Abstract
Background and Aims
Despite vaccination recommendations, hepatitis B (HBV) and D (HDV) coinfections are common in HIV+individuals.
Methods
HBV immunization status (anti‐HBs) as well as HBV (HBsAg/HBV‐DNA) and HDV (anti‐HDV) coinfection rates were assessed in 1870 HIV+individuals at HIV diagnosis (baseline, BL) and last follow‐up (FU).
Results
Sixty‐eight (3.6%) HIV patients were never tested for HBV. At BL, 89/1802 (4.9%) HIV patients were HBV coinfected. Four hundred and fifteen (23.0%) showed virological HBV clearance [HBsAg(‐)/anti‐HBc(+)/anti‐HBs(+)] and 210 (11.7%) presented with anti‐HBc(+) only. Seven hundred and ten (39.4%) were HBV naïve [HBsAg(‐)/anti‐HBs(‐)/anti‐HBc(‐)/HBV‐DNA(‐)], but only 378 (21.0%) received vaccinations with detectable anti‐HBs(+) titres. Among the 89 HBV/HIV‐coinfected patients, only 52 (58.4%) were tested for HDV: 11/49 (22.4%) had anti‐HDV(+) and 3/12 (25.0%) showed HDV‐RNA viraemia. During a median FU of 6.5 (IQR 7.2) years, 44 (4.6%) of the 953 retested BL HBV‐negative patients acquired new HBV infection (including 15/304, 4.9% of vaccinated patients). Of the 89 patients, 22 (24.7%) patients cleared their HBsAg, resulting in 60/1625 (3.7%) HIV/HBV individuals at FU: 34 (56.7%) showed HBV‐DNA suppression and 15 (25.0%) were HBV viraemic, while 12/89 (13.5%) remained without a FU test. Vaccinations induced anti‐HBs(+) in 137 of the retested 649 (21.1%) BL HBV‐naïve patients.
Conclusion
HBV testing is well established among Viennese HIV+patients with HBV coinfection rates around 4%‐5%. HBV vaccinations are insufficiently implemented since anti‐HBs titres were detected in only 21.1% of HBV‐naive HIV(+) patients and new HBV infections occurred in previously vaccinated patients. HDV testing is not systematically performed despite up to 25% of HIV/HBV patients may show HDV coinfection.
Keywords: epidemiology, HBV, HDV, HIV
Abbreviations
- 3TC
lamivudine
- AIDS
acquired immunodeficiency syndrome
- ALT
alanine aminotransferaseanti‐HBc, anti‐hepatitis B core‐antibodies
- anti‐HBs
anti‐hepatitis B surface‐antibodies
- anti‐HDV
anti‐hepatitis D virus‐antibodies
- ART
antiretroviral therapy
- BL
baseline
- cccDNA
covalently closed circular DNA
- CDC
Center for Disease Control and Prevention
- DNA
deoxyribonucleic acid
- FU
follow‐up
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDV
hepatitis D virus
- HIV
human immunodeficiency virus
- IDU
intravenous drug use
- IQR
interquartile range
- MSM
men who have sex with men
- PCR
polymerase chain reaction
- PWIDs
people who inject drugs
- RNA
ribonucleic acid
- TAF
tenofovir alafenamid
- TDF
tenofovir
- WHO
World Health Organization
Lay summary.
HBV testing was regularly performed in our HIV+cohort, yet systematic HBV vaccination remains insufficient. A number of de novo HBV infections occurred in initially HBV‐vaccinated individuals, suggesting reduced immune response to active immunization. HDV testing was not systematically performed.
Key points.
HBV testing was regularly performed in our Viennese HIV+cohort.
HIV/HBV coinfection prevalence was 4‐5% among our cohort.
Systematic HBV vaccination was insufficient with only 21.1% of HBV‐naive HIV(+) patients showing protective anti‐HBs titers.
HDV testing was not systematically performed in our cohort.
1. INTRODUCTION
Chronic hepatitis B virus (HBV) infection affects 257 million people worldwide and is a common cause for cirrhosis and its complications. 1 , 2 The course of HBV‐associated liver disease is accelerated by the presence of HIV infection, leading to a high rate of cirrhosis, hepatic decompensation and hepatocellular carcinoma in the HIV/HBV coinfected population. 1
Among the 37.9 million people living with HIV in 2018, 5%‐20% are estimated to be coinfected with HBV. 1 , 3 Worldwide 15‐20 million people (5% of those infected with HBV) show coinfection with the hepatitis D virus (HDV). 2 However, the percentages of HIV/HBV coinfection exhibit vast geographical differences, and regional risk groups vary. 1 Austria represents a low‐prevalence country for HBV monoinfection as well as for HIV/HBV and HBV/HDV coinfection, 3 , 4 but recent immigration trends and emerging local risk groups may have changed the national epidemiology of HBV infection. While people who inject drugs (PWIDs) used to represent the majority of the Austrian people living with HIV, recent epidemiological analyses identified new risk groups for HIV and viral hepatitis infection, such as men who have sex with men (MSM). 3 , 5
Due to the high global burden of HBV‐associated liver disease, the WHO has set the goal of HBV elimination by 2030. 6 In order to achieve this public health objective, specific consideration and targeting of risk groups are essential. 7 Especially in HIV‐coinfected patients, who require lifelong antiretroviral therapy (ART) in order to preserve immune function and avoid HIV‐associated disease progression, treatment with HBV‐active ART (ie tenofovir or lamivudine containing regimens) is an effective way of reducing plasma HBV‐DNA levels and the risk for progression of liver fibrosis. 1 Moreover, it may also induce liver fibrosis regression and prevent the development of hepatic decompensation, that is the transition from compensated to decompensated cirrhosis. 8 Finally, the use of HBV‐active ART may also have a protective effect regarding de novo HBV infection. 9 However, due to the persistence of cccDNA and HBsAg, HBV viraemia frequently rebounds after treatment cessation. 1 A cure for chronic HBV infection is not available at the moment. In case of HDV co‐ or superinfection, treatment options are even more limited: While interferon‐based therapy has shown some effect on plasma HDV RNA levels and liver disease progression, its application is limited by considerable rates of contraindications and adverse events and the rebound‐rate after cessation of therapy approximates 75%‐80%. 10 , 11 , 12
Considering these preconditions, systematic screening and prophylactic vaccination against HBV infection represent the most crucial strategies in the reduction of HBV‐associated liver disease. 7 In Austria, a governmental programme to prevent vertical HBV transmission has been in place since 1992 for all pregnant women regardless of citizenship. 13 In addition to the implementation of HBV vaccination in the national children's vaccination programme in 1998 (free of charge for all children up to the age of 15 years living in Austria, irrespective of citizenship), vaccination for HBV has been promoted by national campaigns for all adult people living in Austria, especially emphasizing at‐risk key populations like immunocompromised patients. 14 , 15 HIV‐infected individuals are therefore entitled to HBV vaccination, and screening for protective anti‐HBs antibodies or HBV coinfection should be performed in all patients diagnosed with HIV infection. 7 While HBV vaccination for healthcare workers has been provided free of charge since 1991 in Austria, all other adult individuals—including key population groups like HIV(+) patients—have to cover the costs for HBV vaccination themselves despite the recommendations in place. 15 However, the universal healthcare system in Austria entitles all citizens with or without employment to equal medical care, including screening, treatment and follow‐up programmes regarding infectious diseases. While the improvement of the cross‐management concerning disadvantaged groups like PWIDs and prisoners still remains an issue, these populations should be included in disease‐prevention programs and need specific targeting, for example through the involvement of low‐threshold facilities or the national opioid substitution programme in the case of PWIDs. 16
We aimed to investigate epidemiological HBV trends with and without HDV co‐ or superinfection within the Viennese HIV(+) population in order to provide a basis for elimination programmes for viral hepatitis in this special population.
