Abstract
Background and Objectives
Social connectedness has been linked prospectively to cognitive aging, but there is little agreement about the social mechanisms driving this relationship. This study evaluated 9 measures of social connectedness, focusing on 2 forms of social enrichment—access to an expansive and diverse set of loosely connected individuals (i.e., social bridging) and integration in a supportive network of close ties (i.e., social bonding).
Research Design and Methods
This study used egocentric network and cognitive data from 311 older adults in the Social Networks in Alzheimer Disease study. Linear regressions were used to estimate the association between social connectedness and global cognitive function, episodic memory, and executive function.
Results
Measures indicative of social bridging (larger network size, lower density, presence of weak ties, and proportion of non-kin) were consistently associated with better cognitive outcomes, while measures of social bonding (close ties, multiplex support, higher frequency of contact, better relationship quality, and being married) largely produced null effects.
Discussion and Implications
These findings suggest that the protective benefits of social connectedness for cognitive function and memory may operate primarily through a cognitive reserve mechanism that is driven by irregular contact with a larger and more diverse group of peripheral others.
Keywords: Alzheimer’s disease, Cognitive reserve, Social support
Background and Objectives
Given the limited success of drug treatments to prevent or delay cognitive aging, researchers have turned to an examination of social, cognitive, and lifestyle factors to identify candidates for clinical intervention (Ashida & Schafer, 2018; Kempermann et al., 2002). A substantial body of evidence suggests that the extent to which individuals are socially active and connected to others is prospectively linked to the incidence of dementia and the progression of cognitive decline (Fratiglioni et al., 2004; Gow et al., 2013; Kelly et al., 2017; Kuiper et al., 2016; Penninkilampi et al., 2018). However, there have been numerous calls for additional research to identify which aspects of social connectedness are associated with better cognitive function among older adults (Crooks et al., 2008; Gow et al., 2013; Kelly et al., 2017; Kuiper et al., 2016).
This study uses a social network approach to measure social mechanisms of resilience to cognitive aging. Social networks are “structures of relationships linking social actors” (Marsden, 2000, p. 2727). The network perspective is methodologically distinctive in that data are collected on the specific individuals to whom actors are connected, enabling measurement of multiple features of relationships and social processes. After briefly reviewing the current state of the evidence on social connectedness and cognitive aging, we use clinical cognitive and social network data from the Social Networks in Alzheimer Disease (SNAD) study to identify associations between older adults’ personal social network characteristics and multiple indicators of cognitive function and memory. We find that measures of social bridging are consistently associated with better cognitive outcomes, while measures of social bonding produce null effects. In the absence of approved disease-modifying therapies for Alzheimer’s disease (AD), our findings have important implications for preserving or enhancing cognitive health in later life.
Social Connectedness and the Aging Brain
Cognitive function and the incidence and progression of cognitive decline have been associated with social connectedness among older adults (Fratiglioni et al., 2004; Gow et al., 2013; Kelly et al., 2017; Kuiper et al., 2016). Social connectedness has been operationalized using a wide variety of measures that are often treated as similar or unidimensional, but likely reflect distinct social processes. These include number of relationships (Barnes et al., 2004; Bennett et al., 2006; Crooks et al., 2008; Evans et al., 2018), frequency of social contact (Zunzunegui et al., 2003), perceived social support (Seeman et al., 2001), participation in social activities (Barnes et al., 2004; James et al., 2011; Karp et al., 2006; Wang et al., 2002), and subjective loneliness (Donovan et al., 2017; Wilson et al., 2007). This heterogeneity has made it difficult to generate consensus about mechanisms underlying this relationship, which remain unclear and contested (Baumgart et al., 2015; Giles et al., 2012; Gow et al., 2013). In this article, we advance this literature by applying a social network perspective that compares multiple dimensions of connectedness simultaneously.
A Social Network Perspective on Cognitive Aging
All network theories of health and illness share a focus on variation in social ties and interactions, rather than individual characteristics. People are embedded in a personal community—partially of their own creation and nearly unique to them—whose functions, composition, and structure have health consequences. A hallmark of personal social network (i.e., egocentric network) methodology is the collection of more and better data about the structure, function, and composition of individuals’ social ties across a range of different social spheres from the perspective of the focal person or “ego” (e.g., family, friends, coworkers, and neighbors). This is distinct from a whole social network, or sociocentric, approach, which maps connections between all members of one socially or geographically bounded group of actors, focusing on the structure of the group as a whole. In a true personal network approach, alters are individually distinguishable from each other, and characteristics of each alter are collected to provide information about the nature of interactions occurring within the network (McCarty et al., 2019; Perry et al., 2018).
Researchers studying cognitive aging have rarely used a true social network approach (except see Cornwell et al., 2009; Li & Dong, 2018). Instead, participants are typically asked to respond to proxy questions that summarize complex social phenomena using a single measure or scale. A handful of studies have moved the literature forward using proxy network indicators to identify distinct aspects of social connectedness that are protective (Gow et al., 2013; Holtzman et al., 2004; Zunzunegui et al., 2003). For example, in perhaps the most comprehensive comparison of different dimensions of social connectedness in older adults to date, “social networks” were operationalized using (a) a dichotomous indicator of having any friends, (b) number of visual contacts with family members, and (c) number of telephone contacts with family members (Zunzunegui et al., 2003). These were then compared to measures of social integration (i.e., extent to which they help children, family members, and friends) and group participation. Another study aiming to compare the effects of social networks, loneliness, and social support in the Lothian Birth Cohort 1936 operationalized social networks using a count of different kinds of social contacts (Gow et al., 2013).
Though these studies were a vast improvement over those using single measures like living alone or marital status, these proxies for social networks have limitations. Simple summative measures may not capture the richness of older adults’ lives, which are multiplex and heterogeneous (Fiori et al., 2007; Litwin & Shiovitz-Ezra, 2006). First, research suggests that proxy measures map poorly onto true social network measures, reflecting general perceptions instead (Burt, 1987). This is because it is cognitively difficult to accurately aggregate across numerous social relationships. Second, use of proxy measures limits the ability to test alternative hypotheses about social mechanisms (Fratiglioni et al., 2004; Kelly et al., 2017). In contrast, the personal network approach is flexible, offering a large number and variety of measures of social connectedness with which to evaluate hypotheses about underlying social mechanisms. Third, and most importantly, proxies cannot capture structural properties of networks or interactions with less close members of the network (i.e., weaker or more peripheral ties), which are essential for capturing exposure to novel, as opposed to habitual, social stimuli.
