Abstract
To meet the growing demand for chocolate, cocoa (Theobroma cacao) agriculture is expanding and intensifying. Although this threatens tropical forests, cocoa sustainability initiatives largely overlook biodiversity conservation. To inform these initiatives, we analyzed how cocoa agriculture affects bird diversity at farm and landscape scales with a meta‐analysis of 23 studies. We extracted 214 Hedges' g* comparisons of bird diversity and 14 comparisons of community similarity between a forest baseline and 4 farming systems that cover an intensification gradient in landscapes with high and low forest cover, and we summarized 119 correlations between cocoa farm features and bird diversity. Bird diversity declined sharply in low shade cocoa. Cocoa with >30% canopy cover from diverse trees retained bird diversity similar to nearby primary or mature secondary forest but held a different community of birds. Diversity of endemic species, frugivores, and insectivores (agriculture avoiders) declined, whereas diversity of habitat generalists, migrants, nectarivores, and granivores (agriculture associates) increased. As forest decreased on the landscape, the difference in bird community composition between forest and cocoa also decreased, indicating agriculture associates replaced agriculture avoiders in forest patches. Our results emphasize the need to conserve forested landscapes (land sparing) and invest in mixed‐shade agroforestry (land sharing) because each strategy benefits a diverse and distinct biological community.
Keywords: avian, biodiversity, cacao, chocolate, farm, land sparing, meta‐analysis, sustainable, Aves, biodiversidad, cacao, chocolate, conservación de tierras, metaanálisis, sustentable
Short abstract
Article impact statement: Loss of tree and understory plant diversity on cocoa farms and landscapes reduces diversity of endemic, frugivore, and insectivore birds.
Abstract
Impacto de la Intensificación Agrícola del Cacao sobre la Diversidad y Composición de la Comunidad de Aves
Resumen
Para responder a la demanda creciente de chocolate, el cultivo de cacao (Theobroma cacao) se ha expandido e intensificado. Aunque esto es una amenaza para los bosques tropicales, las iniciativas de cacao sustentable en gran medida pasan por alto la conservación de la biodiversidad. Para proporcionar información a estas iniciativas, analizamos como la agricultura del cacao afecta a la diversidad de aves a escala de rancho y de paisaje mediante un metaanálisis de 23 estudios. Extrajimos 214 comparaciones de Hedges g* de la diversidad de aves y 14 comparaciones de la similitud de comunidades entre una línea de base de bosque y 4 sistemas de cultivo que cubren un gradiente de intensificación en paisajes con cobertura de bosque alta a baja, y sintetizamos 119 correlaciones entre características de cultivos de cacao y la diversidad de aves. La diversidad de aves declinó claramente en cultivos con poca sombra. Cultivos con >30% de cobertura de diversos árboles retuvieron una diversidad de aves similar a la de bosques primarios o maduros cercanos, pero presentaron una comunidad diferente. La diversidad de especies endémicas, frugívoras e insectívoras (evasoras de agricultura) declinó, mientras que la diversidad de generalistas de hábitat, migrantes, nectarívoras y granívoras (asociadas a agricultura) incrementó. A medida que decreció el bosque en el paisaje, la diferencia en la composición de la comunidad de aves entre bosque y cacao también decreció, lo que indica que las especies asociadas a la agricultura reemplazaron a las evasoras de la agricultura en los fragmentos de bosque. Nuestros resultados enfatizan la necesidad de conservar paisajes boscosos (conservación de tierras) e invertir en agroforestería de sombra mixta (compartición de tierras) porque cada estrategia beneficia a una comunidad biológica diversa y distinta.
INTRODUCTION
Global biodiversity peaks in tropical regions and is threatened by increasing demand for agricultural commodities (Laurance et al., 2014). With most tropical deforestation driven by agriculture, supply chains face pressure to minimize biodiversity loss while maximizing production and profitability, leading companies to commit to sustainable agricultural practices (Curtis et al., 2018; Rueda et al., 2017). However, such sustainability commitments may not prioritize or ensure tropical biodiversity conservation, given the wide range of environmental, economic, and social issues that fall under the sustainability umbrella (Freidberg, 2017). Even programs that explicitly prioritize biodiversity conservation face challenges to identify best practices because biodiversity responses often vary between regions, landscapes, and farming systems (De Beenhouwer et al. 2013). Quantitative syntheses that account for such variation in responses are critical to guide sustainability initiatives.
Cocoa (Theobroma cacao) is an important tropical commodity farmed on 11.8 million hectares of land, primarily in biodiversity hotspots in West Africa, South America, and Southeast Asia (FAO, 2019) (Figure 1). When cocoa is grown under a native tree canopy, biodiversity of the agroforest can match that of adjacent forest (Faria et al., 2007). Such agroforestry systems contribute to landscape‐level biodiversity by connecting forest patches, facilitating plant and animal dispersal, and providing wildlife habitat (Jose, 2012) and can sustain the productivity of cocoa trees indefinitely (Saj et al., 2017). However, increasing demand for cocoa is driving tropical deforestation (Barima et al., 2016; Kroeger et al., 2017). An estimated 2–3 million ha of tropical forest were converted to cocoa from 1988 to 2008 (Kroeger et al., 2017), and many cocoa agroforestry systems have been intensified through tree reduction or elimination (Clough et al., 2009a). The resulting monocultures tend to collapse under the combined pressures of diseases, pests, and soil degradation, thereby pushing cocoa agriculture into forests with better soils (Clough et al., 2009a; Ruf & Schroth, 2004) and leaving behind impoverished land, biological communities, and human inhabitants (Leakey, 2018).
FIGURE 1.
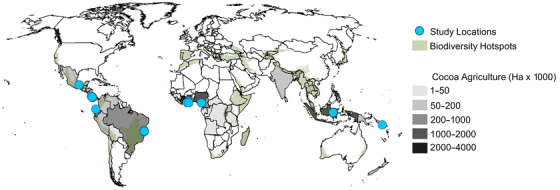
Cocoa agriculture area by country overlaid by biodiversity hotspots and locations of studies used in meta‐analysis of cocoa impacts on bird diversity. Cocoa data from FAOstat (FAO, 2019)
Despite these troubling trends, the cocoa industry is uniquely positioned to adopt and implement global sustainability standards, given high supply chain consolidation (Carodenuto, 2019) and high sustainability certification rates (Uribe‐Leitz & Ruf, 2019). Thirty‐three companies and three governments recently published plans to increase cocoa sustainability and end cocoa‐driven deforestation (Carodenuto, 2019). These plans adopt a sustainable intensification paradigm, calling for increased cocoa yields without additional deforestation or negative environmental impact (Andres & Bhullar, 2016). Intensifying production would theoretically decrease pressure on forests, but explicit biodiversity conservation planning is absent from these plans, especially at the farm level (Cocoa & Forests Initiative, 2019).