2. METHODS
2.1. Study design and patient cohort
This study was performed at the Vienna General Hospital, a large tertiary care including 1874 adult patients with an established diagnosis of HIV attending our HIV and/or liver clinics between 1 January 2014 and 31 December 2016. There were no exclusion criteria. Data acquisition was carried out using the available electronic health records. HBV and HDV serology, as well as HBV‐DNA and HDV‐RNA PCR results, were assessed at first examination after HIV diagnosis (defined as the baseline [BL] date) and at last contact at our institution (defined as follow‐up [FU] date) (see Data S1).
2.2. Ethics statement
The study was approved by the ethics committee of the Medical University of Vienna on 7 July 2017 (EC Number: 1527/2017).
3. RESULTS
3.1. Baseline characteristics of the study population
Of 1889 HIV‐infected patients, 1870 were included. The median age in the study population was 36.5 years (IQR 14.8), 1437 (76.8%) patients were male, and 197 (10.5%) presented with HCV viraemia at BL. Among the detected routes of HIV transmission were intravenous drug use (IDU) in 306 (16.4%) patients, 891 (47.6%) were MSM and 673 (36.0%) cases had other or unknown routes of HIV infection. Among the subgroup of ‘other’ routes of HIV transmission were 568 with heterosexual infection, 15 recipients of blood products, 10 patients with haemophilia, 6 patients with nosocomial infection, 2 patients who had experienced vertical transmission and 72 patients for whom the route of HIV infection could not be determined (Figure 1, Table 1).
FIGURE 1.
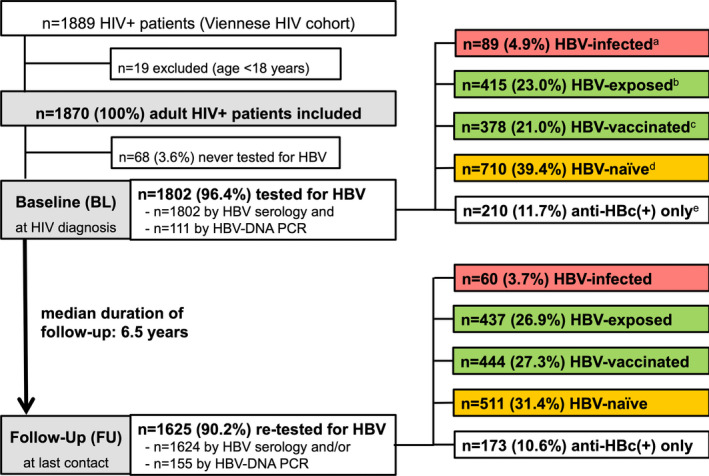
Patient Flow chart. Abbreviations: HIV, human immunodeficiency virus; HBV, hepatitis B virus; BL, baseline; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; FU, follow‐up. adefined as HBsAg(+) and/or HBV DNA(+), anti‐HBc(∓), anti‐HBs(∓). bdefined as HBsAg(‐), anti‐HBc(+), anti‐HBs(+), HBV DNA(‐). cdefined as HBsAg(‐), anti‐HBc(‐), anti‐HBs(+), HBV DNA(‐). ddefined as HBsAg(‐), anti‐HBc(‐), anti‐HBs(‐), HBV DNA(‐). edefined as HBsAg(‐), anti‐HBc(+), anti‐HBs(‐), HBV DNA(‐)
TABLE 1.
Baseline characteristics
| Variable | Overall | PWIDs | MSM | Other |
|---|---|---|---|---|
| Patients (n [%]) | 1870 (100) | 306 (16.4) | 891 (47.6) | 673 (36.0) |
| Sex (n [%]) | ||||
| Male | 1437 (76.8) | 196 (64.1) | 891 (100) | 350 (52.0) |
| Female | 433 (23.2) | 110 (35.9) | 0 (0.0) | 323 (48.0) |
| Age (years) at BL [median (IQR)] | 36.5 (14.8) | 32.1 (12.7) | 37.4 (14.1) | 37.3 (15.9) |
| Advanced Fibrosis at BL (n [%]) according to | ||||
| Transient elastography >9.5kPa | 41 (2.2) | 25 (8.2) | 11 (1.2) | 5 (0.7) |
| Missing values | 1736 (92.8) | 210 (68.6) | 855 (95.6) | 671 (99.7) |
| FIB‐4 score >3.25 a | 72 (3.9) | 22 (7.2) | 23 (2.6) | 27 (4.0) |
| No fibrosis evaluation | 255 (13.6) | 31 (10.1) | 137 (15.4) | 87 (12.9) |
| HCV‐RNA >12 IU/mL at BL (n [%]) | 197 (10.5) | 155 (50.7) | 18 (2.0) | 24 (3.6) |
| Protective anti‐HBs at BL (n [%]) | 793 (42.4) | 108 (35.3) | 464 (52.1) | 221 (32.8) |
| HBsAg(+) at BL (n [%]) | 79 (4.2) | 22 (7.2) | 36 (4.0) | 21 (3.1) |
| HBV‐DNA at BL | ||||
| >12 IU/mL (n [%]) | 38 (2.0) | 11 (3.6) | 16 (1.8) | 11 (1.6) |
| ART (n [%]) | ||||
| No ART at BL, but later | 1799 (96.2) | 296 (96.7) | 849 (95.3) | 653 (97.0) |
| No ART at BL, never ART during FU | 60 (3.2) | 8 (2.6) | 33 (3.7) | 19 (2.8) |
| ART at baseline | 11 (0.6) | 2 (0.7) | 8 (0.9) | 1 (0.1) |
| HIV‐RNA at BL | ||||
| Viral load (log10/mL) [median (IQR)] | 4.6 (1.8) | 4.6 (1.7) | 4.7 (1.8) | 4.6 (1.8) |
| HIV‐RNA suppression (<1.7 log10/mL) (n [%]) | 289 (15.5) | 37 (12.1) | 145 (16.3) | 107 (15.9) |
| Missing values (n [%]) | 49 (2.6) | 4 (1.3) | 34 (3.8) | 11 (1.6) |
| CD4 count at BL (cells/µL) (median [IQR]) | 357 (339.0) | 399 (414.0) | 361 (344.0) | 336 (318.0) |
| Unknown (n [%]) | 40 (2.1) | 2 (0.7) | 27 (3.0) | 11 (1.6) |
| CDC stage at BL (n [%]) | ||||
| A1‐A3 | 1479 (79.1) | 235 (76.8) | 711 (79.8) | 533 (79.2) |
| B1‐B3 | 92 (4.9) | 15 (4.9) | 44 (4.9) | 33 (4.9) |
| C1‐C3 | 139 (7.4) | 26 (8.5) | 57 (6.4) | 56 (8.3) |
| AIDS | 433 (23.2) | 82 (26.8) | 179 (20.0) | 172 (25.6) |
| Unknown | 160 (8.6) | 30 (9.8) | 79 (8.9) | 51 (7.6) |
Abbreviations: AIDS, acquired immune deficiency syndrome; anti‐HBs, anti‐hepatitis B surface antibodies; ART, antiretroviral therapy; BL, baseline; CDC, Centers for Disease Control and Prevention; DNA, deoxyribonucleic acid; FIB‐4, fibrosis‐4 score; FU, follow‐up; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; PWIDs, people who inject drugs; RNA, ribonucleic acid.
Applied upper limit of normal (ULN) for AST and ALT: 35 IU/L (females) and 50 IU/L (males).
The majority of the patients presented at an early stage of HIV infection (CDC stage A in 1479; 79.1%). However, 1227 (65.6%) subjects showed HIV viral loads >4 log10 copies/mL upon HIV diagnosis and 23.2% (433/1870) had AIDS‐defining CD4+ T‐lymphocyte counts below 200 cells/µL. Treatment with combined ART was consecutively initiated in 96.2% (1799/1870) of patients.
