c) represents the horizontal position. The color bar on the right side of the figure represents the intensity of activation in the brain area. The numbers represent different brain regions for ALFF: 1. left middle temporal gyrus, 2. left BA39
FIGURE 4.
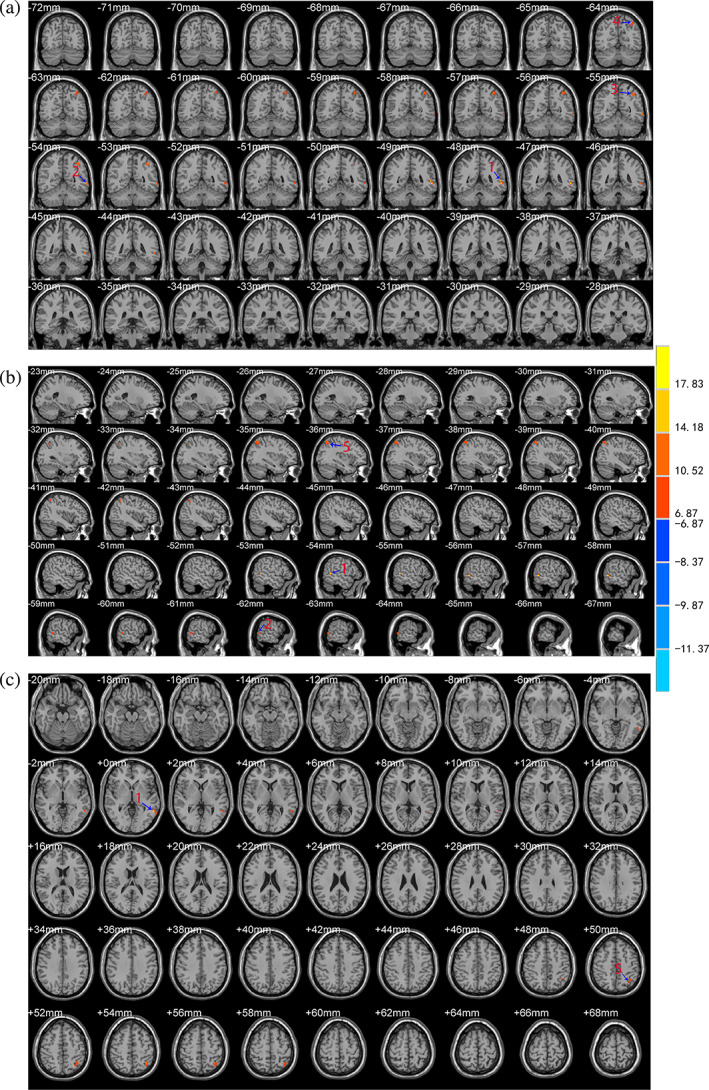
Brain areas with differences in the ReHo values between the two groups after treatment. The letter (a) represents the coronal position of the ReHo analysis results, (b) represents the sagittal position, and (c) represents the horizontal position. The color bar on the right side of the figure represents the intensity of activation in the brain area. The numbers represent different brain regions for ReHo: 1. left middle temporal gyrus (include BA21), 2. left BA37, 3. left BA40, 4. left BA7, 5. left angular gyrus
4. DISCUSSION
In this study, we compared the NIHSS scores between the scalp acupuncture group and the control group and found a significant difference in the NIHSS scores after ISSA treatment between the two groups. This indicated that scalp acupuncture may have an ameliorative effect on the global neurological impairment in stroke patients, which is consistent with the results of previous studies (Wang et al., 2012; You et al., 2018). However, no statistically significant difference in the SFMA scores was observed between the two groups. Based on the Rs‐fMRI, we analyzed the differences in VMHC, ALFF and REHO values between the two groups.
