Abstract
Background
The Central Brain Tumor Registry of the United States (CBTRUS) uses a histology grouping model based on the World Health Organization (WHO) classifications to group records for clinically relevant statistical reporting. Newly identified genetic markers more accurately stratify patients than histology alone and were incorporated into the 2016 update to the WHO Classification.
Methods
CBTRUS and consulting neuropathologists reviewed and aligned histology groupings with the 2016 WHO update. “Obsolete” (terms not currently in use) histology nomenclature along with their International Classification of Disease, Oncology 3rd edition (ICD-O-3) codes were identified, some histologies were reclassified to 2016 WHO, and new codes found in 2016 WHO were incorporated. An evaluation of the frequency of histology codes affected in the realignment process, and incidence and survival pre- and post-realignment was conducted.
Results
After review, 67 codes were noted as obsolete, 51 codes were reclassified, and 12 new codes were incorporated. Histology groups most affected were mesenchymal tumors and neuronal/mixed neuronal-glial tumors. Reorganization resulted in 2588 (0.65%) cases with grouping reassignment or reporting change, indicating that the 2016 WHO Classification revision has impacted the collection and reporting of primary brain and other CNS tumors.
Conclusion
This work demonstrates the need to be responsive to changes in classification and coding in order to ensure the most up-to-date and accurate statistics for brain and CNS tumors. This will require collaboration from all stakeholders within the brain tumor community, so to have the ability to reconcile clinical practices and surveillance requirements.
Keywords: brain tumors, epidemiology, histology, CBTRUS
Prior to 2018, the cancer registration in the United States reported brain and other CNS tumors according to the 2007 WHO Classification of Tumours of the Central Nervous System. The 2016 revision to this classification resulted in significant changes, including the incorporation of some molecular markers into histology nomenclature. While the incorporation of molecular and histologic criteria under “integrated diagnosis” has become the predominant clinical practice, these changes had not been incorporated into the Central Brain Tumor of the United States (CBTRUS) statistical reporting until the publication of its 2021 Report.
Primary brain and spinal CNS tumors, (collectively known as primary brain and other CNS tumors in CBTRUS terminology) both malignant and nonmalignant, are relatively rare. In the US, they account for approximately 4% of all newly diagnosed primary tumors.1 Yet, these tumors represent a disproportionate source of morbidity and mortality. Primary nonmalignant brain and other CNS tumors include those tumors with ICD-O-3 behavior codes/0 (benign) and/1 (borderline) and represent more than half of all brain tumors diagnosed in the US. Due to their location and/or tumor size, these tumors, can cause significant morbidity. There are over 100 histologically distinct types of primary brain and other CNS tumors. Each of these has their spectrum of clinical presentation along with treatments and outcomes. These histologies are reviewed periodically and results are revised by the World Health Organization (WHO) and the classification of these tumors is revised using the International Classification of Diseases for Oncology, third edition (ICD-O-3) assigning histology, behavior, and site codes. While other types of cancer are staged according to the American Joint Commission of Cancer (AJCC) Collaborative Staging (CS) schema, primary brain, and other CNS tumors are not staged in this manner. These tumors are not staged but classified according to the WHO Classification of Tumours of the Central Nervous System2 since 1979, which assigns a grade based on predicted clinical behavior, that has been revised in 1993, 2000, 2007,3 and revised in 2016.4 With the WHO 2016 Classification update, information on molecular markers specific to certain entities has been added into diagnostic criteria for more accurate diagnosis. For example, IDH1/IDH2 mutation is essential diagnostic criteria for both IDH-mutant astrocytoma and IDH-mutant, 1p/19q codeleted oligodendroglioma. These modifications have not been implemented by US central cancer registries (CCR) into collection practices until January 1, 2018. While the lag time between reporting and collecting of data is challenging for clinicians and researchers, the population-based cancer registry data from which CBTRUS prepares its analyses have provided a rich resource for the brain tumor community. It is pertinent to note that unlike many countries governed centrally, the US encompasses many states, the District of Columbia, and some territories, each with their own rules and regulations, some of which govern cancer registration and present a challenge to the timely reporting of cancer statistics. It is also important to note that cancer registration is guided by scientific guidelines for completeness and accuracy to ensure that these data reflect accurate and complete incidence and survival and can be utilized effectively in planning activities related to cancer control and prevention and in research. Cancer registries employ safeguards to ensure the integrity of health data, including technical, physical, and administrative measures to preserve confidentiality of cancer patient information. As part of the WHO classification continuum, a fifth edition of the Classification of CNS Tumours was published in 2021 (reviewed in Ref5) providing additional changes in the diagnostic criteria of CNS tumors with addition of novel tumor types and molecular markers for reporting.
As a unique professional research organization focused exclusively on providing high-quality statistical data on the population-based incidence of primary brain and other CNS tumors in the US,6 CBTRUS has a stake in ensuring cancer registry data are clinically relevant. CBTRUS is the only population-based site-specific registry in the US that works in partnership with a public cancer surveillance organization, the Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries (NPCR). The CBTRUS database is comprised of the largest histology-specific aggregation of population-based data limited to the incidence and survival of primary brain and other CNS tumors in the US and is likely the largest histology-specific aggregation of primary brain and other CNS tumor cases in the world. The CBTRUS database now includes incidence data from all 52 CCR in the US and Puerto Rico from 2000 to 2018 and survival data from 42 CCR (2001–2017 only).1
To ensure that the data are as complete and accurate as possible, surveillance stakeholders in the US employ a system for rating each CCR and routinely conduct quality control checks. As a surveillance partner, CBTRUS reports high-quality data on brain and other CNS tumors with histological specificity useful to the communities it serves using clinically relevant histology groupings corresponding to the WHO classification scheme for tumors of the CNS.7 This practice enables CBTRUS to continue its efforts to provide the most up-to-date population-based incidence available through cancer registration for all primary brain and other CNS tumors by behavior (malignant, nonmalignant), histology, age, sex, race, and ethnicity. As classification and coding schemes are updated over time it becomes necessary for CBTRUS histology schemes to reflect these changes in order to not introduce any bias and misrepresentation in the reported statistics.