According to non‐invasive fibrosis assessment by transient elastography (available in 2.2% [41/1870]) and FIB‐4 score, the vast majority (1502/1615; 93.0%) of the patients did not show advanced liver fibrosis.
3.2. HBV testing
Of the 1870 patients, 1802 (96.4%) received at least 1 HBV test, that is by HBV serology and/or by HBV‐DNA PCR. At BL, all 1802 patients were tested by HBV serology, and 111 (6.2%) received an additional HBV‐DNA PCR. In 36 (32.4%) of the 111 patients who received a PCR test at BL, HBsAg(+) had been detected through HBV serology (Figure 1, Figure 2A).
FIGURE 2.
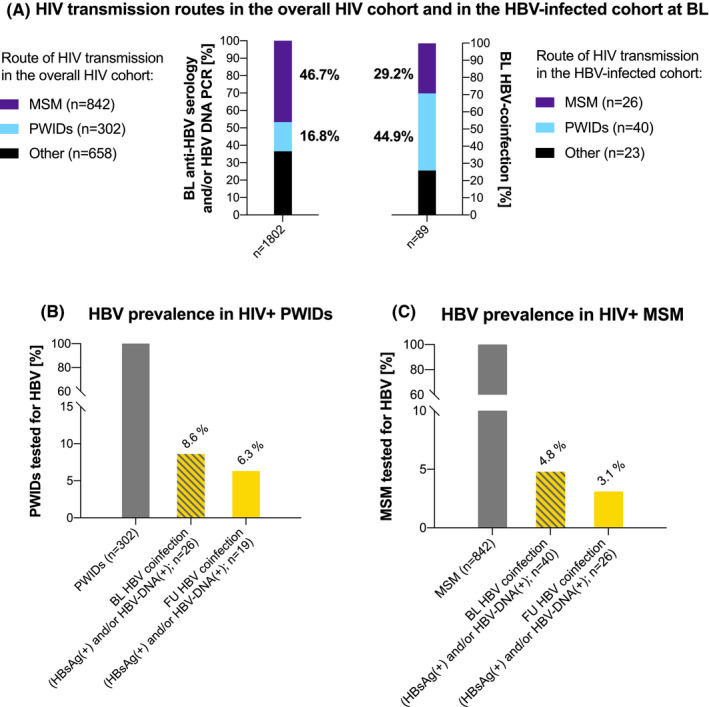
HBV testing and prevalence in the HIV cohort by HIV transmission route. (A) HIV transmission routes in the overall HIV cohort and in the HBV‐infected cohort at BL. (B) HBV prevalence in HIV+PWIDs. (C) HBV prevalence in HIV+MSM. Abbreviations: HBV, hepatitis B virus; HIV, human immunodeficiency virus; BL, baseline; PWIDs, people who inject drugs; MSM, men who have sex with men; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; FU, follow‐up
3.3. HBV coinfection status at BL
Among the 1802 tested at BL, 89 (4.9%) showed HBV coinfection (HBsAg(+) and/or HBV‐DNA(+)): 79 (88.8%) showed [HBsAg(+)] and 38 (42.7%) presented with HBV viraemia (Figure 2A). Of 89 patients, 10 (11.2%) were [HBsAg(‐), HBV‐DNA(+), anti‐HBs(+) and/or anti‐HBc(+)]. The prevalence of HBV coinfection at BL was 26/302 (8.6%) in PWIDs (Figure 2B) and 40/842 (4.8%) in MSM (Figure 2C). Four hundred and fifteen (23.0%; including 67/302 (22.2%) of PWIDs and 220/842 (26.1%) of MSM) and 378 (21.0%; including 41/302 (13.6%) of PWIDs and 244/842 (29.0%) of MSM) patients showed protective anti‐HBs(+) at BL due to previous HBV exposition and HBV vaccination respectively. Seven hundred and ten (39.4%) patients were HBV naïve at BL, including 79/302 (26.2%) of PWIDs and 277/842 (32.9%) of MSM. 210/1802 (11.7%) HIV(+) patients showed only anti‐HBc(+) (Figure 1, Figure 2A‐C).
3.4. HBV coinfection status at FU
Of the 1802 patients, 1625 (90.2%) received at least 1 FU HBV evaluation: 1624 were tested by HBV serology and 155 received an HBV‐DNA PCR. The median FU period was 6.5 years. Overall, 60 (3.7%) patients showed HBV infection at FU, while 437 (26.9%) and 444 (27.3%) had protective anti‐HBs(+) due to previous HBV exposition and HBV vaccination respectively. Five hundred and eleven (31.4%) patients remained HBV naïve, and 173 (10.6%) showed anti‐HBc(+) only at FU (Figure 1, Figure 2, Figure 3A‐B).
FIGURE 3.
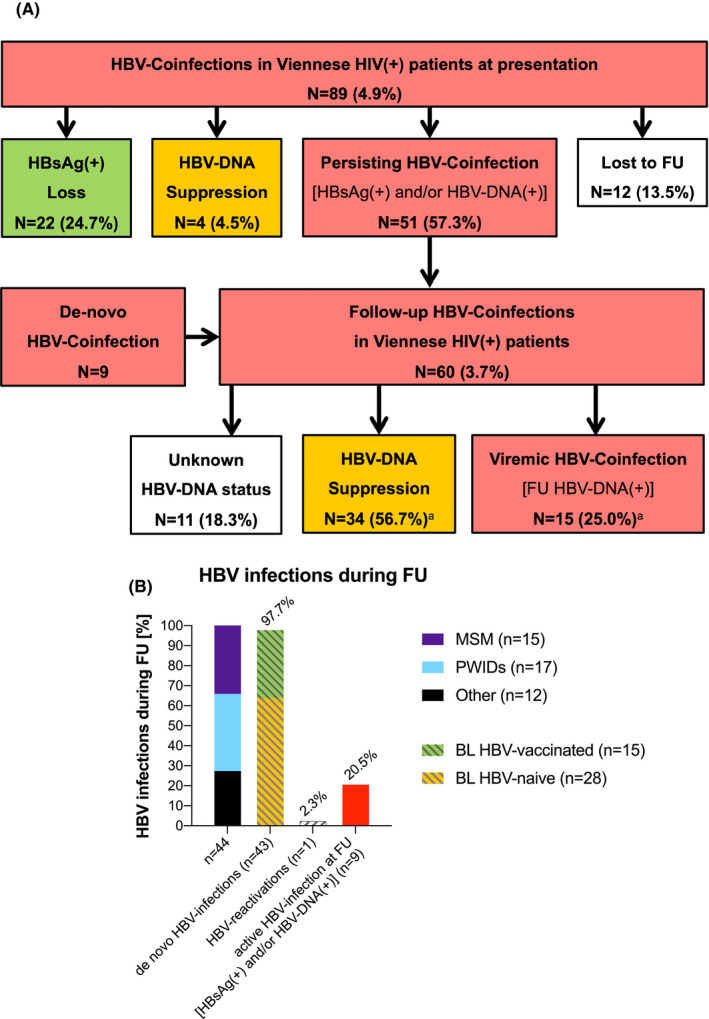
Follow‐up of the HIV‐cohort. (A) FU of BL HBV‐infected patients. (3) HBV infections during FU. Abbreviations: HIV, human immunodeficiency virus; FU, follow‐up; BL, baseline; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; DNA, deoxyribonucleic acid; FU, follow‐up; anti‐HBc, anti‐hepatitis B core antibodies; anti‐HBs, anti‐hepatitis B surface antibodies; MSM, men who have sex with men; PWIDs, people who inject drugs. an=11(18.3%) FU HBsAg(+) patients did not receive a FU PCR test
3.5. FU of BL HBV‐naïve patients
Of the 710 patients who showed [HBsAg(‐), anti‐HBs(‐), anti‐HBc(‐), HBV‐DNA(‐)] at BL, 649 (91.4%) underwent a FU HBV test. One hundred and thirty‐seven (21.1%) patients showed protective anti‐HBs(+) after HBV vaccination while 484 (74.6%) remained HBV naïve. Twenty‐one (3.2%) patients were exposed to HBV during FU: 12 (1.8%) showed [HBsAg(‐), anti‐HBc(+), anti‐HBs(+), HBV‐DNA(‐)] and 9 (1.4%) showed anti‐HBc(+) only at FU. Additionally, 7 (1.1%) patients showed HBsAg(+) and/or HBV‐DNA(+). Overall, the incidence of HBV coinfection among BL HBV‐naïve patients was 28/649 (4.3%) with 10 (13.5%) PWIDs and 12 (4.9%) MSM showing new HBV infection during FU.