Compared with the Western medicine treatment alone, the functional connections between brain regions dominated by bilateral BA6 and BA8 were enhanced after the combined ISSA and Western medicine treatment. Previously, a longitudinal study has shown decreased VMHC value in the homologous sensorimotor cortex of the cerebral hemisphere in the acute phase of stroke (Van Meer et al., 2010). In the sub‐acute phase, the VMHC value in the relevant brain region was gradually increased, and was stabilized during the recovery phase. Notably, the synchronization of functional activities between the bilateral BA6 and BA8 were immediately strengthened after the intervention of scalp acupuncture in the acute phase. BA6 and BA8 together constitute the premotor cortex area (PMA). PMA has a special role in motor learning. It can make corresponding body movements through the memory of visual, auditory, and tactile stimuli (Ramsey et al., 1996). PMA is considered to be an important choice for compensating the primary motor cortex (M1) and other motor‐regulated brain regions because it has extensive spinal cord fibers projection (Dum & Strick, 1991) and more extensive contact with the corpus callosum than M1, which is conducive to bilateral movement control (Boussaoud, Tannegariepy, Wannier, & Rouiller, 2005; Fang, Stepniewska, & Kaas, 2008). Different from the findings of the previous study that the value of VMHC decreased in the motor cortex in the acute phase of stroke, we speculated that scalp acupuncture combined with conventional Western medicine could increase the VMHC value in the PMA. Strengthening the coordination mode of the brain regions relevant to the movement control of the bilateral frontal lobes (BA6 and BA8) may be a compensatory mechanism produced by scalp acupuncture in the acute phase.
In the present study, the ALFF and ReHo values in the left angular gyrus (BA39) and the middle temporal gyrus were positively correlated with the AIS. BA39 is mainly related to cognitive functions like language/semantic processing (Binder, Desai, Graves, & Conant, 2009) and number processing (Dehaene, Piazza, Pinel, & Cohen, 2003). The functions of the middle temporal gyrus (including BA21) are complex and diverse. In addition to participating in the semantic processing of language (Carin, Elizabeth, & Tilo, 2010; Mcdermott, Petersen, Watson, & Ojemann, 2003), it is also involved in the integration of different types of sensory input (e.g., somatosensory, visual and auditory) (Chen et al., 2016), especially in the process of gait initiation, the conflict resolution between the vestibular and other sensory inputs (Mi et al., 2017; Wagner et al., 2008).
The ALFF value was increased in the superior temporal gyrus of the dominant hemisphere. The superior temporal gyrus is the brain region where visual, vestibular and proprioceptive inputs meet (Chiarovano et al., 2016). It participates in the processing of perception (Bangert & Altenmüller, 2003) and simple autonomous movement (Caffarra, Gardini, Vezzadini, Bromiley, & Venner, 2010; Payabvash et al., 2012). Besides, the superior temporal gyrus is also the basic pathway of the language processing network, and participates in language perception and production. It interfaces with the motor planning system for processing language vocabulary (Jungblut, Huber, Mais, et al., 2014), which is one of the components of cognitive ability. The superior temporal gyrus, angular gyrus and middle temporal gyrus are jointly responsible for the integration of hearing and movement. Therefore, we speculated that the enhancement of focal neuronal activity in the middle temporal gyrus, BA39 and superior temporal gyrus was related to the improvement of post‐stroke sensory, motor and speech disorders by scalp acupuncture.
The ReHo value was increased in the fusiform gyrus (BA37), superior marginal gyrus (BA40) and BA7. The fusiform gyrus (BA37) is the most important link in the “what” pathway for object category recognition (Brunyé, Moran, Holmes, Mahoney, & Taylor, 2017). The superior marginal gyrus (BA40) is located in the secondary somatosensory cortex and responds to physical stimulation and completes structural differentiation tasks. It is a vital center responsible for fine motor coordination, complex movements and labor skills (Potok et al., 2019). BA7 combines visual and motor information to complete the visual‐motor coordination function. We speculated that enhanced functional activities among the above‐mentioned brain regions by scalp acupuncture were correlated with improvement of sensory and motor disorders after stroke.
