Abstract
Background
Glioblastoma (GBM) is one of the most common primary brain tumors, which accounts up to 80% of malignant brain tumors and the 5‐year relative survival rate is below 5%. Recent studies showed that the lipid metabolism played an essential role in GBM development. As a peroxisome proliferators‐activated receptors γ (PPAR‐γ) agonist, telmisartan improves the lipid metabolism and has been used to treat hypertension for long time. It has also been shown to have anticancer function, such as in lung cancer and melanoma.
Methods
Incucyte real‐time live cell imaging system was used to assess the effect of telmisartan on glioma cell lines U87 and U251 proliferation. Transwell assay and colony formation assay were conducted to detect the effect of telmisartan on oncogenicity of GBM cell lines. Western blot and immunofluorescence analysis were used to detect the effect of telmisartan on the expression of PPAR‐γ and hydroxyacyl‐coenzyme A dehydrogenase alpha subunit (HADHA).
Results
We demonstrate that telmisartan inhibits two glioma cell lines U87 and U251 proliferation in a time‐ and dose‐dependent manner, and arrests the cell cycle at S phase. We further show that telmisartan decreases the oncogenicity of GBM cell lines. Our data show that telmisartan treatment significantly increases the PPAR‐γ expression level, enhances the lipid oxidation, and upregulates the level of fatty acid oxidation key enzyme HADHA.
Conclusions
Telmisartan inhibits the proliferation and oncogenicity while it also increases the lipid oxidation of human GBM cells.
Keywords: brain tumor, lipid oxidation, metabolism, PPAR‐γ, telmisartan
Telmisartan, a PPAR‐γ agonist, decreases the cell proliferation and inhibits the oncogenicity of human GBM cell lines by inducing S‐phase arrest and increasing the energy expenditure via lipid oxidation.
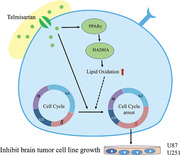
1. INTRODUCTION
As one of the most common and detrimental forms of solid brain tumor, there were over 10,000 new cases reported of glioblastoma (GBM) every year in the United States. Although aggressive multimodal treatment approaches have been applied, including surgery, chemotherapies, and radiotherapies, the overall survival period is reported to be less than 2 years after diagnosis. 1 Brain is a lipid‐rich organ, 2 and the lipid metabolism is closely related to the brain structure, function, and pathological conditions, including Alzheimer disease (AD), Parkinson's diseases, and multiple sclerosis. 3 Specifically, studies have shown that the increased lipid peroxidation was associated with AD, and the lipid oxidation was the major outcome of radical‐mediated injury that oxygenated fatty acids. 4 The recent analysis also showed that the lipid droplets were significantly enriched in the brain tumor tissue from GBM patients compared to the normal controls. 5 The increasing lipid droplets in cancer cells play important roles in facilitating the tumor cells to adapt to the harmful conditions. 6
Telmisartan is an angiotensin II receptor blocker, which functionally affects the lipid metabolism 7 by targeting to the peroxisome proliferators‐activated receptors γ (PPAR‐γ). 8 Telmisartan has been shown to be protective against high lipid‐induced apoptosis via PPAR‐γ. 8 Li et al. demonstrated that telmisartan could inhibit lipid accumulation in the vascular smooth muscle cells by upregulating the PPAR‐γ. 9 Furthermore, telmisartan is also known for its anticancer properties. For instance, telmisartan inhibited the human endometrial cancer cells growth by breaking its DNA double‐strand breaks. 10 It inhibited cholangiocarcinoma tumor growth by blocking its cell proliferation. 11 However, the function of telmisartan in GBM has not been studied.