Recent studies employing a personal social network approach illustrate the promise of this methodology (Ellwardt et al., 2015; Li & Dong, 2018; Sharifian et al., 2020; Sommerlad et al., 2019). For example, a 28-year follow-up study on dementia risk by Sommerlad et al. (2019) adapted a social network index (Berkman & Syme, 1979) to distinguish between the type, number, and frequency of older adults’ interactions with friends and relatives. They found that more frequent social contact was associated with lower dementia risk and better cognitive trajectories, but these findings were driven primarily by interactions with friends and not family members (see also Giles et al., 2012; Katz et al., 2020; Li & Dong, 2018; Sharifian et al., 2020). Along the same lines, a recent study by Ellwardt et al. (2015) found that greater network complexity, measured as irregular contact with a diversity of social roles (Cohen et al., 1997), is associated with better cognitive functioning even after taking network size into account. By explicitly measuring who older adults are interacting with, how often, and what they are doing together, a social network approach can provide novel insight into the specific social mechanisms that help preserve cognitive function.
Mechanisms and Hypotheses
Research on social connectedness and cognitive aging has tended to advocate an implicit or explicit cognitive reserve theory (Stern, 2002, 2009). That is, social integration within enriching social environments provides stimulating experiences that preserve cognitive capabilities despite brain atrophy (Ellwardt et al., 2015; Evans et al., 2018; Hultsch et al., 1999). Prospective studies indicate that up to one third of older adults with no significant cognitive impairment meet full neuropathologic criteria for AD (i.e., significant atrophy or presence of amyloid or tau), suggesting that extensive underlying brain pathology may be overcome by compensatory cognitive/functional processes.
Social networks provide access to different kinds of enrichment, suggesting at least two distinct hypotheses about the types of relationships and interactions that may promote cognitive function in older adults (Li & Dong, 2018; Seeman et al., 2001). First, the social bridging hypothesis suggests that cognitive function may be optimized through access to novel and diverse social stimuli, including a range of ideas, information, activities, verbal and nonverbal social cues, faces, and speech patterns (Coleman, 1988; Cornwell, 2009b). Such enrichment is likely to occur primarily during irregular contact with more peripheral or dissimilar others (i.e., weak ties) which produces eustress (i.e., good stress) due to social learning. Interactions with heterogeneous and weaker ties (e.g., casual friends, neighbors, and coworkers) that are not closely connected to one another are likely to be more cognitively enriching than familiar, repetitive, and comfortable exchanges with immediate family members and other close confidantes. This hypothesis suggests:
H1: Personal social networks that are larger are less densely connected, provide access to weak ties, and contain more non-kin will be associated with better cognitive function among older adults.
Second, the social bonding hypothesis suggests that the enriching functions of social engagement derive from close and supportive connections to others (Berkman & Glass, 2000). Social bonding takes the form of integration, cooperation, meaningful social roles, and exchanges of emotional support and affirmation that result from being embedded in relationships and small groups (Thoits, 2011). Individuals rich in social bonding benefit from a strong informal social safety net that is typically cultivated in the context of intimate relationships with similar others. Social bonding promotes a sense of purpose in life, mattering, and belonging—perceptions that foster behavioral guidance and security, as well as self-esteem and a sense of control or mastery that may reduce the harmful effects of stress on brain health and cognitive function (Cohen, 2004; Thoits, 2011). This hypothesis suggests:
H2: Personal networks that contain closer and higher quality relationships are more supportive and are in regular communication or contact will be associated with better cognitive function among older adults.
Finally, though there is substantial prospective evidence linking social connectedness to cognitive decline, including meta-analyses (Evans et al., 2019; James et al., 2011; Kuiper et al., 2016; Zunzunegui et al., 2003), researchers have also identified the reverse relationship. Specifically, functional limitations associated with cognitive impairment may make it difficult to remain socially active or maintain social relationships (Aartsen et al., 2002, 2004; Biddle et al., 2019; Casey et al., 2020; Cornwell, 2009a). Likewise, older adults with cognitive impairments may become depressed and socially withdraw to avoid the stigma of dementia (Donovan et al., 2016, 2017). A recent longitudinal study employing cross-lagged modeling suggested that the relationship between social connectedness (defined as social network size) and cognitive function is best conceptualized as coconstitutive, with effects in both directions (Casey et al., 2020). In light of these findings, we take multiple steps to reduce the likelihood of reverse causation.
Research Design and Methods
Sample
Data used in this analysis were from the SNAD study, which recruited participants from the Indiana Alzheimer Disease Research Center (IADRC; https://medicine.iu.edu/research/centers-institutes/alzheimers/). The IADRC has collected longitudinal data on a wide variety of clinical measures from a cohort of older adults, in accordance with the National Alzheimer’s Coordinating Center.
The IADRC cohort consisted of roughly 400 older adults made up of cognitively normal participants (CN; n ≈ 200), participants with mild cognitive impairment (MCI; n ≈ 80), participants with AD (n ≈ 120), and participants with other related dementias. Exclusion criteria for the IADRC included history of schizophrenia, bipolar disorder, other major psychiatric disorders, history of cancer with chemotherapy or radiation treatment, traumatic brain injury with loss of consciousness, developmental disabilities, and history of or active alcohol/substance abuse disorders.
Since March 2015, all eligible IADRC participants were approached to voluntarily complete the SNAD protocol (90% response rate). The exclusion criteria for SNAD included (a) IADRC participants who scored less than 10 on the Montreal Cognitive Assessment (MoCA), (b) participants who had a known family history of dominantly inherited dementia genes, and (c) people aged 45 years and less. Data for SNAD were collected face-to-face using computer-assisted personal interviewing.
This analysis drew from 311 participants from SNAD. Of these participants, 60% were women and 83% were White; mean age was 71 years; mean education was 16 years. This sample included 202 cognitively normal participants, 65 with MCI, and 44 with AD. The IADRC Clinical Core determined participant diagnoses using consensus conferences consisting of an interdisciplinary team representing clinical psychiatry, clinical neurology, radiology and imaging sciences, and medical and molecular genetics.
Measures
Dependent variables
General cognitive function was measured using the MoCA (Nasreddine et al., 2005), a brief screening tool for MCI. The MoCA includes items assessing memory, visuospatial ability, executive function, attention, language, and orientation to time and place. Higher scores indicated higher cognitive function. Because participants with very low MoCA scores were excluded from the study, this variable is slightly negatively skewed. To address this skewness, we normalized MoCA and the other cognitive outcomes. Transforming the variable to correct skewness provided consistent results.