Taken together, although ALFF, ReHo and VMHC did not co‐activate in the brain regions, the enhanced functional activities of the local brain regions by ALFF, ReHo and VMHC were closely related to the sensory integration and motor regulation, which were the first two stages of the neural process of controlling spontaneous movement (Kandel, Schwartz, Jessell, Siegelbaum, & Hudspeth, 2013). The first stage was the production of a unified perceptual performance in the sensory process, which is important for the activation of the local brain regions. The second stage was the cognitive process, in which the internal reference system determined how to deal with spontaneous movements. As a result, spontaneous exercise is produced by the brain after selecting the certain exercise plan and making the order to do it (Kandel et al., 2013). It is concluded that scalp acupuncture can regulate movements by strengthening sensory integration through the activation of local brain regions. After receiving enhanced sensory stimulation from local brain regions, PMA can establish a coordination mode of bilateral motor pathways through the enhanced functional connection pathways to generate compensatory pathways.
As indicated by the clinical efficacy indexes, scalp acupuncture combined with Western medicine was able to improve the overall neurological deficit in patients with AIS. However, the recovery of sensation and cognition by scalp acupuncture is in the early stage of spontaneous movement, which may explain the result that there was no significant difference in the motor function score in different groups. From the perspective of TCM, AIS belongs to the category of “stroke disease.” The basic pathogenesis of AIS is the imbalance between Yin and Yang, the disorder of Qi and blood, and blood stasis in the cerebral veins. According to the TCM, the meridians, collaterals and acupuncture points are densely concentrated in the head. The head is also closely related to Qi and blood, viscera and brain. Scalp acupuncture follows the principle of selecting acupoints and making prescriptions, which is to treat the near area in order to regulate the spirit and guide the Qi. In this study, MS5, MS6 and MS7 acupoints were selected for the treatment of AIS according to the International Standardization Scheme for Scalp Acupuncture Points (Min et al., 2007; World Health Organization, 1991; Zhongguo biao zhun chu ban she, 2008). The TCM theory of the functional areas of the cerebral cortex on the scalp believes that MS6 is projected to the precentral gyrus and is responsible for the motor function of the limbs. MS7 is projected to the postcentral gyrus and is responsible for the sensory function of the limbs (Zanardi, Maieron, & Tomasino, 2016). This study showed that scalp acupuncture can increase the activation of functional brain areas (motor and sensory), which is consistent with the theory of TCM. MS5, MS6 and MS7 pass through the Du meridian in TCM, focusing on regulating the mind, which is consistent with the expected results of this study, that is, scalp acupuncture has a more specific effect on the perception and cognition related brain areas.
Based on the above discussion, this study indicated that the ISSA treatment for AIS mainly guide Qi and regulate the mind. Based on the theory of functional reorganization, it is speculated that ISSA intervention can promote the activation of cerebral cortex related to the motor function. Based on the neural theory of motion generation, we speculated that ISSA can strengthen the integration of sensory and cognitive functions and can promote the formation of compensatory motor pathways between hemispheres. At the same time, scalp acupuncture can promote the reorganization of the brain function, which is supported by the theory of nerve regeneration and hemodynamics. Scalp acupuncture has been shown to improve the nervous system defects and promote motor function recovery in stroke patients by increasing blood flow and oxygen supply to the brain (Liu et al., 2018). In addition, scalp acupuncture has been shown to regulate the brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF), as well as proliferation and differentiation of neural stem cells in order to improve the function of nervous system defects after stroke in animal experiments (Tian et al., 2016). Rs‐fMRI is used to detect spontaneous brain functional activity in a resting state. Therefore, in the present study, we believed that scalp acupuncture can regulate the cerebral blood flow in the ischemic brain region and can promote proliferation and differentiation of neural stem cells. However, further Rs‐fMRI studies are needed to evaluate the functional connections in the brain.