Greater clarity about cocoa intensification's effect on biodiversity is needed, given current deforestation rates and projected increases in cocoa demand (Kozicka et al., 2018). Unfortunately, cocoa is poorly represented in quantitative biodiversity reviews compared with coffee and other tropical tree crops (Nájera & Simonetti, 2010; Şekercioḡlu, 2012; De Beenhouwer et al., 2013; Jezee et al., 2017). Case studies show that cocoa management intensity and surrounding forest composition can affect biodiversity (Faria et al., 2007; Bisseleua et al., 2009), yet no study quantitatively synthesizes the impact of cocoa intensification at both farm and landscape scales (e.g., De Beenhouwer et al., 2013). Nearby forest may control the presence of forest‐dependent species in cocoa farms (Clough et al., 2009b), whereas diversity and structure of the tree canopy may affect species diversity at the farm level (Van Bael et al., 2007). Variation in habitat requirements can also lead to major differences in how taxonomic and functional groups respond to intensification (Clough et al., 2009b; Kessler et al., 2009, Newbold et al., 2014). For example, cocoa agroforests can retain the same species richness and abundance of birds as nearby forest (Faria et al., 2007), yet bird species composition may vary dramatically between the two habitats (Greenler & Ebersole, 2015). Global reviews of bird responses to agroforestry suggest that full‐community and insectivore diversity declines across an intensification gradient of forest to agroforest to monoculture, whereas frugivore, nectarivore, and migratory bird diversity is generally greatest in structurally complex agroforestry systems (Nájera & Simonetti, 2010; Şekercioḡlu, 2012). Accounting for such guild‐level responses to landscape and farm management is critical to understand how the proposed sustainable intensification of cocoa will affect biodiversity.
We conducted a quantitative meta‐analysis and review of bird responses to cocoa agriculture that accounted for intensity of farm management and landscape composition. We focused on birds because they are well studied at guild and community levels in cocoa‐growing regions and are strong indicators of ecosystem functioning (Renwick et al., 2012). We tested two hypotheses: diversity of bird communities and some guilds decline over a gradient of agricultural intensification (rustic cocoa, mixed shade cocoa, low shade cocoa, and annual monoculture) relative to nearby native forest, and avian diversity is correlated with both farm‐ and landscape‐scale habitat features. We also compared bird responses to intensification among geographic regions and biodiversity indicator metrics because response to intensification may vary among regions (De Beenhouwer et al., 2013) or the indicator metrics employed in the analysis (Kessler et al., 2009; Santini et al., 2017). We used our results to provide specific recommendations to conserve biodiversity within cocoa agricultural areas.
METHODS
Literature review
We searched relevant Web of Science databases (CABI, ZOOREC, WOS, SCIELO, BCI, BIOSIS, and CCC) for literature from 1995 to 2019 with TS = ((“cocoa” OR “cacao”) AND (avi* OR bird* OR biodivers*)). Databases accessed covered the full time span, except for CCC (1998–present) and SCIELO (2002–present). We reviewed titles and abstracts of the 952 returns and excluded studies that did not report bird community or diversity metrics in a cocoa agricultural system. We reviewed the full text and cited and citing literature of 42 retained articles written in English and Spanish. We identified 4 additional articles that met inclusion criteria and excluded 23 studies because they only reviewed other studies or lacked error estimates for reported biodiversity metrics. Sixteen studies that compared bird biodiversity indicator metrics (e.g., species richness, abundance, diversity, and community similarity indices) between forest and at least one adjacent cocoa system were retained for meta‐analysis. Fourteen studies were retained that reported relationships between bird indicator metrics and continuous habitat covariates at the cocoa farm or landscape scale (see Appendix S1 for PRISMA flow diagram).
Data compilation
We compiled a data set for meta‐analysis with comparisons of bird biodiversity indicator metrics and variances among forest and adjacent agricultural systems (directly adjacent to a few km away) that included cocoa. We retained three biodiversity indicator metrics—species richness, abundance, and Shannon's index—and excluded metrics not reported from multiple studies (e.g., evenness and functional diversity indices). We preferentially retained estimated metrics over observed metrics to account for sampling biases if studies reported both. We used the online Web Plot Digitizer (Rohatgi, 2019) to extract numerical values from figures, including supplemental information and appendices. Nearly, all community similarity analyses reported index values without metrics of variance or ordination analyses with noncomparable axes, so we compiled a separate data set with similarity indices, excluding ordinations. Finally, we compiled a data set with signs and significance values of correlations between bird biodiversity and continuous habitat covariates for qualitative analysis. We determined this data set was inappropriate for meta‐analysis because it lacked comparable correlation coefficients, variance metrics, and habitat covariates assessed across studies. We retained data from farm‐level habitat covariates that described the shade canopy above cocoa––canopy cover, canopy height, tree density, Shannon's index of trees, tree species richness, and vertical structural diversity––and the cocoa understory––leaf litter, herbaceous ground cover, Shannon's index of understory plants, and understory species richness. We also retained landscape‐level covariates that described distance to forest or percent forest on the landscape. We excluded other habitat covariates given low management relevance.
Data classification
For each comparison in the meta‐analysis data set, we classified the bird group studied as full community (i.e., all bird species) or guild (i.e., groups of species with shared characteristics). We excluded guilds based on family, genera, or foraging strata due to low representation (reported in ≤2 studies). The retained foraging guilds were composed of unique species (frugivore, granivore, insectivore, nectarivore, and omnivore), but some species may have overlapped those included in retained life‐history guilds (biome or regional endemics, forest specialists, habitat generalists, and migrants).
We classified land cover as native forest based on author descriptions (including intact primary, disturbed primary, and mature secondary forest) or one of the following agricultural systems: rustic cocoa agroforestry (cocoa under a native shade canopy of retained primary forest trees , typically > 60% canopy cover), mixed cocoa agroforestry (cocoa under a mix of planted shade and fruit trees with some retained forest trees, typically 30–50% canopy cover), low shade cocoa (intensified cocoa plantation with 0–2 species of nitrogen‐fixing, fruit, or timber trees, typically 0–20% canopy cover), or annual monoculture (annual commodity crops cultivated without shade). Cocoa classifications followed Rice and Greenberg (2000), but we divided the planted shade cocoa category into mixed shade (analogous to commercial polyculture [Perfecto et al., 2007]) and low shade, which includes monospecific timber shade and legume service shade (Somarriba & Beer, 2011). Abandoned cocoa was excluded given low industry applicability. We classified landscapes as high forest (>40% primary forest in a 10‐km radius around a study site) or low forest (<39.9% forest) based on author descriptions. If landscape composition was not reported, we extracted the study location from the study text or maps and calculated forest cover in a 10‐km radius around a study location in ESRI ArcMap 10.6. We used the Primary Humid Tropical Forest raster for the year 2001 (Turubanova et al., 2018) and subtracted forest loss pixels from the University of Maryland Forest Loss raster between the year 2000 and the study year (Hansen et al., 2013).