In the current study, we show that telmisartan inhibits GBM cells proliferation by arresting cell cycle at S phase. Furthermore, telmisartan decreases the cell oncogenicity by downregulating the cell migration abilities and colony formation capabilities. We also demonstrate that telmisartan treatment could increase the expression of PPAR‐γ, decrease the overall level of cell oxidation, and upregulate the key enzyme of fatty acid oxidation, hydroxyacyl‐coenzyme A dehydrogenase alpha subunit (HADHA), in GBM cell lines. HADHA, which catalyzes the last three steps of mitochondrial beta‐oxidation of long chain fatty acids, converts medium‐ and long‐chain 2‐enoyl‐CoA compounds into the acetyl‐CoA, when NAD and CoASH are present. 12
2. MATERIALS AND METHODS
2.1. Cell culture
Human glioma cell line U251MG was cultured in Minimum Essential Medium (MEM) containing 10% bovine calf serum, 100 units/ml penicillin, and 50 µg/ml streptomycin. Human glioma cell line U87MG was cultured in MEM containing 10% bovine calf serum, 100 units/ml penicillin, 50 µg/ml streptomycin, and 1 unite/ml nonessential amino acids. Both cell lines were placed in a humidified CO2 incubator (5% CO2, 95% air) at 37°C.
2.2. Cell proliferation assay
To monitor the status of cell proliferation with telmisartan treatment, human glioma cells were plated in 96‐well plates at 37°C. After 12 h, telmisartan was added into the cell culture. For each concentration of telmisartan, there were four replicates. The real‐time status of cells was monitored using Incucyte (Sartorius, USA). Pictures were taken every 3 h up to 72 h. The cell proliferation rate was analyzed by comparing the occupied area (% confluence) of cell images over time.
The EdU cell proliferation kit (Cat# C10310‐3; Ribobio) was used to further confirm the effects of telmisartan on human glioma cell proliferation. A total of 50 µM EdU was added in U87 and U251 cell culture separately and incubated for 2 h. Then the samples were detected and recorded using Beckman CytoFLEX S.
2.3. Flow cytometry analysis of cell cycle
To test the effects of telmisartan on human glioma cell cycle, we collected the 1 × 106 cells and fixed them in ice‐cold 70% EOH. Next, cells were treated with RNase (Absin) at 37°C for 30 min. Before acquisition using flow cytometry, propidium iodide was added into the cell suspension to distinguish the live/dead cells.
2.4. Colony formation assay
Cells were plated in 10‐cm dishes and the media was changed every 4–5 days until the cells became confluent. Cells were then washed with PBS, fixed with Clark's fixative solution, and stained with Giemsa Stain solution (Cat# G1015; Solarbio). Laica DMi8 (Germany) microscope was used to take pictures of the colonies.
2.5. Cell migration assay (transwell assay)
Migration assays were performed, as described previously, 13 in 24‐well transwell plates (Millipore) using uncoated polycarbonate membranes with 8‐mm pores. Serum‐starved cells were harvested and resuspended at a concentration of 1 × 104 cells per 0.2 ml in serum‐free medium containing 10% BSA, and added to the upper chamber. The nonmigrated cells were scraped off the upper side of the filter, and filters were stained with Giemsa Stain solution after 18 h. Picture was taken using Laica DMi8 (Germany).
2.6. Western blotting
Samples from cell lines were collected in RIPA buffer after washing with cold PBS. Primary antibody, anti‐PPAR‐γ, was used at 1:1000 (Santa Cruz, Cat# sc‐271392); secondary antimouse horseradish peroxidase was used at 1:2000 (zsbio, Cat# ZB‐2305). Anti‐tubulin (1:2000, bioss, Cat# bsm‐33034 m) was used as a loading control. Protein Marker was purchased from Applygen (10–180 kDa, Cat# P1103).
2.7. Real‐time PCR
Total mRNA was purified using the RNeasy mini kit (Cat# 74014; Qiagen) and treated with RNase‐Free DNAase (Cat# 79254; Qiagen) for 30 min at room temperature. The High Capacity cDNA Reverse Transcription Kit (Cat# 1725037; Bio‐Rad) was used to synthesize cDNA, and SYBR green real‐time PCR was performed using MyCycler Thermal Cycler.