The revision of the 4th edition of WHO classification scheme was published in 2016, resulting in significant changes to multiple histologies with the addition of molecular markers. These markers provide the ability to distinguish tumor subtypes more accurately and allowed increased precision in diagnosis and prognostication.8 However, their collection in cancer registration practice was challenging as some histologies embedded with biomarkers had not been assigned unique ICD-O-3 codes. As a surveillance partner, CBTRUS successfully petitioned the North American Association of Central Cancer Registries (NAACCR), the surveillance consensus organization in the US to include biomarkers embedded in histologies found in the 2016 revision of WHO classification into the Uniform Data Standards (UDS), which guides US cancer reporting practices. Collection was initiated on January 1, 2018. After achieving UDS approval, CBTRUS and its consulting neuropathologists, reviewed and aligned its Histology Groupings with those found in the updated 2016 WHO Classification. The results of this process are described.
Methods
CBTRUS Data
This study was approved as part of an exempt protocol by the institutional review board of Duke University Health System. Data is directly received through NPCR’s Cancer Surveillance System (NPCR-CSS) through its submission specifications mechanism,9 under a special exclusive agreement with CBTRUS. CBTRUS combines the NPCR data with data obtained from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program.10 All SEER and NPCR data originate from cancer registries who adhere to the UDS for malignant and nonmalignant brain and other CNS tumors as directed by the NAACCR.
Reclassification Process
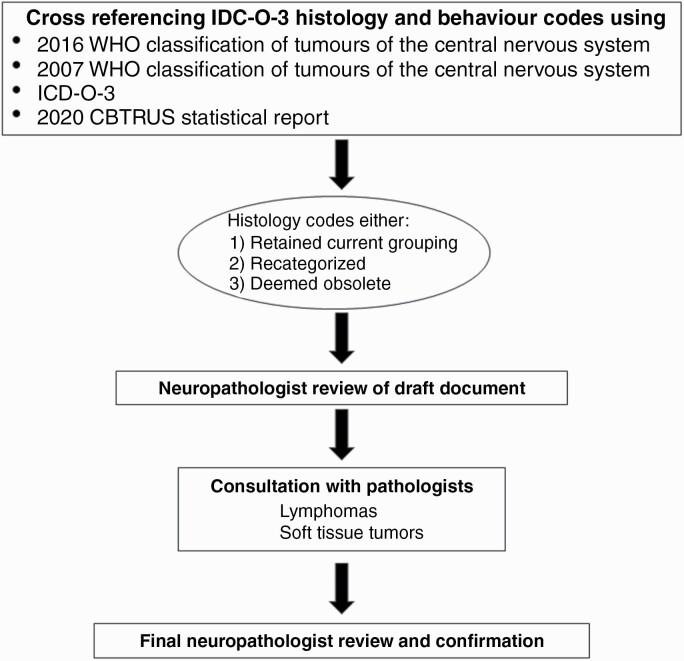
CBTRUS defines glioma as ICD-O-3 histology codes 9380-9384, and 9391-9460, as there is no standard definition for the generic term “glioma”. In addition, CBTRUS only reports lymphomas and hematopoietic neoplasms that arise in the brain and other CNS sites as defined by ICD-O-3 topography codes. The new CBTRUS grouping scheme was developed (Figure 1) by first creating a draft document through cross-referencing all ICD-O-3 histology and behavior codes using four sources: 1) 2016 WHO Classification of Tumours of the Central Nervous System,8 2) 2007 WHO Classification of Tumours of the Central Nervous System,11 3) the ICD-O-3, and 4) Table 2 from the 2020 CBTRUS Statistical Report, Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States, 2013–2017.12 This cross-referencing ensured that there was complete capture of all pertinent brain and other CNS tumor histologies, in Table 2, which served as the base document for realignment of the CBTRUS Histology Groupings with those found in 2016 WHO. Obsolete histology terms found in ICD-O-3 were retained. For this exercise, obsolete in cancer registry data either, as a result of historical recordings or due to time lags in implementing new histopathologies and IDC-O-3 coding, were retained. The working group consisting of the CBTRUS team, and four consulting neuropathologists, reviewed the document, incorporated the histologies listed under the 2016 WHO Histology Groupings, and re-examined each ICD-O-3 code individually. Corresponding changes were made by the CBTRUS team with the neuropathologists providing final confirmation. Additionally, the working group consulted with pathologists with expertise in lymphomas and soft tissue tumors for suggestions with specific 2016 WHO Histology Groupings for these categories. Obsolete histology nomenclatures were identified for future removal and codes were realigned in accordance with the 2016 WHO update. During the review and realignment process histology codes could either 1) retain their current grouping, 2) be recategorized into a more appropriate grouping, or 3) be deemed as obsolete. These obsolete codes are not completely removed from the CBTRUS data as they may still be used despite their obsolescence. Additionally, previously diagnosed tumors are not often reclassified to align with modern coding conventions, and complete removal would result in the loss of these cases. While the annual CBTRUS statistical report includes the most recent 5 years of data, the database itself can be used for projects that span much larger periods of time. The resulting document entitled 2021 CBTRUS Histology Grouping was prepared by the CBTRUS analytic team and approved by the neuropathologists. While additional modifications to the classification scheme for brain and other CNS tumors are found in 2021 WHO, published online in November 2021, (reviewed in Ref5), alignment to histology groupings in this document is outside the scope of this current work.