Finally, we classified each study as belonging to one of three regions: Latin America, Southeast Asia, and West Africa. In total, we identified 214 comparisons of biodiversity indicator metrics between forest and adjacent agriculture and 14 comparisons of bird community similarity between land‐cover classes (Appendix S2).
Data analyses
We calculated a bias‐corrected, Hedges’ g* statistic of effect size (Borenstein et al., 2011) for each comparison between a native forest baseline and an adjacent agricultural system. We tested heterogeneity of the full data set and subsets of bird and habitat groups with fixed‐effect meta‐analysis. The values for I 2 and Q indicate substantial heterogeneity for all groups, justifying analysis with random effects and meta‐regression (Appendix S3). We thus fit sets of linear mixed‐effects models with study as a random effect and inverse‐variance weighted Hedges’ g* as the dependent variable with the packages meta 4.9.9 (Balduzzi et al., 2019) and metafor 2.1.0 (Viechtbauer, 2010) in R (R Core Team, 2017). For all models, we calculated fit statistics with package dmetar 0.0.9 (Harrer et al., 2019), used an Akaike information criteria (ΔAICc) cutoff of 2 to identify supported models (Burnham & Anderson, 2004), and assessed unexplained heterogeneity of supported models with I 2 and QE statistics.
To determine whether diversity of the full bird community varied significantly between each agricultural system and whether response varied among biodiversity metrics, we compared a null model and models with agricultural system, biodiversity metric, and the interaction as independent variables. We pooled biodiversity metrics if the model gave no support for variation in effect size among metrics. We determined whether guilds responded differently to agricultural systems by comparing a set of models with guild, biodiversity metric, agricultural system, and the interactions as independent variables. In this model set, the interaction between agricultural system and guild was supported (Appendix S7), but interaction models did not provide useable confidence intervals around Hedges’ g* estimates around categorical comparisons. We, therefore, fit no‐intercept models for each guild with agricultural system as the predictor to determine whether Hedges’ g* for each category differed from zero. Using the results of this analysis, we classified each guild as an agriculture avoider if Hedges’ g* estimates were >0 at p<0.05 or an agriculture associate if estimates were significantly <0 for at least one agricultural system. These designations indicate average guild trends and do not imply that all species in the guild respond similarly to cocoa agriculture. Next, we determined whether guilds responded differently to cocoa in high and low forest landscapes with an intercept model with landscape composition as the independent variable for full bird communities and agriculture avoider and associate groups. Finally, we determined whether birds responded differently to agriculture among regions by comparing a model set with a null, region, agricultural system, and the interaction as predictors. Negative Hedges’ g* values indicated higher diversity in the agricultural system than the forest baseline.
For the community similarity data set, case studies reported a variety of similarity indices, precluding a direct comparison across all values. Sorensons’ index was the most frequently reported similarity index, and we compared those values with a nonparametric Kruskal–Wallis rank sum test across agricultural systems ordered by intensification. For the habitat correlation data set, we plotted number of case studies reporting positive, negative, or nonsignificant relationships with on‐farm habitat features. Correlations with landscape‐level covariates (e.g., distance to forest) were rarely analyzed, so we report those qualitatively in the Discussion.
RESULTS
Sixteen studies reported comparisons of bird biodiversity metrics between forest and cocoa agriculture (n = 45 comparisons for full bird communities and n = 169 comparisons for guilds). For full bird communities, model selection indicated that biodiversity varied significantly among agricultural systems regardless of the biodiversity indicator metric used (Appendix S4). In the supported model, biodiversity indicator metrics in rustic and mixed cocoa agroforestry systems were similar to nearby forest baselines, whereas low shade cocoa and monoculture had significantly lower biodiversity indicator metrics than forest (Figure 2 & Appendix S5).
FIGURE 2.
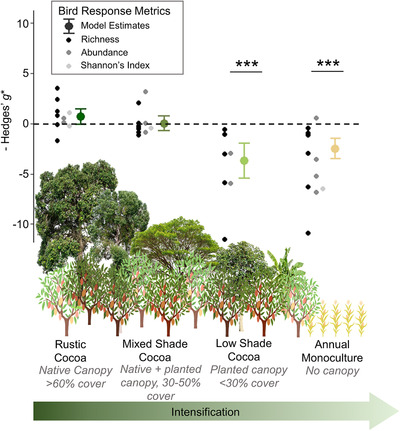
Mean effect size differences in three bird biodiversity metrics between forest and four farming systems of increasing intensity (>0, greater biodiversity in the farm than forest; <0, less biodiversity in the farm than forest; ***, model estimate of Hedges’ g* significantly different from 0 at p< 0.001; model estimate tails, 95% CIs). Data compiled from 16 studies of biodiversity in cocoa.
Community similarity
Seven studies reported 14 comparisons of bird community similarity between native forest and agricultural systems. The Sorensens’ similarity index was used in 67% of comparisons and showed decreasing community similarity relative to forest with increasing land‐use intensification: average Sorensens’ index (SE) = 0.68 (0.10) for rustic shade cocoa, 0.60 (0.14) for mixed shade cocoa, and 0.35 (0.10) for annual monocultures (Appendix S6). A Kruskal–Wallis rank sum test indicated a trend of decreasing community similarity with intensification (χ = 5.54, df = 2, p = 0.06).
Bird guilds
The changes in community similarity were reflected in the divergent responses of bird guilds to cocoa agriculture; both additive and interaction models were supported between guilds and agricultural system (Appendix S7). As for full communities, models testing whether bird response varied with indicator metric were not supported (Appendix S7). The top model showed diversity of endemic birds and frugivores was significantly lower in all agricultural systems than nearby forest baselines (Figure 3a). Insectivore diversity declined significantly in all agricultural systems except rustic cocoa, which maintained similar diversity to forest (Figure 3a). Granivores, generalists, migrants, and nectarivores showed similar or greater diversity in agriculture than forest (Figure 3b). Generalists were more diverse in rustic and mixed shade cocoa agroforests than forest, whereas granivore diversity was greater than forest in mixed shade and monoculture, but not rustic cocoa (Figure 3b). Migrant and nectarivore diversity was only greater than forest in mixed shade cocoa (Figure 3b). We found no support for differences in diversity of forest specialists and omnivores between agricultural systems and forest, although forest specialists were unstudied in low shade cocoa and monoculture (Figure 3c & Appendix S8). Heterogeneity in effect sizes among cases was well explained by the models for endemics and granivores (I 2 < 31%), but all other guilds retained substantial unexplained heterogeneity after models were fit (I 2 > 87%; Appendix S8). High heterogeneity was expected due to differences in sites, data collection methods, analysis methods, and bird community composition, but high heterogeneity supported interpretation of results as general trends rather than precise effect size differences.
FIGURE 3.