3.6. FU of BL HBV‐exposed and HBV‐vaccinated patients
Among 793 patients who showed protective anti‐HBs(+) at BL, no sufficient anti‐HBs(+) titre could be detected in 43 (5.4%) at FU: 29/415 (7.0%) of BL HBV‐exposed patients and 14/378 (3.7%) of BL HBV‐vaccinated patients lost their anti‐HBs(+) during FU. Two of the 378 (0.5%) of BL HBV‐vaccinated patients who lost anti‐HBs(+) during FU showed HBsAg(+) and anti‐HBc(+), respectively, at last contact, and 13/378 (3.4%) BL HBV‐vaccinated patients acquired additional anti‐HBc(+) [HBsAg(‐), anti‐HBc(+), anti‐HBs(+), HBV‐DNA(‐)] indicating HBV exposition during the observation period. At FU, 362/415 (87.2%) of BL HBV‐exposed patients and 276/378 (73.0%) of BL HBV‐vaccinated patients continued to have protective anti‐HBs(+). Of the 793 BL HBV‐immune patients, 98 (12.4%) did not undergo a FU HBV test, including 8 PWIDs and 73 MSM.
3.7. FU of BL HBV‐infected patients
Of the 79 patients who presented with HBsAg(+) HBV infection at BL [HBsAg(+), anti‐HBc(∓), anti‐HBs(∓), HBV‐DNA(∓)], 15 (19.0%) showed HBsAg seroconversion [HBsAg(‐), anti‐HBc(∓), anti‐HBs(+) and HBV‐DNA(‐)] at FU. Additionally, 5/79 were HBV exposed at FU. Of 10 patients with BL [HBsAg(‐), HBV‐DNA(+), anti‐HBs(+) and/or anti‐HBc(+)], 4 showed HBsAg(‐) and HBV‐DNA suppression at FU while one remained HBV viraemic and 5 did not receive a FU PCR test (Figure 3A).
All of the 26 patients who experienced HBsAg loss and/or HBV‐DNA suppression during FU had received HBV‐active ART (1 patient was started on lamivudine (3TC) and switched to tenofovir disoproxil fumarate (TDF)‐based ART, while 25 patients initially received TDF‐based ART: 13/26 were switched to tenofovir alafenamide (TAF)‐based ART and 12/25 had also received 3TC during the FU period; no patient was given entecavir).
Of the 89 patients, 51 (57.3%) continued to show chronic HBV infection at FU (HBsAg(+), anti‐HBc(∓), anti‐HBs(∓), HBV‐DNA(∓)) including 17 PWIDs and 21 MSM. Eleven patients with persisting chronic HBV infection also showed HBV DNA viraemia at FU. Of the 51 patients, 50 (98.0%) had received HBV‐active ART (45 patients received TDF, 30 received TAF and 22 received 3TC) while 1 patient (2.0%) remained without HBV‐active ART.
Of the 89 patients, 11 (12.4%) patients did not receive a FU HBV test, including 4 PWIDs and 4 MSM.
3.8. De novo HBV infections and HBV reactivations during FU
During a median FU of 6.4 (IQR 7.2) years, 43 new cases of HBV exposition were documented via serology (n = 42) or HBV‐DNA PCR (n = 1) among the 953/1088 retested individuals who did not show HBV infection at BL: 28/649 (4.3%) BL HBV‐naïve patients and 15/304 (4.9%) BL HBV‐vaccinated patients. Therefore, HBV incidence within our study population was 4.5% (43/953). Sixteen of the patients with de novo HBV infection were PWIDs, and 15 were MSM. At FU, 8 of the 44 patients who experienced HBV exposition during the observation period showed active HBV infection, defined as [HBsAg(+) and/or HBV DNA(+)] (Figure 3A).
One of the 199 (0.5%) BL anti‐HBc(+) only patients presented with HBV reactivation [HBsAg(+) and HBV DNA(+)] at FU.
3.9. HDV testing and HBV/HDV coinfection status
Fifty‐two (58.4%) of the 89 BL HBV‐infected [HBsAg(+), anti‐HBc(∓), anti‐HBs(∓), HBV‐DNA(∓)] patients were tested for HDV coinfection at least once, including 15 PWIDs and 23 MSM. At BL, 49 patients received an HDV serology, and 12 were tested by HDV‐RNA PCR. In 58.3% of the cases, HDV‐RNA PCR was performed following anti‐HDV(+). Of the 49 patients, 11 (22.4%) presented with anti‐HDV(+) at BL and 3/12 (25.0%) anti‐HDV(+) patients showed HDV‐RNA viraemia. Nine of the 11 BL HBV/HDV‐coinfected patients were PWIDs and 1 was MSM. Of the 11 patients, 7 (63.6%) were Austrian or German, and 1 (9.1%) each were from Romania, Russia, Iran and Cameroon (Figure 4).
FIGURE 4.
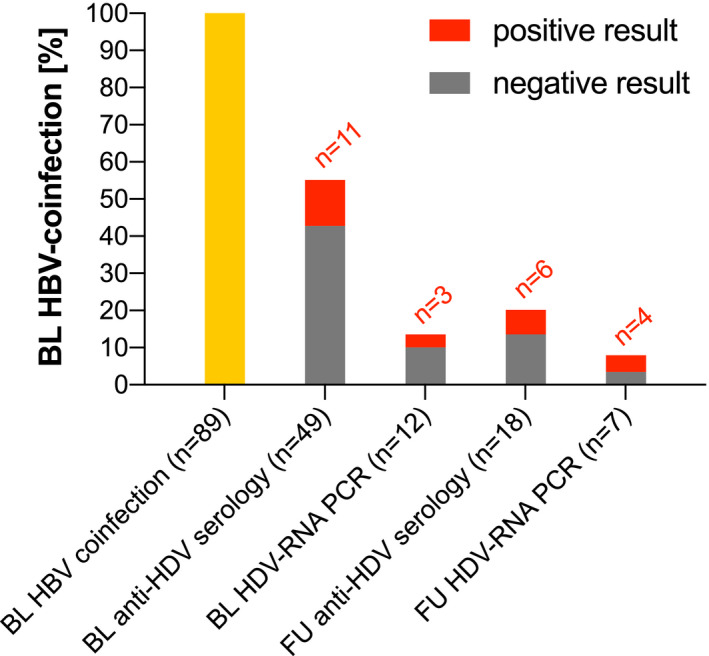
HDV testing in HBV/HIV patients at BL and FU. Abbreviations: HDV, hepatitis delta virus; HBV, hepatitis B virus; HIV, human immunodeficiency virus; BL, baseline; FU, follow‐up; RNA, ribonucleic acid; PCR, polymerase chain reaction
Among the 60 patients who showed HBV infection at FU, 18 were retested for HDV by serology and 7 received a FU HDV RNA PCR after having been tested for HDV at BL already: The 6 patients who showed anti‐HDV at FU and the four who showed HDV viraemia had already been detected at BL. Two of the three BL HDV‐viraemic patients continued to show HDV‐RNA(+), and one did not receive a FU HDV RNA PCR. No new HDV infections were documented during FU.