2.8. Lipid peroxidation assay
To image the lipid oxidation in live cells, image‐iT Lipid Peroxidation kit (Cat# C10445; Lifetechnology) was used. As protocol suggests, the image‐iT Lipid Peroxidation kit is based on BODIPY 581/591 reagent and is a sensitive fluorescent reporter for lipid oxidation. The fluorescence shifts from red to green of the phenybutadiene segment of the fluorophore, providing a radiometric indication of lipid peroxidation by traditional and high‐content microscopy. U87 cells were planted in a 96‐well plate. After overnight incubation, telmisartan was added into the cell culture (final concentration was 50 µM) and left for 2 days. The 10‐µM lipid peroxidation sensor was added into the complete growth medium at 37°C for 30 min. DMSO was used as negative control. Pictures were taken using Laica DMi8 (Germany).
2.9. Lipid oxidation assay
The lipid oxidation assay was performed by the fatty acid oxidation assay kit (Cat#118183, Abcam). Cells treated with telmisartan and vehicle control DMSO for 48 h were collected, harvested and fixed/permeabilized in suspension, stained with ACADM, ACADVL, and HADHA antibodies separately for 1 h, and then stained with secondary FITC antibody for another 1 h. The expression levels of ACADM, ACADVL, and HADHA were determined using flow cell cytometry (Beckman CytoFLEX S).
2.10. Statistical analysis
One‐way ANOVA and two‐tailed Student's t‐test were performed using GraphPad Prism. The values were considered significantly different when p < 0.05. Data are presented as mean ± SD.
3. RESULTS
3.1. Telmisartan attenuates the proliferation of human glioma cells
To investigate the effects of telmisartan on human glioma cells growth, different concentrations of telmisartan were added into the cell culture and the cells were monitored using real‐time Incucyte system. Our results showed that telmisartan reduces the proliferation of two human brain tumor cell lines in a dose‐dependent manner (Figure 1A–D). Specifically, adding 50 µM telmisartan could significantly decrease the cell proliferation (Figure 1A–D; Videos S1 and S2 [U87 cells with vehicle or with 50 µM telmisartan, separately]; Videos S3 and S4 [U251 cells with vehicle or with 50 µM telmisartan, separately]).
FIGURE 1.
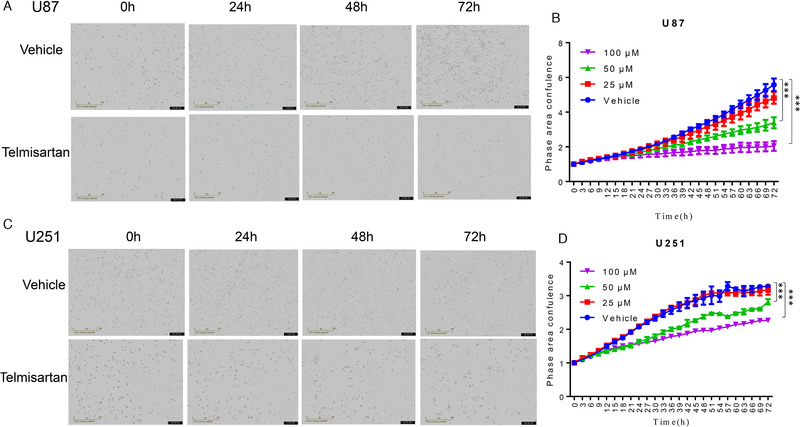
The effect of telmisartan on GBM cells proliferation in a dose‐ and time‐dependent manner. (A) Pictures taken at various time points (0, 24, 48, and 72 h) after adding telmisartan (50 µM) and vehicle (DMSO) in U87 cell lines. (B) The effects of different concentrations of telmisartan on U87 cell proliferation. (C) Pictures taken at various time points (0, 24, 48, and 72 h) after adding telmisartan (50 µM) and vehicle (DMSO) in U251 cell lines. (D) The effects of different concentrations of telmisartan on U251 cell proliferation. Each concentration of telmisartan had four replicates. *** p < 0.001
To further confirm the function of 50 µM telmisartan on cell proliferation, the EdU method has been used. The flow cell cytometry results confirmed that after telmisartan treatment, the percentage of EdU + U87 cells was decreased from 24.25% to 21.16% and the percentage of EdU + U251 cells was decreased from 41.44% to 38.78% (Figure 2A).