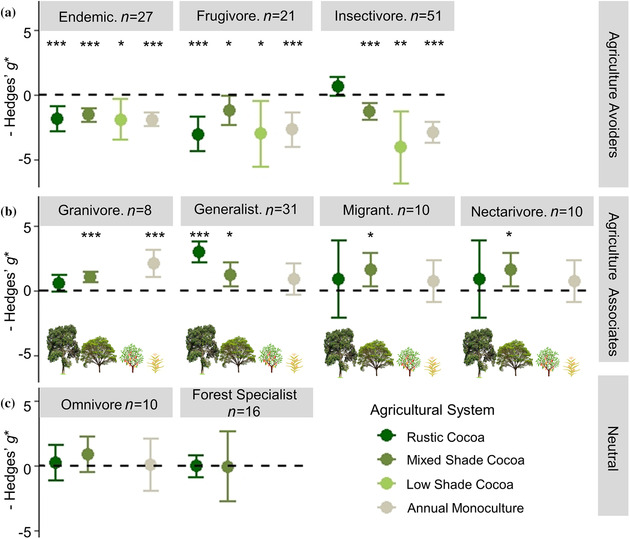
Mean effect size differences in biodiversity metrics between forest and four farming systems of increasing intensity for nine bird guilds classified as (a) agriculture avoiders (endemic species, frugivores, and insectivores) when biodiversity is lower in agricultural systems than forest, (b) agriculture associates (habitat generalists, migrants, nectarivores, and granivores) when biodiversity is greater in agricultural systems than forest, and (c) neutral when no difference was recorded between forest and agriculture (>0, greater biodiversity in cocoa system than forest; <0, less biodiversity in cocoa system than forest; asterisks, estimated Hedges’ g* significantly different from 0 at *p< 0.05, **p< 0.01, or ***p < 0.001; model estimate tails, 95% CIs). Data compiled from 16 studies of biodiversity in cocoa.
Farm‐level habitat
Fourteen studies reported relationships between on‐farm habitat features and bird diversity. Full bird communities and agriculture avoiders (endemics, frugivores, and insectivores) were well studied (n = 51 and n = 44 cases, respectively) compared with agriculture associates (habitat generalists, migrants, nectarivores, and granivores, n = 24). Diversity of full bird communities was positively correlated with canopy metrics in 70% of studied cases, understory metrics in 60% of cases, and with both tree and understory plant diversity in all cases (Figure 4a). Understory plant metrics were poorly studied compared with canopy metrics across bird groups, although understory plant richness and diversity were positively correlated with all bird groups in all studied cases (Figure 4). Canopy tree richness and vertical structural diversity were also positively correlated with diversity for all bird groups (Figure 4). Basal area was the only canopy metric that never positively correlated with bird diversity, and it was negatively associated with agriculture associate diversity in half of the cases (Figure 4).
FIGURE 4.
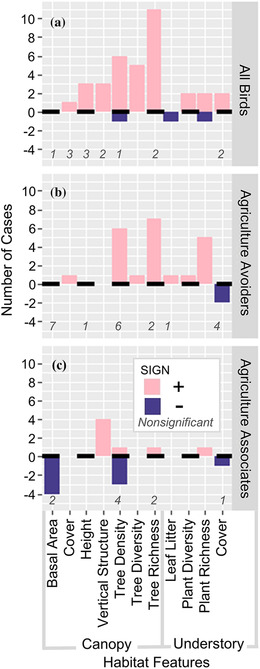
Number of correlations (significant at p< 0.05) between bird biodiversity metrics and continuous habitat features managed on cocoa farms for (a) all birds, (b) guilds that avoid agriculture (endemics, frugivores, and insectivores), and (c) guilds associated with agriculture (generalists, migrants, granivores, and nectarivores) (>0, positive relationship; <0, negative relationship; italicized numbers, nonsignificant cases). Data compiled from 14 studies of biodiversity in cocoa.
Landscape effects
Magnitude of effect size differences in bird diversity between cocoa systems and forest was conditional on landscape composition for both agriculture avoider and agriculture associate groups (Figure 5 & Appendix S9). Relative to nearby forest baselines, agriculture associates gained significantly more diversity in agricultural systems when forest comprised >40% of the landscape composition (Figure 5 & Appendix S10). Agriculture avoiders showed the opposite pattern, with significantly lower diversity in cocoa relative to forest when landscapes were highly forested (Figure 5). Landscape was not a supported covariate for full bird communities (Figure 5 & Appendix S9).
FIGURE 5.
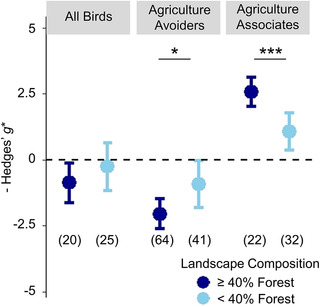
Mean effect size differences in bird biodiversity metrics between forest and cocoa agriculture for all birds, guilds that avoid agriculture (endemics, frugivores, and insectivores), and agriculture associates (granivores, generalists, migrants, and nectarivores) in landscapes with high (>40%) and low (<40%) amounts of primary forest in a 10‐km radius around the study site (asterisks, estimated Hedges’ g* values significantly different between landscapes for each group at *p< 0.05 and ***p< 0.001; whiskers, 95% CIs around model estimates). Data compiled from 16 studies of biodiversity in cocoa.
Geographic region
Region was a supported covariate in models to explain bird responses to cocoa agriculture for full bird communities but not for agriculture avoider or associate groups (Appendix S11). However, estimates of mean effect size differences in full bird community metrics between forest and agriculture did not show meaningful, regional differences for full bird communities (Appendix S12). For full communities, sampling of agricultural systems was uneven between regions, with rustic cocoa primarily studied in Latin America, low shade cocoa studied exclusively in Southeast Asia, and West Africa underrepresented in general (Appendix S12).
DISCUSSION
Our results showed that intensification of cocoa agroforestry into low or no shade systems caused large losses in bird diversity, whereas mixed shade and rustic cocoa agroforestry maintained bird diversity similar to forest. However, some forest species were lost in all cocoa agricultural systems because even rustic cocoa retained only an average of 68% community similarity to nearby forests. Differences in community composition resulted from divergent responses of bird guilds to cocoa intensification. Relative to forest, the diversity of endemic, frugivore, and insectivore birds declined in rustic and mixed shade cocoa, whereas generalist, migrant, nectarivore, and granivore diversity increased. This compensatory mechanism appeared absent in low shade cocoa plantations, given large declines in bird diversity relative to native forest. Low shade, intensified cocoa, therefore, possessed low biodiversity conservation potential, whereas intermediate to high shade systems may support a diverse but distinct biological community relative to native forest.