Of the 89 BL HIV(+) HBV‐infected patients, 37 (41.6%) were never tested for HDV coinfection, including 11 PWIDs and 17 MSM. 2 (4.5%) of the 44 patients with de novo HBV infection during FU were tested by HDV serology at last contact and showed [anti‐HDV(‐)].
3.10. Severity of HBV‐related liver disease at BL and last FU
In 77 of the 89 (86.5%) patients who presented with HBV infection at BL, alanine aminotransferase (ALT) was available. Of the 77 patients, 12 (15.6%) showed an ALT level >2x ULN, suggesting hepatitis, while 49 (63.6%) had an ALT level within the normal range. Among patients with HBV infection at BL, median time to FU was 7.8 years, at which 55 (80.9%) of them showed an ALT level within the normal range.
Among 74 patients for whom a FIB‐4 score was available at BL, 6 (8.1%) showed a FIB‐4 score >3.25, including 1 patient with HBV/HCV coinfection, indicating advanced liver fibrosis. Significant liver fibrosis was ruled out in 52 (70.3%) based on a FIB‐4 score <1.45 at BL. At FU, 2 (3.1%) of the 65 patients who had a FIB‐4 score re‐evaluation showed values suggestive of advanced liver fibrosis while advanced liver fibrosis was ruled out in 50 (76.9%) patients (Figure 5A‐B).
FIGURE 5.
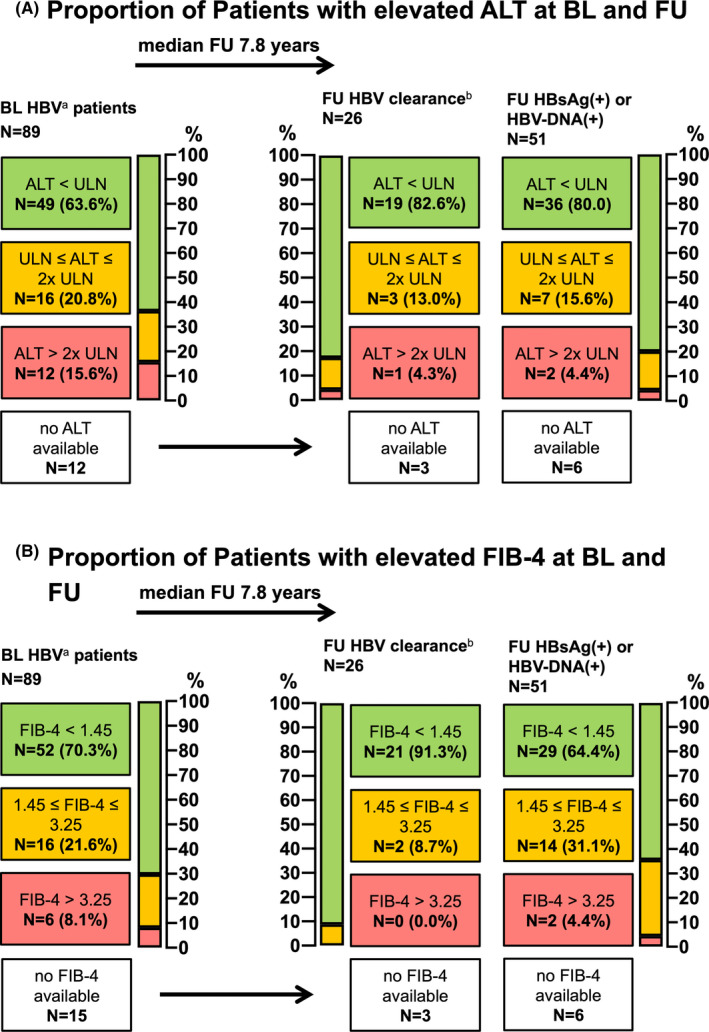
Evaluation of biochemical liver damage and advanced fibrosis by ALT‐levels and FIB‐4 score in HIV+patients with HBV coinfection at baseline. (A) Proportion of Patients with elevated ALT at BL and FU; (B) Proportion of Patients with elevated FIB‐4 at BL and FU; Abbreviations: ALT, alanine aminotransferase (ULN <35 U/L (f); <50 U/L (m)); BL, baseline; FU, follow‐up; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; DNA, deoxyribonucleic acid; ULN, upper limit of normal; FIB‐4, fibrosis‐4 Score. adefined as HBsAg(+) and/or HBV DNA(+), anti‐HBc(∓), anti‐HBs(∓). bdefined as HBsAg(‐), anti‐HBc(∓), anti‐HBs(∓), HBV DNA(‐)
4. DISCUSSION
In line with the WHO goal of eliminating viral hepatitis until 2030, 6 , 7 we aimed to analyse epidemiological trends of HBV and HDV coinfection in our large Viennese HIV(+) patient cohort in order to pave the path for future elimination programmes. To our knowledge, there are no recent Central European data, specifically none from Austria, describing the current epidemiological situation regarding HBV and HDV coinfection in HIV patients. A Canadian Study found an HBV coinfection prevalence of 6.1% in their HIV cohort, with an especially high proportion of MSM (25.8%) among HIV/HBV‐coinfected individuals, while the prevalence of HBV coinfection in a French HIV cohort was 4.5%. 17 , 18 While modern direct‐acting antivirals have revolutionized HCV therapy and now facilitate cure rates of up to 100% in chronically HCV‐infected patients, 19 chronic HBV infection remains a major public health burden and often requires lifelong medical treatment. 1 Screening and treatment uptake are crucial steps in HCV elimination, yet vaccination and protective anti‐HBs titre monitoring currently remain the most important approach in HBV elimination. French data showed a prevalence of protective anti‐HBs(+) in 60.0% of the assessed HIV(+) subjects, while similar data from Austrian HIV patients are missing. 18 In HIV(+) patients, the progression to HBV‐associated cirrhosis is accelerated as compared to HBV‐monoinfected patients. 20 Therefore, HBV vaccination is particularly important in these patients, which is also emphasized in current guidelines. 7 , 14 , 21 Active HBV vaccination also prevents infection with HDV, where currently only very limited treatment options are available. 12 However, with new HDV‐active treatments on the horizon, the assessment of HDV epidemiology—especially in the HIV population that is at high risk for rapid disease progression—has gained importance: HDV coinfection prevalence may be as high as 15.4% among HBV/HIV‐coinfected individuals, as suggested by the Swiss HIV Cohort Study and may be even higher in Austria, according to our data. 22
Potential differences in HBV/HDV coinfection epidemiology among our Viennese HIV(+) cohort as compared to other countries may be attributable to the distinct constitution of our study population: While median age and sex distribution were similar to those reported in British Columbian, French and Swiss cohorts, 17 , 18 , 22 and the percentage of patients with a history of IDU (17.0%) was comparable to the Swiss HIV Cohort Study (16.5%), the percentage of MSM (47.6%) was noticeably higher in our study population as compared to the Canadian cohort (19.3%). Interestingly, the composition of our study cohort with regard to ethnicity varied strikingly from the Swiss report: While the Swiss HIV cohort mainly consisted of north‐western European patients (57.5%), our study population was of predominantly central European (66.7%) descent. The percentages of patients born in sub‐Saharan Africa or Southeast Asia were slightly higher in the Swiss cohort (19.8% and 5.4%, respectively) as opposed to our study (9.6% and 1.7%, respectively), while the percentages of patients with Mediterranean or south‐eastern European background were similar between the Swiss (10.7%) and our Viennese (8.8%) cohort. We furthermore observed a slightly higher percentage of patients from former USSR states in our cohort. These variations in study population composition seem to be mainly due to geographical reasons, including the fact that our cohort consists of an exclusively urban HIV(+) population which may explain the high percentage of MSM among our study population. Accordingly, the pronounced differences in ethnicity between our study population and other international cohorts underline the importance of understanding regional differences in epidemiological data on HIV/HBV/HDV coinfection in order to support effective elimination programmes.