FIGURE 2.
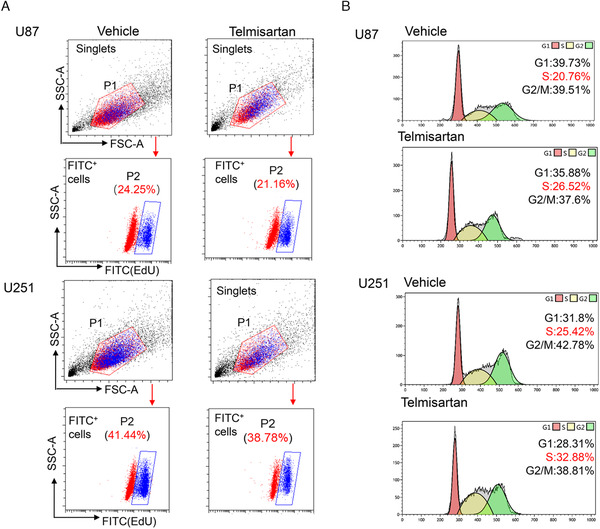
Telmisartan inhibits GBM cell proliferation and arrests cell cycle at S phase. (A) FACS quantification of the percentage of EdU (FITC+) cells after 48 h of telmisartan addition in both U87 and U251 GBM cell lines. The singlets were gated first and then the FITC+ cells were gated. The percentages of FITC+ cells were listed above the gated cell populations. (B) FACS analysis of cell cycle. The red peaks represent cells in G1 phase, the yellow peaks represent cells in S phase, and the green peaks represent cells in G2/M phase. The percentage of cells in each phase was listed on the right side for each group
3.2. Telmisartan decreases cell proliferation by arresting cell cycle in S phase
We next investigated whether telmisartan could affect the cell cycle of human glioma cells. Two days after telmisartan treatment, flow cell cytometry results showed that 26.52% of U87 cells were arrested in S phase and the percentage of untreated U87 cells was 20.76%. The same trend was also observed in U251 cell lines: the percentage of U251 cells in S phase was 32.88% after telmisartan treatment and only 25.42% were untreated (Figure 2B).
3.3. Telmisartan inhibits the oncogenicity of human glioma cells
To confirm that the telmisartan decreases the human glioma cells proliferation and arrests the cells in S phase, we next sought to determine whether this drug could inhibit the oncogenicity of human glioma cells using colony formation assay and transwell assay. Our data showed after 50 µM telmisartan treatment, the capabilities of colony formation were significantly decreased (Figure 3A). At the same time, the migration abilities of U87 were downregulated after adding telmisartan (Figure 3B).
FIGURE 3.
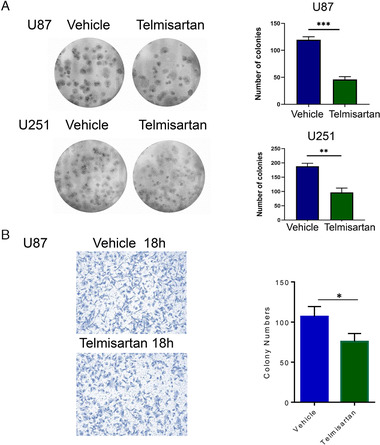
Telmisartan decreases the oncogenicity of GBM cell lines. (A) Cells with the telmisartan treatment have less colonies formations compared to the vehicle groups. Left panel shows the representative colony formation pictures of telmisartan treatment group and control group. Colonies were grown for 3 weeks, and then foci were counted and photographed. The right panel shows the statistical analysis. Each experiment had three replicates. The error bar represents SD; **p < 0.01 and ***p < 0.001. (B) Pictures taken after 18 h of transwell assay, and the statistical results, *p < 0.05
3.4. Telmisartan increases the lipid oxidation of GBM cells
Tumor cells usually have abnormal metabolism. As telmisartan has been broadly used to treat high blood pressure, we were aiming to investigate the function of telmisartan on cell metabolism. Our data showed that with 50 µM telmisartan, the level of PPAR‐γ was increased compared to the control group (Figure 4A). The overall fatty acid oxidation was increased using the image‐iT Lipid Peroxidation kit. When the lipid is oxidized, the fluorescence will shift from red to green. Our results showed that telmisartan increased the lipid oxidation in human glioma U87 cells (Figure 4B). We further confirmed that the expression level of key enzyme involved in fatty acid oxidation, HADHA, is increased (Figure 4C).
