Abstract
Intramuscular testosterone undecanoate is indicated as testosterone replacement in adult males with a deficiency in or absence of endogenous testosterone (hypogonadism). Intramuscular testosterone undecanoate 750 mg is approved to be administered at initiation and at 4 weeks, followed by a maintenance dose every 10 weeks. However, a more frequent maintenance regimen may improve symptom management of low testosterone at the end of each dosing interval. The current objective was to develop a population pharmacokinetic (PK) model for intramuscular testosterone undecanoate 750 mg and to perform PK simulations to assess the impact of an 8‐week maintenance regimen on testosterone exposure. A 1‐compartment model with first‐order absorption and first‐order elimination best described the PK of testosterone undecanoate. The model included time‐dependent suppression and gradual recovery of endogenous testosterone production during testosterone undecanoate administration. Significant covariates included body weight and sex hormone–binding globulin level. With the final PK model, simulations were performed to evaluate the impact of an 8‐week vs a 10‐week maintenance regimen on testosterone exposure. The 8‐week testosterone undecanoate regimen had a predicted 11% increase in average concentration and last observed concentration during a dosing interval before a subsequent dose and a 5% increase in maximum concentration. This translated into an ≈10% increase in the percentage of patients predicted to have a last observed concentration during a dosing interval before a subsequent dose >300 ng/dL, minimal change in the percentage of patients with average concentration in the normal range, and a low likelihood of maximum concentration >2500 ng/dL. These simulations suggest that more frequent administration of intramuscular testosterone undecanoate may be beneficial in some patients. Further clinical evaluation of an 8‐week dose regimen is warranted.
Keywords: hypogonadism, modeling, population pharmacokinetics, simulation, testosterone, undecanoate
Male hypogonadism, an impairment of testosterone production or other physiological activity of the gonads, can be due to a primary testicular disorder (primary hypogonadism) or secondary to dysfunction of the hypothalamic‐pituitary‐testicular axis (hypogonadotropic hypogonadism). 1 Both primary and secondary hypogonadism are characterized by low levels of serum testosterone (eg, total testosterone <300 ng/dL), with clinical signs and symptoms such as impairment of spermatogenesis, low libido, increased adiposity, and low bone mineral density. 1 , 2 , 3 Guidelines recommend the use of testosterone therapy in men with hypogonadism to manage symptoms of testosterone deficiency and maintain secondary sex characteristics. 1
There are several testosterone formulations available, including oral tablets, topical creams and gels, dissolvable subcutaneous implants, and short‐ and long‐acting injections. The long‐acting injectable formulation of testosterone undecanoate (17β‐undecanoyloxy‐4‐androsten‐3‐one) is an ester prodrug formed by acylation of testosterone with undecanoic acid, an 11‐carbon straight chain fatty acid side chain. When administered intramuscularly (IM), testosterone undecanoate provides a longer effective half‐life, thereby allowing less frequent administration than required for short‐acting testosterone injections. 1 , 4 A formulation of testosterone undecanoate (Nebido 1000 mg/4 mL, solution for injection; Bayer Pharma AG, Berlin, Germany) was first approved in the European Union (EU) in 2003 for the treatment of male hypogonadism, with a dosing regimen of 1000 mg administered every 10 to 14 weeks. In 2014, IM testosterone undecanoate (Aveed; Endo Pharmaceuticals Inc., Malvern, Pennsylvania) was approved by the US Food and Drug Administration (FDA) as a testosterone replacement therapy in adult men with primary hypogonadism or hypogonadotropic hypogonadism (congenital or acquired). 5 The FDA‐approved dosing regimen for testosterone undecanoate differs from the EU formulation, with a 750‐mg dose administered IM at initiation of therapy, at 4 weeks, and every 10 weeks thereafter as maintenance therapy. Data from an open‐label study of 117 men with hypogonadism showed that this testosterone undecanoate dosing regimen maintained mean testosterone levels in the normal physiologic range (300‐1000 ng/dL) for 94% of patients, with only 5.1% of patients having a mean testosterone concentration during a dosing interval (Cavg) <300 ng/dL, and 0.9% of patients having a Cavg >1000 ng/dL. 5 , 6 In some patients, breakthrough symptoms of low testosterone levels have been reported at the end of each testosterone undecanoate 10‐week dosing interval. 7 This suggests that the optimal maintenance dosing frequency of testosterone undecanoate administered IM may be <10 weeks for some patients. A shorter dosing maintenance interval could provide a higher trough testosterone concentration and potentially reduce the incidence of breakthrough symptoms.