Against current recommendations for this risk group including the Austrian vaccination plan, 7 , 14 , 21 systematic HBV vaccination has not yet been implemented in the Viennese HIV(+) cohort: Only 21.1% of the retested BL HBV‐naive patients developed vaccination‐induced anti‐HBs(+) titres during FU, while 74.6% of the patients were not vaccinated and remained HBV naive. Furthermore, 7.7% of the patients who showed protective anti‐HBs(+) at BL lost their anti‐HBs titres during FU. Interestingly, only 84/183 (45.9%) patients who would have been eligible for HBV vaccination free of charge according to the national children's vaccination programme showed protective anti‐HBs at BL (83/84 were born in Austria, 1/84 had immigrated to Austria after the implementation of the vaccination plan in 1998). While we cannot prove that the remaining 99/183 (54.1%) patients who would have been eligible for HBV vaccination according to the children's vaccination plan but did not show anti‐HBs(+) upon HIV diagnosis had received an HBV vaccination, it seems likely that at least some of these patients may have lost their pre‐existing anti‐HBs(+), potentially due to HIV‐associated immunosuppression. 15 124/762 (16.3%) patients who had immigrated to Austria and were not eligible for HBV vaccination according to the children's vaccination programme showed protective anti‐HBs(+) at BL. Since this is a retrospective study analysing data from two defined points in time (ie HIV diagnosis and last follow‐up) for each patient, we could only analyse vaccination status according to HBsAg/anti‐HBs serostatus but we do not have any data regarding the specific time of vaccination.
However, these findings highlight the importance of follow‐up anti‐HBs titre determinations after HBV vaccination for early detection of the reduction or loss of anti‐HBs titres before HBV coinfection can occur and for following the recommendations for booster doses for this risk group according to the Austrian vaccination plan, and for identification of potential non‐responders to HBV vaccination. Since HIV and HBV share the same transmission routes, counselling on HBV transmission and prophylactic measures aside from HBV vaccination are essential for all HIV(+) patients.
In order to facilitate HBV vaccination in case of HBV‐negative serostatus or to allow initiation of HBV‐active ART in case of HIV/HBV coinfection as early as possible, HBV screening should be performed at the time of HIV diagnosis. At BL, 96.4% of our study population received a screening HBV‐serology, and 6.2% were additionally tested via HBV DNA‐PCR at BL, indicating a sufficient implementation of HBV screening at our clinic. However, 3.6% of our HIV(+) patients were never tested for HBV: In 48 of these 68 (70.6%) patients, the initiation of ART was documented in our HIV clinic's medical records, while in 20 (29.4%) cases no such documentation was available. Considering that many of these HBV‐untested patients did not have any additional medical records at our hospital, these patients may have only received an HIV test at the emergency clinic or non‐elective admission at our centre, while further medical workup may have been performed at a different centre following the communication of the positive HIV test result.
We previously reported a relevant impact of high‐risk sex practices and IDU concerning HCV transmission in Viennese HIV+MSM. 4 While we consider IDU as a major risk factor for the transmission of HBV among HIV+MSM as well, this effect may not appear as pronounced as in HIV/HCV coinfection as 185/432 (42.8%) BL HBsAg(‐) MSM had protective anti‐HBs(+) after previous vaccination: 23/891 (2.6%) MSM reported IDU, including 12 (1.3%) who had received opioid agonist therapy at some point. Among 432 MSM with BL HBsAg(‐) and HBV‐DNA PCR(‐) who received a FU HBV evaluation, 15 (3.5%) showed HBV infection during FU. One (6.7%) of these 15 patients reported IDU. Furthermore, one case of persisting HDV viraemia between BL and FU was observed within the MSM cohort, but this patient had no known history of or current IDU. However, there may be a much higher rate of unreported recreational drug use within our MSM cohort that could not be taken into consideration due to the retrospective study design. Importantly, n = 16 (14.4%) de novo HBV infections and one (0.9%) HBV‐reactivation (BL anti‐HBc(+) only) occurred among 111 BL HBsAg(‐) and HBV‐DNA PCR(‐) PWIDs who were retested for HBV at FU. These differences in HBV infection rate during FU between MSM and PWIDs may be attributable to the higher percentage of protective anti‐HBs(+) after previous vaccination in the MSM cohort (42.8%) vs in the PWID cohort (33.3%).
We furthermore assessed the strategies used for counselling and HBV‐care after diagnosis of HIV/HBV coinfection at our centre: At BL, 4.9% HBV coinfections were detected among our Viennese HIV(+) population, 97.4% of which subsequently received an HBV‐active ART regimen. Due to the retrospective design of the study, the reason why 2/78 patients with a FU evaluation remained without HBV‐active ART remains unclear. However, we cannot exclude that these patients have received HBV‐active ART at another centre or have refused to start ART for personal reasons. 19.0% of BL HIV/HBV‐coinfected patients achieved HBsAg seroconversion under treatment with HBV‐active ART, suggestive of acute HBV infection at BL with clearance during FU.
Based on our findings, the most important measures to improve HBV care for HIV(+) patients in Vienna include systematic vaccination of all HBV‐naive HIV(+) patients. Besides, intensified FU evaluation of HBV‐vaccinated patients regarding their anti‐HBs titres is essential, since our data suggest that protective anti‐HBs(+) titres may often remain at or decline to insufficient levels in HIV(+) patients, resulting in HBV susceptibility despite previous vaccination. For n = 503 HIV+patients who showed HBsAg(‐) at BL as well as at FU, anti‐HBs quantification was performed at least once between 1 January 2015 and last FU: Among n = 238/503 patients who showed anti‐HBs(‐) at FU, 86 (36.1%) had a positive anti‐HBs result (median anti‐HBs titre: 250 (95% CI: 389.3‐655.0) U/mL) available during the observation period (median time period between anti‐HBs quantification and last FU: 9.0 (IQR 9.4) months). While these results do not to prove an accelerated anti‐HBs decline in HIV+patients, it may be assumed. Our results go in line with previous findings of reduced HBV vaccine effectiveness in HIV(+) patients, likely related to HIV‐induced CD4 cell depletion. 23 HBV surveillance by serology may, therefore, also be reasonable in HIV(+) patients with previous HBV exposure. 24 48.6% of the anti‐HBc(+) only patients at BL also tested positive for anti‐HCV(+), potentially suggesting serological interactions of HCV and HBV coinfections in HIV(+) patients, while the shared modes of disease transmission also need to be considered. 2 , 25 , 26 However, isolated anti‐HBc(+) have been frequently reported in HIV(+) patients and may indicate occult hepatitis B with the potential of HBV‐transmission, especially in HIV/HCV‐coinfected individuals. 27 , 28 One BL anti‐HBc(+) only patient showed HBV viraemia and HBsAg(+) at FU, which was recorded as HBV‐reactivation. However, since no HBV genotyping was performed in this patient, we cannot exclude HBV reinfection or de novo HBV infection following the potentially unspecific BL isolated anti‐HBc(+) result. Further studies are needed to assess the clinical relevance of the serological constellation of only anti‐HBc(+). However, testing for anti‐HCV, HCV‐RNA and HBV‐DNA in cases of anti‐HBc(+) only HIV(+) patients seems reasonable. 29
While the prevalence and incidence of HBV/HDV coinfection among HIV(+) remain mostly unclear, the HDV prevalence among HBV patients has been estimated from 13.0% in HBsAg carriers to 26.8% in patients with HBV‐related fulminant hepatitis, cirrhosis or hepatocellular carcinoma respectively. 30 Despite the low HBV prevalence in central Europe, systematic HDV screening revealed a high prevalence among chronically infected hepatitis B patients: Analyses performed within the Swiss HIV Cohort Study showed HDV coinfection rates of up to 18% according to HDV serology in HIV/HBV‐coinfected patients, most of which also showed HDV viraemia (59% of all anti‐HDV(+) who underwent HDV‐RNA PCR testing). 22