FIGURE 4.
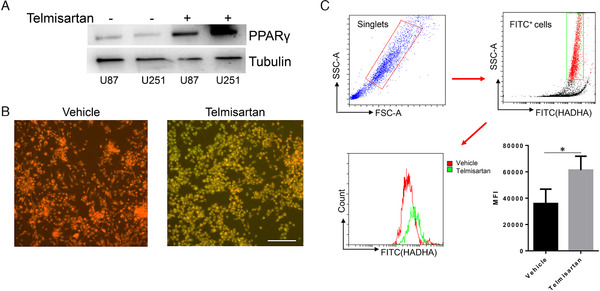
Telmisartan increases the PPAR‐γ expression, enhances the overall lipid oxidation, and upregulates the key enzyme, HADHA. (A) Western blots show with telmisartan treatment, the protein levels of PPAR‐γ are increased in both U87 and U251 cells. Tubulin is served as a control. (B) The representative fluoresces pictures show the overall lipid oxidation of U87 cells with telmisartan treatment or with DMSO only. (C) FACS quantifications of HADHA expression level. Cells are first gated as singlets and then HADHA+ (FITC+) cells. Red line strands for cells treated with vehicle (DMSO) and green line strands for cell treated with telmisartan. The bar chat shows the mean fluoresce intensity (MFI); the error bar represents SD; *p < 0.05
4. DISCUSSION
GBM multiform is one of the primary brain tumors with extremely low survival rate 14 and very poor treatment outcomes. 15 Telmisartan has been shown to have anticancer properties in various types of cancer, such as melanoma, 16 colon cancer, 17 and lung cancer. 18 Furthermore, it has already been applied to treat brain‐related diseases. For instance, Wei et al. showed that telmisartan relived the cerebral edema induced by cold brain injury by blocking the NLRP3 inflammasome. 19 Telmisartan was also shown to prevent recurrent stroke and cardiovascular events. 20 However, until now, there is very rare evidence to show the function of telmisartan on GBM. Therefore, the aim of present research was to investigate the effects of telmisartan on GBM.