This study is different from the molecular biology research, not from the perspective of structural repair, nor from the perspective of pathway activation or inhibition, but from the perspective of the brain function to directly observe the mechanism of scalp acupuncture for AIS treatment in vivo. In this study, VMHC, ALFF and ReHo were used to investigate the mechanism of scalp acupuncture treatment in AIS. This study may provide a scientific basis for the clinical application of ISSA in the treatment of AIS, and may also provide a preliminary research basis for further animal experiments. There are some limitations in this study. One of the limitations is that there were fewer subjects, and the other is the lack of comparison with healthy subjects. The lack of evaluation of sensory and cognitive impairment can lead to the failure of detailed clinical evidence for brain region changes. Further studies with larger sample sizes and more optimized research designs are needed to confirm our findings.
5. CONCLUSION
ISSA treatment can strengthen regional brain functional activities as well as functional connections between cerebral hemispheres related to sensory integration, language processing, and motor coordination in patients with middle cerebral artery AIS.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Huacong Liu: Data curation (lead); investigation (lead); project administration (lead); supervision (lead); validation (equal); visualization (equal); writing – original draft (lead). Yijing Jiang: Investigation (equal); project administration (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (lead). Ningning Wang: Investigation (equal); project administration (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal). Han Yan: Writing – review and editing (equal). Lanpin Chen: Formal analysis (equal); resources (equal). Jingchun Gao: Formal analysis (equal); resources (equal). Jiping Zhang: Resources (equal). Shanshan Qu: Resources (equal). Songyan Liu: Resources (equal). Gang Liu: Conceptualization (equal); investigation (equal); methodology (equal); project administration (lead); supervision (lead). Yong Huang: Conceptualization (equal); funding acquisition (supporting); investigation (equal); methodology (equal); project administration (lead); supervision (lead). Junqi Chen: Conceptualization (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); supervision (lead).
ETHICS STATEMENT
The study was approved by the Ethics Committee of the China‐Japan Union Hospital (Jilin University, China) (No.: 2016ks043) and registered in the Clinical Trial Center (No.: ChiCTR‐IOR‐15007672).
ACKNOWLEDGMENTS
All authors thank for the technical support from all colleagues in the imaging department and TCM Department of China‐Japan Union Hospital, Jilin University. The preprint is at the research square: https://www.researchsquare.com/article/rs-104143/v1. This study was funded by the National Natural Science Foundation of China, No. 81403455 (to Junqi Chen), the Program for College Students' innovation and entrepreneurship training of Southern Medical University in 2019, No.: S201912121172 (to Junqi Chen) and the Second Batch of Research and Study Programs for Outstanding Clinical Talents of Traditional Chinese Medicine in Guangdong province, No.: 2017‐267 (to Yong Huang).
Liu, H. , Jiang, Y. , Wang, N. , Yan, H. , Chen, L. , Gao, J. , Zhang, J. , Qu, S. , Liu, S. , Liu, G. , Huang, Y. , & Chen, J. (2021). Scalp acupuncture enhances local brain regions functional activities and functional connections between cerebral hemispheres in acute ischemic stroke patients. The Anatomical Record, 304(11), 2538–2551. 10.1002/ar.24746
Huacong Liu is the first author, and Yijing Jiang and Ningning Wang are the co‐first authors.
Funding information The Second Batch of Research and Study Programs for Outstanding Clinical Talents of Traditional Chinese Medicine in Guangdong province, Grant/Award Number: 2017‐267; National Natural Science Foundation of China, Grant/Award Number: 81403455; Program for College Students’ Innovation and Entrepreneurship Training of Southern Medical University in 2019, Grant/Award Number: S201912121172
Contributor Information
Huacong Liu, Email: smuhuacong@163.com.
Yijing Jiang, Email: 290658478@qq.com.
Ningning Wang, Email: wisemandgut@126.com.
Han Yan, Email: nydyanhan2018@163.com.
Lanpin Chen, Email: 1543203392@qq.com.
Jingchun Gao, Email: 702169148@qq.com.