Our study cohort represents HIV(+) patients living in an urban setting in central Europe, where migration is an ongoing phenomenon. While the majority of the BL anti‐HDV(+) subjects were Austrian or German, 4/11 (36.4%) reported a personal history of migration from Romania, Russia, Iran and Cameroon. However, bigger studies are needed in order to assess geographical distribution of HDV and HDV‐infected patients' descent, as the small sample of HDV‐infected patients among our HIV(+) cohort does not allow for a representative analysis of geographical origin. Although HDV testing was not systematically performed in our cohort, nearly one third of HIV/HBV patients showed HDV coinfection. Our data suggest a similar anti‐HDV prevalence as described in the literature of 13.0%‐26.8% with HDV viraemia rates of up to 59.0%, 22 , 30 yet screening for HDV coinfection has not been sufficiently implemented in HIV/HBV care: In our subpopulation of HBV/HIV‐coinfected patients, only 30.0% were tested for anti‐HDV, yet 33.3% were HDV viraemic. Since treatment options for HDV are limited and viral recurrence is common upon cessation of interferon‐based treatment, HBV prophylaxis by vaccination remains the most effective and important measure in order to reduce HDV transmission and HDV‐associated morbidity and mortality. However, bulevirtide, a new agent for the treatment of chronic hepatitis D in adults with compensated liver disease has recently received approval by the European Medicines Agency, 31 , 32 , 33 , 34 and thus, the identification of HDV‐infected patients will be even more crucial from now on. While HIV/HBV‐coinfected individuals showed higher rates of thrombocytopenia (68.4%) suggestive of portal hypertension, hyperbilirubinaemia (23.5%) suggestive of hepatic decompensation, and death (9.2%) as compared to the overall HIV(+) cohort, the HIV/HBV/HDV‐coinfected subgroup presented with even more pronounced rates of suspected portal hypertension (80.0%), hepatic decompensation (53.3%) and death (20.0%) (see Table S1). HIV‐associated mortality was most relevant across all groups (overall HIV(+) cohort vs HIV/HBV vs HIV/HBV/HDV), yet 1 (33.3%) of the 3 deaths among the HIV/HBV/HDV‐coinfected subgroup was clearly liver related. These results go in line with data from the Swiss HIV Cohort Study, 22 yet larger studies and sample sizes are needed to validate these findings and to draw firm conclusions. However, these outcome data strongly suggest a clinical relevance of HBV/HDV coinfection in HIV.
Our study has several limitations—first, the retrospective design of the study. Second, due to the absence of standardized testing protocols and heterogeneous schedules of clinical visits, not all parameters of interest could be retrieved in every patient. Furthermore, some patients were lost to FU, and diagnostic procedures performed at other medical institutions could not be taken into consideration. While our aim was to provide numbers on HBV/HDV coinfection among HIV patients to inform future elimination strategies, it is evident that due to geographical, legal and social differences between Austria and other countries, each country will likely have to adapt their approach and find an individually suitable strategy to reach the HBV elimination goal.
However, fostering vaccination programme is an effective measure to support the elimination goal, and this paper demonstrated that it is critical to mandate vaccination not only for early childhood but also for adults in high‐risk groups.
However, we believe that our results still illustrate important epidemiological trends of HBV and HDV coinfection in the Viennese HIV(+) cohort.
While there are various reports on HIV/HBV and HIV/HBV/HDV epidemiology, there are – to our knowledge—no current data from Central Europe or from Austria available, making this the first study to report on current Austrian HIV/HBV/HDV epidemiology trends. Since organizational, legal and reimbursement structures vary greatly between countries, each country or geographical region will need to adapt the course of action suggested here and find an individually suitable approach for HBV prevention and care. However, from our data, we conclude that in order to reach the WHO goal of HBV elimination until 2030 in Austria, it is essential to continue systematic screening for HBV in at‐risk populations, maintain HBV surveillance including retesting for HBsAb in HIV(+) subjects who received HBV vaccinations, and greatly improve HBV vaccination coverage in HIV(+) patients. Especially the increasing of immunization rates may be achieved by raising awareness in health professionals caring for HIV(+) patients and by lowering the access barrier to prevention programs for disadvantaged groups of society, for example, by integrating vaccination programmes into low‐threshold facilities for PWIDs. Furthermore, the insufficient screening for HDV coinfection in HIV/HBV‐infected individuals needs to be addressed and should be overcome by integrating HDV screening into regular HIV surveillance. As HIV patients are managed by several different medical disciplines in Austria (including infectiologists, pulmologists, addiction medicine specialists or dermatologists), a reasonable way to ensure HDV screening in those at risk through HBV infection may be the integration of reflex anti‐HDV assessment in case of first HBsAg(+) and/or HBV‐DNA(+) tests in a given laboratory.
In summary, this study describes epidemiological trends of HBV and HDV coinfection at the largest Austrian HIV treatment centre comprising patients with different transmission risk behaviour (PWIDs, MSM, recipients of blood products, etc). While HBV testing is well established among Viennese HIV+patients, HBV vaccinations are insufficiently implemented with vaccination rates of only 21.1%. The HBV coinfection rate among Viennese HIV+patients slightly decreased from 4.9% to 3.7% during the study observation period. HDV testing is not systematically performed despite the fact that as many as one‐third of HIV/HBV patients may show HDV coinfection. Strengthening the HBV vaccination programme currently represents the most efficient route to reach the WHO goal of viral hepatitis elimination, and it will be meaningful to mandate vaccination not only for early childhood but also for adults in the high‐risk groups. Our data demonstrate the importance of HBV/HDV surveillance and prophylaxis in the special risk group of HIV+patients, and will support future prospective studies aimed at viral hepatitis elimination.
CONFLICTS OF INTEREST
Schmidbauer C. received travel support from Gilead, Abbvie, and Gebro and speaking honoraria from Abbvie. Chromy D. received payments for consulting from MSD, Abbvie, and Gilead as well as travel support from Abbvie and Gilead. Schmidbauer VU has nothing to disclose. Schwarz M. received travel support from MSD, Sandoz and BMS; and speaking honoraria from BMS. Jachs M. has nothing to disclose. Bauer D. received travel support from Gilead and Abbvie and speaker fees from Abbvie. Binter T., Apata M. and Nguyen D. have nothing to disclose. Mandorfer M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Collective Acumen, Gilead, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb, and Gilead. Simbrunner B. received travel support from AbbVie and Gilead. Rieger A., Mayer F., Breuer M., Strassl R. Schmidt R. and Holzmann H. have nothing to disclose. Trauner M. served as speaker for BMS, Falk Foundation, Gilead, Intercept and MSD; advisory boards for Albireo, BiomX, Falk Pharma GmbH, Genfit, Gilead, Intercept, Jannsen, MSD, Novartis, Phenex and Regulus. He further received travel grants from Abbvie, Falk, Gilead and Intercept and research grants from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda. He is also co‐inventor of patents on the medical use of norUDCA. Gschwantler M. received grant support from Abbvie, Gilead and MSD; speaking honoraria from Abbvie, Gilead, Intercept, and MSD; consulting/advisory board fees from Abbvie, Gilead, Intercept and MSD; and travel support from Abbvie and Gilead. Reiberger T received grant support from Abbvie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche.
ETHICS STATEMENT
The study was approved by the ethics committee of the Medical University of Vienna on July 7, 2017 (EC Number: 1527/2017).
PATIENT CONSENT STATEMENT
We performed a retrospective data analysis that was approved by the local ethics committee (see above). No study‐related interventions were performed and all data were analysed anonymously. Therefore, the ethics committee waived the necessity of obtaining patient consent.
Supporting information
Table S1
Data S1
ACKNOWLEDGEMENTS
Nothing to disclose.