To explore the potential dosing flexibility of testosterone undecanoate and to gain further insight into the impact of a shorter maintenance dosing interval on the pharmacokinetics (PK) of testosterone undecanoate, a population PK model was developed to assess the impact of an alternative testosterone undecanoate dosing regimen (750 mg at start of therapy, at 4 weeks, and then every 8 weeks as maintenance) on PK parameters of testosterone exposure and the percentage of patients achieving testosterone concentrations within the normal physiologic range.
Methods
Study Design and Data Set
The population PK model was developed using data from a phase 3, single‐arm, open‐label study that has been previously described (Study IP157‐001, Part C of a 5‐part study; ClinicalTrials.gov identifier: NCT00467870). 6 , 8 The study protocol was approved by a central institutional review board (Schulman Associates, Cincinnati, Ohio), and followed Good Clinical Practice and principles outlined in the Declaration of Helsinki. All patients provided written informed consent.
This multicenter study was conducted at the following locations: Alpha Clinical Research, LLC (Clarksville, Tennessee); Anne Arundel Urology (Annapolis, Maryland); California Professional Research (Newport Beach, California); Carolinas Research (Charlotte, North Carolina); Center for Urologic Research of Western New York (Williamsville, New York); Connecticut Clinical Research Center (Middlebury, Connecticut); Diabetes & Glandular Disease Research Associates (San Antonio, Texas); HOPE Research Institute (Phoenix, Arizona); Hudson Valley Urology 1 (Poughkeepsie, New York); Institute of Advanced Urology (Los Angeles, California), Medical and Clinical Research Associates, LLC (Bay Shore, New York), Medical Research Associates of Nashville (Nashville, Tennessee); Mobley Research (Houston, Texas); Myron Murdock, MD (Greenbelt, Maryland); Northeast Indiana Research LLC (Fort Wayne, Indiana); Regional Urology (Shreveport, Louisiana); South Florida Medical Research (Aventura, Florida); Swansea Family Practice (Swansea, Massachusetts); Tampa Bay Urology (Tampa, Florida); Texas Urology (Carrollton, Texas); the Clinical Trials Unit, Johns Hopkins University (Baltimore, Maryland); University of Oklahoma Medical Center (Edmond, Oklahoma); University Urology Associates (New York, New York); Urologic Surgery, PC (Bala Cynwyd, Pennsylvania); Urological Sciences Research Foundation (Culver City, California); Urological Surgeons of Long Island (Garden City, New York); Urology Associates of North Texas–Research Department (Arlington, Texas); Urology Centers of Alabama, PC (Homewood, Alabama); Urology Consultants (San Antonio, Texas); Urology Research (Aurora, Colorado); and Western New York Urology Associates (Orchard Park, New York).
Men with hypogonadism received testosterone undecanoate 750 mg in 3 mL of castor oil and benzyl benzoate (250 mg/mL) injected IM into the buttocks at baseline, at 4 weeks, and then every 10 weeks for up to 84 weeks. For patients who crossed over to another part of the study (Part D) and received subcutaneous dosing of testosterone undecanoate, data for time points following the first subcutaneous dose were excluded from the analysis. The original study PK population was defined as patients weighing ≥65 kg, without other testosterone therapy use, and with ≥4 serum total testosterone concentration values during the third injection interval.
Blood samples were collected in the morning before injection 3 (week 14), and on days 4, 7, 11, 14, 21, 28, 42, and 56, and on day 70 before injection 4 (week 24; day 0 of injection 4). For injection 4, blood samples were collected on days 4, 7, 11, and 42 after injection 4, and before injection 5 (week 34). As published previously, serum testosterone concentrations were measured using validated methods that involved liquid chromatography with tandem mass spectrometry (lower limit of quantification, 20.0 ng/dL). 8
Population PK Modeling
A structural model was developed using a stepwise approach by evaluating 1‐ and 2‐compartment models with features added sequentially (eg, compartments, variance terms, covariates). Interindividual variability in model parameters was evaluated and was assumed to follow a log‐normal distribution. Additive, proportional, and additive and proportional combined residual error models were evaluated. Following development of the structural model, covariates were evaluated using a forward addition and backward elimination approach. During the forward addition approach, covariates were added to PK parameters 1 covariate at a time. A minimum decrease in the objective function value (OFV) of ≥3.841 (P ≤ .05) and/or improvement in diagnostic plots were required for covariate retention. At each step, the most significant covariate was retained. Stepwise addition was repeated until there were no additional significant parameter/covariate combinations to be added to the model. Subsequently, the backward elimination procedure was implemented with each covariate removed from the model 1 at a time. The covariate contributing the least to the fit (smallest increase in OFV upon elimination) was removed, and the model was rerun. A minimum increase of 6.635 in the OFV (P ≤ .01) was needed for retention of covariates in all backward elimination steps with 1 eliminated parameter. The stepwise elimination of covariates was repeated (maintaining removal of previous covariates) until there were no additional parameter/covariate combinations to be removed from the model.