Fig. 1.
Workflow of alignment of CBTRUS histology with groupings.
Table 2.
Changes to the Central Brain Tumor Registry of the United States (CBTRUS), Brain and Other Central Nervous System Tumor Histology Groupings
| Histology | Coding Changes | ||
|---|---|---|---|
| New/added | Recategorized or Removed From Group | Obsolete | |
| Diffuse Astrocytic and Oligodendroglial Tumors | |||
| Diffuse astrocytoma | 9381 | None | 9410, 9420, 9442/1 |
| Anaplastic astrocytoma | None | None | None |
| Glioblastoma | 9445 | None | None |
| Oligodendroglioma | None | None | None |
| Anaplastic oligodendroglioma | None | None | 9460 |
| Oligoastrocytic tumors | None | None | 9382 |
| Other Astrocytic Tumors | |||
| Pilocytic astrocytoma | 9425 | None | None |
| Unique astrocytoma variants | 9431 | 9381 | None |
| Ependymal tumors | 9396 | 9391 (site C75.1 for /1) | None |
| Other Gliomas | |||
| Glioma malignant, NOS | 9385 | 9431, 9432 | None |
| Other neuroepithelial tumors | None | 9363 | 9423 |
| Neuronal and Mixed Neuronal- Glial Tumors | 9490, 9509 | None | 8680, 8681, 8690 |
| Choroid Plexus Tumors | None | None | None |
| Tumors of The Pineal Region | None | None | 9360 (recode cases to 9361) |
| Embryonal Tumors | 9475, 9476, 9477, 9478 | 9490 | 8963, 9364 (recode to 9473/3) 9471 (recode to 9470/3), 9472 (recode to 9470/3), 9480 (recode to 8800/3) |
| Tumors of Cranial and Paraspinal Nerves | |||
| Nerve sheath tumors | None | None | None |
| Other tumors of cranial and paraspinal nerves | 9563 | None | None |
| Tumors of Meninges | |||
| Meningioma | 9535 | None | 9539 |
| Mesenchymal tumors | 8710, 8711, 8810, 8821, 8825, 8840, 9120, 9125, 9130, 9131, 9133, 9161, 9220, 9231, 9240, 9243, 9370–9372 | None | 8710 (recode to 8800/3), 8711 (recode to 8693/1), 8830, 8831, 8850, 8853, 8854, 8857, 8902, 8935, 8990, 9040, 9130, 9131, 9150 |
| Primary melanocytic lesions | None | None | 8771 (remove from data, not in SEER site/type validation list) |
| Other neoplasms related to the meninges | None | 9161, 9220, 9231, 9240, 9243, 9370–9372, 9535 | None |
| Lymphomas and Hematopoietic Neoplasms | |||
| Lymphoma | 9727, 9750, 9751, 9755, 9823, 9826, 9827, 9832, 9837, 9861, 9866, 9930, 9970 | None | 9653–9655, 9667, 9670, 9671, 9675, 9690 (recode all to 9590/3) |
| Other hematopoietic neoplasms | None | 9727, 9750, 9751, 9755, 9823, 9826, 9827, 9832, 9837, 9861, 9866, 9930, 9970 | 9740, 9750, 9753, 9754, 9860 |
| Germ Cell Tumors | 9084/3 | 8020 | 8440 |
| Tumors of Sellar Region | |||
| Tumors of the pituitary | 9391/1 (Site C75.1 only), 9432, 9580, | None | 8040, 8140, 8146, and 8260 (for sites other than C75.1 remove from data), 8040, 8140, 8146 and 8260 (recode to 8272/0 if site C75.1) |
| Craniopharyngioma | None | None | None |
| Unclassified Tumors | |||
| Hemangioma | None | 9120, h9125, 9130, 9131 | None |
| Neoplasm, unspecified | 8020 | None | None |
| All other | 8963, 9084/0, 9363 | 8710, 8711, 8811, 8840, 9580 | 8320 |
Case Counts & Incidence Statistics
CBTRUS data was used to generate case counts and average annual age-adjusted incidences rates (AAIR) by histology groupings and classification scheme for cases diagnosed between 2014 and 2018. AAIR’s were generated per 100 000 population and age-adjusted to the 2000 US Standard Population. Associated 95% confidence intervals (CI) were generated per the methodology described in Tiwari et al.13 Case counts and incidence statistics were calculated using SEER*Stat 8.3.9.14 For incidence data, counts and rates are not presented when fewer than 16 cases were reported for the specific category.
Survival Statistics
Median survival and Kaplan–Meier estimates for primary malignant brain and other CNS tumors were generated from the NPCR survival database from 2001 to 2017. Median survival and corresponding 95% CI were estimated in months for histology groupings and classification schemes. Additionally, Kaplan–Meier survival curves were generated by classification schemes for select histologies. All cases were restricted to first sequence tumors, and histological or radiographic confirmation. NAACCR data item #1787, survival months presumed alive, was used to ascertain follow-up information. For survival data, median survival is not presented when fewer than 50 events (deaths) were reported for the specific category. Survival analyses and figures were generated in R 4.0.5 using the following packages: flextable, officer, orca, plotly, sf, survminer, tigris, and tidyverse.