Habitat structure of the shade canopy and understory positively predicted bird diversity on cocoa farms in most cases. Positive correlations with canopy tree density, richness, and diversity were particularly well documented for full communities and agriculture avoider guilds, as was understory plant richness for avoider guilds. Interestingly, canopy cover was a poorly studied predictor of bird diversity, despite being an evaluation metric in several third‐party sustainability certifications (Waldron et al., 2015; Newsom et al., 2017). All but two studies showed linear relationships between bird diversity and canopy metrics, but in Ecuador, bird species richness peaked in cocoa with 35–40% shade cover (Waldron et al., 2012), whereas >20% native tree cover in Indonesian cocoa was required to retained forest birds (Sodhi et al., 2005). Although the relative importance of distinct canopy metrics varies among locations (Clough et al., 2011; Van Bael et al., 2007), most studies agreed that bird diversity increased linearly with the richness, diversity, and density of canopy trees. This finding stresses the conservation importance of managing cocoa farms as multispecies and multistrata agroforestry systems.
Landscape composition around cocoa farms affected the bird guild diversity relationships between cocoa and nearby forest. The diversity differences in agriculture associate and agriculture avoider guilds between cocoa and forest were less pronounced in landscapes with low forest composition than landscapes with high forest. In cocoa farms within highly forested landscapes, agriculture avoider guilds had significantly less diversity than in forest, whereas agriculture associate guilds showed greater diversity. Because the diversity relationship between cocoa and forest did not vary with landscape for the full bird community, these findings suggest that agriculture avoiders were lost from the forest patches in low forest landscapes and replaced by agriculture associates. The alternative explanation—agriculture avoiders preferentially select cocoa farms in low‐forest landscapes and vice versa—is improbable given the habitat correlations we documented between agriculture avoider guilds and tree diversity and density. These findings highlight the importance of conserving not just forest patches, but highly forested landscapes for guilds that generally avoid cocoa agriculture. Cocoa industry commitments to zero‐deforestation support this conservation action (Carodenuto, 2019) and could maximize their impact by focusing on landscapes that retain at least 40% forest.
Findings from case studies further support the importance of highly forested landscapes for guilds that avoid cocoa agriculture. In Brazil, forest specialists, frugivores, and insectivores declined sharply in landscapes with less than approximately 50% primary forest cover (Morante‐Filho et al., 2016), whereas approximately 74% forest was required to retain those guilds in Cameroon (Kupsch et al., 2019). Research from Ghana and Indonesia reported linear declines in those guilds and surprisingly nectarivores (which we found had equal or greater diversity in cocoa than forest) as distance to forest increases (Clough et al., 2009b; Clough et al., 2011; Deikumah et al., 2017). Given differences in the methods and spatial scales of these studies, we recommend further research to quantify the interactions and critical thresholds among forest cover, cocoa cover, and biodiversity. Such research should focus on landscapes with 40–74% cover of natural forest because ecological integrity of the forest becomes uncertain in those landscapes (Morante‐Filho et al., 2016, Kupsch et al., 2019). Such research would help tailor industry zero‐deforestation or reforestation commitments to the scales and landscape configurations with greatest potential to conserve endemic, at‐risk, and forest‐dependent species.
The conservation value of cocoa agriculture depends on the diversity, composition, and fitness of the biological community that occupies it. As with shade coffee, mixed shade cocoa retained a greater diversity of migratory birds than forest (Philpott et al., 2008). In the Americas, migratory birds have declined continuously since at least 1970, making them a group of conservation concern (Rosenberg et al., 2019). Migratory bird abundance and species richness in cocoa was positively correlated with vertical canopy structure (Estrada & Coates‐Estrada, 2005), indicating that cocoa agroforestry can be managed to conserve members of this guild and other species of conservation concern with similar habitat requirements. However, agroforests may contain ecological traps (Sánchez–Clavijo et al., 2020); thus, future studies that quantify the survival and demographics of the species or guilds that occupy these agroforests would greatly increase understanding of the potential benefits and consequences they pose to biodiversity.
Among guilds that avoided agriculture, frugivores and endemic species were particularly sensitive to cocoa agriculture. Frugivore diversity in other tropical agroforestry systems can exceed the diversity in forest (Nájera & Simonetti, 2010; Şekercioḡlu, 2012), suggesting that frugivores are uniquely sensitive to cocoa agriculture, perhaps due to their species composition in cocoa regions or unique habitat features of cocoa agroforests. Insectivores, which provide important pest regulation services (Van Bael et al., 2007), maintained similar diversity in rustic cocoa and forest. However, understory insectivores are highly sensitive to tropical forest disturbance (Şekercioḡlu, 2012; Van Bael et al., 2007), suggesting that species composition within the guild likely shifted between forest and rustic plantations. Agriculture avoider diversity was also positively associated with manageable features of cocoa farming systems, including understory plant diversity and richness. However, active weed suppression on cocoa farms may hinder the adoptability of recommendations to retain understory plants, and off‐farm conservation areas are likely required to maintain a diverse understory bird assemblage. Forest specialists were remarkably underrepresented in our metanalyses as an artifact of the guilds that the case studies reported on. Although we found no difference in their diversity between forest and rustic cocoa, broader reviews show that agricultural intensification drives biodiversity loss of forest specialists (De Beenhouwer et al., 2013), which indicates that caution should be taken not to overinterpret this result.
Our results reveal two complimentary strategies by which cocoa sustainability initiatives can support biodiversity conservation. The first is targeting zero‐deforestation commitments at highly forested landscapes that still retain high diversity of species and guilds that generally avoid cocoa agriculture or agricultural landscapes. The ambitious zero‐deforestation commitments by industry groups have already started the process and achieved government support in several countries that lead global cocoa production (Carodenuto, 2019; Cocoa & Forests Initiative, 2019). Achieving similar commitments in other cocoa‐producing countries and, most importantly, achieving successful and permanent implementation of these policies should continue to be priorities for all participants in cocoa supply chains.
The second strategy is protecting and implementing rustic and mixed shade agroforestry systems that maintain a diversity of tree species and vegetation structures. This strategy conserves different birds than zero‐deforestation policies and may be at odds with the industry push toward sustainable intensification. Cocoa agricultural intensification has led to the widespread prevalence of cocoa monocultures with low biodiversity value (Clough et al., 2009a), and it is currently unclear if sustainable intensification commitments will change that trend. Prior industry efforts to maximize cocoa yields have been criticized for overlooking farmer initiatives to diversify production within the farming area that could increase the ecological diversity and economic viability of the farm unit (Mithöfer et al., 2017). Indeed, industry and government extension programs can drive canopy tree cover well below optimal levels for cocoa production (Waldron et al., 2015; Asare et al., 2019), with negative impacts for farmers and biodiversity. In contrast to intensification, farm‐diversification programs can reincorporate timber species and native trees that produce nontimber products with market value into cocoa agroforests, which provides mutual benefits for farmers and biodiversity (Sonwa et al., 2014).