Baseline demographics of age, albumin level, body weight, body mass index (BMI), race, and sex hormone–binding globulin (SHBG) levels were evaluated for inclusion as covariates on clearance (CL/F) and volume of distribution (V/F); body weight and BMI were also evaluated on rate and extent of absorption. Relationships for continuous covariates were modeled according to a power model with centering by the population median of the respective covariate. Categorical covariates were modeled using a proportional model structure.
Models were evaluated at each stage of development based on diagnostic plots, parameter precision, and physiologic plausibility of parameter estimates. The precision of final parameter estimates was reported as the relative standard error based on nonlinear mixed‐effects modeling (NONMEM) output. In addition, central tendency and precision of parameter estimates were assessed by nonparametric bootstrap. For the bootstrap analysis, 2000 replicate data sets were generated with a patient as the sampling unit. Each replicate data set was fitted to the final model to obtain parameter estimates. Median parameter estimates and empirical confidence intervals (2.5th and 97.5th percentiles) were calculated across bootstrap fits. Parameter estimates from the final model were compared with bootstrap estimates as a further evaluation of model performance. A visual predictive check was performed to assess the ability of the final model to adequately characterize central tendency and variability of observed testosterone data. Using the final structural model, 1000 simulated data sets were generated, and observed data were overlaid with the median and 95% prediction interval of simulated testosterone concentrations.
Simulation of Dose Regimens
The final model was used to perform simulations to assess the alternative dosing regimen (a dose at baseline, a dose 4 weeks later, and then a dose every 8 weeks) compared with the currently approved dosing regimen (a dose at baseline, a dose 4 weeks later, and then a dose every 10 weeks). A total of 500 studies were simulated for the 10‐week and 8‐week testosterone undecanoate dosing regimens. Testosterone concentrations were simulated every 48 hours following doses 1, 2, 3, 4, and 10. Each of the 500 study simulations included 117 patients with demographics identical to those of patients in the original study (Study IP157‐001, Part C). Significant covariate effects, interindividual variability for PK parameters, and residual variability were included in the simulations.
Software
Population PK analyses were performed using the NONMEM software version 7.3 (ICON Development Solutions, Gaithersburg, Maryland) using the first‐order conditional estimation method with interaction. Bootstrapping and visual predictive checks were conducted using Perl‐speaks‐NONMEM version 4.6.0. 9 Data formatting, creation of simulation data sets, and postprocessing of simulations were performed using R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Graphical analyses and diagnostic plots to support model development were generated using R package Xpose 4 version 4.5.3 or Xpose version 0.4.3. 10
Results
Population PK Model
Development of the population PK model included data from 130 men with hypogonadism enrolled in the original study, who had 2360 total testosterone concentration measurements (Table 1). 8 A total of 117 of the 130 patients enrolled in the original study were included in the PK population (ie, original study PK population; Table 1), which was matched in size and demographics for the 500 study simulations as part of the final model validation. The final population PK model was a 1‐compartment model with first‐order elimination and first‐order absorption of testosterone (Figure 1). During model development, conditional weighted residuals vs time plots indicated a time‐dependent bias in testosterone exposure that was not adequately explained by repeat‐dose accumulation of testosterone. Previous research found that administration of exogenous testosterone is associated with an initial suppression of endogenous testosterone production, followed by a time‐dependent recovery. 11 Time‐dependence of testosterone exposure was therefore accounted for with an acute suppression and gradual recovery of the apparent zero‐order production rate of testosterone (R):
Parameters Rb and Rss represent apparent baseline (predose) and steady‐state testosterone production rate, respectively, whereas K1 and K2 represent first‐order rate constants for acute suppression and gradual recovery of endogenous testosterone production, respectively.
Table 1.