The Craft Story 21 is a paragraph story recall test that assesses episodic memory (Craft et al., 1996). The examiner read a story aloud and then asked the participant to repeat the details of the story in the same words read by the examiner or in his/her own words. Points for verbatim (exact content words) and paraphrase recall (similar contextual story units) were summed separately and yielded substantively identical results. After approximately 20 min, the participant was asked to recall the story again. For this study, we used the delayed paraphrase scores for their proven validity in measuring global cognition and memory (Dodge et al., 2020). Higher scores indicated better episodic memory. This outcome is normalized.
The Trail Making Test is a timed neuropsychological test consisting of two parts, A and B. The test taker must draw a line connecting 25 sequentially numbered circles (Part A) or alternating numbers and letters (Part B). Both parts assess motor speed and visual attention, and the more difficult Part B also tests executive function (Gaudino et al., 1995). We used Part B because it is more sensitive to impairment (Ashendorf et al., 2008). The test is scored using the time to completion, so lower scores signify better executive function. To maintain consistent direction with the other cognitive outcomes, we reverse-coded and normalized the Trails B score for regression models such that higher scores indicate better executive function.
Independent variables
Egocentric social network data were collected using an expanded PhenX Social Network Battery tailored to the case of dementia (Hamilton et al., 2011). For this protocol, interviewers elicited names of individuals in a participant’s social network that was activated in the past 6 months for discussions about important matters and health matters using items called name generators (Perry et al., 2018). After network members’ names were provided, questions were asked about each person in the network. This process yielded structural, functional, and compositional variables about a person’s core discussion network.
Measures to assess social bridging included network size, density, presence of weak ties, and proportion of non-kin. Network size was measured by the number of unique people mentioned in response to name generators. Network density was the mean of the closeness of ties between each network member in a participant’s network, which ranged from 0 to 3. Response categories were “don’t know each other,” “not very close,” “sort of close,” and “very close.” Higher scores signified higher strength of ties between network members. The presence of weak ties in the network was assessed using the minimum value of the strength of ties between a participant and each of his/her network members, which had a potential range of 1–10 on a sliding scale (i.e., no response categories). Proportion of non-kin was measured by calculating the proportion of network members who were not identified as family members by birth or marriage. These included friends, neighbors, coworkers, clergy, and other types of non-kin.
Measures operationalizing social bonding included number of very close ties, support multiplexity, mean frequency of contact, quality of social relationships, and marital status. Number of close ties was a count of the number of network members that participants reported being “very close” to, as opposed to “sort of close” or “not very close.” Support multiplexity was measured by averaging the number of different support functions provided (out of five) by all network members, ranging from 0 to 5. Support functions assessed included listening, caring, providing advice, help with chores and other tasks, and giving or loaning money. Mean frequency of contact was computed by averaging the frequency of contact between a participant and all network members, which ranged from 1 to 3 (corresponding to “hardly ever,” “occasionally,” and “often”). Quality of social relationships was measured by taking the average of all nonmissing values for three items in the Quality of Life in Alzheimer’s Disease scale (Logsdon et al., 1999). Items included in the subscale included relationships with family members, relationships with a marital or romantic partner (or if none, the person they are closest to), and relationships with friends. The resulting index ranged from 1 to 4 (corresponding to “poor,” “fair,” “good,” or “excellent”) with higher values indicating better quality. Marital status was computed as a binary indicator, with 1 equal to married and 0 equal to never married, divorced, separated, or widowed.
Controls
Depressive symptoms were measured using the 15-item Geriatric Depression Scale (Sheikh & Yesavage, 1986). Self-rated health was assessed using a single item with four response categories ranging from “poor” to “excellent.” Impairment in instrumental activities of daily living was measured using the Functional Activities Questionnaire and binarized to indicate no impairment versus any impairment (Pfeffer et al., 1982). We controlled for these indicators of health status to reduce the potential confounding effect of functional decline and social withdrawal (i.e., reverse causality). Furthermore, we controlled for gender (woman or man), race (person of color or White), age in years, and education in years, which may be correlated both with social network characteristics and cognitive performance.
Analysis
We first conducted bivariate statistics by diagnostic group and created ego network visualizations using ideal types (i.e., mean bridging measures by group). Because it is not possible to depict partial nodes (i.e., people), we rounded to whole numbers where necessary (e.g., in depicting network size). Next, all nine network variables were separately entered into linear regression models to predict cognitive function, episodic memory, and executive function, controlling for gender, race, age, education, depressive symptoms, self-rated health, and functional limitations. This strategy avoids multicollinearity, as these measures are moderately to highly correlated within the bridging or bonding domain. This process was repeated for all three cognitive outcomes. All network variables and outcomes were standardized to facilitate interpretation. Full regression results are presented in Supplementary Table 1.
Cases with missing data on outcomes were excluded from each model. Missing data on independent variables and controls were handled by using full information maximum likelihood in structural equation models (Enders & Bandalos, 2001). Based on the variable with the most missing data (i.e., relationship satisfaction), the missingness pattern by diagnostic group is 31/202 = 15% for CN, 8/65 = 12% for MCI, and 7/44 = 16% for AD. Missingness did not differ significantly across diagnostic group. All analyses were computed using Stata 16. It is important to declare that this was an exploratory study. As such, no adjustments for multiple testing were made, and additional dedicated studies are needed to confirm the results (Althouse, 2016; Feise, 2002).
In addition to the main findings, we also conducted sensitivity analyses to assess whether our results were robust to adjustments for reverse causation. First, we controlled for depressive symptoms, self-rated health, and functional limitations to reduce the confounding influence of social withdrawal or declining social activity secondary to cognitive impairment. Second, we conducted longitudinal regression analyses using two waves of data collected 1 year apart on a smaller subsample of participants. These predicted global cognitive function, episodic memory, and executive function at the 12-month follow-up using baseline social network characteristics and controlling for baseline cognitive outcomes. Collection of these data is in progress, but this provides a very conservative preliminary indication of a prospective association given the short duration of follow-up.