Jiping Zhang, Email: zhangjp611@163.com.
Shanshan Qu, Email: 344914598@qq.com.
Songyan Liu, Email: liu_sy@jlu.edu.cn.
Gang Liu, Email: lg2781@smu.edu.cn.
Yong Huang, Email: nanfanglihuang@163.com.
Junqi Chen, Email: meixibao@126.com.
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed in the current study are not publicly available because the original data contains personal information of patients.
REFERENCES
- Bangert, M. , & Altenmüller, E. O. (2003). Mapping perception to action in piano practice: A longitudinal DC‐EEG study. BMC Neuroscience, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, X. , Li, L. , Liu, H. , Shi, H. F. , Xu, S. , Wang, M. H. , & Xie, J. Y. (2021). Effect of acupuncture combined with rehabilitation on cognitive and motor functions in poststroke patients. Anatomical Record, 1–7. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Desai, R. H. , Graves, W. W. , & Conant, L. L. (2009). Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud, D. , Tannegariepy, J. , Wannier, T. , & Rouiller, E. M. (2005). Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: A multiple retrograde tracing study. BMC Neuroscience, 6(1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunyé, T. T. , Moran, J. M. , Holmes, A. , Mahoney, C. R. , & Taylor, H. A. (2017). Non‐invasive brain stimulation targeting the right fusiform gyrus selectively increases working memory for faces. Brain and Cognition, 113, 32–39. [DOI] [PubMed] [Google Scholar]
- Caffarra, P. , Gardini, S. , Vezzadini, G. , Bromiley, A. , & Venner, A. (2010). The ideation of movement is supported by fronto‐temporal cortical regions involved in the retrieval of semantic knowledge. Acta Biomed, 81(1), 21–29. [PubMed] [Google Scholar]
- Carin, W. , Elizabeth, J. , & Tilo, K. (2010). Heterogeneity of the left temporal lobe in semantic representation and control: Priming multiple versus single meanings of ambiguous words. Cerebral Cortex, 2010(4), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Sun, D. , Shi, Y. , Jin, W. , Wang, Y. , Xi, Q. , & Ren, C. (2019). Dynamic alterations in spontaneous neural activity in multiple brain networks in subacute stroke patients: A resting‐state fMRI study. Frontiers in Neuroscience, 12, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Sun, D. , Shi, Y. , Jin, W. , Wang, Y. , Xi, Q. , & Ren, C. (2020). Altered static and dynamic voxel‐mirrored homotopic connectivity in subacute stroke patients: A resting‐state fMRI study. Brain Imaging and Behavior, 15(1), 389–400. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Li, C. , Zhai, J. , Wang, A. , Song, Q. , Liu, Y. , … Zhang, X. (2016). Altered resting‐state signals in patients with acute stroke in or under the thalamus. Neuroscience Bulletin, 32(6), 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarovano, E. , Vidal, P. , Magnani, C. , Lamas, G. , Curthoys, I. S. , & de Waele, C. (2016). Absence of rotation perception during warm water caloric irrigation in some seniors with postural instability. Frontiers in Neurology, 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Society of Neurology, Cerebrovascular Disease Group of Chinese Society Neurology . (2015). Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. Chinese Journal of Neurology, 48(4), 246–257. [Google Scholar]
- Dehaene, S. , Piazza, M. , Pinel, P. , & Cohen, L. (2003). Three parietal circuits for number processing. Cognitive Neuropsychology, 20(3–6), 487–506. [DOI] [PubMed] [Google Scholar]
- Desmond, J. E. , & Glover, G. H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. Journal of Neuroscience Methods, 118(2), 115–128. [DOI] [PubMed] [Google Scholar]