Demographicsand Baseline Characteristics for Enrolled and PK Populations
| Enrolled Population | PK Population | |
|---|---|---|
| Parameter | (n = 130) a | (n = 117) |
| Age, y, mean (SD) | 54.2 (10.2) | 54.6 (10.0) |
| Range | 24‐75 | 30‐75 |
| Race, n (%) | ||
| White | 97 (74.6) | 87 (74.4) |
| Black | 16 (12.3) | 14 (12.0) |
| Other | 17 (13.1) | 16 (13.7) |
| Weight, kg, mean (SD) | 101.2 (18.0) | 101.6 (17.4) |
| BMI, kg/m2, mean (SD) | 32.0 (5.4) | 32.0 (5.2) |
| Albumin level, g/dL, mean (SD) b | 4.2 (0.3) | 4.2 (0.3) |
| SHBG, nmol/L, mean (SD) c | 20.9 (8.9) | 20.8 (8.5) |
BMI, body mass index; PK, pharmacokinetic; SHBG, sex hormone–binding globulin.
Some data reported in Morgentaler et al. 6
Potentially clinically significant laboratory value for albumin was defined in original study protocol as ≤2.5 g/dL. Normal range is typically 3.5 to 5.5 g/dL.
Normal range is typically 16.5−55.9 nmol/L in men aged 20 to 49 years and 19.3−76.4 nmol/L in men aged >49 years.
Figure 1.
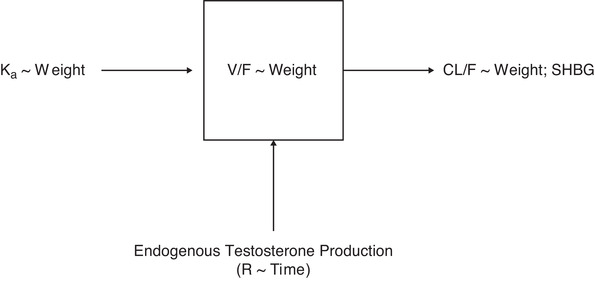
Final model structure for testosterone undecanoate intramuscular injection. CL/F, apparent clearance; Ka, first‐order absorption rate constant; R, zero‐order production rate; SHBG, sex hormone–binding globulin; V/F, apparent volume of distribution; ∼ indicates “is a function of.”
Interindividual variability was included on CL/F and first‐order absorption rate constant (Ka) according to an exponential error model. The model did not support estimation of interindividual variability on V/F. Residual variability was characterized with an additive and proportional combined residual error model. Patient weight and SHBG levels were significant covariates in the final model. A decrease in SHBG levels was associated with an increase in CL/F; a decrease in weight was associated with a decrease in CL/F and V/F, but an increase in Ka.
The NONMEM relative standard error estimates for all parameters in the final population PK model were <18%, indicating that parameters were estimated with good precision (Table 2). In addition, parameter estimates from the NONMEM output were in close agreement with estimates obtained from the bootstrap procedure (Table 2), and the visual predictive checks for the final population PK model showed that predictions were consistent with observed data (Figure 2). In the original study, a co‐primary end point was evaluated to establish treatment success and was based on PK assessments. 6 Achieving treatment success was based on the percentage of patients achieving a 10‐week Cavg and maximum observed testosterone concentration (Cmax) during a dosing interval within prespecified ranges following testosterone undecanoate injection/dose 3. 6 The required percentage of patients with Cavg in normal range was ≥65%, with a point estimate of ≥75%. 6 A Cmax of 2500 ng/dL was considered the maximum testosterone undecanoate concentration within acceptable limits. The model‐predicted percentages of patients meeting prespecified ranges were compared with observed data across PK parameters and indicated that the final PK model was capable of reproducing exposure trends and associated variability consistent with observed data (Table 3).
Table 2.