Potential trade‐offs between cocoa production and shade cover may limit industry interest in retaining and implementing rustic and mixed shade cocoa agroforestry systems. This trepidation may be unfounded, however, because case studies suggest that 30–60% shade tree cover optimizes the trade‐off between cocoa yields and biodiversity conservation (Bisseleua et al., 2009; Waldron et al., 2015; Jezee et al., 2017; Blaser et al., 2018). Similarly, a canopy cover of 30–40% either optimizes or negligibly affects cocoa yields in many regions (Clough et al., 2011; Blaser et al., 2018; Asare et al., 2019), although careful configuration of shade trees and farmer extension programs may be required to achieve maximum yield benefits (Waldron et al., 2015; Andres & Bhullar, 2016). Additionally, mixed and rustic shade agroforestry systems provide greater ecological resilience and ecosystem services than low shade cocoa (Jacobi et al., 2015; Mortimer et al., 2018). As cocoa industry groups and national governments launch programs to meet zero‐deforestation and sustainable intensification commitments, a focus on incorporating a diversity of trees into the cocoa farm system will be critical to linking the conservation of on‐farm biodiversity with landscape‐level biodiversity.
Supporting information
Appendix S1: PRISMA flow diagram for study selection
Appendix S2: Number of paired comparisons of species richness, abundance, and diversity index metrics for bird functional groups between human altered habitats and adjacent native forest. Data compiled from 16 peer‐reviewed articles.
Appendix S3: Test of heterogeneity among effect sizes for the full data set, species groupings of either full community or all guilds, and the full bird community with each of four habitat comparisons to forest.
Appendix S4: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture for full bird communities.
Appendix S5: Model estimates for the top model selected in Appendix S2.
Appendix S6: Bird community similarity index values between native forest and three agricultural systems of increasing intensity, compiled from seven studies.
Appendix S7: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S8: Model estimates for linear mixed effects models comparing mean effect size (Hedges’ g*) of bird response metrics between forest and four agricultural systems for each of nine avian functional groups.
Appendix S9: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S10: Model estimates for linear mixed effects models comparing mean effect size (Hedges’ g*) of bird response metrics between forest and agricultural systems for models with landscape as an independent variable.
Appendix S11: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S12: Mean effect size differences in bird biodiversity response metrics between forest and four agriculture systems for studies of full bird communities in the three study regions.
Additional information is available online in the Supporting Information section at the end of the online article. Queries (other than absence of the material) should be directed to the corresponding author. All data files, R codes, and case study metadata are available from https://osf.io/fd6kc/.
Bennett, R.E. , Sillett, T.S. , Rice, R.A. , Marra, P.P. . (2022). Impact of cocoa agricultural intensification on bird diversity and community composition. Conservation Biology, 36, e13779. 10.1111/cobi.13779
Article impact statement: Loss of tree and understory plant diversity on cocoa farms and landscapes reduces the diversity of endemic, frugivore, and insectivore birds.
LITERATURE CITED
- Andres, C. , & Bhullar, G. S . (2016). Sustainable intensification of tropical agro‐ecosystems: Need and potentials. Frontiers in Environmental Science, 4, 10.3389/fenvs.2016.00005. [DOI] [Google Scholar]
- Asare, R. , Markussen, B. , Asare, R. A. , Anim‐Kwapong, G. , & Ræbild, A . (2019). On‐farm cocoa yields increase with canopy cover of shade trees in two agro‐ecological zones in Ghana. Climate and Development, 11, 435–445. 10.1080/17565529.2018.1442805 [DOI] [Google Scholar]
- Balduzzi, S. , Rücker, G. , & Schwarzer, G . (2019). How to perform a meta‐analysis with R: A practical tutorial. Evidence‐Based Mental Health, 22, 153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barima, Y. S. S. , Kouakou, A. T. M. , Bamba, I. , Sangne, Y. C. , Godron, M. , Andrieu, J. , & Bogaert, J . (2016). Cocoa crops are destroying the forest reserves of the classified forest of Haut‐Sassandra (Ivory Coast). Global Ecology and Conservation, 8, 85‐98. [Google Scholar]
- Bisseleua, D. H. B. , Missoup, A. D. , & Vidal, S . (2009). Biodiversity conservation, ecosystem functioning, and economic incentives under cocoa agroforestry intensification. Conservation Biology, 23, 1176‐1184. [DOI] [PubMed] [Google Scholar]
- Blaser, W. J. , Oppong, J. , Hart, S. P. , Landolt, J. , Yeboah, E. , & Six, J . (2018). Climate‐smart sustainable agriculture in low‐to‐intermediate shade agroforests. Nature Sustainability, 1, 234‐239. [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. , & Rothstein, H. R . (2011). Introduction to meta‐analysis. United Kingdom: John Wiley & Sons. [Google Scholar]
- Burnham, K. P. , & Anderson, D. R . (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261‐304. [Google Scholar]
- Carodenuto, S . (2019). Governance of zero deforestation cocoa in West Africa: New forms of public–private interaction. Environmental Policy and Governance, 29, 55‐66. [Google Scholar]
- Clough, Y. , Faust, H. , & Tscharntke, T . (2009a). Cacao boom and bust: Sustainability of agroforests and opportunities for biodiversity conservation. Conservation Letters, 2, 197‐205. [Google Scholar]
- Clough, Y. , Putra, D. D. , Pitopang, R. , & Tscharntke, T . (2009b). Local and landscape factors determine functional bird diversity in Indonesian cacao agroforestry. Biological Conservation, 142, 1032‐1041. [Google Scholar]
- Clough, Y. , Barkmann, J. , Juhrbandt, J. , Kessler, J. , Wanger, T. C. , Anshary, A. , Buchori, D. , Cicuzza, D. , Darras, K. , Putra, D. D. , Erasmi, S. , Pitopang, R. , Schmidt, C. , Schulze, C. H. , Seidel, D. , Steffan‐Dewenter, I. , Stenchly, K. , Vidal, S. , Weist, M. , Wielgoss, A. & Tscharntke, T. (2011). Combining high biodiversity with high yields in tropical agroforests. Proceedings of the National Academy of Sciences, 108, 8311‐8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocoa & Forests Initiative . (2019). Summary of company initial action plans for Cote d'Ivoire. Cocoa & Forests Initiative. [Google Scholar]
- Curtis, P. G. , Slay, C. M. , Harris, N. L. , Tyukavina, A. , & Hansen, M. C . (2018). Classifying drivers of global forest loss. Science, 361, 1108‐1111. [DOI] [PubMed] [Google Scholar]
- De Beenhouwer, M. , Aerts, R. , & Honnay, O . (2013). A global meta‐analysis of the biodiversity and ecosystem service benefits of coffee and cacao agroforestry. Agriculture, Ecosystems & Environment, 175, 1‐7. [Google Scholar]
- Deikumah, J. P. , Kwafo, R. , & Konadu, V. A . (2017). Land use types influenced avian assemblage structure in a forest–agriculture landscape in Ghana. Ecology and Evolution, 7, 8685‐8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, A. , & Coates‐Estrada, R . (2005). Diversity of Neotropical migratory landbird species assemblages in forest fragments and man‐made vegetation in Los Tuxtlas, Mexico. Biodiversity & Conservation, 14, 1719‐1734. [Google Scholar]
- FAO (Food and Agriculture Organization) . (2019). Food and agriculture data, 2019. Rome: FAO. [Google Scholar]
- Faria, D. , Paciencia, M. L. , Dixon, M. , Laps, R. R. , & Baumgarten, J . (2007). Ferns, frogs, lizards, birds and bats in forest fragments and shade cacao plantations in two contrasting landscapes in the Atlantic forest, Brazil. Biodiversity and Conservation, 16, 2335‐2357. [Google Scholar]
- Freidberg, S . (2017). Big food and little data: The slow harvest of corporate food supply chain sustainability initiatives. Annals of the American Association of Geographers, 107, 1389‐1406. [Google Scholar]
- Greenler, S. , & Ebersole, J. (2015). Bird communities in tropical agroforestry ecosystems: An underappreciated conservation resource. Agroforestry Systems, 89, 691–704. [Google Scholar]
- Hansen, M. C. , Potapov, P. V. , Moore, R. , Hancher, M. , Turubanova, S. A. , Tyukavina, A. , Thau, D. , Stehman, S. V. , Goetz, S. J. , Loveland, T. R. , Kommareddy, A. , Egorov, A. , Chini, L. , Justice, C. O. , & Townshend, J. R. G. (2013). High‐resolution global maps of 21st‐century forest cover change. Science, 342, 850‐853. [DOI] [PubMed] [Google Scholar]
- Harrer, M. , Cuijpers, P. , Furukawa, T. & Ebert, D. D (2019). dmetar: Companion R package for the guide 'Doing meta‐analysis in R'. R package version 0.0.9000. http://dmetar.protectlab.org.