- Dum, R. P. , & Strick, P. L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. The Journal of Neuroscience, 11(3), 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, P. , Stepniewska, I. , & Kaas, J. H. (2008). Corpus callosum connections of subdivisions of motor and premotor cortex, and frontal eye field in a prosimian primate, Otolemur garnetti . The Journal of Comparative Neurology, 508(4), 565–578. [DOI] [PubMed] [Google Scholar]
- Hegyi, G. , & Szigeti, G. P. (2012). Rehabilitation of stroke patients using yamamoto new scalp acupuncture: A pilot study. Journal of Alternative and Complementary Medicine, 18(10), 971–977. [DOI] [PubMed] [Google Scholar]
- Hu, H. , Chung, C. , Liu, T. J. , Chen, R. C. , Chen, C. H. , Chou, P. , … Tsuei, J. J. (1993). A randomized controlled trial on the treatment for acute partial ischemic stroke with acupuncture. Neuroepidemiology, 12(2), 106–113. [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Yi, L. , Cai, S. , & Zhang, L. (2019). Ischemic stroke in Pontine and Corona Radiata: Location specific impairment of neural network investigated with resting state fMRI. Frontiers in Neurology, 10, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, B. B. , Haker, E. , von Arbin, M. , Britton, M. , Långström, G. , Terént, A. , … Swedish Collaboration on Sensory Stimulation After Stroke . (2001). Acupuncture and transcutaneous nerve stimulation in stroke rehabilitation: A randomized, controlled trial. Stroke, 32(3), 707–713. [DOI] [PubMed] [Google Scholar]
- Jorgensen, H. S. , Nakayama, H. , Raaschou, H. O. , Vive‐Larsen, J. , Støier, M. , & Olsen, T. S. (1995). Outcome and time course of recovery in stroke. Part II: Time course of recovery. The copenhagen stroke study. Archives of Physical Medicine and Rehabilitation, 76(5), 406–412. [DOI] [PubMed] [Google Scholar]
- Jungblut, M. , Huber, W. , Mais, C. , & Schnitker, R. (2014). Paving the way for speech: Voice‐training‐induced plasticity in chronic aphasia and apraxia of speech – Three single cases. Neural Plasticity, 2014, 841982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel, E. , Schwartz, J. H. , Jessell, T. , Siegelbaum, S. , & Hudspeth, A. J. (2013). Principles of neural science (5th ed.). New York, NY: McGraw‐Hill Professional. [Google Scholar]
- Kjendahl, A. , Salistrom, S. , Osten, P. E. , Stanghelle, J. K. , & Borchgrevink, C. F. (1997). A one year follow‐up study on the effects of acupuncture in the treatment of stroke patients in the subacute stage: A randomized, controlled study. Clinical Rehabilitation, 11(3), 192–200. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Guan, L. , Wang, Y. , Xie, C.‐L. , Lin, X.‐M. , & Zheng, G.‐Q. (2012). History and mechanism for treatment of intracerebral hemorrhage with scalp acupuncture. Evidence‐Based Complementary and Alternative Medicine, 2012, 895032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Zhang, X. , & Nie, K. (2018). Effect of electro‐scalp acupuncture on acute ischemic stroke: a randomized, single blind, trial. J Tradit Chin Med., 38(1), 95–100. [PubMed] [Google Scholar]
- Mcdermott, K. B. , Petersen, S. E. , Watson, J. M. , & Ojemann, J. G. (2003). A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia, 41(3), 293–303. [DOI] [PubMed] [Google Scholar]
- Mi, T. , Mei, S. , Liang, P. , Gao, L.‐L. , Li, K.‐C. , Wu, T. , & Chan, P. (2017). Altered resting‐state brain activity in Parkinson's disease patients with freezing of gait. Scientific Reports, 7(1), 16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, Y. J. , Yao, H. H. , Shao, S. J. , He, X. W. , Wang, H. S. , Yan, Z. G. , & Wei, J. (2007). Brief analysis on scientifity of the international scalp acupuncture. Zhongguo Zhen Jiu, 27(8), 612–616. [PubMed] [Google Scholar]