Results
Bivariate Associations by Clinical Diagnosis
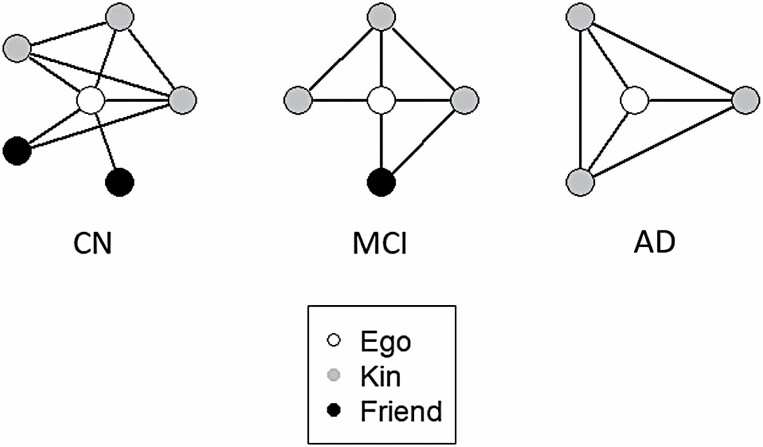
Table 1 indicates statistically significant differences across diagnostic groups. Overall, cognitively normal participants reported core networks that were larger, more loosely connected, and more diverse than participants with MCI and early-stage AD. On average, cognitively normal participants reported having 5.39 (SD = 2.46) network members while participants with MCI and AD reported having 4.69 (SD = 2.51) and 3.48 (SD = 1.87) network members (F = 25.62; df = 2; p = .000), respectively. The proportion of non-kin was higher (0.38 vs. 0.31 vs. 0.20; F = 10.47; df = 2; p = .001), while network density (1.64 vs. 1.92 vs. 2.16; F = 17.31; df = 2; p = .000) and minimum tie strength (6.52 vs. 7.18 vs. 8.23; F = 20.83; df = 2; p = .000) were lower among cognitively normal participants compared to those with MCI and AD, respectively. Cognitively normal participants reported better quality of social relationships (3.42 vs. 3.26 vs. 3.14; F = 9.75; df = 2; p = .002) and more close ties (3.77 vs. 3.55 vs. 2.95; F = 5.76; df = 2; p = .017) than those with MCI and early-stage AD, but there were no significant differences in other indicators of social bonding across diagnostic group. Figure 1 offers a visual typology of personal networks across the three groups using group means to create ideal types.
Table 1.
Sample Descriptive Statistics by Diagnostic Group (N = 311)
| Independent variables | CN (N = 202) | MCI (N = 65) | AD (N = 44) | |
|---|---|---|---|---|
| Mean (SD) or proportion | Mean (SD) or proportion | Mean (SD) or proportion | F or χ 2 | |
| Demographics | ||||
| Male | 0.30 | 0.52 | 0.50 | 13.29** |
| Age | 69.73 (8.60) | 73.98 (8.53) | 71.89 (10.13) | 5.21* |
| White | 0.77 | 0.88 | 0.84 | 4.00 |
| Education | 16.44 (2.61) | 16.37 (2.55) | 15.07 (3.43) | 6.69* |
| Health status | ||||
| Depressive symptoms | 1.47 (1.78) | 2.83 (2.35) | 3.23 (3.20) | 35.29*** |
| Self-rated health | 3.12 (0.74) | 2.79 (0.70) | 2.62 (0.83) | 16.17*** |
| Functional limitations | 0.22 | 0.77 | 0.95 | 114.96*** |
| Cognitive function | ||||
| Global cognitive function | 25.80 (3.03) | 22.33 (2.94) | 16.95 (5.03) | 265.34*** |
| Episodic memory | 15.19 (3.57) | 8.40 (4.74) | 4.25 (5.47) | 313.54*** |
| Executive function | 80.89 (47.31) | 113.31 (61.50) | 195.57 (96.28) | 102.63*** |
| Social bridging | ||||
| Network size | 5.39 (2.46) | 4.69 (2.51) | 3.48 (1.87) | 25.62*** |
| Network density | 1.64 (0.72) | 1.92 (0.75) | 2.16 (0.65) | 17.31*** |
| Minimum strength of tie | 6.52 (2.42) | 7.18 (2.20) | 8.23 (1.66) | 20.83*** |
| Proportion of non-kin | 0.38 (0.28) | 0.31 (0.28) | 0.20 (0.25) | 10.47** |
| Social bonding | ||||
| Number of close ties | 3.77 (2.00) | 3.55 (2.20) | 2.95 (1.86) | 5.76* |
| Support multiplexity | 3.04 (0.73) | 2.98 (0.69) | 3.33 (0.86) | 1.50 |
| Mean frequency of contact | 2.64 (0.30) | 2.72 (0.27) | 2.66 (0.49) | 0.38 |
| Quality of social relationships | 3.42 (0.49) | 3.26 (0.53) | 3.14 (0.68) | 9.75** |
| Married | 0.71 | 0.78 | 0.72 | 0.89 |
Note: CN = cognitively normal; MCI = mild cognitive impairment; AD = Alzheimer’s disease.
*p < .05, **p < .01, ***p < .001.
Figure 1.
Typical core network structure and composition by clinical diagnosis. Note: CN = cognitively normal; MCI = mild cognitive impairment; AD = Alzheimer’s disease. For CN, MCI, and AD, network size and proportion of non-kin decrease, whereas the proportion of alters close to other alters increases, respectively, with additional pathology. This figure is a visual representation of network ideal types by a diagnosis that shows trends in the data and is informed by mean and modal scores.
Multivariate Regression Models
Table 2 presents results from linear regressions predicting three standardized cognitive function outcomes (i.e., global cognitive function, episodic memory, and executive function), controlling for gender, age, race, education, depressive symptoms, self-rated health, and functional limitations. With respect to variables operationalizing social bridging, we found support for all indicators in the expected direction. Specifically, larger network size and lower network density and minimum tie strength were associated with better global cognitive function, episodic memory, and executive function (p < .05). The proportion of non-kin in the network was positively associated with episodic memory (p = .002), but not significantly related to global cognitive function nor executive function.
Table 2.
Regression of Cognitive Function on Network Characteristics
| Independent variables | Global cognitive function (N = 310) | Episodic memory (N = 311) | Executive function (N = 292) |
|---|---|---|---|
| β (SE) | β (SE) | β (SE) | |
| Social bridging | |||
| Network size | 0.20** (0.07) | 0.30*** (0.07) | 0.15* (0.07) |
| Network density | −0.21** (0.07) | −0.21* (0.08) | −0.19* (0.08) |
| Minimum tie strength | −0.28*** (0.06) | −0.31*** (0.07) | −0.17* (0.08) |
| Proportion of non-kin | 0.10 (0.08) | 0.17* (0.08) | 0.06 (0.08) |
| Social bonding | |||
| Number of close ties | 0.07 (0.06) | 0.18* (0.07) | 0.06 (0.07) |
| Support multiplexity | −0.12 (0.09) | −0.23** (0.07) | −0.15 (0.10) |
| Mean frequency contact | −0.03 (0.10) | −0.16 (0.09) | −0.01 (0.09) |
| Quality of social relationships | 0.09 (0.07) | 0.03 (0.09) | 0.28** (0.10) |
| Married | 0.08 (0.18) | 0.09 (0.20) | 0.07 (0.18) |
Notes: The table presents fully standardized coefficients with standard errors in parentheses. All predictors were modeled separately, controlling for gender, age, race, education, and depressive symptoms, self-rated health, and functional limitations.