Results and Discussion
As part of the CBTRUS realignment process based on the 2007 WHO classification scheme, unique ICD-O-3 histology codes were placed into 29 histology groupings (Suppl Table 1). During the revision process 15 of these had realignment changes and 1 grouping (other tumors of the meninges) was removed, resulting in 28 histology groupings (Tables 1 and 2). Broader histology groups were re-organized to align with the WHO 2016 classification. The largest of these changes was reclassification of glioma histologies from “tumors of the neuroepithelial tissue” to “diffuse astrocytic and oligodendroglial tumors”, “other astrocytic tumors”, “ependymal tumors”, and “other gliomas”. Histology codes that were added in the 2016 WHO Classification of Tumours of the Central Nervous System and incorporated into US cancer registration in diagnosis year 2018 were added to their appropriate groupings. After the CBTRUS realignment process, 55 ICD-O-3 histology codes were determined to be obsolete (Suppl Table 2), 37 ICD-O-3 histology codes were reclassified (Suppl Table 3), and 12 new ICD-O-3 histology codes were added (Suppl Table 4).
Table 1.
Central Brain Tumor Registry of the United States (CBTRUS) Brain and Other Central Nervous System Tumor Histology Groupings, 2021 Implementation
| Histology Grouping | ICD-O-3a Histology Codesb | ICD-O-3 Histology and Behavior Codec | |
|---|---|---|---|
| Malignant | Nonmalignant | ||
| Diffuse Astrocytic and Oligodendroglial Tumors | |||
| Diffuse astrocytomad | 9381, 9400, 9410, 9411, 9420, 9442 | 9381/3, 9400/3, 9410/3, 9411/3, 9420/3 | 9442/1 |
| Anaplastic astrocytomad | 9401 | 9401/3 | None |
| Glioblastomad | 9440, 9441, 9442/3, 9445f | 9440/3, 9441/3, 9442/3, 9445/3 | None |
| Oligodendrogliomad | 9450 | 9450/3 | None |
| Anaplastic oligodendrogliomad | 9451, 9460 | 9451/3, 9460/3 | None |
| Oligoastrocytic tumorsd | 9382 | 9382/3 | None |
| Other Astrocytic Tumors | |||
| Pilocytic astrocytomad | 9421, 9425c | 9421/1, 9425/3 | None |
| Unique astrocytoma variantsd | 9384, 9424, 9431c | 9424/3 | 9384/1, 9431/1 |
| Ependymal tumors d | 9383, 9391 (excluding site C75.1 for behavior /1), 9392- 9394, 9396c | 9391/3, 9392/3, 9393/3, 9396/3 | 9383/1, 9391/1 (excluding site C75.1), 9394/1 |
| Other Gliomas | |||
| Glioma malignant, NOSd | 9380, 9385c | 9380/3, 9385/3 | None |
| Other neuroepithelial tumorsd | 9423, 9430, 9444 | 9423/3, 9430/3 | 9444/1 |
| Neuronal and Mixed Neuronal-glial Tumors d | 8680, 8681, 8690, 8693, 9412, 9413, 9442/1, 9490, 9492 (excluding site C75.1), 9493, 9505, 9506, 9509c, 9522 (site C30.0 only), 9523 (site C30.0 only) | 8680/3, 8693/3, 9490/3, 9505/3, 9509/3, 9522/3 (site C30.0 only), 9523/3 (site C30.0 only) | 8680/0,1, 8681/1, 8690/1, 8693/1, 9412/1, 9413/0, 9442/1, 9490/0, 9492/0 (excluding site C75.1), 9493/0, 9505/0,1, 9506/1, 9509/1 |
| Choroid Plexus Tumors | 9390 | 9390/3 | 9390/0,1 |
| Tumors of the Pineal Region | 9360, 9361, 9362, 9395c | 9362/3, 9395/3 | 9360/1, 9361/1 |
| Embryonal Tumors | 8963, 9364, 9470-9478c, 9480, 9500, 9501/3, 9502/3, 9508 | 8963/3, 9364/3, 9470/3, 9471/3, 9472/3, 9473/3, 9474/3, 9475/3, 9476/3, 9477/3, 9478/3, 9480/3, 9500/3, 9501/3, 9502/3, 9508/3 | None |
| Tumors of Cranial and Paraspinal Nerves | |||
| Nerve sheath tumors | 9540, 9541, 9550, 9560, 9561, 9570, 9571 | 9540/3, 9560/3, 9561/3, 9571/3 | 9540/0,1, 9541/0, 9550/0, 9560/0,1, 9570/0, 9571/0 |
| Other tumors of cranial and paraspinal nerves | 9562, 9563 | None | 9562/0, 9563/0 |
| Tumors of Meninges | |||
| Meningioma | 9530–9535, 9537- 9539 | 9530/3, 9538/3, 9539/3 | 9530/0,1, 9531/0, 9532/0, 9533/0, 9534/0, 9535/0, 9537/0, 9538/1, 9539/1 |