Parameter Estimates for Final Testosterone PK Model
| Characteristics | NONMEM, Estimate (% RSE) | Bootstrap, a Median (95%CI) |
|---|---|---|
| PK parameters b | ||
| CL/F, L/h | 197 (6.9) | 198 (174 to 227) |
| V/F, L | 12 300 (13.7) | 12 400 (9620 to 16 500) |
| Ka, L/h | 0.001 (8.6) | 0.001 (0.001 to 0.002) |
| Rb, mg/h | 0.445 (8.5) | 0.446 (0.378 to 0.531) |
| Rss, mg/h | 0.572 (9.6) | 0.575 (0.483 to 0.697) |
| K1, L/h | 10 FIXED | 10 FIXED |
| K2, L/h | 0.000727 (15.9) | 0.000733 (0.000535 to 0.000982) |
| Weight, CL/F (power) | 0.75 FIXED | 0.75 FIXED |
| SHBG, CL/F (power) | –0.219 (17.9) | –0.219 (–0.299 to –0.144) |
| Weight, Ka (power) | –1.83 (12.0) | –1.83 (–2.27 to –1.38) |
| Weight, V/F (power) | 1.0 FIXED | 1.0 FIXED |
| Interindividual variability CL/F, % CV | 20.0 (10.0) c | 19.7 (16.4 to 23.9) |
| Interindividual variability Ka, % CV | 47.1 (9.4) c | 46.5 (38.6 to 56.8) |
| Proportional residual error, % | 18.8 (6.5) | 18.7 (16.4 to 21.1) |
| Additive residual error SE, ng/dL | 56.8 (9.3) | 56.7 (45.6 to 66.5) |
CI, confidence interval; CL/F, clearance; CV, coefficient of variation; Ka, first‐order absorption rate constant; K1, first‐order rate constant for suppression of endogenous testosterone production; K2, first‐order rate constant for recovery of endogenous testosterone production; NONMEM, nonlinear mixed‐effects modeling; PK, pharmacokinetic; Rb, apparent baseline endogenous testosterone production rate; RSE, relative standard error; Rss, apparent steady‐state endogenous testosterone production rate; SHBG, sex hormone–binding globulin; V/F, apparent volume of distribution.
Of 2000 bootstrap replicates, 5 runs with minimization terminated were skipped when calculating the bootstrap results.
Assuming weight of 101 kg and SHBG concentration of 20 nmol/L.
The relative standard errors for interindividual variability estimates are reported on the approximate standard deviation scale (SE/variance estimate)/2.
Figure 2.
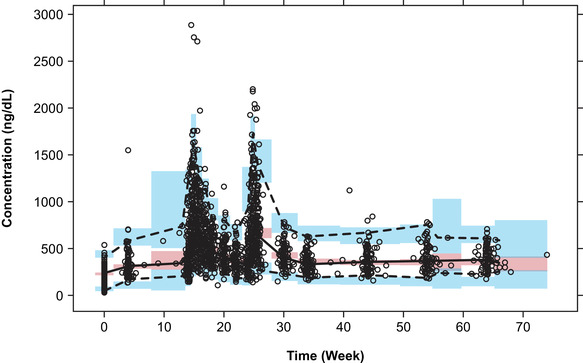
Prediction‐corrected visual predictive check for total testosterone conducting 1000 simulations with the final pharmacokinetic model. Circles, observed individual data; dashed and solid black lines: 2.5th, 50th, and 97.5th percentiles of the observed data; blue and red shaded areas, median and 95% prediction interval of simulated data.
Table 3.
Comparison of Simulated and Observed Percentage of Patients Within Prespecified Ranges for Testosterone Undecanoate Injection/Dose 3
| Patients Within Range | |||
|---|---|---|---|
| Parameter | Concentration Range, ng/dL | Simulated, % (95%PI) a | Observed, b % (95%CI) |
| Cavg | 300‐1000 | 94.0 (89.7‐98.3) | 94.0 (89.7‐98.3) |
| <300 | 6.0 (1.7‐10.3) | 5.1 (1.1‐9.1) | |
| >1000 | 0.0 (0.0‐0.9) | 0.9 (0.0‐2.5) | |
| Cmax | <1500 | 94.9 (90.6‐98.3) | 92.3 (87.5‐97.1) |
| 1800‐2500 | 1.7 (0.0‐4.3) | 0.0 | |
| >2500 | 0.0 (0.0‐0.9) | 0.0 | |
| Ctrough | <300 | 38.5 (29.5‐47.0) | 44.8 (31.3‐58.3) |
Cavg, average testosterone concentration during a dosing interval; Cmax, maximum observed testosterone concentration during a dosing interval; Ctrough, last observed testosterone concentration during a dosing interval prior to a subsequent dose; CI, confidence interval; PI, prediction interval.
Percent‐within‐range values were calculated for each simulated study and were reported as the median percentage across 500 simulations.
Observed results from original study.