*p < .05, **p < .01, ***p < .001.
Indicators of social bonding were inconsistently associated with cognitive function. Number of very close ties was weakly and positively related to better episodic memory (p = .014), but not to global cognitive function nor executive function. Higher support multiplexity was associated with worse episodic memory (p = .002), contrary to our hypothesis, but not to global cognitive function nor executive function. This suggests that participants who received multiple kinds of emotional and instrumental support from their network members, on average, had worse episodic memory. Reporting higher quality social relationships was associated with better executive function (p = .004). No significant association was identified for mean frequency of contact or marital status for any cognitive outcome.
Sensitivity Analyses
We conducted multivariate regression analyses predicting cognitive outcomes at 12-month follow-up (n = 168), adjusting for baseline cognition and other control variables (Supplementary Table 2). These analyses confirmed significant findings for change in global cognitive function. Specifically, for measures of social bridging, we found support for all indicators in the expected direction. Specifically, larger network size and proportion of non-kin, and lower network density and minimum tie strength, were significantly associated with better global cognitive function (p < .05). No measures of social bonding yielded significant associations with global cognitive function. Analyses of change in episodic memory and executive function produced null results. This may indicate that the main findings reflect reverse causation. However, we observed very little change in episodic memory or executive function over the 1-year period, so there was minimal variation in T2 outcomes to explain once T1 cognitive function was controlled. The coefficients for T1 cognitive function in these models approach 1.00 (B = 0.87 and B = 0.91 for episodic memory and executive function, respectively). Together, robustness to conservative controls for health status and functional limitations, combined with promising preliminary findings on the relationship between social network bridging and change in global cognitive function, are suggestive of the hypothesized directionality for social bridging.
Discussion and Implications
The goal of this study was to evaluate a range of measures of social connectedness to determine which may be the most promising candidates for preserving cognitive function in older adults. We focused on two distinct forms of social enrichment, social bridging and social bonding. In linear regression models adjusting for gender, age, race, education, and depressive symptoms, measures indicative of social bridging were consistently associated with global cognitive function, episodic memory, and executive function in the hypothesized direction. In contrast, measures of social bonding were largely unrelated to cognitive function in this sample of older adults. These results were robust to controls for demographics and several indicators of health status that might drive any reverse causation, including depressive symptoms, self-rated health, and functional limitations. We also observed preliminary evidence for an association between social bridging and lower levels of decline in global cognitive function. These findings suggest that the protective benefits of social connectedness for cognitive function and memory may operate primarily through a cognitive reserve mechanism that is driven by irregular contact with a larger and more diverse group of peripheral others.
Bridging interactions likely provide exposure to social stimuli that require higher-order cognitive processes, such as novel ideas, information, and activities. However, social bridging may also present opportunities for more basic forms of sensory information processing and social learning, including unfamiliar faces and speech patterns, verbal and nonverbal social cues, and interactions requiring theory of mind (i.e., the ability to predict and interpret the behavior and mental state of others). More broadly, social bridging has been linked to a variety of positive outcomes, including well-being (Bath & Deeg, 2005; Harris & Thoresen, 2005), life expectancy (Rostila, 2010), and good health and independence in late adulthood (Cornwell, 2009b, 2011).
The biological mechanism underlying the association between social bridging and cognitive function and memory may be an accumulation of cognitive reserve. Broadly, the cognitive reserve hypothesis suggests that individual variations in enrichment experiences across the life course provide different degrees of reserve against age-related neuropathology (Ellwardt et al., 2015; Evans et al., 2018; Katzman, 1993; Stern, 2006, 2009). Prospective studies indicate that up to 25% of older adults with unimpaired neuropsychological functioning meet full pathological criteria for AD (Esiri et al., 2001), suggesting that extensive underlying neuropathology may be masked by compensatory functional processes. This explanation is consistent with research suggesting that older adults with a larger number of friends and a more diverse set of associates are disproportionately resilient to the cognitive effects of neurodegeneration (Kelly et al., 2017; Pillai & Verghese, 2009).
While we did not find much direct support for the social bonding hypothesis, there are at least two potential explanations that leave open the possibility that close and supportive networks are beneficial for cognitive aging. First, social bonding may be relatively distal in etiological pathways, operating through more proximate and subjective psychosocial resources such as feelings of mattering, self-esteem, mastery, and security (Bandura, 2001; Cohen, 2004; Thoits, 2011). Given that these psychosocial resources are influenced by a wide range of experiences and structural conditions, the “signal” from social network measures of social bonding may be weak absent information about key mediators. Second, social bonding may play a stress-buffering role in cognitive aging processes (Uchino, 2006). Social support and integration have been associated with less negative appraisals of stressors, more active and effective coping, and downregulation of the hypothalamic–pituitary–adrenal response (Heinrichs et al., 2003; Unger et al., 1997; Wilson et al., 2007). If true, social bonding would only be positively related to cognitive function in the context of stress exposure. Because we were unable to test these alternative explanations with available data, our study stops short of implying that social bonding is unrelated to cognitive aging.
Our findings have important implications for preserving or enhancing cognitive health in late life. There are currently no approved disease-modifying therapies for AD and related dementias, and existing interventions have had limited effectiveness in delaying the onset of cognitive decline or slowing its progression (Cummings et al., 2018). This underscores the urgent need for a paradigm shift toward potential upstream mechanisms and approaches to dementia prevention that operate over the life course. Maintaining cognitive function through social stimulation is a promising and modifiable target that has numerous other physical and psychological benefits (Smith & Christakis, 2008).
This study has multiple limitations. Foremost among these is the cross-sectional nature of the main findings, which make it impossible to eliminate reverse causation as a competing explanation for our findings. We took several steps to address this issue, including controlling for the confounding effects of health status and conducting preliminary longitudinal analyses using a subset of participants with two waves of data. Additionally, there is ample prospective evidence for the proposed interpretation of findings (Evans et al., 2018; Kuiper et al., 2016), and some research has explicitly ruled out reverse causation in the case of social engagement or connectedness and cognition (Ertel et al., 2008; James et al., 2011). Nonetheless, it is inappropriate to make inferences about causal direction.