| Mesenchymal tumors | 8324, 8710, 8711, 8800–8806, 8810, 8811, 8815, 8821, 8824, 8825, 8830, 8831, 8835, 8836, 8840, 8850–8854, 8857, 8861, 8870, 8880, 8890, 8897, 8900–8902, 8910, 8912, 8920, 8921, 8935, 8990, 9040, 9120, 9125, 9130, 9131, 9133, 9136, 9150, 9161, 9170, 9180, 9210, 9220, 9231, 9240, 9241, 9243, 9260, 9370–9373 | 8710/3, 8711/3, 8800/3, 8801/3, 8802/3, 8803/3, 8804/3, 8805/3, 8806/3, 8810/3, 8811/3, 8815/3c, 8825/3, 8830/3, 8840/3, 8850/3, 8851/3, 8852/3, 8853/3, 8854/3, 8857/3, 8890/3, 8900/3, 8901/3, 8902/3, 8910/3, 8912/3, 8920/3, 8921/3, 8935/3, 8990/3, 9040/3, 9120/3, 9130/3, 9150/3, 9170/3, 9180/3, 9220/3, 9231/3, 9240/3, 9243/3, 9260/3, 9370/3, 9371/3, 9372/3 | 8324/0, 8711/0, 8800/0, 8810/0, 8811/0, 8815/0,1c, 8821/1, 8824/0,1, 8825/0,1, 8830/0,1, 8831/0, 8835/1, 8836/1, 8840/0, 8850/0,1, 8851/0, 8852/0, 8854/0, 8857/0, 8861/0, 8870/0, 8880/0, 8890/0,1, 8897/1, 8900/0, 8920/1, 8935/0,1, 8990/0,1, 9040/0, 9120/0, 9125/0, 9130/0,1, 9131/0, 9136/1, 9150/0,1, 9161/0,1, 9170/0, 9180/0, 9210/0, 9220/0, 9241/0, 9373/0 |
| Primary melanocytic lesions | 8720, 8728, 8770 | 8720/3, 8728/3, 8770/3 | 8728/0,1, 8770/0 |
| Other neoplasms related to the meninges | None | None | None |
| Lymphomas and Hematopoietic Neoplasms | |||
| Lymphoma | 9590, 9591, 9596, 9650–9655, 9659, 9661–9665, 9667, 9670, 9671, 9673, 9675, 9680, 9684, 9687, 9688, 9690, 9691, 9695, 9698, 9699, 9701, 9702, 9705, 9712, 9714, 9715, 9719, 9724, 9727–9729, 9735, 9737, 9738, 9750, 9751, 9755, 9756, 9811–9819, 9823, 9826, 9827, 9831, 9832, 9837, 9861, 9866, 9930, 9965, 9966, 9967, 9970, 9971, 9975 | 9590/3, 9591/3, 9596/3, 9650/3, 9651/3, 9652/3, 9653/3, 9654/3, 9655/3, 9659/3, 9661/3, 9662/3, 9663/3, 9664/3, 9665/3, 9667/3, 9670/3, 9671/3, 9673/3, 9675/3, 9680/3, 9684/3, 9687/3, 9688/3, 9690/3, 9691/3, 9695/3, 9698/3, 9699/3, 9701/3, 9702/3, 9705/3, 9712/3, 9714/3, 9715/3, 9719/3, 9724/3, 9727/3, 9728/3, 9729/3, 9735/3, 9737/3, 9738/3, 9750/3, 9751/3, 9755/3, 9756/3, 9811/3, 9812/3, 9813/3, 9814/3, 9815/3, 9816/3, 9817/3, 9818/3, 9819/3, 9823/3, 9826/3, 9827/3, 9831/3, 9837/3, 9861/3, 9866/3, 9930/3, 9965/3, 9966/3, 9967/3, 9971/3, 9975/3 | 9750/1, 9751/1, 9766/1, 9970/1 |
| Other hematopoietic neoplasms | 9731, 9733, 9734, 9740, 9741, 9749, 9752–9754, 9757–9758, 9759, 9760, 9766, 9860, | 9731/3, 9733/3, 9734/3, 9740/3, 9741/3, 9749/3, 9753/3, 9754/3, 9756/3, 9757/3, 9758/3, 9759/3, 9760/3, 9766/3, 9823/3, 9826/3, 9827/3, 9832/3, 9860/3, | 9740/1, 9752/1, 9753/1, 9766/1 |
| Germ Cell Tumors | 8440, 9060, 9061, 9064, 9065, 9070–9072, 9080–9083, 9084/3 9085, 9100, 9101 | 8440/3, 9060/3, 9061/3, 9064/3, 9065/3, 9070/3, 9071/3, 9072/3, 9080/3, 9081/3, 9082/3, 9083/3, 9084/3, 9085/3, 9100/3, 9101/3 | 8440/0, 9080/0,1 |
| Tumors of Sellar Region | |||
| Tumors of the pituitary | 8040 (site C75.1 only), 8140 (site C75.1 only), 8146 (site C75.1 only), 8246, 8260 (site C75.1 only), 8270–8272, 8280, 8281, 8290, 8300, 8310, 8323, 9391/1 (site C75.1 only), 9432c (site C75.1 only), 9492 (site C75.1 only), 9580, 9582 | 8140/3, 8246/3, 8260/3, 8270/3, 8272/3, 8280/3, 8281/3, 8290/3, 8300/3, 8310/3, 8323/3, 9580/3 | 8040/0,1, 8140/0,1, 8146/0, 8260/0, 8270/0, 8271/0, 8272/0, 8280/0, 8281/0, 8290/0, 8300/0, 8310/0, 8323/0, 9391/1 (site C75.1 only), 9432/1, 9492/0 (site C75.1 only), 9580/0, 9582/0 |
| Craniopharyngioma | 9350–9352 | None | 9350/1, 9351/1, 9352/1 |
| Unclassified Tumors | |||
| Hemangioma | 9121–9123, 9133, 9140 | 9133/3, 9140/3 | 9121/0, 9122/0, 9123/0, 9133/1 |
| Neoplasm, unspecified | 8000–8005, 8010, 8020, 8021 | 8000/3, 8001/3, 8002/3, 8003/3, 8004/3, 8005/3, 8010/3, 8020/3, 8021/3 | 8000/0,1, 8001/0,1, 8005/0, 8010/0 |
| All other | 8320, 8452, 8713, 8896, 8963, 8980, 9084/0, 9173, 9363, 9503 | 8320/3, 8452/3, 8896/3, 8980/3, 9503/3 | 8452/1, 8713/0, 9084/0, 9173/0, 9363/0 |
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NOS, not otherwise specified.