- Jacobi, J. , Schneider, M. , Bottazzi, P. , Pillco, M. , Calizaya, P. , & Rist, S . (2015). Agroecosystem resilience and farmers’ perceptions of climate change impacts on cocoa farms in Alto Beni, Bolivia. Renewable Agriculture and Food Systems, 30, 170‐183. [Google Scholar]
- Jezeer, R. E. , Verweij, P. A. , Santos, M. J. , & Boot, R. G. A. (2017). Shaded coffee and cocoa–double dividend for biodiversity and small‐scale farmers. Ecological Economics, 140, 136‐145. [Google Scholar]
- Jose, S . (2012). Agroforestry for conserving and enhancing biodiversity. Agroforestry Systems, 85, 1‐8. [Google Scholar]
- Kessler, M. , Abrahamczyk, S. , Bos, M. , Buchori, D. , Putra, D. D. , Gradstein, S. R. , Höhn, P. , Kluge, J. , Orend, F. , Pitopang, R. , Saleh, S. , Schulze, C. H. , Sporn, S. G. , Steffan‐Dewenter, I. , Tjitrosoedirdjo, S. S. , & Tscharntke, T. (2009). Alpha and beta diversity of plants and animals along a tropical land‐use gradient. Ecological Applications, 19, 2142‐2156. [DOI] [PubMed] [Google Scholar]
- Kozicka, M. , Tacconi, F. , Horna, D. , & Gotor, E . (2018). Forecasting cocoa yields for 2050. Rome: Biodiversity, International. [Google Scholar]
- Kroeger, A. , Bakhtary, H. , Haupt, F. , & Streck, C . (2017). Eliminating deforestation from the cocoa supply chain. Washington, DC: World Bank. [Google Scholar]
- Kupsch, D. , Vendras, E. , Ocampo‐Ariza, C. , Batáry, P. , Motombi, F. N. , Bobo, K. S. , & Waltert, M . (2019). High critical forest habitat thresholds of native bird communities in Afrotropical agroforestry landscapes. Biological Conservation, 230, 20‐28. [Google Scholar]
- Laurance, W. F. , Sayer, J. , & Cassman, K. G . (2014). Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Evolution, 29, 107‐116. [DOI] [PubMed] [Google Scholar]
- Leakey, R. R. B . (2018). Converting ‘trade‐offs’ to ‘trade‐ons’ for greatly enhanced food security in Africa: Multiple environmental, economic and social benefits from ‘socially modified crops’. Food Security, 10, 505‐524. [Google Scholar]
- Mithöfer, D. , Roshetko, J. M. , Donovan, J. A. , Nathalie, E. , Robiglio, V. , Wau, D. , Sonwa, D. J. , & Blare, T . (2017). Unpacking ‘sustainable’ cocoa: Do sustainability standards, development projects and policies address producer concerns in Indonesia, Cameroon and Peru? International Journal of Biodiversity Science, Ecosystem Services & Management, 13, 444‐469. [Google Scholar]
- Morante‐Filho, J. C. , Arroyo‐Rodríguez, V. , & Faria, D . (2016). Patterns and predictors of β‐diversity in the fragmented Brazilian Atlantic forest: A multiscale analysis of forest specialist and generalist birds. Journal of Animal Ecology, 85, 240‐250. [DOI] [PubMed] [Google Scholar]
- Mortimer, R. , Saj, S. , & David, C . (2018). Supporting and regulating ecosystem services in cacao agroforestry systems. Agroforestry Systems, 92:1639‐1657. [Google Scholar]
- Nájera, A. , & Simonetti, J. A . (2010). Enhancing avifauna in commercial plantations. Conservation Biology, 24, 319‐324. [DOI] [PubMed] [Google Scholar]
- Newbold, T. , Hudson, L. N. , Phillips, H. R. P. , Hill, S. L. L. , Contu, S. , Lysenko, I. , Blandon, A. , Butchart, S. H. M. , Booth, H. L. , Day, J. , Palma, A. D. , Harrison, M. L. K. , Kirkpatrick, L. , Pynegar, E. , Robinson, A. , Simpson, J. , Mace, G. M. , … Purvis, A. (2014). A global model of the response of tropical and sub‐tropical forest biodiversity to anthropogenic pressures. Proceedings of the Royal Society B: Biological Sciences, 281, 10.1098/rspb.2014.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom, D. , Milder, J. C. , & Bare, M . (2017). Towards a sustainable cocoa sector: Effects of SAN/Rainforest Alliance certification on farmer livelihoods and the environment. Rainforest Alliance. [Google Scholar]
- Perfecto, I. , Armbrecht, I. , Philpott, S. M. , Soto‐Pinto, L. , & Dietsch, T. V . (2007). Shaded coffee and the stability of rainforest margins in northern Latin America. Pages 225–261 in Tscharntke T., Leuschner C., Zeller M., Guhardja E., Bidin A. editors. Stability of tropical rainforest margins. Springer, Berlin. [Google Scholar]
- Philpott, S. M. , Arendt, W. J. , Armbrecht, I. , Bichier, P. , Diestch, T. V. , Gordon, C. , Greenberg, R. , Perfecto, I. , Reynoso‐Santos, R. , Soto‐Pinto, L. , Tejeda‐Cruz, C. , Williams‐Linera, G. , Valenzuela, J. , & Zolotoff, J. M. (2008). Biodiversity loss in Latin American coffee landscapes: Review of the evidence on ants, birds, and trees. Conservation Biology, 22, 1093‐1105. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing.