In addition, our data are not representative. Rather, they are based on a convenience sample of participants in an NIH-funded ADRC. As such, participants are more likely to be White, live in a metropolitan area, and have higher socioeconomic status relative to the general population. It is possible that these characteristics of our sample yielded an underestimate of the association between social network characteristics and cognition, given the relatively lower risk for dementia in this subpopulation.
The main strength of this study is the personal social network approach to data collection and measurement, which makes it possible to disaggregate dimensions of social connectedness and empirically link specific social processes to outcomes. In doing so, we have responded to calls for additional research on the mechanisms underlying the relationship between social connectedness and cognitive aging (Bielak, 2010; Kelly et al., 2017; Kuiper et al., 2016). Our analysis of nine measures of social connectedness and three cognitive outcomes provides unique insight into two distinct social mechanisms—social bridging and bonding. We generated new evidence that personal social networks that are larger, less densely connected, and that contain weaker ties to non-kin are associated with better cognitive function among older adults, making them a promising target for individual- and population-level health policies and programs.
Supplementary Material
Acknowledgments
We would like to thank faculty and staff at the Indiana Alzheimer Disease Research Center, the Indiana University Department of Sociology, the Indiana University Sociomedical Sciences Institute, the Indiana Consortium for Mental Health Services Research, and the Indiana University Network Science Institute for their contributions to project conceptualization and data collection. Thanks, especially, to Evan Finley, Bernice Pescosolido, Erin Pullen, Kate Eddens, Alex Capshew, Tugce Duran, Mary Austrom, Sujuan Gao, Shannon Risacher, Andrew Saykin, and Frederick Unverzagt.
Contributor Information
Brea L Perry, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
William R McConnell, Department of Sociology, Florida Atlantic University, Boca Raton, Florida, USA.
Siyun Peng, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
Adam R Roth, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
Max Coleman, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
Mohit Manchella, Department of Biology, University of Southern Indiana, Evansville, USA.
Meghann Roessler, Department of Biology, University of Dayton, Dayton, Ohio, USA.
Heather Francis, Kinsey Institute, Indiana University, Bloomington, Indiana, USA.
Hope Sheean, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
Liana A Apostolova, Radiology and Imaging Sciences, Indiana University, Indianapolis, Indiana, USA.
Funding
This work was supported by the National Institutes of Health through the National Institute on Aging (grant numbers 5R01AG057739, 5R01AG0709315, R01AG070931, and P30AG010133) and by an Indiana University Collaborative Research Grant through the Vice President for Research. This project also received support from the Indiana Clinical and Translational Sciences Institute funded in part by (grant number UL1TR002529) the National Institutes of Health, National Center for Advancing Translational Sciences, and Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
References
- Aartsen, M. J., Smits, C. H., van Tilburg, T., Knipscheer, K. C., & Deeg, D. J. (2002). Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57(2), 153–162. doi: 10.1093/geronb/57.2.p153 [DOI] [PubMed] [Google Scholar]
- Aartsen, M. J., Van Tilburg, T., Smits, C. H., & Knipscheer, K. C. (2004). A longitudinal study of the impact of physical and cognitive decline on the personal network in old age. Journal of Social and Personal Relationships, 21(2), 249–266. doi: 10.1177/0265407504041386 [DOI] [Google Scholar]
- Althouse, A. D. (2016). Adjust for multiple comparisons? It’s not that simple. The Annals of Thoracic Surgery, 101(5), 1644–1645. doi: 10.1016/j.athoracsur.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Ashendorf, L., Jefferson, A. L., O’Connor, M. K., Chaisson, C., Green, R. C., & Stern, R. A. (2008). Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Archives of Clinical Neuropsychology, 23(2), 129–137. doi: 10.1016/j.acn.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida, S., & Schafer, E. J. (2018). Social networks, social relationships, and their effects on the aging mind and brain. In Rizzo M., Anderson Steven, & Fritzsch Bernd (Eds.), The Wiley handbook on the aging mind and brain (pp. 19–36). Wiley. [Google Scholar]
- Bandura, A. (2001). Social cognitive theory: An agentic perspective. Annual Review of Psychology, 52, 1–26. doi: 10.1146/annurev.psych.52.1.1 [DOI] [PubMed] [Google Scholar]
- Barnes, L. L., Mendes de Leon, C. F., Wilson, R. S., Bienias, J. L., & Evans, D. A. (2004). Social resources and cognitive decline in a population of older African Americans and whites. Neurology, 63(12), 2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3 [DOI] [PubMed] [Google Scholar]
- Bath, P. A., & Deeg, D. (2005). Social engagement and health outcomes among older people: Introduction to a special section. European Journal of Ageing, 2(1), 24–30. doi: 10.1007/s10433-005-0019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, M., Snyder, H. M., Carrillo, M. C., Fazio, S., Kim, H., & Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s & Dementia, 11(6), 718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., & Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. The Lancet. Neurology, 5(5), 406–412. doi: 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- Berkman, L., & Glass, T. (2000). Social integration, social networks, social support, and health. In Berkman L. & Kawachi I. (Eds.), Social epidemiology (pp. 137–173). Oxford University Press. [Google Scholar]
- Berkman, L. F., & Syme, S. L. (1979). Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. American Journal of Epidemiology, 109(2), 186–204. doi: 10.1093/oxfordjournals.aje.a112674 [DOI] [PubMed] [Google Scholar]
- Biddle, K. D., d’Oleire Uquillas, F., Jacobs, H. I. L., Zide, B., Kirn, D. R., Rentz, D. M., Johnson, K. A., Sperling, R. A., & Donovan, N. J. (2019). Social engagement and amyloid-β-related cognitive decline in cognitively normal older adults. The American Journal of Geriatric Psychiatry, 27(11), 1247–1256. doi: 10.1016/j.jagp.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak, A. A. (2010). How can we not ‘lose it’ if we still don’t understand how to ‘use it’? Unanswered questions about the influence of activity participation on cognitive performance in older age—A mini-review. Gerontology, 56(5), 507–519. doi: 10.1159/000264918 [DOI] [PubMed] [Google Scholar]
- Burt, R. S. (1987). A note on the general social survey’s ersatz network density item. Social Networks, 9(1), 75–85. doi: 10.1016/0378-8733(87)90019-0 [DOI] [Google Scholar]