aInternational Classification of Diseases for Oncology, 3rd Edition, 2000. World Health Organization, Geneva, Switzerland.
bSee the CBTRUS website for additional information about the specific histology codes included in each group: http://www.cbtrus.org.
cAdded starting with diagnosis year 2018.
dAll or some of this histology is included in the CBTRUS definition of gliomas, including ICD-O-3 histology codes 9380–9384, 9391–9460.
In order to evaluate the potential effect of 2016 WHO classification changes on statistical reporting, counts and incidence rates were generated for the 2014–2018 CBTRUS data under both CBTRUS Grouping schemes. There were 431 590 total cases of primary brain and other CNS tumors diagnosed in the US between 2014 and 2018 that were included in this analysis. Overall, the realignment process affected a very small number of cases in the CBTRUS data. A grouping reassignment change occurred for 1.3% (5624) of cases, while 0.4% (1741) of cases were assigned to codes now considered to be obsolete. Importantly, the new realignment process allows the addition of new histology codes that incorporate molecular biomarker data that will allow CBTRUS to provide information on these clinically relevant molecular histologies as part of the routine statistical reporting process. There were 498 cases with newly added codes, representing 0.12% of the total data. The largest group of these cases was aligned within the broader histology group diffuse astrocytic and oligodendroglial tumors.
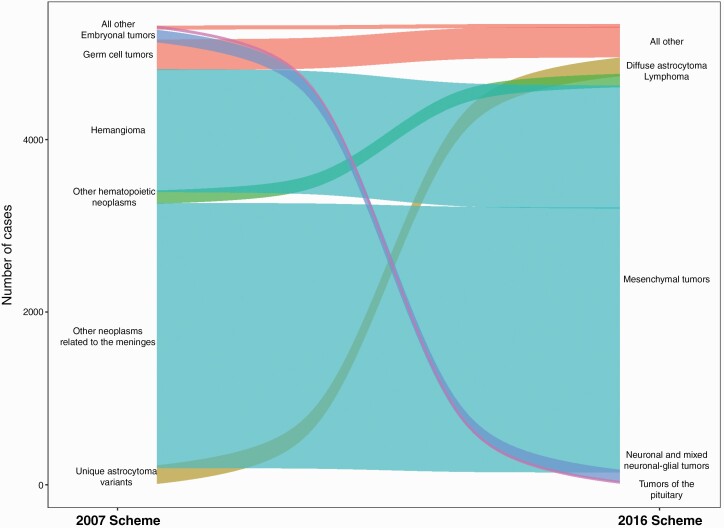
For the 15 groups that had realignment changes, the greatest effects were seen in rarer histology groups, such as other neuroepithelial tumors (3 cases), mesenchymal tumors (4,489 cases), other hematopoietic neoplasms, and “all other” tumors (343 cases), which had the largest change in absolute numbers of cases (Suppl Fig 1). Among these, mesenchymal tumors had the most cases impacted by realignment, of which many had been previously categorized as other neoplasms related to the meninges or hemangioma (Figure 2, discussed below). While these changes appear to be dramatic, when one looks at the absolute number of cases impacted, we find that the net number of cases impacted by these changes are generally small. Considering the large size of the CBTRUS data, it is unlikely that most of these changes will have a substantial effect on case reporting.
Fig. 2.
Changes in histology codes between categories, CBTRUS Statistical Report: US Cancer Statistics-NPCR and SEER, 2014–2018.
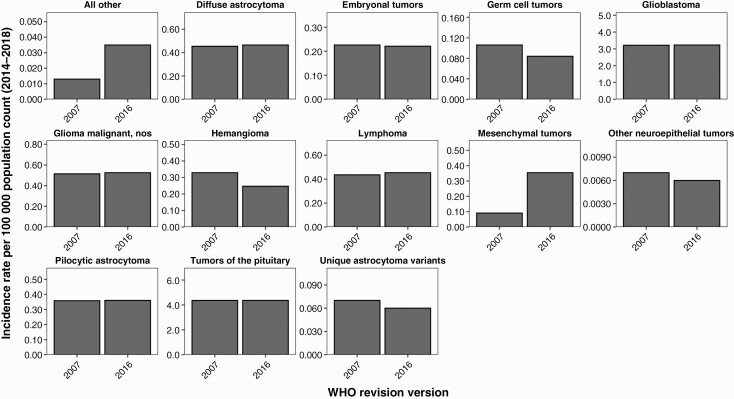
To assess the potential effect of these realignment changes on CBTRUS reporting of age-adjusted incidence rates, we generated incidence rates for the 2014–2018 data under both histology grouping schemes. For several histologies, AAR was also affected by realignment changes. For mesenchymal tumors, the AAIR changed from 0.091 per 100 000 (95% CI: 0.086–0.096) in the 2007 classification to 0.354 (95% CI: 0.345–0.363) in the 2016 classification (Figure 3; Suppl Table 5), or 3.8-fold increase. Tumors classified as “all other” saw an incidence change from 0.013 per 100 000 (95% CI: 0.011–0.014) in the 2007 classification scheme to 0.035 per 100 000 (95% CI: 0.032–0.038) in to 2016 scheme (Figure 3; Suppl Table 5), or a 2.7-fold increase. In most other CBTRUS histology groupings, the changes in incidence and survival between realignment schemes are marginal (Figure 3; Suppl Table 5). As CBTRUS has previously shown, realignment changes in both WHO diagnostic criteria and histologic grouping can have significant effects on incidence rates.15–17 Incidence of the histology codes affected by these grouping changes as well as the overall groupings is very low. This large proportion change led to the appearance of dramatic changes in incidence based on realignment, but these changes are very minimal in the scheme of the complete CBTRUS data.