Simulations
A total of 500 studies were simulated, with each study matching the size and demographics of the original study (n = 117). Results from the simulations for testosterone undecanoate injection/doses 1, 2, 3, and 4 for the 10‐week and 8‐week regimens are presented in Table S1. The PK model predicted that testosterone exposures would be increased for the 8‐week testosterone undecanoate regimen compared with the 10‐week testosterone undecanoate regimen (Table 4). Both Cavg and the last observed testosterone concentration during a dosing interval before a subsequent dose (Ctrough) would be increased by 11% for the 8‐week regimen, whereas Cmax would be increased to a lesser extent (5%). Despite the trend for increased exposure with the 8‐week testosterone undecanoate regimen, the 95% confidence intervals for Cmax and Ctrough ratios included unity, whereas the lower bound of the 95% confidence interval for Cavg was greater than unity (Table 4).
Table 4.
Comparison of Simulated Exposures for the 8‐Week and 10‐Week Regimens Following Testosterone Undecanoate Injection/Dose 10
| Median (95%CI) | |||
|---|---|---|---|
| Parameter (ng/dL) | 8‐Week Regimen | 10‐Week Regimen | Ratio |
| Cmax | 978.2 (923.3‐1037.1) | 934.2 (880.9‐998.0) | 1.05 (0.96‐1.14) |
| Cavg | 564.5 (540.5‐595.4) | 510.1 (485.5‐535.1) | 1.11 (1.04‐1.19) |
| Ctrough | 391.3 (362.6‐421.1) | 351.9 (325.8‐379.6) | 1.11 (0.99‐1.24) |
Cavg, average testosterone concentration during a dosing interval; Cmax, maximum observed testosterone concentration during a dosing interval; Ctrough, last observed testosterone concentration during a dosing interval prior to a subsequent dose.
The estimates of the higher percentage of patients achieving testosterone exposures within prespecified ranges for testosterone undecanoate injection/dose 10 were consistent with a relative increase in testosterone exposures for the 8‐week regimen compared with the 10‐week regimen (Table 5). The estimated percentage of patients with Cavg in the 300 to 1000 ng/dL range increased only slightly, from 97.4% with the 10‐week regimen to 98.3% for the 8‐week regimen. Similar trends were observed for Cmax; however, importantly, the 8‐week regimen was not associated with an increase in the percentage of patients with Cmax >2500 ng/dL. The 8‐week regimen had the greatest predicted impact on Ctrough. The percentage of patients with Ctrough >300 ng/dL increased from 66.7% with the 10‐week regimen to 76.1% with the 8‐week regimen.
Table 5.
Percentage of Patients Predicted to Achieve Prespecified Exposure Ranges for the Testosterone Undecanoate 8‐Week and 10‐Week Regimens (Injection/Dose 10)
| Patients, % (95%PI) | |||
|---|---|---|---|
| Parameter | Concentration Range, ng/dL | 10‐Week Dosing | 8‐Week Dosing |
| Cavg | 300‐1000 | 97.4 (94.0‐100) | 98.3 (94.9‐100) |
| <300 | 1.7 (0.0‐5.1) | 0.9 (0.0‐2.6) | |
| >1000 | 0.0 (0.0‐1.7) | 0.9 (0.0‐3.4) | |
| Cmax | 1300‐1500 | 8.5 (4.3‐12.8) | 9.4 (5.1‐14.5) |
| 1800‐2500 | 3.0 (0.9‐6.8) | 3.4 (0.9‐6.8) | |
| <1500 | 90.6 (86.3‐94.9) | 88.9 (84.6‐94.0) | |
| <1300 | 82.1 (76.1‐88.0) | 79.5 (74.4‐85.5) | |
| >2500 | 0.0 (0.0‐1.7) | 0.0 (0.0‐1.7) | |
| Ctrough | >300 | 66.7 (59.0‐75.2) | 76.1 (67.5‐83.8) |
Cavg, average testosterone concentration during a dosing interval; Cmax, maximum observed testosterone concentration during a dosing interval; Ctrough, last observed testosterone concentration during a dosing interval prior to a subsequent dose; PI, prediction interval.