- NIH Consens Statement . 1997. Acupuncture, 15(5), 1–34. [PubMed]
- Payabvash, S. , Souza, L. C. S. , Kamalian, S. , Wang, Y. , Passanese, J. , Kamalian, S. , … Lev, M. H. (2012). Location‐weighted CTP analysis predicts early motor improvement in stroke: A preliminary study. Neurology, 78(23), 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok, W. , Maskiewicz, A. , Kroliczak, G. , & Marangon, M. (2019). The temporal involvement of the left supramarginal gyrus in planning functional grasps: A neuronavigated TMS study. Cortex, 111, 16–34. [DOI] [PubMed] [Google Scholar]
- Ramsey, N. F. , Tallent, K. A. , Van Gelderen, P. , Frank, J. A. , Moonen, C. T. , & Weinberger, D. R. (1996). Reproducibility of human 3D fMRI brain maps acquired during a motor task. Human Brain Mapping, 4(2), 113–121. [DOI] [PubMed] [Google Scholar]
- Sallstrom, S. , Kjendahl, A. , Osten, P. E. , Stanghelle, J. K. , & Borchgrevink, C. F. (1995). Acupuncture therapy in stroke during the subacute phase. A randomized controlled trial. Tidsskr Nor Laegeforen, 115(23), 2884. [PubMed] [Google Scholar]
- Tian, L. , Wang, J. H. , Sun, R. J. , Zhang, X. H. , Yuan, B. , & du, X. Z. (2016). Development of researches on scalp acupuncture for ischemic stroke. Zhen Ci Yan Jiu, 41(1), 87–89. [PubMed] [Google Scholar]
- Van Meer, M. P. , Van Marel, K. , Wang, K. , Otte, W. M. , el Bouazati, S. , Roeling, T. A. P. , … Dijkhuizen, R. M. (2010). Recovery of sensorimotor function after experimental stroke correlates with restoration of resting‐state interhemispheric functional connectivity. The Journal of Neuroscience, 30(11), 3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, J. , Stephan, T. , Kalla, R. , Brückmann, H. , Strupp, M. , Brandt, T. , & Jahn, K. (2008). Mind the bend: Cerebral activations associated with mental imagery of walking along a curved path. Experimental Brain Research, 191(2), 247–255. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Shen, J. , Wang, X. M. , Fu, D. L. , Chen, C. Y. , Lu, L. Y. , … Zheng, G. Q. (2012). Scalp acupuncture for acute ischemic stroke: A meta‐analysis of randomized controlled trials. Evidence‐Based Complementary and Alternative Medicine, 2012(2), 480950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Murray, V. , Berge, E. , & del Zoppo, G. J. (2014). Thrombolysis for acute ischaemic stroke. The Cochrane Database of Systematic Reviews, 2014(7), CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (1991). A proposed standard international acupuncture nomenclature. Report of a WHO Scientific Group.
- World Health Organization . (2002). Acupuncture: Review and analysis reports on.
- Xu, M. , Li, D. , & Zhang, S. (2018). Acupuncture for acute stroke. The Cochrane Database of Systematic Reviews, 2018(3), CD003317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, Y. N. , Song, M. Y. , Park, G. C. , Na, C. S. , Han, J. Y. , Cho, M. R. , & Kim, J. H. (2018). Meta‐analysis on randomized controlled trials for scalp acupuncture treatment of stroke: A systematic review. Journal of Traditional Chinese Medicine, 38, 465–479. [PubMed] [Google Scholar]
- Zanardi, R. , Maieron, M. , & Tomasino, B. (2016). Modulation of hand motor‐related area during motor imagery and motor execution before and after middle 2/5 of the MS6 line scalp acupuncture stimulation: An fMRI study. Brain and Cognition, 103, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhongguo biao zhun chu ban she . 2008. Standardized manipulations of acupuncture and moxibustion GB/T‐21709 ‐ Part 2: Scalp Acupuncture.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the current study are not publicly available because the original data contains personal information of patients.