In the current study, using two GBM cell lines, U87 and U251, our data demonstrated that telmisartan inhibited the human glioma cells proliferation by both the real‐time cell monitor systems—Incucyte and EdU method. Forty‐eight hours after adding telmisartan, the percentage of proliferation cells was dramatically decreased compared to the control group. We also showed that after adding telmisartan, both U87 and U251 cells were arrested at S phase (Figure 2). This results were consistent with pervious findings that telmisartan could induce cell cycle arrest and inhibit the proliferation of hepatocellular carcinoma cells 21 and endothelial cell. 22 In esophageal squamous cell carcinoma, telmisartan induced the cells’ arrest at S phase due to the decreased expression of cyclin A2. 23
In addition, we demonstrated that telmisartan decreased the oncogenicity of both U87 and U251 cell lines, with downregulated colony formation capabilities and the abilities of cell migration (Figure 3). The similar phenomenon has been shown in NSCLC A548 cells—telmisartan inhibited the cell migration by regulating the PI3K/AKT signaling pathway. 24 Similar results were also found in immune cells. Walcher et al. showed that telmisartan could inhibit the CD4+ cells migration via PPAR‐γ. 25 This cell migration inhibition function of telmisartan was not limited in vitro, it also worked in vivo. Matsui et al. showed that telmisartan treatment inhibited the KYSE180 tumor cells migration in nude mice. 23
As a PPAR‐γ agonist, telmisartan has been reported to play its multifunction directly via PPAR‐γ. Our data showed that telmisartan could elevate the PPAR‐γ expression (Figure 4A). Indeed, telmisartan treatment has been reported to enhance the expression of PPAR‐γ both in vivo and in vitro. For instance, in A549 cell line, telmisartan presented its anticancer function by increasing the level of PPAR‐γ, which further inhibited the ICAM‐1 and MMP‐9. 26 Goyal et al. showed that diabetic rats with telmisartan (10 mg/kg/day) had much higher PPAR‐γ expression compared to control group, which may protect the myocardium from ischemic injury in experimental rats. 27
Brain is a lipid‐rich organ. 28 In a recent report, it was found that the hydrolysis of lipids, such as triglycerides and lipid droplets, could release fatty acids as the major energy reservoir for GBM tumors. 29 The lipid accumulation and oxidation are closely related to the development of GBM. Taïb et al. showed that pharmacologic inhibitor of monoacylglycerol lipase attenuates GBM proliferation. 5 PPAR‐γ is a lipid‐sensitive nuclear receptor, which is mainly involved in the regulation of the adiposeness and lipid biosynthesis. 30 Furthermore, PPAR‐γ was shown to have a direct function in fatty acid metabolism. For instance, activation of PPAR‐γ with pioglitazone reversed metabolic changes that occur with pulmonary arterial hypertension (PAH). 31 Specifically, pioglitazone, the PPAR‐γ agonist, reversed PAH and prevented heart failure via fatty acid oxidation. 1 Therefore, we questioned that whether telmisartan, a PPAR‐γ agonist, could regulate the lipid (fatty acid) metabolism. To answer this question, we used the image‐iT Lipid Peroxidation kit in the present study. The data showed that with telmisartan treatment, the fluorescence shifted from red to green as the lipid is oxidized (Figure 4B). Sugimoto et al. showed that telmisartan could increase fatty acid oxidation in skeletal muscle via PPAR‐γ‐dependent pathway. 32 They also demonstrated that the increased fatty acid oxidation induced by telmisartan could be blocked by adding PPAR‐γ antagonist GW9662. 32 Furthermore, Li et al. provided in vivo data that after 4 weeks treatment of telmisartan, the spontaneously hypertensive rat had reduced fatty acid levels. 33
Based on these findings, we next investigated which enzyme might play the essential role in telmisartan‐induced fatty acid oxidation. Fatty acid β‐oxidation metabolic pathway regulates the energy homeostasis. 34 Oxidation of fatty acids occurs inside the mitochondria to degrade the acyl‐CoA esters into units of acetyl‐CoA. Our results showed that HADHA catalyzes the hydration of enoyl‐CoA and increases the dehydrogenation of 3‐hydroxyacyl CoA compounds (Figure 4C), suggesting it might be the reason that caused the overall upregulated lipid oxidation. This finding is consistent with published data by Villa et al. They performed RNA sequencing using telmisartan‐treated rat with model of glomerulonephritis, and their results showed that HADHA was increased by 1.7‐fold in high‐telmisartan group compared to the hydrochlorothiazide/hydralazine‐treated group. 35
5. CONCLUSION
In summary, our current study demonstrated that telmisartan inhibited the proliferation of GBM cells and arrested the cell cycle at S phase. The treatment of telmisartan decreased the oncogenicities of both U87 and U251 cells by downregulating their colony formation and migration capabilities. Considering brain is a lipid‐rich organ and lipid metabolism is a key regulator for GBM, we further confirmed that telmisartan treatment increased the PPAR‐γ expression in GBM cell lines and enhanced the overall lipid oxidation level, which may contribute to the upregulation of the key enzyme in fatty acid oxidation, HADHA.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
YW, TRZ, and CL contributed to cell experiments, study analysis, and data analysis. YW, JG, BHX, and LXX contributed to the conception, design of the project, and writing of the manuscript.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 82001248 to YW; No. 81972966 to LXX) and Peking University Third Hospital Talent Program C (No. BYSYZD2019047 to YW).