Fig. 3.
Incidence differences between schemes by histology, CBTRUS Statistical Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
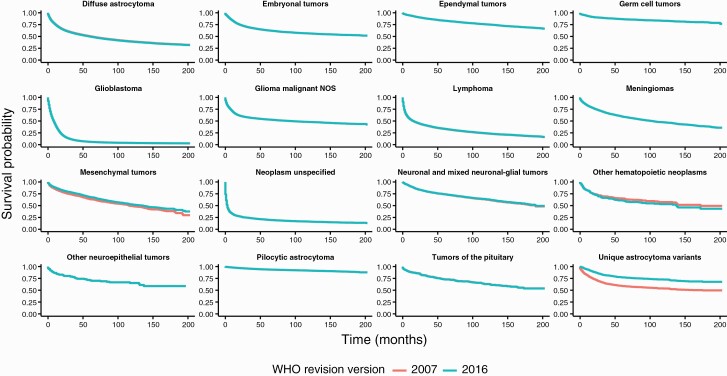
In order to assess the potential effect of the 2016 WHO classification changes on CBTRUS reporting of overall survival, we generated median survival times for the 2014–2018 data under both CBTRUS Histology Grouping schemes. Median survival for each grouping was assessed based on the 2007 classification scheme and the 2016 classification scheme using data from the years 2001 to 2017 in the NPCR data for malignant histology codes only (ICD-O-3 behavior code/3). Overall, there were no substantial changes in median survival under the two schemes. Median survival for mesenchymal tumors had a modest change between grouping schemes (2007 Classification: 116 months (95% CI: 106–136); 2016 Classification: 136 months (95% CI: 118–148)) (Suppl Table 6). Median survival for other hematopoietic neoplasms decreased from 172 months to 138 months (upper bound of the confidence interval not able to be calculated for either estimate). In most other histology groupings, the changes in survival between CBTRUS Histology Grouping schemes are marginal (Figure 4; Suppl Table 6) due to the available follow-up period of 16 years, and the extended survival period observed in nonmalignant tumors. CBTRUS has previously shown that changes in WHO classification can have significant effects on reported survival, particularly when the reclassified histology codes have different clinical behavior than other histology codes included in the grouping.18
Fig. 4.
Survival differences between schemes by histology, CBTRUS Statistical Report: US Cancer Statistics—NPCR, 2001–2017.
Limitations
There were several limitations to our ability to evaluate differences in incidence and survival patterns under the CBTRUS Histology Grouping scheme based on the 2007 as opposed to the 2016 WHO Classification of Tumours of the Central Nervous System. The first of these is that the data chosen for the period chosen for our analyses began in 2014, which predates the release of the 2016 scheme. Many of the classification changes and molecular markers implemented in this scheme were already in use in diagnostic procedure, so it is likely that diagnostic pattern changes can be observed prior to 2016. The second of these is that new histologies introduced in the 2016 WHO classification were not assigned codes until diagnosis year 2018, and as a result newly created entities diagnosed in years 2016–2017 may have been assigned incorrect codes. One inherent limitation of cancer registry data is that there is no central pathology review of assigned diagnosis found in the patient’s record. Histology reflects what is assigned by the diagnosing pathologist based on the prevailing criteria used in that diagnostic facility at the time. This may lead to some centers adopting criteria prior to release of official changes, such as academic centers which may be more likely to adopt new procedures based on recent scientific developments for assays performed in house, while community hospitals may experience delays in adoption of classification changes. All of these factors may limit our ability to accurately measure the true population-level effect of classification changes.