Schmidbauer C, Chromy D, Schmidbauer VU, et al. Epidemiological trends of HBV and HDV coinfection among Viennese HIV+ patients. Liver Int. 2021;41:2622–2634. 10.1111/liv.15018
Handling Editor: Junko Tanaka
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV‐hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS Lond Engl. 2017;31(15):2035‐2052. 10.1097/QAD.0000000000001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steffen G, Sperle I, Leendertz SA, et al. The epidemiology of hepatitis B, C and D in Germany: a scoping review. PLoS One. 2020;15(3):e0229166. 10.1371/journal.pone.0229166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Mendoza C. UNAIDS update global HIV numbers. AIDS Rev. 2019;21:170‐171. [PubMed] [Google Scholar]
- 4. Leierer G, Rappold M, Strickner S, Zangerle R. 35th report of the Austrian HIV cohort study. 2018. [Google Scholar]
- 5. Schmidbauer C, Chromy D, Schmidbauer V, et al. Epidemiological trends in HCV transmission and prevalence in the Viennese HIV+ population. Liver Int Off J Int Assoc Study Liver. Published online February 4, 2020. doi: 10.1111/liv.14399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chromy D, Schmidt R, Mandorfer M, et al. HCV‐RNA is commonly detectable in rectal and nasal fluids of patients with high viremia. Clin Infect Dis Off Publ Infect Dis Soc Am. Published online September 28, 2019. doi: 10.1093/cid/ciz948 [DOI] [PubMed] [Google Scholar]
- 7. Popping S, Bade D, Boucher C, et al. The global campaign to eliminate HBV and HCV infection: international viral hepatitis elimination meeting and core indicators for development towards the 2030 elimination goals. J Virus Erad. 2019;5(1):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM. High value care task force of the American College of Physicians and the centers for disease control and prevention. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the centers for disease control and prevention. Ann Intern Med. 2017;167(11):794‐804. 10.7326/M17-1106 [DOI] [PubMed] [Google Scholar]
- 9. Mandorfer M, Simbrunner B. Prevention of first decompensation in advanced chronic liver disease. Clin Liver Dis. Published online 2020. [DOI] [PubMed] [Google Scholar]
- 10. Heuft MM, Houba SM, van den Berk GEL, et al. Protective effect of hepatitis B virus‐active antiretroviral therapy against primary hepatitis B virus infection. AIDS Lond Engl. 2014;28(7):999‐1005. 10.1097/QAD.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 11. Krause A, Haberkorn U, Mier W. Strategies for the treatment of HBV/HDV. Eur J Pharmacol. 2018;833:379‐391. 10.1016/j.ejphar.2018.06.030 [DOI] [PubMed] [Google Scholar]
- 12. Soriano V, Sherman KE, Barreiro P. Hepatitis Delta and HIV Infection. AIDS Lond Engl. Published online January 24, 2017. doi: 10.1097/QAD.0000000000001424 [DOI] [PubMed] [Google Scholar]
- 13. Botelho‐Souza LF, Vasconcelos MPA, dos Santos ADO, Salcedo JMV, Vieira DS. Hepatitis delta: virological and clinical aspects. Virol J. 2017;14(1):177. 10.1186/s12985-017-0845-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipp A. Der österreichische Mutterkindpass als Präventivtool für Kinder. Beteiligungsraten, Stichprobenanalysen und Gedanken zu einer möglichen Kosten‐Nutzen‐Evaluierung. Published online June 17, 2013.
- 15. EACS . European AIDS clinical society guidelines 2019. In: European AIDS Clinical Society (EACS). 2019. http://www.eacsociety.org
- 16. Bundesministerium Soziales, Gesundheit, Pflege und Konsumentenschutz . Impfplan Österreich 2021. Published online Jänner 2021.
- 17. Schmidbauer C, Schubert R, Schütz A, et al. Directly observed therapy for HCV with glecaprevir/pibrentasvir alongside opioid substitution in people who inject drugs‐first real world data from Austria. PLoS One. 2020;15(3):e0229239. 10.1371/journal.pone.0229239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKee G, Butt ZA, Wong S, et al. Syndemic characterization of HCV, HBV, and HIV co‐infections in a large population based cohort study. EClinicalMedicine. 2018;4–5:99‐108. 10.1016/j.eclinm.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohseni‐Zadeh M, Rey D, Batard M‐L, et al. Inadequate vaccination coverage in a French cohort of HIV positive patients. Med Mal Infect. 2010;40(12):683‐690. 10.1016/j.medmal.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 20. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461‐511. 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 21. Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Human immunodeficiency virus and hepatotropic viruses co‐morbidities as the inducers of liver injury progression. World J Gastroenterol. 2019;25(4):398‐410. 10.3748/wjg.v25.i4.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 23. Béguelin C, Moradpour D, Sahli R, et al. Hepatitis delta‐associated mortality in HIV/HBV‐coinfected patients. J Hepatol. 2017;66(2):297‐303. 10.1016/j.jhep.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 24. Saco TV, Strauss AT, Ledford DK. Hepatitis B vaccine nonresponders. Ann Allergy Asthma Immunol. 2018;121(3):320‐327. 10.1016/j.anai.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 25. Huang Y‐C, Hsieh S‐M, Sheng W‐H, et al. Serological responses to revaccination against HBV in HIV‐positive patients born in the era of nationwide neonatal HBV vaccination. Liver Int Off J Int Assoc Study Liver. Published online February 15, 2018. doi: 10.1111/liv.13721 [DOI] [PubMed] [Google Scholar]
- 26. Neifer S, Molz B, Sucker U, Kreuzpaintner E, Weinberger K, Jilg W. High percentage of isolated anti‐HBc‐positive persons among prisoners. Gesundheitswesen Bundesverb Arzte Offentlichen Gesundheitsdienstes Ger. 1997;59(6):409‐412. [PubMed] [Google Scholar]
- 27. Weber B, Melchior W, Gehrke R, Doerr HW, Berger A, Rabenau H. Hepatitis B virus markers in anti‐HBc only positive individuals. J Med Virol. 2001;64(3):312‐319. 10.1002/jmv.1052 [DOI] [PubMed] [Google Scholar]
- 28. Chang JJ, Mohtashemi N, Bhattacharya D. Significance and management of isolated hepatitis B core antibody (Anti‐HBc) in HIV and HCV: strategies in the DAA Era. Curr HIV/AIDS Rep. 2018;15(2):172‐181. 10.1007/s11904-018-0379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Q, Klenerman P, Semmo N. Significance of anti‐HBc alone serological status in clinical practice. Lancet Gastroenterol Hepatol. 2017;2(2):123‐134. 10.1016/S2468-1253(16)30076-0 [DOI] [PubMed] [Google Scholar]
- 30. Azadmanesh K, Mohraz M, Aghakhani A, et al. Occult hepatitis B virus infection in HIV‐infected patients with isolated hepatitis B core antibody. Intervirology. 2008;51(4):270‐274. 10.1159/000160217 [DOI] [PubMed] [Google Scholar]
- 31. Miao Z, Zhang S, Ou X, et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. Published online November 28, 2019. doi: 10.1093/infdis/jiz633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol. 2016;65(3):490‐498. 10.1016/j.jhep.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 33. Loglio A, Ferenci P, Uceda Renteria SC, et al. Excellent safety and effectiveness of high‐dose myrcludex‐B monotherapy administered for 48 weeks in HDV‐related compensated cirrhosis: a case report of 3 patients. J Hepatol. 2019;71(4):834‐839. 10.1016/j.jhep.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 34. Rizzetto M, Niro GA. Myrcludex B, a novel therapy for chronic hepatitis D? J Hepatol. 2016;65(3):465‐466. 10.1016/j.jhep.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 35. European Medicines Agency. Hepcludex: EPAR‐Public Assessment Report . Published online 2020. https://www.ema.europa.eu/en/documents/assessment‐report/hepcludex‐epar‐public‐assessment‐report_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