- Casey, A.-N. S., Liu, Z., Kochan, N. A., Sachdev, P. S., & Brodaty, H. (2020). Cross-lagged modeling of cognition and social network size in the Sydney Memory and Ageing Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, gbaa193. doi: 10.1093/geronb/gbaa193 [DOI] [PubMed] [Google Scholar]
- Cohen, S. (2004). Social relationships and health. The American Psychologist, 59(8), 676–684. doi: 10.1037/0003-066X.59.8.676 [DOI] [PubMed] [Google Scholar]
- Cohen, S., Doyle, W. J., Skoner, D. P., Rabin, B. S., & Gwaltney, J. M.Jr. (1997). Social ties and susceptibility to the common cold. Journal of the American Medical Association, 277(24), 1940–1944. doi: 10.1001/jama.1997.03540480040036 [DOI] [PubMed] [Google Scholar]
- Coleman, J. S. (1988). Social capital in the creation of human capital. American Journal of Sociology, 94, S95–S120. doi: 10.1086/228943 [DOI] [Google Scholar]
- Cornwell, B. (2009a). Good health and the bridging of structural holes. Social Networks, 31(1), 92–103. doi: 10.1016/j.socnet.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, B. (2009b). Network bridging potential in later life: Life-course experiences and social network position. Journal of Aging and Health, 21(1), 129–154. doi: 10.1177/0898264308328649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, B. (2011). Independence through social networks: Bridging potential among older women and men. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(6), 782–794. doi: 10.1093/geronb/gbr111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, B., Schumm, L. P., Laumann, E. O., & Graber, J. (2009). Social networks in the NSHAP Study: Rationale, measurement, and preliminary findings. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64(Suppl. 1), 47–55. doi: 10.1093/geronb/gbp042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft, S., Newcomer, J., Kanne, S., Dagogo-Jack, S., Cryer, P., Sheline, Y., Luby, J., Dagogo-Jack, A., & Alderson, A. (1996). Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiology of Aging, 17(1), 123–130. doi: 10.1016/0197-4580(95)02002-0 [DOI] [PubMed] [Google Scholar]
- Crooks, V. C., Lubben, J., Petitti, D. B., Little, D., & Chiu, V. (2008). Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health, 98(7), 1221–1227. doi: 10.2105/AJPH.2007.115923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J., Ritter, A., & Zhong, K. (2018). Clinical trials for disease-modifying therapies in Alzheimer’s disease: A primer, lessons learned, and a blueprint for the future. Journal of Alzheimer’s Disease, 64(s1), S3–S22. doi: 10.3233/jad-179901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge, H. H., Goldstein, F. C., Wakim, N. I., Gefen, T., Teylan, M., Chan, K. C., Kukull, W. A., Barnes, L. L., Giordani, B. Hughes, T. M., & Kramer, J. H. 2020. Differentiating among stages of cognitive impairment in aging: Version 3 of the Uniform Data Set (UDS) neuropsychological test battery and MoCA index scores. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 6(1), p.e12103. doi: 10.1001/jamapsychiatry.2016.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, N. J., Okereke, O. I., Vannini, P., Amariglio, R. E., Rentz, D. M., Marshall, G. A., Johnson, K. A., & Sperling, R. A. (2016). Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry, 73(12), 1230–1237. doi: 10.1001/jamapsychiatry.2016.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, N. J., Wu, Q., Rentz, D. M., Sperling, R. A., Marshall, G. A., & Glymour, M. M. (2017). Loneliness, depression and cognitive function in older U.S. adults. International Journal of Geriatric Psychiatry, 32(5), 564–573. doi: 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwardt, L., Van Tilburg, T. G., & Aartsen, M. J. (2015). The mix matters: Complex personal networks relate to higher cognitive functioning in old age. Social Science & Medicine (1982), 125, 107–115. doi: 10.1016/j.socscimed.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Enders, C. K., & Bandalos, D. L. (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457. doi: 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Ertel, K. A., Glymour, M. M., & Berkman, L. F. (2008). Effects of social integration on preserving memory function in a nationally representative US elderly population. American Journal of Public Health, 98(7), 1215–1220. doi: 10.2105/AJPH.2007.113654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri, M. M., Matthews, F., Brayne, C., Ince, P. G., Matthews, F. E., Xuereb, J. H., Broome, J. C., McKenzie, J., Rossi, M., & McKeith, I. G. (2001). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet, 357(9251):169–75. doi: 10.1016/s0140-6736(00)03589-3 [DOI] [PubMed] [Google Scholar]
- Evans, I. E., Llewellyn, D. J., Matthews, F. E., Woods, R. T., Brayne, C., Clare, L., & CFAS-Wales Research Team. (2018). Social isolation, cognitive reserve, and cognition in healthy older people. PloS One, 13(8), e0201008. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, I. E. M., Martyr, A., Collins, R., Brayne, C., & Clare, L. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise, R. J. (2002). Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology, 2, 8. doi: 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori, K. L., Smith, J., & Antonucci, T. C. (2007). Social network types among older adults: A multidimensional approach. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62(6), 322–330. doi: 10.1093/geronb/62.6.p322 [DOI] [PubMed] [Google Scholar]
- Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet. Neurology, 3(6), 343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Gaudino, E. A., Geisler, M. W., & Squires, N. K. (1995). Construct validity in the Trail Making Test: What makes Part B harder? Journal of Clinical and Experimental Neuropsychology, 17(4), 529–535. doi: 10.1080/01688639508405143 [DOI] [PubMed] [Google Scholar]
- Giles, L. C., Anstey, K. J., Walker, R. B., & Luszcz, M. A. (2012). Social networks and memory over 15 years of follow-up in a cohort of older Australians: Results from the Australian Longitudinal Study of Ageing. Journal of Aging Research, 2012, 856048. doi: 10.1155/2012/856048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow, A. J., Corley, J., Starr, J. M., & Deary, I. J. (2013). Which social network or support factors are associated with cognitive abilities in old age? Gerontology, 59(5), 454–463. doi: 10.1159/000351265 [DOI] [PubMed] [Google Scholar]
- Hamilton, C. M., Strader, L. C., Pratt, J. G., Maiese, D., Hendershot, T., Kwok, R. K., Hammond, J. A., Huggins, W., Jackman, D., Pan, H., Nettles, D. S., Beaty, T. H., Farrer, L. A., Kraft, P., Marazita, M. L., Ordovas, J. M., Pato, C. N., Spitz, M. R., Wagener, D., … Haines, J. (2011). The PhenX Toolkit: Get the most from your measures. American Journal of Epidemiology, 174(3), 253–260. doi: 10.1093/aje/kwr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. H., & Thoresen, C. E. (2005). Volunteering is associated with delayed mortality in older people: Analysis of the longitudinal study of aging. Journal of Health Psychology, 10(6), 739–752. doi: 10.1177/1359105305057310 [DOI] [PubMed] [Google Scholar]