Conclusions
Prior to 2021, the WHO Classification of Tumours of the CNS was updated in 20073 and 2016,4 but these updated schemes were not fully implemented by US CCRs until collection year 2018 (included for the first time in the 2021 data release). Changes in clinical practices significantly impact the collection and reporting of CNS and brain tumors. Therefore, it is critical that cancer registration stays responsive to these changes to ensure that the statistics produced from the data reflect real-world practice and ensure that the changes do not introduce any bias and misrepresentation in the reported statistics. The realigning of the CBTRUS histology grouping system with the 2016 WHO Classification of Tumours of the CNS demonstrates that changes in clinical practices can impact the collection and reporting of CNS tumors. While small overall changes to histology classification can have a large proportion impact on incidence rates of rare histologies or survival patterns within heterogeneous histology groups, we did not identify substantial changes as a result of this realignment process. These findings highlight the need for collaboration and cooperation between clinical and cancer registration stakeholders so that CCRs can be responsive to changes in classification and coding and thereby provide statistics that reflect their real-world use. Reconciling clinical practice and surveillance requirements requires the collaborative efforts described in this report from all stakeholders in neuro-oncology for success. We expect the publication of the 2021 WHO Classification will incur additional updating of the CBTRUS 2021 Histology Groupings and that an additional realignment of histologies and ICD-O-3 codes will be needed in the future. The separation of IDH-mutant and IDH-wild type gliomas will likely be incorporated in further realignments. Further, diffuse midline glioma must be in its own category, per 2016 and 2021 WHO, and further recodes may result in this being listed separately. The addition of additional molecular markers will require further analysis and potential recoding and analysis as these changes become implemented. These changes will take time as CCRs move from implementing 2016 WHO to implementing 2021 WHO. CBTRUS plans to continue working with the UDS Committee to include 2021 WHO Classification changes into cancer collection practices, while being mindful that any implementation will be bound by the UDS timeline and plans to provide analysis documenting their impact on population-based statistics of primary brain and other CNS tumors in the US in future reports once the WHO 2021 scheme begins to be implemented and applied.
Supplementary Material
Acknowledgments
The CBTRUS data presented in this report were provided through an agreement with the Centers for Disease Control’s National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No:00002, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Contributor Information
Kristin A Waite, Division of Cancer Epidemiology and Genetics, Trans-Divisional Research Program, National Cancer Institute, Bethesda, Maryland, USA.
Gino Cioffi, Division of Cancer Epidemiology and Genetics, Trans-Divisional Research Program, National Cancer Institute, Bethesda, Maryland, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA.
Nirav Patil, University Hospitals, Cleveland, Ohio.
Daniel J Brat, Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Janet M Bruner, Department of Pathology, MD Anderson Cancer Center, Houston, Texas, USA.
Roger E McLendon, Department of Pathology, Duke University Medical Center Durham , North Carolina, USA; Department of Neurosurgery, Duke University, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, North Carolina, USA.
Tarik Tihan, Department of Pathology, Division of Neuropathology, University of California San Francisco, San Francisco, California, USA.
Quinn T Ostrom, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, North Carolina, USA; Department of Neurosurgery, Duke University, Durham, North Carolina, USA.
Jill S Barnholtz-Sloan, Division of Cancer Epidemiology and Genetics, Trans-Divisional Research Program, National Cancer Institute, Bethesda, Maryland, USA; Center for Biomedical Informatics & Information Technology, National Cancer Institute, Bethesda, Maryland, USA.
Funding
CBTRUS receives funding through the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No:00002; the American Brain Tumor Association; Novocure; the Musella Foundation; The Sontag Foundation; National Brain Tumor Society; the Uncle Kory Foundation; the Pediatric Brain Tumor Foundation; and the Zelda Dorin Tetenbaum Memorial Fund as well as private and in kind donations. The research services of Jill S. Barnholtz-Sloan, Ph.D., Kristin Waite, Ph.D., and Gino Gioffi, M.P.H. were provided by the Division of Cancer Epidemiology and Genetics of the National Cancer Institute (NCI).
Conflict of interest statement. The authors do not have any conflicts of interest to declare. JBS is a full-time employee of the NIH/NCI. KW and GC are full-time contractors of the NIH/NCI.
References
- 1. Ostrom Q, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous (CNS) tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(12 Suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kleihues P, Cavenee W, eds. Tumours of the Nervous System: World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 3. Louis D, Wiestler O, Cavanee W, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 5. Louis DN, Perry A, Wesseling P, et al. . The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kruchko C, Ostrom QT, Gittleman H, Barnholtz-Sloan JS. The CBTRUS story: providing accurate population-based statistics on brain and other central nervous system tumors for everyone. Neuro Oncol. 2018; 20(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). National Program of Cancer Registries Cancer Surveillance System Rational and Approach. http://www.cdc.gov/cancer/npcr/pdf/npcr_css.pdf. 1999 [cited 2020 July 21, 2020]. Accessed July 3, 2021.
- 8. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC). National Program of Cancer Registries Cancer Surveillance System Rationale and Approach 1999 [July 21, 2020]. http://www.cdc.gov/cancer/npcr/pdf/npcr_css.pdf. Accessed July 3, 2021.
- 10. National Cancer Institute. Overview of the SEER Program [July 21, 2020]. http://seer.cancer.gov/about/overview.html. Accessed July 3, 2021.
- 11. Louis DN, Ohgaki H, Wiestler OD, et al. . P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):54797–54547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrom QT, Patil N, Cioffi G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020; 22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006; 15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 14. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.3.9: National Cancer Institute, DCCPS, Surveillance Research Program; 2020 [April 7, 2021]. www.seer.cancer.gov/seerstat. Accessed July 3, 2021.
- 15. McCarthy BJ, Propp JM, Davis FG, Burger PC. Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology. 2008; 30(1):34–44. [DOI] [PubMed] [Google Scholar]
- 16. Khanna V, Achey RL, Ostrom QT, et al. . Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neuro Oncol. 2017; 135(3):433–441. [DOI] [PubMed] [Google Scholar]
- 17. Achey RL, Khanna V, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence and survival trends in oligodendrogliomas and anaplastic oligodendrogliomas in the United States from 2000 to 2013: a CBTRUS Report. J Neuro Oncol. 2017; 133(1):17–25. [DOI] [PubMed] [Google Scholar]
- 18. Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020; 22(2):298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.