Discussion
Male hypogonadism can result in a myriad of clinical signs and symptoms, including decreased libido, increased adiposity, and low bone mineral density. 1 , 2 , 3 A goal of testosterone therapy in men with hypogonadism is to help normalize total testosterone levels and improve symptoms associated with androgen deficiency. 1 , 3 Data used to develop the current population PK model were part of a long‐term study to evaluate the safety and PK of testosterone undecanoate 750 mg administered IM in male patients with hypogonadism (aged 24‐75 years) with testosterone undecanoate administration at baseline, at 4 weeks, and then every 10 weeks for up to 84 weeks. 6 , 8 The currently approved 10‐week dose regimen of testosterone undecanoate maintains a Cavg of testosterone levels within the normal range for most patients (94% in the normal range following testosterone undecanoate injection/dose 3). 5 , 6 However, individual patient characteristics may negatively impact testosterone levels (eg, high BMI, low SHBG levels), 12 and trough testosterone levels may decline in some patients by the end of a testosterone undecanoate 10‐week dosing interval. This trough level may result in undesirable breakthrough clinical symptoms associated with low testosterone levels. 7 Thus, treatment with a higher testosterone dose (ie, 1000 mg [Nebido]) 13 or introduction of a testosterone undecanoate regimen with a shorter dosing interval (eg, 8 weeks vs 10 weeks) may be appropriate for some patients.
The objective of this population PK analysis was to assess the potential impact of a more frequent dose regimen of testosterone undecanoate (8 weeks vs 10 weeks) on exposure parameters of clinical interest. A more frequent dosing regimen was expected to provide additional accumulation and less washout of testosterone between doses, thus increasing the percentage of patients having trough testosterone concentrations within the normal range. The population PK model of testosterone undecanoate in men with hypogonadism was described by a 1‐compartment model with first‐order absorption into the central compartment and first‐order elimination from the central compartment. Administration of exogenous testosterone has been shown to suppress endogenous testosterone initially, but later enable gradual recovery of endogenous testosterone production. 11 Consistent with this phenomenon, the final PK model characterized apparent endogenous testosterone production as a time‐dependent zero‐order input into the central compartment, with an initial suppression following the first dose of testosterone and a gradual return of production over time.
During structural model development, the impact of BMI and body weight was evaluated on CL/F and V/F, and body weight was selected for inclusion in the model, consistent with standard allometric principles. 14 A small fraction of testosterone circulates unbound (≈2%) and is metabolically active, with the remaining circulating testosterone primarily bound to SHBG or albumin. 15 SHBG has a high affinity for testosterone, and increasing levels of SHBG can restrict the availability of circulating testosterone for hepatic extraction. 16 , 17 Consistent with its physiologic role, SHBG was found to be a significant covariate on CL/F in the current population PK model (ie, lower SHBG levels were associated with increased CL/F). Given that testosterone undecanoate is administered IM, it was hypothesized that the rate of absorption of the depot IM injection could be dependent on body weight (ie, muscle mass). During covariate analysis, weight was found to be a significant covariate on Ka, with lower body weight being associated with more rapid absorption.
Greater accumulation and higher overall testosterone exposures were predicted for the 8‐week maintenance regimen compared with the FDA‐approved 10‐week regimen. These findings are consistent with the long apparent elimination half‐life of testosterone undecanoate following IM injection and the anticipated accumulation associated with more frequent administration. 4 Approximately 10% more patients achieved trough testosterone concentrations in the normal range (>300 ng/dL) for the 8‐week regimen compared with the 10‐week regimen. Minimal differences were observed in the percentage of patients with Cavg in the normal range between the 2 dosing regimens. Importantly, there was no predicted increase in the percentage of patients with Cmax >2500 ng/dL. The simulations suggest that an 8‐week maintenance regimen of testosterone undecanoate may effectively elevate trough testosterone concentration into the normal range in some patients. As such, a more frequent dosing regimen may be beneficial in reducing hypogonadal symptoms at the end of the maintenance dosing interval.
Limitations include that Cmax and Ctrough were determined based on single observations per simulated patient, whereas Cavg was a composite parameter dependent on multiple observations. As such, it is likely that the apparent discrepancy in significance for these parameters is due to greater variability associated with Cmax and Ctrough estimates relative to Cavg. In addition, the clinical impact of the predicted Ctrough elevations cannot be inferred directly from the current analysis. A prospective study would be needed to assess individual PK variability among a real‐world population of males with hypogonadism and to evaluate the impact of an 8‐week maintenance regimen on clinical symptoms.