Wang Y, Zhang T, Li C, Guo J, Xu B, Xue L. Telmisartan attenuates human glioblastoma cells proliferation and oncogenicity by inducing the lipid oxidation. Asia-Pac J Clin Oncol. 2022;18:217–223. 10.1111/ajco.13574
Yan Wang and Tengrui Zhang contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Legchenko E, Chouvarine P, Borchert P, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018;10(438):eaao0303. [DOI] [PubMed] [Google Scholar]
- 2. Norris C, Fong B, MacGibbon A, McJarrow P. Analysis of phospholipids in rat brain using liquid chromatography‐mass spectrometry. Lipids. 2009;44(11):1047‐1054. [DOI] [PubMed] [Google Scholar]
- 3. Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidol. 2007;2(4):403‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montine TJ, Neely MD, Quinn JF, et al. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002;33(5):620‐626. [DOI] [PubMed] [Google Scholar]
- 5. Taïb B, Aboussalah AM, Moniruzzaman M, et al. Lipid accumulation and oxidation in glioblastoma multiforme. Sci Rep. 2019;9(1):19593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Q, Luo Q, Halim A, Song G. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer Lett. 2017;401:39‐45. [DOI] [PubMed] [Google Scholar]
- 7. Kang C, Yijun L, Jingtao D, et al. Effects of telmisartan on lipid metabolisms and proinflammatory factors secretion of differentiated 3T3‐L1 adipocytes. J Renin Angiotensin Aldosterone Syst. 2015;16(4):1061‐1068. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Xue J, Li Y, Zhou X, Qiao S, Han D. Telmisartan protects against high glucose/high lipid‐induced apoptosis and insulin secretion by reducing the oxidative and ER stress. Cell Biochem Funct. 2019;37(3):161‐168. [DOI] [PubMed] [Google Scholar]
- 9. Li BH, Liao SQ, Yin YW, et al. Telmisartan‐induced PPARγ activity attenuates lipid accumulation in VSMCs via induction of autophagy. Mol Biol Rep. 2015;42(1):179‐186. [DOI] [PubMed] [Google Scholar]
- 10. Koyama N, Nishida Y, Ishii T, Yoshida T, Furukawa Y, Narahara H. Telmisartan induces growth inhibition, DNA double‐strand breaks and apoptosis in human endometrial cancer cells. PLoS One. 2014;9(3):e93050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samukawa E, Fujihara S, Oura K, et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int J Oncol. 2017;51(6):1674‐1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miklas JW, Clark E, Levy S, et al. TFPa/HADHA is required for fatty acid beta‐oxidation and cardiolipin re‐modeling in human cardiomyocytes. Nat Commun. 2019;10(1):4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen J, Xiong K, Aili A, et al. PEX5, a novel target of microRNA‐31‐5p, increases radioresistance in hepatocellular carcinoma by activating Wnt/β‐catenin signaling and homologous recombination. Theranostics. 2020;10(12):5322‐5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasilev A, Sofi R, Rahman R, Smith SJ, Teschemacher AG, Kasparov S. Using light for therapy of glioblastoma multiforme (GBM). Brain Sci. 2020;10(2):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu W, Wang W, Li H, et al. Single‐cell atlas reveals complexity of the immunosuppressive microenvironment of initial and recurrent glioblastoma. Front Immunol. 2020;11:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grahovac J, Srdić‐Rajić T, Francisco Santibañez J, Pavlović M, Čavić M, Radulović S. Telmisartan induces melanoma cell apoptosis and synergizes with vemurafenib. Cancer Biol Med. 2019;16(2):247‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ozeki K, Tanida S, Morimoto C, et al. Telmisartan inhibits cell proliferation by blocking nuclear translocation of ProHB‐EGF C‐terminal fragment in colon cancer cells. PLoS One. 2013;8(2):e56770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang XB, Cai JH, Yang YY, et al. Telmisartan attenuates kidney apoptosis and autophagy‐related protein expression levels in an intermittent hypoxia mouse model. Sleep Breath. 2019;23(1):341‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei X, Hu CC, Zhang YL, Yao SL, Mao WK. Telmisartan reduced cerebral edema by inhibiting NLRP3 inflammasome in mice with cold brain injury. J Huazhong Univ Sci Technolog Med Sci. 2016;36(4):576‐583. [DOI] [PubMed] [Google Scholar]