- Renwick, A. R. , Johnston, A. , Joys, A. , Newson, S. E. , Noble, D. G. , & Pearce‐Higgins, J. W . (2012). Composite bird indicators robust to variation in species selection and habitat specificity. Ecological Indicators, 18, 200‐207. [Google Scholar]
- Rice, R. A. , & Greenberg, R . (2000). Cacao cultivation and the conservation of biological diversity. AMBIO: A Journal of the Human Environment, 29, 167‐173. [Google Scholar]
- Rohatgi, A . (2019). WebPlotDigitizer, version 4.2.
- Rosenberg, K. V. , Dokter, A. M. , Blancher, P. J. , Sauer, J. R. , Smith, A. C. , Smith, P. A. , Stanton, J. C. , Panjabi, A. , Helft, L. , Parr, M. , & Marra, P. P. (2019). Decline of the North American avifauna. Science, 366, 120‐124. [DOI] [PubMed] [Google Scholar]
- Rueda, X. , Garrett, R. D. , & Lambin, E. F . (2017). Corporate investments in supply chain sustainability: Selecting instruments in the agri‐food industry. Journal of Cleaner Production, 142, 2480‐2492. [Google Scholar]
- Ruf, F. , & Schroth, G . (2004). Chocolate forests and monocultures: A historical review of cocoa growing and its conflicting role in tropical deforestation and forest conservation. Pages 107–134 in Schroth G., Izac A.M.N., Vasconcelos H.L, Gascon C., Fonseca G.A.B. da, Harvey C.A. editors. Agroforestry and biodiversity conservation in tropical landscapes. Washington, DC: Island Press. [Google Scholar]
- Saj, S. , Jagoret, P. , Etoa, L. E. , Fonkeng, E. E. , Tarla, J. N. , Nieboukaho, J. D. , & Sakouma, K. M . (2017). Lessons learned from the long‐term analysis of cacao yield and stand structure in central Cameroonian agroforestry systems. Agricultural Systems, 156, 95‐104. [Google Scholar]
- Sánchez‐Clavijo, L. M. , Bayly, N. J. , & Quintana‐Ascencio, P. F . (2020). Habitat selection in transformed landscapes and the role of forest remnants and shade coffee in the conservation of resident birds. Journal of Animal Ecology, 89, 553‐564. [DOI] [PubMed] [Google Scholar]
- Santini, L. , Belmaker, J. , Costello, M. J. , Pereira, H. M. , Rossberg, A. J. , Schipper, A. M. , Ceaușu, S. , Dornelas, M. , Hilbers, J. P. , Hortal, J. , Huijbregts, M. A. J. , Navarro, L. M. , Schiffers, K. H. , Visconti, P. , & Rondinini, C. (2017). Assessing the suitability of diversity metrics to detect biodiversity change. Biological Conservation, 213, 341‐350. [Google Scholar]
- Şekercioḡlu, C. H . (2012). Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. Journal of Ornithology, 153, 153‐161. [Google Scholar]
- Sodhi, N. S. , Koh, L. P. , Prawiradilaga, D. M. , Tinulele, I. , Putra, D. D. , & Tan, T. H . (2005). Land use and conservation value for forest birds in Central Sulawesi (Indonesia). Biological Conservation, 122, 547‐558. [Google Scholar]
- Somarriba, E. , & Beer, J . (2011). Productivity of Theobroma cacao agroforestry systems with timber or legume service shade trees. Agroforestry Systems, 81, 109‐121. [Google Scholar]
- Sonwa, D. J. , Weise, S. F. , Schroth, G. , Janssens, M. J. , & Shapiro, H. Y . (2014). Plant diversity management in cocoa agroforestry systems in West and Central Africa—effects of markets and household needs. Agroforestry Systems, 88, 1021‐1034. [Google Scholar]
- Turubanova, S. , Potapov, P. V. , Tyukavina, A. , & Hansen, M. C . (2018). Ongoing primary forest loss in Brazil, Democratic Republic of the Congo, and Indonesia. Environmental Research Letters, 13, 10.1088/1748-9326/aacd1c. [DOI] [Google Scholar]
- Uribe‐Leitz, E. , & Ruf, F . (2019). Cocoa certification in West Africa: The need for change. Pages 435–461 in Schmidt M., Giovannucci D., Palekhov D., Hansmann B. editors. Sustainable global value chains. Cham: Springer. [Google Scholar]
- Van Bael, S. A. , Bichier, P. , Ochoa, I. , & Greenberg, R . (2007). Bird diversity in cacao farms and forest fragments of western Panama. Biodiversity and Conservation, 16, 2245‐2256. [Google Scholar]
- Viechtbauer, W . (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1‐48. [Google Scholar]
- Waldron, A. R. , Justicia, R. , & Smith, L. E . (2015). Making biodiversity‐friendly cocoa pay: Combining yield, certification, and REDD for shade management. Ecological Applications, 25, 361‐372. [DOI] [PubMed] [Google Scholar]
- Waldron, A. , Justicia, R. , Smith, L. , & Sanchez, M . (2012). Conservation through chocolate: A win‐win for biodiversity and farmers in Ecuador's lowland tropics. Conservation Letters, 5, 213‐221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: PRISMA flow diagram for study selection
Appendix S2: Number of paired comparisons of species richness, abundance, and diversity index metrics for bird functional groups between human altered habitats and adjacent native forest. Data compiled from 16 peer‐reviewed articles.
Appendix S3: Test of heterogeneity among effect sizes for the full data set, species groupings of either full community or all guilds, and the full bird community with each of four habitat comparisons to forest.
Appendix S4: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture for full bird communities.
Appendix S5: Model estimates for the top model selected in Appendix S2.
Appendix S6: Bird community similarity index values between native forest and three agricultural systems of increasing intensity, compiled from seven studies.
Appendix S7: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S8: Model estimates for linear mixed effects models comparing mean effect size (Hedges’ g*) of bird response metrics between forest and four agricultural systems for each of nine avian functional groups.
Appendix S9: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S10: Model estimates for linear mixed effects models comparing mean effect size (Hedges’ g*) of bird response metrics between forest and agricultural systems for models with landscape as an independent variable.
Appendix S11: Model selection for linear mixed‐effects models comparing mean effect size (Hedges’ g*) between forest and cocoa agriculture.
Appendix S12: Mean effect size differences in bird biodiversity response metrics between forest and four agriculture systems for studies of full bird communities in the three study regions.
Additional information is available online in the Supporting Information section at the end of the online article. Queries (other than absence of the material) should be directed to the corresponding author. All data files, R codes, and case study metadata are available from https://osf.io/fd6kc/.