Conclusions
Predicted outcomes based on the population PK model indicated that, in men with hypogonadism, an 8‐week maintenance regimen with testosterone undecanoate 750 mg administered IM would increase the percentage of those with testosterone Ctrough concentrations >300 ng/dL, with minimal impact on Cmax, compared with a 10‐week maintenance regimen. Similar to a 10‐week maintenance regimen, there was no increase in calculated Cmax values >2500 ng/dL with the 8‐week regimen. This PK modeling study supports that more frequent administration of testosterone undecanoate may be beneficial for reducing fluctuations in serum testosterone levels and elevating testosterone concentrations at the end of each maintenance dosing interval while maintaining acceptable levels of overall testosterone exposure. Further clinical evaluation of a testosterone undecanoate 8‐week dosing interval as a maintenance regimen will be needed to assess the clinical relevance of these predictions.
Disclosures
M.B. and L.C. are employees of Nuventra, LLC, which conducted this analysis with funding from Endo Pharmaceuticals Inc. S.V., Q.X., and Y.H. are employees of Endo Pharmaceuticals Inc. T.P. is a former employee of and current consultant for Endo Pharmaceuticals Inc. A.W.P. is an advisor and speaker for Endo Pharmaceuticals Inc. and receives research and fellowship support from Endo Pharmaceuticals Inc. A.W.P. is a speaker for Bayer AG, founder/currently in a leadership role at Woven Health and in a leadership role at Vault Health.
Data Accessibility Statement
Data will not be made available.
Funding
Data analyses, conducted by Nuventra, LLC, and technical editorial and medical writing assistance, provided by Synchrony Medical Communications, LLC, were supported by Endo Pharmaceuticals Inc.
Supporting information
Supporting material
Acknowledgments
Technical editorial and medical writing assistance was provided, under the direction of the authors, by Anginelle Alabanza, Nuventra Pharma Sciences, and Mary Beth Moncrief, PhD, Synchrony Medical Communications, LLC, West Chester, Pennsylvania.
References
- 1. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715‐1744. [DOI] [PubMed] [Google Scholar]
- 2. Marcelli M, Mediwala SN. Male hypogonadism: a review. J Investig Med. 2020;68(2):335‐356. [DOI] [PubMed] [Google Scholar]
- 3. Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA Guideline. J Urol. 2018;200(2):423‐432. [DOI] [PubMed] [Google Scholar]
- 4. Zhang GY, Gu YQ, Wang XH, Cui YG, Bremner WJ. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl. 1998;19(6):761‐768. [PubMed] [Google Scholar]
- 5. AVEED® (testosterone undecanoate) injection, for intramuscular use CIII [package insert]. Malvern, PA: Endo Pharmaceuticals Inc.; 2018.. [Google Scholar]
- 6. Morgentaler A, Dobs AS, Kaufman JM, et al. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. J Urol. 2008;180(6):2307‐2313. [DOI] [PubMed] [Google Scholar]
- 7. Krakowsky Y, Conners W, Davidson E, Rawji A, Morgentaler A. Initial clinical experience with testosterone undecanoate therapy (AVEED) in men with testosterone deficiency in the United States. Urology. 2017;109:27‐31. [DOI] [PubMed] [Google Scholar]
- 8. Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long‐acting testosterone undecanoate injections in hypogonadal men: an 84‐week phase III clinical trial. J Androl. 2010;31(5):457‐465. [DOI] [PubMed] [Google Scholar]
- 9. Lindbom L, Pihlgren P, Jonsson EN. PsN‐Toolkit—a collection of computer intensive statistical methods for non‐linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241‐257. [DOI] [PubMed] [Google Scholar]
- 10. Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujioka M, Shinohara Y, Baba S, Irie M, Inoue K. Acute suppression of endogenous testosterone levels by exogenous testosterone in normal men. Life Sci. 1987;41(8):945‐949. [DOI] [PubMed] [Google Scholar]
- 12. Lamm S, Chidakel A, Bansal R. Obesity and hypogonadism. Urol Clin North Am. 2016;43(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 13. Nebido® (testosterone undecanoate) [EU essential information]. Berlin, Germany: Bayer AG; 2018. [Google Scholar]
- 14. Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 15. Shea JL, Wong PY, Chen Y. Free testosterone: clinical utility and important analytical aspects of measurement. Adv Clin Chem. 2014;63:59‐84. [DOI] [PubMed] [Google Scholar]
- 16. Baird DT, Horton R, Longcope C, Tait JF. Steroid dynamics under steady‐state conditions. Recent Prog Horm Res. 1969;25:611‐664. [DOI] [PubMed] [Google Scholar]
- 17. Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab. 2006;91(11):4669‐4675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Data Availability Statement
Data will not be made available.