- 20. Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oura K, Tadokoro T, Fujihara S, et al. Telmisartan inhibits hepatocellular carcinoma cell proliferation in vitro by inducing cell cycle arrest. Oncol Rep. 2017;38(5):2825‐2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siragusa M, Sessa WC. Telmisartan exerts pleiotropic effects in endothelial cells and promotes endothelial cell quiescence and survival. Arterioscler Thromb Vasc Biol. 2013;33(8):1852‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsui T, Chiyo T, Kobara H, et al. Telmisartan inhibits cell proliferation and tumor growth of esophageal squamous cell carcinoma by inducing s‐phase arrest in vitro and in vivo. Int J Mol Sci. 2019;20(13):3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang S, Wang Y. Telmisartan inhibits NSCLC A549 cell proliferation and migration by regulating the PI3K/AKT signaling pathway. Oncol Lett. 2018;15(4):5859‐5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walcher D, Hess K, Heinz P, et al. Telmisartan inhibits CD4‐positive lymphocyte migration independent of the angiotensin type 1 receptor via peroxisome proliferator‐activated receptor‐gamma. Hypertension. 2008;51(2):259‐266. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Chen L, Yu P, Liu B, Zhu J, Yang Y. Telmisartan exerts anti‐tumor effects by activating peroxisome proliferator‐activated receptor‐γ in human lung adenocarcinoma A549 cells. Molecules. 2014;19(3):2862‐2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goyal SN, Bharti S, Bhatia J, Nag TC, Ray R, Arya DS. Telmisartan, a dual ARB/partial PPAR‐γ agonist, protects myocardium from ischaemic reperfusion injury in experimental diabetes. Diabetes Obes Metab. 2011;13(6):533‐541. [DOI] [PubMed] [Google Scholar]
- 28. Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta Neurol Taiwan. 2009;18(4):231‐241. [PubMed] [Google Scholar]
- 29. Wu X, Geng F, Cheng X, et al. Lipid droplets maintain energy homeostasis and glioblastoma growth via autophagic release of stored fatty acids. iScience. 2020;23(10):101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grygiel‐Górniak B. Peroxisome proliferator‐activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J. 2014;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim GB. Treatment of PAH with a PPARγ agonist. Nat Rev Cardiol. 2018;15(7):381. [DOI] [PubMed] [Google Scholar]
- 32. Sugimoto K, Kazdová L, Qi NR, et al. Telmisartan increases fatty acid oxidation in skeletal muscle through a peroxisome proliferator‐activated receptor‐gamma dependent pathway. J Hypertens. 2008;26(6):1209‐1215. [DOI] [PubMed] [Google Scholar]
- 33. Li YQ, Ji H, Zhang YH, Ding DY, Ye XL. Metabolic effects of telmisartan in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(4):264‐270. [DOI] [PubMed] [Google Scholar]
- 34. Debard C, Laville M, Berbe V, et al. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia. 2004;47(5):917‐925. [DOI] [PubMed] [Google Scholar]
- 35. Villa L, Boor P, Konieczny A, et al. Effects and mechanisms of angiotensin II receptor blockade with telmisartan in a normotensive model of mesangioproliferative nephritis. Nephrol Dial Transplant. 2011;26(10):3131‐3143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