- Heinrichs, M., Baumgartner, T., Kirschbaum, C., & Ehlert, U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. doi: 10.1016/s0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Holtzman, R. E., Rebok, G. W., Saczynski, J. S., Kouzis, A. C., Wilcox Doyle, K., & Eaton, W. W. (2004). Social network characteristics and cognition in middle-aged and older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59(6), 278–284. doi: 10.1093/geronb/59.6.p278 [DOI] [PubMed] [Google Scholar]
- Hultsch, D. F., Hertzog, C., Small, B. J., & Dixon, R. A. (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14(2), 245–263. doi: 10.1037//0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- James, B. D., Wilson, R. S., Barnes, L. L., & Bennett, D. A. (2011). Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society, 17(6), 998–1005. doi: 10.1017/S1355617711000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp, A., Paillard-Borg, S., Wang, H. X., Silverstein, M., Winblad, B., & Fratiglioni, L. (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders, 21(2), 65–73. doi: 10.1159/000089919 [DOI] [PubMed] [Google Scholar]
- Katz, B., Turney, I., Lee, J. H., Amini, R., Ajrouch, K., & Antonucci, T. (2020). Race/ethnic differences in social resources as cognitive risk and protective factors. Research in Human Development, 17(1), 57–77. doi: 10.1080/15427609.2020.1743809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman, R. (1993). Education and the prevalence of dementia and Alzheimer’s disease. Neurology, 43(1), 13–20. doi: 10.1212/wnl.43.1_part_1.13 [DOI] [PubMed] [Google Scholar]
- Kelly, M. E., Duff, H., Kelly, S., McHugh Power, J. E., Brennan, S., Lawlor, B. A., & Loughrey, D. G. (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann, G., Gast, D., & Gage, F. H. (2002). Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Annals of Neurology, 52(2), 135–143. doi: 10.1002/ana.10262 [DOI] [PubMed] [Google Scholar]
- Kuiper, J. S., Zuidersma, M., Zuidema, S. U., Burgerhof, J. G., Stolk, R. P., Oude Voshaar, R. C., & Smidt, N. (2016). Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology, 45(4), 1169–1206. doi: 10.1093/ije/dyw089 [DOI] [PubMed] [Google Scholar]
- Li, M., & Dong, X. (2018). Is social network a protective factor for cognitive impairment in US Chinese older adults? Findings from the PINE Study. Gerontology, 64(3), 246–256. doi: 10.1159/000485616 [DOI] [PubMed] [Google Scholar]
- Litwin, H., & Shiovitz-Ezra, S. (2006). Network type and mortality risk in later life. The Gerontologist, 46(6), 735–743. doi: 10.1093/geront/46.6.735 [DOI] [PubMed] [Google Scholar]
- Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L. (1999). Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging, 5, 21–32. doi: 10.1037/t03352-000 [DOI] [Google Scholar]
- Marsden, P. V. (2000). Social networks. Encyclopedia of Sociology, 4, 2727–2735. [Google Scholar]
- McCarty, C., Lubbers, M. J., Vacca, R., & Molina, J. L. (2019). Conducting personal network research: A practical guide. Guilford Publications. [Google Scholar]
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Penninkilampi, R., Casey, A. N., Singh, M. F., & Brodaty, H. (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 66(4), 1619–1633. doi: 10.3233/JAD-180439 [DOI] [PubMed] [Google Scholar]
- Perry, B. L., Pescosolido, B. A., & Borgatti, S. P. (2018). Egocentric network analysis: Foundations, methods, and models. Cambridge University Press. [Google Scholar]
- Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H.Jr, Chance, J. M., & Filos, S. (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 37(3), 323–329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- Pillai, J. A., & Verghese, J. (2009). Social networks and their role in preventing dementia. Indian Journal of Psychiatry, 51(Suppl. 1), S22–S28. [PMC free article] [PubMed] [Google Scholar]
- Rostila, M. (2010). Birds of a feather flock together—And fall ill? Migrant homophily and health in Sweden. Sociology of Health & Illness, 32(3), 382–399. doi: 10.1111/j.1467-9566.2009.01196.x [DOI] [PubMed] [Google Scholar]
- Seeman, T. E., Lusignolo, T. M., Albert, M., & Berkman, L. (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology, 20(4), 243–255. doi: 10.1037//0278-6133.20.4.243 [DOI] [PubMed] [Google Scholar]
- Sharifian, N., Kraal, A. Z., Zaheed, A. B., Sol, K., & Zahodne, L. B. (2020). Longitudinal associations between contact frequency with friends and with family, activity engagement, and cognitive functioning. Journal of the International Neuropsychological Society, 26(8), 815–824. doi: 10.1017/S1355617720000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, J. I., & Yesavage, J. A. (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In Brink T. L. (Ed.), Clinical gerontology: A guide to assessment and intervention (pp. 165–173). The Haworth Press. [Google Scholar]
- Smith, K. P., & Christakis, N. A. (2008). Social networks and health. Annual Review of Sociology, 34(1), 405–429. doi: 10.1146/annurev.soc.34.040507.134601 [DOI] [Google Scholar]
- Sommerlad, A., Sabia, S., Singh-Manoux, A., Lewis, G., & Livingston, G. (2019). Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Medicine, 16(8), e1002862. doi: 10.1371/journal.pmed.1002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. doi: 10.1017/s1355617702813248 [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2006). Cognitive reserve and Alzheimer disease. Alzheimer’s Disease and Associated Disorders, 20, 112–117. doi: 10.1097/01.wad.0000213815.20177.19 [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits, P. A. (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52(2), 145–161. doi: 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Uchino, B. N. (2006). Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29(4), 377–387. doi: 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Unger, J. B., Johnson, C. A., & Marks, G. (1997). Functional decline in the elderly: Evidence for direct and stress-buffering protective effects of social interactions and physical activity. Annals of Behavioral Medicine, 19(2), 152–160. doi: 10.1007/BF02883332 [DOI] [PubMed] [Google Scholar]
- Wang, H. X., Karp, A., Winblad, B., & Fratiglioni, L. (2002). Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. American Journal of Epidemiology, 155(12), 1081–1087. doi: 10.1093/aje/155.12.1081 [DOI] [PubMed] [Google Scholar]
- Wilson, R. S., Krueger, K. R., Arnold, S. E., Schneider, J. A., Kelly, J. F., Barnes, L. L., Tang, Y., & Bennett, D. A. (2007). Loneliness and risk of Alzheimer disease. Archives of General Psychiatry, 64(2), 234–240. doi: 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- Zunzunegui, M. V., Alvarado, B. E., Del Ser, T., & Otero, A. (2003). Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58(2), 93–100. doi: 10.1093/geronb/58.2.s93 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.